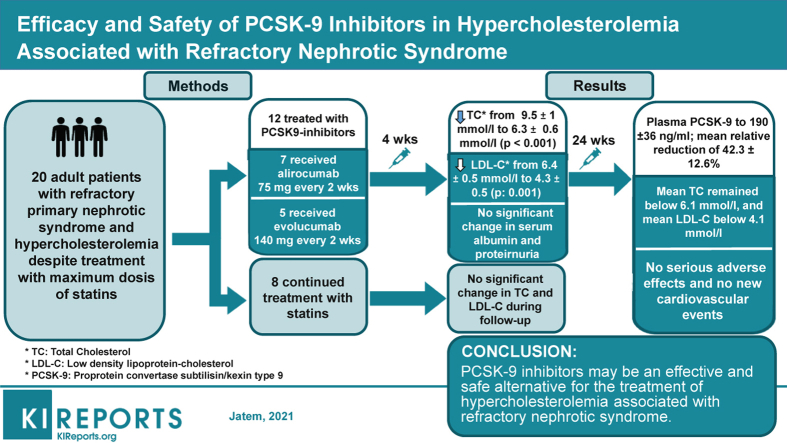
Abstract
Introduction
Treatment of hypercholesterolemia in refractory nephrotic syndrome remains a therapeutic challenge. There is not enough evidence supporting the efficacy of statins, and these drugs can be associated with an increased incidence of adverse effects. Herein we summarize our clinical experience with 12 patients suffering from refractory nephrotic syndrome with associated vascular disease and uncontrolled hypercholesterolemia despite treatment with statins who were treated with proprotein convertase subtilisin kexin 9 (PCSK9) inhibitors.
Methods
Twelve adult patients with primary nephrotic syndrome refractory to multiple lines of immunosuppressive treatment who suffered from clinical atheromatous vascular disease were treated with PCSK9 inhibitors according to the prescription guidelines for secondary prevention of cardiovascular events. Eight patients with refractory nephrotic syndrome without vascular disease treated with atorvastatin comprised the control group.
Results
Four weeks after treatment with PCSK9 inhibitors, a statistically significant decrease in total cholesterol and low-density lipoprotein cholesterol (LDL-C) levels was observed without significant changes in serum albumin levels or proteinuria. The mean LDL-C decrease was 36.8% ± 4.9% mmol/L at 4 weeks and remained unchanged throughout the follow-up period. In the control group, there were no significant changes in the levels of total cholesterol or LDL-C during the follow-up period. At the diagnosis of nephrotic syndrome, plasma PCSK9 levels were 334 ± 40 ng/mL and correlated significantly with serum LDL-C levels (r = 0.49, P = 0.023). Six months after starting treatment with PCSK9 inhibitors, plasma PCSK9 levels were significantly reduced to values of 190 ± 36 ng/mL (P = 0.001) with a mean relative reduction of 42.3% ± 12.6%. No local adverse effects were seen at the injection site and no significant changes were seen in the levels of transaminase, creatine phosphokinase, or aldolase.
Conclusion
PCSK9 inhibitors may be an effective and safe alternative for the treatment of hypercholesterolemia associated with refractory nephrotic syndrome.
Keywords: dyslipidemia, hypercholesterolemia, nephrotic syndrome, PSCK9 inhibitors
Graphical abstract
Dyslipemia, hypercholesterolemia, hypertriglyceridemia, and hypoalphalipoproteinemia are major risk factors for cardiovascular disease and are associated with higher mortality.1
Hypercholesterolemia is one of the metabolic consequences of nephrotic syndrome and follows a parallel course to the activity of kidney disease.2 While kidney disease responds to treatment, patients are usually exposed to high cholesterol levels for a short period of time. When patients with nephrotic syndrome do not respond to treatment, it usually causes progressive renal failure and a definitive loss of renal function requiring renal replacement therapy.3 In these cases, hypercholesterolemia is present through the whole clinical course of the disease, which usually lasts several years, and has been recognized as a cardiovascular risk factor.3 Controlling cholesterol levels in these patients is often difficult. Treatment consists of statins taken at the maximum tolerated doses. However, there are cases in which statins do not allow adequate control of cholesterol levels or cause muscle or liver toxicity, and in these cases it is necessary to consider other options.2 The current evidence indicates that hypercholesterolemia associated with nephrotic syndrome is caused by the combination of an increase in production and a reduction in the clearance of low-density lipoprotein cholesterol (LDL-C) and apolipoprotein B100.4, 5, 6 The underlying mechanism involves the positive regulation of 3-hydroxy-3-methyl-glutaryl coenzyme A reductase and the decrease in the elimination of LDL caused by proprotein convertase subtilisin kexin 9 (PCSK9)–mediated degradation of the LDL receptor.4 Conceptually, the positive regulation of PCSK9 and its central role in the increase in LDL cholesterol4,5 suggest that PCSK9 inhibitors may be effective in the treatment of patients with nephrotic-mediated hypercholesterolemia. However, clinical trials evaluating the efficacy of monoclonal antibodies against PCSK9 have not included patients with nephrotic syndrome. The available evidence supporting their potential benefits in this context is still limited.7, 8, 9
Herein we summarize our clinical experience with 12 patients with refractory nephrotic syndrome, associated clinical vascular disease, and uncontrolled hypercholesterolemia despite the association of statins who were treated with PCSK9 inhibitors.
Methods
Between April 2017 and May 2018, 12 patients with nephrotic syndrome refractory to immunosuppressive treatment recommended by current clinical guidelines, concomitant clinical atheromatous vascular disease, and LDL-C levels >150 mg/dl despite treatment with maximum doses of atorvastatin (80 mg/day) received treatment with PCSK9 inhibitors.
Nephrotic syndrome was defined as the presence of hypoalbuminemia <3.5 g/dl associated with 24-hour proteinuria ≥3.5 g/1.73 m2 and edema. Refractoriness to treatment was defined as the persistence of nephrotic syndrome after having exhausted all the treatment options recommended in the currently available guidelines.10
A history of vascular comorbidity, including previous episodes of ischemic stroke, ischemic heart disease, or lower limb arterial ischemia was obtained from the patients’ clinical records.
Patient Assessment Before the Start of Treatment with PCSK9 Inhibitors
In all patients, the results of total cholesterol, LDL-C, creatinine, and proteinuria values before the diagnosis of nephrotic syndrome were available. We recorded the evolution of the levels of total cholesterol and LDL-C, albumin, and proteinuria from the time of the diagnosis of nephrotic syndrome to the start of treatment with PCSK9 inhibitors. Before treatment with PCSK9 inhibitors, statin treatment compliance was assessed using the Morinsky–Green test.11
Patient Follow-Up After Starting Treatment with PCSK9 Inhibitors
Seven patients received treatment with alirocumab at a dose of 75 mg every 2 weeks and 5 with evolucumab at a dose of 140 mg every 2 weeks. Atorvastatin was withdrawn at the time treatment with PCSK9 inhibitors began. The minimum follow-up time after the start of treatment was 12 months and the maximum 32 months. Clinical and biochemical controls are reported monthly during the first 6 months, at the end of the first year, and at the last available follow-up. At each control, the levels of total cholesterol, LDL-C, albumin, proteinuria, creatine phosphokinase, muscle aldolase, PCSK9, aspartate transaminase, and alanine aminotransferase were measured.
The control group included 8 patients with refractory nephrotic syndrome, defined with the same criteria stated above, for whom treatment with PCSK9 inhibitors was not authorized because they lacked a history of clinical vascular disease and who continued treatment with atorvastatin at doses of 80 mg/day. In this group of patients, the minimum follow-up after diagnosis was 21 months and the maximum 38 months. Most of the patients in this group were young and relatively healthy at the time of diagnosis of nephrotic syndrome, and cholesterol, serum albumin, and 24-hour proteinuria measurements before this period were not available.
Laboratory Methods
Serum creatinine was measured by the isotope dilution mass spectrometry–traceable compensated method (Hitachi Modular P-800; Roche Diagnostics, Mannheim, Germany). The estimated glomerular filtration rate was calculated using the Chronic Kidney Disease Epidemiology Collaboration equation.12 For lipid measurement, blood was collected from the antecubital vein in glass tubes with no additives after an overnight fast of 12 hours. High-density lipoprotein cholesterol and LDL-C were isolated by sequential ultracentrifugation. In the 2 lipoprotein subfractions, cholesterol was determined using enzymatic methods (CHOD-PAP; Boehringer Mannheim, Mannheim, Germany). Albumin concentration was determined using the green bromocresol method. Plasma PCSK9 concentrations were quantified using plasma samples obtained at the time of diagnosis of nephrotic syndrome and 6 months after starting treatment with PCSK9 inhibitors, using sandwich enzyme-linked immunosorbent assays according to the manufacturer’s instructions (R&D Systems, Minneapolis, MN, USA).
Ethics Approval
The prescription of PCSK9 inhibitors was carried out according to their authorized therapeutic indications. The study was approved by Hospital Universitari Vall d′Hebron ethics committee.
Statistics
Results are expressed as mean and standard deviations for normally distributed variables or median and quartiles for non-normally distributed variables. Qualitative variables are represented as percentages. Quantitative changes in biochemical variables over time were calculated by the Friedman analysis for repeated measures.
Results
Table 1 summarizes the clinical and the demographic characteristics of patients and the biochemical data corresponding to the last control before starting treatment with PCSK9 inhibitors. Before the diagnosis of nephrotic syndrome, all patients suffered hypertension and were treated with angiotensin II blockers, 2 patients had type 2 diabetes mellitus treated with oral antidiabetic drugs, and all patients received treatment with antiplatelet drugs. All patients had hypercholesterolemia and were treated with statins, with total cholesterol values of 4.98 ± 0.8 mmol/L and LDL-C of 2.6 ± 0.5 mmol/L. Table 2 summarizes the clinical and the demographic characteristics of control group and their biochemical data at diagnosis. Patients in the control group were significantly younger (66 ± 5.4 vs. 31.1 ± 10.5 years of age, P = 0.001) and had significantly lower creatinine levels (0.87 ± 0.13 vs. 1.2 ± 0.31 mg/dl, P = 0.006) than those treated with PCSK9 inhibitors.
Table 1.
Demographic, clinical characteristics, and biochemical values of patients at last follow-up before starting treatment with PCSK9 inhibitors
| Patient no. | Age, y | G | Timea | KD | Cr | eGFR | Alb | TC | LDL-C | Pr | Previous IS treatment | Treatment | Vascular disease |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 66 | F | 38 | FSGS | 1.3 | 53 | 2.3 | 9.7 | 6.1 | 12 | Corticosteroids 6 months, cyclosporine A 12 months, and mycophenolate mofetil 6 months | Atorvastatin 80 mg plus gemfibrozil 600 mg | Ischemic cerebrovascular disease |
| 2 | 68 | M | 43 | FSGS | 1.6 | 42 | 2.1 | 10.6 | 6.3 | 9.5 | Corticosteroids 6 months, cyclosporine A 6 months, and mycophenolate mofetil 9 months | Atorvastatin 80 mg plus ezetimibe 10 mg | Ischemic heart disease |
| 3 | 70 | M | 37 | FSGS | 0.9 | >90 | 2.2 | 10.9 | 7.1 | 8.4 | Corticosteroids 6 months and cyclosporine A 6 months | Atorvastatin 80 mg plus ezetimibe 10 mg | Ischemic heart disease and peripheral vascular disease |
| 4 | 76 | M | 30 | MN | 1.2 | 54 | 2.4 | 11.2 | 7 | 11 | Corticosteroids plus tacrolimus 12 months, cyclophosphamide 6 months, and rituximab 2 doses 1 gr | Atorvastatin 80 mg plus ezetimibe 10 mg | Ischemic heart disease |
| 5 | 74 | M | 39 | MN | 0.7 | >90 | 2.3 | 8.8 | 6.5 | 12.5 | Corticosteroids and tacrolimus 15 months, cyclophosphamide 6 months, and rituximab | Atorvastatin 80 mg plus ezetimibe 10 mg | Ischemic cerebrovascular disease and ischemic heart disease |
| 6 | 59 | M | 26 | FSGS | 0.9 | >90 | 1.9 | 9 | 6.8 | 9.7 | Corticosteroids 6 months, tacrolimus 12 months, and mycophenolate mofetil 6 months | Atorvastatin 80 mg plus ezetimibe 10 mg | Ischemic heart disease |
| 7 | 61 | M | 25 | FSGS | 1.3 | 67 | 2.1 | 9.9 | 7.1 | 13.1 | Corticosteroids 6 months, tacrolimus 12 months, and mycophenolate mofetil 6 months | Atorvastatin 80 mg plus ezetimibe 10 mg | Ischemic cerebrovascular disease |
| 8 | 67 | M | 31 | FSGS | 1.4 | 65 | 2.4 | 9.6 | 6.3 | 8.5 | Corticosteroids plus tacrolimus 12 months and rituximab 4 doses 1 gr | Atorvastatin 80 mg plus ezetimibe 10 mg | Ischemic heart disease |
| 9 | 63 | M | 32 | MN | 1.3 | 72 | 2.2 | 8.9 | 5.8 | 7.9 | Corticosteroids plus tacrolimus 12 months and rituximab 4 doses 1 gr | Atorvastatin 80 mg | Ischemic cerebrovascular disease |
| 10 | 60 | M | 28 | FSGS | 1.8 | 58 | 2.3 | 9.1 | 6.1 | 11 | Corticosteroids 6 months and cyclosporine A 9 months | Atorvastatin 80 mg | Ischemic cerebrovascular disease and peripheral vascular disease |
| 11 | 71 | F | 29 | MN | 0.9 | 85 | 2 | 8 | 6.2 | 12.8 | Corticosteroids plus tacrolimus 9 months, cyclophosphamide 6 months, and rituximab 4 doses 1 gr | Atrovastatin 80 mg plus ezetimibe 10 mg | Ischemic heart disease |
| 12 | 64 | M | 41 | MN | 1.1 | 81 | 2.1 | 8.2 | 5.9 | 17.5 | Corticosteroids plus tacrolimus 9 months, cyclophosphamide 6 months, and rituximab 4 doses 1 gr | Atorvastatin 80 mg | Ischemic heart disease |
Alb, serum albumin (g/dL); Cr, serum creatinine; eGFR, estimated glomerular filtration rate (ml/min/1.73 m2); F, female; FSGS, focal and segmental glomerulosclerosis; G, gender; IS, immunosuppressor; KD, kidney disease; LDL-C, low-density lipoprotein cholesterol (mmol/L); M, male; MN, membranous nephropathy; Pr, proteinuria (g/day); TC, total cholesterol (mmol/L).
Months from diagnosis of nephrotic syndrome.
Table 2.
Demographic, clinical, and biochemical characteristics of the control group
| Patient no. | Age, y | G | Timea | KD | Cr | eGFR | Alb | TC | LDL-C | Pr | Previous IS treatment | Treatment | Vascular disease |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 46 | M | 30 | MN | 0.80 | >90 | 2.2 | 9.5 | 6.9 | 11.1 | Corticosteroids 4 months, cyclosporine A 10 months, cyclophosphamide 6 months, mycophenolate mofetil 6 months, and rituximab 3 doses 1 gr | Atorvastatin 80 mg | No |
| 2 | 35 | M | 33 | FSGS | 0.84 | >90 | 1.9 | 9.4 | 5.8 | 12.8 | Corticosteroids 6 months, cyclosporine A 12 months, and mycophenolate mofetil 6 months | Atrovastatin 80 mg plus ezetimibe 10 mg | No |
| 3 | 41 | M | 38 | FSGS | 0.90 | >90 | 2 | 8.5 | 6.4 | 12 | Corticosteroids 6 months and cyclosporine A 6 months | Atorvastatin 80 mg | No |
| 4 | 36 | F | 30 | MCD | 0.75 | >90 | 2.4 | 8.1 | 5.5 | 8.3 | Corticosteroids 6 months, tacrolimus 6 months, and rituximab 2 doses 1 gr | Atorvastatin 80 mg | No |
| 5 | 19 | F | 29 | FSGS | 0.91 | >90 | 2 | 10.6 | 5.6 | 7.2 | Corticosteroids 6 months, cyclosporine A 6 months, and rituximab 2 doses 1 gr | Atorvastatin 80 mg | No |
| 6 | 23 | M | 36 | MCD | 1.03 | >90 | 2.3 | 9.9 | 6.7 | 8.3 | Corticosteroids 6 months, mycophenolate mofetil 6 months, and rituximab 2 doses 1 gr | Atorvastatin 80 mg | No |
| 7 | 32 | F | 27 | FSGS | 0.85 | >90 | 2.1 | 10.8 | 6.8 | 7.9 | Corticosteroids 6 months, tacrolimus 6 months, and mycophenolate mofetil 6 months | Atorvastatin 80 mg | No |
| 8 | 17 | M | 21 | FSGS | 1.12 | 82 | 2.3 | 9.1 | 6.9 | 6.7 | Corticosteroids plus tacrolimus 6 months and rituximab 2 doses 1 gr | Atrovastatin 80 mg plus ezetimibe 10 mg | No |
Alb, serum albumin (g/dL); Cr, serum creatinine; eGFR, estimated glomerular filtration rate (ml/min/1.73 m2); F, female; FSGS, focal and segmental glomerulosclerosis; G, gender; IS, immunosuppressor; KD, kidney disease; LDL-C, low-density lipoprotein cholesterol (mmol/L); M, male; MCD, minimal change disease; MN, membranous nephropathy; Pr, proteinuria (g/day); TC, total cholesterol (mmol/L).
Months from diagnosis of nephrotic syndrome.
After the diagnosis of nephrotic syndrome and before starting treatment with PCSK9 inhibitors, no significant differences were observed in total cholesterol (9.49 ± 1 vs. 9.4 ± 0.9 mmol/L, P = 0.99), LDL-C 6.5 ± 0.5 vs. 6.3 ± 0.6 mmol/L, P = 0.67), albumin (2.13 ± 0.12 vs. 2.3 ± 0.27 g/dl, P = 0.11), or proteinuria (8.8 ± 1.1 vs. 9.8 ± 3.7 g/day, P = 0.48) between patients treated with PCSK9 inhibitors and the control group.
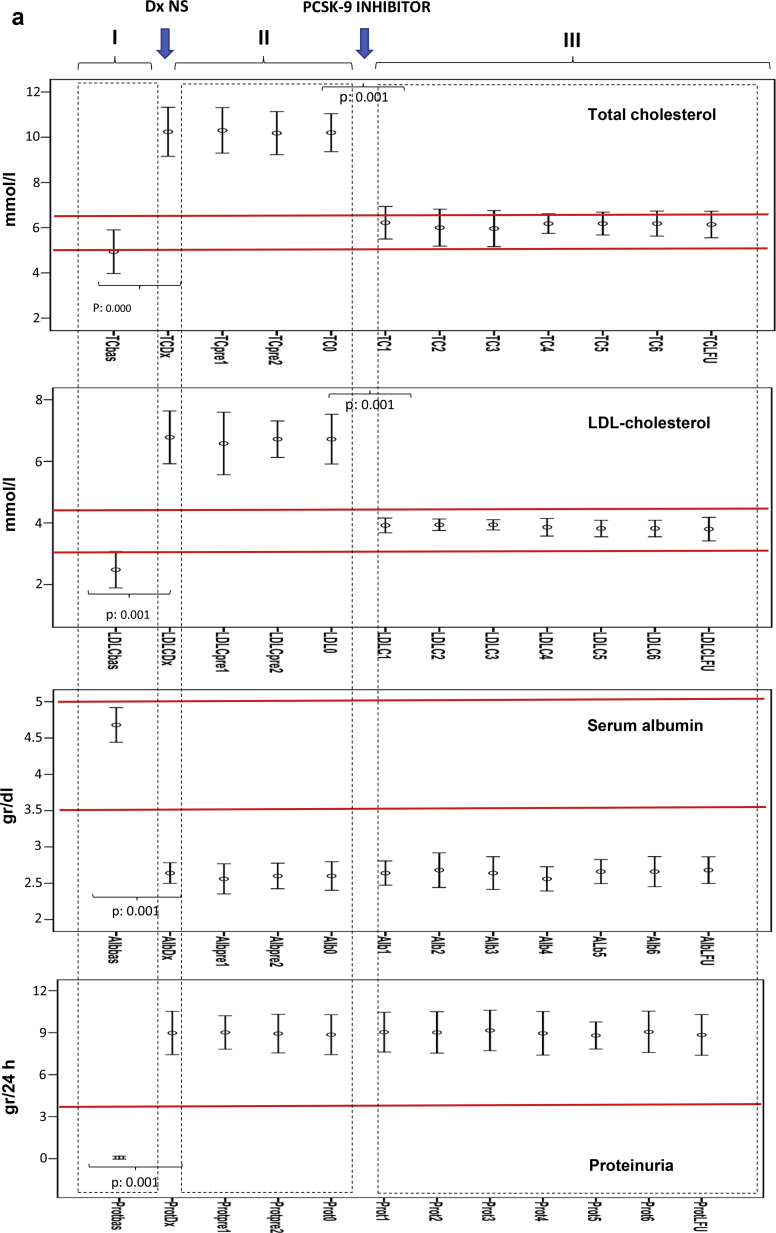
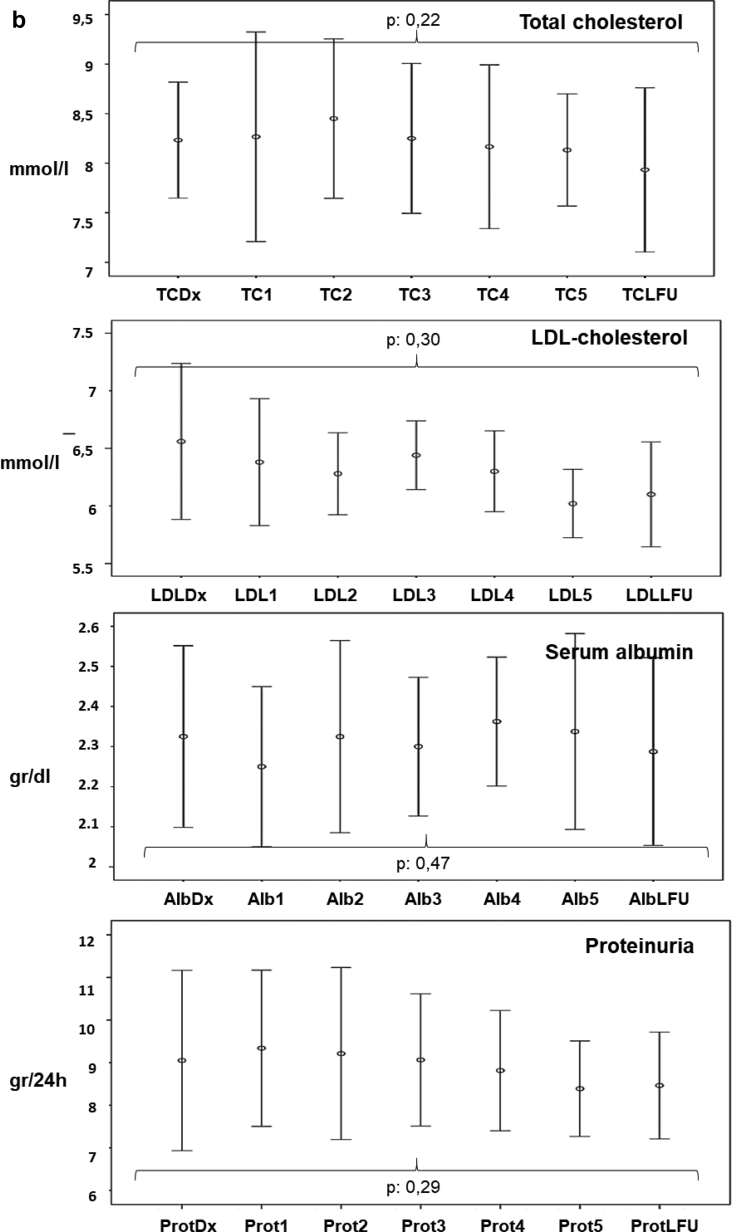
Figure 1a shows the serum levels of total cholesterol, LDL-C, albumin, and proteinuria (I) before the diagnosis of nephrotic syndrome, (II) between the diagnosis of nephrotic syndrome and the start of treatment with PCSK9 inhibitors, and (III) along the follow-up period after starting treatment with PCSK9 inhibitors. Figure 1b shows the evolution of total cholesterol, LDL-C, albumin, and proteinuria after the diagnosis of nephrotic syndrome in patients in the control group.
Figure 1.
(a) Evolution of serum levels of total cholesterol (TC), low-density lipoprotein cholesterol (LDL-C), albumin, and proteinuria along follow-up in patients treated with proprotein convertase subtilisin kexin 9 (PCSK9) inhibitors. Results are shown as error bars representing the means and 95% confidence intervals. (I) Before the diagnosis of nephrotic syndrome. (II) Between the diagnosis of nephrotic syndrome and the start of treatment with PCSK9 inhibitors. (III) Along the follow-up period after starting treatment with PCSK9 inhibitors. (b) Evolution of serum levels of TC, LDL- C, albumin, and proteinuria along follow-up in control group. Results are shown as error bars representing the means and 95% confidence intervals. TC and LDL-C measured in millimoles per liter, serum albumin measured in grams per deciliter, and proteinuria measured in grams per day. The values correspond to the monthly controls carried out after the diagnosis of nephrotic syndrome and at the last available follow-up. Normal value ranges for each parameter are shown by the red lines. 0, At the start of treatment with PCSK-9 inhibitors, 1–6 monthly values after starting treatment with PCSK9 inhibitors; bas, before the diagnosis of nephrotic syndrome; dx, at diagnosis of nephrotic syndrome, pre1 and pre2 during follow-up after the diagnosis of nephrotic syndrome and before starting treatment with PCSK9 inhibitors; dx NS, time of diagnosis of nephrotic syndrome; LFU, last follow-up.
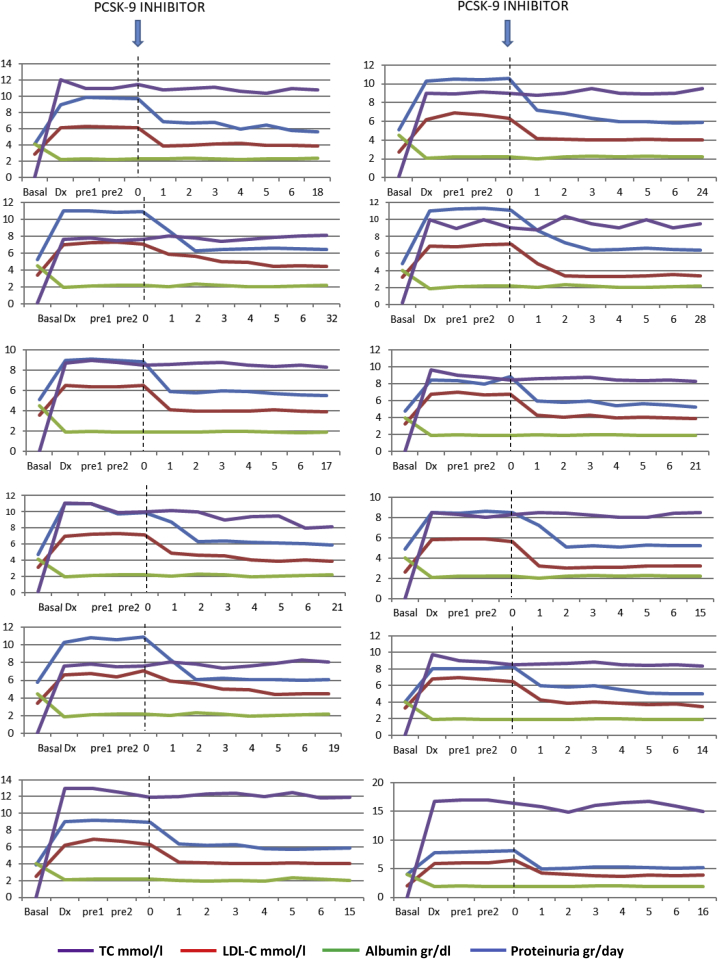
The evolution of the levels of total cholesterol, LDL-C, albumin, and proteinuria for each patient is shown in Figure 2. In patients treated with PCSK9 inhibitors, a significant increase in the levels of total cholesterol from 4.9 ± 0.8 mmol/L to 9.5 ± 1 mmol/L (P < 0.001) and of those of LDL-C from 2.6 ± 0.5 mmol/L to 6.4 ± 0.5 mmol/L (P < 0.001) were observed after the diagnosis of nephrotic syndrome. During the period of time between the diagnosis of nephrotic syndrome and the start of treatment with PCSK9 inhibitors, total cholesterol, LDL-C, serum albumin, and urinary protein excretion did not undergo significant changes. One month after treatment with PCSK9 inhibitors, a statistically significant decrease in both total cholesterol and LDL-C levels was observed, with a decrease in total cholesterol from 9.5 ± 1 mmol/L to 6.3 ± 0.6 mmol/L (P < 0.001) and a decrease in LDL-C from 6.4 ± 0.5 mmol/L to 4.3 ± 0.5 mmol/L (P = 0.001), without significant changes in serum albumin levels or proteinuria. The mean decrease in LDL-C was 36.8% ± 4.9% at 4 weeks and remained unchanged throughout the follow-up period.
Figure 2.
Individual evolution of the levels of total cholesterol (TC), low-density lipoprotein cholesterol (LDL-C), albumin, and proteinuria along follow-up. 0, At the start of treatment with proprotein convertase subtilisin kexin 9 (PCSK9) inhibitors, 1–6 monthly values after starting treatment with PCSK9 inhibitors; basal, before the diagnosis of nephrotic syndrome; dx, at diagnosis of nephrotic syndrome, pre1 and pre2 during follow-up after the diagnosis of nephrotic syndrome and before starting treatment with PCSK9 inhibitors; LFU: last follow-up.
After diagnosis of nephrotic syndrome, plasma PCSK9 levels were 334 ± 40 ng/mL and correlated significantly with serum LDL-C levels (r = 0.49, P = 0.023).
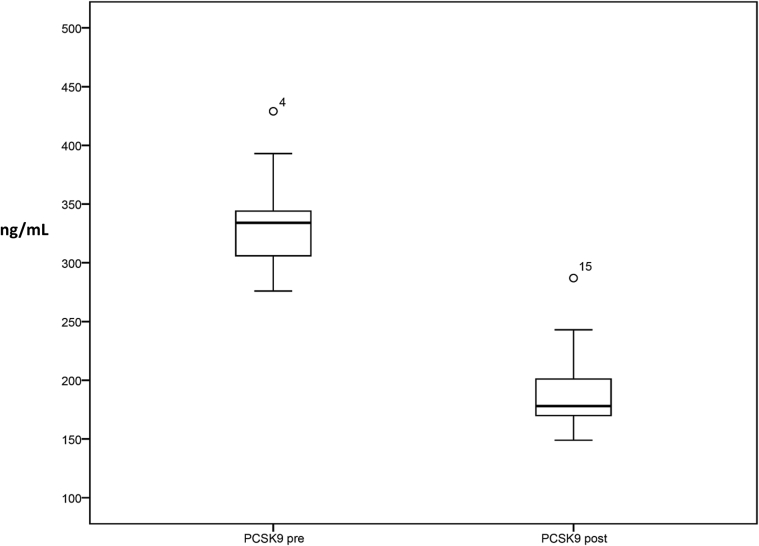
Six months after statin treatment with PCSK9 inhibitors, plasma PCSK9 levels were significantly reduced to values of 190 ± 36 ng/ml with a mean relative reduction of 42.3% ± 12.6% (Figure 3). In contrast, in the control group there were no significant changes in the levels of total cholesterol or LDL-C during the follow-up period. Plasma PCSK9 levels were not measured in patients in the control group.
Figure 3.
Serum proprotein convertase subtilisin kexin 9 (PCSK9) levels before and after treatment with PCSK9 inhibitors. PCSK9 pre, Plasma levels of PCSK9 at the time of diagnosis of nephrotic syndrome and before starting treatment with PCSK9 inhibitors; PCSK9 post, plasma levels of PCSK9 6 months after starting treatment with PCSK9 inhibitors and atorvastatin withdrawal.
No local adverse effects were seen at the injection site and no significant changes were seen in the levels of transaminase, creatine phosphokinase, or aldolase. No adverse effects were reported in any of the patients treated. No new cardiovascular event or death form any cause was observed during the follow-up period.
Discussion
The group of patients described above represent the limitations in treating severe hypercholesterolemia associated with nephrotic syndrome that is refractory to immunosuppressive treatment. Treatment guidelines recommend that these patients be treated with statins at the maximum tolerated doses.2,3 However, a systematic review from the Cochrane database including 5 randomized clinical trials enrolling 203 patients concluded that there is not enough evidence supporting the efficacy of statins in the treatment of nephrotic hypercholesterolemia.13 In addition, statins are not exempt of potential toxicity, especially when the nephrotic syndrome is associated with a reduced glomerular filtration rate.14, 15, 16 In these cases, other treatment options are necessary. At present, PCSK9 inhibitors are not approved for the treatment of hypercholesterolemia associated with refractory nephrotic syndrome. Therefore, unless patients with nephrotic syndrome suffer from clinical atheromatous vascular disease and morbidity, the only option available for them would be the treatment with LDL apheresis.5,6,17,18 However, LDL apheresis is expensive and requires extracorporeal circuits, anticoagulation, and often central venous catheters subject to infection or vascular thrombosis risk, which are especially relevant in this context because of the hypercoagulability and malnutrition status associated with nephrotic syndrome.2
All patients treated with PCSK9 inhibitors had a history of clinical ischemic cardiovascular disease and before the diagnosis of nephrotic syndrome were treated with statins with proper control of the LDL-C values. After the diagnosis of nephrotic syndrome and the evidence of no response to immunosuppressive treatment, their risk profile increased significantly, as LDL-C levels could not be adequately controlled with standard of care measures. After the diagnosis of nephrotic syndrome, plasma PCSK9 levels were increased and correlated significantly with LDL-C levels. This increase could not only be caused by the nephrotic syndrome itself4,6 but also by the high-dose atorvastatin treatment prescribed.19 After starting treatment with PCSK9 inhibitors there was a significant reduction in both total and LDL-C levels and consequently in their vascular risk profile. The decrease in LDL-C levels ran in parallel with the decrease in serum PCSK9 levels which could be related to the effect of PCSK9 inhibitors, together with the withdrawal of atorvastatin treatment.19 This reduction was maintained along the whole observation period with no evidence of adverse effects.
PCSK9 levels are increased in patients with nephrotic syndrome, and it has been proposed as one of the key events in the pathogeny of dyslipidemia associated with this condition. PSCK9 levels are directly correlated with proteinuria and decrease with the remission of nephrotic syndrome.4 No significant change was observed in proteinuria with treatment with PCSK9 inhibitors. Our findings suggest, at least in the group of patient treated, that PCSK9 dysregulation is secondary to the nephrotic syndrome in question, and along the resulting dyslipidemia, does not behave as an amplifying factor of filtration barrier dysfunction.
Our findings indicate that PCSK9 inhibitors may be an effective and safe option for treatment of hypercholesterolemia associated with refractory nephrotic syndrome.
Disclosure
All the authors declared no competing interests.
Author Contributions
AS and JL designed the study, performed the statistical analyses, and wrote the final version of the manuscript. EJ, BM, and FT-B contributed to the discussion and critically reviewed the final version of the manuscript.
References
- 1.Scandinavian Simvastatin Survival Study Group Randomised trial of cholesterol lowering in 4444 patients with coronary heart disease: the Scandinavian Simvastatin Survival Study (4S) Lancet. 1994;344:1383–1389. [PubMed] [Google Scholar]
- 2.Chapter 2: General principles in the management of glomerular disease. Kidney Int Suppl. 2012;2:156–162. doi: 10.1038/kisup.2012.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Radhakrishnan J., Appel A.S., Valeri A., Appel G.B. The nephrotic syndrome, lipids, and risk factors for cardiovascular disease. Am J Kidney Dis. 1993;22:135–142. doi: 10.1016/s0272-6386(12)70179-8. [DOI] [PubMed] [Google Scholar]
- 4.Haas M.E., Levenson A.E., Sun X. The role of proprotein convertase subtilisin/kexin type 9 in nephrotic syndrome-associated hypercholesterolemia. Circulation. 2016;134:61–72. doi: 10.1161/CIRCULATIONAHA.115.020912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Liu S., Vaziri N.D. Role of PCSK9 and IDOL in the pathogenesis of acquired LDL receptor deficiency and hypercholesterolemia in nephrotic syndrome. Nephrol Dial Transplant. 2014;29:538–543. doi: 10.1093/ndt/gft439. [DOI] [PubMed] [Google Scholar]
- 6.Jin K., Park B.S., Kim Y.W., Vaziri N.D. Plasma PCSK9 in nephrotic syndrome and in peritoneal dialysis: a cross-sectional study. Am J Kidney Dis. 2014;63:584–589. doi: 10.1053/j.ajkd.2013.10.042. [DOI] [PubMed] [Google Scholar]
- 7.Kohli M, Patel K, MacMahon Z, et al. Pro-protein subtilisin kexin-9 (PCSK9) inhibition in practice: lipid clinic experience in 2 contrasting UK centres [e-pub ahead of print]. Int J Clin Pract.https://doi.org/10.1111/ijcp.13032. Accessed October 17, 2020. [DOI] [PubMed]
- 8.Awanami Y., Fukuda M., Nonaka Y. Successful treatment of a patient with refractory nephrotic syndrome with PCSK9 inhibitors: a case report. BMC Nephrol. 2017;18:221. doi: 10.1186/s12882-017-0644-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.ClinicalTrials.gov website Effect of PCSK9-antibody (alirocumab) on dyslipidemia secondary to nephrotic syndrome. https://clinicaltrials.gov/ct2/show/NCT03004001 Available at:
- 10.Kidney Disease: Improving Global Outcomes (KDIGO) Glomerulonephritis Work Group KDIGO clinical practice guideline for Glomerulonephritis. Kidney Int Suppl. 2012;2:139–274. [Google Scholar]
- 11.Morisky D.E., Green L.W., Levine D.M. Concurrent and predictive validity of a self-reported measure of medication adherence. Med Care. 1986;24:67–74. doi: 10.1097/00005650-198601000-00007. [DOI] [PubMed] [Google Scholar]
- 12.Levey A.S., Stevens L.A., Schmid C.H. A new equation to estimate glomerular filtration rate. Ann Intern Med. 2009;150:604–612. doi: 10.7326/0003-4819-150-9-200905050-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kong X., Yuan H., Fan J., Li Z., Wu T., Jiang L. Lipid-lowering agents for nephrotic syndrome. Cochrane Database Syst Rev. 2013;10:CD005425. doi: 10.1002/14651858.CD005425.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jose J., Saravu K., Shastry B.A. Atorvastatin-induced early-onset rhabdomyolysis in a patient with nephrotic syndrome. Am J Health Syst Pharm. 2007;64:726–729. doi: 10.2146/ajhp060241. [DOI] [PubMed] [Google Scholar]
- 15.SEARCH Collaborative Group. Link E., Parish S. SLCO1B1 variants and statin-induced myopathy-a genomewide study. N Engl J Med. 2008;359:789–799. doi: 10.1056/NEJMoa0801936. [DOI] [PubMed] [Google Scholar]
- 16.Cholesterol Treatment Trialists’ (CTT) Collaboration. Herrington W.G., Emberson J. Impact of renal function on the effects of LDL cholesterol lowering with statin-based regimens: a meta-analysis of individual participant data from 28 randomised trials. Lancet Diabetes Endocrinol. 2016;4:829–839. doi: 10.1016/S2213-8587(16)30156-5. [DOI] [PubMed] [Google Scholar]
- 17.Muso E., Mune M., Hirano T. Immediate therapeutic efficacy of low-density lipoprotein apheresis for drug-resistant nephrotic syndrome: evidence from the short-term results from the POLARIS study. Clin Exp Nephrol. 2015;19:379–386. doi: 10.1007/s10157-014-0996-8. [DOI] [PubMed] [Google Scholar]
- 18.Muso E., Mune M., Hirano T. A prospective observational survey on the long-term effect of LDL apheresis on drug-resistant nephrotic syndrome. Nephron Extra. 2015;5:58–66. doi: 10.1159/000437338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Careskey H.E., Davis R.A., Alborn W.E., Troutt J.S., Cao G., Konrad R.J. Atorvastatin increases human serum levels of proprotein convertase subtilisin/kexin type 9. J Lipid Res. 2008;49:394–398. doi: 10.1194/jlr.M700437-JLR200. [DOI] [PubMed] [Google Scholar]