Abstract
Introduction
Familial IgA nephropathy (IgAN) has been widely reported. However, its clinicohistologic characteristics and long-term prognosis are not clear.
Methods
A total of 348 familial IgAN cases from 167 independent families were recruited and their clinicohistologic characteristics as well as lifetime risk of end-stage renal disease (ESRD) were compared to 1116 sporadic IgAN patients from the same geographic region.
Results
Of all familial IgAN patients, 60 (17%) came from 32 single-generation (SG; all affected individuals are siblings) families, whereas 286 (82%) came from 134 multiple-generation (MG; affected individuals were present in at least 2 consecutive generations) families. The lifetime ESRD risk was significantly higher in familial patients than sporadic ones after adjusting by gender (hazard ratio [HR]=1.40, 95% confidence interval [CI]: 1.12–1.74, P = 0.004), with 5 years younger in median ESRD age (60 years vs. 65 years in familial and sporadic cases separately). Interestingly, among familial patients, we found cases from SG families (vs. MG families: HR = 2.62, 95% CI: 1.59–4.31, P < 0.001) or with early onset (onset age <30 years) (vs. late onset: HR = 4.79, 95% CI: 3.16–7.26, P < 0.001) had higher lifetime ESRD risk. Furthermore, among sporadic patients, men had lower estimated glomerular filtration rate (eGFR), higher urine protein, higher Oxford T score, and higher risk for life span ESRD compared with women (male vs. female, 25% vs. 17%, P = 0.003) whereas these gender differences were not seen in familial patients.
Conclusion
Familial IgAN cases had poorer renal outcomes and less gender differences compared with sporadic cases. These findings provide evidence that familial disease represent a distinct subtype of more progressive IgAN. Early diagnosis could improve the prognosis of cases with familial IgAN.
Keywords: familial IgA nephropathy, end-stage renal disease, gender, lifetime risk, multiple-generation, single-generation
Graphical abstract
IgAN is the most common form of primary glomerulonephritis worldwide and accounts for up to 54% of all the primary glomerulonephritis in China.1 Registry data suggest that the prevalence of IgAN varies among populations with different ancestries,2 with a higher prevalence in Asians than other populations. Furthermore, relatively more severe clinical manifestations and worse outcomes were reported in East Asians with IgAN.3 Familial aggregation of IgAN has been widely reported around the world since the first familial cases of IgAN were described in the late 1970s.4 Subsequent studies suggested that familial disease might represent up to 10%–15% of all IgAN cases, suggesting that mechanism of IgAN has a strong genetic component.5 Several linkage loci have been subsequently identified based on family studies of IgAN although causal variants underlying these loci are not clear, highlighting potential genetic heterogeneity of the disease.6, 7, 8 Further insights came from genome-wide association studies, which have identified more than 20 common susceptibility loci to date.9, 10, 11, 12, 13, 14
The clinical outcome of IgAN is highly variable. Dozens of clinical risk factors have been identified, and clinical risk models have been established to predict the risk for disease progression in IgAN patients.15, 16, 17, 18, 19, 20, 21, 22, 23 For example, a recent multicenter study established and validated 2 risk equations for predicting risk of ESRD at the time of biopsy, identifying age, gender, eGFR, hemoglobin, urine protein excretion, and Oxford M and T scores as important predictors of renal outcomes.23 Treatment with renin-angiotensin system blockade (RASB) has been demonstrated to reduce proteinuria and delay the progression of kidney disease in IgAN.24,25 The renoprotective effect of RASB may come from the control of blood pressure and inhibition of the synthesis and secretion of cytokines, such as transforming growth factor–β1.26 Recently, 2 randomized clinical trials tested the effects of glucocorticoid treatment in IgAN with persistent proteinuria and both demonstrated reduction in proteinuria with steroids, but at the cost of serious adverse events.27,28
Importantly, the above studies recruited predominantly sporadic patients and it is not clear whether these findings also generalized to familial IgAN. Schena et al.29 reported that renal prognosis was significantly less favorable in Italian patients with familial IgAN, but another Italian study found similar renal outcomes between familial and sporadic IgAN.30 It is also unclear what the differences in clinical, pathologic, and prognostic features among patients with familial and sporadic IgA nephropathy are. Our study aims to characterize the clinical features and long-term renal outcomes in familial forms of IgAN based on an extended cohort of Chinese IgAN patients.
Materials and Methods
Study Design
All patients enrolled in this study were self-reported Chinese Han and were diagnosed and treated at Ruijin Hospital between 1990 and 2017. The diagnosis of IgAN was made by a kidney biopsy documenting positive mesangial staining for IgA of at least 2+ on a scale from 0 to 3+ by immunofluorescence. Electronic microscopy was performed routinely for each patient with a renal biopsy. Skin collagen type IV alpha 5 chains staining, kidney biopsy collagen type IV alpha 3/4/5 chains staining, hearing tests, and plasma alpha-galactosidase tests were carried out for all familial IgAN patients to exclude other hereditary kidney diseases such as Alport syndrome, thin basement membrane disease, or Fabry disease. Patients who were secondary to systemic diseases such as systemic lupus erythematosus or Henoch-Schonlein purpura were excluded from the study. Family disease screening was conducted among all available family members by routine urinalysis, urine microalbumin-to-creatinine ratio test, and renal function tests. IgAN families were ascertained by having at least 2 affected family members of whom at least 1 was diagnosed as IgAN by renal biopsy.6 A family member was regarded as affected if he or she was diagnosed with IgAN by a renal biopsy, had ESRD with unknown cause, or had unexplained hematuria (≥5 red blood cells per high-power field) or proteinuria (defined as proteinuria equal to or higher than ++ or a urine albumin-to-creatinine ratio higher than 90 mg/g) on at least 3 occasions. SG families had affected family members only in a single generation, suggesting a recessive hereditary pattern. MG families were defined by affected family members in at least 2 consecutive generations indicating a dominant hereditary pattern. Family members older than 18 years with eGFR higher than 60 ml/min per 1.73 m2 and without urinary abnormalities were classified as unaffected. In addition, those with normal urinary analysis and eGFR higher than 60 ml/min per 1.73 m2 yet younger than 18 years, or for whom clinical data were not available, were classified as affection status unknown. Patients with biopsy-confirmed IgAN but without family history of kidney diseases were defined as sporadic IgAN patients.
This study was approved by the Institutional Review Board of Ruijin Hospital, Shanghai Jiao Tong University School of Medicine, and was in accordance with the principles of the Helsinki Declaration. Written informed consent was obtained from all participants.
Data Collection and Outcome Measurement
Data were collected including demographic (date of birth, gender, family history), clinical and laboratory variables (i.e., age of onset, onset symptoms, history of hematuria, blood pressure, blood routine test, urinalysis, serum creatinine, albumin), Oxford MEST-C score (if renal biopsy was done), and treatment strategies (e.g., angiotensin-converting enzyme inhibitor or angiotensin receptor blocker, steroid, other immunosuppressant, and renal replacement therapy). eGFR was calculated by using the Chronic Kidney Disease Epidemiology Collaboration (CKD-EPI) equation.31 Chronic kidney disease was classified based on the Kidney Disease: Improving Global Outcomes (KDIGO) practice guidelines.
Patient Classification and Treatment
Familial IgAN patients were categorized into the following groups based on renal function at disease onset: preserved eGFR group (eGFR ≥ 60 ml/min per 1.73 m2) and nonpreserved eGFR group (eGFR < 60 ml/min per 1.73 m2). In addition to supportive therapy, patients with hypertension and/or proteinuria more than 0.5 g/d were treated with RASB (including angiotensin-converting enzyme inhibitor or angiotensin receptor blocker). Steroid would be added if patients had persistent proteinuria more than 1 g/d after treatment with RASB for at least 3 months. Immunosuppressive agents would be used if patients presented with rapidly progressive glomerulonephritis or nephrotic syndrome. Patients were divided into 2 groups according to disease onset age: early-onset were patients with disease onset at age <30 years; late-onset were patients with disease onset at age ≥30 years.
Statistical Methods
The normally distributed continuous variables were summarized as means ± SDs, whereas non-normally distributed continuous variables were summarized as median (range). All categorical variables were summarized as frequencies and percentages (%). The primary outcome was defined as time to lifetime ESRD. Statistical methods for censored time-to-event data were used with a Kaplan-Meier plot and the log-rank test. Median outcome-free age was reported with 95% CIs. Associations between familial IgAN and sporadic IgAN patients and lifetime renal survival rate were estimated by multivariate Cox regression analysis. The HR was calculated with 95% CIs. All tests were 2-sided, with P <0.05 considered statistically significant. Survival analysis was performed using survival package, version 2.40-1 (R v.3.2.2).
Results
Pedigree Structures and Disease Transmission Patterns
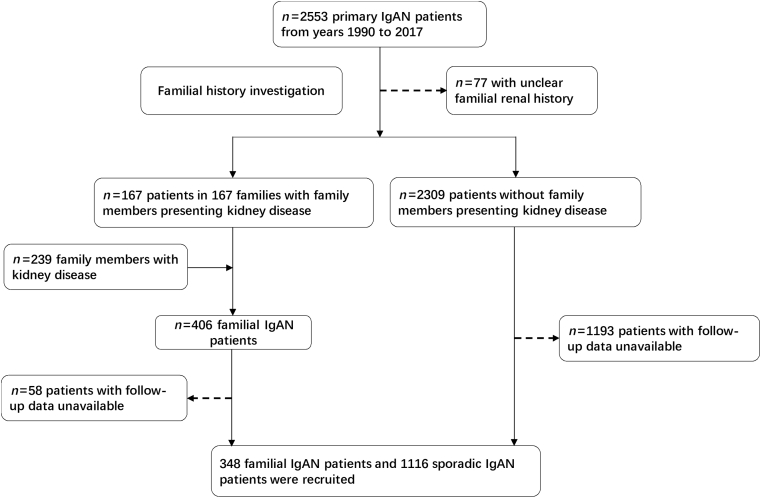
We screened 2553 primary IgAN patients diagnosed in our center between the years 1990 and 2017. After familial investigation, we found out that 167 IgAN patients had family members presenting kidney disease and 2309 IgAN patients showed no affecting family members, the other 77 patients could not provide clear familial renal history and had been excluded from the study. Based on our familial investigation, 239 family members from the 167 IgAN families were defined as affected and were also included in our following study. Then 58 familial and 1193 sporadic IgAN patients were also excluded through further investigation because of unavailable follow-up data. Finally, there were 348 familial IgAN patients from 167 independent IgAN families and 1116 sporadic IgAN patients enrolled in this study (Figure 1). Totally, 198 (57%) familial patients were diagnosed by kidney biopsy; others were diagnosed by clinical manifestations of renal dysfunction, that is, proteinuria, hematuria, chronic kidney disease stage 3 or 4, or ESRD with unknown cause (Supplementary Table S1).
Figure 1.
Workflow of screening IgA nephropathy (IgAN) patients.
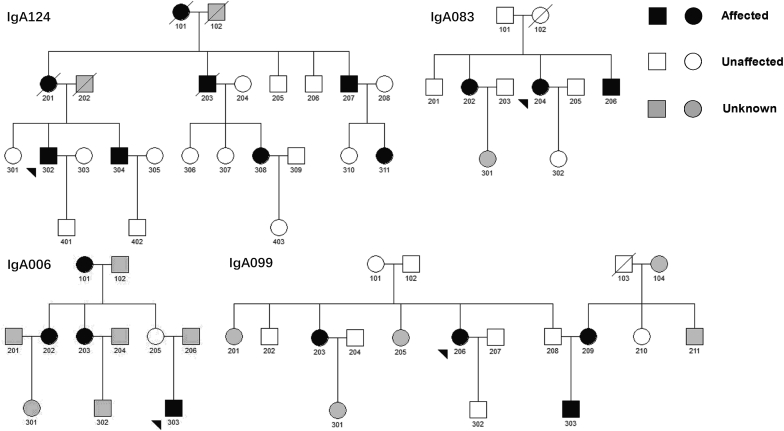
Of all included familial patients, 60 (17%) came from 32 SG families, whereas 286 (82%) came from 134 MG families. Only 1 family was skipped generation (the 2 patients were grandmother and granddaughter, respectively), which was excluded from the subsequent SG versus MG analysis. The smallest family involved 2 affected siblings, whereas the largest family included 8 affected relatives across 3 generations. Interestingly, 2 of all 134 MG families had bilateral transmission; that is, both parents were affected in these 2 IgAN families. There was a total of 178 transmissions, 79 of which (44%) were paternal transmission and 99 (56%) maternal transmission. We observed evidence for incomplete penetrance in 30 of 134 MG families (22%), where a total of 30 obligate carriers under a dominant inheritance model exhibited no clinical features of kidney disease (e.g., unaffected father of a proband with an affected paternal uncle or aunt, or unaffected mother of a proband with an affected maternal uncle or aunt) (Figure 2).
Figure 2.
Pedigree structures and disease transmission patterns of IgA nephropathy families.
Clinical Characteristics
Clinical characteristics of patients with familial and sporadic disease are summarized in Table 1. Around 40% of familial IgAN patients were early onset, which was higher than sporadic cases as 32% (P = 0.003). Compared with sporadic IgAN patients, familial IgAN patients had a younger onset age and lower proteinuria level at the time of disease onset. Oxford MEST-C scores were evaluated and compared among 713 sporadic and 166 familial IgAN patients. Results showed that familial patients had less severe histologic damage, as evidenced by lower Oxford M (familial vs. sporadic IgAN patients: 23% vs. 36%, P = 0.002), E (familial vs. sporadic IgAN patients: 19% vs. 33%, P < 0.001), S (familial vs. sporadic IgAN patients: 68% vs. 80%, P = 0.001), and T (familial vs. sporadic IgAN patients: 25% vs. 45%, P < 0.001) scores. The onset characteristics and severity of histology indicated that most familial cases were diagnosed younger and at an earlier disease stage. Besides, steroid or immunosuppressant treatment was less commonly used in familial compared with sporadic IgAN patients (19% vs. 54%, P < 0.001) (Table 1). Besides, we have observed those patients who did not use steroid or immunosuppressive agents at diagnosis, and finally found that 79 (23%) familial IgAN patients versus 695 (62%) sporadic IgAN patients received steroids or immunosuppressive agents treatment during the whole disease course.
Table 1.
Characteristics of familial IgA nephropathy and sporadic IgA nephropathy patients at time of diagnosis
| Parameters | Familial IgAN (n = 348) | Sporadic IgAN (n = 1116) | P value |
|---|---|---|---|
| Male sex | 175 (50) | 593 (53) | 0.35 |
| Age at disease onset, yr, mean ± SD | 35 ± 15 | 37 ± 12 | 0.03 |
| Early-onset patients | 137 (40) | 358 (32) | 0.003 |
| Proteinuria, g/24 h, median (range) | 0.55 (0.03–4.14) | 1.46 (0.02–19.48) | <0.001 |
| Steroids and/or immunosuppressants | 67 (19) | 608 (54) | <0.001 |
| Severity of disease at diagnosis | 0.32 | ||
| Preserved eGFR | 201 (58) | 612 (55) | |
| Nonpreserved eGFR | 147 (42) | 504 (45) | |
| Oxford MEST-C | n = 166 | n = 713 | |
| M1 | 38 (23) | 257 (36) | 0.002 |
| E1 | 31 (19) | 236 (33) | <0.001 |
| S1 | 113 (68) | 571 (80) | 0.001 |
| T1/2 | 41 (25) | 323 (45) | <0.001 |
| C1/2 | 73 (44) | 348 (49) | 0.31 |
eGFR, estimated glomerular filtration rate; IgAN, IgA nephropathy.
Values are n (%), unless otherwise noted.
Early-onset were patients with disease onset at age <30 years. Late-onset were patients with disease onset at age ≥30 years. Proteinuria at disease onset was presented with median (range) and transformed by log when compared in the 2 groups. Preserved eGFR group: patients with eGFR ≥60 ml/min per 1.73 m2. Nonpreserved eGFR group: patients with eGFR <60 ml/min per 1.73 m2.
Oxford MEST-C score: M, mesangial hypercellularity; E, presence of endocapillary proliferation; S, segmental glomerulosclerosis/adhesion; T, severity of tubular atrophy/interstitial fibrosis; C, presence of crescent.
Tests with P <0.05 are indicated in bold.
Comparison of Lifetime ESRD Between Familial and Sporadic Patients
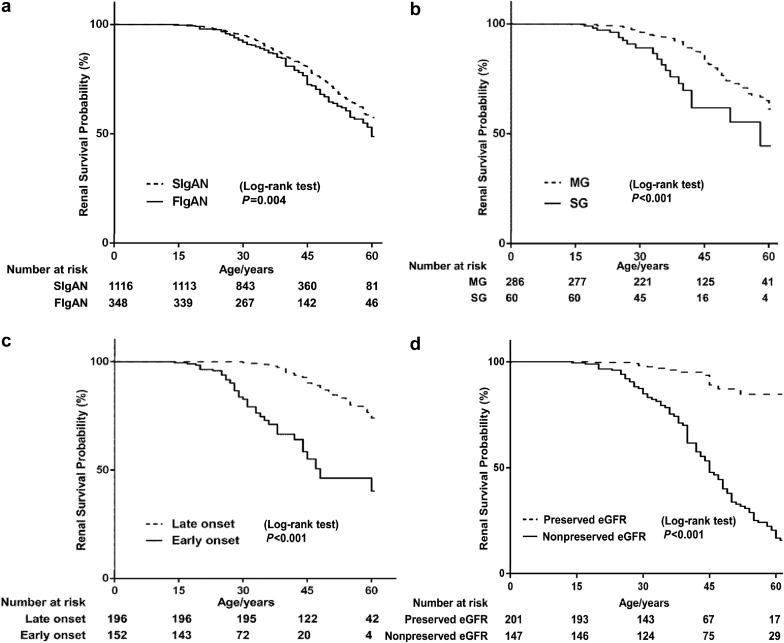
The primary renal endpoint was age at ESRD defined by the need for renal replacement therapy (dialysis or transplantation). In total, 120 (34%) of familial IgAN patients and 235 (21%) of sporadic IgAN patients progressed to ESRD. The median age at the time of ESRD was 60 years of age (95% CI: 55–63) for familial cases and 65 years of age (95% CI: 61–71) for sporadic cases. After adjusting by gender, the overall lifetime risk of ESRD was 40% higher for patients with familial disease compared with sporadic disease (HR = 1.40, 95% CI: 1.12–1.74, P = 0.004) (Figure 3a). We then compared the life span renal survival rate in the subgroup of sporadic and familial IgAN patients treated with steroids and immunosuppressive treatment. Results showed that 80 of 695 sporadic patients (12%) reached ESRD during lifetime, whereas 23 of 79 familial cases (29%) reached ESRD during the lifetime (P < 0.001). The median age of ESRD was 58 for familial cases versus 71 for sporadic cases. The overall lifetime ESRD risk was 1.46 higher for steroids- and immunosuppressive-treated patients with familial disease than sporadic disease (HR = 2.46, 95% CI: 1.54–3.93, P < 0.001) (Supplementary Table S2).
Figure 3.
Kaplan-Meier renal survival curves of patients with IgA nephropathy patients according to (a) familial history in the whole cohort, and (b) inheritance mode, (c) early or late onset, and (d) onset symptoms among familial IgA nephropathy patients. eGFR, estimated glomerular filtration rate; FIgAN, familial IgA nephropathy; MG, multiple-generation; SG, single-generation; SIgAN, sporadic IgA nephropathy.
We further analyzed the life span ESRD among familial IgAN cases in different subgroups. Interestingly, familial cases from SG families had a higher lifetime risk for ESRD than the ones from MG families (HR = 2.62, 95% CI: 1.59–4.31, P < 0.001) after adjusting for gender, onset age, and onset symptoms (Figure 3b). Moreover, we found that early age at disease onset was a risk factor for lifetime ESRD (HR = 4.79, 95% CI: 3.16–7.26, P < 0.001) (Figure 3c). At last, as expected, familial cases with nonpreserved eGFR at diagnosis had a much higher risk of lifetime ESRD compared to familial cases with preserved eGFR (nonpreserved eGFR group vs. preserved eGFR group, HR = 6.32, 95% CI: 3.73–10.72, P < 0.001) (Figure 3d). We then compared the difference in treatment with RASB or steroid or immunosuppressive therapy between the nonpreserved eGFR and preserved eGFR groups. Familial IgAN patients with nonpreserved eGFR were less likely to have RAAS blockade treatment compared to those with preserved eGFR (preserved eGFR vs. nonpreserved eGFR patients, 42% vs. 24%, P < 0.001), whereas no significant difference was seen when comparing steroid/immunosuppressive treatment (Supplementary Table S3). That was reasonable, as the use of RASB was limited among patients with severe renal dysfunction.
Subgroup Analysis for Lifetime ESRD Between Familial and Sporadic Patients
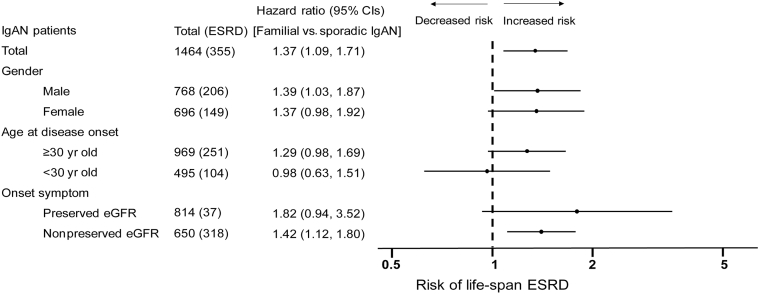
We then analyzed the association between family history and IgAN prognosis in different subgroups. The results confirmed that the increased risk of ESRD was true in most subgroups. Interestingly, in the early disease onset group, we found the ESRD risk was almost equal between the familial and sporadic cases (HR = 0.98, 95% CI: 0.63–1.51, P = 0.92) (Figure 4). We further analyzed the lifetime ESRD risk between familial and sporadic IgAN patients diagnosed by renal biopsy (Supplementary Figure S1a) or by clinical features (Supplementary Figure S1b). As we described before, familial IgAN patients had milder renal lesion than sporadic cases, so the Cox regression analyses of sporadic IgAN and familial patients diagnosed by renal biopsy was adjusted by CLINPATH risk score (an equation composed of clinical and pathologic characters from Xie et al.23). The results of the 2 subgroups were similar to the analyses of the total sporadic and familial IgAN patients.
Figure 4.
Cox regression analysis for risk of lifetime end-stage renal disease (ESRD) among familial IgA nephropathy (IgAN) and sporadic IgAN patients in different subgroups. CIs, confidence intervals.
Gender Difference in Sporadic and Familial Patients
In total, there are 768 (52%) men and 696 (48%) women in this cohort. Among them, 207 men (27%) and 148 women (21%) reached ESRD during their lifetimes. Among sporadic IgAN patients, men had lower eGFR, higher urine protein, and more patients with Oxford T score. In addition men had a higher risk score than women (CLIN: 0.24 vs. 0.15; CLINPATH: 0.24 vs. 0.16) based on risk score calculating equations,23 indicating higher risk for renal function progression in sporadic males. Accordingly, sporadic male patients were associated with a higher risk for lifetime ESRD compared with sporadic female patients (25% vs. 17%, P = 0.003). Nevertheless, the gender difference for ESRD was not found in familial IgAN patients (Table 2).
Table 2.
Gender difference among familial or sporadic IgA nephropathy patients at time of diagnosis
| Parameters | Familial IgAN (n = 348) |
Sporadic IgAN (n = 1116) |
||||
|---|---|---|---|---|---|---|
| Male | Female | P value | Male | Female | P value | |
| Total patients | 175 (50) | 173 (50) | — | 593 (53) | 523 (47) | — |
| Age at disease onset, yr, mean ± SD | 33±15 | 36±15 | 0.08 | 37±13 | 36±12 | 0.07 |
| eGFR, ml/min per 1.73 m2, mean ± SD | 88±42 | 84±36 | 0.48 | 60±35 | 72±37 | <0.001 |
| Proteinuria, g/24 h, median (range) | 0.70 (0.03–0.66) | 0.67 (0.04–4.14) | 0.62 | 1.60 (0.02–13.44) | 1.33 (0.02–19.48) | 0.01 |
| Steroid and/or immunosuppressant | 34 (19) | 33 (19) | 0.93 | 315 (53) | 290 (55) | 0.54 |
| CLIN risk score,a mean ± SD | 0.15±0.21 | 0.11±0.16 | 0.23 | 0.24±0.29 | 0.15±0.23 | <0.001 |
| CLINPATH risk score,b mean ± SD | 0.08±0.16 | 0.10±0.21 | 0.56 | 0.24±0.31 | 0.16±0.26 | <0.001 |
| Onset symptoms | ||||||
| Preserved eGFR | 97 (56) | 104 (60) | 0.50 | 291 (49) | 321 (61) | <0.001 |
| Nonpreserved eGFR | 78 (44) | 69 (40) | 302 (51) | 202 (39) | ||
| Oxford MESTC score | ||||||
| M1 | 22 (27) | 16 (19) | 0.20 | 150 (39) | 107 (33) | 0.11 |
| E1 | 14 (17) | 17 (20) | 0.65 | 111 (29) | 125 (38) | 0.01 |
| S1 | 57 (70) | 56 (66) | 0.53 | 305 (79) | 266 (82) | 0.35 |
| T1/2 | 18 (22) | 23 (27) | 0.47 | 198 (51) | 125 (38) | 0.001 |
| C1/2 | 38 (47) | 35 (42) | 0.45 | 187 (48) | 161 (49) | 0.78 |
| Lifetime ESRD | 62 (36) | 58 (34) | 0.71 | 145 (25) | 90 (17) | 0.003 |
| <20 yr | 5 (3) | 2 (1) | 0.40 | 6 (1) | 4 (1) | 0.75 |
| 20–40 yr | 26 (15) | 20 (12) | 0.36 | 71 (12) | 43 (8) | 0.04 |
| >40 yr | 31 (18) | 36 (21) | 0.38 | 68 (12) | 43 (8) | 0.07 |
eGFR, estimated glomerular filtration rate; ESRD, end-stage renal disease; IgAN, IgA nephropathy.
CLIN risk score and CLINPATH risk scores were based on equations from Xie et al.23
Values are n (%), unless otherwise noted.
Preserved eGFR group: patients with eGFR ≥60 ml/min per 1.73 m2 at the time of disease onset. Nonpreserved eGFR group: patients with eGFR <60 ml/min per 1.73 m2 at the time of disease onset.
Oxford MEST-C score: M, mesangial hypercellularity; E, presence of endocapillary proliferation; S, segmental glomerulosclerosis/adhesion; T, severity of tubular atrophy/interstitial fibrosis; C, presence of crescent.
Tests with P <0.05 are indicated in bold.
CLIN risk score:
1 – 0.9515ˆexp{–0.5364∗(Gender – 1.5) – 0.0382∗(Age – 36.5) – 0.0459∗(eGFR – 74.7) +0.1913∗[ln(UP) – 0.12] – 0.1736∗(Hb – 12.9)}.
CLINPATH risk score:
1 – 0.9725ˆexp{–0.0323∗(Age – 37.3) – 0.0567∗(eGFR – 72.5) + 0.6351∗(M – 0.39) +0.7452∗(T – 0.53)}.
Discussion
Worldwide reports of familial aggregation strongly suggest genetic factors involved in the pathogenesis of IgAN.4,32, 33, 34 However, pathogenic genetic variants underlying familial IgAN have not been identified to date although several loci have been linked to this trait based on linkage studies performed in recent decades. Thus, it is still not clear whether the familial form of IgAN is a distinct subtype of IgAN and what the clinical and prognostic features of familial IgAN are. In this study, we enrolled an extended cohort of 348 familial and 1116 sporadic IgAN patients. Under family analysis, we found that the paternal transmission and maternal transmissions were similar (44% vs. 56%), indicated an underlying genetic pattern that was consistent with autosomal inheritance. We also found evidence for incomplete penetrance for around 22%, which has been reported before. We found that the lifetime risk of ESRD is higher in familial IgAN compared with sporadic IgAN. Compared with sporadic IgAN patients, familial IgAN patients were associated with earlier diagnosis likely because of greater disease awareness and more active disease screening. Even within familial IgAN patients, we found that relatively early diagnosis (eGFR ≥60 ml/min per 1.73 m2 at diagnosis) could improve the renal prognosis compared with late diagnosis (eGFR <60 ml/min per 1.73 m2 at diagnosis) by decreasing the risk of lifetime ESRD. Despite relatively earlier diagnosis, we observed that ESRD occurred 5 years younger in familial cases compared with sporadic cases in the lifetime, indicating a faster progression of renal dysfunction. Furthermore, our study showed that familial IgAN patients with an SG involvement (suggesting a recessive inheritance) had a higher risk of ESRD when compared to patients from families with multigenerational disease transmission (suggesting a dominant transmission). Moreover, we found that male sex was associated with a higher risk for lifetime ESRD compared with female in sporadic IgAN patients, whereas the gender difference for risk of ESRD was not found in familial IgAN patients. Our study is the largest one in this field to our knowledge; our findings suggested that familial cases might represent a distinct subtype of more progressive IgAN.
Contradictory results came from the previous studies on the characteristics of familial IgAN. Two prior studies detected no difference in the laboratory and histologic features between familial IgAN and sporadic patients,29,35 whereas another study showed a higher level of serum creatinine, proteinuria, and more severe histologic lesions in familial disease.30 In this study, we found that familial IgAN patients tend to have milder clinical manifestations and Oxford MEST-C scores at the time of diagnosis, which suggests that familial IgAN patients are diagnosed earlier in their clinical course. Histologic findings in renal biopsy specimens were also reported to be milder in familial IgAN patients in a prior study30: sporadic IgAN patients had mild renal lesions in 31.2% of cases, moderate in 40.5%, and severe in 28.1%, whereas familial IgAN showed mild lesions in 48.9% of cases, moderate in 28.1%, and severe in 22.9%. This might be explained by an earlier kidney disease awareness in family members, and a greater likelihood to perform renal biopsies in early stages of renal histologic lesions by nephrologists when a familial history of kidney diseases was detected.
A prior small study29 of 32 Italian familial IgAN cases and 25 ethnically and geographically matched sporadic IgAN patients suggested faster renal progression in familial disease, with a 41% renal survival rate in familial IgAN patients versus 94% in sporadic patients within 20 years of disease onset. A larger study30 recruited 589 sporadic patients and 96 patients with familial IgA nephropathy. The authors found that the familial form tended to be less advanced renal disease and was diagnosed earlier than the sporadic form, which was similar to the findings of our study. However, the authors reported similar prognosis in familial versus sporadic disease after adjustment for clinical and pathologic factors at the time of biopsy. The discordant results of the above 2 studies might be due to limited sample size and heterogeneity of genetic causes within IgAN families.36 Another reason might be the indication of renal biopsy, which is largely different around the world. Thus, using life span ESRD analysis could overcome the influence of discrepancy of indications of renal biopsy compared with using ESRD from renal biopsy as the study endpoint. The primary outcome of this study was the lifetime risk of ESRD, which is different from previous studies. We found that familial IgAN leads to an earlier lifetime ESRD, with an average of 5 years earlier of familial cases compared with sporadic cases. Subgroup analysis confirmed that the associations between familial IgAN and increased risk of lifetime ESRD were true in most subgroups except in the early disease onset subgroup. The reasons for this might be the relatively low risk of ESRD in younger IgAN patients, and genetic factors might also be involved in the mechanism of younger sporadic IgAN patients. Meanwhile, there is no evidence that the IgAN with early disease onset is the same disease as the ones with late disease onset. Our finding was similar to the report by Schena,29 which also suggested an unfavorable outcome of familial compared with sporadic IgAN. Furthermore, the differences in lifetime ESRD risk between cases from SG versus MG families suggest potential heterogeneity of genetic mechanisms that underlie familial IgAN. The relatively poor prognosis of SG familial IgAN patients suggested poor prognosis of a recessive inheritance which is consistent with other monogenic renal diseases such as Alport syndrome.
Several studies showed that a faster decline in eGFR over time resulted in a higher risk of ESRD in men compared to women with chronic kidney disease.23,37, 38, 39, 40 That female sex usually confers a relatively better kidney prognosis might be attributed to the effect of hormones, including endogenous estrogens and/or the testosterone, as well as gender differences in nitric oxide metabolism and lifestyle. However, studies on gender differences in familial IgAN are lacking. Unlike Alport syndrome, the inheritance mode of familial IgAN remains largely unclear. It is generally believed that IgAN is a complex disease, meaning that both genetic and environmental factors determine the occurrence of the disease, which often does not comply with the classic Mendelian genetic law. The finding of incomplete penetrance in this study also supports this view. From the pedigree analysis, families with affected individuals from both SG and MG suggest that dominant and recessive inheritance may coexist, and that different susceptible genes may be involved in the disease. Paternal and maternal inheritance are basically the same, suggesting that sex-linked inheritance may not be the main inheritance method. Here we found that male sporadic IgAN patients had severer onset symptoms and a higher risk for ESRD than female sporadic IgAN patients, whereas no gender difference was found in familial IgAN patients, which also supported that familial IgAN might represent a distinct subtype of IgAN. There were several limitations in our study. First, it is a retrospective study. Second, this study included patients with a single race of Chinese Han population. At last, some family members of this study may remain undiagnosed because of inadequate screening, and some affected family members may be misdiagnosed because of phenocopy and inability to perform kidney biopsy for all affected individuals.
In conclusion, based on a large collection of Chinese families with IgAN, we found that familial cases generally had poorer renal outcomes and less gender differences compared with sporadic IgAN cases. These findings provide evidence that familial disease may represent a distinct subtype of more progressive IgAN.
Disclosure
All the authors declared no competing interests.
Acknowledgments
This work was supported by grants from the National Natural Science Foundation of China (nos. 81870460, 81570598, 81370015), Science and Technology Innovation Action Plan of Shanghai Science and Technology Committee (no. 17441902200), Shanghai Municipal Education Commission, Gaofeng Clinical Medicine Grant (no. 20152207), Shanghai Jiao Tong University School of Medicine, Multi-Center Clinical Research Project (no. DLY201510), Shanghai Health and Family Planning Committee Hundred Talents Program (no. 2018BR37), Shanghai Jiao Tong University “Jiaotong Star” Plan Medical Engineering Cross Research Key Project (no. YG2019ZDA18), and Shanghai Municipal Key Clinical Specialty (No. shslczdzk02502).
Footnotes
Table S1. Diagnostic criteria of familial IgA nephropathy patients.
Table S2. Cox regression analyses for sporadic and familial IgA nephropathy patients with steroid and immunosuppressive treatments.
Table S3. Comparison of renin-angiotensin system blockade and steroid/immunosuppressive treatment among familial IgA nephropathy patients with preserved eGFR and nonpreserved eGFR.
Figure S1. Cox regression analysis for risk of lifetime ESRD among sporadic IgAN patients and familial IgAN diagnosed by renal biopsy (A) and by clinical features (B) in different subgroups.
STROBE Statement.
Supplementary Material
Table S1. Diagnostic criteria of familial IgA nephropathy patients.
Table S2. Cox regression analyses for sporadic and familial IgA nephropathy patients with steroid and immunosuppressive treatments.
Table S3. Comparison of renin-angiotensin system blockade and steroid/immunosuppressive treatment among familial IgA nephropathy patients with preserved eGFR and nonpreserved eGFR.
Figure S1. Cox regression analysis for risk of lifetime ESRD among sporadic IgAN patients and familial IgAN diagnosed by renal biopsy (A) and by clinical features (B) in different subgroups.
STROBE Statement.
References
- 1.Xie J., Chen N. Primary glomerulonephritis in mainland China: an overview. Contrib Nephrol. 2013;181:1–11. doi: 10.1159/000348642. [DOI] [PubMed] [Google Scholar]
- 2.Xie J., Shapiro S., Gharavi A. Genetic studies of IgA nephropathy: what have we learned from genome-wide association studies. Contrib Nephrol. 2013;181:52–64. doi: 10.1159/000348652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Barbour S.J., Cattran D.C., Kim S.J. Individuals of Pacific Asian origin with IgA nephropathy have an increased risk of progression to end-stage renal disease. Kidney Int. 2013;84:1017–1024. doi: 10.1038/ki.2013.210. [DOI] [PubMed] [Google Scholar]
- 4.Tolkoff-Rubin N.E., Cosimi A.B., Fuller T., Rublin R.H., Colvin R.B. IGA nephropathy in HLA-identical siblings. Transplantation. 1978;26:430–433. doi: 10.1097/00007890-197812000-00014. [DOI] [PubMed] [Google Scholar]
- 5.Kiryluk K., Julian B.A., Wyatt R.J. Genetic studies of IgA nephropathy: past, present, and future. Pediatr Nephrol. 2010;25:2257–2268. doi: 10.1007/s00467-010-1500-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gharavi A.G., Yan Y., Scolari F. IgA nephropathy, the most common cause of glomerulonephritis, is linked to 6q22-23. Nat Genet. 2000;26:354–357. doi: 10.1038/81677. [DOI] [PubMed] [Google Scholar]
- 7.Bisceglia L., Cerullo G., Forabosco P. Genetic heterogeneity in Italian families with IgA nephropathy: suggestive linkage for two novel IgA nephropathy loci. Am J Hum Genet. 2006;79:1130–1134. doi: 10.1086/510135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Paterson A.D., Liu X.Q., Wang K. Genome-wide linkage scan of a large family with IgA nephropathy localizes a novel susceptibility locus to chromosome 2q36. J Am Soc Nephrol. 2007;18:2408–2415. doi: 10.1681/ASN.2007020241. [DOI] [PubMed] [Google Scholar]
- 9.Feehally J., Farrall M., Boland A. HLA has strongest association with IgA nephropathy in genome-wide analysis. J Am Soc Nephrol. 2010;21:1791–1797. doi: 10.1681/ASN.2010010076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gharavi A.G., Kiryluk K., Choi M. Genome-wide association study identifies susceptibility loci for IgA nephropathy. Nat Genet. 2011;43:321–327. doi: 10.1038/ng.787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yu X.Q., Li M., Zhang H. A genome-wide association study in Han Chinese identifies multiple susceptibility loci for IgA nephropathy. Nat Genet. 2011;44:178–182. doi: 10.1038/ng.1047. [DOI] [PubMed] [Google Scholar]
- 12.Kiryluk K., Li Y., Scolari F. Discovery of new risk loci for IgA nephropathy implicates genes involved in immunity against intestinal pathogens. Nat Genet. 2014;46:1187–1196. doi: 10.1038/ng.3118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Saka S., Hirawa N., Oka A. Genome-wide association study of IgA nephropathy using 23 465 microsatellite markers in a Japanese population. J Hum Genet. 2015;60:573–580. doi: 10.1038/jhg.2015.88. [DOI] [PubMed] [Google Scholar]
- 14.Li M., Foo J.N., Wang J.Q. Identification of new susceptibility loci for IgA nephropathy in Han Chinese. Nat Commun. 2015;6:7270. doi: 10.1038/ncomms8270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Barbour S.J., Espino-Hernandez G., Reich H.N. The MEST score provides earlier risk prediction in IgA nephropathy. Kidney Int. 2016;89:167–175. doi: 10.1038/ki.2015.322. [DOI] [PubMed] [Google Scholar]
- 16.Bartosik L.P., Lajoie G., Sugar L., Cattran D.C. Predicting progression in IgA nephropathy. Am J Kidney Dis. 2001;38:728–735. doi: 10.1053/ajkd.2001.27689. [DOI] [PubMed] [Google Scholar]
- 17.Berthoux F., Mohey H., Laurent B. Predicting the risk for dialysis or death in IgA nephropathy. J Am Soc Nephrol. 2011;22:752–761. doi: 10.1681/ASN.2010040355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Goto M., Wakai K., Kawamura T. A scoring system to predict renal outcome in IgA nephropathy: a nationwide 10-year prospective cohort study. Nephrol Dialysis Transplant. 2009;24:3068–3074. doi: 10.1093/ndt/gfp273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ouyang Y., Xie J., Yang M. Underweight is an independent risk factor for renal function deterioration in patients with IgA nephropathy. PLoS One. 2016;11:e0162044. doi: 10.1371/journal.pone.0162044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Xie J., Kiryluk K., Wang W. Predicting progression of IgA nephropathy: new clinical progression risk score. PLoS One. 2012;7:e38904. doi: 10.1371/journal.pone.0038904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yang M., Xie J.Y., Ouyang Y. ABO blood type is associated with renal outcomes in patients with IgA nephropathy. Oncotarget. 2017;8:73603–73612. doi: 10.18632/oncotarget.20701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Shi M., Ouyang Y., Yang M. IgA nephropathy susceptibility loci and disease progression. Clin J Am Soc Nephrol. 2018;13:1330–1338. doi: 10.2215/CJN.13701217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Xie J., Lv J., Wang W. Kidney failure risk prediction equations in IgA nephropathy: a multicenter risk assessment study in Chinese patients. Am J Kidney Dis. 2018;72:371–380. doi: 10.1053/j.ajkd.2018.01.043. [DOI] [PubMed] [Google Scholar]
- 24.Coppo R., Peruzzi L., Amore A. IgACE: a placebo-controlled, randomized trial of angiotensin-converting enzyme inhibitors in children and young people with IgA nephropathy and moderate proteinuria. J Am Soc Nephrol. 2007;18:1880–1888. doi: 10.1681/ASN.2006040347. [DOI] [PubMed] [Google Scholar]
- 25.Li P.K., Leung C.B., Chow K.M. Hong Kong study using valsartan in IgA nephropathy (HKVIN): a double-blind, randomized, placebo-controlled study. Am J Kidney Dis. 2006;47:751–760. doi: 10.1053/j.ajkd.2006.01.017. [DOI] [PubMed] [Google Scholar]
- 26.Park H.C., Xu Z.G., Choi S. Effect of losartan and amlodipine on proteinuria and transforming growth factor-beta1 in patients with IgA nephropathy. Nephrol Dialysis Transplant. 2003;18:1115–1121. doi: 10.1093/ndt/gfg090. [DOI] [PubMed] [Google Scholar]
- 27.Lv J., Zhang H., Wong M.G. Effect of oral methylprednisolone on clinical outcomes in patients with IgA nephropathy: the TESTING randomized clinical trial. JAMA. 2017;318:432–442. doi: 10.1001/jama.2017.9362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rauen T., Eitner F., Fitzner C. Intensive supportive care plus immunosuppression in IgA nephropathy. N Engl J Med. 2015;373:2225–2236. doi: 10.1056/NEJMoa1415463. [DOI] [PubMed] [Google Scholar]
- 29.Schena F.P., Cerullo G., Rossini M. Increased risk of end-stage renal disease in familial IgA nephropathy. J Am Soc Nephrol. 2002;13:453–460. doi: 10.1681/ASN.V132453. [DOI] [PubMed] [Google Scholar]
- 30.Izzi C., Ravani P., Torres D. IgA nephropathy: the presence of familial disease does not confer an increased risk for progression. Am J Kidney Dis. 2006;47:761–769. doi: 10.1053/j.ajkd.2006.01.010. [DOI] [PubMed] [Google Scholar]
- 31.Levey A.S., Stevens L.A., Schmid C.H. A new equation to estimate glomerular filtration rate. Ann Intern Med. 2009;150:604–612. doi: 10.7326/0003-4819-150-9-200905050-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Julian B.A., Quiggins P.A., Thompson J.S. Familial IgA nephropathy. Evidence of an inherited mechanism of disease. N Engl J Med. 1985;312:202–208. doi: 10.1056/NEJM198501243120403. [DOI] [PubMed] [Google Scholar]
- 33.Sabatier J.C., Genin C., Assenat H. Mesangial IgA glomerulonephritis in HLA-identical brothers. Clin Nephrol. 1979;11:35–38. [PubMed] [Google Scholar]
- 34.Scolari F., Amoroso A., Savoldi S. Familial clustering of IgA nephropathy: further evidence in an Italian population. Am J Kidney Dis. 1999;33:857–865. doi: 10.1016/s0272-6386(99)70417-8. [DOI] [PubMed] [Google Scholar]
- 35.Julian B.A., Woodford S.Y., Baehler R.W. Familial clustering and immunogenetic aspects of IgA nephropathy. Am J Kidney Dis. 1988;12:366–370. doi: 10.1016/s0272-6386(88)80026-x. [DOI] [PubMed] [Google Scholar]
- 36.Wyatt R.J., Julian B.A. IgA nephropathy. N Engl J Med. 2013;368:2402–2414. doi: 10.1056/NEJMra1206793. [DOI] [PubMed] [Google Scholar]
- 37.Norris K.C., Greene T., Kopple J. Baseline predictors of renal disease progression in the African American Study of Hypertension and Kidney Disease. J Am Soc Nephrol. 2006;17:2928–2936. doi: 10.1681/ASN.2005101101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Menon V., Wang X., Sarnak M.J. Long-term outcomes in nondiabetic chronic kidney disease. Kidney Int. 2008;73:1310–1315. doi: 10.1038/ki.2008.67. [DOI] [PubMed] [Google Scholar]
- 39.Ricardo A.C., Yang W., Sha D. Sex-related disparities in CKD progression. J Am Soc Nephrol. 2019;30:137–146. doi: 10.1681/ASN.2018030296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Rheault M.N. Women and Alport syndrome. Pediatr Nephrol. 2012;27:41–46. doi: 10.1007/s00467-011-1836-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.