Abstract
Direct-current (DC) cardioversion is effective at terminating arrhythmias in an emergency. During treatment, energy delivery synchronizing with the QRS complex is essential to avoid ventricular fibrillation (VF) caused by a shock on the T wave, which is the vulnerable period of ventricular repolarization. However, distinguishing the QRS from the T wave is difficult in some patients with abnormal, irregular, and varying QRS complexes. We report the case of a 45-year-old man who had iatrogenic VF caused by inappropriate synchronization with the T wave during cardioversion of pre-excited atrial fibrillation due to high ventricular rates and varying R wave amplitude affected by an accessory pathway.
<Learning objective: During direct-current cardioversion, energy delivery synchronizing with the QRS complex is essential to avoid ventricular fibrillation (VF) caused by a shock on the T wave. However, distinguishing the QRS from the T wave is difficult in some patients with abnormal, irregular, and varying QRS complexes. We report a case of iatrogenic VF caused by failed synchronization with the R wave in a patient with pre-excited atrial fibrillation (AF). Clinicians managing pre-excited AF should be aware of the possibility of iatrogenic VF triggered by cardioversion.>
Keywords: Pre-excited atrial fibrillation, Cardioversion, Iatrogenic ventricular fibrillation, Wolff–Parkinson–White syndrome
Introduction
Direct-current (DC) cardioversion is effective at terminating arrhythmias in an emergency. During treatment, energy delivery synchronizing with the QRS complex is essential to avoid ventricular fibrillation (VF) caused by a shock on the T wave, which is the vulnerable period of ventricular repolarization. However, distinguishing the QRS from the T wave is difficult in some patients with abnormal, irregular, and varying QRS complexes.
Case report
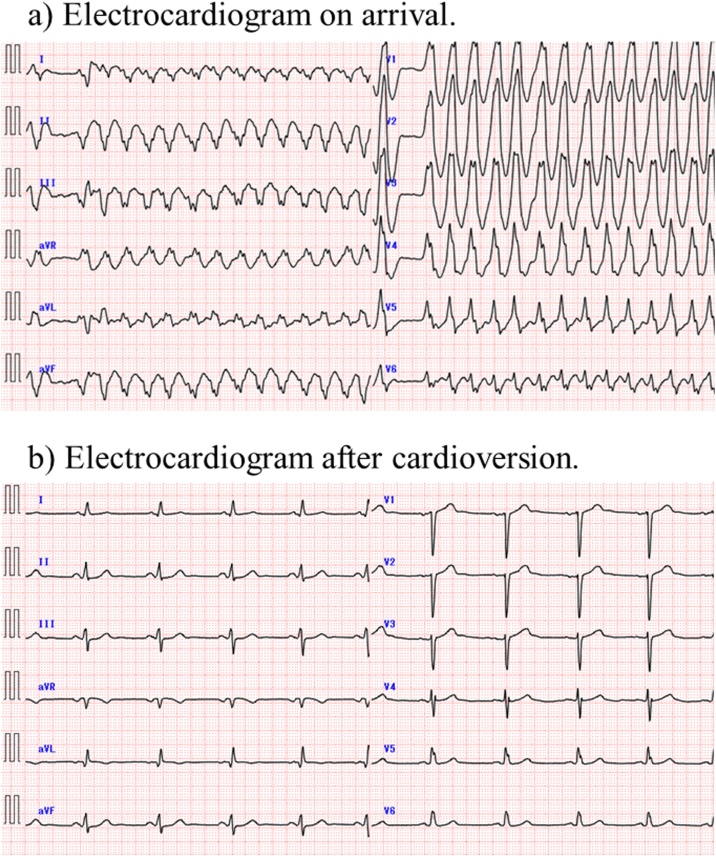
A 45-year-old man with a previous history of broad anterior myocardial infarction presented to our emergency room with palpitations and dizziness. His blood pressure was 129/78 mmHg and his pulse rate was 168/min. Electrocardiography (ECG) on arrival demonstrated wide QRS tachycardia with irregular R-R intervals (Fig. 1a). He received 100 mg of intravenous lidocaine, but there was no change in heart rate or QRS morphology. He was then sedated by intravenous propofol and a 100-J shock was performed with synchronization on. The rhythm reverted to sinus rhythm, but a typical delta wave was not observed on ECG (Fig. 1b). We later confirmed that his previous 12-lead ECG, when he had myocardial infarction treated, did not show a typical delta wave.
Fig. 1.
Electrocardiography. (a) Electrocardiography on arrival showed wide QRS tachycardia with irregular R-R intervals. (b) Electrocardiography after cardioversion showed normal sinus rhythm and the delta wave was not recognized.
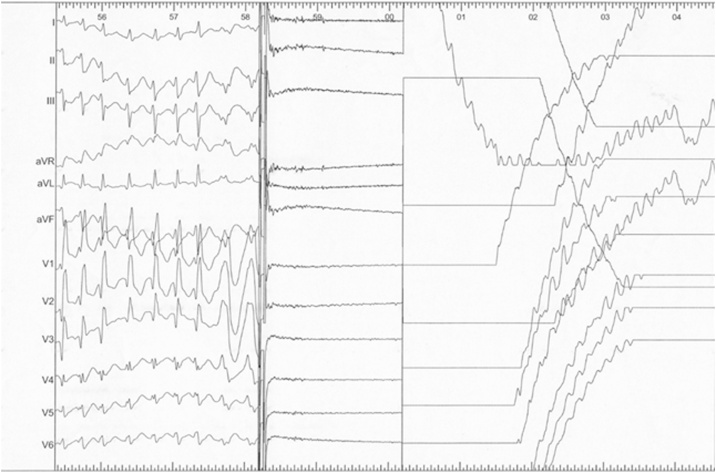
We performed staged electrophysiological study (EPS) and catheter ablation for symptomatic wide QRS tachycardia. In the EPS, a left posterolateral accessory pathway was identified as the earliest site of ventricular pre-excitation, which manifested more clearly by left atrial pacing from an electrode catheter in the coronary sinus. No ventricular tachycardia was induced by the ventricular program stimulation. Furthermore, we administered isoproterenol to provoke arrhythmia, and atrial fibrillation (AF) developed spontaneously. The width of QRS varied irregularly beat by beat. The wide QRS morphology was similar to the initial ECG of wide QRS tachycardia, which was documented in the emergency room. When a synchronized 100-J shock was delivered, VF was induced by the shock on the T wave due to inappropriate synchronization of the external defibrillator (Fig. 2). Thereafter, the rhythm reverted to sinus rhythm spontaneously during the preparation of extra defibrillation. The left posterolateral accessory pathway was then ablated, after which the patient did well. We did not treat AF, but the recurrence of AF has never been observed after the ablation.
Fig. 2.
Electrocardiography during catheter ablation showed that the R wave amplitude decreased with wider QRS complexes in several beats just before the shock, and cardioversion for atrial fibrillation-induced ventricular fibrillation.
Discussion
In patients with ventricular pre-excitation, AF can cause rapid ventricular activation via the accessory pathway and lead to irregular wide-QRS tachycardia with varying QRS widths. This is known as pre-excited AF.
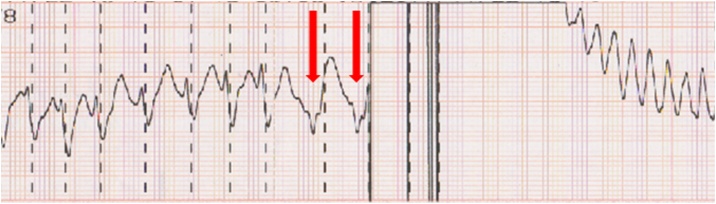
In this case, the R wave amplitude decreased with wider QRS complexes in several beats just before the shock that induced VF. This change in QRS morphology may be explained by the rate of ventricular activation via the accessory pathway increasing with varying QRS widths. On review of the printout from the defibrillator monitor, the shock was delivered at the beginning of the T wave because of failure to recognize the decreased amplitude of the R wave, although the preceding beats exhibited a synchronized line on the R wave (Fig. 3). Therefore, it would have been difficult to avoid the VF induction because of the sudden change in QRS morphology immediately before the shock delivery in this case. However, we could have avoided it if we had taken into consideration that the AF tachycardia could potentially change into the wide QRS tachycardia as was seen at the first cardioversion in the emergency room.
Fig. 3.
Telemetry strip showing inappropriate synchronization and shock on the T wave because of the failure to recognize the decreased amplitude of the R wave (red arrow), although the preceding beats exhibited the synchronized line on the R wave, which induced ventricular fibrillation.
We found 5 previous reports before 2017 [1], [2], [3], [4], [5], one in 2018 [6], and one in 2019 [7] demonstrating that iatrogenic VF was caused by synchronization failure during electrical cardioversion of pre-excited AF in patients with Wolff–Parkinson–White syndrome. In the reports in 2018 and 2019, inappropriate synchronization with the T wave induced VF, as in the present case. These previous reports emphasized the importance of careful selection of ECG leads and gain before delivering a DC shock. In the present case, we assessed the accuracy of synchronization with the R wave just before defibrillation, but we were unable to avoid shocking on the T wave because of the abrupt decline in R wave amplitude in several beats just before the shock. Even if the other available limb leads (lead I and lead III) were selected for synchronization with the R wave, we would not have avoided the inappropriate shock because the decrease in R wave amplitude was observed in leads I, II, and III (Fig. 2). It would be useful to change the positions of limb electrodes so that the R wave amplitude is high enough to secure the appropriate synchronization, especially when pre-excited AF is present. To the best of our knowledge, this is the first report of an abrupt decline in R wave amplitude leading to failed synchronization with the R wave, which caused VF due to shocking on the T wave just before defibrillation.
Of note, during a rapid and irregular rhythm in patients with pre-excited AF, even a shock appropriately synchronized with the QRS complex has the potential to induce VF if delivered after a short R-R interval (≤300 ms) following a long R-R interval, especially if lower-energy shocks are used [8], [9]. This occurs when one region of the ventricles is still repolarizing because of the regional delay after the long-short sequence while the remaining myocardium begins to depolarize. In previous reports [2], [3], [5], [6], [7], VF was induced by synchronous T-wave shocks with energy lower than 100 J. Selecting a higher energy may be helpful in preventing VF induction [5]. However, in another report [6], VF was induced by a 360-J shock, suggesting that high-energy shocks do not necessarily compensate for poor synchronization.
Conclusion
Appropriate synchronization of cardioversion with the QRS is essential, but it is sometimes difficult in patients with pre-excited AF because the QRS-T complex may change morphology abruptly. Cardioversion of pre-excited AF may induce VF due to failure to synchronize with the R wave. Clinicians managing pre-excited AF should be aware of the possibility of developing VF.
Conflicts of interest
All of the authors have no conflicts of interest to disclose.
References
- 1.Ebrahimi R., Rubin S.A. Electrical cardioversion resulting in death from synchronization failure. Am J Cardiol. 1994;74:100–102. doi: 10.1016/0002-9149(94)90504-5. [DOI] [PubMed] [Google Scholar]
- 2.Adlam D., Azeem T. Ventricular fibrillation during electrical cardioversion of pre-excited atrial fibrillation. Postgrad Med J. 2003;79:297–299. doi: 10.1136/pmj.79.931.297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Xavier L.C., Memon A. Synchronized cardioversion of unstable supraventricular tachycardia resulting in ventricular fibrillation. Ann Emerg Med. 2004;44:178–180. doi: 10.1016/j.annemergmed.2004.03.036. [DOI] [PubMed] [Google Scholar]
- 4.O’Connell M., Bernard A. A serious cause of panic attack. Case Rep Emerg Med. 2012;2012:393275. doi: 10.1155/2012/393275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Taylor N. Direct current cardioversion causing ventricular fibrillation in Wolff–Parkinson–White syndrome. Emerg Med Australas. 2013;25:612–614. doi: 10.1111/1742-6723.12150. [DOI] [PubMed] [Google Scholar]
- 6.Kaufmann M.R., McKillop M.S., Burkart T.A., Panna M., Conti J.B., Miles W.M. Iatrogenic ventricular fibrillation after direct-current cardioversion of preexcited atrial fibrillation caused by inadvertent T-wave synchronization. Tex Heart Inst J. 2018;45:39–41. doi: 10.14503/THIJ-16-6162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Danon A., Militianu A., Schliamser J.E. Cardioversion of pre-excited atrial fibrillation leading to ventricular fibrillation — case report and review of literature. Am J Emerg Med. 2019;37:1539–1543. doi: 10.1016/j.ajem.2019.05.031. [DOI] [PubMed] [Google Scholar]
- 8.Ayers G.M., Alferness C.A., Ilina M., Wagner D.O., Sirokman W.A., Adams J.M. Ventricular proarrhythmic effects of ventricular cycle length and shock strength in a sheep model of transvenous atrial defibrillation. Circulation. 1994;89:413–422. doi: 10.1161/01.cir.89.1.413. [DOI] [PubMed] [Google Scholar]
- 9.Barold H.S., Wharton J.M. Ventricular fibrillation resulting from synchronized internal atrial defibrillation in a patient with ventricular preexcitation. J Cardiovasc Electrophysiol. 1997;8:436–440. doi: 10.1111/j.1540-8167.1997.tb00809.x. [DOI] [PubMed] [Google Scholar]