Abstract
Introduction
Establishing the frequency and nature of arrhythmias in hemodialysis (HD) is an important step in improving outcomes of these patients. We undertook this systematic review and meta-analysis to characterize arrhythmia frequency in maintenance HD patients.
Methods
We identified studies on arrhythmias in adult patients on maintenance HD detected via implantable loop recorders (ILRs). Studies included were in English and reported ILR-detected arrhythmia incidence in HD patients. Data were extracted by one author using electronic spreadsheets and verified by a second author. Random effects models were used for pooled inferences. The I2 statistic was used to quantify heterogeneity.
Results
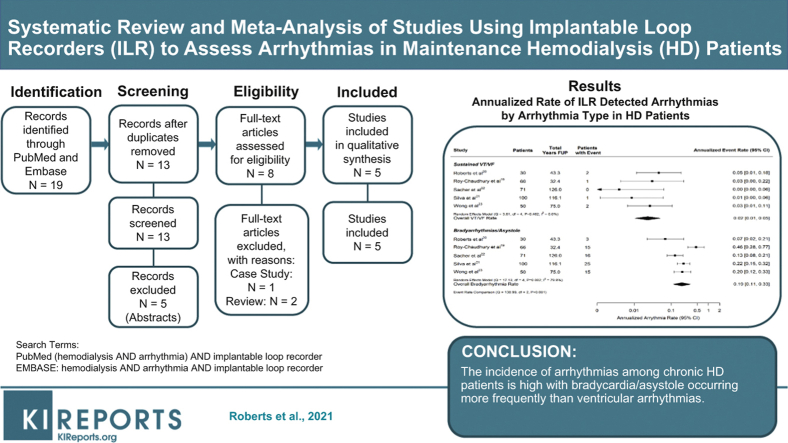
Five studies qualified for inclusion (317 patients). The overall estimates for the annualized rate of death and sudden cardiac death (SCD) was 0.14 (95% confidence interval [CI]: 0.11–0.18) and 0.06 (95% CI: 0.03–0.10), respectively. Across all 5 studies, the combined annualized rate of patients experiencing at least 1 bradycardia/asystole event was 0.19 (95% CI: 0.11–0.33) but heterogeneity was high (I2 = 79.8%). The average annualized rate of sustained ventricular tachycardia (VT) or ventricular fibrillation (VF) episodes (0.02, 95% CI: 0.01–0.05) was significantly lower (P < 0.001) than the rate of bradycardia/asystole reported in the same patients. Incidence of atrial fibrillation (AF) varied significantly across the studies (from 0.07 to 0.83 patients per year) reflecting variable definitions (new-onset vs. total number of episodes).
Conclusion
The incidence of arrhythmias among chronic HD patients is high, with bradycardia/asystole occurring more frequently than ventricular arrhythmias. Additional studies to refine estimates particularly of AF are needed.
Keywords: asystole, bradycardia, hemodialysis, implantable loop recorder, sudden cardiac death
Graphical abstract
Maintenance dialysis patients have a greatly increased risk of dying compared to patients with preserved kidney function with mortality rates exceeding 180 per 1000 patient-years in the most recent United States Renal Data System Report.1 The majority of deaths are attributed to cardiovascular disease, with sudden cardiovascular death and arrhythmia frequently implicated and accounting for 40% of deaths with known cause.1 Similar rates of sudden death have been reported in large clinical trials enrolling dialysis patients, confirming that sudden death is a critical problem for the dialysis population, particularly among those receiving chronic HD.2,3
Although this high risk of cardiovascular and sudden death is now widely recognized, it is not fully explained by traditional cardiovascular risk factors.4,5 The etiology of sudden death, in particular, remains incompletely understood. A clear relationship between the timing of dialysis sessions and episodes of sudden death6,7 as well as studies demonstrating associations with serum or dialysate electrotype concentrations8, 9, 10, 11 have strongly implicated arrhythmia as the principal cause of sudden deaths on dialysis. However, sudden deaths are frequently unwitnessed, and the only autopsy study to investigate the etiology of sudden death in chronic dialysis patients included only 35 patients and identified dissecting aortic aneurysm rather than primary arrhythmia as the most frequent sudden death trigger.12
Establishing whether HD induces potentially fatal arrhythmias, how frequently they occur, and the nature of those arrhythmias, particularly the terminal rhythms underlying sudden death events, would be an important step in improving outcomes for patients on dialysis. Documentation of premature ventricular or atrial contractions, peri-dialytic changes in electrocardiographic morphology, and changes in heart rate variability13, 14, 15, 16, 17, 18 has motivated the need for cardiac monitoring, but until recently, the use of surface electrocardiographs or Holter monitoring technology limited the realistic duration of monitoring to periods of time that were insufficiently long to reliably capture the occurrence of arrhythmia or SCD.
Contemporary ILRs have enabled minimally invasive and comprehensive capture of arrhythmias for periods up to several years. A number of studies have now leveraged this technology to better characterize the occurrence of arrhythmias in the setting of HD, with each study reporting a high incidence of cardiac arrythmias.19, 20, 21, 22, 23 However, the challenges inherent to recruiting patients for observational research using an ILR required stringent recruitment criteria and limited cohort size in these studies. As a result, CIs around estimated rates were wide and the overall generalizability of the findings were uncertain. We undertook this meta-analysis of studies using ILR technology to characterize arrhythmia in maintenance HD patients, summarize available data, describe the populations studied, and refine estimates of arrhythmia incidence among maintenance HD patients.
Materials and Methods
Search Strategy, Study Selection, and Data Extraction
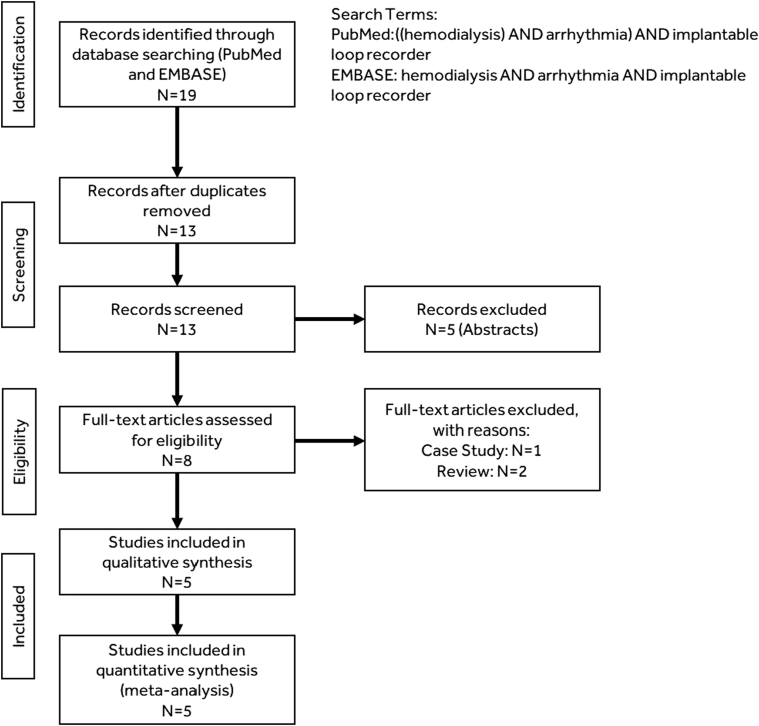
This meta-analysis was performed in compliance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) statement. In accordance with PRISMA guidelines, this meta-analysis was registered in PROSPERO (Registration no. CRD42019130461). A systematic review and meta-analysis were conducted following a literature search of PubMed and Embase to identify publications on arrhythmias in HD patients detected via ILR. Search dates included all articles published between January 1, 2000, and March 20, 2019. Search terms included: “Hemodialysis” AND “Arrhythmia” AND “Implantable loop recorder.”
Titles and abstracts were retrieved and reviewed. Articles were included if they reported ILR-detected arrhythmia incidence in HD patients and were in the English language. Case reports, conference abstracts, editorials/commentary/letters, and review articles were excluded. Final determination on article inclusion was assessed by 2 authors via a consensus process (KS and LCJ). Data were extracted by one author (KS or LCJ) and verified by a second author (KS or LCJ).
Quality Review
A modified Newcastle-Ottawa scale was used to assess study quality.24 The Newcastle-Ottawa scale is a system to characterize quality of studies included in systematic reviews and meta-analyses. Each study is assessed with 8 questions categorized into 3 groups: study group selections, group comparability, and the ascertainment of the outcomes of interest. Two authors (TAM and BW) independently reviewed all manuscripts for study quality, with differences adjudicated by contacting the manuscript authors for clarification and then reaching consensus. Because all studies were observational cohort studies without controls, the comparability metric within the scale was not applicable and was not included in our modified scoring system.
Meta-analysis
Continuous variables were summarized by extracting means, SDs, and sample sizes from reports. Mean and SD were estimated from the reported medians and interquartile range if necessary.24 Numerators and denominators were extracted to estimate overall proportions and CIs for dichotomous variables. To provide summary estimates, random effects models were used to estimate overall summary statistics across studies where individual studies were treated as a random variable.
The number of patient deaths, SCD, clinically relevant VT or VF, bradycardia, asystole, and AF per study definition were extracted from each report, and the event rate per year of follow-up was estimated. Total follow-up time was computed by multiplying the average follow-up time in years by the number of patients studied for each study. A continuity correction of 0.5 was added to studies where zero events were reported. A mixed effect model treating each study as a random effect and type of arrhythmia as a fixed effect was used to test the difference between rates of bradycardia/asystole and VT or VF events.
Cochran’s Q test for heterogeneity was performed for each model to determine if the variability in outcomes was larger than expected by sampling variability. The I2 statistic was used to quantify the magnitude of heterogeneity.25 Tests for heterogeneity between studies for most outcomes of interest were highly significant; thus, random effects models were used to better incorporate study heterogeneity when making pooled inferences. Mixed effects models were employed for meta-regression to evaluate the impact of study level and aggregated patient-level data on the difference in annualized bradycardia/asystole rates. Reporting bias was assessed visually by plotting each report’s log event rate versus its standard error (funnel plot).
The metafor package for R (www.r-project.org) was used for all analyses.26 P values < 0.05 were considered significant.
Results
Selected Studies
As shown in Figure 1, we identified 19 publications via PubMed and Embase searches, of which 5 qualified for inclusion.19, 20, 21, 22, 23 Characteristics of the included studies are displayed in Table 1. The total population included 317 patients with end-stage renal disease implanted with REVEAL XT/LINQ (Medtronic Inc., Fridley, MN) devices (4 studies) or CONFIRM (Abbott, Inc., Abbott Park, IL) devices (1 study). Initial enrollment ranged from 2009 to 2013 and average follow-up within study ranged from 5.9 to 21.3 months with a total of 392.8 years of total patient follow-up. Study quality range from 5 to 6 on the Newcastle-Ottawa (NOS) 6-point scale.
Figure 1.
Publication selection.
Table 1.
Characteristics of the included studies
| Characteristic | Roberts et al.20 | Roy-Chaudhury et al.19 | Sacher et al.22 | Silva et al.21 | Wong et al.23 |
|---|---|---|---|---|---|
| Country | United Kingdom | United States, India | France | Brazil | Australia |
| Years | 2011–2014 | 2013–2014 | 2010–2014 | 2009–2010 | 2012–2014 |
| Follow-up, mo | 17.2 ± 12.3 | 5.9 ± 0.7 | 21.3 ± 6.9 | 13.9 ± 4.2 | 18.0 ± 4.0 |
| Patients, n | 30 | 66 | 71 | 100 | 50 |
| Device episodes adjudication | Yes (authors) | Yes (core lab) | Yes (authors) | Yes (authors) | Yes (authors) |
| Bradycardia definition | ≤30 bpm for ≥4 beats | ≤40 bpm for ≥6 sec | ≤30 bpm for ≥4 beats | <40 bpm | ≤40 bpm for ≥4 beats |
| Asystole definition | Pause ≥ 3 s | Pause ≥ 3 s | Pause ≥ 3 s | Pause ≥ 3 s | Pause ≥ 3 s |
| VT/VF definition | 12 beats >162 bpm | ≥130 bpm for ≥30 s or VF | ≥150 bpm for ≥30 s or VF | VT > 30 s or VF | ≥125 bpm for ≥30 s or VF |
| AF definition | New-onset ILR-detected AF | ILR-detected AF (new-onset and total) | ILR-detected AF (new-onset and total) | ILR-detected AF | ILR-detected AF (new-onset and total) |
| NOS scorea | 5/6 | 6/6 | 6/6 | 6/6 | 5/6 |
| ILR device | Reveal XT | Reveal XT or Reveal LINQ | Reveal XT | Reveal XT | Confirm |
AF, atrial fibrillation; bpm, beats per minute; ILR; implantable loop recorder; NOS, Newcastle-Ottawa scale; VT, ventricular tachycardia; VF, ventricular fibrillation.
Three items’ metrics within the scale were not applicable and were not included in our modified scoring system.
Baseline characteristics were reported in all 5 studies (Table 2), though the number and types of baseline characteristics reported varied greatly. Average age was 62.9 years (95% CI: 58.8–67.0). Patients started dialysis on average 46.0 months (95% CI: 31.1–60.8) prior to study.
Table 2.
Baseline characteristics
| Baseline characteristic | Roberts et al.20 | Roy-Chaudhury et al.19 | Sacher et al.22 | Silva et al.21 | Wong et al.23 | n | Value (95% CI) |
|---|---|---|---|---|---|---|---|
| Age, yr | 67.8 ± 12.1 | 56.3 ± 12.2 | 65 ± 8.6 | 59.0 ± 8.8 | 67.0 ± 11.0 | 317 | 62.9 (58.8–67.0) |
| Male, % | 60.0 | 69.7 | 73.2 | 65.0 | 72.0 | 317 | 68.7 (63.7–73.8) |
| Mean months since dialysis initiation | 45 ± 40 | 35.6 ± 37.3a | 25.6 ± 32.7a | 53.8 ± 30.0 | 72 ± 48 | 317 | 46.0 (31.1–60.8) |
| Diabetes, % | 36.7 | 63.6 | 59.1 | 70.0 | 58.0 | 317 | 58.8 (48.5–69.2) |
| Hyperlipidemia, % | — | 60.6 | — | 54.0 | 58.0 | 216 | 57.0 (50.4–63.6) |
| Hypertension, % | — | 84.8 | 84.1 | 97.0 | 86.0 | 287 | 88.8 (80.8–96.7) |
| Heart rate, bpm | 73 ± 12 | — | 71 ± 13 | 73 ± 15.2 | — | 201 | 72.2 (70.3–74.1) |
| PR interval, ms | 174 ± 44 | — | 185 ± 34 | 173 ± 24 | — | 201 | 177.6 (168.9–186.3) |
| QRS, ms | 102 ± 22 | — | 94 ± 22 | 91 ± 18 | — | 201 | 94.8 (89.6–100.0) |
| LVEF, % | 55 ± 8 | 56.7 ± 3.8a | 61 ± 11 | 59.5 ± 10.8 | 35%–40%: 4% 40%–59%: 36% >60%: 58% |
295 | 58.0 (55.7–60.3)b |
| Serum potassium, mmol/l | 4.9 ± 0.5 | 4.8 ± 0.9a | 4.7 ± 0.7 | — | — | 167 | 4.8 (4.7–4.9) |
| Hemoglobin, g/dl | 11.8 ± 1.3 | 10.7 ± 1.1a | 11.2 ± 1.2 | — | — | 167 | 11.2 (10.6–11.8) |
LVEF, left ventricular ejection fraction; PR, interval duration on ECG; QRS, interval on baseline ECG.
N, sample size of reported data.
Dashes indicate variables that were not reported.
Mean and SD estimated from median and interquartile range.
Pooled estimate for LVEF did not include data from Wong et al.23
Deaths
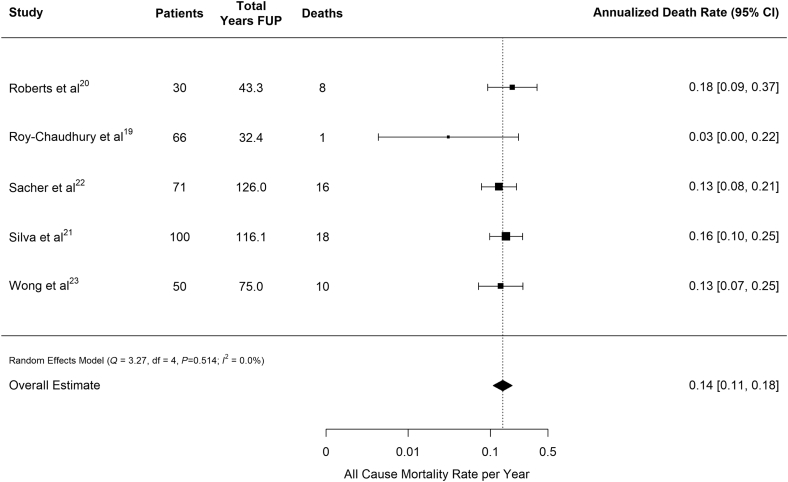
Deaths from any cause totaled 53 and ranged from 1 to 18 patients, with corresponding annualized death rates ranging from 0.03 to 0.18 deaths per patient-year of follow-up. Across all 5 studies, the overall estimate for the rate of death was 0.14 (95% CI: 0.11–0.18) deaths per patient-year of follow-up (Table 3, Figure 2).
Table 3.
Results of the included studies
| Outcome: events (patients) | Roberts et al.20 | Roy-Chaudhury et al.19 | Sacher et al.22 | Silva et al.21 | Wong et al.23 |
|---|---|---|---|---|---|
| Patients (number) | 30 | 66 | 71 | 100 | 50 |
| Deaths | 8 | 1 | 16 | 18 | 10 |
| SCD | 2 | 0 | 4 | 7 | 8 |
| Bradycardia | — | 1461 (13) | — | — | 1031 (15) |
| Asystole | — | 14 (6) | — | — | 180 (14) |
| Bradycardia/ asystole | 3 (3) | 1475 (15) | 64 (16) | 155 (25) | — |
| VT/VF | 2 (2) | 1 | 0 | 1 | 10 (2) |
| AF | 188 (3) | 4419 (27); new onset in 22 | NR (21); new onset in 14 | 42 (13) | 4749 (21); new onset in 14 |
| AF annualized rate∗ (95% CI) | 0.07 (0.02–0.21) | 0.68 (0.45–1.03) | 0.11 (0.07–0.19) | 0.11 (0.07–0.19) | 0.19 (0.11–0.32) |
| Intervention | 3 pacemakers | 5 pacemakers | 3 pacemakers | 1 pacemaker | 1 pacemaker, 1 ICD |
AF, atrial fibrillation; CI, confidence interval; ICD, implantable cardioverter defibrillator; NR, not reported; SCD, sudden cardiac death; VF, ventricular fibrillation; VT, ventricular tachycardia.
New-onset AF used when available.
Figure 2.
Annualized death rate for any cause pooled across studies. Vertical line indicates pooled estimate across studies. FUP, follow-up.
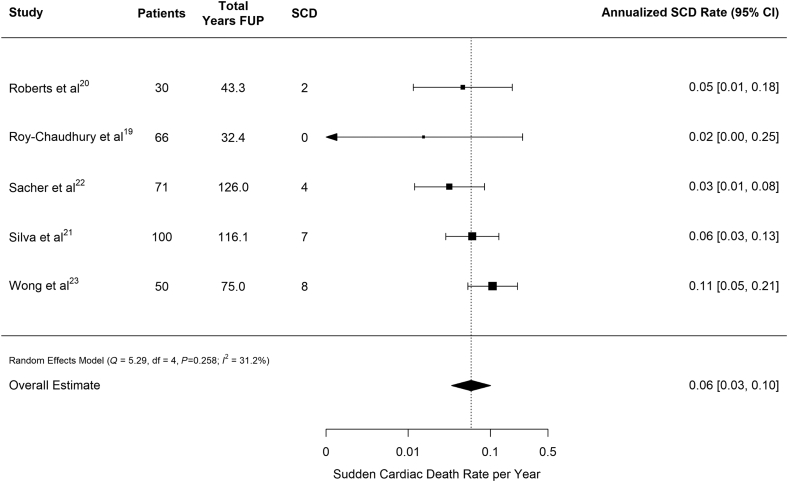
Of 53 total deaths reported, 21 were considered SCD. However, only 2 studies provided a definition for SCD,19,21 with both defining SCD as sudden, unexpected death within 1 hour of symptom onset or unwitnessed, unexpected death without obvious noncardiac cause in patients known to be well within the past 24 hours. The overall rate of SCD was 0.06 (95% CI: 0.03–0.10) SCDs per patient-year of follow-up (Table 3, Figure 3). Cardiac-related deaths made up 24 (45.3%) of the deaths reported, and half of these (n = 12) were associated with bradycardia preceding death. Thus, 12 of 21 SCDs (57%) were due to bradycardia. However, for several patients the cardiac tracings were not available at the time of death. Of those in which preterminal recordings were available, 12 of 16 (75%) were due to bradycardia.
Figure 3.
Annualized sudden cardiac death rate pooled across studies. Vertical line indicates pooled estimate across studies. FUP, follow-up; SCD, sudden cardiac death.
Arrhythmias
Bradycardia and sustained VT or VF definitions differed among the 5 studies. Specifically, 2 studies defined bradycardia as episodes where the heart rate was ≤30 beats per minute (bpm) for at least 4 consecutive beats,22,27 whereas the remaining 3 studies defined bradycardia as episodes with a heart rate <40 bpm (Table 1).19,21,23 The definition of asystole was consistent across all studies. Three studies provided counts of general bradyarrhythmias that combined episodes of bradycardia and asystole,19,21,22 whereas access to the patient-level data for a fourth study allowed for a combined count of bradycardia and asystole.20 The fifth study did not report the number of unique patients with bradycardia or asystole; thus, the number of patients with bradycardia were used in the analysis.23
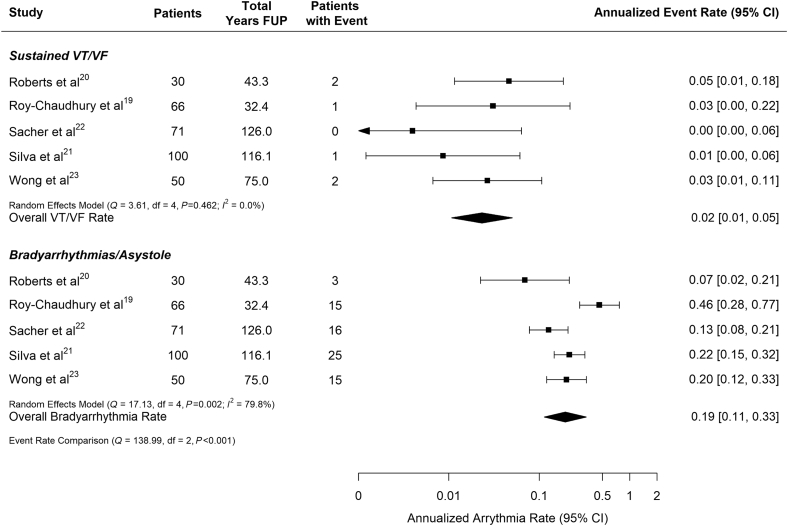
The total number of bradycardia/asystole episodes varied greatly between studies, ranging from 3 to 1475. Two studies reported more than 1000 episodes in 15 patients19,23—suggesting variation in reporting or episode count that was highly clustered within certain subjects (Table 3). Across all studies, the combined annualized rate (number of patients with at least 1 episode per patient-year) of patients experiencing at least 1 bradycardia/asystole event was 0.19 (95% CI: 0.11–0.33) (Figure 4). There was a high degree of heterogeneity in the annualized rate of patients with bradycardia/asystole (I2 = 79.8%), which ranged from 0.07 patients per year to 0.46 patients per year.19,20 Some of the heterogeneity between studies could be attributed to the bradycardia definition employed (0.11 patients per year when a 30-bpm cutoff was employed vs. 0.27 patients per year when a 40-bpm cutoff was used, P = 0.04). The incidence was robust in comparison to other arrhythmias necessitating a therapeutic device, with 13 of the 14 implanted cardiovascular implanted electronic devices being pacemakers (Table 3). After adjusting for the heart rate cutoff, neither the average age of study participants nor the percentage of study participants reporting a history of diabetes was associated with annualized bradycardia/asystole rate (Supplementary Figure S1; P = 0.18 and P = 0.56, respectively).
Figure 4.
Annualized rate of arrhythmias by arrhythmia type pooled across studies. Vertical line indicates pooled estimate across studies. FUP, follow-up; VF, ventricular fibrillation; VT, ventricular tachycardia.
No more than 2 patients with at least 1 sustained VT or VF episode were observed in any single study, with Sacher et al. observing no sustained VT or VF episodes in their cohort of 71 patients monitored for a total of 126 patient-years (Table 3).21 The annualized rate of patients with sustained VT or VF ranged from 0 patients per year to 0.05 patients per year.21,19 Figure 4 shows that across all 5 studies the average annualized rate of patients with sustained VT or VF episodes was 0.02 (95% CI: 0.01–0.05) and was significantly lower than the rate of bradycardia/asystole reported in these same patients (P < 0.001).
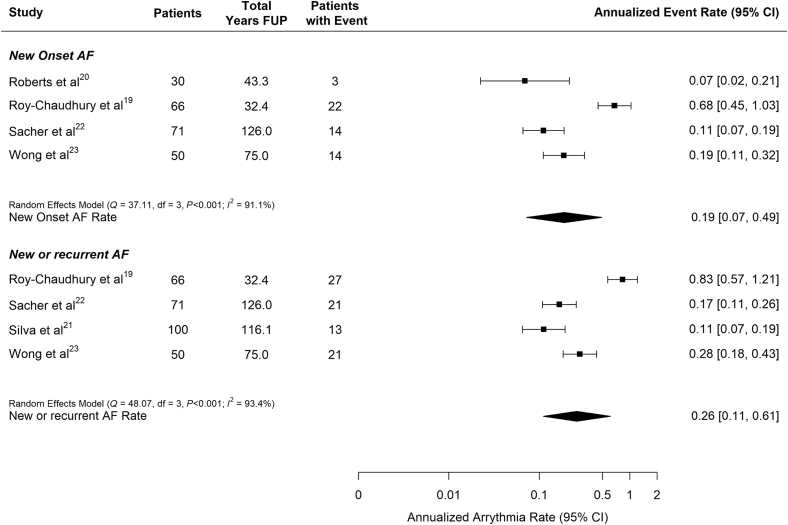
The rate of patients with newly reported and new or recurrent AF during the follow-up period ranged from 0.07 to 0.68 patients per year and from 0.11 to 0.83 patients per year, respectively (Table 3, Figure 5). However, although the algorithms used to detect AF were similar across studies, they differed in reporting new-onset AF, total AF, or nonspecific origination of AF (Table 1). Because of these methodologic differences, we chose not to report a pooled estimate for the rate of AF occurrence.
Figure 5.
Annualized rate of atrial fibrillation (AF) pooled across studies by arrhythmia type pooled across studies. Top panel: new-onset AF. Bottom panel: new or recurrent any AF identified by implantable loop recorder (ILR). The study by Roberts et al.20 provided data only for new-onset AF. The study by Silva et al.21 provided data only for all ILR-detected AF and did not clearly distinguish between de novo and recurrent AF. FUP, follow-up.
Examination of the funnel plots (Supplementary Figure S2) did not indicate concern for publication bias. However, the annualized rate of patients with both new AF and with new or recurrent AF was higher than expected in Roy-Chaudhury et al.19 relative to the other reports.
Discussion
This meta-analysis examined studies that investigated the incidence of arrhythmias in patients with chronic kidney disease on HD using an ILR. ILRs are unique, providing continuous ECG monitoring for changes in rate (bradycardia, asystole and tachycardia) and rhythm (i.e., AF). The current generation of ILRs are implanted by inserting the device subcutaneously on the left side of the chest and has been shown to be acceptable to HD patients.1 Importantly, the use of ILRs as a continuous monitoring modality provided substantial insight into cardiac events in HD patients.
All-cause mortality was similar across the included studies, at a calculated rate 0.14 deaths per patient-year of follow-up (Figure 2). Although there was moderate variation in rates across the studies (Figure 3), 40% of deaths were characterized as SCD. Overall, the incidence of SCD was 0.06 (0.03–0.10), which is comparable to the US Renal Data System reported rate of cardiac arrest and ventricular arrhythmias among HD patients of 0.048 events per patient-year.1 Thus, although the reported studies may systematically undercount the incidence of arrhythmia during the early months of dialysis, the high all-cause mortality and SCD rates provide reassurance that the populations studied are unlikely to be healthier than typical prevalent HD populations. The observed heterogeneity in sudden death and the various arrhythmias may, in part, be explained by study design: Roy-Chaudhury et al. had the shortest average follow-up, reporting rates during the first 6 months of implantation.20 Comparatively, the other 4 studies included in the analysis examined arrhythmias for the duration of device longevity, which could reach up to 3 years. Additional heterogeneity can be attributed to the variation in definitions of SCD. Furthermore, the ILR was not always retrieved posthumously to allow for the mechanism of death to be clearly determined.
There is a broad perception that many cases of SCD could be aborted or treated with appropriate therapy such as pacemakers, implantable cardiac defibrillators (ICDs), or antiarrhythmic therapy. This issue was recently tested directly in the ICD2 study that examined the use of prophylactic ICDs in HD patients and was stopped early because of futility, with no difference in the ICD and usual care arms.28 It should be noted that despite the efficacy of ICDs in preventing SCD in individuals with preserved kidney function, most HD patients do not meet the criteria for ICD implantation.27 Our results provide a potential explanation for this finding, suggesting that serious and fatal arrhythmias in HD patients are much more likely to represent conduction defects (bradycardia/asystole) than shockable tachyarrhythmias, which is echoed in this report’s finding that 50% of cardiac deaths were preceded by a bradycardia or pause episode. Further emphasizing this hypothesis is the finding that 13 of the 14 patients implanted with a pacing or defibrillating device over the follow-up of their respective study received a pacemaker, whereas only 1 received an ICD. However, it is important to recognize that all patients will have an arrhythmia as their terminal event that may be asystole or VF at the time of death. It is possible that a number of the documented arrhythmias resulting in death in the included studies were merely an observation of terminal events, rather than causative, malignant arrythmias. This may be particularly pertinent when considering agonal events and progressive bradycardia/asystole that were seen in 10 of the reported cases. However, because ILRs should generally have sufficient memory to capture events of VT or fibrillation that subsequently progress to asystole, our data do imply that primary tachyarrhythmia leading to cardiac arrest were rare in the populations included in these studies. Our data suggest that devices with combined pacing and defibrillation capacity may be worth studying in the HD population despite the apparent lack of benefit of ICDs in the ICD2 trial.
We found that the incidence of ventricular arrhythmias was low, which was surprising considering that previous reports demonstrated an association between renal insufficiency and ICD shocks.29,30 Nevertheless, analysis of these data indicates a relatively high rate of bradycardia/asystole events across the population instead. Furthermore, the use of implanted therapeutic cardiac devices may have resulted in some underestimation of the total number of bradycardia/asystole events. This high incidence of bradycardia/asystole is perhaps not surprising when considering the potential mechanism of bradycardia/asystole in this population with the potential for cardiac specialized conduction system fibrosis and calcification along with the potential added impact of autonomic dysfunction.
AF incidence was high when compared to a normal population, although notable variation existed across the studies. The prevalence of AF across the HD population in the US Renal Data System population was 19.6% in 2016,3 whereas the reported incidence in clinical studies is in the region of 20%.31, 32, 33, 34 Importantly, it is not clear which patients experienced a new diagnosis of AF and which were previously diagnosed as only some studies indicated new versus established AF. This high incidence of AF is not unexpected, as the ILR provides complete disclosure on arrhythmias whether symptomatic or asymptomatic. The use of ILRs in other patient populations has also demonstrated a high prevalence of AF in patients with no previous history. For example, the REVEAL AF study detected a 40% prevalence of AF during 30 months of ILR monitoring among patients at high risk, but without known history, of AF.35 Similarly, AF was detected in 30% of cryptogenic stroke patients in the CRYSTAL AF study after 36 months of follow-up,36 and in 34% of older adults with stroke or AF risk factors who were monitored for 16 months on average in the ASSERT-II study.37 The aggregated results suggest that subclinical AF has a similarly high prevalence in the HD population as in other high-risk populations, and that further studies are needed to determine the association of monitor-detected AF with clinical events and to test potential treatments (e.g., anticoagulation) for ILR-detected AF.
An important goal of our study was to understand the characteristics of the HD populations observed in ILR studies. In the included manuscripts, 317 patients were enrolled between 2009 and 2013 with 392 years of patient follow-up. Although the mean age of the population studied was slightly younger than the average HD patient represented in many Western dialysis populations,2 many other patient characteristics were consistent with previous investigations of HD populations. This analysis captured patients with an extended time since HD initiation (average of 46.0 months), which is important because the first year on HD carries with it a high rate of morbidity and mortality when compared to an established, well-controlled HD population. US Renal Data System figures indicate that the incidence of death on HD drops dramatically from month 2 to month 12.3 Therefore, if a high proportion of early deaths on HD are attributable to sudden death, the prevalent patients enrolled in the ILR studies are likely to be enriched for individuals with a lower probability to experience serious cardiac arrhythmias than the average dialysis patient. Thus, estimates from these studies, including our summary estimates, may underestimate the true rate of arrhythmia in the dialysis population. One important, yet underinvestigated, metric that would provide greater insight into patient risk is the temporal association among HD therapy and cardiac events. Unfortunately, only 2 studies, Roy-Chaudhury et al. and Wong et al., reported this outcome. Although their work is complementary and consistent with increased risks of peridialytic risk of arrhythmia, particularly during the long interval, differences in methods of reporting and time periods investigated precluded the calculation of summary estimates. The clinical implications of this important question point to the need for more research to better understand this association.
We also identified significant variation among the baseline demographics of the populations examined in this meta-analysis. It is possible that this heterogeneity accounts for the significant variation in outcomes among different groups, that is, SCD, bradycardia/asystole, ventricular arrhythmias, and AF. It is also possible that differences in patient population, dialysis practices, electrolyte disturbances, and duration of time on HD allows for variation in the incidence of arrhythmias. Our inability to provide separate summary estimates for asystole and bradycardia due to their joint reporting in several trials and instead having to report the overall incidence of conduction defects (combined bradycardia and asystole) also provided analytic challenges. Additionally, differences in device programming and sensing across various models, including algorithms, and episode reporting introduces variability that is difficult to capture in this type of summary analysis. Finally, the clustering of events required that we estimate the rate of detection of at least 1 event occurring in an individual patient rather than the rate of arrhythmias.
Our findings should be interpreted within the context of the strengths and weaknesses of our analysis as well as the underlying studies. In addition to providing a useful overview of the similarities and differences in the included study populations and arrhythmia definitions used, our meta-analysis provides the first comprehensive overview of arrhythmia rates in the HD population that provides more accurate and generalizable estimates than any single trial. The rate of SCD is comparable to reported populations; however, this analysis revealed a higher than expected prevalence of bradycardia/asystole events as a potential underlying cause of SCD, challenging the original emphasis on ventricular arrhythmias in this patient population. Furthermore, AF is also highly prevalent and much more common than previous registry data suggest. These findings provide a framework for future studies to investigate not only the role of bradycardia/asystole in HD patients but the efficacy of therapeutic strategies such as the use of potassium binders or manipulation of dialysate potassium, calcium, magnesium, or bicarbonate rates to reduce morbidity and mortality in HD patients.
Disclosure
DMC has received research support from Medtronic and has consulted to Zoll Medical, Amgen, Astra Zeneca, Jansen, Merck, Gilead, Glaxo Smith Kline, NovoNordisk, Merck, and Daichi Sankyo (Travel support only). He has received research support from Janssen, Gilead, Amgen (Pending), Novo Nordisk, and Bioporto. KS and LCJ are employees and shareholders of Medtronic, Inc. PRR has received consultancy payments and research funding from Medtronic and Boston Scientific. BMW has received consultancy payments and research funding from Boston Scientific. The other author declared no competing interests.
Footnotes
Figure S1. Annualized rate of patients with bradycardia/asystole by age (A) and percentage of study population with diabetes (B) and heart rate cutoff.
Figure S2. Funnel plots used to examine potential bias among the studies included in this analysis.
PRISMA Checklist.
Supplementary Material
Figure S1. Annualized rate of patients with bradycardia/asystole by age (A) and percentage of study population with diabetes (B) and heart rate cutoff.
Figure S2. Funnel plots used to examine potential bias among the studies included in this analysis.
PRISMA Checklist.
References
- 1.US Renal Data System . National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases; Bethesda, MD: 2018. USRDS 2018 Annual Data Report: Atlas of End-Stage Renal Disease in the United States. [Google Scholar]
- 2.Evolve Trial Investigators. Chertow G.M., Block G.A. Effect of cinacalcet on cardiovascular disease in patients undergoing dialysis. N Engl J Med. 2012;367:2482–2494. doi: 10.1056/NEJMoa1205624. [DOI] [PubMed] [Google Scholar]
- 3.Wanner C., Krane V., Marz W. Atorvastatin in patients with type 2 diabetes mellitus undergoing hemodialysis. N Engl J Med. 2005;353:238–248. doi: 10.1056/NEJMoa043545. [DOI] [PubMed] [Google Scholar]
- 4.Weiner D.E., Tighiouart H., Elsayed E.F. The Framingham predictive instrument in chronic kidney disease. J Am Coll Cardiol. 2007;50:217–224. doi: 10.1016/j.jacc.2007.03.037. [DOI] [PubMed] [Google Scholar]
- 5.Himmelfarb J., Stenvinkel P., Ikizler T.A. The elephant in uremia: oxidant stress as a unifying concept of cardiovascular disease in uremia. Kidney Int. 2002;62:1524–1538. doi: 10.1046/j.1523-1755.2002.00600.x. [DOI] [PubMed] [Google Scholar]
- 6.Foley R.N., Gilbertson D.T., Murray T. Long interdialytic interval and mortality among patients receiving hemodialysis. N Engl J Med. 2011;365:1099–1107. doi: 10.1056/NEJMoa1103313. [DOI] [PubMed] [Google Scholar]
- 7.Bleyer A.J., Russell G.B., Satko S.G. Sudden and cardiac death rates in hemodialysis patients. Kidney Int. 1999;55:1553–1559. doi: 10.1046/j.1523-1755.1999.00391.x. [DOI] [PubMed] [Google Scholar]
- 8.Pun P.H., Lehrich R.W., Honeycutt E.F. Modifiable risk factors associated with sudden cardiac arrest within hemodialysis clinics. Kidney Int. 2011;79:218–227. doi: 10.1038/ki.2010.315. [DOI] [PubMed] [Google Scholar]
- 9.Pun P.H., Horton J.R., Middleton J.P. Dialysate calcium concentration and the risk of sudden cardiac arrest in hemodialysis patients. Clin J Am Soc Nephrol. 2013;8:797–803. doi: 10.2215/CJN.10000912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Adam W.R. Plasma and dialysate potassium concentrations and haemodialysis associated mortality. Nephrology (Carlton) 2013;18:655–656. doi: 10.1111/nep.12140. [DOI] [PubMed] [Google Scholar]
- 11.Bommer J., Locatelli F., Satayathum S. Association of predialysis serum bicarbonate levels with risk of mortality and hospitalization in the Dialysis Outcomes and Practice Patterns Study (DOPPS) Am J Kidney Dis. 2004;44:661–671. [PubMed] [Google Scholar]
- 12.Takeda K., Harada A., Okuda S. Sudden death in chronic dialysis patients. Nephrol Dial Transplant. 1997;12:952–955. doi: 10.1093/ndt/12.5.952. [DOI] [PubMed] [Google Scholar]
- 13.Shimada K., Tomita T., Kamijo Y. Hemodialysis-induced P-wave signal-averaged electrocardiogram alterations are indicative of vulnerability to atrial arrhythmias. Circ J. 2012;76:612–617. doi: 10.1253/circj.cj-11-1000. [DOI] [PubMed] [Google Scholar]
- 14.Szabo Z., Kakuk G., Fulop T. Effects of haemodialysis on maximum P wave duration and P wave dispersion. Nephrol Dial Transplant. 2002;17:1634–1638. doi: 10.1093/ndt/17.9.1634. [DOI] [PubMed] [Google Scholar]
- 15.Steinberg J.S., Zelenkofske S., Wong S.C. Value of the P-wave signal-averaged ECG for predicting atrial fibrillation after cardiac surgery. Circulation. 1993;88:2618–2622. doi: 10.1161/01.cir.88.6.2618. [DOI] [PubMed] [Google Scholar]
- 16.Fukunami M., Yamada T., Ohmori M. Detection of patients at risk for paroxysmal atrial fibrillation during sinus rhythm by P wave-triggered signal-averaged electrocardiogram. Circulation. 1991;83:162–169. doi: 10.1161/01.cir.83.1.162. [DOI] [PubMed] [Google Scholar]
- 17.Ferrario M., Moissl U., Garzotto F. Effects of fluid overload on heart rate variability in chronic kidney disease patients on hemodialysis. BMC Nephrol. 2014;15:26. doi: 10.1186/1471-2369-15-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hou S., McElroy P.A., Nootens J. Safety and efficacy of low-potassium dialysate. Am J Kidney Dis. 1989;13:137–143. doi: 10.1016/s0272-6386(89)80132-5. [DOI] [PubMed] [Google Scholar]
- 19.Roy-Chaudhury P., Tumlin J.A., Koplan B.A. Primary outcomes of the Monitoring in Dialysis Study indicate that clinically significant arrhythmias are common in hemodialysis patients and related to dialytic cycle. Kidney Int. 2018;93:941–951. doi: 10.1016/j.kint.2017.11.019. [DOI] [PubMed] [Google Scholar]
- 20.Roberts P.R., Zachariah D., Morgan J.M. Monitoring of arrhythmia and sudden death in a hemodialysis population: The CRASH-ILR Study. PloS One. 2017;12 doi: 10.1371/journal.pone.0188713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Silva R.T., Martinelli Filho M., Peixoto Gde L. Predictors of arrhythmic events detected by implantable loop recorders in renal transplant candidates. Arq Bras Cardiol. 2015;105:493–502. doi: 10.5935/abc.20150106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sacher F., Jesel L., Borni-Duval C. Cardiac rhythm disturbances in hemodialysis patients: early detection using an implantable loop recorder and correlation with biological and dialysis parameters. JACC Clin Electrophysiol. 2018;4:397–408. doi: 10.1016/j.jacep.2017.08.002. [DOI] [PubMed] [Google Scholar]
- 23.Wong M.C., Kalman J.M., Pedagogos E. Temporal distribution of arrhythmic events in chronic kidney disease: Highest incidence in the long interdialytic period. Heart Rhythm. 2015;12:2047–2055. doi: 10.1016/j.hrthm.2015.06.033. [DOI] [PubMed] [Google Scholar]
- 24.Wan X., Wang W., Liu J. Estimating the sample mean and standard deviation from the sample size, median, range and/or interquartile range. BMC Med Res Methodol. 2014;14:135. doi: 10.1186/1471-2288-14-135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Higgins J.P., Thompson S.G., Deeks J.J. Measuring inconsistency in meta-analyses. BMJ. 2003;327:557–560. doi: 10.1136/bmj.327.7414.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Viechtbauer W. Conducting meta-analyses in R with the metafor package. J Stat Softw. 2010;36:1–48. [Google Scholar]
- 27.Roberts P.R., Green D. Arrhythmias in chronic kidney disease. Heart. 2011;97:766–773. doi: 10.1136/hrt.2010.208587. [DOI] [PubMed] [Google Scholar]
- 28.Jukema J.W., Timal R.J., Rotmans J.I. Prophylactic use of implantable cardioverter-defibrillators in the prevention of sudden cardiac death in dialysis patients. Circulation. 2019;139:2628–2638. doi: 10.1161/CIRCULATIONAHA.119.039818. [DOI] [PubMed] [Google Scholar]
- 29.Robin J., Weinberg K., Tiongson J. Renal dialysis as a risk factor for appropriate therapies and mortality in implantable cardioverter-defibrillator recipients. Heart Rhythm. 2006;3:1196–1201. doi: 10.1016/j.hrthm.2006.06.013. [DOI] [PubMed] [Google Scholar]
- 30.Hreybe H., Ezzeddine R., Bedi M. Renal insufficiency predicts the time to first appropriate defibrillator shock. Am Heart J. 2006;151:852–856. doi: 10.1016/j.ahj.2005.06.042. [DOI] [PubMed] [Google Scholar]
- 31.Goldstein B.A., Arce C.M., Hlatky M.A. Trends in the incidence of atrial fibrillation in older patients initiating dialysis in the United States. Circulation. 2012;126:2293–2301. doi: 10.1161/CIRCULATIONAHA.112.099606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Konigsbrugge O., Posch F., Antlanger M. Prevalence of atrial fibrillation and antithrombotic therapy in hemodialysis patients: cross-sectional results of the Vienna InVestigation of AtriaL Fibrillation and Thromboembolism in Patients on HemoDIalysis (VIVALDI) PloS One. 2017;12 doi: 10.1371/journal.pone.0169400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wetmore J.B., Ellerbeck E.F., Mahnken J.D. Atrial fibrillation and risk of stroke in dialysis patients. Ann Epidemiol. 2013;23:112–118. doi: 10.1016/j.annepidem.2012.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Winkelmayer W.C., Patrick A.R., Liu J. The increasing prevalence of atrial fibrillation among hemodialysis patients. J Am Soc Nephrol. 2011;22:349–357. doi: 10.1681/ASN.2010050459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Reiffel J.A., Verma A., Kowey P.R. Incidence of previously undiagnosed atrial fibrillation using insertable cardiac monitors in a high-risk population: the REVEAL AF study. JAMA Cardiol. 2017;2:1120–1127. doi: 10.1001/jamacardio.2017.3180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sanna T., Diener H.C., Passman R.S. Cryptogenic stroke and underlying atrial fibrillation. N Engl J Med. 2014;370:2478–2486. doi: 10.1056/NEJMoa1313600. [DOI] [PubMed] [Google Scholar]
- 37.Healey J.S., Alings M., Ha A. Subclinical atrial fibrillation in older patients. Circulation. 2017;136:1276–1283. doi: 10.1161/CIRCULATIONAHA.117.028845. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.