Introduction
Autosomal recessive polycystic kidney disease (ARPKD) is a rare form of fibrocystic kidney disease that affects approximately 1 in 20,000 live births.1,2 The classic presentation is with prominent hepatic impairment caused by hepatic fibrosis in addition to renal impairment that is more aggressive than that seen in autosomal dominant polycystic kidney disease (ADPKD). Caroli syndrome is the eponym given to the combination of ARPKD, hepatic fibrosis, portal hypertension, and intrahepatic bile duct dilatation. It is seen in ≤50% of cases of ARPKD.3
Although cerebral aneurysms are seen in 8% to 12% of patients with ADPKD,4, 5, 6 only a handful of cases have been described in patients with ARPKD. The widely held belief that all patients with ARPKD progress to end-stage kidney disease by early adolescence is incorrect. Approximately one third of patients present after 20 years of age,7 by which time they are at risk of having developed severe extrarenal manifestations, such as intracranial aneurysms. Physicians should be aware of the possibility of this diagnosis in adults and know when to suspect it.
We present a 29-year-old woman with ARPKD who was found to have 8 cerebral aneurysms diagnosed during the investigation of postpartum headaches.
Case Presentation
A 29-year-old woman presented to her general practitioner with increasing headaches over the preceding weeks.
She had a history of ARPKD that had been diagnosed in infancy. She initially presented with bilateral inguinal hernia at 4 months of age. Abdominal ultrasound revealed bilaterally enlarged cystic kidneys. Her brother also had renal impairment and was subsequently diagnosed with ARPKD; both of her parents had normal kidney function. Her liver function tests were normal, and both ultrasound and magnetic resonance imaging revealed a normal appearing liver without evidence of portal hypertension. Imaging of the patient’s renal tract when she was 28 years of age revealed bilaterally enlarged kidneys at 13.5 cm and 13.4 cm, respectively. She had slowly progressive chronic kidney disease with the most recent serum creatinine before presentation being 140 μmol/L. Her blood pressure had been elevated since childhood but was well controlled on lisinopril. Genetic testing revealed compound heterozygous mutations in PKHD1, confirming the diagnosis of ARPKD.
Five months before the discovery of the cerebral aneurysms, during her first pregnancy, the patient required an emergency caesarean section for severe pre-eclampsia at 35 weeks’ gestation. She had been hypertensive throughout pregnancy requiring intensive monitoring. Surgery was complicated by postoperative aspiration and pulmonary edema necessitating intubation and admission to the intensive care unit; she recovered completely within 2 weeks and her child suffered no complications. During the same pregnancy, the patient presented with unilateral upper motor neuron seventh nerve palsy and was treated for Bell’s palsy. She received a 5-day course of oral prednisolone and valacyclovir with complete resolution of paralysis. A magnetic resonance imaging scan of the brain performed at that time did not reveal any abnormalities. Magnetic resonance angiography (MRA), which likely would have shown aneurysms, was not performed at this point.
The patient had mild headaches throughout her pregnancy that became more prominent in the postpartum period. They were global in nature with steadily increasing severity since childbirth. There were no neurologic deficits on examination and the patient was normotensive. The patient’s general practitioner arranged for an outpatient MRA scan that revealed 6 aneurysms involving the Circle of Willis without evidence of rupture.
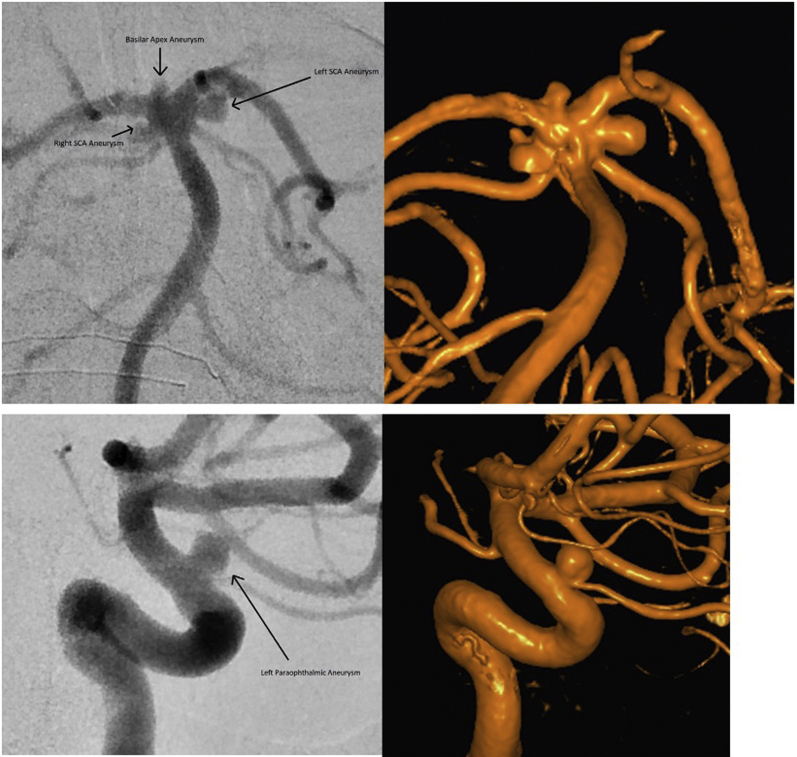
She was urgently referred for neurosurgical opinion and subsequent digital subtraction angiography showed 8 intracranial aneurysms involving the right middle cerebral artery, left internal carotid artery, and basilar artery. The largest aneurysm was 4.3 mm and was located in the left internal carotid artery paraopthalmic segment (Figure 1).
Figure 1.
Labeled 2- and 3-dimensional angiographic images of the basilar termination and intracranial internal carotid artery. SCA, superior cerebellar artery.
The patient’s case was discussed at a multidisciplinary neurovascular meeting. The aneurysms were assessed according to their location, morphology, and treatment options. Endovsacular management was favored over microsurgical clipping. The basilar and middle cerebral artery aneurysms were felt to be best managed by observation. Endovascular intervention with coiling or flow-diverting stent to manage the 4-mm internal carotid artery aneurysm was thought preferable to an observational approach. However, after further discussions between the neurosurgeons, nephrologists, and the patient, she elected not to proceed with any intervention given the young age of her child and her desire for more children in the near future. A decision was made only to proceed to intervention if there is an increase in the size of the aneurysms on follow-up MRA. The patient remains well 8 months later but still has mild, intermittent headaches that are thought to be migrainous and not related to her aneurysms.
Discussion
To our knowledge, this is only the seventh reported case of intracranial aneurysms in a patient with ARPKD. There are also reports of 2 patients with ARPKD found to have extracranial aneurysms.8 These cases are summarized in Table 1. As in this case, 3 were diagnosed in early adulthood and the remainder were diagnosed during childhood (range 2–36 years of age). Four of the cases presented with aneurysm rupture while the others were diagnosed during the investigation of neurologic symptoms. Two of the patients died (ages 10 and 29 years). In those who survived or who had aneurysms diagnosed without rupture, all patients underwent a full recovery or remained well.
Table 1.
Reported cases of aneurysms in patients with ARPKD (n = 9)
| Study | Country | Gender | Age, yrs | Intra- or extracranial | Artery involved | Multiple or single | Size (widest diameter) | Genetic testing | Rupture | Intervention | Outcome |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Gately et al., 2020 (current study) | Australia | F | 29 | Intracranial | R middle cerebral | Multiple | 2 mm | Compound heterozygote PKHD1 variants [p.(Thr36Met) and p.(Ser1156Leu)] | No | No | Asymptomatic |
| R middle cerebral | 2 mm | ||||||||||
| R middle cerebral | 1.5 mm | ||||||||||
| L internal carotid | 4.3 mm | ||||||||||
| L internal carotid | 2.9 mm | ||||||||||
| Basilar apex | 2.3 mm | ||||||||||
| L superior cerebellar | 3.3 mm | ||||||||||
| R superior cerebellar | 2 mm | ||||||||||
| Helal et al.,S15 2019 | Tunisia | M | 29 | Intracranial | Bilateral (not specified) | Multiple | 9.1 mm | NS | Yes | Endovascular | Died |
| Perez et al.,S3 2018 | U.S. | M | 10 | Intracranial | L anterior communicating | Single | NS | Compound heterozygote PKHD1 variants (NS) | Yes | Extraventricular drain, coiling, craniectomy | Died |
| Elchediak et al.,8 2017 | U.S. | M | 36 | Extracranial | Aorta | Multiple | 3.1 cm | NS | Yes | No | Complete recovery |
| Common iliac bilaterally | 1.9 cm | ||||||||||
| Celiac | 1.2 cm | ||||||||||
| Elchediak et al.,8 2017 | U.S. | F | 17 | Extracranial | Gastric Splenic |
Multiple | NS | NS | Yes | Endoscopy, gastrectomy | Died |
| Chalhoub et al.,S16 2013 | Lebanon | M | 21 | Intracranial | R anterior choroid | Single | 5.7 mm | NS | Yes | Clipping | Complete recovery |
| Lilova and Petkov,9 2001 | Bulgaria | F | 2 | Intracranial | Middle cerebral Posterior cerebral |
Multiple | NS | NS | No | No | Asymptomatic |
| Neumann et al.,S17 1999 | Germany | F | 31 | Intracranial | R middle cerebral | Multiple | 12 mm | NS | No | Clipping | Asymptomatic |
| L anterior communicating | 8 mm |
||||||||||
| L posterior communicating | 4 mm |
||||||||||
| L anterior choroid | 3 mm |
||||||||||
| De Blasi et al.,S2 1997 | France | M | 12 | Intracranial | L basilar tip | Multiple | 5.8 mm | NS | Yes | Coiling and clipping | Complete recovery |
| L sylvian artery | 4 mm |
ARPKD, autosomal recessive polycystic kidney disease; F, female; L, left; M, male; NS, not specified; R, right; US, United States.
To date, our patient has remained well without intervention upon the aneurysms. Only 1 other case was managed conservatively, the case of a 2-year-old girl who presented with dizziness and vomiting and was found to have multiple aneurysms of her middle and posterior cerebral arteries bilaterally. She remained well for 2 years postdiagnosis with no neurologic sequelae.9
The young age at which some of these aneurysms are diagnosed is remarkable. Pediatric aneurysms are rare, with only 0.5% to 4.6% of aneurysms thought to occur in those <18 years of age.S1 Three of these cases were diagnosed under 18 years of age.9,S2,S3 Interestingly, 5 of these cases of intracranial aneurysms were diagnosed with multiple aneurysms—a much higher proportion than one would expect in the general population. In those without ARPKD, approximately 30% of patients who present with hemorrhage caused by a ruptured aneurysm have >1 aneurysm and <10% of patients have ≥3 aneurysms.S4 Multiple aneurysms are more common in patients with ADPKD, and we hypothesize that the same may also be true for patients with ARPKD.4 Multiple intracranial aneurysms have been associated with higher mortality and, historically, a neurosurgical or endovascular management approach has been pursued for patients with unruptured aneurysms.S5,S6 However, these procedures are not without risks, and a meta-analysis has shown significant morbidity (6.7%) and mortality (1.7%) complicating surgical intervention.S7 Management decisions should be tailored to the individual patient dependent upon aneurysm size, location, number, and the patient’s premorbid condition. We advocate for a multidisciplinary approach to all management decisions involving the radiology, neurointerventional, and neurosurgical teams.
Even in patients with ADPKD there is not uniform consensus on who to screen for aneurysms.S8 Most groups advocate for screening in patients with a family history of aneurysmal rupture or in those with high-risk occupations, but a trend toward screening more patients is emerging. No such recommendations exist for patients with ARPKD. The decision to screen patients with ARPKD assumes increased importance given the young age of these patients and their potential requirement for transplantation given that roughly half will progress to end-stage kidney disease during childhood.S9,S10
The biologic plausibility for the potential association between ARPKD and aneurysm formation is supported by mouse models that have shown that the defective gene responsible for ARPKD, PKHD1, is highly expressed in the large arteries, such as the thoracic and abdominal aorta.S11 A previous report of 2 patients with ARPKD and multiple extracranial aneurysms postulated that vascular integrity may be compromised in those with ARPKD.8 A 36-year old male nonsmoker with ARPKD was found to have an infrarenal aortic aneurysm and dissection, an exceptionally rare finding in someone of this age.S12 In the second case, a 17-year-old female died after massive upper gastrointestinal bleeding related to portal hypertension and gastric aneurysms. Histology of these aneurysms was suggestive of developmental defects in the arterial wall as opposed to the typical arterial weakening of vessel walls.
Conclusion
The possibility that these cases of intracranial aneurysms in ARPKD represent pure coincidence must be considered. However, it is conceivable that an increasing number of intracranial aneurysms will be discovered as outcomes improve and patients survive into adulthood. Most of these cases have been reported in the last 10 years. The considerable increase in the utility of advanced cranial diagnostic imaging could also be contributing to the increasing detection rate. The high proportion of multiple intracranial aneurysms found in these patients also suggests that a distinct pathophysiologic mechanism for aneurysm development may exist.
MRA offers the potential for noninvasive screening of patients without the detrimental effects of radiation exposure. Earlier detection will likely lead to better outcomes given that ruptured aneurysms can lead to death in approximately 30% of cases.S13 Even in patients who survive, significant neurologic morbidity often complicates recovery.S14 This potential benefit must be weighed against the psychological impact that aneurysm diagnosis will have on the patient and their family, the significant risks of intervention, and the economic impact of widespread screening (Table 2).
Table 2.
Teaching points
|
|
|
Disclosure
All the authors declared no competing interests.
Acknowledgments
We have obtained written informed consent from the patient reported herein.
Footnotes
Supplementary References.
Supplementary Material
Supplementary References.
References
- 1.Guay-Woodford L.M., Bissler J.J., Braun M.C. Consensus expert recommendations for the diagnosis and management of autosomal recessive polycystic kidney disease: report of an international conference. J Pediatr. 2014;165:611–617. doi: 10.1016/j.jpeds.2014.06.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zerres K., Hansmann M., Mallmann R., Gembruch U. Autosomal recessive polycystic kidney disease. Problems of prenatal diagnosis. Prenat Diagn. 1988;8:215–229. doi: 10.1002/pd.1970080308. [DOI] [PubMed] [Google Scholar]
- 3.Guay-Woodford L.M., Desmond R.A. Autosomal recessive polycystic kidney disease: the clinical experience in North America. Pediatrics. 2003;111(5 pt 1):1072–1080. doi: 10.1542/peds.111.5.1072. [DOI] [PubMed] [Google Scholar]
- 4.Pirson Y., Chauveau D., Torres V. Management of cerebral aneurysms in autosomal dominant polycystic kidney disease. J Am Soc Nephrol. 2002;13:269–276. doi: 10.1681/ASN.V131269. [DOI] [PubMed] [Google Scholar]
- 5.Perrone R.D., Malek A.M., Watnick T. Vascular complications in autosomal dominant polycystic kidney disease. Nat Rev Nephrol. 2015;11:589–598. doi: 10.1038/nrneph.2015.128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sanchis I.M., Shukoor S., Irazabal M.V. Presymptomatic screening for intracranial aneurysms in patients with autosomal dominant polycystic kidney disease. Clin J Am Soc Nephrol. 2019;14:1151–1160. doi: 10.2215/CJN.14691218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Adeva M., El-Youssef M., Rossetti S. Clinical and molecular characterization defines a broadened spectrum of autosomal recessive polycystic kidney disease (ARPKD) Medicine (Baltimore) 2006;85:1–21. doi: 10.1097/01.md.0000200165.90373.9a. [DOI] [PubMed] [Google Scholar]
- 8.Elchediak D.S., Cahill A.M., Furth E.E., Kaplan B.S., Hartung E.A. Extracranial aneurysms in 2 patients with autosomal recessive polycystic kidney disease. Case Rep Nephrol Dial. 2017;7:34–42. doi: 10.1159/000475492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lilova M.I., Petkov D.L. Intracranial aneurysms in a child with autosomal recessive polycystic kidney disease. Pediatr Nephrol. 2001;16:1030–1032. doi: 10.1007/s004670100019. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.