Abstract
Introduction
Immune checkpoint inhibitors (ICIs) are increasingly used to treat cancers. Kidney immune-related adverse events (IRAEs) are now well recognized, with the incidence of IRAEs ranging from 2% to 5%. Most of the initial data related to kidney IRAEs have focused on acute interstitial nephritis (AIN). There are minimal data on the types and relative frequencies of glomerular diseases associated with ICIs, their treatment, and outcomes.
Methods
We performed a systematic review and meta-analysis of all biopsy-proven published cases/series of glomerular pathology associated with ICIs. We searched the MEDLINE, EMBASE, and Cochrane databases from inception to February 2020. We abstracted patient-level data, including demographics, cancer and ICI therapy details, and characteristics of kidney injury.
Results
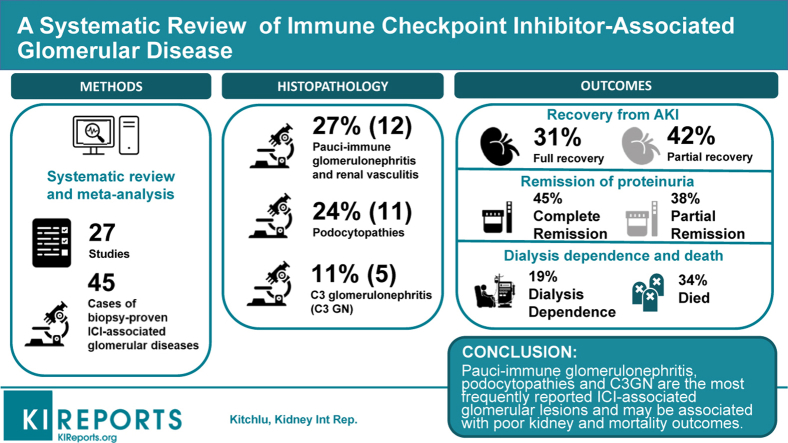
After screening, 27 articles with 45 cases of biopsy-confirmed ICI-associated glomerular disease were identified. Several lesion types were observed, with the most frequent being pauci-immune glomerulonephritis (GN) and renal vasculitis (27%), podocytopathies (24%), and complement 3 GN (C3GN; 11%). Concomitant AIN was reported in 41%. Most patients had ICIs discontinued (88%), and nearly all received corticosteroid treatment (98%). Renal replacement therapy (RRT) was required in 25%. Most patients had full (31%) or partial (42%) recovery from acute kidney injury (AKI), although 19% remained dialysis-dependent, and approximately one-third died. Complete or partial remission of proteinuria was achieved in 45% and 38%, respectively.
Conclusion
Multiple forms of ICI-associated glomerular disease have been described. Pauci-immune GN, podocytopathies, and C3GN are the most frequently reported lesions. ICI-associated glomerular disease may be associated with poor kidney and mortality outcomes. Oncologists and nephrologists must be aware of glomerular pathologies associated with ICIs and consider obtaining a kidney biopsy specimen when features atypical for AIN are present.
Graphical abstract
ICI therapies are now widely used in an increasing number of cancer types.1 These agents are humanized antibodies that inhibit down-regulatory receptors on T cells (such as cytotoxic T-lymphocyte antigen 4 [CTLA-4] and programmed cell death 1 [PD-1] and its ligand, PD-L1).2 By blocking negative costimulatory pathways, ICI therapies allow T cells to remain activated, thereby enhancing the antitumor immune response. ICIs are now approved for use in melanoma, small cell and non–small cell lung cancer, renal cell carcinoma, and urothelial carcinoma, among others.3, 4, 5, 6, 7, 8 However, despite benefits with respect to progression-free and overall survival, upregulation of the immune system has been associated with a wide spectrum of systemic IRAEs.9,10
Kidney IRAEs are increasingly recognized,11, 12, 13, 14, 15, 16, 17, 18 with a reported incidence of 2% to 5% in clinical trial recipients19; however, this proportion may be greater in the real-world use of these agents.20 Most of the initial data related to kidney IRAE has focused on AIN, which is often responsive to treatment with steroids, as the predominant kidney lesion associated with ICIs.19,21, 22, 23, 24, 25, 26, 27 As such, current American Society of Clinical Oncology guidelines state that in patients with suspected kidney IRAEs, “reflex kidney biopsy should be discouraged until corticosteroid treatment has been attempted,”28 ostensibly because of the assumption that most patients will have AIN.
However, there is increasing recognition of the possible association between glomerular disease and ICIs, with reports describing both nephrotic and nephritic presentations. Although these presentations are relatively rare compared to AIN, much less is known about their clinical features, implications on cancer therapy, and outcomes—both oncologic and renal.
We conducted a systematic review and meta-analysis of reported cases of glomerular disease associated with ICI therapy, with the objectives of characterizing the clinical features, describing the current management strategies, and estimating the associated outcomes.
Methods
This review was performed following the guidelines of Meta-analysis of Observational Studies in Epidemiology and reported using Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines (see the Supplementary Material).29
Literature Sources and Search Strategy
We used a search strategy developed with a health information specialist (T.K.), to search MEDLINE, the Cochrane Central Registry of Controlled Trials, and EMBASE from inception to February 2020 (Literature Search Strategy reported in the Supplementary Material).
Study Selection
We included all case reports, series, cohort studies, or clinical trials that reported ≥1 patients who developed a glomerular disease in association with receipt of any ICI (including CTLA-4, PD-1, and PD-L1 pathway agents). We excluded articles that did not report specific instances of glomerular disease (e.g., clinical trials that reported IRAEs by organ system without further details) or those that did not report new cases (e.g., review articles). Given the importance of kidney biopsy specimens in confirming the presence and type of glomerular disease, cases that did not include renal pathology findings were excluded. Three nephrologists (A.K., K.D.J., R.W.) scanned titles and abstracts for initial selection. Selected articles were reviewed in full by multiple reviewers (A.K., K.D.J., S.W., P.D., Z.H., K.H., R.W.), and independently assessed for eligibility and causality by multiple reviewers (A.K., P.D.). Discrepancies were resolved by consensus.
Causality Assessment and Assessment of Quality and Bias
We used the World Health Organization–Uppsala Monitoring Center causality assessment system to assess the likelihood of a causal relationship between ICI and glomerular disease for all eligible reports. The World Health Organization–Uppsala Monitoring Center causality assessment system is a validated and widely accepted method of pharmacovigilance that uses prespecified criteria to categorize the causal link between a drug and an adverse event into 1 of 6 discrete levels of certainty (certain, probable/likely, possible, unlikely, conditional/unclassified, and unassessable/unclassifiable).30 Quality assessment of the case reports and case series was done using the tool developed by Murad et al.31 The quality of the case-control and cohort studies was assessed using the Newcastle-Ottawa Scale.32
Data Abstraction
For each included study, we performed a standardized patient-level data abstraction from each of the included citations using prespecified parameters of interest: patient demographics, cancer diagnosis, ICI treatment characteristics, laboratory data, glomerular disease–related descriptors, immunosuppressive treatment, and outcomes (for AKI, proteinuria, and mortality). Published articles and supplementary materials were reviewed for relevant data. Corresponding authors were contacted to request data that were unavailable from these sources.
Statistical Analyses
We reported descriptive statistics related to the prespecified parameters of interest. We expressed continuous variables as the mean (standard deviation [SD]) or median (25th–75th percentile), and categorical variables as a percentage. Univariate logistic regression was used to assess for risk factors for a composite endpoint of end-stage kidney disease (ESKD) or death as an exploratory analysis. Analyses were performed using SAS software (version 9.4; SAS Institute, Cary, NC). P < 0.05 was considered statistically significant.
Results
Search Results
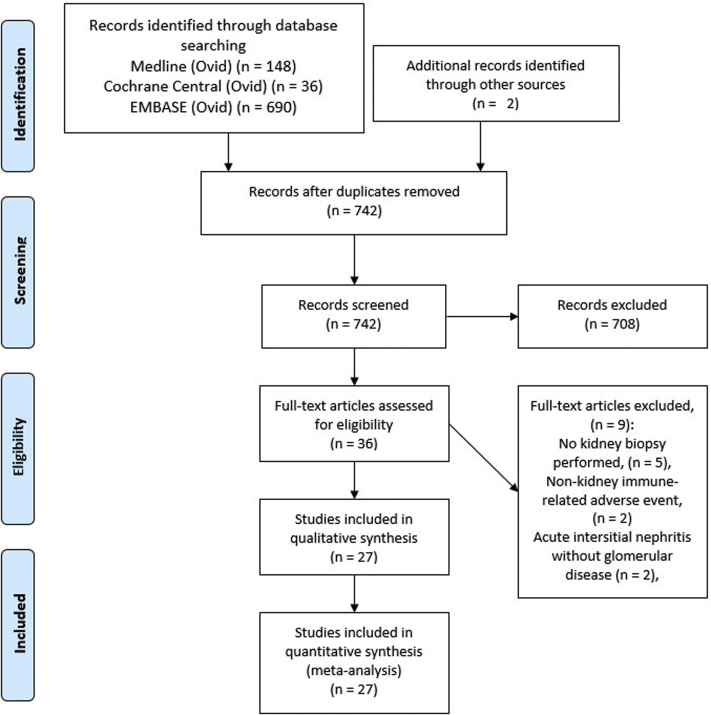
The search strategy yielded a total of 742 citations from MEDLINE (Ovid), the Cochrane Central Registry of Controlled Trials (Ovid), and EMBASE (Ovid). Title and abstract screening resulted in 36 articles subject to detailed review. The 708 excluded citations comprised nonrelevant citations, review articles, clinical trials (those without reported glomerular lesions), and case reports of AIN and other nonglomerular lesions. Eleven citations were discrepant among title and abstract reviewers and these were resolved by consensus (via teleconference).
After full review, 27 articles were retained for inclusion and abstraction of data (Figure 1, PRISMA diagram).19,20,33, 34, 35, 36, 37, 38, 39, 40, 41, 42, 43, 44, 45, 46, 47, 48, 49, 50, 51, 52, 53, 54, 55 From these articles, 45 patients with ICI-associated, biopsy-confirmed glomerular disease were identified (Supplementary Table S1). Most cases met World Health Organization–Uppsala Monitoring Center case causality criteria for the probable or certain categories (80%, Supplementary Table S1). Quality assessment of the case reports and case series showed that most studies were of fair or good quality. The observational studies by Cortazar et al.36 (case-control study) and Seethapathy et al.20 (cohort study) were determined to be of high quality (scored as >7; Supplementary Table S2 and Supplementary Table S3).
Figure 1.
Preferred Reporting Items for Systematic Reviews and Meta-Analyses diagram of search strategy results and screening.
Aggregate Data of Patients with ICI-Associated Glomerular Disease
The median age of patients with glomerular disease after ICI use was 63 years (IQR 56–72 years) and 75% were male (Table 1). Various primary cancer sites were represented, with melanoma (38%) and lung (24%) being the most frequent. A majority (81%) of reported cases were associated with PD-1/PD-L1 pathway checkpoint inhibitor use.
Table 1.
Characteristics of reported patients with glomerular disease associated with immune checkpoint inhibitors (N = 45)
| Characteristic | IQR or n (%)a |
|---|---|
| Age, yrs (IQR) | 63 (55.8–72.3) |
| Sex, n (%) | |
| Female | 10 (25.0) |
| Male | 30 (75.0) |
| Primary cancer site, n (%) | |
| Lung cancer | 10 (23.8) |
| Lymphoma | 3 (7.1) |
| Melanoma | 16 (38.1) |
| Renal cell carcinoma | 6 (14.3) |
| Other cancers | 7 (16.7) |
| Immunotherapy, n (%) | |
| CTLA-4 agents | 9 (21.4) |
| PD-1/PD-L1 agents | 34 (81.0) |
| Ipilimumab | 5 (11.9) |
| Nivolumab | 15 (35.7) |
| Pembrolizumab | 15 (35.7) |
| Tremelimumab | 2 (4.8) |
| Combination: ipilimumab plus nivolumab | 4 (9.5) |
| Other | 1 (2.4) |
| Glomerular disease type, n (%) | |
| Amyloid A amyloidosis | 4 (8.9) |
| Anti-GBM | 3 (6.7) |
| C3GN | 5 (11.1) |
| FSGS | 2 (4.4) |
| Immunoglobulin A nephropathy | 4 (8.9) |
| Immune-complex GN | 2 (4.4) |
| Lupus-like nephritis | 1 (2.2) |
| Minimal change disease | 9 (20.0) |
| Membranous nephropathy | 1 (2.2) |
| Pauci-immune GN/renal vasculitis | 12 (26.7) |
| Thrombotic microangiopathy | 2 (4.4) |
| Glomerular disease characteristics | |
| Time from therapy start to glomerular disease, days (IQR) | 93 (43.5–212) |
| Baseline serum creatinine, mg/day (IQR) | 0.95 (0.78–1.20) |
| Peak serum creatinine, mg/day (IQR) | 3.4 (2.25–3.36) |
| RRT requirement during AKI, n (%) | 10 (25) |
| Peak proteinuria, g/day (IQR) | 8.25 (4.33–13.0) |
| Other IRAEs, n (%) | 5 (16.1) |
| Associated tubulointerstitial nephritis, n (%) | 17 (40.5) |
| Treatment characteristics | |
| Immune checkpoint inhibitor discontinuation, n (%) | 37 (88.1) |
| Corticosteroid use, n (%) | 39 (97.5) |
| Corticosteroid taper duration, days (IQR) | 90 (60–120) |
| Immune checkpoint inhibitor rechallenge, n (%) | 4 (8.9) |
| Recurrence of glomerular disease after rechallenge, n (%) | 2 (4.4) |
| Outcomes, n (%) | |
| AKI outcome (n = 36) | |
| Full recovery | 11 (30.6) |
| Partial recovery | 15 (41.7) |
| No recovery (non-ESKD) | 3 (8.3) |
| ESKD | 7 (19.4) |
| Proteinuria outcome (n = 29) | |
| Complete remission | 13 (44.8) |
| Partial remission | 11 (37.9) |
| No remission | 5 (17.2) |
| Cancer outcome (n = 32) | |
| Stable/remission | 16 (50.0) |
| Progression | 5 (15.6) |
| Death | 11 (34.4) |
AKI, acute kidney injury; C3GN, complement 3 glomerulonephritis; CTLA-4, cytotoxic T-lymphocyte antigen 4; ESKD, end-stage kidney disease; FSGS, focal segmental glomerulosclerosis; GBM, glomerular basement membrane; GN, glomerulonephritis; IgA, immunoglobulin A; IQR, interquartile range; IRAEs, immune-related adverse events;
PD-1, programmed cell death 1; PD-L1, programmed cell death ligand 1; RRT, renal replacement therapy.
Data are shown as median (IQR) for continuous variables and n (%).
Percentages for each characteristic are calculated as a proportion of the number of patients with data available.
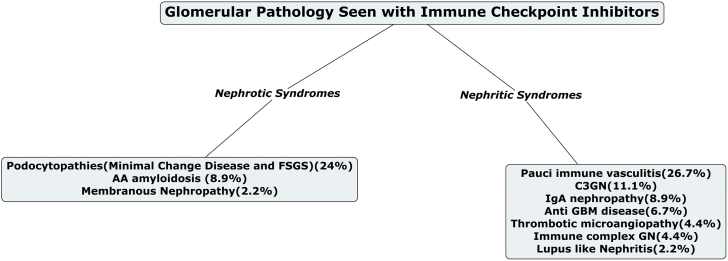
Several types of glomerular lesions were observed, with the most frequent being pauci-immune GN and renal vasculitis (27%), minimal change disease (MCD; 20%), and C3 GN (11%; Table 2, Figure 2). Of note, to our knowledge, all reported cases yielded by our search represented newly diagnosed (i.e., de novo) glomerular disease rather than flares of previously diagnosed disease. Time from ICI initiation to the recognition of glomerular disease was variable, with a median of 93 days (IQR 44–212 days). Most patients had normal baseline kidney function (median serum creatinine 0.95 mg/dl [0.78–1.2 mg/dL]). Peak serum creatinine and proteinuria associated with glomerular injury were 3.4 mg/dl (2.3–3.5 mg/dl) and 8.3 g/day (4.3–13.0 g/day), respectively. Twenty-five percent of patients received RRT during the AKI episode. Concomitant AIN was reported among 41%. Nonsteroidal anti-inflammatory drug use was noted in 5 patients (11%) and proton-pump inhibitor use was reported in 9 patients (20%).
Table 2.
Acute kidney injury characteristics and outcomes in 3 most commonly reported immune checkpoint inhibitor–associated glomerular diseases
| Glomerular diseases | IQR or n (%)a |
|---|---|
| Pauci-immune glomerulonephritis and renal vasculitis (n = 12) | |
| Peak SCr, mg/dl (IQR) | 4.75 (4.49–5.80) |
| Time to glomerular disease diagnosis, days (IQR) | 73 (53–102) |
| RRT requirement during AKI, n (%) | 3 (33) |
| ANCA serology positivity, n (%) | 2 (20) |
| Duration of post–biopsy procedure follow-up reported, months (IQR) | 5 (3.25–7.5) |
| AKI outcome, n (%) | |
| Complete recovery | 4 (40) |
| Partial recovery | 5 (50) |
| No recovery/ESKD | 1 (10) |
| Cancer outcome, n (%) | |
| Stable/remission | 4 (50) |
| Death | 4 (50) |
| Podocytopathies (minimal change disease/FSGS; n = 11) | |
| Peak SCr, mg/dl (IQR) | 0.99 (0.76–1.2) |
| Time to glomerular disease diagnosis, days (IQR) | 52 (28–420) |
| RRT requirement during AKI, n (%) | 2 (22) |
| Peak proteinuria, g/day (IQR) | 10.3 (9.0–19.0) |
| Post-treatment proteinuria, g/day (IQR) | 0.29 (0.05–1.97) |
| Duration of post–biopsy procedure follow-up reported, months (IQR) | 5.5 (4.0–10.25) |
| AKI outcome, n (%) | |
| Complete recovery | 2 (29) |
| Partial recovery | 3 (43) |
| No recovery/ESKD | 1 (14) |
| Cancer outcome, n (%) | |
| Stable/remission | 3 (37.5) |
| Progression/death | 5 (62.5) |
| C3 glomerulonephritis (n =5) | |
| Peak SCr, mg/dl (IQR) | 3.13 (2.55–3.48) |
| Time to glomerular disease diagnosis, days (IQR) | 58 (53–68) |
| RRT requirement during AKI, n (%) | 0 (0) |
| Duration of post–biopsy procedure follow-up reported, months (IQR) | 4 (3.25–8.0) |
| Abnormal serum complement level(s), low C3, low C4 = 1, low C3, normal C4 = 1, n (%) | 2 (40) |
| AKI outcome, n (%) | |
| Complete recovery | 1 (25) |
| Partial recovery | 3 (75) |
| No recovery/ESKD | 0 (0) |
| Cancer outcome, n (%) | |
| Stable/remission | 2 (50) |
| Death | 2 (50) |
AKI, acute kidney injury; ANCA, antinuclear cytoplasmic antibody; ESKD, end-stage kidney disease; FSGS, focal segmental glomerulosclerosis; IQR, interquartile range; RRT, renal replacement therapy; SCr, serum creatinine.
Data are shown as median (IQR) for continuous variables and n (%).
Percentages for each characteristic are calculated as a proportion of the number of patients with data available.
Figure 2.
The various nephrotic and nephritic syndromes associated with immunotherapy. AA, amyloid A; C3GN, complement 3 glomerulonephritis; FSGS, focal segmental glomerulosclerosis; GBM, glomerular basement membrane; GN, glomerulonephritis; IgA, immunoglobulin A.
Most patients had ICIs discontinued (88%) and nearly all received corticosteroid treatment (98%). Most patients had either complete (31%) or partial (42%) recovery from AKI. Complete or partial remission of proteinuria was achieved in 45% and 38% of patients, respectively. The proportion with ESKD, however, was substantial at 19%, and approximately one third of all patients died.
Rechallenge was reported in only 4 patients, with 2 of these demonstrating recurrence of glomerular disease. These 4 patients included 2 with MCD (1 of whom had recurrence upon rechallenge; the other had no recurrence while maintained on prednisone 10 mg/day), 1 with C3 GN (with recurrence upon rechallenge despite a switch from pembrolizumab to nivolumab), and 1 with renal vasculitis (who had progressed to ESKD and had ICI switch from nivolumab/ipilimumab to pembrolizumab).
Characteristics, Treatment Patterns, and Outcomes of Specific Glomerular Lesions
Pauci-immune GN and renal vasculitis was the most frequently reported type of glomerular lesion associated with ICI use (n =12, summarized in Table 3). Median peak serum creatinine in these cases was 4.75 mg/dl (IQR 4.49–5.80 mg/dl), and 3 of 12 required RRT. Antineutrophil cytoplasmic antibody (ANCA) serology was positive in only 2 patients. All patients received corticosteroid therapy and 4 received initial pulse steroid treatment, with the remainder receiving prednisone 1 to 2 mg/kg/day. Rituximab was used in 4 patients, and 2 received cyclophosphamide. One patient progressed to ESKD, and death occurred in 4 of the 8 patients with available mortality data (Table 3).
Table 3.
Summary of published reports of pauci-immune glomerulonephritis and renal vasculitis associated with immune checkpoint inhibitor therapy
| Reference | Age, yrs | Sex | Cancer site | ICI(s) received | Baseline SCr (mg/dL) | Peak SCr (mg/dL) | ANCA serology | Initial corticosteroid therapy | Other immunosuppressive treatment | AKI outcome | Cancer outcome |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Heo et al.,39 2017 | 56 | M | Thymoma | Pembrolizumab | 0.78 | 4.49 RRT |
Positive | Methylprednisolone 500 mg IV daily × 3 d, then oral methylprednisolone | Cyclophosphamide | Partial | Stable |
| Cho et al.,35 2018 | NA | NA | Thymoma | Pembrolizumab | NA | NA | Positive | Corticosteroid, NOS | Cyclophosphamide | Partial | Stable |
| Gallan et al.,37 2019 | 68 | M | Melanoma | Nivolumab | 1.2 | 1.7 | NA | NA | NA | NA | Death |
| Gallan et al.,37 2019 | 71 | F | Lung adenocacrcinoma | Pembrolizumab | 0.8 | 0.8 | Negative | Pulse steroid, then high-dose oral steroid | None | NA | NA |
| Gallan et al.,37 2019 | 75 | F | Non–small cell lung carcinoma | Nivolumab | 1.1 | 6.1 | Negative | Corticosteroid, NOS | None | Partial | NA |
| Gallan et al.,37 2019 | 63 | F | Melanoma | Nivolumab | 0.9 | 8.9 RRT |
Negative | Pulse steroid, then oral steroid | IMCgp100a | Complete | NA |
| Lemoine et al.,46 2019 | 70 | M | Melanoma | Ipilimumab | 1.7 | 5.8 | Negative | Prednisone 1 mg/kg ×1 mo then tapered over 4 wks | None | Partial | Death |
| Mamlouk et al.,47 2019 | 41 | M | Squamous cell lung carcinoma | Nivolumab | 0.8 | 4.52 | Negative | Prednisone 1 mg/kg | Rituximab (1 dose) | Complete | Death |
| Mamlouk et al.,47 2019 | 75 | M | Renal cell carcinoma | Tremelimumab | 1.8 | 4.75 | NA | Methylprednisolone 2 mg/kg | Rituximab weekly ×4 doses, plasmapheresis daily ×5 sessions |
Partial | Stable |
| Mamlouk et al.,47 2019 | 69 | F | Melanoma | Ipilimumab plus nivolumab | 1.4 | 4.9 | Negative | Prednisone 1 mg/kg | Rituximab × 1 | Complete | Stable |
| Cortazar et al.,36 2020 | 41 | M | Squamous cell lung carcinoma | Nivolumab | NA | NA | Negative | Corticosteroid, NOS | Rituximab | Full | Death |
| Person et al.,48 2020 | 55 | M | Melanoma | Ipilimumab plus nivolumab | 1.2 | 1.7 RRT |
Negative | Methylprednisolone 200 mg IV daily | MMF, infliximab (for colitis) | ESKD | NA |
ANCA, antineutrophil cytoplasmic antibody; ESKD, end-stage kidney disease; F, female; ICI, immune checkpoint inhibitor; IV, intravenous; M, male; MMF, mycophenolate mofetil; NA, not available; NOS, not otherwise specified; RRT, renal replacement therapy.
IMCgp100 is an investigational drug that refocuses a T cell against the gp100 protein in uveal melanoma cells.
Podocytopathies, including MCD and focal segmental glomerulosclerosis, were the next most common lesions, with 11 published cases (Table 4). These patients had relatively preserved renal function but profound nephrotic-range proteinuria with median 10.3 g/day (IQR 9.0–19.0 g/day). Prednisone 1 to 2 mg/kg/day was used predominantly, with only 1 patient receiving pulse steroids. Complete or partial remission of proteinuria was achieved in 8 patients, with a median post-treatment proteinuria of 0.29 g/day (IQR 0.05–1.97 g/d). One patient progressed to ESKD, and 5 patients had cancer progression or death.
Table 4.
Summary of published reports of podocytopathies (minimal change disease and focal segmental glomerulosclerosis) associated with immune checkpoint inhibitor therapy
| Reference | Age, yrs | Sex | Cancer site | ICI(s) received | Baseline SCr, mg/dl | Peak SCr, mg/dl | Peak proteinuria, g/day | Post-treatment proteinuria, g/day | Initial corticosteroid therapy | Corticosteroid taper, days | AKI outcome | Proteinuria outcome | Cancer outcome |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Minimal change disease | |||||||||||||
| Bickel et al.,34 2016 | 62 | M | Mesothelioma | Pembrolizumab | GFR 90 | GFR 27 | 19 | 0 | Prednisone 1 mg/kg | 70 | Full | Remission | NA |
| Kidd and Gizaw,43 2016 | 55 | M | Melanoma | Ipilimumab | 1.2 | 5.2 | 9 | NA | Prednisone 2 mg/kg | NA | Full | Remission | Stable |
| Kitchlu et al.,45 2017 | 43 | M | Hodgkin lymphoma | Pembrolizumab | 0.76 | 3.93 | 10.3 | 3.1 | Prednisone 2 mg/kg × 14 d, then prednisone 1 mg/kg | 180 | Partial | Partial | Death |
| Kitchlu et al.,45 2017 | 45 | M | Melanoma | Ipilimumab | 0.68 | 0.8 | 9.5 | 0.39 | Prednisone 1 mg/kg taper over 4 mo | 120 | NA | Remission | Death |
| Gao et al.,38 2018 | 40 | M | Hodgkin lymphoma | SHR-1210 (anti-PD-1) | 0.77 | NA | 30 | 0.18 | Prednisone 1 mg/kg | 56 | NA | Remission | Stable |
| Izzedine et al.,41 2019 | NA | NA | Melanoma | Pembrolizumab | NA | NA | 6 | NA | NA | NA | ESKD | No improvement | Death |
| Izzedine et al.,41 2019 | NA | NA | Ileal neuroendocrine tumor | Pembrolizumab | NA | 1.65 | 3.5 | NA | NA | NA | No improvement | NA | NA |
| Saito et al.,52 2019 | 79 | M | Lung adenocarcinoma | Pembrolizumab | NA | NA | 13.8 | 0 | Prednisone 40 mg/d × 2 weeks, then 10 mg/d | 56 | NA | Remission | Stable |
| Cortazar et al.,36 2020 | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA |
| Focal segmental glomerulosclerosis | |||||||||||||
| Daanen et al.,53 2017 | 62 | M | Renal cell carcinoma | Nivolumab | 1.2 | 4.8 | 22 | 2.5 | Methylprednisolone 1 g × 3 d, then 60 mg/d | NA | Partial | Partial | Death |
| Mamlouk et al.,47 2019 | 74 | M | Renal cell carcinoma | Nivolumab | 1.6 | 2.73 | UPC 0.38 | NA | Prednisone 0.8 mg/kg | NA | Partial | Partial | Progression |
AKI, acute kidney injury; ESKD, end-stage kidney disease; GFR, glomerular filtration rate; ICI, immune checkpoint inhibitor; M, male; NA, not available; PD-1, programmed cell death 1; SCr, serum creatinine; UPC, urine protein creatinine ratio.
There were 5 cases of C3GN (Table 2) with a median peak serum creatinine 3.13 mg/dl (IQR 2.55–3.48 mg/dl). Two cases had low serum complement values, and 4 received prednisone therapy (1 mg/kg/day), 1 patient had complete recovery of AKI, and 3 had partial recovery. One patient (who had achieved partial recovery) was rechallenged with recurrence of disease.
Because of the small number of total reported patients, the planned univariate logistic regression for predictors of ESKD or death can only be considered an exploratory analysis, and this did not demonstrate significant associations (Supplementary Table S4).
Discussion
ICIs have revolutionized the treatment of patients with cancer in the last decade. In case reports and series, the most common histopathologic finding in patients who develop ICI-related AKI is AIN; however, acute tubular injury has also been described in a significant number of patients.56 Glomerular diseases with several different pathologies have been reported sporadically in the last few years. Our study is the first systematic review and meta-analysis specifically looking at all published series of glomerular pathology associated with ICI therapy and their outcomes.
Both nephrotic and nephritic presentations were associated with ICI therapy, with the most common pathologic findings being acute pauci-immune GN/vasculitis (27%), MCD (20%), and C3 GN (11%; Figure 2). Of the 12 published cases of pauci-immune GN, ANCA serologies were positive in only 2 patients, suggesting that most cases were ANCA-negative vasculitis. Dialysis was required in 25% of the patients with vasculitis with 1 patient progressing to ESKD. Treatment of renal vasculitis is challenging, let alone in the setting of ICI. All patients received steroids, although other treatment modalities were variable. Mortality was high in this group. In ICI-induced AIN, the use of proton-pump inhibitors may be a risk factor20,36; however, none typically associated with vasculitides (e.g., hydralazine) were noted in these 12 patients.
Podocytopathies, including MCD and focal segmental glomerulosclerosis, were the next most common lesions, with 11 published cases. More than 60% of these cases achieved partial or complete remission with drug discontinuation and corticosteroid therapy. Interestingly, C3GN was the third most common disease finding, with most patients achieving complete remission with steroids alone. This may suggest that in some patients with ICI-related glomerular disease (in which the kidney IRAE may be responsive to treatment), rechallenge with ICI can potentially be considered if the cancer response has been favorable and in the absence of other available treatment options. Interestingly, immunoglobulin A nephropathy and amyloid A amyloidosis have also been associated with ICI therapy, with the latter resulting in poor outcomes among these reported cases. Additional data are needed to determine whether ICI rechallenge may be associated with a greater risk in these glomerular pathologies (along with pauci-immune GN and renal vasculitis).
Initial reports suggested that anti–CTLA-4 agents may be more likely to cause glomerular diseases than PD-1/PD L1 inhibitors.19,43,45,57 Based on our analysis, there appears to be no such distinction. In fact, in the last few years, more glomerular diseases have been associated with PD-1/PD L-1 inhibitor therapy than CTLA-4 inhibitor therapies. Men seem to predominate over women in our analysis. This may be related to the increased prevalence of some skin cancers in males.58,59
ICI-associated AIN usually occurs 12 to 14 weeks after ICI initiation.36 Importantly AKI can develop earlier (especially when CTLA-4 and PD-1 signaling blockade are combined) or later (even after several months to over a year after ICI treatment discontinuation).60 The median time to initiation of ICI to diagnosis of glomerular disease was 3 months. Glomerular disease occurred as late as 7 months into the ICI therapy, and in a few cases, even months after it had been discontinued. This may be related to delayed recognition of glomerular disease relative to AIN. While the latter would be noted via routine serum creatinine testing with each cycle of ICI therapy, glomerular disease may only be recognized once symptoms manifest (i.e., edema, worsening hypertension, and foamy urine) or when serum creatinine increases substantially. The variety of glomerular pathologies noted in this review (particularly with frequent instances of podocytopathy) suggests that urinalysis and quantitative measures of proteinuria (e.g., urine albumin-/protein-to-creatinine ratios) may be beneficial before ICI initiation and as part of monitoring in select patients. In the various types of glomerular pathology observed, the severity of AKI varied, but most of the cases described were Kidney Disease: Improving Global Outcomes (KDIGO) AKI stages 2 and 3 at the time of diagnosis. Concomitant AIN was frequent (41%) in the cases reported, suggesting that AKI may be mediated both by glomerular injury as well and tubulointerstitial damage. Most patients with glomerular diseases had good baseline kidney function (with median serum creatinine 0.95 mg/dl). However, this may in part be related to the exclusion of patients with chronic kidney disease from trials involving novel cancer therapies.61 As ICI therapy becomes more widespread in real-world populations, patients with chronic kidney disease (and even those with pre-existing glomerular disease) may be at risk for poor outcomes.
The management of ICI-associated AIN includes intravenous or oral corticosteroids in addition to holding ICI therapy (and proton-pump inhibitor or other AIN-associated concomitant treatments). Standard steroid dosing of prednisone 0.8 to 1 mg/kg/day (or equivalent), with a maximum dose of 60 to 80 mg/day for stage 1 or 2 AKI has been shown to provide good renal response.28,36 The addition of intravenous steroids for 2 to 3 days before oral steroids has been noted in patients with stage 3 AKI who need dialysis. Eight to 12 weeks of steroid taper is often required to adequately treat and prevent relapse. In the reported cases of ICI-associated glomerular diseases, most patients had ICI discontinued (88%) and nearly all received corticosteroid treatment (98%). Most patients had either complete (31%) or partial (42%) recovery from AKI, although the incidence of ESKD was greater (19%) when compared with published data from ICI-induced AIN cases (9% in the largest series). Cortazar et al.36 showed in the largest series of ICI-induced AIN that failure to improve renal function had a higher mortality. In our analysis, >30% of the patients died either of cancer progression or worsening of kidney function. The high rate of ESKD and death may reflect the complex nature of treating a rare glomerular disease and cancer at the same time.
ICIs may be the only therapeutic option available to effectively treat and maintain tumor remission in certain cancers. A key concern is the safety of ICI rechallenge after an episode of AKI, particularly those with stage 2 or 3 AKI. Cortazar et al.36 rechallenged 31 patients with the same ICI with 40% receiving concomitant corticosteroids at the time of rechallenge. Recurrence of ICI-associated AKI occurred in 23% of rechallenged patients, with a shorter latency period between the initial AKI episode and the rechallenge. Importantly, all but 1 patient had complete or partial recovery of kidney function after rechallenge. Similarly, Manohar et al.62 described 4 patients who underwent rechallenge after removing AIN-associated drugs in 3 of the 4 patients. Rechallenge was done along with a low dose of corticosteroid therapy (prednisone 10-20 mg/day) in 3 of 4 patients.
In glomerular diseases, rechallenge may not be as simple. In our analysis of the cases, rechallenge was reported in only 4 patients, with 2 of these demonstrating recurrence of glomerular disease. The rechallenge approach used in ICI-associated AIN may not be applicable in cases of systemic vasculitis and other severe nephritic syndromes (particularly those in whom remission was not achieved), but may be safer to do in cases of nephrotic syndromes with MCD and focal segmental glomerulosclerosis. Given the small number of cases in total, we cannot make any specific recommendations of ICI rechallenge in patients with ICI-induced GNs. An individualized approach might be preferred.
Another important topic that has been debated is the need for obtaining a kidney biopsy specimen for ICI-associated kidney injuries.63, 64, 65 The American Society of Clinical Oncology and the National Comprehensive Cancer Network guidelines recommend the following: if an alternative cause of AKI is not identified, clinicians should proceed directly with immunosuppressive therapy without a kidney biopsy specimen unless a moderate/severe life-threatening renal IRAE (defined as Common Terminology Criteria for Adverse Events Grade ≥2–3 is present).28,66 In the largest multicenter series by Cortazar et al.,36 a kidney biopsy specimen was obtained in only 43% of patients. Perhaps not unexpectedly, there were only 4 reported glomerular disease cases within this study. Given that glomerular diseases might be present in certain cases (with or without concomitant AIN), obtaining a kidney biopsy specimen may be indicated in many more cases. This may be particularly important in patients with significant or new-onset proteinuria, urinary sediment atypical for AIN, and those who do not respond to typical AIN corticosteroid dosing. Given the rise in the number of glomerular lesions reported with ICI therapy, a prompt nephrology evaluation and obtaining a kidney biopsy specimen may be prudent in many of these patients.
In our exploratory logistic regression analyses of possible predictors of ESKD and death, we did not find statistically significant associations, despite the inclusion of variables that ostensibly would be associated with this outcome (e.g., peak creatinine and proteinuria). This is likely because of the small numbers within our pooled cases (i.e., 15 patients of 45 reaching the composite endpoint of ESKD or death) and the lack of sufficient statistical power to detect associations. However, it is also possible that cancer-specific variables would be more likely to predict disease progression and death, particularly as even patients with severe AKI and high-grade proteinuria had resolution of these issues after ICI cessation and corticosteroid therapy. Similarly, additional data are needed to clarify the potential association between drugs such as nonsteroidal anti-inflammatory drugs and proton-pump inhibitors (which have been implicated in ICI-related AIN)20,36 and ICI-related glomerular disease.
The pathophysiology linking ICI use and these multiple forms of glomerular pathology remains unclear. One may speculate that derangements in T cell activity may lead to inflammatory processes that may underlie certain glomerular diseases. For example, T cell overactivity is associated with the propagation of the inflammatory response in ANCA-associated GN and vasculitis. In the pathogenesis of ANCA-associated vasculitis, CD8+ T cells, which express the CTLA-4 receptor, may activate polymorphonuclear cells. Polymorphonuclear cell activation leads to the exposure of proteinase-3 and myeloperoxidase antigens on the surface of the polymorphonuclear cells; these antigens may then react with ANCA.67 Interestingly, we observed that only 2 of 12 reported patients had ANCA positivity. We may speculate that one possible reason is that the available assays may not detect certain epitopes to which pathogenic ANCA reacts68; however, further research is needed to elucidate this. Similarly, it is remains unclear why cases of C3GN were prominent in our findings. We may conjecture that ICIs may induce autoantibodies similar to C3 and C5 nephritic factors. Also, it has been shown that PD-L1 blockade causes a significant amount of C5a production, which could conceivably lead to glomerular inflammation.69 Of note, in the small number of reported patients with C3GN, 4 responded to steroids (which is atypical of most other forms of C3GN). Steroids and immunosuppressive therapy may possibly decrease the generation of C3a and C5a by inhibiting the production of autoantibodies.70 In these patients, it is possible that stopping the ICI and administering steroids halted autoantibody production and the fixing of complement. At present, this remains entirely speculative and additional research into the mechanisms by which ICIs mediate glomerular disease may provide insight into the pathogeneses of these conditions, which remain poorly understood.
This is the first study to analyze all published cases and case series of glomerular pathology associated with ICI therapy. Strengths include the use of a comprehensive search strategy, based on PRISMA recommendations. We also limited cases to peer-reviewed publications with a reported kidney biopsy specimen, allowing us to include cases in which glomerular injury was convincingly attributable to ICI therapy. We were able to include all reported forms of glomerular pathology and to comment on treatment strategies and outcomes. Given that biopsy specimens for kidney IRAE are infrequently obtained, even with numerous participating sites, cohort studies or even large case series of ICI-associated glomerular disease may not be feasible in the near future. As such, pooling available case reports and series is likely a necessary approach to obtaining a reasonable number of cases from which to draw conclusions.
This review does, however, have important limitations. First, as our review includes only reported cases of glomerular disease with ICIs; we are unable to make any specific estimates as to the overall (or lesion-specific) incidence among patients receiving ICIs. Moreover, given the variability in glomerular diseases, case presentations, treatment approaches, and outcomes, conclusions may be difficult to generalize. To this end, we attempted to group cases of the most commonly reported disease types. Given that the disease-specific numbers were quite small, our ability to comment on therapeutic strategies and outcomes is limited. For the same reasons, our exploratory analyses to assess predictors of ESKD/death were restricted to univariate analyses, and likely had limited ability to detect associations. Also, while we presume that the included glomerular disease cases purported to be associated with ICI use (based on time-course and disease presentation) are true kidney IRAEs, it is possible that some cases simply revealed coexisting glomerular disease or disease related to malignancy itself. Lastly, missing data, and nonuniform reporting of case descriptors, pathologic findings, and outcomes may also have reduced our ability to assess risk factors and estimate outcomes.
In conclusion, myriad forms of glomerular disease have been reported in association with ICI therapy and these pathologies may present distinctly from the more frequently observed AIN-type of immune-mediated nephrotoxicity. Pauci-immune GN/renal vasculitis, podocytopathies, and C3GN appear to be the most frequently reported types of glomerular lesions. Glomerular disease associated with ICI use portends poor kidney and cancer outcomes in many cases. Oncologists and nephrologists must be aware of the potential for glomerular injury associated with these agents and consider the judicious use of obtaining a kidney biopsy specimen when clinically indicated.
Disclosure
KDJ is a consultant for Astex Pharmaceuticals and Natera and receives fees from Uptodate.com outside of the submitted work and also receives honorarium from the International Society of Nephrology and American Society of Nephrology. SW has received an honorarium from Retrophin, Inc. All the other authors declared no competing interests.
Acknowledgments
We thank Edgar Lerma, Clinical Professor of Medicine with the Section of Nephrology at University of Illinois at Chicago, for his assistance with the visual abstract.
Author Contributions
AK, ZH, KDJ, and RW had full access to all of the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis. AK, KDJ, and RW are responsible for the concept and design. AK, KDJ, RW, PD, ZH, SW, and KH had roles in the acquisition, analysis, and interpretation of data. AK, KDJ, and RW drafted the manuscript. AK, KDJ, RW, PD, ZH, SW, and KH were responsible for critical revision of the manuscript for important intellectual content. AK and ZH conducted the statistical analysis.
Footnotes
PRISMA Checklist.
Literature Search Strategy.
Table S1. Summary of all reported cases of ICI-associated glomerular disease and causality assessment.
Table S2. Quality assessment of case reports and series of immune checkpoint inhibitor-associated glomerular disease.
Table S3. Newcastle-Ottawa scale for non-randomized studies for immune checkpoint inhibitor-associated glomerular disease.
Table S4. Exploratory univariate logistic regression for predictor of end-stage renal disease or death in patients with glomerular disease associated with immune checkpoint inhibitors.
Contributor Information
Abhijat Kitchlu, Email: abhijat.kitchlu@uhn.ca.
Kenar D. Jhaveri, Email: kjhaveri@northwell.edu.
Supplementary Material
PRISMA Checklist.
Literature Search Strategy.
Table S1. Summary of all reported cases of ICI-associated glomerular disease and causality assessment.
Table S2. Quality assessment of case reports and series of immune checkpoint inhibitor-associated glomerular disease.
Table S3. Newcastle-Ottawa scale for non-randomized studies for immune checkpoint inhibitor-associated glomerular disease.
Table S4. Exploratory univariate logistic regression for predictor of end-stage renal disease or death in patients with glomerular disease associated with immune checkpoint inhibitors.
References
- 1.Darvin P., Toor S.M., Sasidharan Nair V. Immune checkpoint inhibitors: recent progress and potential biomarkers. Exp Mol Med. 2018;50:1–11. doi: 10.1038/s12276-018-0191-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Weber J. Immune checkpoint proteins: a new therapeutic paradigm for cancer--preclinical background: CTLA-4 and PD-1 blockade. Semin Oncol. 2010;37:430–439. doi: 10.1053/j.seminoncol.2010.09.005. [DOI] [PubMed] [Google Scholar]
- 3.Callahan M.K., Kluger H., Postow M.A. Nivolumab plus ipilimumab in patients with advanced melanoma: updated survival, response, and safety data in a phase I dose-escalation study. J Clin Oncol. 2018;36:391–398. doi: 10.1200/JCO.2017.72.2850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Flippot R., Escudier B., Albiges L. Immune checkpoint inhibitors: toward new paradigms in renal cell carcinoma. Drugs. 2018;78:1443–1457. doi: 10.1007/s40265-018-0970-y. [DOI] [PubMed] [Google Scholar]
- 5.Garon E.B., Rizvi N.A., Hui R. Pembrolizumab for the treatment of non-small-cell lung cancer. N Engl J Med. 2015;372:2018–2028. doi: 10.1056/NEJMoa1501824. [DOI] [PubMed] [Google Scholar]
- 6.Ghatalia P., Plimack E.R. Integration of immunotherapy into the treatment of advanced urothelial carcinoma. J Natl Compr Canc Netw. 2020;18:355–361. doi: 10.6004/jnccn.2020.7539. [DOI] [PubMed] [Google Scholar]
- 7.Motzer R.J., Escudier B., McDermott D.F. Nivolumab versus everolimus in advanced renal-cell carcinoma. N Engl J Med. 2015;373:1803–1813. doi: 10.1056/NEJMoa1510665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yokota K., Uchi H., Uhara H. Adjuvant therapy with nivolumab versus ipilimumab after complete resection of stage III/IV melanoma: Japanese subgroup analysis from the phase 3 CheckMate 238 study. J Dermatol. 2019;46:1197–1201. doi: 10.1111/1346-8138.15103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Postow M.A., Sidlow R., Hellmann M.D. Immune-related adverse events associated with immune checkpoint blockade. N Engl J Med. 2018;378:158–168. doi: 10.1056/NEJMra1703481. [DOI] [PubMed] [Google Scholar]
- 10.Abdel-Wahab N., Shah M., Lopez-Olivo M.A. Use of immune checkpoint inhibitors in the treatment of patients with cancer and preexisting autoimmune disease: a systematic review. Ann Intern Med. 2018;168:121–130. doi: 10.7326/M17-2073. [DOI] [PubMed] [Google Scholar]
- 11.Izzedine H., Mateus C., Boutros C. Renal effects of immune checkpoint inhibitors. Nephrol Dial Transplant. 2017;32:936–942. doi: 10.1093/ndt/gfw382. [DOI] [PubMed] [Google Scholar]
- 12.Jhaveri K.D., Wanchoo R. Adverse events associated with immune checkpoint inhibitors. JAMA. 2019;321:1218–1219. doi: 10.1001/jama.2018.22114. [DOI] [PubMed] [Google Scholar]
- 13.Mamlouk O., Abudayyeh A. Cancer immunotherapy and its renal effects. Journal of Onco-Nephrology. 2019;3:151–159. [Google Scholar]
- 14.Murakami N., Motwani S., Riella L.V. Renal complications of immune checkpoint blockade. Curr Probl Cancer. 2017;41:100–110. doi: 10.1016/j.currproblcancer.2016.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Perazella M.A. Checkmate: kidney injury associated with targeted cancer immunotherapy. Kidney Int. 2016;90:474–476. doi: 10.1016/j.kint.2016.05.024. [DOI] [PubMed] [Google Scholar]
- 16.Perazella M.A., Shirali A.C. Immune checkpoint inhibitor nephrotoxicity: what do we know and what should we do? Kidney Int. 2020;97:62–74. doi: 10.1016/j.kint.2019.07.022. [DOI] [PubMed] [Google Scholar]
- 17.Sury K., Perazella M.A., Shirali A.C. Cardiorenal complications of immune checkpoint inhibitors. Nat Rev Nephrol. 2018;14:571–588. doi: 10.1038/s41581-018-0035-1. [DOI] [PubMed] [Google Scholar]
- 18.Wanchoo R., Karam S., Uppal N.N. Adverse renal effects of immune checkpoint inhibitors: a narrative review. Am J Nephrol. 2017;45:160–169. doi: 10.1159/000455014. [DOI] [PubMed] [Google Scholar]
- 19.Cortazar F.B., Marrone K.A., Troxell M.L. Clinicopathological features of acute kidney injury associated with immune checkpoint inhibitors. Kidney Int. 2016;90:638–647. doi: 10.1016/j.kint.2016.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Seethapathy H., Zhao S., Chute D.F. The incidence, causes, and risk factors of acute kidney injury in patients receiving immune checkpoint inhibitors. Clin J Am Soc Nephrol. 2019;14:1692–1700. doi: 10.2215/CJN.00990119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Belliere J., Meyer N., Mazieres J. Acute interstitial nephritis related to immune checkpoint inhibitors. Br J Cancer. 2016;115:1457–1461. doi: 10.1038/bjc.2016.358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bottlaender L., Breton A.L., de Laforcade L. Acute interstitial nephritis after sequential ipilumumab - nivolumab therapy of metastatic melanoma. J Immunother Cancer. 2017;5:57. doi: 10.1186/s40425-017-0261-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.de Sousa Oliveira D., Mesquita J.L., Garcia Y.D.O. Interstitial nephritis associated with nivolumab in a patient with hodgkin lymphoma. Rev Assoc Med Bras. 2019;68:934–936. doi: 10.1590/1806-9282.65.7.934. [DOI] [PubMed] [Google Scholar]
- 24.Georgianos P.I., Vaios V., Leontaridou E. Acute interstitial nephritis in a patient with non-small cell lung cancer under immunotherapy with nivolumab. Case Rep Nephrol. 2019;2019:3614980. doi: 10.1155/2019/3614980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Manohar S., Albright R.C. Interstitial nephritis in immune checkpoint inhibitor therapy. Kidney Int. 2019;96:252. doi: 10.1016/j.kint.2018.11.009. [DOI] [PubMed] [Google Scholar]
- 26.Shirali A.C., Perazella M.A., Gettinger S. Association of acute interstitial nephritis with programmed cell death 1 inhibitor therapy in lung cancer patients. Am J Kidney Dis. 2016;68:287–291. doi: 10.1053/j.ajkd.2016.02.057. [DOI] [PubMed] [Google Scholar]
- 27.Thajudeen B., Madhrira M., Bracamonte E. Ipilimumab granulomatous interstitial nephritis. Am J Ther. 2015;22:e84–e87. doi: 10.1097/MJT.0b013e3182a32ddc. [DOI] [PubMed] [Google Scholar]
- 28.Brahmer J.R., Lacchetti C., Schneider B.J. Management of immune-related adverse events in patients treated with immune checkpoint inhibitor therapy: American Society of Clinical Oncology clinical practice guideline. J Clin Oncol. 2018;36:1714–1768. doi: 10.1200/JCO.2017.77.6385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Moher D., Liberati A., Tetzlaff J. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. 2009;6 doi: 10.1371/journal.pmed.1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.World Health Organization website The use of the WHO-UMC system for standardised case causality assessment. https://www.who.int/medicines/areas/quality_safety/safety_efficacy/WHOcausality_assessment.pdf Available at:
- 31.Murad M.H., Sultan S., Haffar S. Methodological quality and synthesis of case series and case reports. BMJ Evid Based Med. 2018;23:60–63. doi: 10.1136/bmjebm-2017-110853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.The Ottawa Hospital Researh Institute website. Wells G.A., Shea B., O’Connell D. The Newcastle-Ottawa Scale (NOS) for assessing the quality of nonrandomised studies in meta-analyses. http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp Available at:
- 33.Ashour T., Nakhoul G., Patil P. Immune check point inhibitor-associated glomerulonephritis. Kid Int Rep. 2019;4:355–359. doi: 10.1016/j.ekir.2018.10.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bickel A., Koneth I., Enzler-Tschudy A. Pembrolizumab-associated minimal change disease in a patient with malignant pleural mesothelioma. BMC Cancer. 2016;16:656. doi: 10.1186/s12885-016-2718-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Cho J., Kim H.S., Ku B.M. Pembrolizumab for patients with refractory or relapsed thymic epithelial tumor: an open-label phase II trial. J Clin Oncol. 2019;37:2162–2170. doi: 10.1200/JCO.2017.77.3184. [DOI] [PubMed] [Google Scholar]
- 36.Cortazar F.B., Kibbelaar Z.A., Glezerman I.G. Clinical features and outcomes of immune checkpoint inhibitor-associated AKI: a multicenter study. J Am Soc Nephrol. 2020;31:435–446. doi: 10.1681/ASN.2019070676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gallan A.J., Alexander E., Reid P. Renal vasculitis and pauci-immune glomerulonephritis associated with immune checkpoint inhibitors. Am J Kidney Dis. 2019;74:853–856. doi: 10.1053/j.ajkd.2019.04.016. [DOI] [PubMed] [Google Scholar]
- 38.Gao B., Lin N., Wang S. Minimal change disease associated with anti-PD1 immunotherapy: a case report. BMC Nephrol. 2018;19:156. doi: 10.1186/s12882-018-0958-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Heo M.H., Kim H.K., Lee H. Antineutrophil cytoplasmic antibody-associated rapid progressive glomerulonephritis after pembrolizumab treatment in thymic epithelial tumor: a case report. J Thorac Oncol. 2017;12:e103–e105. doi: 10.1016/j.jtho.2017.03.011. [DOI] [PubMed] [Google Scholar]
- 40.Irifuku T., Satoh A., Tani H. Acute tubulointerstitial nephritis and IgM deposits on glomerular capillary walls after immunotherapy with nivolumab for metastatic renal cell carcinoma. CEN Case Rep. 2020;9:48–54. doi: 10.1007/s13730-019-00424-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Izzedine H., Mathian A., Champiat S. Renal toxicities associated with pembrolizumab. Clin Kidney J. 2019;12:81–88. doi: 10.1093/ckj/sfy100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Jung K., Zeng X., Bilusic M. Nivolumab-associated acute glomerulonephritis: a case report and literature review. BMC Nephrol. 2016;17:188. doi: 10.1186/s12882-016-0408-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kidd J.M., Gizaw A.B. Ipilimumab-associated minimal-change disease. Kidney Int. 2016;89:720. doi: 10.1016/j.kint.2015.11.028. [DOI] [PubMed] [Google Scholar]
- 44.Kishi S., Minato M., Saijo A. IgA nephropathy after nivolumab therapy for postoperative recurrence of lung squamous cell carcinoma. Intern Med. 2018;57:1259–1263. doi: 10.2169/internalmedicine.9814-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kitchlu A., Fingrut W., Avila-Casado C. Nephrotic syndrome with cancer immunotherapies: a report of 2 cases. Am J Kidney Dis. 2017;70:581–585. doi: 10.1053/j.ajkd.2017.04.026. [DOI] [PubMed] [Google Scholar]
- 46.Lemoine M., Dilly B., Curie A. Ipilimumab-induced renal granulomatous arteritis: a case report. BMC Nephrol. 2019;20:366. doi: 10.1186/s12882-019-1552-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Mamlouk O., Selamet U., Machado S. Nephrotoxicity of immune checkpoint inhibitors beyond tubulointerstitial nephritis: single-center experience. J Immunother Cancer. 2019;7:2. doi: 10.1186/s40425-018-0478-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Person F., Chahoud-Schriefer T., Fehrle W. Severe acute kidney injury due to nivolumab/ipilimumab-induced granulomatosis and fibrinoid vascular necrosis. J Immunother. 2020;43:29–31. doi: 10.1097/CJI.0000000000000296. [DOI] [PubMed] [Google Scholar]
- 49.Takahashi N., Tsuji K., Tamiya H. Goodpasture’s disease in a patient with advanced lung cancer treated with nivolumab: an autopsy case report. Lung Cancer. 2018;122:22–24. doi: 10.1016/j.lungcan.2018.05.015. [DOI] [PubMed] [Google Scholar]
- 50.Ville S., Kandel-Aznar C., Fremeaux-Bacchi V. C3 glomerulonephritis in a patient treated with anti-PD-1 antibody. Eur J Cancer. 2020;125:46–48. doi: 10.1016/j.ejca.2019.11.011. [DOI] [PubMed] [Google Scholar]
- 51.Oki R., Hirakawa Y., Kimura H. Renal effects after pembrolizumab treatment for non-small cell lung carcinoma: a case report. Intern Med. 2020;59:977–981. doi: 10.2169/internalmedicine.3928-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Saito S., Kadota T., Gochi M. Re-administration of pembrolizumab with prednisolone after pembrolizumab-induced nephrotic syndrome. Eur J Cancer. 2020;126:74–77. doi: 10.1016/j.ejca.2019.12.015. [DOI] [PubMed] [Google Scholar]
- 53.Daanen R.A., Maas R.J.H., Koornstra R.H.T. Nivolumab-associated nephrotic syndrome in a patient with renal cell carcinoma: a case report. J Immunother. 2017;40:345–348. doi: 10.1097/CJI.0000000000000189. [DOI] [PubMed] [Google Scholar]
- 54.Lapman S., Whittier W.L., Parikh R. Immune checkpoint inhibitor–associated renal amyloid A amyloidosis: a case series and review of the literature. Journal of Onco-Nephrology. 2020;4 2399369320907598. [Google Scholar]
- 55.Sammartino C., Goodman D., Flanagan G. Anti-GBM disease following CTLA4 blockade in a patient with metastatic melanoma. NDT Plus. 2009;3:135–137. [Google Scholar]
- 56.Sprangers B. Pembrolizumab-related renal toxicities: diagnosis first, treatment later. Clin Kidney J. 2019;12:78–80. doi: 10.1093/ckj/sfy114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Fadel F., El Karoui K., Knebelmann B. Anti-CTLA4 antibody-induced lupus nephritis. N Engl J Med. 2009;361:211–212. doi: 10.1056/NEJMc0904283. [DOI] [PubMed] [Google Scholar]
- 58.Chen J., Shih J., Tran A. Gender-based differences and barriers in skin protection behaviors in melanoma survivors. J Skin Cancer. 2016;2016:3874572. doi: 10.1155/2016/3874572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Smalley K.S. Why do women with melanoma do better than men? Elife. 2018;7 doi: 10.7554/eLife.33511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Jhaveri K.D., Perazella M.A. Adverse events associated with immune checkpoint blockade. N Engl J Med. 2018;378:1163. doi: 10.1056/NEJMc1801663. [DOI] [PubMed] [Google Scholar]
- 61.Kitchlu A., Shapiro J., Amir E. Representation of patients with chronic kidney disease in trials of cancer therapy. JAMA. 2018;319:2437–2439. doi: 10.1001/jama.2018.7260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Manohar S., Ghamrawi R., Chengappa M. acute interstitial nephritis and checkpoint inhibitor therapy. Kidney360. 2020;1:16. doi: 10.34067/KID.0000152019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Eijgelsheim M., Sprangers B. Kidney biopsy should be performed to document the cause of immune checkpoint inhibitor–associated acute kidney injury: pro. Kidney360. 2020;1:158. doi: 10.34067/KID.0001192019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Gutgarts V., Glezerman I.G. Kidney biopsy should be performed to document the cause of immune checkpoint inhibitor–associated acute kidney injury: con. Kidney360. 2020;1:162. doi: 10.34067/KID.0000132020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Perazella M.A. Kidney biopsy should be performed to document the cause of immune checkpoint inhibitor–associated acute kidney injury: commentary. Kidney360. 2020;1:166. doi: 10.34067/KID.0001072019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Thompson J.A., Schneider B.J., Brahmer J. Management of immunotherapy-related toxicities, version 1.2019. J Natl Compr Canc Netw. 2019;17:255–289. doi: 10.6004/jnccn.2019.0013. [DOI] [PubMed] [Google Scholar]
- 67.Martinez Valenzuela L., Bordignon Draibe J., Fulladosa Oliveras X. T-lymphocyte in ANCA-associated vasculitis: what do we know? A pathophysiological and therapeutic approach. Clin Kidney J. 2019;12:503–511. doi: 10.1093/ckj/sfz029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Roth A.J., Ooi J.D., Hess J.J. Epitope specificity determines pathogenicity and detectability in ANCA-associated vasculitis. J Clin Invest. 2013;123:1773–1783. doi: 10.1172/JCI65292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Zha H., Han X., Zhu Y. Blocking C5aR signaling promotes the anti-tumor efficacy of PD-1/PD-L1 blockade. Oncoimmunology. 2017;6 doi: 10.1080/2162402X.2017.1349587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Smith R.J.H., Appel G.B., Blom A.M. C3 glomerulopathy - understanding a rare complement-driven renal disease. Nat Rev Nephrol. 2019;15:129–143. doi: 10.1038/s41581-018-0107-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.