Key Points
Question
Is maternal oxygen supplementation at the time of delivery associated with improved umbilical artery gas measures and neonatal outcomes?
Findings
In this systematic review and meta-analysis of 16 randomized clinical trials, peripartum maternal oxygen supplementation was associated with an improvement in umbilical artery Pao2 but no significant difference in umbilical artery pH compared with room air. Other umbilical artery gas measures, rates of neonatal intensive care unit admission, and Apgar scores were similar between the oxygen and room air groups.
Meaning
This systematic review and meta-analysis found no association between maternal oxygen supplementation and a clinically relevant improvement in umbilical artery pH or other neonatal outcomes.
Abstract
Importance
Supplemental oxygen is commonly administered to pregnant women at the time of delivery to prevent fetal hypoxia and acidemia. There is mixed evidence on the utility of this practice.
Objective
To compare the association of peripartum maternal oxygen administration with room air on umbilical artery (UA) gas measures and neonatal outcomes.
Data Sources
Ovid MEDLINE, Embase, Scopus, ClinicalTrials.gov, and Cochrane Central Register of Controlled Trials were searched from February 18 to April 3, 2020. Search terms included labor or obstetric delivery and oxygen therapy and fetal blood or blood gas or acid-base imbalance.
Study Selection
Studies were included if they were randomized clinical trials comparing oxygen with room air at the time of scheduled cesarean delivery or labor in patients with singleton, nonanomalous pregnancies. Studies that did not collect paired umbilical cord gas samples or did not report either UA pH or UA Pao2 results were excluded.
Data Extraction and Synthesis
Data were extracted by 2 independent reviewers. The analysis was stratified by the presence or absence of labor at the time of randomization. Data were pooled using random-effects models.
Main Outcomes and Measures
The primary outcome for this review was UA pH. Secondary outcomes included UA pH less than 7.2, UA Pao2, UA base excess, 1- and 5-minute Apgar scores, and neonatal intensive care unit admission.
Results
The meta-analysis included 16 randomized clinical trials (n = 1078 oxygen group and n = 974 room air group). There was significant heterogeneity among the studies (I2 = 49.88%; P = .03). Overall, oxygen administration was associated with no significant difference in UA pH (weighted mean difference, 0.00; 95% CI, −0.01 to 0.01). Oxygen use was associated with an increase in UA Pao2 (weighted mean difference, 2.57 mm Hg; 95% CI, 0.80-4.34 mm Hg) but no significant difference in UA base excess, UA pH less than 7.2, Apgar scores, or neonatal intensive care unit admissions. Umbilical artery pH values remained similar between groups after accounting for the risk of bias, type of oxygen delivery device, and fraction of inspired oxygen. After stratifying by the presence or absence of labor, oxygen administration in women undergoing scheduled cesarean delivery was associated with increased UA Pao2 (weighted mean difference, 2.12 mm Hg; 95% CI, 0.09-4.15 mm Hg) and a reduction in the incidence of UA pH less than 7.2 (relative risk, 0.63; 95% CI, 0.43-0.90), but these changes were not noted among those in labor (Pao2: weighted mean difference, 3.60 mm Hg; 95% CI, −0.30 to 7.49 mm Hg; UA pH<7.2: relative risk, 1.34; 95% CI, 0.58-3.11).
Conclusions and Relevance
This systematic review and meta-analysis suggests that studies to date showed no association between maternal oxygen and a clinically relevant improvement in UA pH or other neonatal outcomes.
This systematic review and meta-analysis examines randomized clinical trials on the use of oxygen vs room air in women in labor or undergoing cesarean delivery.
Introduction
Maternal oxygen supplementation is a widely used intrauterine resuscitation technique recommended by the American College of Obstetricians and Gynecologists for the management of abnormal fetal heart rate tracings.1 In the presence of fetal heart rate patterns that may represent fetal hypoxia,2 oxygen is administered to the mother with the intent of increasing placental oxygen transfer and preventing neonatal acidemia. This use of oxygen for fetal resuscitation is so widespread that 2 of 3 women in labor in the US will receive oxygen at some point during labor.3
Umbilical artery (UA) gas samples obtained at the time of delivery provide an objective assessment of in utero fetal oxygenation and metabolic status.4 Umbilical artery gas analysis provides measurements of pH, Pao2, Paco2, and base excess. These measurements are used by clinicians to assess neonatal acidemia and estimate short- and long-term morbidity.5,6
A 2016 Cochrane review of 2 trials investigating the use of intrapartum oxygen for intrauterine resuscitation concluded that there was insufficient evidence to evaluate the effectiveness of oxygen in that setting.7 A separate 2012 Cochrane review specifically assessing the use of supplemental oxygen at the time of cesarean delivery (CD) concluded that oxygen was associated with higher maternal and neonatal blood gas values with otherwise no evidence of clinical benefit or harm.8 Subsequent to these Cochrane reviews, additional trials showing mixed results in investigation of peripartum oxygen administration have been published.9,10,11,12,13,14 Although some studies suggested fetal benefit with increased UA Pao2 and UA pH levels,10,15 others demonstrated harm with oxygen, including a higher proportion of neonates with acidemia and requiring delivery room resuscitation compared with those exposed to room air.16 Moreover, the timing and setting of oxygen administration varied among these studies, with some evaluating oxygen during labor and others in the absence of labor. This distinction is important because the physiologic characteristics of placental oxygen transfer and, hence, UA gases may differ based on the presence or absence of regular uterine contractions.
The objectives of this study were to synthesize data from randomized clinical trials comparing peripartum oxygen supplementation with room air and investigate the association between oxygen administration at the time of labor or planned CD and UA gas measures and other neonatal outcomes.
Data Sources
This study followed the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) reporting guideline for meta-analyses and the Cochrane Handbook for Systematic Reviews of Interventions.17 We used an a priori research protocol that defined the research question, inclusion and exclusion criteria, population, exposures, and risk of bias criteria.
The literature was searched using strategies created by a medical librarian (M.M.D.) for administration of maternal oxygen during delivery and umbilical cord gas measures. The search strategies were implemented in Ovid MEDLINE 1946-, Embase 1947-, Scopus, ClinicalTrials.gov, and Cochrane Central Register of Controlled Trials, without a language limit, and were established using a combination of standardized terms and key words including, but not limited to, labor or obstetric delivery and oxygen therapy and fetal blood or blood gas or acid-base imbalance. The search syntax appears in the eAppendix in the Supplement. A sensitive search filter was used to limit for randomized clinical trials. All databases were searched from February 18 to April 3, 2020.
Study Selection
Studies were included if they were randomized clinical trials comparing maternal oxygen supplementation with room air at the time of delivery in patients with singleton, nonanomalous pregnancies. All routes and doses of oxygen delivery were included. Studies that did not collect paired cord gas samples or did not have either UA pH or UA Pao2 results were excluded. We excluded nonrandomized studies, case reports or series, and studies published only in abstract form. The primary outcome for this review was UA pH. Secondary outcomes were UA pH less than 7.2, UA pH less than 7.1, UA Pao2, UA base excess, 1- and 5-minute Apgar scores, neonatal intensive care unit admission, and oxidative stress markers.
Titles and abstracts from the initial search result were independently reviewed by 2 of us (N.R. and L.A.T.). Full-text articles were obtained if there was uncertainty about inclusion based on the abstract. Discrepancies were resolved by the senior author (M.G.T.). Two of us (N.R. and L.A.T.) independently abstracted data into standard extraction forms. Discrepancies in data abstraction were resolved by discussion or by the senior author. Details regarding oxygen administration were collected. Nasal cannulas and simple face masks were categorized as low-flow devices, whereas nonrebreathers, Venturi masks, and anesthetic masks were considered high-flow devices.
We categorized studies as low or high risk for bias using 3 factors considered most likely to limit the validity of study results18,19,20: valid randomization method, loss to follow-up less than 15%, and analysis using the intention-to-treat principle. All 3 criteria had to be met for a study to be labeled as low risk for bias. Studies that did not have adequate information to determine the answers to the above criteria were considered to be at high risk for bias. Valid randomization included the use of random number tables, computer-generated sequences, and other accepted methods of random allocation.
Statistical Analysis
Mean values were estimated from median values using the method published by Hozo et al.21 Umbilical artery Pao2 results reported in kilopascals were converted to millimeters of mercury using the formula millimeters of mercury = kilopascals × 7.50. The results for each outcome were stratified by the presence or absence of labor (labor or scheduled CD). We performed additional stratified analyses by risk of bias, fraction of inspired oxygen (Fio2), and type of oxygen delivery device for the primary outcome.
The Higgins I2 test and Cochrane Q test were used to quantify and assess heterogeneity.22 Heterogeneity was considered significant at P < .10 for the Q tests or I2 > 30%. Using random-effects models, raw data from the studies were pooled to obtain relative risks (RRs) with 95% CI for categorical outcomes and weighted mean difference (WMD) with 95% CI for continuous outcomes. Random-effects models were used because of the significant heterogeneity among the studies. To assess for publication bias, we visually inspected funnel plots and performed the Egger test for small study effect.23 A 2-sided P < .05 was considered statistically significant for pooled analyses. Statistical analyses were performed using Stata, version 16.1 (StataCorp LLC).
Results
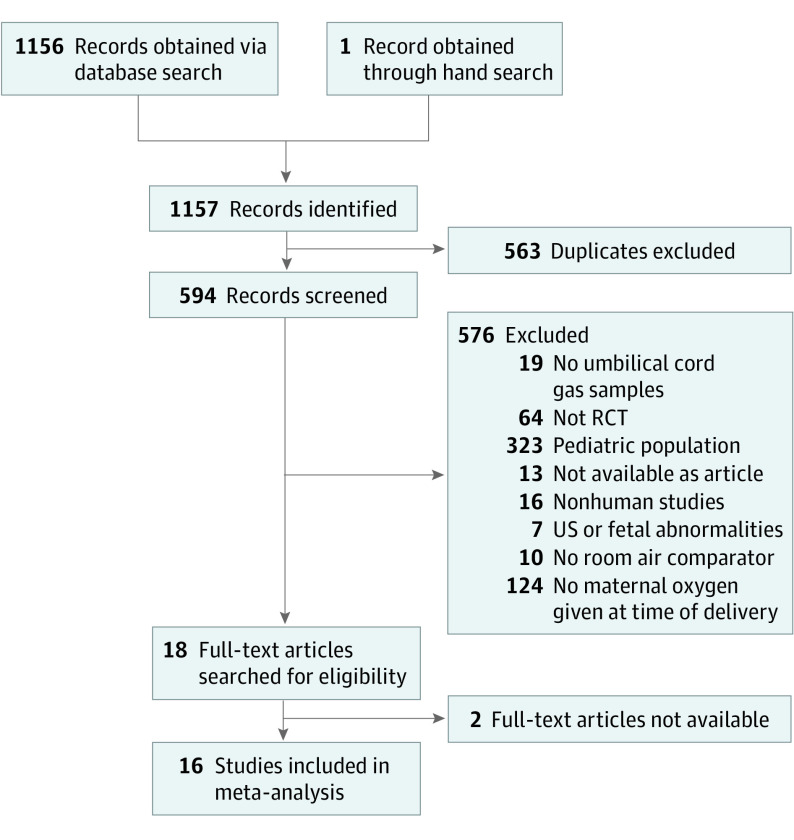
A total of 1156 references were obtained from the initial electronic database search. One additional reference was obtained from a hand search of the citations. After removing 563 duplicates, a total of 594 references were screened. We eliminated 576 of these references for not being relevant or meeting exclusion criteria. Of the 18 full-text articles searched for eligibility, 2 did not have full text available for review, and 16 were included in the final meta-analysis (Figure 1).
Figure 1. Randomized Clinical Trials Included in the Meta-analysis.
RCT indicates randomized clinical trial; US, ultrasonography.
Ten trials were performed in patients undergoing scheduled CD with regional anesthesia10,14,24,25,26,27,28,29,30,31 and 4 trials were performed in women in labor.11,12,13,16 One trial included both scheduled CD and emergent CD during labor,9 and another trial15 only included patients undergoing emergency CD during labor. In both of these trials, data from the patients undergoing emergency CD were abstracted and categorized in the subgroup of labor. Detailed information on the characteristics of the included studies is provided in Table 1. A total of 1078 patients were randomized to the oxygen group (622 at time of scheduled CD and 456 in labor) and 974 patients were randomized to the room air group (561 at the time of a scheduled CD and 413 during labor).
Table 1. Characteristics of Randomized Clinical Trials Comparing Peripartum Maternal Oxygen Supplementation With Room Air.
| Source | Country | Inclusion criteria | Exclusion criteria | Patient population | Exposure (No. of patients) | Risk of bias | Outcomes |
|---|---|---|---|---|---|---|---|
| Ahuja et al,9 2018 | India | ASA I-II, term, elective or emergency CD with subarachnoid block | ASA >II, preoperative receipt of oxygen, multiple gestation, no regional anesthesia, BMI>45, arterial injury, arterial disease, prematurity, fetal anomaly | Labor followed by emergent CD, scheduled CD | Venturi mask, 50% (30 [initial labor], 30 [scheduled CD]), room air (30) | Low | UA malondialdehyde,a UV malondialdehyde, UA total antioxidant status, UV total antioxidant status, cord gases |
| Biswas et al,10 2019 | India | ASA I-II, term, elective CD with subarachnoid block | Fetal anomaly, maternal medical problem affecting placental perfusion, skin incision to delivery >30 min, uterine incision to delivery >5 min | Scheduled CD | Nasal cannula (61), room air (66) | High | UA Pao2,a cord gases, neonatal outcomes |
| Castro et al,25 2009 | Brazil | Term, scheduled CD | Maternal disease that affects fetal oxygenation (eg, preeclampsia, hypertension, and diabetes), intraoperative events that compromise fetal oxygenation | Scheduled CD | Nonrebreather, 60% (12), room air (8) | Low | Maternal Pao2,a UA Pao2 |
| Cogliano et al,26 2002 | UK | Elective CD with spinal anesthesia | Age <18 y, height <152 cm, weight >85 kg, history of fetal compromise, coexisting medical condition, non-English speaking | Scheduled CD | Nasal cannula (23), simple face mask, 40% (23), room air (23) | High | UV Pao2,a cord gas measures, Apgar scores, patient comfort |
| Gunaydin et al,27 2011 | Turkey | Elective CD with spinal anesthesia, term, singleton, vertex | Not specified | Scheduled CD | Nasal cannula, 32% (30), simple face mask, 30% (30), room air (30) | Low | Maternal and neonatal cerebral oximetry,a Apgar scores, cord gas measures |
| Khaw et al,29 2002 | China | ASA I-II, no labor, term, elective CD with spinal anesthesia | Not specified | Scheduled CD | Venturi mask, 60% (22), room air (22) | Low | Maternal and UA malondialdehyde,a cord gas measures, Apgar scores, maternal arterial blood gas measures |
| Khaw et al,28 2004 | China | ASA I-II, no labor term, elective CD with spinal anesthesia | Not specified | Scheduled CD | Venturi mask, 40% (44), Venturi mask, 60% (60), room air (55) | High | UV oxygen content,a Apgar scores, UA pH, uterine incision to delivery time, UV Pao2, oxyhemoglobin saturation |
| Khaw et al,15 2009 | China | ASA I-II, term, singleton, emergent CD after labor with regional anesthesia | Fetal anomaly, fetal growth restriction, preeclampsia, general anesthesia for fetal compromise | Labor followed by emergent CD | Venturi mask, 60% (61), room air (64) | High | UV oxygen content,a umbilical 8-isoprostane, uterine incision to delivery time, UA and UV Pao2 |
| Moors et al,11 2020 | Netherlands | Term, labor, singleton, cephalic, age >18 y, abnormal fetal heart rate in second stage of labor | Tobacco, drug, alcohol use; cardiac disease; pulmonary disease; diabetes; hyperthyroidism; anemia; corticosteroid, antihypertensive, or magnesium treatment; medications associated with free radicals; congenital malformation; infection in labor; prolonged bradycardia; normal or preterminal fetal heart rate pattern | Labor | Nonrebreather, 80% (57), room air (60) | High | Change of fetal heart rate pattern,a Apgar scores, cord gas measures, NICU admission, perinatal death |
| Qian et al,12 2017 | China | Age <35 y, term, singleton, nulliparous, cephalic, spontaneous or induction of labor, normal fetal heart rate pattern in first stage of labor, normal labor at onset of second stage | Pregestational and gestational diabetes, hypertension, preeclampsia, eclampsia, oligohydramnios, abruption, fetal growth restriction, cephalopelvic disproportion, meconium, tachysystole, oxygen in first stage of labor, history of prior uterine incision, respiratory or cardiovascular disease, anemia, fever, disorder in oxygen saturation, hypotension, tobacco and alcohol use | Labor | Nasal cannula (219), room air (224) | Low | UA pH,a maternal satisfaction, mode of delivery, fetal heart rate pattern, tachysystole, hypotension, meconium, use of oxytocin, duration of second stage of labor, maternal arterial blood gas measures, cord gas measure, postpartum hemoglobin, neonatal outcomes |
| Raghuraman et al,13 2018 | US | Term, singleton, admitted for labor or induction of labor, category II requiring resuscitation | Fetal anomalies, multiple gestation, maternal hypoxia | Labor | Nonrebreather, 80% (48), room air (51) | Low | UA lactate,a cord gas measures, CD for nonreassuring fetal status, operative vaginal delivery |
| Ramanathan et al,24 1982 | US | Term, elective repeat CD, epidural anesthesia without obstetric complications | None specified | Scheduled CD | Anesthetic face mask with circle absorber, 47% (10), anesthetic face mask with circle absorber, 74% (10), anesthetic face mask with circle absorber, 100% (10), room air (10) | High | UA and UV Pao2, maternal arterial blood gases, fetal and maternal oxyhemoglobin saturation |
| Simon et al,14 2018 | US | Term, singleton, age >18 y, planned CD, with regional anesthesia | Chronic hypertension, maternal lung disease, preeclampsia, fetal growth restriction, fetal anomalies, multiple gestation, breech | Scheduled CD | Simple face mask (33), room air (32) | Low | UA pH, cord gas measures,a composite maternal complications, composite neonatal complications |
| Siriussawakul et al,31 2014 | Thailand | Term, singleton, adult, elective CD with spinal anesthesia | Maternal hypoxemia, diabetes, hypertension, heart disease, obesity, previa, premature rupture of membranes, fetal growth restriction | Scheduled CD | Nasal cannula (163), room air (163) | Low | Maternal oxygen saturation,a cord gas measures |
| Thorp et al,16 1995 | US | Spontaneous or induced labor, normal labor at onset of second stage | Maternal respiratory disease, diabetes, hypertension, preeclampsia, no significant fetal heart rate tracing abnormalities | Labor | Simple face mask, 81% (41), room air (44) | Low | UA pH,a cord gas measures |
| Palacio et al,30 2008 | Spain | Term, received prenatal care, ASA I, CD under spinal | Fetal anomalies, maternal health problems | Scheduled CD | Venturi mask, 40% (62), room air (62) | High | Maternal pulse oximetry,a cord gas measures |
Abbreviations: ASA, American Society of Anesthesiologists; BMI, body mass index (calculated as weight in kilograms divided by height in meters squared); CD, cesarean delivery; Fio2, fraction of inspired oxygen; UA, umbilical artery; UV, umbilical vein.
Primary outcome identified in the study.
Eight trials were considered at low risk for bias.9,12,13,14,16,27,29,31 Three studies did not report or lacked valid randomization methods.10,24,26 Only 1 trial had loss to follow-up greater than 15%.11 Four trials did not perform or specifically report an intention-to-treat analysis.15,24,28,30
Fourteen trials reported results for UA pH—the primary outcome of this review.7,9,10,11,12,13,14,16,25,26,27,29,30,31 One of these trials9 had a cohort of patients randomized at the time of the scheduled CD and another cohort randomized at the time of an emergent CD during labor. There was significant heterogeneity between studies (I2 = 49.88%; P = .03). Overall, there was no significant difference in mean UA pH between the room air and oxygen groups (15 studies: WMD, 0.00; 95% CI, −0.01 to 0.01). The mean difference in UA pH between the oxygen and room air groups did not appear to be impacted by the presence (5 studies: WMD, −0.01; 95% CI, −0.03 to 0.00) or absence (10 studies: WMD, 0.00; 95% CI, −0.01 to 0.01) of labor (Figure 2). There was no evidence of publication bias from visual inspection of the funnel plot and the Egger test (P = .45) (eFigure 1 in the Supplement).
Figure 2. Maternal Oxygen Supplementation vs Room Air and Umbilical Artery (UA) pH.
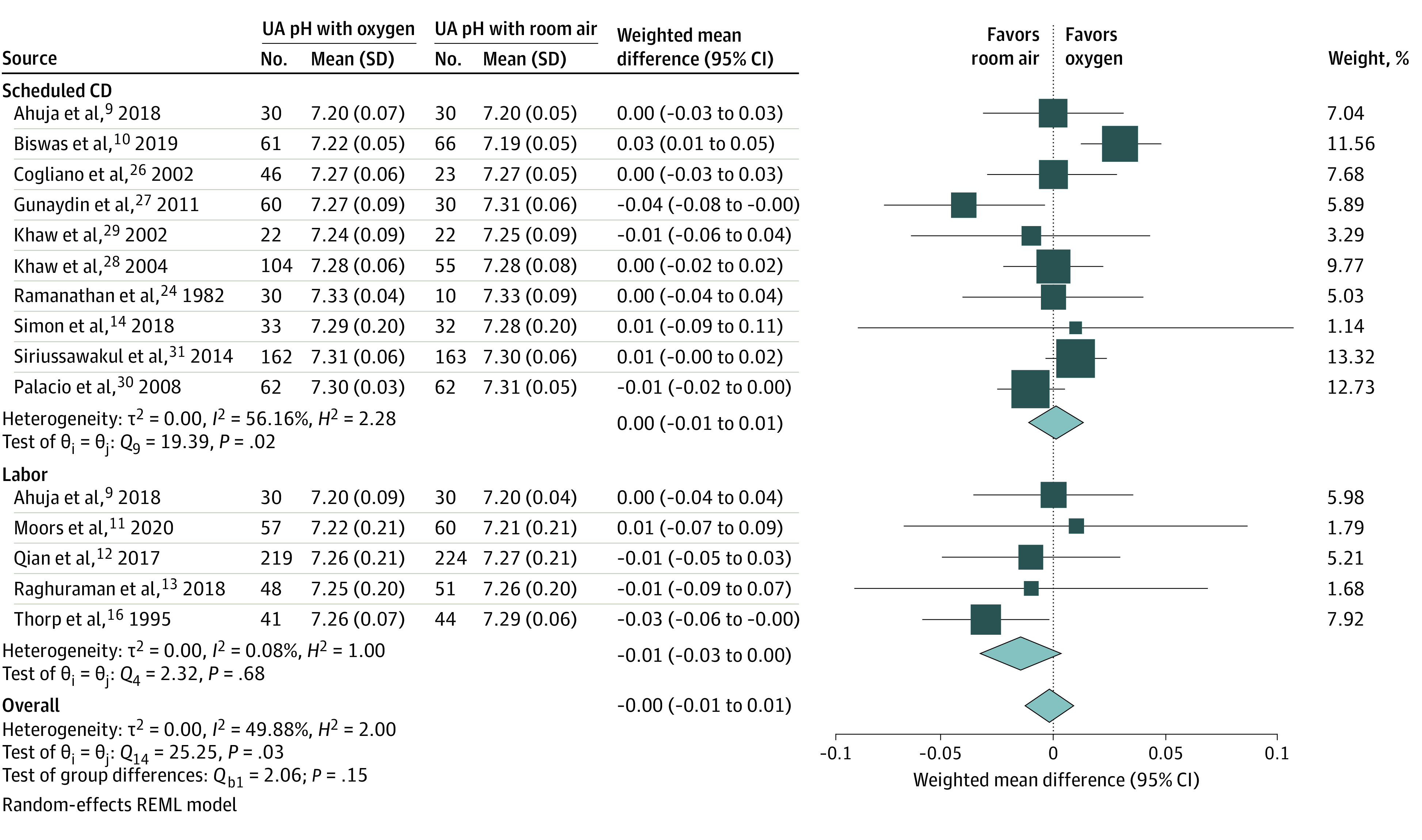
Pooled relative risk estimates for the association between oxygen or room air and UA pH stratified by the presence or absence of labor. CD indicates cesarean delivery; REML, residual maximum likelihood.
After stratifying by risk of bias, Fio2, and oxygen delivery device, heterogeneity was reduced for studies that used high-flow oxygen devices (I2 = 0.11%; P = .93). In stratified analysis, there remained no significant difference in UA pH between oxygen and room air at the time of a scheduled CD or during labor (eTable in the Supplement).
Six studies reported UA pH less than 7.2 as an outcome.10,12,14,15,16,28 Overall, there was no significant difference in UA pH less than 7.2 between oxygen and room air groups (6 studies: relative risk [RR], 0.87; 95% CI, 0.58-1.32). After stratifying by the presence or absence of labor, oxygen administration was associated with a reduction in the risk of UA pH less than 7.2 at the time of scheduled CD (3 studies: RR, 0.63; 95% CI, 0.43-0.90) with no evidence of heterogeneity (I2 = 0.00%; P = .62). In contrast, there was no significant difference in UA pH less than 7.2 between oxygen and room air among women in labor (3 studies: RR, 1.34; 95% CI, 0.58-3.11) (eFigure 2 in the Supplement). In the 3 studies that reported UA pH less than 7.1 as an outcome,11,12,16 there was no significant difference between the oxygen and room air groups (3 studies: RR, 3.16; 95% CI, 0.64-15.50).
Thirteen studies reported the outcome of UA Pao2. Nine included patients with a scheduled CD,10,14,24,25,26,27,29,30,31 3 included women who were in labor,13,15,16 and 1 included both scheduled CD and labor groups that were analyzed separately.9 There was significant heterogeneity among all studies (I2 = 90.37%; P < .01). Overall, oxygen administration was associated with an increase in UA Pao2 compared with room air (14 cohorts: WMD, 2.57 mm Hg; 95% CI, 0.80-4.34 mm Hg). After the results were stratified by the presence or absence of labor, the significant association between oxygen and UA Pao2 was limited to women undergoing scheduled CD (10 studies: WMD, 2.12 mm Hg; 95% CI, 0.09-4.15 mm Hg), but not among those during labor (4 studies: WMD, 3.60 mm Hg; 95% CI, −0.30 to 7.49 mm Hg) (eFigure 3 in the Supplement).
Eleven studies reported results for the outcome of UA base excess. Six were in women with scheduled CD,14,24,27,29,30,31 4 were in women in labor,11,12,13,16 and 1 included both groups.9 There was significant heterogeneity between studies (I2 = 94.63%; P < .01). There was no significant difference in UA base excess between the oxygen and room air groups overall (12 studies: WMD, −0.13; 95% CI, −0.74 to 0.49) and after stratifying by the presence or absence of labor (scheduled CD, 7 studies: WMD, −0.54; 95% CI, −1.49 to 0.41; labor, 5 studies: WMD, −0.21; 95% CI, −0.16 to 0.58) (eFigure 4 in the Supplement). Table 2 summarizes the pooled results for all UA gas measures.
Table 2. Pooled and Stratified Results of the Effect of Maternal Oxygen Supplementation vs Room Air on UA Gas Measures.
| Characteristic | No. of studies | No. of patients | Measure of effect | Effect size (95% CI) | I2 value | P value | |
|---|---|---|---|---|---|---|---|
| Oxygen | Room air | ||||||
| All studies | |||||||
| UA pH | 15 | 1005 | 902 | WMD | 0.00 (−0.01 to 0.01) | 49.88 | .03 |
| UA pH <7.2 | 6 | 512 | 487 | RR | 0.87 (0.58-1.32) | 34.87 | .11 |
| UA pH <7.1a | 3 | RR | 3.16 (0.64-15.50) | 0.00 | >.99 | ||
| UA Pao2 | 13 | 698 | 635 | WMD | 2.57 (0.80-4.34) | 90.37 | .005 |
| UA base excess | 11 | 794 | 758 | WMD | −0.13 (−0.74 to 0.49) | 94.63 | <.001 |
| Scheduled CD | |||||||
| UA pH | 10 | 610 | 493 | WMD | 0.00 (−0.01 to 0.01) | 56.16 | .02 |
| UA pH <7.2 | 3 | 198 | 155 | RR | 0.63 (0.43-0.90) | 0.00 | .62 |
| UA Pao2 | 10 | 518 | 446 | WMD | 2.12 (0.09-4.15) | 84.33 | <.001 |
| UA base excess | 7 | 399 | 349 | WMD | −0.54 (−1.49 to 0.41) | 88.12 | <.001 |
| Labor | |||||||
| UA pH | 5 | 395 | 409 | WMD | −0.01 (−0.03 to 0.00) | 0.08 | .68 |
| UA pH <7.2 | 3 | 260 | 268 | RR | 1.34 (0.58-3.11) | 57.53 | .10 |
| UA Pao2 | 4 | 180 | 189 | WMD | 3.60 (−0.30 to 7.49) | 94.14 | <.001 |
| UA base excess | 5 | 395 | 409 | WMD | 0.21 (−0.16 to 0.58) | 78.51 | <.001 |
Abbreviations: CD, cesarean delivery; RR, relative risk; UA, umbilical artery; WMD, weighted mean difference.
All 3 trials in women in labor.
Seven studies reported 1- and 5- minute Apgar scores,9,10,11,27,28,29,31 1 of which included both women in labor and those scheduled for CD and analyzed the results separately.9 Overall, there were no significant differences in Apgar scores between groups. However, after stratifying by the presence or absence of labor, infants of mothers receiving oxygen during scheduled CD had slightly lower 1-minute Apgar scores than those whose mothers were receiving room air (6 studies: WMD, −0.20; 95% CI, −0.40 to −0.01). There was no statistically significant difference in 5-minute Apgar scores. All Apgar scores were similar in the oxygen and room air groups among infants of mothers who were in labor (Table 3; eFigure 5 and eFigure 6 in the Supplement).
Table 3. Pooled and Stratified Results for the Effect of Maternal Oxygen Supplementation vs Room Air on Neonatal Outcomes.
| Characteristic | No. of studies | No. of patients | Measure of effect | Effect size (95% CI) | I2 value | P value | |
|---|---|---|---|---|---|---|---|
| Oxygen | Room air | ||||||
| All studies | |||||||
| 1-min Apgar score | 8 | 526 | 456 | WMD | −0.13 (−0.30 to 0.04) | 71.68 | .001 |
| 5-min Apgar score | 8 | 526 | 456 | WMD | −0.12 (−0.27 to 0.04) | 99.91 | <.001 |
| NICU admission | 4 | 333 | 340 | RR | 0.87 (0.44-1.73) | 0.00 | .64 |
| Scheduled CD | |||||||
| 1-min Apgar score | 6 | 439 | 366 | WMD | −0.20 (−0.40 to −0.01) | 62.60 | .02 |
| 5-min Apgar score | 6 | 439 | 366 | WMD | −0.16 (−0.36 to 0.04) | 99.30 | <.001 |
| NICU admission | 1 | 30 | 30 | RR | 1.00 (0.02-48.82) | NA | NA |
| Labor | |||||||
| 1-min Apgar score | 2 | 87 | 90 | WMD | 0.08 (−0.02 to 0.19) | 0.00 | .32 |
| 5-min Apgar score | 2 | 87 | 90 | WMD | −0.12 (−0.27 to 0.04) | 0.01 | >.99 |
| NICU admission | 3 | 303 | 310 | RR | 0.87 (0.44-1.73) | 0.00 | .43 |
Abbreviations: CD, cesarean delivery; NA, not applicable; NICU, neonatal intensive care unit; RR, relative risk; WMD, weighted mean difference.
Neonatal intensive care unit admission was reported in 4 studies,9,11,12,16 3 of which were performed in women during labor. There was no significant difference in the rate of neonatal intensive care unit admission between all oxygen and room air groups (4 studies: RR, 0.87; 95% CI, 0.44-1.73), patients undergoing scheduled CD (1 study: RR, 1.00; 95% CI, 0.02-48.82), or patients in labor (3 studies: RR, 0.87; 95% CI, 0.44-1.73) (Table 3; eFigure 7 in the Supplement).
The association between oxygen administration and oxidative stress was investigated in 4 studies.9,11,15,28 Maternal and/or UA malondialdehyde was the most commonly studied marker among these trials. Use of oxygen was associated with an increase in maternal malondialdehyde levels (3 studies: WMD, 0.37μM; 95% CI, 0.26-0.48μM) and no significant difference in the UA malondialdehyde level (4 studies: WMD, 0.16μM; 95% CI, −0.18 to 0.50μM).
Discussion
The results of this systematic review and meta-analysis suggest that maternal oxygen supplementation at the time of delivery yields no substantial difference in UA pH compared with room air, despite an increase in UA Pao2. The UA pH remained similar between the oxygen and room air groups even after accounting for risk of bias, use of low-flow devices, or Fio2 less than 60%. Oxygen supplementation appeared to lower rates of UA pH less than 7.2 and increase UA Pao2 compared with room air at the time of a scheduled CD. Trials including women in labor and data on neonatal outcomes were limited. One-minute Apgar scores were marginally lower in infants whose mothers were receiving oxygen at the time of a scheduled CD; however, the mean difference between oxygen and room air was less than 1 point. There were no statistically significant differences in other secondary outcomes. There was significant interstudy heterogeneity for most of the outcomes.
Similar to our results, a 2016 Cochrane review on supplemental oxygen at the time of elective CD with the use of regional anesthesia reported that oxygen administration was associated with a higher UA Pao2 with no difference in UA pH.7 Contrary to the Cochrane review’s finding that Apgar scores were similar between the oxygen and room air groups, we observed a lower 1-minute Apgar score in infants exposed to oxygen at the time of a scheduled CD. We suspect that this lower score may be a spurious finding, particularly because it is inconsistent with the finding of oxygen administration reducing the incidence of UA pH less than 7.2 in the same cohort. Furthermore, a mean difference of 0.20 in 1-minute Apgar scores is unlikely to be clinically relevant, particularly when 5-minute Apgar scores were similar between groups.
A 2012 Cochrane review of 2 trials comparing room air with oxygen in women in labor found that oxygen was associated with an increased risk of UA pH less than 7.2 with no significant differences in UA oxygen content or Apgar scores.8 Our review found no significant differences in UA pH less than 7.2 between oxygen and room air groups in women during labor. Although the overall number of trials in women during labor is limited, our review included 5 additional trials9,11,12,13,15 in this group that were published subsequent to the 2012 Cochrane review and therefore provides a more comprehensive analysis of UA gases after oxygen exposure.
We stratified our analysis by the presence or absence of labor and observed that oxygen administration at the time of scheduled CD was associated with increased UA Pao2 and a lower rate of UA pH less than 7.2. Spinal anesthesia at the time of CD has been associated with acute hypotension in 70% to 80% of patients.32 The development of hypotension may result in decreased uteroplacental perfusion and impaired maternal-fetal gas exchange that women who receive epidural anesthesia during labor are less likely to experience. A meta-analysis comparing epidural, spinal, and general anesthesia reported that UA pH and base excess were significantly lower after spinal anesthesia than after general or epidural anesthesia.33 Furthermore, the choice of vasopressor (ephedrine vs phenylephrine) to treat hypotension might play a role because ephedrine is associated with stimulation of fetal metabolism and, consequently, fetal acidemia.34,35 Administration of oxygen to women may therefore improve fetal oxygenation and prevent acidemia in the setting of spinal anesthesia–associated hypotension. However, it remains to be determined whether such improvement will be noticeable in the current paradigm of preventing hypotension with a continuous phenylephrine infusion.36
Pooled results from all of the studies in this review showed increased UA Pao2 but no significant differences in UA pH with the use of oxygen. Umbilical artery Pao2 has been shown to be a poor estimator of neonatal morbidity37 because the Pao2 evaluated in a cord blood gas represents dissolved oxygen in the sample and does not reflect the amount of oxygen that is bound to hemoglobin.38 Therefore, hypoxia or inadequate tissue oxygenation cannot be inferred from dissolved oxygen content alone. Prolonged tissue hypoxia leads to anaerobic metabolism, resulting in decreased pH, which is why UA pH ultimately serves as a better marker for prediction of neonatal morbidity. An intervention that increases the Pao2 without concomitantly increasing the pH has limited clinical benefit, particularly because hyperoxemia is associated with the production of free radicals and oxidative cell damage in adults and neonates.3,39,40
Strengths and Limitations
The strengths of this review include adherence to an a priori study protocol, inclusion of only randomized clinical trials, and assessment of heterogeneity for the primary outcome by using stratified analyses to address factors such as route and dose of oxygen administration. To our knowledge, this is the first meta-analysis to combine data from all trials investigating the utility of peripartum oxygen administration for fetal benefit.
This review has limitations. First, using our preestablished criteria, 50% of the studies were at high risk of bias. In a sensitivity analysis excluding these studies, we found no significant difference in the UA pH between groups. Another limitation is the heterogeneity of all the studies, particularly with regard to the way oxygen was administered. To account for the heterogeneity, we used random-effects models and performed stratified analyses by type of oxygen delivery device and Fio2. Despite pooling data from existing trials, it is possible that our analysis was underpowered to detect differences in UA pH or other UA gas outcomes. Furthermore, only 1 trial in this review assessed oxygen administration for category II fetal tracings, which is the most common indication for oxygen use in labor and delivery settings.13 Data on short- and long-term neonatal outcomes were limited, with few trials presenting results for neonatal intensive care unit admissions and Apgar scores. Although these 2 outcomes are commonly used to gauge neonatal risk, they poorly correlate with high-acuity illness, asphyxia, and long-term neurologic morbidity.41,42,43,44,45
Conclusions
The results of this systematic review and meta-analysis suggest that peripartum maternal oxygen supplementation is not associated with a clinically relevant improvement in the UA pH or other neonatal outcomes. However, the published studies on this topic are heterogeneous, lack important data on the association between oxygen supplementation and clinically relevant neonatal sequelae, and largely did not assess oxygen use for abnormal fetal heart rate tracings. A large, adequately powered trial is needed to investigate the effect of maternal oxygen supplementation in response to fetal heart rate tracings on short- and long-term neonatal morbidity. In the interim, prolonged oxygen use should be limited given lack of proven benefit and potential risk of harm. Future studies should also assess the optimal dose, duration, and route of peripartum oxygen administration.
eTable. Pooled and Stratified Analyses for the Effect of Oxygen Versus Room Air on Umbilical Artery pH
eFigure 1. Funnel Plot: The Effect of Maternal Oxygen Administration Versus Room Air on Umbilical Artery pH
eFigure 2. Forest Plot: Effect of Maternal Oxygen Supplementation Versus Room Air on Umbilical Artery pH<7.2
eFigure 3. Forest Plot: Effect of Maternal Oxygen Supplementation Versus Room Air on Umbilical Artery pO2 (mmHg)
eFigure 4. Forest Plot: Effect of Maternal Oxygen Supplementation Versus Room Air on Umbilical Artery Base Excess
eFigure 5. Forest Plot: Effect of Maternal Oxygen Supplementation Versus Room Air on 1 Minute Apgar Score
eFigure 6. Forest Plot: Effect of Maternal Oxygen Supplementation Versus Room Air on 5 Minute Apgar Score
eFigure 7. Forest Plot: Effect of Maternal Oxygen Supplementation Versus Room Air on NICU Admission
eAppendix. Search Syntax
eReferences
References
- 1.American College of Obstetricians and Gynecologists . ACOG Practice Bulletin No. 106: Intrapartum fetal heart rate monitoring: nomenclature, interpretation, and general management principles. Obstet Gynecol. 2009;114(1):192-202. doi: 10.1097/AOG.0b013e3181aef106 [DOI] [PubMed] [Google Scholar]
- 2.Macones GA, Hankins GD, Spong CY, Hauth J, Moore T. The 2008 National Institute of Child Health and Human Development workshop report on electronic fetal monitoring: update on definitions, interpretation, and research guidelines. Obstet Gynecol. 2008;112(3):661-666. doi: 10.1097/AOG.0b013e3181841395 [DOI] [PubMed] [Google Scholar]
- 3.Hamel MS, Anderson BL, Rouse DJ. Oxygen for intrauterine resuscitation: of unproved benefit and potentially harmful. Am J Obstet Gynecol. 2014;211(2):124-127. doi: 10.1016/j.ajog.2014.01.004 [DOI] [PubMed] [Google Scholar]
- 4.Tuuli MG, Stout MJ, Shanks A, Odibo AO, Macones GA, Cahill AG. Umbilical cord arterial lactate compared with pH for predicting neonatal morbidity at term. Obstet Gynecol. 2014;124(4):756-761. doi: 10.1097/AOG.0000000000000466 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Malin GL, Morris RK, Khan KS. Strength of association between umbilical cord pH and perinatal and long term outcomes: systematic review and meta-analysis. BMJ. 2010;340:c1471. doi: 10.1136/bmj.c1471 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.ACOG Committee on Obstetric Practice . ACOG Committee Opinion No. 348, November 2006: Umbilical cord blood gas and acid-base analysis. Obstet Gynecol. 2006;108(5):1319-1322. doi: 10.1097/00006250-200611000-00058 [DOI] [PubMed] [Google Scholar]
- 7.Chatmongkolchart S, Prathep S. Supplemental oxygen for caesarean section during regional anaesthesia. Cochrane Database Syst Rev. 2016;3:CD006161. doi: 10.1002/14651858.CD006161.pub3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fawole B, Hofmeyr GJ. Maternal oxygen administration for fetal distress. Cochrane Database Syst Rev. 2012;12:CD000136. doi: 10.1002/14651858.CD000136.pub2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ahuja V, Gombar S, Jaswal S, et al. Effect of maternal oxygen inhalation on foetal free radical activity: a prospective, randomized trial. Acta Anaesthesiol Scand. 2018;62(1):26-37. doi: 10.1111/aas.13007 [DOI] [PubMed] [Google Scholar]
- 10.Biswas J, Choudhury A, Das S, Mukhopadhyay P, Pal A, Jana D. Analysis of neonatal outcome with supplemental oxygen to mother during elective cesarean section under spinal anesthesia: a prospective randomized controlled trial. Anesth Essays Res. 2019;13(3):577-582. doi: 10.4103/aer.AER_71_19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Moors S, Bullens LM, van Runnard Heimel PJ, et al. The effect of intrauterine resuscitation by maternal hyperoxygenation on perinatal and maternal outcome: a randomized controlled trial. Am J Obstet Gynecol MFM. Published online May 1, 2020. doi: 10.1016/j.ajogmf.2020.100102 [DOI] [PubMed] [Google Scholar]
- 12.Qian G, Xu X, Chen L, et al. The effect of maternal low flow oxygen administration during the second stage of labour on umbilical cord artery pH: a randomised controlled trial. BJOG. 2017;124(4):678-685. doi: 10.1111/1471-0528.14418 [DOI] [PubMed] [Google Scholar]
- 13.Raghuraman N, Wan L, Temming LA, et al. Effect of oxygen vs room air on intrauterine fetal resuscitation: a randomized noninferiority clinical trial. JAMA Pediatr. 2018;172(9):818-823. doi: 10.1001/jamapediatrics.2018.1208 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Simon VBF, Fong A, Nageotte MP. Supplemental oxygen study: a randomized controlled study on the effect of maternal oxygen supplementation during planned cesarean delivery on umbilical cord gases. Am J Perinatol. 2018;35(1):84-89. doi: 10.1055/s-0037-1606184 [DOI] [PubMed] [Google Scholar]
- 15.Khaw KSW, Wang CC, Ngan Kee WD, et al. Supplementary oxygen for emergency caesarean section under regional anaesthesia. Br J Anaesth. 2009;102(1):90-96. doi: 10.1093/bja/aen321 [DOI] [PubMed] [Google Scholar]
- 16.Thorp JAT, Trobough T, Evans R, Hedrick J, Yeast JD. The effect of maternal oxygen administration during the second stage of labor on umbilical cord blood gas values: a randomized controlled prospective trial. Am J Obstet Gynecol. 1995;172(2, pt 1):465-474. doi: 10.1016/0002-9378(95)90558-8 [DOI] [PubMed] [Google Scholar]
- 17.Cumpston M, Li T, Page MJ, et al. Updated guidance for trusted systematic reviews: a new edition of the Cochrane Handbook for Systematic Reviews of Interventions. Cochrane Database Syst Rev. 2019;10:ED000142. doi: 10.1002/14651858.ED000142 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lewkowitz AK, Gupta A, Simon L, et al. Intravenous compared with oral iron for the treatment of iron-deficiency anemia in pregnancy: a systematic review and meta-analysis. J Perinatol. 2019;39(4):519-532. doi: 10.1038/s41372-019-0320-2 [DOI] [PubMed] [Google Scholar]
- 19.Eskew AM, Bedrick BS, Hardi A, et al. Letrozole compared with clomiphene citrate for unexplained infertility: a systematic review and meta-analysis. Obstet Gynecol. 2019;133(3):437-444. doi: 10.1097/AOG.0000000000003105 [DOI] [PubMed] [Google Scholar]
- 20.Tuuli MG, Frey HA, Odibo AO, Macones GA, Cahill AG. Immediate compared with delayed pushing in the second stage of labor: a systematic review and meta-analysis. Obstet Gynecol. 2012;120(3):660-668. doi: 10.1097/AOG.0b013e3182639fae [DOI] [PubMed] [Google Scholar]
- 21.Hozo SP, Djulbegovic B, Hozo I. Estimating the mean and variance from the median, range, and the size of a sample. BMC Med Res Methodol. 2005;5:13. doi: 10.1186/1471-2288-5-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Higgins JP, Thompson SG. Quantifying heterogeneity in a meta-analysis. Stat Med. 2002;21(11):1539-1558. doi: 10.1002/sim.1186 [DOI] [PubMed] [Google Scholar]
- 23.Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ. 1997;315(7109):629-634. doi: 10.1136/bmj.315.7109.629 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ramanathan S, Gandhi S, Arismendy J, Chalon J, Turndorf H. Oxygen transfer from mother to fetus during cesarean section under epidural anesthesia. Anesth Analg. 1982;61(7):576-581. doi: 10.1213/00000539-198207000-00005 [DOI] [PubMed] [Google Scholar]
- 25.Castro CH, Cruvinel MG, Carneiro FS, Silva YP, Cabral AC, Bessa RC. Correlation between the inspired fraction of oxygen, maternal partial oxygen pressure, and fetal partial oxygen pressure during cesarean section of normal pregnancies [in Portuguese]. Rev Bras Anestesiol. 2009;59(4):452-460. doi: 10.1590/S0034-70942009000400007 [DOI] [PubMed] [Google Scholar]
- 26.Cogliano MSG, Graham AC, Clark VA. Supplementary oxygen administration for elective Caesarean section under spinal anaesthesia. Anaesthesia. 2002;57(1):66-69. doi: 10.1046/j.1365-2044.2002.02327.x [DOI] [PubMed] [Google Scholar]
- 27.Gunaydin B, Nas T, Biri A, Koc E, Koc A, McCusker K. Effects of maternal supplementary oxygen on the newborn for elective cesarean deliveries under spinal anesthesia. J Anesth. 2011;25(3):363-368. doi: 10.1007/s00540-011-1123-6 [DOI] [PubMed] [Google Scholar]
- 28.Khaw KSNK, Ngan Kee WD, Lee A, et al. Supplementary oxygen for elective Caesarean section under spinal anaesthesia: useful in prolonged uterine incision-to-delivery interval? Br J Anaesth. 2004;92(4):518-522. doi: 10.1093/bja/aeh092 [DOI] [PubMed] [Google Scholar]
- 29.Khaw KSW, Wang CC, Ngan Kee WD, Pang CP, Rogers MS. Effects of high inspired oxygen fraction during elective caesarean section under spinal anaesthesia on maternal and fetal oxygenation and lipid peroxidation. Br J Anaesth. 2002;88(1):18-23. doi: 10.1093/bja/88.1.18 [DOI] [PubMed] [Google Scholar]
- 30.Palacio F, Ortiz-Gómez JR, Fornet I, Morillas P, Bermejo L, López A. Is oxygen therapy truly useful and necessary during elective cesarean section under spinal anesthesia? [Spanish]. Rev Esp Anestesiol Reanim. 2008;55(10):597-604. doi: 10.1016/S0034-9356(08)70670-4 [DOI] [PubMed] [Google Scholar]
- 31.Siriussawakul A, Triyasunant N, Nimmannit A, et al. Effects of supplemental oxygen on maternal and neonatal oxygenation in elective cesarean section under spinal anesthesia: a randomized controlled trial. Biomed Res Int. 2014;2014:627028. doi: 10.1155/2014/627028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lee JE, George RB, Habib AS. Spinal-induced hypotension: incidence, mechanisms, prophylaxis, and management: summarizing 20 years of research. Best Pract Res Clin Anaesthesiol. 2017;31(1):57-68. doi: 10.1016/j.bpa.2017.01.001 [DOI] [PubMed] [Google Scholar]
- 33.Reynolds F, Seed PT. Anaesthesia for caesarean section and neonatal acid-base status: a meta-analysis. Anaesthesia. 2005;60(7):636-653. doi: 10.1111/j.1365-2044.2005.04223.x [DOI] [PubMed] [Google Scholar]
- 34.Ngan Kee WD, Khaw KS, Tan PE, Ng FF, Karmakar MK. Placental transfer and fetal metabolic effects of phenylephrine and ephedrine during spinal anesthesia for cesarean delivery. Anesthesiology. 2009;111(3):506-512. doi: 10.1097/ALN.0b013e3181b160a3 [DOI] [PubMed] [Google Scholar]
- 35.Singh PM, Singh NP, Reschke M, Ngan Kee WD, Palanisamy A, Monks DT. Vasopressor drugs for the prevention and treatment of hypotension during neuraxial anaesthesia for caesarean delivery: a bayesian network meta-analysis of fetal and maternal outcomes. Br J Anaesth. 2020;124(3):e95-e107. doi: 10.1016/j.bja.2019.09.045 [DOI] [PubMed] [Google Scholar]
- 36.Kinsella SM, Carvalho B, Dyer RA, et al. ; Consensus Statement Collaborators . International consensus statement on the management of hypotension with vasopressors during caesarean section under spinal anaesthesia. Anaesthesia. 2018;73(1):71-92. doi: 10.1111/anae.14080 [DOI] [PubMed] [Google Scholar]
- 37.Raghuraman N, Temming LA, Stout MJ, Macones GA, Cahill AG, Tuuli MG. Umbilical cord oxygen content and neonatal morbidity at term. Am J Perinatol. 2018;35(4):331-335. doi: 10.1055/s-0037-1607318 [DOI] [PubMed] [Google Scholar]
- 38.Loscalzo J. Hypoxia and cyanosis. In: Kasper D, Fauci A, Hauser S, Longo D, Jameson JL, Loscalzo J, eds. Harrison's Principles of Internal Medicine. 19th ed. McGraw-Hill Education; 2014. [Google Scholar]
- 39.Damiani E, Donati A, Girardis M. Oxygen in the critically ill: friend or foe? Curr Opin Anaesthesiol. 2018;31(2):129-135. doi: 10.1097/ACO.0000000000000559 [DOI] [PubMed] [Google Scholar]
- 40.Dias-Freitas F, Metelo-Coimbra C, Roncon-Albuquerque R Jr. Molecular mechanisms underlying hyperoxia acute lung injury. Respir Med. 2016;119:23-28. doi: 10.1016/j.rmed.2016.08.010 [DOI] [PubMed] [Google Scholar]
- 41.Committee on Obstetric Practice American Academy of Pediatrics—Committee on Fetus and Newborn . Committee Opinion No. 644: the Apgar score. Obstet Gynecol. 2015;126(4):e52-e55. doi: 10.1097/AOG.0000000000001108 [DOI] [PubMed] [Google Scholar]
- 42.Ehrenstein V. Association of Apgar scores with death and neurologic disability. Clin Epidemiol. 2009;1:45-53. doi: 10.2147/CLEP.S4782 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Schmidt B, Kirpalani H, Rosenbaum P, Cadman D. Strengths and limitations of the Apgar score: a critical appraisal. J Clin Epidemiol. 1988;41(9):843-850. doi: 10.1016/0895-4356(88)90100-X [DOI] [PubMed] [Google Scholar]
- 44.Schulman J, Braun D, Lee HC, et al. Association between neonatal intensive care unit admission rates and illness acuity. JAMA Pediatr. 2018;172(1):17-23. doi: 10.1001/jamapediatrics.2017.3913 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ziegler KA, Paul DA, Hoffman M, Locke R. Variation in NICU admission rates without identifiable cause. Hosp Pediatr. 2016;6(5):255-260. doi: 10.1542/hpeds.2015-0058 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eTable. Pooled and Stratified Analyses for the Effect of Oxygen Versus Room Air on Umbilical Artery pH
eFigure 1. Funnel Plot: The Effect of Maternal Oxygen Administration Versus Room Air on Umbilical Artery pH
eFigure 2. Forest Plot: Effect of Maternal Oxygen Supplementation Versus Room Air on Umbilical Artery pH<7.2
eFigure 3. Forest Plot: Effect of Maternal Oxygen Supplementation Versus Room Air on Umbilical Artery pO2 (mmHg)
eFigure 4. Forest Plot: Effect of Maternal Oxygen Supplementation Versus Room Air on Umbilical Artery Base Excess
eFigure 5. Forest Plot: Effect of Maternal Oxygen Supplementation Versus Room Air on 1 Minute Apgar Score
eFigure 6. Forest Plot: Effect of Maternal Oxygen Supplementation Versus Room Air on 5 Minute Apgar Score
eFigure 7. Forest Plot: Effect of Maternal Oxygen Supplementation Versus Room Air on NICU Admission
eAppendix. Search Syntax
eReferences