Abstract
A 40-year-old woman, known type 2 diabetes mellitus, obese (100 kg), on insulin (80 units), was under treatment for unstable proliferative diabetic retinopathy with extramacular tractional retinal detachment (TRD) in the left eye. At 1-year follow-up, she developed progression of TRD involving the macula in the left eye for which she was advised surgery. She did not follow-up for 6 months during which time she had adopted a coconut oil-rich ketogenic diet. She reported losing 25 kg body weight along with reversal of diabetes (Glycosylated Haemglobin (HbA1C) of 5.3% without insulin) in the interim. During this 2-year follow-up visit, it was found that there was complete resolution of macular detachment due to TRD in the left eye with stable vision. This was attributed to the impactful ketogenic diet.
Keywords: obesity (nutrition), retina
Background
Systemic control plays an important role in the prevention and progression of diabetic retinopathy (DR). The protective roles of glycaemic and hypertension control in preventing complications of DR have been established.1 2
Obesity is a known risk factor, causing insulin resistance and progression of diabetes mellitus (DM) with its associated complications.3 Physical exercise and dietary modifications have long been considered integral to the management plan for individuals with DM.4 These measures can cause weight loss and, in turn, improve insulin sensitivity and control of DM.
The role of keto diet in reversing complications of DM such as diabetic nephropathy has been reported.5 However, the impact of keto diet on DR reversal is not well known. We present a case of reversal of DM and associated diabetic tractional retinal detachment (TRD) in a woman who adopted a coconut oil-rich diet to control her obesity and DM.
Case presentation
A 40-year-old woman with type 2 DM presented to the retina clinic with complaints of defective vision in both eyes of 1-year duration. She was a known diabetic on insulin for 10 years and on medications for hypertension and hypothyroidism for 5 years. She was obese by definition of adult body mass index, weighing 100 kg (220 pounds) and was on insulin 80 units along with oral hypoglycaemic agents tab metformin 1500 mg daily in divided doses for her DM control. She had been diagnosed elsewhere as having proliferative diabetic retinopathy (PDR) and had got pan retinal photocoagulation (PRP) laser treatment done in both eyes to reduce the risk of vision loss precipitated by uncontrolled DM.
On examination, her best corrected visual acuity (BCVA) was 20/100, N18 and 20/125, N36 in the right and left eye, respectively. The fundus revealed PDR with old laser scars and the presence of fresh vitreous haemorrhage and diabetic macular oedema (DME) in both eyes (left>right). Optical coherence tomography (OCT) shows DME in both eyes (figure 1A, B).
Figure 1.
(A, B) Optical coherence tomography (OCT) shows diffuse cystic macular oedema in both eyes.
Treatment
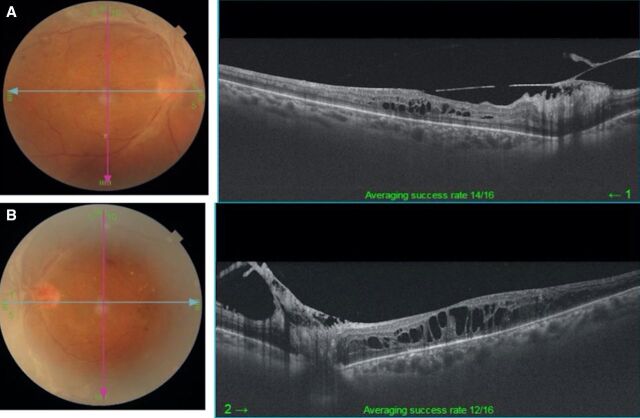
During the following 10 months, she underwent two more sessions of filling PRP laser in both eyes, injection of intravitreal antivascular endothelial growth factor bevacizumab (1.25 mg in 0.05 mL) along with anterior retinal cryotherapy in the left eye for the management of DME and unstable PDR with recurrent episodes of vitreous haemorrhage (left>right eye). At the 1-year follow-up, she presented with further diminution of vision in her left eye. On examination, her BCVA was 20/50, N8 and 20/125, <N36 in the right and left eye, respectively. Her right eye showed PDR s/p PRP with presence of diffuse DME (figure 2A) while there was progression of TRD involving the macula in the left eye (figure 2B). She was advised surgical intervention for the management of TRD in the left eye.
Figure 2.
(A) Optical coherence tomography (OCT) shows diffuse cystic diabetic macular oedema in the right eye. (B) OCT shows progression of tractional retinal detachment involving the macula in the left eye.
Outcome and follow-up
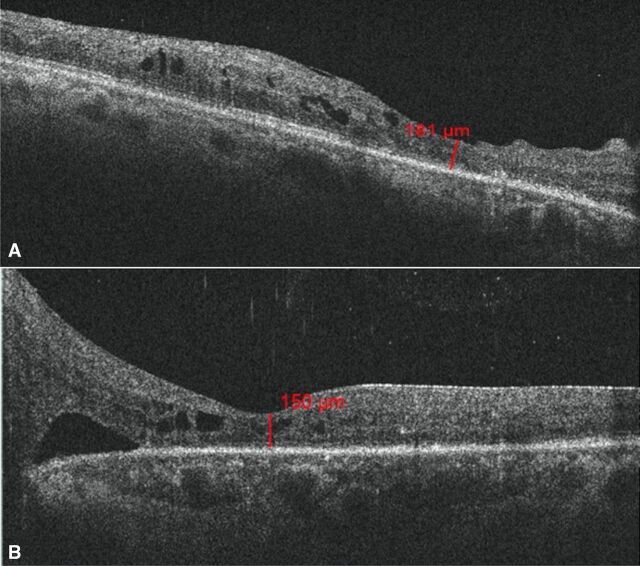
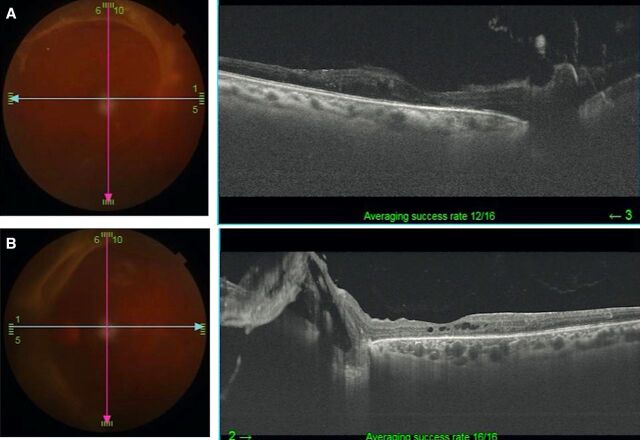
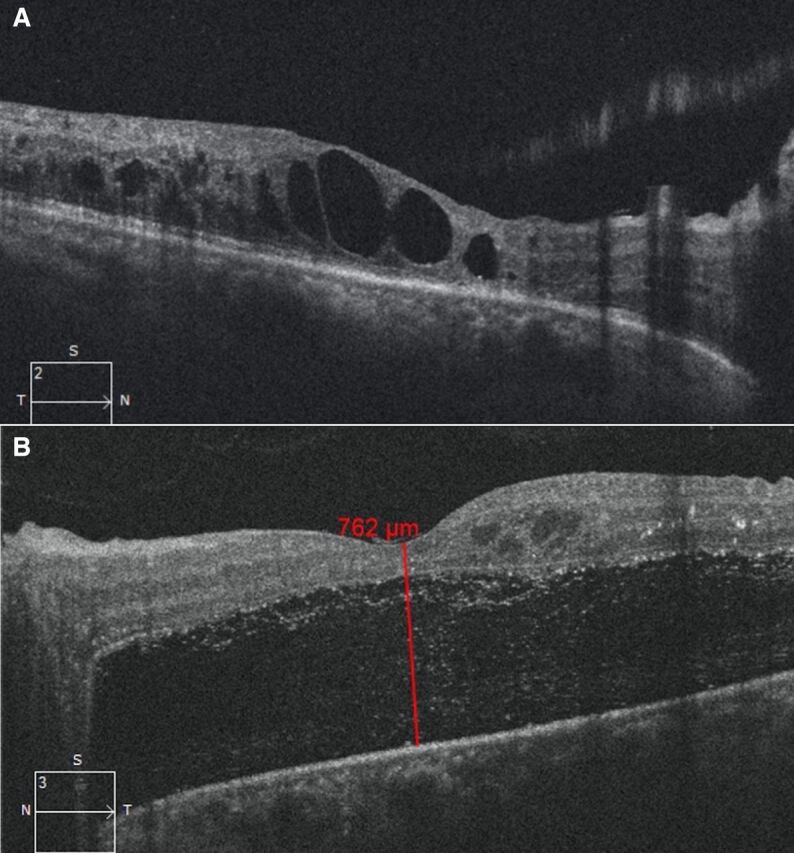
After advising surgical intervention for her left eye, she did not follow-up for 6 months. During that time, she had adopted a coconut oil-rich keto diet under supervision by her physician. She stated that as a result of having forfeited her regular diet in favour of the keto diet, she had lost over 25 kg of body weight and experienced good control of glycaemic lipid and ocular parameters resulting in reversal of DM (table 1). Indeed, there was improvement in BCVA of 20/50, N6 and 20/125, N18 in the right and left eye, respectively. The OCT of the right eye showed reduction in macular oedema, while there clearly was a spontaneous resolution of the TRD in the left eye (figure 3A, B). At the 2-year follow-up, she was maintaining BCVA in both eyes along with a stable PDR (figure 4A, B) with the OCT showing resolution of the macular oedema in the right eye as well as the TRD in the left eye (figure 5A, B).
Table 1.
Biochemical and ocular changes before and after keto diet
| Biochemistry test or medication dosage | Before dietary intervention | After dietary intervention |
| Fasting Blood Sugar (FBS) | 250 mg/dL | 70 mg/dL |
| Post Prandial Blood Sugar (PPBS) | 350 mg/dL | 120 mg/dL |
| HbA1c (Glycosylated Haemoglobin) | 7.1% | 5.3% |
| Insulin | 80 IU | Nil |
| Metformin | 1500 mg | 500 mg |
| Visual acuity | Right eye: 20/50, N8 Left eye: 20/125, <N36 |
Right eye: 20/50, N6 Left eye: 20/125, N18 |
| Optical coherence tomography (central macular thickness) | Right eye: 200 u Left eye: 762 u |
Right eye: 181 u Left eye: 150 u |
Figure 3.
(A) Optical coherence tomography (OCT) shows resolution of diabetic macular oedema in the right eye post keto diet, at 6-month follow-up. (B) OCT shows spontaneously resolving tractional retinal detachment in the left eye post keto diet, at 6-month follow-up.
Figure 4.
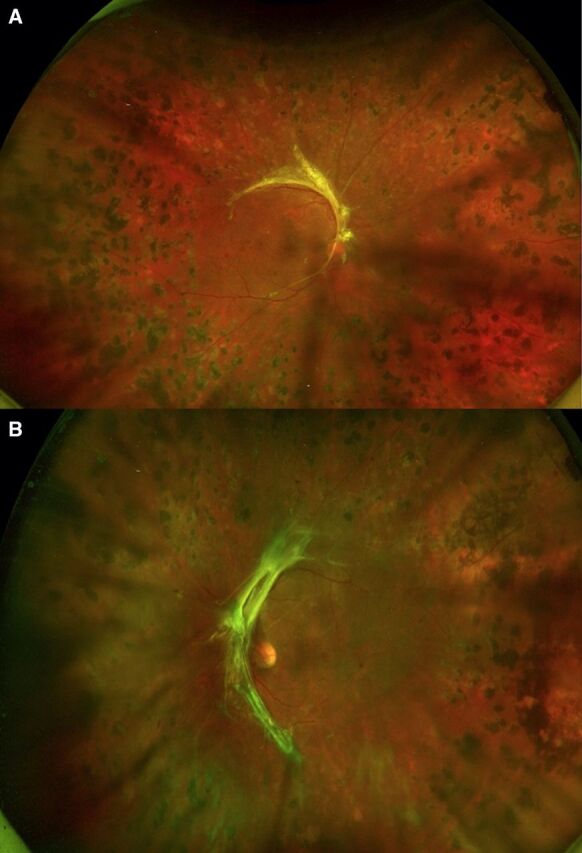
(A, B) Fundus photographs show stable proliferative diabetic retinopathy in both eyes at 2-year follow-up.
Figure 5.
(A) Resolution of diabetic macular oedema in the right eye at 2-year follow-up. (B) Complete resolution of tractional retinal detachment in the left eye at 2-year follow-up.
Discussion
The keto diet with its role in the reversal of DM and impact on glycaemic control has been well reported. The ketogenic diet, which is high in fat, adequate in protein and low in carbohydrates, results in a greater circulation of ketone bodies by a process of ketogenesis. This, in turn, facilitates energy utilisation by burning fat and causing weight loss.6 7 The keto diet considerably reduces glycosylated Haemoglobin (HbA1c) levels, thereby helping to delay the microvascular and neurogenic complications of diabetes. The keto diet is also proposed as an adjunct to pharmacologic therapy in type 1 DM.8 It has been reported that a keto diet and the associated low carbohydrate eating patterns leads to a reversal of diabetes and now the diet has been recommended as a dietary option in both type 1 and type 2 DM.9–13 Our patient showed reversal of DM after adopting the keto diet under supervision by physician, with no side effects such as deranged renal function. It is important to note that patients will need antidiabetic medication management during the keto diet in order to prevent the risk of hypoglycaemic. Reducing sugars in the diet cannot only eliminate the requirement of medications but also insulin. However, careful monitoring of blood sugars is required on a periodical basis to ascertain this. Phased manner of withdrawal of medications has been recommended instead of abrupt termination of medications.10 11
Our patient had put herself on a keto diet rich in coconut oil. Sheela et al had earlier studied the effect of unprocessed coconut oil and lauric acid in animal models, viz, Wistar rats, by the in silico (virtual screening or computer modelling of human disease using bioinformatic tools) gene expression analysis and molecular docking method, concluding that coconut phytocompounds reduce the activity of both aldose reductase enzyme and sorbitol dehydrogenase. Also, the phenolic bioactive contents of coconut oil such as ferulic acid, catechins, kaempferol and its glycosides have been individually proven to be antidiabetic and lipid lowering, in addition to bestowing anticancer properties.12 The Malmö diet and Cancer cohort study found that intake of saturated fat from dairy products, coconut oil and palm kernel oil were associated with lower diabetes risk.13
Globally, there is increasing evidence to link diet and DR. Wong et al studied the association of Mediterranean diet with DR, concluding that dietary fibre, oily fish and the Mediterranean diet per se (rich in vegetables, fruits, grains, nuts, beans, seeds and olive oil, but moderate in protein) are together beneficial against developing DR disease, while high caloric intake was linked to a greater risk of developing DR.14 Eva et al concluded that the consumption of more than four diet soft drink cans a week resulted in a twofold increase in PDR in individuals with DM.15 This risk was attributed to the adverse effects of the artificial sweeteners present in these diet drinks. Schulze et al have shown that caramel colouring of both diet and regular soft drinks may increase the levels of proinflammatory advanced glycation‐end products.16
Our patient reported having resorted to the keto diet for 3 months under the supervision of her diabetologist when she also regularly used unprocessed coconut oil in the diet. She ascribed her weight loss of nearly 25 kg to the dietary intervention. It is relevant to note that when adopting the keto/very low-carbohydrates diets, close medical supervision is essential for individuals with atherosclerotic cardio vascular disease, risk of atrial fibrillation (AF), or the presence or history of heart failure, kidney disease or liver disease.17
Long term use of low carbohydrate diet has been associated with an increased incidence of cardiovascular events.18 19 Zhang et al reported an increased risk of incident AF with low-carbohydrate intake in a prospective cohort study involving 13 385 participants with a median follow-up of 22.5 years. The study results showed that low‐carbohydrate diets were associated with increased risk of incident AF, regardless of the type of protein or fat used to replace the carbohydrate.18 A metanalysis of eleven randomised clinical trials comparing low-carbohydrate versus low-fat diets showed that low carbohydrate diets resulted in greater weight loss but also an increase in atherogenic low density lipoprotein (LDL) cholesterol levels. The beneficial changes of low-carbohydrate diets must be weighed against the possible detrimental effects of increased LDL cholesterol on cardiovascular health.20
Low-carbohydrate diets with high protein intake can have adverse effects on kidney function through renal hyperfiltration with the worst outcomes occurring in individuals with chronic kidney disease (CKD).21–23 In a metanalysis of low-carbohydrate diet trials among people with type 2 DM, without specified CKD (whose baseline renal function is unknown), the diet was neither harmful nor beneficial.22 24 Consumption of red meat protein intake was associated with a higher risk of developing incident CKD than plant based protein.25
These low carbohydrate diets can result in severe hypoglycaemic episodes and hence early deprescription in the form of the rapid titration of insulin dosage, especially in insulin dependent type 2 DM patients, has been advocated. They should have immediate access to a diabetes nurse/physician/primary care doctor who has experience with low carbohydrate diets.26 Caution should be exercised while following these diets in people with CKD, disordered eating patterns and in pregnant women. Further research is required before such dietary recommendations can be made for these subgroups.9 Long term of use of a low-carbohydrate diet with an increase in the intake of fat especially animal fats has been associated with a rising risk of all-cause mortality. However, those that favoured plant-derived protein and fat intake from sources such as vegetables, nuts, peanut butter and whole-grain breads were associated with lower mortality rates, suggesting that the source of food can modify the association between carbohydrate intake and mortality.27
Zarnowski et al proposed that kynurenic acid has neuroprotective effects and can play a possibly beneficial role in patients taking ketogenic diet.28 It is produced by kynurenic metabolic pathways. Studies on rats that have been fed a long standing ketogenic diet have indicated that kynurenic acid can have a neuroprotective effect on rat retinal ganglion cells.29 The role of other ketone bodies such as acetoacetate and beta hydroxy butyrate in enhancing the effect of kynurenic acid by attenuating the effects of glutamate have been reported. However, larger clinical studies in humans are required to assess the role of such products in treating glaucoma and other retinal degenerative diseases.30
Following keto diet and significant weight loss, our patient had improved vision, there was resolution of the macular oedema and spontaneous resolution of the tractional detachment without requiring surgical intervention. This spontaneous reversal of tractional detachment might be partly due to the occurrence of posterior vitreous detachment, though that phenomenon is rare in individuals with DM. Hence, we believe that the improvement in her ocular condition may have been due to the good systemic control achieved by mechanisms involved in the keto diet, the presence of phytocompounds in unprocessed coconut oil and weight loss.
To conclude, systemic control is an important dimension of managing complications of DR in individuals with DM. Careful consideration of dietary interventions under the supervision of a physician may have favourable results and, therefore, should be kept in mind while managing ocular complications of DM.
Patient’s perspective.
I was having eye complications of diabetes mellitus for the last 4 years now. I faced a lot of difficulties about my vision. I realised that my obesity was the main culprit for my uncontrolled diabetes and related eye complications. I underwent keto dietary intervention under the supervision of my diabetes doctor. By God’s grace, after adhering to the keto diet, I lost a significant amount of my body weight. I am happy that after going on the keto diet, I now feel far healthier, what with the reversal of my Diabetes and also the improvement in my vision without needing surgical intervention to stabilise my vision.
Learning points.
Rare occurrence of spontaneous reversal of tractional retinal detachment was noted following the adoption of a keto diet by a patient with diabetes mellitus (DM) and diabetic retinopathy (DR).
When adopted under the supervision of a physician, dietary interventions such as the keto diet can have a positive impact on the management of DM and DR.
Coconut oil in association with the keto diet might play a role in reversal of DM due to the antidiabetic properties of its lauric acid component, helping reduce ocular complications secondary to diabetes.
Footnotes
Contributors: PC - did lietraure search and wrote manuscript draft; RPK - managed the case, review and edited the draft and wrote final version.
Funding: This study was funded by Hyderabad Eye Research Foundation.
Competing interests: None declared.
Patient consent for publication: Obtained.
Provenance and peer review: Not commissioned; externally peer reviewed.
References
- 1.Stratton IM, Adler AI, Neil HA, et al. Association of glycaemia with macrovascular and microvascular complications of type 2 diabetes (UKPDS 35): prospective observational study. BMJ 2000;321:405–12. 10.1136/bmj.321.7258.405 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Tight blood pressure control and risk of macrovascular and microvascular complications in type 2 diabetes: UKPDS 38. UK prospective diabetes Study Group. BMJ 1998;317:703–13. [PMC free article] [PubMed] [Google Scholar]
- 3.Watada H. Clinical evidence regarding factors linking metabolic abnormal obesity to pancreatic β-cell dysfunction. J Diabetes Investig 2020;11:798–800. 10.1111/jdi.13264 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Williams A, Radford J, O'Brien J, et al. Type 2 diabetes and the medicine of exercise: the role of general practice in ensuring exercise is part of every patient's plan. Aust J Gen Pract 2020;49:189–93. 10.31128/AJGP-09-19-5091 [DOI] [PubMed] [Google Scholar]
- 5.Poplawski MM, Mastaitis JW, Isoda F, et al. Reversal of diabetic nephropathy by a ketogenic diet. PLoS One 2011;6:e18604. 10.1371/journal.pone.0018604 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Samaha FF, Iqbal N, Seshadri P, et al. A low-carbohydrate as compared with a low-fat diet in severe obesity. N Engl J Med 2003;348:2074–81. 10.1056/NEJMoa022637 [DOI] [PubMed] [Google Scholar]
- 7.Leonetti F, Campanile FC, Coccia F, et al. Very low-carbohydrate ketogenic diet before bariatric surgery: prospective evaluation of a sequential diet. Obes Surg 2015;25:64–71. 10.1007/s11695-014-1348-1 [DOI] [PubMed] [Google Scholar]
- 8.Feinman RD, Pogozelski WK, Astrup A, et al. Dietary carbohydrate restriction as the first approach in diabetes management: critical review and evidence base. Nutrition 2015;31:1–13. 10.1016/j.nut.2014.06.011 [DOI] [PubMed] [Google Scholar]
- 9.Evert AB, Dennison M, Gardner CD, et al. Nutrition therapy for adults with diabetes or prediabetes: a consensus report. Diabetes Care 2019;42:731–54. 10.2337/dci19-0014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zinn C, Rush A, Johnson R. Assessing the nutrient intake of a low-carbohydrate, high-fat (LCHF) diet: a hypothetical case study design. BMJ Open 2018;8:e018846. 10.1136/bmjopen-2017-018846 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Westman EC, Tondt J, Maguire E, et al. Implementing a low-carbohydrate, ketogenic diet to manage type 2 diabetes mellitus. Expert Rev Endocrinol Metab 2018;13:263–72. 10.1080/17446651.2018.1523713 [DOI] [PubMed] [Google Scholar]
- 12.Sheela DL, Nazeem PA, Narayanankutty A, et al. Coconut phytocompounds inhibits polyol pathway enzymes: implication in prevention of microvascular diabetic complications. Prostaglandins Leukot Essent Fatty Acids 2017;127:20–4. 10.1016/j.plefa.2017.10.004 [DOI] [PubMed] [Google Scholar]
- 13.Ericson U, Hellstrand S, Brunkwall L, et al. Food sources of fat may clarify the inconsistent role of dietary fat intake for incidence of type 2 diabetes. Am J Clin Nutr 2015;101:1065–80. 10.3945/ajcn.114.103010 [DOI] [PubMed] [Google Scholar]
- 14.Wong MYZ, REK M, Fenwick EK, et al. Dietary intake and diabetic retinopathy: A systematic review. PLoS One 2018;13:e0186582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fenwick EK, Gan AT, Man RE, et al. Diet soft drink is associated with increased odds of proliferative diabetic retinopathy. Clin Exp Ophthalmol 2018;46:767–76. 10.1111/ceo.13154 [DOI] [PubMed] [Google Scholar]
- 16.Schulze MB, Manson JE, Ludwig DS, et al. Sugar-Sweetened beverages, weight gain, and incidence of type 2 diabetes in young and middle-aged women. JAMA 2004;292:927–34. 10.1001/jama.292.8.927 [DOI] [PubMed] [Google Scholar]
- 17.Kirkpatrick CF, Bolick JP, Kris-Etherton PM, et al. Review of current evidence and clinical recommendations on the effects of low-carbohydrate and very-low-carbohydrate (including ketogenic) diets for the management of body weight and other cardiometabolic risk factors: a scientific statement from the National lipid association nutrition and lifestyle Task force. J Clin Lipidol 2019;13:689–711. 10.1016/j.jacl.2019.08.003 [DOI] [PubMed] [Google Scholar]
- 18.Zhang S, Zhuang X, Lin X, et al. Low-Carbohydrate Diets and Risk of Incident Atrial Fibrillation: A Prospective Cohort Study. J Am Heart Assoc 2019;8:e011955. 10.1161/JAHA.119.011955 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lagiou P, Sandin S, Lof M, et al. Low carbohydrate-high protein diet and incidence of cardiovascular diseases in Swedish women: prospective cohort study. BMJ 2012;344:e4026. 10.1136/bmj.e4026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mansoor N, Vinknes KJ, Veierød MB, et al. Effects of low-carbohydrate diets V. low-fat diets on body weight and cardiovascular risk factors: a meta-analysis of randomised controlled trials. Br J Nutr 2016;115:466–79. 10.1017/S0007114515004699 [DOI] [PubMed] [Google Scholar]
- 21.Friedman AN. High-Protein diets: potential effects on the kidney in renal health and disease. Am J Kidney Dis 2004;44:950–62. 10.1053/j.ajkd.2004.08.020 [DOI] [PubMed] [Google Scholar]
- 22.Mitchell NS, Scialla JJ, Yancy WS. Are low-carbohydrate diets safe in diabetic and nondiabetic chronic kidney disease? Ann N Y Acad Sci 2020;1461:25–36. 10.1111/nyas.13997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Knight EL, Stampfer MJ, Hankinson SE, et al. The impact of protein intake on renal function decline in women with normal renal function or mild renal insufficiency. Ann Intern Med 2003;138:460–7. 10.7326/0003-4819-138-6-200303180-00009 [DOI] [PubMed] [Google Scholar]
- 24.Suyoto PST. Effect of low-carbohydrate diet on markers of renal function in patients with type 2 diabetes: a meta-analysis. Diabetes Metab Res Rev 2018;34:e3032. 10.1002/dmrr.3032 [DOI] [PubMed] [Google Scholar]
- 25.Haring B, Selvin E, Liang M, et al. Dietary protein sources and risk for incident chronic kidney disease: results from the Atherosclerosis risk in communities (ARIC) study. J Ren Nutr 2017;27:233–42. 10.1053/j.jrn.2016.11.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kelly T, Unwin D, Finucane F. Low-Carbohydrate diets in the management of obesity and type 2 diabetes: a review from clinicians using the approach in practice. Int J Environ Res Public Health 2020;17:2557. 10.3390/ijerph17072557 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Seidelmann SB, Claggett B, Cheng S, et al. Dietary carbohydrate intake and mortality: a prospective cohort study and meta-analysis. Lancet Public Health 2018;3:e419–28. 10.1016/S2468-2667(18)30135-X [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zarnowski T, Tulidowicz-Bielak M, Kosior-Jarecka E, et al. A ketogenic diet may offer neuroprotection in glaucoma and mitochondrial diseases of the optic nerve. Med Hypothesis Discov Innov Ophthalmol 2012;1:45–9. [PMC free article] [PubMed] [Google Scholar]
- 29.Zarnowski T, Choragiewicz TJ, Schuettauf F, et al. Ketogenic diet attenuates NMDA-induced damage to rat's retinal ganglion cells in an age-dependent manner. Ophthalmic Res 2015;53:162–7. 10.1159/000379753 [DOI] [PubMed] [Google Scholar]
- 30.Zarnowski T, Tulidowicz-Bielak M, Zarnowska I, et al. Kynurenic acid and neuroprotective activity of the ketogenic diet in the eye. Curr Med Chem 2017;24:3547–58. 10.2174/0929867324666170509120257 [DOI] [PubMed] [Google Scholar]