Abstract
Objectives
This study evaluates the impact of frailty, which is a state of increased vulnerability to stressors, on 30-day and 1-year mortality among elderly patients with community-acquired pneumonia (CAP). The main hypothesis is that frailty is an independent predictor of prognosis in elderly CAP patients.
Design
Prospective, observational, follow-up cohort study.
Setting
A 2000-bed tertiary care hospital in Beijing, China.
Participants
Consecutive CAP patients aged ≥65 years admitted to the geriatric department of our hospital between September 2017 and February 2019.
Main outcome measures
The primary outcomes were all-cause mortality at 30 days and 1 year after hospital admission. The impact of frailty (defined by frailty phenotype) on 30-day and 1-year mortality of elderly patients with CAP was assessed by Cox regression analysis.
Results
The cohort included 256 patients. The median (IQR) age was 86 (81–90) years, and 180 (70.3%) participants were men. A total of 171/256 (66.8%) patients were frail. The prevalence of frailty was significantly associated with older age, female gender, lower body mass index, comorbidities, limitations in activities of daily living (ADLs) and poor nutritional status. Frail participants were significantly more likely to have severe CAP (SCAP) than non-frail counterparts (28.65% vs 9.41%, p<0.001). The 1-year mortality risk was approximately threefold higher in frail patients (adjusted HR, 2.70; 95% CI, 1.69 to 4.39) than non-frail patients. Subgroup analysis of patients with SCAP showed that the 1-year mortality risk was approximately threefold higher in the frail group (adjusted HR, 2.87; 95% CI, 1.58 to 4.96) than in the non-frail group. The association between frailty and 30-day mortality was not significant.
Conclusions
These findings suggest that frailty is strongly associated with SCAP and higher 1-year mortality in elderly patients with CAP, and frailty should be detected early to improve the management of these patients.
Keywords: geriatric medicine, internal medicine, respiratory infections, respiratory medicine (see thoracic medicine)
Strengths and limitations of this study.
This is a prospective cohort study, included the comprehensive scope of prognostic factors (frailty, age, disability, malnutrition, comorbidities and disease severity) for patients with community-acquired pneumonia (CAP).
Results of this study may contribute to a better understanding of the risk profile of frail older adults hospitalised with CAP.
Frailty was defined by Fried’s frailty phenotype, which is well validated and simple to use.
The study was conducted in a single centre and evaluated a small number of endpoints.
The study included a small sample of older patients and did not evaluate the association between frailty and CAP in outpatients.
Introduction
Community-acquired pneumonia (CAP) is the most prevalent infectious disease in the elderly and is associated with high rates of mortality, morbidity and high costs worldwide.1–4 In the USA, the incidence of CAP in the age group 65–79 years is 63 cases per 10 000 adults and increases to 164.3 cases per 10 000 adults in the age group >80 years.5 Mortality from CAP increases with age. In 2012, the average mortality from pneumonia was 17.46 cases per 100 000 in all age groups in China, 23.55 cases per 100 000 in the age group 65–69 years and nearly 36 times higher in the age group >85 years.6
Studies have shown that age, functional status, comorbidities and malnutrition are strongly associated with poor prognosis in CAP patients,4 6–10 and higher mortality in elderly patients with CAP underscores the need to identify novel, modifiable risk factors for poor outcomes. Particular attention has recently been directed to frail elderly patients. Frailty is associated with disabilities, comorbidities and old age and is defined as a cumulative decline in multiple organ systems and loss of physiological reserves, increasing the vulnerability to adverse outcomes, including falls, hospitalisation and mortality.11–13 Moreover, frailty is an independent risk factor for mortality in patients with acute and chronic diseases.14 15 A European multicenter study assessed the impact of frailty (measured by the Clinical Frailty Scale) on intensive care unit admission and 30-day mortality in 5021 elderly patients and observed that frailty was found in 43% of these patients and was independently related to 30-day survival (HR, 1.54; 95% CI, 1.38–1.73, frail vs non-frail patients).16 A study evaluated the relationship between frailty and CAP in older patients and found that frailty was associated with increased hospitalisation at 28 days after CAP diagnosis17 and significantly predicted the risk of 1-month mortality.18 Therefore, we hypothesised that frailty is positively correlated with CAP severity and mortality in elderly patients.
The objective of this study was to assess the prevalence of frailty in older patients with CAP, the association between frailty and CAP severity and 30-day and 1-year mortality. This information may be useful for the early stratification of high-risk patients and the hierarchical management of modifiable factors to improve the prognosis of elderly patients with CAP.
Methods
Study design
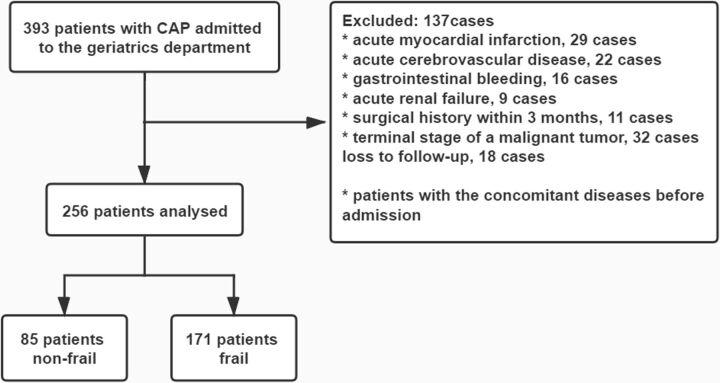
We prospectively and consecutively enrolled elderly patients (aged ≥65 years) diagnosed with CAP in the geriatric department of our institution from September 2017 to February 2019. The exclusion criteria were complications, such as acute myocardial infarction, acute cerebrovascular disease, gastrointestinal bleeding or acute renal failure, surgical history within 3 months and terminal stage of a malignant tumour (figure 1).
Figure 1.
Flowchart of patients excluded/included for the frailty assessment analyses in community-acquired pneumonia (CAP) cohort.
CAP was defined as pneumonia acquired outside the hospital by an immunocompetent individual. The criteria for diagnosing CAP were community onset and the presence of new infiltrates on chest X-ray or CT scan together with at least one of the following conditions: (1) new or increased cough (productive, non-productive or accompanied by a change in sputum characteristics) with or without dyspnoea, chest pain or haemoptysis; (2) fever; (3) rales and/or signs of consolidation and (4) peripheral white blood count (WBC) count >10 × 109/L or <4 × 109/L with or without an increase in immature forms. Differential diagnosis included tuberculosis, lung cancer, non-infectious pulmonary interstitial disease, pulmonary oedema, atelectasis, pulmonary embolism, pulmonary eosinophilic infiltration and pulmonary vasculitis. The patients with these diagnoses were excluded.6
Baseline characteristics
The following parameters were evaluated within 24 hours of admission: age, sex, body mass index (BMI), smoking history, procalcitonin, high-sensitivity C reactive protein, WBC, haemoglobin, alanine aminotransferase, albumin, prealbumin, serum creatinine and blood urea nitrogen (BUN). A comprehensive geriatric assessment (CGA), including frailty, comorbidities and functional and nutritional status, was conducted by trained evaluators within 24 hours of admission.
Severity of CAP
Severe CAP (SCAP) was diagnosed according to criteria established by the Infectious Diseases Society of America and the American Thoracic Society,19 and at least one major criterion or three minor criteria should be satisfied. The major criteria were the need for mechanical ventilation or the diagnosis of septic shock. The minor criteria were respiratory rate >130 breaths/min, arterial oxygen pressure/fraction of inspired oxygen ratio <250 mm Hg, multilobar infiltrates, confusion, BUN level >120 mg/dL, leucopenia resulting from infection, thrombocytopenia, hypothermia or hypotension requiring aggressive fluid resuscitation.
Assessment of frailty
Frailty was assessed using the following frailty phenotype criteria: unintentional weight loss (4.5 kg in the past 12 months), self-reported exhaustion, weakness (reduced grip strength), slow walking speed and low physical activity.20 The cut-off values were consistent with the original criteria. Each construct was considered present (score of 1) or absent (score of 0). The total score ranged from 0 to 5. As established previously, the presence and absence of frailty were defined as a score of ≥3 and ≤2, respectively.
Covariates
Comorbidity was evaluated with the Charlson Comorbidity Index (CCI).21 The CCI is the sum of the weighted scores of the following chronic medical conditions: ischaemic heart disease, congestive heart failure, cerebrovascular accident, peripheral vascular diseases, chronic lung diseases, diabetes, dementia, connective tissue diseases, renal diseases, liver diseases, hemiplegia, solid or haematological malignancy and acquired immunodeficiency disease. A score of 1, 2, 3 or 6 is assigned to each disease, depending on severity.
Functional status was assessed by evaluating patients’ ability to perform ADLs using the Barthel Index.22
Nutritional status was assessed using the Mini Nutritional Assessment-Short Form questionnaire.23 This questionnaire evaluates BMI, weight loss in the past 3 months, dietary changes, stress or acute illness, degree of mobility and neuropsychiatric diseases. The total score is 14 points, and scores of 0–7 indicate malnutrition.
Follow-up and outcomes
All patients were followed for 1 year after hospital admission. Deaths were confirmed through surveillance or official death records. The primary outcomes were all-cause mortality at 30 days and 1 year after admission.
Statistical analysis
Statistical analyses were performed using IBM SPSS software V.23.0. Continuous variables were expressed as the means±SD or IQR and compared using Student’s t-test or the Mann-Whitney U test. Categorical variables were compared using the χ2 test or Fisher’s exact test. The association between baseline frailty status and 30-day and 1-year all-cause mortality was analysed using univariate and multivariate Cox proportional hazards regression models, and the results were expressed as HR and 95% CI. A two-tailed p value of less than 0.05 was considered to indicate a statistically significant difference.
Patient and public involvement
Patients and the public were not involved in the study design, recruitment or execution, and the participants were not to be involved in disseminating the results. The study results will be provided to patients on request, and aggregated data will be reported in project reports and research publications and meetings.
Results
In our cohort, 393 older patients with CAP were admitted to our department during the study period. Of these, 119 patients were excluded because of concomitant diseases, and 18 were lost to follow-up (figure 1). A total of 256 patients with CAP aged 65–99 years were included in the final analysis. Demographic characteristics are presented in table 1. The median age of the cohort was 86 (IQR, 81–90) years, and 180 (70.3%) patients were men. A total of 171 (66.8%) participants were frail, 71 (27.7%) patients were malnourished and 57 (22.3%) participants had SCAP. All-cause mortality at 30 days and 1 year was 5.5% and 16.8%, respectively.
Table 1.
Baseline characteristics of the study population according to frailty status
| Characteristics | Total cohort | Non-frail | Frail† | P value |
| N (%) | 256 | 85 (33.2) | 171 (66.8) | |
| Age, median (IQR) | 86 (81–90) | 83 (75–87) | 88 (84–91) | <0.001* |
| Sex, n (%) | ||||
| Female | 76 (29.7) | 17 (20.0) | 59 (34.5) | 0.017* |
| Male | 180 (70.3) | 68 (80.0) | 112 (65.5) | |
| Smoking status, n (%) | ||||
| Current | 29 (11.3) | 11 (12.9) | 18 (10.5) | 0.288 |
| Previous | 71 (27.7) | 28 (32.9) | 43 (25.1) | |
| Never | 156 (60.9) | 46 (54.1) | 110 (64.3) | |
| BMI (kg/m2), mean (SD) | 22.9 (4.3) | 24.2 (3.8) | 22.3 (4.4) | 0.001* |
| Barthel Index, median (IQR) | 65 (40–95) | 95 (85–100) | 50 (20–70) | <0.001* |
| CCI, median (IQR) | 4 (3–5) | 3 (2–4) | 4 (3–6) | <0.001* |
| MNA-SF, median (IQR) | 10 (7–12) | 12 (11–14) | 9 (7–11) | <0.001* |
| Malnutrition, n (%)‡ | 71 (27.7) | 6 (7.1) | 65 (38.0) | |
| SCAP§, n (%) | 57 (22.3) | 8 (9.41) | 49 (28.65) | <0.001* |
| PCT (ng/mL), median (IQR) | 0.28 (0.21–0.44) | 0.27 (0.20–0.37) | 0.29 (0.21–0.55) | 0.149 |
| hs-CRP (mg/L), median (IQR) | 25.89 (5.79–61.41) | 19.63 (4.19–54.29) | 27.08 (7.56–62.43) | 0.129 |
| WBC (×109/L), median (IQR) | 7.65 (5.57–11.06) | 7.02 (5.64–9.01) | 8.02 (5.51–12.33) | 0.043* |
| HGB (g/L), median (IQR) | 121 (108–133) | 127 (120–136) | 115 (100–128) | <0.001* |
| Scr (μmol/L), median (IQR) | 76.9 (61.6–96.8) | 78.6 (67.4–90.5) | 75.2 (59.5–101.6) | 0.72 |
| BUN (mmol/L), median (IQR) | 5.98 (4.58–7.94) | 5.99 (4.30–7.01) | 5.98 (4.79–8.65) | 0.039* |
| ALT (U/L), median (IQR) | 14 (10–22) | 15 (11–22) | 13 (10–21) | 0.058 |
| ALB (g/L), mean (SD) | 32.46 (4.39) | 34.38 (4.22) | 31.50 (4.16) | <0.001* |
| PA (g/L), mean (SD) | 16.92 (6.19) | 18.74 (6.41) | 15.98 (5.88) | 0.001* |
| 30-day mortality, n (%) | 14 (5.5) | 0 (0) | 14 (8.19) | 0.015* |
| 1-year mortality, n (%) | 43 (16.8) | 0 (0) | 43 (25.15) | <0.001* |
*P<0.05.
†Frailty was defined as Fried phenotype scores ≥3.
‡Malnutrition was defined as MNA-SF scores ≤7.
§SCAP was defined by IDSA/ATS criteria (2007).
ALB, albumin; ALT, alanine aminotransferase; BMI, body mass index; BUN, blood urea nitrogen; CCI, Charlson’s Comorbidity Index; HGB, haemoglobin; hs-CRP, high sensitivity C reactive protein; MNA-SF, Mini Nutritional Assessment-Short Form; PA, prealbumin; PCT, procalcitonin; SCAP, severe community-acquired pneumonia; Scr, serum creatinine; WBC, white blood cell.
Frailty was significantly associated with older age, female gender, lower BMI, limitations in ADLs, comorbidities and poor nutritional status. Frail participants were significantly more likely to have SCAP than non-frail counterparts (28.65% vs 9.41%, p<0.001). Thirty-day and one-year mortality was significantly higher in frail patients (0 vs 8.19%, p=0.015; and 0 vs 25.25%, p<0.001, respectively). The results of Cox proportional hazards regression are presented in table 2. SCAP, frailty, malnutrition and CCI were significantly associated with 30-day mortality in the univariate analysis, but only SCAP remained significant in the multivariate analysis (adjusted HR, 30.60; 95% CI, 3.77 to 248.06). The factors associated with 1-year mortality in the multivariable analysis were SCAP (adjusted HR, 7.68; 95% CI, 3.79 to 15.58), frailty (adjusted HR, 2.70; 95% CI, 1.69 to 4.39) and CCI (adjusted HR, 1.19; 95% CI, 1.05 to 1.34).
Table 2.
Factors associated with 30-day and 1-year mortality in patients with CAP in Cox proportional hazards regression analyses (n=256)
| Variable | Univariate | Multivariate | ||||
| HR | 95% CI | P value | HR | 95% CI | P value | |
| 30-day mortality | ||||||
| SCAP | 52.01 | 6.80 to 398.09 | <0.001 | 30.60 | 3.77 to 248.06 | 0.001* |
| BI | 0.99 | 0.97 to 1.00 | 0.101 | _ | _ | _ |
| BMI | 0.97 | 0.86 to 1.09 | 0.609 | _ | _ | _ |
| Malnutrition | 0.84 | 0.73 to 0.97 | 0.015 | 1.11 | 0.90 to 1.35 | 0.330 |
| Frailty | 2.58 | 1.42 to 4.69 | 0.002 | 1.83 | 0.88 to 3.81 | 0.108 |
| CCI | 1.41 | 1.19 to 1.67 | <0.001 | 1.19 | 0.99 to 1.43 | 0.069 |
| 1-year mortality | ||||||
| SCAP | 13.32 | 6.81 to 26.04 | <0.001 | 7.68 | 3.79 to 15.58 | <0.001* |
| BI | 0.98 | 0.97 to 0.99 | <0.001 | 1.01 | 0.99 to 1.02 | 0.352 |
| BMI | 0.93 | 0.87 to 1.00 | 0.038 | 1.07 | 0.98 to 1.16 | 0.128 |
| Malnutrition | 0.78 | 0.72 to 0.85 | <0.001 | 0.94 | 0.81 to 1.10 | 0.424 |
| Frailty | 3.41 | 2.32 to 5.03 | <0.001 | 2.70 | 1.69 to 4.39 | <0.001* |
| CCI | 1.40 | 1.26 to 1.55 | <0.001 | 1.19 | 1.05 to 1.34 | <0.001* |
Data are estimated HR and 95% CIs of the explanatory variables in the 30-day and 1-year mortality group. Data were adjusted for age, sex, disability, malnutrition, comorbidities and the severity of CAP.
*P<0.05.
BI, Barthel Index; BMI, body mass index; CAP, community-acquired pneumonia; CCI, Charlson’s Comorbidity Index; SCAP, severe community-acquired pneumonia.
Subgroup analysis of patients with SCAP by frailty status showed that 8 (14%) patients were non-frail and 49 patients (86%) were frail. The frail group presented significantly worse functional and nutritional status and higher 30-day and 1-year mortality (see online supplemental table). In the multivariate analysis of patients with SCAP, 1-year mortality risk was approximately threefold higher in the frail group (adjusted HR, 2.87; 95% CI, 1.58 to 4.96) than in the non-frail group, and 1-year mortality risk was 16% higher among those with more comorbidities (adjusted HR, 1.16; 95% CI, 1.01 to 1.34) (table 3). Only CCI was significantly correlated with 30-day mortality (HR, 1.21; 95% CI, 1.02 to 1.43).
Table 3.
Factors associated with 30-day and 1-year mortality in patients with severe community-acquired pneumonia in Cox proportional hazards regression analyses (n=57)
| Variable | Univariate | Multivariate | ||||
| HR | 95% CI | P value | HR | 95% CI | P value | |
| 30-day mortality | ||||||
| BI | 1.00 | 0.99 to 1.02 | 0.899 | _ | _ | _ |
| BMI | 1.04 | 0.92 to 1.18 | 0.507 | _ | _ | _ |
| Malnutrition | 0.73 | 0.24 to 2.23 | 0.581 | _ | _ | _ |
| Frailty | 26.61 | 0.04 to 16 017.0 | 0.315 | _ | _ | _ |
| CCI | 1.21 | 1.02 to 1.43 | 0.028 | 1.21 | 1.02 to 1.43 | 0.028* |
| 1-year mortality | ||||||
| BI | 0.99 | 0.98 to 1.00 | 0.046 | 1.00 | 0.99 to 1.02 | 0.875 |
| BMI | 0.99 | 0.92 to 1.08 | 0.854 | _ | _ | _ |
| Malnutrition | 0.86 | 0.77 to 0.97 | 0.010 | 1.05 | 0.89 to 1.25 | 0.545 |
| Frailty | 2.82 | 1.70 to 4.68 | <0.001 | 2.87 | 1.58 to 4.96 | <0.001* |
| CCI | 1.24 | 1.10 to 1.40 | 0.001 | 1.16 | 1.01 to 1.34 | 0.034* |
Data are estimated HR and 95% CIs of the explanatory variables in the 1-year mortality group. Data were adjusted for age, sex, disability, malnutrition and comorbidities.
*P<0.05.
BI, Barthel Index; BMI, body mass index; CCI, Charlson’s Comorbidity Index.
bmjopen-2020-038370supp001.pdf (67.8KB, pdf)
Discussion
The results showed that frail older patients with CAP were significantly more likely to have SCAP and had a significantly higher 30-day and 1-year mortality risk than non-frail patients. In addition, frailty was an independent risk factor for 1-year mortality after adjusting for age, sex, disability, malnutrition, comorbidities and CAP severity but was not an independent risk factor for 30-day mortality.
The incidence of CAP increases with age, and CAP increases the risk of morbidity and mortality. Mortality is significantly higher in elderly patients with CAP than in the general population.4 9 All-cause mortality at 30 days and 1 year among elderly patients with CAP in our cohort was 5.5% and 16.8%, respectively. Known independent prognostic factors for CAP mortality are age, comorbidities, functional status, microbial aetiology and early antibiotic treatment.9 10 24 25 However, some studies reported that age per se was not an independent predictor of CAP mortality in the elderly population with pneumonia.26 27 Frailty is an age-related disease associated with disabilities, comorbidities and advanced age, and is characterised by a decline in physiological functions across multiple organ systems and increased vulnerability to stressors,20 28 29 which increases the risk of adverse health outcomes, including falls, disability, hospitalisation, institutionalisation and death. Various conditions, including malnutrition, sarcopenia, gait impairment, chronic inflammation, polypharmacy, cardiovascular changes and morbidity, may cause frailty.30–32 Accordingly, in our sample, all patients who died within 30 days or 1 year were frail.
Frailty was more prevalent in women in our study, which is consistent with a previous study.30 In addition, frail patients had significantly older age, more comorbidities, poor nutritional and functional status and limited ability to perform ADLs. Poor nutritional status, including hypoalbuminemia, hypoproteinemia, malnourishment and a low nutritional score, is a strong predictor of mortality in CAP patients.33 34 However, in our cohort, there was no significant association between nutritional status and 30-day or 1-year mortality in the multivariate analysis (adjusted HR, 1.11; 95% CI, 0.90 to 1.35; adjusted HR, 0.94; 95% CI, 0.81 to 1.10, respectively). Some studies have shown that the definition, diagnosis and treatment of frailty and malnutrition overlap.30 32 35 Malnutrition is a physiological condition that predisposes to the occurrence and development of frailty.31 In our study, frailty was defined as a syndrome that affects multiple organ systems and was a strong predictor of long-term mortality, and this multidimensional nature may explain why frailty, but not malnutrition and disability, was significantly associated with higher mortality in the multivariate analysis.
Our results indicated that frailty was common in elderly patients with CAP (prevalence of 66.8%) and was closely linked to pneumonia severity; furthermore, frailty increased 1-year mortality risk nearly threefold compared with the absence of frailty (adjusted HR, 2.70; 95% CI, 1.69 to 4.39). A study evaluated patient outcomes at 1 year after the diagnosis of CAP and showed that age ≥65 years, nursing home residency and comorbidity were positively associated with 1-year mortality.36 Another study found that the risk of CAP mortality was more strongly correlated with underlying diseases than with CAP severity.25 However, these studies did not assess the effect of frailty on CAP prognosis. The results of our study demonstrated that frailty was independently associated with 1-year mortality after adjusting for risk factors, suggesting that frailty could accurately predict adverse outcomes.
Our findings suggest that frail patients are more likely to have SCAP, and frailty is positively correlated to the risk of 1-year mortality. Since the clinical presentation of pneumonia may be atypical in the elderly, clinicians should suspect pneumonia in the presence of symptoms such as falls, altered mental status, fatigue, delirium and anorexia to avoid complications associated with delayed diagnosis and therapy.37 With respect to clinical decision-making and planning of health services, it is important to identify frailty in elderly CAP patients to better stratify the risk of adverse outcomes and implement treatments tailored to individual needs, weighing up the risks and benefits of invasive diagnostic procedures and therapies as well as taking into account end of life care needs for individuals with advanced frailty. A Korean study found that frailty was independently associated with do-not-resuscitate orders and healthcare transitions, even after adjusting for sepsis and pneumonia severity.38 An international multidisciplinary group proposed a CGA39 adapted to the emergency department context to assess frailty in elderly patients.16 40 Although many physicians and intensivists do not currently perform CGAs in critically ill patients with infectious diseases, demographic shifts may require addressing this issue promptly. Moreover, frailty is a dynamic state and can be reversed.41 Preventing frailty is possible, especially during onset, and the early diagnosis of this condition is crucial to improve therapeutic efficacy and the postdischarge management of elderly patients with CAP.11
This study has limitations. First, the results were not compared with data from other hospitals and regions. Second, the number of patients and endpoint events was small. Third, the study did not evaluate the association between frailty and CAP in outpatients; therefore, the results cannot be generalised to other patient groups.
This study adjusted for confounding factors and demonstrated that frailty strongly affected the long-term prognosis of CAP patients. These data can contribute to the long-term management of frailty in CAP patients.
Conclusions
Frailty is very common in elderly patients with CAP and increases the risk of 1-year mortality from CAP and SCAP. Our findings suggest that frailty should be evaluated in routine clinical practice to improve the postdischarge management of older patients with CAP.
Supplementary Material
Footnotes
Contributors: JL, WT, YS and CJ conceived and designed the study. JL and WT collected data. WT, YS and CJ analysed and interpreted data. JL and WT wrote the manuscript. All authors approved the final manuscript.
Funding: This work was supported by the Beijing Municipal Administration of Hospitals Clinical Medicine Development of Special Funding Support (Process No. ZYLX201838) and the Beijing Friendship Hospital of Capital Medical University Hospital Startup Funding (Process No. yyqdkt2017-37).
Competing interests: None declared.
Patient consent for publication: Parental/guardian consent obtained.
Ethics approval: This study was approved by the research ethics committee of Beijing Friendship Hospital and Capital Medical University and conformed to the ethical guidelines of the Declaration of Helsinki (Project No. 2018-P2-138-01). All patients provided informed consent before the commencement of the study.
Provenance and peer review: Not commissioned; externally peer reviewed.
Data availability statement: Data are available upon reasonable request to corresponding author (e-mail: twybzx@163.com).
Supplemental material This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.
References
- 1.Prina E, Ranzani OT, Torres A. Community-acquired pneumonia. Lancet 2015;386:1097–108. 10.1016/S0140-6736(15)60733-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Song J-H, Huh K, Chung DR. Community-acquired pneumonia in the Asia-Pacific region. Semin Respir Crit Care Med 2016;37:839–54. 10.1055/s-0036-1592075 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chalmers J, Campling J, Ellsbury G, et al. Community-acquired pneumonia in the United Kingdom: a call to action. Pneumonia 2017;9:15. 10.1186/s41479-017-0039-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cillóniz C, Rodríguez-Hurtado D, Torres A. Characteristics and management of community-acquired pneumonia in the era of global aging. Med Sci 2018;6:35. 10.3390/medsci6020035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jain S, Self WH, Wunderink RG, et al. Community-acquired pneumonia requiring hospitalization among U.S. adults. N Engl J Med Overseas Ed 2015;373:415–27. 10.1056/NEJMoa1500245 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cao B, Huang Y, She D-Y, et al. Diagnosis and treatment of community-acquired pneumonia in adults: 2016 clinical practice guidelines by the Chinese thoracic Society, Chinese medical association. Clin Respir J 2018;12:1320–60. 10.1111/crj.12674 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Almirall J, Serra-Prat M, Bolíbar I, et al. Risk factors for community-acquired pneumonia in adults: a systematic review of observational studies. Respiration 2017;94:299–311. 10.1159/000479089 [DOI] [PubMed] [Google Scholar]
- 8.Yeo HJ, Byun KS, Han J, et al. Prognostic significance of malnutrition for long-term mortality in community-acquired pneumonia: a propensity score matched analysis. Korean J Intern Med 2019;34:841–9. 10.3904/kjim.2018.037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cillóniz C, Polverino E, Ewig S, et al. Impact of age and comorbidity on cause and outcome in community-acquired pneumonia. Chest 2013;144:999–1007. 10.1378/chest.13-0062 [DOI] [PubMed] [Google Scholar]
- 10.Klausen HH, Petersen J, Lindhardt T, et al. Outcomes in elderly Danish citizens admitted with community-acquired pneumonia. regional differencties, in a public healthcare system. Respir Med 2012;106:1778–87. 10.1016/j.rmed.2012.08.010 [DOI] [PubMed] [Google Scholar]
- 11.Hoogendijk EO, Afilalo J, Ensrud KE, et al. Frailty: implications for clinical practice and public health. The Lancet 2019;394:1365–75. 10.1016/S0140-6736(19)31786-6 [DOI] [PubMed] [Google Scholar]
- 12.Chang S-F, Lin P-L. Frail phenotype and mortality prediction: a systematic review and meta-analysis of prospective cohort studies. Int J Nurs Stud 2015;52:1362–74. 10.1016/j.ijnurstu.2015.04.005 [DOI] [PubMed] [Google Scholar]
- 13.Dent E, Martin FC, Bergman H, et al. Management of frailty: opportunities, challenges, and future directions. The Lancet 2019;394:1376–86. 10.1016/S0140-6736(19)31785-4 [DOI] [PubMed] [Google Scholar]
- 14.Costa D, Aladio M, Girado CA, et al. Frailty is independently associated with 1-year mortality after hospitalization for acute heart failure. Int J Cardiol Heart Vasc 2018;21:103–6. 10.1016/j.ijcha.2018.10.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Walker SR, Gill K, Macdonald K, et al. Association of frailty and physical function in patients with non-dialysis CKD: a systematic review. BMC Nephrol 2013;14:228. 10.1186/1471-2369-14-228 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Flaatten H, De Lange DW, Morandi A, et al. The impact of frailty on ICU and 30-day mortality and the level of care in very elderly patients (≥ 80 years). Intensive Care Med 2017;43:1820–8. 10.1007/s00134-017-4940-8 [DOI] [PubMed] [Google Scholar]
- 17.Millett ERC, De Stavola BL, Quint JK, et al. Risk factors for hospital admission in the 28 days following a community-acquired pneumonia diagnosis in older adults, and their contribution to increasing hospitalisation rates over time: a cohort study. BMJ Open 2015;5:e008737. 10.1136/bmjopen-2015-008737 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pilotto A, Dini S, Daragjati J, et al. Combined use of the multidimensional prognostic index (Mpi) and procalcitonin serum levels in predicting 1-month mortality risk in older patients hospitalized with community-acquired pneumonia (CAP): a prospective study. Aging Clin Exp Res 2018;30:193–7. 10.1007/s40520-017-0759-y [DOI] [PubMed] [Google Scholar]
- 19.Mandell LA, Wunderink RG, Anzueto A, et al. Infectious diseases Society of America/American thoracic Society consensus guidelines on the management of community-acquired pneumonia in adults. Clin Infect Dis 2007;44 Suppl 2:S27–72. 10.1086/511159 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fried LP, Tangen CM, Walston J, et al. Frailty in older adults: evidence for a phenotype. J Gerontol A Biol Sci Med Sci 2001;56:M146–57. 10.1093/gerona/56.3.M146 [DOI] [PubMed] [Google Scholar]
- 21.Charlson ME, Pompei P, Ales KL, et al. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis 1987;40:373–83. 10.1016/0021-9681(87)90171-8 [DOI] [PubMed] [Google Scholar]
- 22.Mahoney FI, Barthel DW. Functional evaluation: the BARTHEL index. Md State Med J 1965;14:61–5. [PubMed] [Google Scholar]
- 23.Rubenstein LZ, Harker JO, Salvà A, et al. Screening for undernutrition in geriatric practice: developing the short-form mini-nutritional assessment (MNA-SF). J Gerontol A Biol Sci Med Sci 2001;56:M366–72. 10.1093/gerona/56.6.M366 [DOI] [PubMed] [Google Scholar]
- 24.Luna CM, Palma I, Niederman MS, et al. The impact of age and comorbidities on the mortality of patients of different age groups admitted with community-acquired pneumonia. Ann Am Thorac Soc 2016;13:1519–26. 10.1513/AnnalsATS.201512-848OC [DOI] [PubMed] [Google Scholar]
- 25.Weir DL, Majumdar SR, McAlister FA, et al. The impact of multimorbidity on short-term events in patients with community-acquired pneumonia: prospective cohort study. Clin Microbiol Infect 2015;21:264.e7–264.e13. 10.1016/j.cmi.2014.11.002 [DOI] [PubMed] [Google Scholar]
- 26.Chen J-H, Chang S-S, Liu JJ, et al. Comparison of clinical characteristics and performance of pneumonia severity score and CURB-65 among younger adults, elderly and very old subjects. Thorax 2010;65:971–7. 10.1136/thx.2009.129627 [DOI] [PubMed] [Google Scholar]
- 27.Simonetti AF, Viasus D, Garcia-Vidal C, et al. Management of community-acquired pneumonia in older adults. Ther Adv Infect Dis 2014;2:3–16. 10.1177/2049936113518041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Morley JE, Vellas B, van Kan GA, et al. Frailty consensus: a call to action. J Am Med Dir Assoc 2013;14:392–7. 10.1016/j.jamda.2013.03.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Dent E, Martin FC, Bergman H, et al. Management of frailty: opportunities, challenges, and future directions. Lancet 2019;394:1376–86. 10.1016/S0140-6736(19)31785-4 [DOI] [PubMed] [Google Scholar]
- 30.Collard RM, Boter H, Schoevers RA, et al. Prevalence of frailty in community-dwelling older persons: a systematic review. J Am Geriatr Soc 2012;60:1487–92. 10.1111/j.1532-5415.2012.04054.x [DOI] [PubMed] [Google Scholar]
- 31.Lorenzo-López L, Maseda A, de Labra C, et al. Nutritional determinants of frailty in older adults: a systematic review. BMC Geriatr 2017;17:108. 10.1186/s12877-017-0496-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Jeejeebhoy KN, Malnutrition JKN. Malnutrition, fatigue, frailty, vulnerability, sarcopenia and cachexia: overlap of clinical features. Curr Opin Clin Nutr Metab Care 2012;15:213–9. 10.1097/MCO.0b013e328352694f [DOI] [PubMed] [Google Scholar]
- 33.Yeo HJ, Byun KS, Han J, et al. Prognostic significance of malnutrition for long-term mortality in community-acquired pneumonia: a propensity score matched analysis. Korean J Intern Med 2019;34:841–9. 10.3904/kjim.2018.037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Washio M, Kondo K, Fujisawa N, et al. Hypoalbuminemia, influenza vaccination and other factors related to the development of pneumonia acquired outside hospitals in southern Japan: a case-control study. Geriatr Gerontol Int 2016;16:223–9. 10.1111/ggi.12456 [DOI] [PubMed] [Google Scholar]
- 35.Laur CV, McNicholl T, Valaitis R, et al. Malnutrition or frailty? overlap and evidence gaps in the diagnosis and treatment of frailty and malnutrition. Appl Physiol Nutr Metab 2017;42:449–58. 10.1139/apnm-2016-0652 [DOI] [PubMed] [Google Scholar]
- 36.Wesemann T, Nüllmann H, Pflug MA, et al. Pneumonia severity, comorbidity and 1-year mortality in predominantly older adults with community-acquired pneumonia: a cohort study. BMC Infect Dis 2015;15:2. 10.1186/s12879-014-0730-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Schoevaerdts D, Sibille F-X, Gavazzi G. Infections in the older population: what do we know? Aging Clin Exp Res 2019;25. 10.1007/s40520-019-01375-4 [DOI] [PubMed] [Google Scholar]
- 38.Choi J-Y, Kim S-W, Yoon S-J, et al. Impact of frailty on do-not-resuscitate orders and healthcare transitions among elderly Koreans with pneumonia. Clin Interv Aging 2018;13:2237–45. 10.2147/CIA.S181400 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.González-Castillo J, Martín-Sánchez FJ, Llinares P, et al. Guidelines for the management of community-acquired pneumonia in the elderly patient. Rev Esp Quimioter 2014;27:69–86. [PubMed] [Google Scholar]
- 40.Rubenstein LZ, Stuck AE, Siu AL, et al. Impacts of geriatric evaluation and management programs on defined outcomes: overview of the evidence. J Am Geriatr Soc 1991;39:8S–16. 9. 10.1111/j.1532-5415.1991.tb05927.x [DOI] [PubMed] [Google Scholar]
- 41.Hoogendijk EO, Afilalo J, Ensrud KE, et al. Frailty: implications for clinical practice and public health. Lancet 2019;394:1365–75. 10.1016/S0140-6736(19)31786-6 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
bmjopen-2020-038370supp001.pdf (67.8KB, pdf)