Abstract
The effectiveness of reticulocyte hemoglobin content (CHr) had been reported to detect early functional iron deficiency especially among Chronic kidney disease (CKD) patients. CHr is more superior to classic biochemical indices in reflecting transient iron-deficiency status, therefore improving diagnosis and treatment. This study was conducted to determine the sensitivity of CHr in the diagnosis of functional iron deficiency (FID) in hemodialyzed patients. One hundred hemodialyzed patients along with 60 healthy controls were recruited and blood specimens were collected. Venous blood was used for hematological and biochemical investigations collected via 3 ml lavender-top tubes for hematological tests including CBC, blood film, ESR and CHr, and red-top tube for biochemical tests including TIBC, SF and CRP. A statistically significant decrease was noted in CHr values between hemodialysis patients and the control group (24.8 ± 2.0 pg vs. 30.9 ± 1.3 pg, p<0.001). CHr values showed a significant correlations with RBCs, Hb- hemoglobin, Hct- hematocrit level, MCV- mean corpuscular volume, MCH- mean corpuscular hemoglobin, MCHC, RDW- red cell distribution width , SI-Serum Iron, TIBC- Total iron binding capacity and TSAT- Transferrin saturation. The present study showed that CHr in comparison to the conventional hematological and biochemical markers commonly used to diagnose iron deficiency.
Keywords: reticulocytes, hemoglobin, hemodialysis
1. Introduction
Iron is a very important substance, which was needed by the body to transport oxygen to tissues. Majority of the body iron (2/3) is in the red blood cells (RBC), and about 1/3 is stored in the liver, spleen and bone marrow. Iron is transported into the circulation by transferrin(Knutson, 2017). Patients on hemodialysis have lower intestinal iron absorption and greater iron loss(Rostoker et al., 2016). Levels of ingested iron vary from very small amounts up to 200 mg as that was seen in end-stage renal disease (ESRD) patients given supplements(Rostoker et al., 2016). In ESRD though, there is little iron absorption, as 1 to 2% of this stored iron(Zumbrennen-Bullough and Babitt, 2014). Some studies showed that despite therapy with recombinant human erythropoietin (rHuEPO) in hemodialysis (HD) patients, there would still be iron deficiency anemia in as much as 49.1% of HD patients(Kuo, 2018, Chavers, 2004). Thus, there is an utmost need for compensation of iron in HD patients
In Chronic kidney disease (CKD) patients on HD, annual iron loss can amount to 2 to 4 g or even more. Correction of anemia in hemodialysis patients with Recombinant human erythropoietin(rHuEPO) can be infuriating, if iron is insufficient(Alves, 2015). Thus, it is wise to establish the diagnosis of iron deficiency in CKD patients on HD. However, there is no ideal test for monitoring iron storage. Currently, iron status is evaluated by serum ferritin levels (SF) and transferring saturation (TSAT). Although serum ferritin is still a reliable indicator of iron confession in most cells(Nakanishi, 2010), its value is limited because ferritin is an acute-phase reactant(Kell and Pretorius, 2014) and the extracellular ferritins are raised in incendiary or malicious disease(Wang, 2010).
Other biomarkers of body iron stores were assessed including transferrin saturation (TSAT), percentage of hypochromic erythrocytes (HYPO) and hemoglobin content of reticulocytes (HCr) (Buttarello, 2016, Cai, 2017). In one study, TSAT showed most suitability to determine iron stores for optimal IV iron therapy (Buttarello, 2016). However, more studies showed that both TSAT and ferritin as poor indicators of body iron load particularly in hemodialysis patients (Diebold and Kistler, 2019, Ali Rafi, 2007, Wish, 2018).
The usefulness of CHr has been reported to identify initial functional iron shortage particularly amongst CKD patients who are on EPO therapy (Kuo, 2018, G, 2011, Hayat et al., 2008, Reddy et al., 2013). Increasing evidence showed that CHr is more superior than classic biochemical indices in reflecting transient iron-deficiency status, thus improving diagnosis and treatment (G, 2011, Hayat et al., 2008, Reddy et al., 2013). This study was conducted to determine the sensitivity of CHr in the diagnosis of functional iron deficiency (FID) in hemodialyzed patients.
2. Materials and Methods
One hundred adults (>18 years old), comprised of 51 males and 49 females were recruited from the hemodialysis unit of Prince Sultan ibn Abdulaziz King Fahad Hospital in Jizan, Saudi Arabia. Eligibility criteria included the age criteria more than 18 years old, and those undergone hemodialysis treatment for more than 3 months, the EPO dosing for at least three months with no changes in EPO dosing for at least 4 weeks. All patients received EPO 2,000-6,000 units three times a week.
A control group was selected from blood donors from the same institution comprising of 60 healthy individuals (43 males and 17 females). All were with normal complete blood count (CBC) and biochemical iron parameters. Informed and written consents were taken from all participants, both hemodialysis and control group.
Blood specimens were collected from all patients. Venous blood was used for all hematological and biochemical investigations and collected via 3 ml lavender-top tubes for hematological tests including CBC, blood film, ESR and CHr, and red-top tube for biochemical tests including TIBC, SF and CRP.
2.1. Reticulocyte hemoglobin content measurement
CHr was measured on the ADVIA 2120 hematology analyzer (Siemens Diagnostic Solutions, Tarrytown, New York). Measurement was carried out by fluorescence flow cytometry as the CHr which stains cells according to their RNA content (Fishbane et al., 1997).
ESR was measured by Westergren using Sedplast ESR system (Polymedco, Cortlandt Manor, NY). Serum iron, serum iron binding capacity were measured automatically using Siemens Flex reagent cartridge DF49A on Dade Behring Dimension RXL clinical chemistry autoanalyser. Serum ferritin was measured using Modular Analytic E170 analyzer. CRP was measured by NycoCard CRP single test from Axis-shield.
2.2. Statistical Analysis
Data analysis was performed using Statistical Package for Social Sciences version 18.1 (SPSS Inc, Chicago, Il, USA)(Corp., 2010). All normal distributions of continuous variables were reported as mean ± standard deviation. Correlation coefficient was used to analyze the degrees of association between two variables (Pearson correlation coefficient with p value and 95% confidence interval for r). Regression analysis were performed to describe the relationship between two variables and to predict one variable from another. P values <0.05 were considered statistically significant for all analyses.
3. Results
There were 100 hemodialysis patients, 51 (51%) males and 49 (49%) females. Mean age of the patients was 46.9 ± 9.9 years. Mean CHr was 24.8 ± 2.0 pg. Mean TSAT was 17.4 ± 8.1%. Mean TIBC was 45.7 ± 7.7 umol/L. Mean hematological and biochemical parameters in these patients are shown in Table 1. Most of the patients had transferring saturation (TSAT) <20% and serum ferritin (SF) of >100 ug/L. Mean CRP was 11.1 ± 4.0 mg/L. Mean EPO dose was 3393.3 ± 1328.2. (Table 1)
Table 1.
Comparison of mean results of haematological and biochemical parameters in CRF and control groups.
| Variables | Control | CRF | T test |
|---|---|---|---|
| Mean ± SD | Mean ± SD | p values | |
| WBC, x103/ul | 6.3 ± 1.9 | 5.7 ± 1.8 | <0.001 |
| RBC, x106/ul | 4.7 ± 0.5 | 3.7 ± 0.4 | <0.001 |
| Hb in g/dl | 14.1 ± 1.4 | 9.6 ± 0.8 | <0.001 |
| Hct in % | 41.6 ± 4.1 | 29.0 ± 2.6 | <0.001 |
| MCV in fL | 87.1 ± 2.9 | 80.4 ± 5.9 | <0.001 |
| MCH in pg | 29.7 ± 1.0 | 26.6 ± 2.3 | <0.001 |
| MCHC in g/dl | 34.3 ± 1.5 | 32.0 ± 1.5 | <0.001 |
| RDW in % | 13.2 ± 0.5 | 15.1 ± 1.7 | <0.000 |
| Plt, x103/ul | 26.2 ± 4.8 | 21.3 ± 9.1 | <0.001 |
| CHr in pg | 30.9 ± 1.3 | 24.8 ± 2.0 | <0.001 |
| SI in umol/L | 20.9 ± 2.9 | 10.1 ± 5.1 | <0.001 |
| TIBC in umol/L | 42.8 ± 4.5 | 45.7 ± 7.7 | 0.004 |
| TSAT in % | 46.4 ± 6.0 | 17.4 ± 8.1 | <0.001 |
| SF in ug/L | 9.7 ± 1.2 | 52.6 ± 31.6 | <0.001 |
In contrast to the control group, hemodialysis patients had significantly lower (Hb), (RBC), (Hct), (MCV), (MCH), (MCHC), (SI) and (TSAT)(Karagülle, 2013). A statistically significant decrease was noted in CHr values between hemodialysis patients and the control group (24.8 ± 2.0 pg vs. 30.9 ± 1.3 pg, p<0.001). (Table 1)
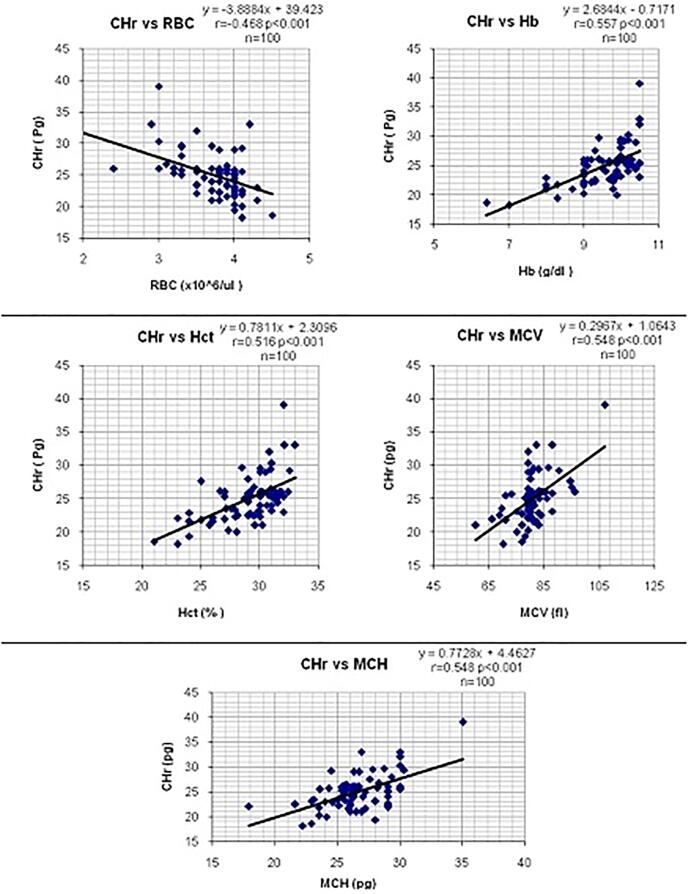
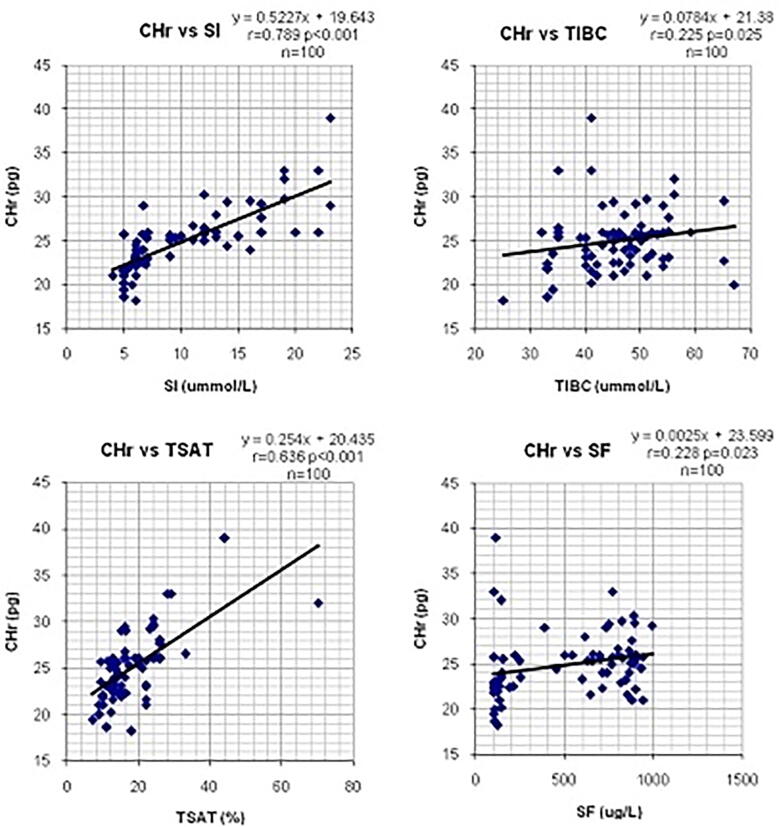
The correlations between CHr values and the mean results of hematological and biochemical parameters of iron status in hemodialysis patients were calculated and shown in Table 2. CHr values showed a significant negative correlation. There were significant positive correlations seen between CHr and SI (r=0.79, p<0.001), TIBC (r=0.225, p=0.025) and TSAT (r=0.64, p<0.001). (Table 2, Figure 1, Figure 2)
Table 2.
Correlations between CHr values and the mean results of hematological and biochemical parameters for iron status in CRF patients.
| Variables | CHr | ||
|---|---|---|---|
| Correlation | DF= n-2 | p values | |
| RBCs | 0.468- | 98 | <0.001 |
| Hb | 0.557 | 98 | <0.001 |
| Hct | 0.516 | 98 | <0.001 |
| MCV | 0.548 | 98 | <0.001 |
| MCH | 0.548 | 98 | <0.001 |
| MCHC | 0.300 | 98 | 0.003 |
| RDW | -0.56 | 98 | <0.001 |
| SI | 0.789 | 98 | <0.001 |
| TIBC | 0.225 | 98 | 0.025 |
| TSAT | 0.636 | 98 | <0.001 |
| SF | 0.228 | 98 | 0.023 |
Figure 1.
Correlations between CHr values and haematological parameters in CRF patients.
Figure 2.
Li near regression analysis showing the correlations between CHr values and biochemical parameters in CRF patients.
4. Discussion
In this study, hemodialysis patients had significantly lower HB, RBC, Hct. MCV, MCH, MCHC, SI, TSAT and, higher levels of RDW, TIBC and SF compared to the control group. There was significantly lower level of CHr in hemodialyszed patients compared to the control group. The mean CHr in our study group was 24.8 ± 2.0 pg, which is in congruence to the values reported by Fishbane et al. (1997) (27.5 ± 2.8 pg) done in 164 stable hemodialyzed patients but, is lower than the values reported by Fukui et al. (2002) (mean of 31.0 pg), Kim et al. (2008) (29.9 ± 1.9 pg), Miyata (2003) (33.1 ± 1.5 pg), Buttarello (2010) (mean of 30.6 pg) and Urrechaga (2009) (31.6 ± 3.5 pg).
This study also showed the inverse correlation between CHr and Total RBC count. This is in agreement with Mittman’s report Neal Mittman et al., 1997, who suggested that the erythropoeitic stimulus of routinely administered rHuEPO may result in functional iron deficiency. The positive correlations that existed between CHr and Hb, Hct, SI, TSAT and SF, making CHr as a potential marker for monitoring renal anemia especially in dialyzed patients Neal Mittman et al., 1997.
The hemodialysis procedure is linked to an increased risk of inflammation thus elevations of CRP levels, which somewhat increases when patients start HD. However, CRP responds only in acute phases of inflammation but it subsides quickly in concentration Gabay and Kushner, 1999, thus making CHr as a promising test to measure ID in hemodialysis patients (Fishbane et al., 1997, Hurrell, 2012, Saito, 2014, Menno, 1998, Nairz, 2016, Wessling-Resnick, 2010, Kotze1 et al., 2009, Van Wyck, 1989, Tarng, 1999, Muñoz, 2008).
The present study showed that CHr in comparison to the conventional hematological and biochemical markers commonly used to diagnose iron deficiency yielded a good and acceptable correlation. CHr can be a very good predictor of iron deficiency and enable to diagnose iron deficiency anemia more rapidly during a routine blood testing with little incremental cost. Our study confirms that CHr is not influenced by the inflammatory process that exists during hemodialysis.
Acknowledgement
This work was supported by the College of Medicine Research Center, Deanship of Scientific Research, King Saud University, Riyadh, Saudi Arabia.
References
- Knutson M.D. Iron transport proteins: Gateways of cellular and systemic iron homeostasis. J Biol Chem. 2017;292(31):12735–12743. doi: 10.1074/jbc.R117.786632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rostoker G., Vaziri N.D., Fishbane S. Iatrogenic Iron Overload in Dialysis Patients at the Beginning of the 21st Century. Drugs. 2016;76(7):741–757. doi: 10.1007/s40265-016-0569-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zumbrennen-Bullough K., Babitt J.L. The iron cycle in chronic kidney disease (CKD): from genetics and experimental models to CKD patients. Nephrol Dial Transplant. 2014;29(2):263–273. doi: 10.1093/ndt/gft443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuo K.L. Association of Anemia and Iron Parameters With Mortality Among Patients Undergoing Prevalent Hemodialysis in Taiwan: The AIM - HD Study. J Am Heart Assoc. 2018;7(15) doi: 10.1161/JAHA.118.009206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chavers B.M. Prevalence of anemia in erythropoietin-treated pediatric as compared to adult chronic dialysis patients. Kidney Int. 2004;65(1):266–273. doi: 10.1111/j.1523-1755.2004.00357.x. [DOI] [PubMed] [Google Scholar]
- Alves M.T. Resistance of dialyzed patients to erythropoietin. Rev Bras Hematol Hemoter. 2015;37(3):190–197. doi: 10.1016/j.bjhh.2015.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakanishi T. Importance of ferritin for optimizing anemia therapy in chronic kidney disease. Am J Nephrol. 2010;32(5):439–446. doi: 10.1159/000320733. [DOI] [PubMed] [Google Scholar]
- Kell D.B., Pretorius E. Serum ferritin is an important inflammatory disease marker, as it is mainly a leakage product from damaged cells. Metallomics. 2014:748–773. doi: 10.1039/c3mt00347g. [DOI] [PubMed] [Google Scholar]
- Wang W. Serum ferritin: Past, present and future. Biochim Biophys Acta. 2010;1800(8):760–769. doi: 10.1016/j.bbagen.2010.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buttarello M. Evaluation of the hypochromic erythrocyte and reticulocyte hemoglobin content provided by the Sysmex XE-5000 analyzer in diagnosis of iron deficiency erythropoiesis. Clin Chem Lab Med. 2016;54(12):1939–1945. doi: 10.1515/cclm-2016-0041. [DOI] [PubMed] [Google Scholar]
- Cai Jie. Evaluation of the Efficiency of the Reticulocyte Hemoglobin Content on Diagnosis for Iron Deficiency Anemia in Chinese Adults. Nutrients. 2017;2017:9. doi: 10.3390/nu9050450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diebold M., Kistler A.D. Evaluation of iron stores in hemodialysis patients on maintenance ferric Carboxymaltose dosing. BMC Nephrol. 2019;20(1):76. doi: 10.1186/s12882-019-1263-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ali Rafi A.K. Mohammed Abdelrahman, Monitoring Iron status in End-Stage Renal Disease Patients on Hemodialysis. Saudi J Kidney Dis Transplant. 2007;18(1):73–78. [PubMed] [Google Scholar]
- Wish J.B. Positive Iron Balance in Chronic Kidney Disease: How Much is Too Much and How to Tell? Am J Nephrol. 2018;47(2):72–83. doi: 10.1159/000486968. [DOI] [PubMed] [Google Scholar]
- G, T., Renal anemia: a nephrologist’s view. HIPPOKRATIA 2011: p. 39-43. [PMC free article] [PubMed]
- Hayat A., Haria D., Salifu M.O. Erythropoietin stimulating agents in the management of anemia of chronic kidney disease. Patient Preference and Adherence. 2008;2:195–200. doi: 10.2147/ppa.s2356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reddy G.C., Devaki R., Rao P. IRON INDICES IN PATIENTS WITH FUNCTIONAL ANEMIA IN CHRONIC KIDNEY DISEASE. The Journal of the International Federation of Clinical Chemistry and Laboratory Medicine. 2013;24:3–4. [PMC free article] [PubMed] [Google Scholar]
- Fishbane S. Reticulocyte hemoglobin content in the evaluation of iron status of hemodialysis patients. Kidney Int. 1997;52(1):217–222. doi: 10.1038/ki.1997.323. [DOI] [PubMed] [Google Scholar]
- Corp., I., IBM SPSS Statistics for Windows, Version 19.0, 2010, IBM Corp.: Armonk, NY:.
- Karagülle Mustafa. Clinical Signifi cance of Reticulocyte Hemoglobin Content in the Diagnosis of Iron Defi ciency Anemia. Turk J Hematol. 2013;30:153–156. doi: 10.4274/Tjh.2012.0107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukui, Y., et al., Reticulocyte hemoglobin content as a marker of iron status in patients receiving maintenance hemodialysis. Clin Exp Nephrol (2002) 6:147–153, 2002. 6: p. 147-153. [DOI] [PubMed]
- Kim J.M., Ihm C.H., Kim H.J. Evaluation of reticulocyte haemoglobin content as marker of iron deficiency and predictor of response to intravenous iron in haemodialysis patients. Int J Lab Hematol. 2008;30(1):46–52. doi: 10.1111/j.1751-553X.2007.00901.x. [DOI] [PubMed] [Google Scholar]
- Miyata K.M.A.H.Y. Assessment of iron deficiency in chronic hemodialysis patients: investigation of cutoff values for reticulocyte hemoglobin content. Clin Exp Nephrol. 2003;7:52–57. doi: 10.1007/s101570300007. [DOI] [PubMed] [Google Scholar]
- Buttarello M. Diagnosis of iron deficiency in patients undergoing hemodialysis. Am J Clin Pathol. 2010;133(6):949–954. doi: 10.1309/AJCPQAX0JFHFS0OA. [DOI] [PubMed] [Google Scholar]
- Urrechaga E., Borque L., Escanero J.F. Potential utility of the new Sysmex XE 5000 red blood cell extended parameters in the study of disorders of iron metabolism. Clin Chem Lab Med. 2009;47(11):1411–1416. doi: 10.1515/CCLM.2009.301. [DOI] [PubMed] [Google Scholar]
- Neal Mittman, M., Rajanna Sreedhara, MD, Robert Mushnick, MD, Jyoti Chattopadhyay, PhD, and P. David Zelmanovic, Mehdi Vaseghi, MD, and Morrell M. Avram, MD, FACP, Reticulocyte Hemoglobin Content Predicts Functional Iron Deficiency in Hemodialysis Patients Receiving rHuEP0. American Journal of Kidney Diseases,, 1997. 30(6): p. 912-922. [DOI] [PubMed]
- GABAY, C. and I. KUSHNER, Acute Phase proteins and other systemic responses to inflammation. The New England Journal of Medicine, 1999. Volume 340 Number 6 · 453: p. 449-454. [DOI] [PubMed]
- Hurrell R.F. Influence of inflammatory disorders and infection on iron absorption and efficacy of iron-fortified foods. Nestle Nutr Inst Workshop Ser. 2012;70:107–116. doi: 10.1159/000337673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saito, H., METABOLISM OF IRON STORES. Nagoya J. Med. Sci, 2014. 76: p. p. 235 ~ 254. [PMC free article] [PubMed]
- Menno P. Kooistra':“, E.C.N., Ad van Es3 and N.M.M.-B. , Albert Struyvenberg/ and Joannes J. M. Marxv”, Iron absorption in erythropoietin-treated haemodialysis patients:effects of iron availability, inflammation and aluminium. Nephrol Dial Transplant 1998. 13: p. 82-88. [DOI] [PubMed]
- Nairz M. Iron deficiency or anemia of inflammation? : Differential diagnosis and mechanisms of anemia of inflammation. Wien Med Wochenschr. 2016;166(13–14):411–423. doi: 10.1007/s10354-016-0505-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wessling-Resnick M. Iron homeostasis and the inflammatory response. Annu Rev Nutr. 2010;30:105–122. doi: 10.1146/annurev.nutr.012809.104804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MJ Kotze1, D.v.V., SJ van Rensburg2, R Erasmus, PATHOGENIC MECHANISMS UNDERLYING IRON DEFICIENCY AND IRON OVERLOAD:NEW INSIGHTS FOR CLINICAL APPLICATION. The Journal of the International Federation of Clinical Chemistry and Laboratory Medicine, 2009. 20(2): p. 108-123. [PMC free article] [PubMed]
- Van Wyck D.B. Iron status in patients receiving erythropoietin for dialysis-associated anemia. Kidney Int. 1989;35(2):712–716. doi: 10.1038/ki.1989.43. [DOI] [PubMed] [Google Scholar]
- Tarng D.-C. Erythropoietin hyporesponsiveness: From iron deficiency to iron overload. Kidney International. 1999;55:S107–S118. [PubMed] [Google Scholar]
- Muñoz M. Efficacy and safety of intravenous iron therapy as an alternative/adjunct to allogeneic blood transfusion. Vox Sang. 2008;94(3):172–183. doi: 10.1111/j.1423-0410.2007.01014.x. [DOI] [PubMed] [Google Scholar]