Abstract
The aim of this study was to investigate the antihyperlipidemic potential of Diosmin (DS) in mice fed with a high-fat diet (HFD). Animals were divided in five groups (n = 6). The total duration of the study was 90 days split into two intervals. During the first 45-day interval, mice were administered with HFD, whereas during the second 45-day interval they were co-administered HFD plus DS or the standard drug atorvastatin. DS was administered at the dose of 100 and 200 mg/kg;p.o. DS treatment to HFD-induced hyperlipidemic mice caused significant decrements in the levels of total cholesterol, triglycerides, LDL-C and VLDL-C. Moreover, DS resulted in significant increase in the levels of HDL-C and improvements in total protein levels, whereas it caused remarkable decreases in SGOT, SGPT and ALP enzymatic activities in hyperlipidemic mice. Histopathological examination of hyperlipidemic mice revealed a disorganized hepatic tissue, fatty changes, and mononuclear cell infiltration, which were all ameliorated by DS administration. The results revealed that DS possesses potential ameliorating benefits again.st hyperlipidemia induced by HFD on lipid profile, liver function enzymes and hepatic histoarchitecture. Further investigations are highly recommended and clinical trials are warranted in order to assess the efficacy and to fully dissect the mode-of-action underpinning the observed antihyperlipidemic effect of DS.
Keywords: Antihyperlipidemic, Diosmin, Lipid profile, Liver function, Hepatic histology, Mice
1. Introduction
Globally, diabetes mellitus (DM) and myocardial infarction and cardiovascular diseases (CVDs) are responsible for significant disease burden and therefore considered the most reported health complication. They are highly associated with hyperlipidemia that is characterized by the increase in serum lipid profile indices, namely triglycerides (TG), low-density lipoprotein cholesterol (LDL-C) and total cholesterol (TC), and thus is considered one of the primary risk factors leading to CVDs. Hyperlipidemia is directly correlated with a prominent metabolic dysregulation in the affected patients (Alamgeer et al., 2014, Ansarullah et al., 2009). It could also result in serious cardiac pathologies such as atherosclerosis, due to the fact that hyperlipidemia is associated with increased serum TG and TC; thus, it has been documented as a prevalent susceptibility marker of atherosclerotic heart disease (Surya et al., 2017). Hence, the abovementioned changes in association with declining high-density lipoprotein cholesterol (HDL-C) levels eventually result in hyperlipidemia, thereby causing initiation of advanced cardiac pathological conditions (Ghule et al., 2006). Moreover, the interfering harmful effect of high-fat diet (HFD) with the process of lipid metabolism in the liver is the primary factor responsible for the development of nonalcoholic fatty liver disease (NAFLD) (Altunkaynak, 2005, Kameshwara et al., 2013). Currently available practical strategies to counter hyperlipidemia include the reduction in both productions of lipids as well as their gut absorption using synthetic therapeutic agents such as statins, fibrates, and bile acid sequestrants. However, the use of these synthetic agents might be manifested with series of negative and recurrent side effects, most notably myopathy, rhabdomyolysis, and elevated risk of gallstone formation. Hence, developing novel and effective antihyperlipidemic therapeutic agents with zero or minimal undesired side effects is urgently required (Aladaileh et al., 2019). The opportunity of treating these variety of pathological conditions using herbal products and medicinal plants is emerging as new rising trend in recent years as they are poised as safer, well-tolerated therapeutic strategy with minimal side effects in comparison to synthetic drugs (Javed et al., 2009). In recent times, a tremendous growing interest has been shown by researchers in nutraceuticals and herbal therapies with proven health benefits, as the World Health Organization (WHO) estimated that population trust rate is around 80% on the usage of herbal medicine in treating various pathological conditions and diseases (Vogel, 2007). Numerous studies have reported the use of medicinal plants in traditional medicine as alternative approach over the past three decades have shown their ability and efficacy to generate reliable data emphasizing the utility of plant-derived products as valuable pharmacological resources (Aladaileh et al., 2019, Ashour et al., 2016, El-Shemy et al., 2009).
Flavonoids are plant-based natural products that exert multiple therapeutic and biological activities including antioxidant and free radical-scavenging effects, as well as antihyperglycemic, anti-inflammatory, anti-atherosclerosis, anticarcinogenic, antihyperammonemia, nephroprotective, and hepatoprotective actions (Mahmoud et al., 2019). Their primary mod-of-action (MOA) is believed to proceed via its potent free-radical scavenging, quenching oxygen and hydrogen-donating antioxidant activities, thereby blocking its determental cellular effects and preventing cell injury. Flavonoids have been reported to exhibit potent direct scavenging activities against reactive oxygen species (ROS) and reactive nitrogen species (RNS), which is ascribed for the presence of OH groups. Its antioxidant capacity has been shown to increase proportional to the number of OHs in the molecular structure (Hernández-Aquino and Muriel, 2018, Mulvihill et al., 2016). In this context, diosmin (DS) is a naturally-occuring flavone glycoside (diosmetin 7-O-rutinoside; PubChem CID 5281613) in the Rutaceae family that is predominantly present in the pericarp of several citrus fruits (Imam et al., 2015). DS have been reported to possess a plethora of pharmacological and therapeutic benefits in various pathological conditions including treatment of cardiovascular diseases, particularly chronic venous insufficiency (CVI) that is defined as a progressive morphological and functional abnormalities of the venous system (Feldo et al., 2018). Moreover, DS has been reported to exert an anti-hyperglycemic effect through the adrenal gland, thereby leading to enhancement of the β-endorphin secretion and attenuation of hepatic gluconeogenesis in streptozotocin (STZ)-induced diabetic rats (Hsu et al., 2017). Moreover, it has also been reported that DS has a favorable effect on delaying cataracts formation in the lenses of STZ-induced diabetic rats, which has been postulated to take place via counteracting the increased oxidative stress associated with diabetic cataractogenesis (Wojnar et al., 2017). Furthermore, DS has been shown to exhibit capacities to stall lipopolysaccharide-induced lung injury via downregulation of tumor necrosis factor alpha (TNF-α) and subunit p65 of nuclear-factor-kappa-B transcription complex (NF-κB-p65) expressions (Imam et al., 2015). Additionally, there are reports that confirm its cytoprotective effects including cardiac protective potential by virtue of its antioxidant and hypolipidemic mechanisms (Queenthy and John, 2013, Senthamizhselvan et al., 2014), as well as demonstrated hepatoprotective actions (Abdel-Reheim et al., 2017, Ali et al., 2018, Hassan et al., 2018) in different models. Besides, recent studies has an ameliorative effect against ulcerative colitis (Shalkami et al., 2018). To this end, non-human primate (NHP) models to study complex diseases are becoming increasingly important and invaluable for the analyses of abnormalities in lipid profile and the underlying metabolic defects manifested as insulin insensitivity that is associated with the development of the characteristic dyslipidemia seen in type 2 DM (Aladaileh et al., 2019, Ansarullah et al., 2009, Ghule et al., 2006, Javed et al., 2009, Kameshwara et al., 2013, Sikarwar and Patil, 2012, Surya et al., 2017). There is an increasing dependence and need for in vivo non-human primates (NHP) models in biomedical research, particularly in studies of metabolic defects such as DM and dyslipidemia because of their genetic and physiological resemblance to humans, and being and ideal mimic for both high cholesterol and HFD-induced hypercholesterolemia and hyperlipidemia (Prior et al., 2017). In most cases, NHPs are the relevant species for testing studies of biotherapeutics, where the use of other non-rodent species is unsuitable, provided that international regulations ensuring the animal safety are withheld and implementing the 3Rs principal (i.e., replacement, refinement and reduction) (Prior et al., 2017).
Thus, based on our current knowledge of the above literature, this study was designed to explore the ameliorative and hepatoprotective effect of DS in hyperlipidemic mousce model. The significance of the results was evaluated by measuring the key lipid profile indices, including total cholesterol, triglycerides, LDL-C, VLDL-C, HDL-C and the liver function enzyme activities of serum glutamic oxaloacetic transaminase (SGOT), serum glutamic pyruvic transaminase (SGPT) and alkaline phosphatase (ALP), and total protein in serum samples collected from hyperlipidemic rats induced by feeding on high-fat diet. Moreover, the outcome of the research conducted was compared with atorvastatin, as a standard cholesterol-lowering drug that blocks the production of cholesterol and with proper controls. We show evidence that DS possesses significant desirable effects on lowering lipid profiles, ameliorating liver functions as well as its histoarchitecture.
2. Materials and methods
2.1. Drugs and chemicals
DS and atorvastatin were obtained from Emcure Pharmaceuticals Ltd., India. The chemicals and diagnostic kits were procured from Span Diagnostics, India. All other chemicals were of analytical grade. HFD pellets were obtained from Inveniolife Technology Pvt. Ltd. The diet contains 19% protein, 17.50% fat, 3.50% fiber, 3.50% ashes, vitamins and minerals. Normal diet was also obtained from the same company which contains 16.50% protein, 8% fiber, 8% ashes, 2% fat, 1%NaCl, vitamins and minerals.
2.2. Acute oral toxicity study
The mice were kept on overnight fasting prior to the drug administration following Organisation for Economic Co-operation and Development (OECD) 423 guidelines for testing of the oral acute toxicity of chemicals (OECD, 2002). Mice were administered with a single oral dose of DS at a dose rate of 2000 mg/kg, b.w. of DS. Post DS treatment, diet was suspended for 3–4 h. This was followed by their strict periodic observation at regular intervals (OECD, 2002).
2.3. Animals Ethics and growth conditions
Adult healthy male Swiss Albino mice with weights in the range of 25–30 g were housed in polypropylene cages under standard lighting (12 h day/night cycle) and relative humidity (50 ± 15%) conditions in temperature-controlled rooms (22 ± 1 °C) as previously described (Aboul-Soud et al., 2011). Animals were housed in sanitized polypropylene cages and there were allowed free access to feed on standard mice pellet diet and water ad libitum. All animals were allowed to acclimatize to the abovementioned laboratory environment for 10 days prior to the actual treatments. All the experimental usage of animals was carried out as per the guidelines of Committee for the Purpose of Control And Supervision of Experiments on Animals (CPCSEA) and the experimental protocol was approved by the Institutional Animal Ethics Committee (IAEC) (Ref. No. F4/CIPT/ADMIN/2018-19/004).
2.4. Experimental design
Thirty normal healthy male Swiss Albino mice were kept separately in 5 random groups (n = 6).
Group 1: Control diet + 0.3% of aqueous carboxymethylcellulose (CMC, as vehicle)
Group 2: HFD + 0.3% CMC (vehicle)
Group 3: HFD + Atorvastatin (10 mg/kg; p.o.)
Group 4: HFD + DS (100 mg/kg; p.o.)
Group 5: HFD + DS (200 mg/kg; p.o.)
The total duration of the study was 90 days. During the first 45-day interval, animals of all groups were treated with HFD, whereas during the consequent 45-day interval animals were administered HFD along with either the standard cholesterol-lowering drug atorvastatin or DS (Jain et al., 2010, Lu et al., 2010, Sikarwar and Patil, 2012).
2.5. Blood collection
Mice were kept fasting for 24 hrs and blood samples were extracted via retro-orbital sinus puncture. This was followed by centrifugation at 2500 rpm for 15 min. The obtained serum was used for the biochemical analysis of lipid profile indices and liver function enzymes.
2.6. Biochemical and histopathological studies
The standard diagnostic kits were used for estimation of lipid profile indices as well as liver function enzymes including serum total cholesterol, total triglyceride, LDL, VLDL, HDL, SGOT, SGPT, serum ALP, and total protein levels. At the end of the experimental period and after blood sampling, animals were sacrificed under anesthesia and the liver was removed as previously described (Aboul-Soud et al., 2011). For histopathological examination, the hepatic tissue samples were first rinsed in phosphate-buffered saline (PBS), then fixed in 10% neutral buffered formalin, standardized dehydration in ascending grades of alcohol, and paraffin-embedding procedures were conducted. Microtomy of paraffin-embedded ultrathin sections (5 µm thickness) was carried out and slides were stained by hematoxylin-eosin (H & E) dyes. The sections were analyzed using an Olympus light microscope (Olympus, BX51, Tokyo, Japan) with an attached photograph machine (Olympus E-330, Olympus Optical Co. Ltd.) Five slides were examined for each liver and sections were screened for liver injury and hepatotoxicity.
2.7. Statistical analyses
Results were expressed as Mean ± SEM. The statistical analyses were performed by GraphPad prism 8.0 software. The one-way analysis of variance (ANOVA) test was utilized for the comparisons. This was followed by multiple comparisons with Dunnett’s Multiple Comparisons Test using Graph Pad Prism version 8.1. P < 0.05 signified statistically significant result.
3. Results and discussion
3.1. Acute oral dose toxicity
In the current study, safety of the acute oral dose toxicity for diosmin (DS) was confirmed at a dose rate of 2000 mg/kg; p.o, as no mortality was observed throughout the course of the entire experiment of 90 days. Hence, dose ranges of 200 and 100 mg/kg; p.o. was selected for this investigation.
3.2. Effects of DS on lipid profile in hyperlipidemic mice
Abnormal lipid profiles are one of the most prevalent pathological complications in DM that are estimated to be present in 40% of diabetic patients. DM is characterized by hyperglycaemia, hypercholesterolemia and hypertriglyceridemia caused by defects in insulin secretion superseded by dysfunctional organ performances and failures particularly heart and arteries, kidneys eyes and neurons (Duckworth et al., 2009). Hyperlipidemia is a reflection of an increase in cholesterol level, both total cholesterol (TC) and low-density lioporotein (LDL), which plays an essential role in the production of reactive oxygen species (ROS) leading to a subsequent oxidative stress (OS) and the stimulation of the lipid peroxidation. Moreover, the elevated levels of ROS result in severe cellular damage that is primarily caused by the oxidation of cellular components, including DNA and mitochondrial membrane (Jain et al., 2010, Lu et al., 2010, Yazdanparast et al., 2008). OS has been documented to play a pivotal role in the pathophysiology and progression of diverse human diseases including CVI, CVD, and DM (Feldo et al., 2018, Mahmoud et al., 2019, Mahmoud et al., 2012).
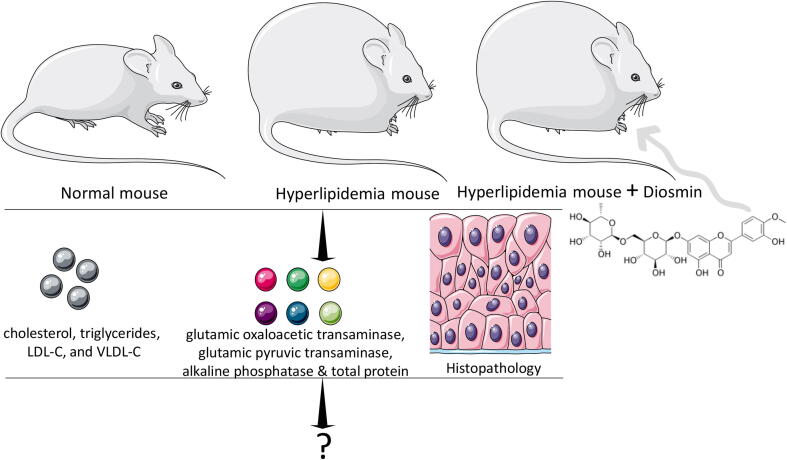
To this end, the proved potent antioxidant properties of natural flavonoids such as DS have been shown to prevent and/or attenuate the pathological development for adverse conditions (Feldo et al., 2018, Mahmoud et al., 2019). Moreover, risks for onset of advance cardiac pathologies like atherosclerosis is also associated with chronic hyperlipidemia (Martins and Redgrave, 2004, Mbikay, 2012). In this present study, the consequences of hyperlipidemia were followed in mice fed with high-fat diet (HFD) by analyzing serum TC, triglycerides (TG), LDL-C, and VLDL-C was monitored as outlined in the Fig. 1.
Fig. 1.
Representation for the drug treatment and analysis with hyperlipidemia. Both normal and hyperlipidemia mice are shown.
For the lipid profile analysis, the levels of TC, TG, HDL-C, LDL-C and VLDL-C were monitored and results were depicted in Table 1.
Table 1.
Effect of DS on lipid profile in HFD induced hyperlipidemia in mice.
| Groups | Treatment | Total Cholesterol (mg/dL) | Triglycerides (mg/dL) | HDL-C (mg/dL) | LDL-C (mg/dL) | VLDL-C (mg/dL) |
|---|---|---|---|---|---|---|
| I | Normal Control | 59.22±0.5 | 65.838±1.1 | 37.754±0.2 | 8.31±0.5 | 13.164±0.2 |
| II | HFD Control | 200.04±0.7a | 101.872±7.7a | 20.83±1.1a | 158.846±1.9a | 20.372±1.5a |
| III | HFD + Atorvastatin (10 mg/kg;p.o) | 129.12±3.3b | 72.118±7.0b | 32.15±1.1b | 82.552±5.1b | 14.418±1.4b |
| IV | HFD + DS (100 mg/kg;p.o) | 153.26±7.0b | 67.576±3.1b | 29.628±0.3c | 110.13±7.4b | 13.51±0.6b |
| V | HFD + DS (200 mg/kg;p.o) | 133.57±6.8b | 64.018±3.2b | 32.23±1.2b | 88.544±6.1b | 12.798±0.6b |
Values are expressed as mean ± SEM (n = 6). ap < 0.01 considered statistically significant as compared to normal control group; bp < 0.01 and cp < 0.05 considered statistically significant when compared to HFD control group.
The proper controls were followed with the normal mouse, HFD control and to compare the DS treatment, with a standard cholesterol-lowering drug, atorvastatin was administrated to mice. The decrement in the total cholesterol was noticed at comparable rate with atorvastatin (10 mg) in the mice groups administered with 200 mg of DS. Interestingly, with 100 mg of DS also significant level of reduction in the total cholesterol was found. These results were further supported by the improved TG, HDL-C, LDL-C and VLDL-C levels as compared to hyperlipidemia in mice. Overall, the treatment with DS significantly reversed the changes recorded in mice fed with HFD. HFD caused significant (ap < 0.01) increase in serum total cholesterol, triglycerides, LDL-C, and VLDL-C and decrease in HDL-C. Treatment with DS resulted in significant (bp < 0.01) decline in total cholesterol, triglycerides, LDL-C, and VLDL-C. DS treatment was also resulted in significant rise in the levels of HDL-C. Standard drug atorvastatin showed a more prominent effect than DS (Table 1). Moreover, the rise in HDL-C was revealed in a dose-depended manner and administration of DS at a dose of 200 mg/kg; p.o. outshined DS at the dose rate of 100 mg/kg; p.o.
3.3. Effects of DS on SGOT, SGPT, ALP, and total protein in hyperlipidemia mice model
Liver function enzymatic activities and total protein were monitored in control and HFD mice treated along with either atorvastatin or DS at different doses for comparison (Table 2). In HFD-induced hyperlipidemia mice group, a significant rise in liver function enzymatic activities (SGOT, SGPT, and ALP) was observed. Evidently, significant and normalizing effects have been noticed with the administration of DS and comparable with the atorvastatin-treated group. An equal performance of DS (at 200 mg) with atorvastatin was at dose of 20-fold higher than the level of atorvastatin (10 mg). Moreover, A significant effect was also registered with the 100 mg of DS, however, 200 mg was shown to be the perfect dose against hyperlipidemia. Interestingly, SGPT and ALP activities were found to be better respond to the lowering effect of DS than atorvastatin. Treatment with DS (100 and 200 mg/kg;p.o) showed a significant (cp < 0.01) decrease in SGOT and SGPT levels. ALP enzyme activities also showed significant (dp < 0.05) decrease upon treatment with DS. On the other hand, a decrease in the total protein level was observed in HDF induced hyperlipidemic mice that however, got reversed upon treatment with DS. Further, treatment with atorvastatin also improved the levels of SGOT, SGPT, ALP, and total protein in HFD induced hyperlipidemia in mice (Table 2).
Table 2.
Effect of DS on SGOT, SGPT, ALP, and total protein in HFD induced hyperlipidemia in mice.
| Groups | Treatment | SGOT (IU/L) | SGPT (IU/L) | ALP (KA/100 ml) | Total protein (gm/dL) |
|---|---|---|---|---|---|
| I | Normal Control | 24.256 ± 1.0 | 23.67 ± 1.0 | 4.71 ± 0.3 | 7.824 ± 0.3 |
| II | HFD Control | 50.64 ± 1.6a | 61.984 ± 0.9a | 8.742 ± 2.0b | 6.41 ± 0.1 |
| III | HFD + Atorvastatin (10 mg/kg;p.o.) | 33.098 ± 1.5c | 35.249 ± 2.5c | 4.256 ± 0.4d | 8.354 ± 0.7 |
| IV | HFD + DS (100 mg/kg;p.o.) |
38.778 ± 2.2c | 41.348 ± 4.0c | 5.55 ± 0.5 | 8.294 ± 0.7 |
| V | HFD + DS (200 mg/kg;p.o.) |
32.26 ± 3.4c | 29.836 ± 2.7c | 2.742 ± 0.5c | 8.256 ± 0.6 |
Values are expressed as mean ± SEM (n = 6). ap < 0.01 and bp < 0.05 considered statistically significant as compared to normal control group; cp < 0.01 and dp < 0.05 considered statistically significant when compared to the HFD control group.
3.4. Histopathological examination of liver tissues
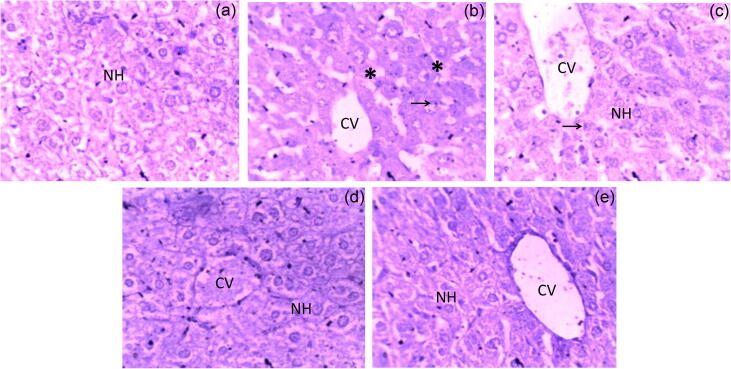
The hepatic tissue samples of control mice showed central vein, bile duct and hepatocytes arranged in the cords, whereas in HFD-induced hyperlipidemic mice it showed disorganized hepatic tissue, fatty changes, and condensed nuclei (Fig. 2). Mice treated with atorvastatin exhibited improvement in cellular histoarchitectural morphology, but hepatocytes with condensed nuclei were observed, whereas DS-treated mice acquired distinct quassi-normal histopathology profile (Fig. 2).
Fig. 2.
Histopathological examination of mice liver tissues (H&E stain × 400). (a) Normal control; (b) HFD control; (c) HFD + Atorvastatin (10 mg/kg;p.o.); (d) HFD + DS (100 mg/kg;p.o.); (e) HFD + DS (200 mg/kg;p.o.). (NH – Normal hepatocytes, CV – Central vein, * – Fatty Changes, and → Condensed nucleus).
The prevalence rate of NAFLD globally ranges from 10 −35%, where it depends on various population studies and their approach using different diagnostic tools. Meanwhile, the incidence rate in United States is reported to be 75%, which is highest among all chronic liver diseases (El-Sheekh et al., 2014). It has been documented, by earlier studies, that HFD is associated with abnormalities in lipid profile indices and metabolism, and is considered the primary causative factor accountable for the progression to NAFLD (Altunkaynak, 2005, Čonková et al., 2001, Kameshwara et al., 2013). Our results revealed that mice fed with HFD for 90 days exhibited hepatic mutilation, as evidenced by biochemical and histopathology results. However, treatment with DS showed a transitory or momentary reduction in the activities of the enzymes, confirming a protective potential of DS against hyperlipidemia. Taken together, it could be concluded from above results that DS possess potent antihyperlipidemic activity and also hepatoprotective properties as evidenced by the attenuated HFD-induced NAFLD.
Hyperlipidemia is a condition of increased total cholesterol, low and very low density lipoprotein cholesterol level, as this situation is associated with disorders in lipid metabolism which indeed leads to cardiovascular diseases. The World Health Organization (WHO) has already reported that 40% of the world population is suffering from high plasma cholesterol levels, which is alarming the danger bells as it is regarded as the most important cause of the mortality (Organization, 2019). Meta-analysis work has documented that around 23.6% of the general African adult population is suffering from dyslipidemia condition (Noubiap et al., 2018), whereas, it has been reported that 31% of the Asian population are at high risk due to hyperlipidemia (Poh et al., 2018).
Indeed, there are several treatment options and medications are in use to treat this conditions but are limited to chemical drugs. Today, it’s trending to use herbal medicine as an alternative treatment which has long history of usage and coming up with more options in treatment methods. Recently, Chinese scientific researchers have come up with positive reports revealing interventional targets for treating hyperlidemia and postulated fours different lipid-lowering mechanisms-of-action of bioactive compounds derived from herbal medicine (Ji et al., 2019). Recently, plant products and their derivatives have been extensively employed as precious resource for medical and pharmaceutical agents in diverse therapeutic protocols both in experimental animals and in humans (Ashour et al., 2016, El-Shemy et al., 2009, Mahmoud et al., 2019, Mahmoud et al., 2012). However, limited knowledge about the toxicity and the pronounced side effects observed for ethnomedical herbal products highlighting the urgent need for studies primarily focusing on the purified active principals as they exhibit lesser toxicity with more pharmacological values compared with their crude unpurified counterparts. In this context, conforming with our findings on DS, the antioxidant activity of hesperidin, another flavonoid that is mainly present in lemons and oranges, has been reported against hyperglycemia-induced OS in HFD/streptozotocin-induced diabetic rats (Mahmoud et al., 2012). Hespiridin has been reported to significantly lowered lipid peroxidation and stimulated the antioxidant capacity in the form of reduced glutathione (GSH), vitamin C, and vitamin E, as well as instigated a notable enhancement in the activity of antioxidant enzymes in type 2 diabetic rats (Mahmoud et al., 2012). A recent study has reported that the use of DS in combination with hesperidin alleviates exhibits notable neuroprotective and antihyperalgesic effects in rats, suggesting that this combination can be used in treating experimental chronic neuropathic pain (Carballo-Villalobos et al., 2016). A recent study on spirulina, biomass of cyanobacteria (blue-green algae) known to be used as a dietary supplement worldwide, have reported a significant beneficial effect on lowering total cholesterol, triglycerides, systolic and diastolic blood pressure, and more notably have been associated with elevation in HDL levels (Torres-Duran et al., 2007).
4. Conclusion
The current study was performed to assess the antihyperlipidemic potential of DS in mice fed with HFD for the first 45 days and the consequent 45 days were fed with HFD plus the drugs. The DS administration to hyperlipidemic mice displayed a significant improvement in investigated lipid profile parameters including total cholesterol, triglycerides, LDL-C, VLDL-C and HDL-C in HFD. Moreover, an improvement has been recorded in liver enzyme activities (serum glutamic oxaloacetic transaminase, serum glutamic pyruvic transaminase, and alkaline phosphatase) and total protein in the serum. Furthermore, evidence was obtained from histopathological examination that the displayed disorganized hepatic tissue, fatty changes, and mononuclear cell infiltration exhibited in HFD-fed mice could be ameliorated by DS administration. In conclusion, beneficial antihyperlipidemic and hepatoprotective effects of DS that were comparable to that obtained with atorvastatin, the standard cholesterol-lowering drug that blocks the production of cholesterol, have been reported.
Acknowledgment
Authors are grateful to the Researchers Supporting Project No. (RSP-2020-161) King Saud University, Riyadh, Saudi Arabia.
Footnotes
Peer review under responsibility of King Saud University.
Contributor Information
Sayeed Mohammed Firdous, Email: firdous.oncology@gmail.com.
Mourad A.M. Aboul-Soud, Email: maboulsoud@ksu.edu.sa.
References
- Abdel-Reheim M.A., Messiha B.A.S., Abo-Saif A.A. Hepatoprotective effect of diosmin on iron-induced liver damage. Int. J. Pharmacol. 2017;13:529–540. doi: 10.3923/ijp.2017.529.540. [DOI] [Google Scholar]
- Aboul-Soud M.A.M., Al-Othman M.A., El-Desoky G.E., Al-Othman Z.A., Kareem Y., Javed A., Al-Khedhairy A.A. Hepatoprotective effects of vitamin E/selenium against malathion-induced injuries on the antioxidant status and apoptosis-related gene expression in rats. J. Toxicol. Sci. 2011;36:285–296. doi: 10.2131/jts.36.285. [DOI] [PubMed] [Google Scholar]
- Aladaileh S.H., Saghir S.A.M., Murugesu K., Sadikun A., Ahmad A., Kaur G., Mahmoud A.M., Murugaiyah V. Antihyperlipidemic and antioxidant effects of averrhoa carambola extract in high-fat diet-fed rats. Biomedicines. 2019;7 doi: 10.3390/biomedicines7030072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alamgeer, Ghuffar A., Ahmad T., Mushtaq M.N. Antihyperlipidemic effect of Berberis orthobotrys in hyperlipidemic animal models. Bangladesh J. Pharmacol. 2014;9:377–382. doi: 10.3329/bjp.v9i3.19922. [DOI] [Google Scholar]
- Ali F.E.M., Azouz A.A., Bakr A.G., Abo-youssef A.M., Hemeida R.A.M. Hepatoprotective effects of diosmin and/or sildenafil against cholestatic liver cirrhosis: the role of Keap-1/Nrf-2 and P38-MAPK/NF-κB/iNOS signaling pathway. Food Chem. Toxicol. 2018;120:294–304. doi: 10.1016/j.fct.2018.07.027. [DOI] [PubMed] [Google Scholar]
- Altunkaynak Z. Effects of high fat diet induced obesity on female rat livers (a histochemical study) Eur. J. Gen. Med. 2005;2:100–109. [Google Scholar]
- Ansarullah A., Jadeja R.N., Thounaojam M.C., Patel V., Devkar R.V., Ramachandran A.V. Antihyperlipidemic potential of a polyherbal preparation on triton WR 1339 (Tyloxapol) induced hyperlipidemia: a comparison with lovastatin. Int. J. Green Pharm. 2009;3 [Google Scholar]
- Ashour A.E., Ahmed A.F., Kumar A., Zoheir K.M.A., Aboul-Soud M.A., Ahmad S.F., Attia S.M., Abd-Allah A.R.A., Cheryan V.T., Rishi A.K. Thymoquinone inhibits growth of human medulloblastoma cells by inducing oxidative stress and caspase-dependent apoptosis while suppressing NF-κB signaling and IL-8 expression. Mol. Cell. Biochem. 2016;416:141–155. doi: 10.1007/s11010-016-2703-4. [DOI] [PubMed] [Google Scholar]
- Carballo-Villalobos A., González-Trujano M., Pellicer F., López-Muñoz F. Antihyperalgesic effect of hesperidin improves with diosmin in experimental neuropathic pain. Biomed Res. Int. 2016;2016 doi: 10.1155/2016/8263463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Čonková E., Laciaková A., Pástorová B., Seidel H., Kováč G. The effect of zearalenone on some enzymatic parameters in rabbits. Toxicol. Lett. 2001;121:145–149. doi: 10.1016/S0378-4274(01)00312-5. [DOI] [PubMed] [Google Scholar]
- Duckworth W., Abraira C., Moritz T., Reda D., Emanuele N., Reaven P.D., Zieve F.J., Marks J., Davis S.N., Hayward R., Warren S.R., Goldman S., McCarren M., Vitek M.E., Henderson W.G., Huang G.D. Glucose control and vascular complications in veterans with type 2 diabetes. N. Engl. J. Med. 2009;360:129–139. doi: 10.1056/NEJMoa0808431. [DOI] [PubMed] [Google Scholar]
- El-Sheekh M.M., Hamad S.M., Gomaa M. Protective effects of Spirulina on the liver function and hyperlipidemia of rats and human. Brazilian Arch. Biol. Technol. 2014;57:77–86. doi: 10.1590/S1516-89132014000100012. [DOI] [Google Scholar]
- El-Shemy H., Aboul-Soud M., Nassr-Allah A., Aboul-Enein K., Kabash A., Yagi A. Antitumor properties and modulation of antioxidant enzymes activity by aloe vera leaf active principles isolated via supercritical carbon dioxide extraction. Curr. Med. Chem. 2009;17:129–138. doi: 10.2174/092986710790112620. [DOI] [PubMed] [Google Scholar]
- Feldo M., Woźniak M., Wójciak-Kosior M., Sowa I., Kot-Waśik I., Aszyk J., Bogucki J., Zubilewicz T., Bogucka-Kocka A. Influence of diosmin treatment on the level of oxidative stress markers in patients with chronic venous insufficiency. Oxid. Med. Cell. Longev. 2018;2018 doi: 10.1155/2018/2561705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghule B.V., Ghante M.H., Saoji A.N., Yeole P.G. Hypolipidemic and antihyperlipidemic effects of Lagenaria siceraria (Mol.) fruit extracts. Indian J. Exp. Biol. 2006 [PubMed] [Google Scholar]
- Hassan A., Thabet N., Abdel-Rafei M. Hyaluronan as a mediator for the hepatoprotective effect of diosmin/hesperidin complex - PubMed. Pak. J. Pharm. Sci. 2018;31:1191–1201. [PubMed] [Google Scholar]
- Hernández-Aquino E., Muriel P. Beneficial effects of naringenin in liver diseases: molecular mechanisms. World J. Gastroenterol. 2018 doi: 10.3748/wjg.v24.i16.1679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsu C.C., Lin M.H., Cheng J.T., Wu M.C. Antihyperglycaemic action of diosmin, a citrus flavonoid, is induced through endogenous β-endorphin in type I-like diabetic rats. Clin. Exp. Pharmacol. Physiol. 2017;44:549–555. doi: 10.1111/1440-1681.12739. [DOI] [PubMed] [Google Scholar]
- Imam F., Al-Harbi N.O., Al-Harbi M.M., Ansari M.A., Zoheir K.M.A., Iqbal M., Anwer M.K., Al Hoshani A.R., Attia S.M., Ahmad S.F. Diosmin downregulates the expression of T cell receptors, pro-inflammatory cytokines and NF-κB activation against LPS-induced acute lung injury in mice. Pharmacol. Res. 2015;102:1–11. doi: 10.1016/j.phrs.2015.09.001. [DOI] [PubMed] [Google Scholar]
- Jain P.G., Patil S.D., Haswani N.G., Girase M.V., Surana S.J. Atividade hipolipidemica de Moringa oleifera Lam., Moringaceae, na hiperlipidemia induzida por dieta rica em gordura em ratos albinos. Brazilian J. Pharmacogn. 2010;20:969–973. doi: 10.1590/S0102-695X2010005000038. [DOI] [Google Scholar]
- Javed I., Zia-Ur-Rahman, Khan M.Z., Muhammad F., Aslam B., Iqbal Z., Sultan J.I., Ahmad I. Antihyperlipidaemic efficacy of Trachyspermum ammi in albino rabbits. Acta Vet. Brno. 2009;78:229–236. doi: 10.2754/avb200978020229. [DOI] [Google Scholar]
- Ji X., Shi S., Liu B., Shan M., Tang D., Zhang W., Zhang Y., Zhang L., Zhang H., Lu C., Wang Y. Bioactive compounds from herbal medicines to manage dyslipidemia. Biomed. Pharmacother. 2019 doi: 10.1016/j.biopha.2019.109338. [DOI] [PubMed] [Google Scholar]
- Kameshwara S., Jothimaniv R., Senthilkum R., Kothai A.R. Anti-obesity and hypolipidemic activity of methanol extract of tecoma stans flowers on atherogenic diet induced obesity in rats. Pharmacologia. 2013;4:77–81. doi: 10.5567/pharmacologia.2013.77.81. [DOI] [Google Scholar]
- Lu B., Xia D., Huang W., Wu X., Zhang Y., Yao Y. Hypolipidemic effect of bamboo shoot oil (P. pubescens) in Sprague-Dawley rats. J. Food Sci. 2010;75:H205–H211. doi: 10.1111/j.1750-3841.2010.01716.x. [DOI] [PubMed] [Google Scholar]
- Mahmoud A.M., Ashour M.B., Abdel-Moneim A., Ahmed O.M. Hesperidin and naringin attenuate hyperglycemia-mediated oxidative stress and proinflammatory cytokine production in high fat fed/streptozotocin-induced type 2 diabetic rats. J. Diabetes Complications. 2012;26:483–490. doi: 10.1016/j.jdiacomp.2012.06.001. [DOI] [PubMed] [Google Scholar]
- Mahmoud, A.M., Hernández Bautista, R.J., Sandhu, M.A., Hussein, O.E., 2019. Beneficial effects of citrus flavonoids on cardiovascular and metabolic health. Oxid. Med. Cell. Longev. https://doi.org/10.1155/2019/5484138. [DOI] [PMC free article] [PubMed]
- Martins I.J., Redgrave T.G. Obesity and post-prandial lipid metabolism. Feast or famine? J. Nutr. Biochem. 2004 doi: 10.1016/j.jnutbio.2003.10.006. [DOI] [PubMed] [Google Scholar]
- Mbikay, M., 2012. Therapeutic potential of Moringa oleifera leaves in chronic hyperglycemia and dyslipidemia: A review. Front. Pharmacol. 3 MAR. https://doi.org/10.3389/fphar.2012.00024. [DOI] [PMC free article] [PubMed]
- Mulvihill E.E., Burke A.C., Huff M.W. Citrus flavonoids as regulators of lipoprotein metabolism and atherosclerosis. Annu. Rev. Nutr. 2016 doi: 10.1146/annurev-nutr-071715-050718. [DOI] [PubMed] [Google Scholar]
- Noubiap J.J., Bigna J.J., Nansseu J.R., Nyaga U.F., Balti E.V., Echouffo-Tcheugui J.B., Kengne A.P. Prevalence of dyslipidaemia among adults in Africa: a systematic review and meta-analysis. Lancet. Glob. Heal. 2018;6:e998–e1007. doi: 10.1016/S2214-109X(18)30275-4. [DOI] [PubMed] [Google Scholar]
- OECD, 2002. Test No. 423: Acute Oral toxicity - Acute Toxic Class Method. Oecd Guidel. Test. Chem. 1–14. https://doi.org/10.1787/9789264071001-en.
- Organization, W.H., 2019. WHO | Cholesterol. Global Health Observatory (GHO) data. WHO.
- Poh K.K., Ambegaonkar B., Baxter C.A., Brudi P., Buddhari W., Chiang F.T., Horack M., Jang Y., Johnson B., Lautsch D., Sawhney J.P.S., Vyas A., Yan B.P., Gitt A.K. Low-density lipoprotein cholesterol target attainment in patients with stable or acute coronary heart disease in the Asia-Pacific region: results from the Dyslipidemia International Study II. Eur. J. Prev. Cardiol. 2018;25:1950–1963. doi: 10.1177/2047487318798927. [DOI] [PubMed] [Google Scholar]
- Prior H., Sewell F., Stewart J. Overview of 3Rs opportunities in drug discovery and development using non-human primates. Drug. Discov. Today Dis. Model. 2017 doi: 10.1016/j.ddmod.2017.11.005. [DOI] [Google Scholar]
- Queenthy S.S., John B. Diosmin exhibits anti-hyperlipidemic effects in isoproterenol induced myocardial infarcted rats. Eur. J. Pharmacol. 2013;718:213–218. doi: 10.1016/j.ejphar.2013.08.031. [DOI] [PubMed] [Google Scholar]
- Senthamizhselvan O., Manivannan J., Silambarasan T., Raja B. Diosmin pretreatment improves cardiac function and suppresses oxidative stress in rat heart after ischemia/reperfusion. Eur. J. Pharmacol. 2014;736:131–137. doi: 10.1016/j.ejphar.2014.04.026. [DOI] [PubMed] [Google Scholar]
- Shalkami A.S., Hassan M.I.A., Bakr A.G. Anti-inflammatory, antioxidant and anti-apoptotic activity of diosmin in acetic acid-induced ulcerative colitis. Hum. Exp. Toxicol. 2018;37:78–86. doi: 10.1177/0960327117694075. [DOI] [PubMed] [Google Scholar]
- Sikarwar M.S., Patil M.B. Antihyperlipidemic activity of Salacia chinensis root extracts in triton-induced and atherogenic diet-induced hyperlipidemic rats. Indian J. Pharmacol. 2012;44:88–92. doi: 10.4103/0253-7613.91875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Surya S., Arun Kumar R., Carla B., Sunil C. Antihyperlipidemic effect of Ficus dalhousiae miq. stem bark on Triton WR-1339 and high fat diet-induced hyperlipidemic rats. Bull. Fac Pharmacy, Cairo Univ. 2017;55:73–77. doi: 10.1016/j.bfopcu.2016.10.003. [DOI] [Google Scholar]
- Torres-Duran P.V., Ferreira-Hermosillo A., Juarez-Oropeza M.A. Antihyperlipemic and antihypertensive effects of Spirulina maxima in an open sample of mexican population: a preliminary report. Lipids Health Dis. 2007;6 doi: 10.1186/1476-511X-6-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vogel, C., 2007. Medicinal Plants in Tropical Countries. Traditional UseExperience Facts By Markus S. Mueller, M.D. (German Institute for Medical Mission) and Ernst Mechler, Ph.D. (University of Tuebingen). Thieme, New York. 2005. viii + 168 pp. 16 × 24 cm. $109.95. ISBN 1-58890-253-6. J. Nat. Prod. 70, 1068–1068. https://doi.org/10.1021/np0781458.
- Wojnar W., Kaczmarczyk-Sedlak I., Zych M. Diosmin ameliorates the effects of oxidative stress in lenses of streptozotocin-induced type 1 diabetic rats. Pharmacol. Reports. 2017;69:995–1000. doi: 10.1016/j.pharep.2017.04.005. [DOI] [PubMed] [Google Scholar]
- Yazdanparast R., Bahramikia S., Ardestani A. Nasturtium officinale reduces oxidative stress and enhances antioxidant capacity in hypercholesterolaemic rats. Chem. Biol. Interact. 2008;172:176–184. doi: 10.1016/j.cbi.2008.01.006. [DOI] [PubMed] [Google Scholar]