Abstract
Cellular elements of maturing brain are vulnerable to insults, which lead to neurodevelopmental defects. There are no established treatments at present. Here we examined the efficacy of selective adenosine A2A receptor inhibitor SCH58261 to combat brain injury, particularly oligodendrocyte (OL) lineage cells, in young rats. Wistar rats (n = 24, 6.5 days old) were randomly divided into equal groups of four. The sham (SHAM) group received no treatment, the vehicle (VEHICLE) group received 0.1% dimethylsufoxide, the injury (INJ) group was exposed to oxygen-glucose deprivation insult, and the injury+SCH58261 (INJ+SCH58261) group was exposed to the insult and received 1 μM SCH58261. Immunocytochemical experiments revealed that there was a significant reduction in the populations of mature OL (MBP+ OLs) and immature OL precursors (NG2+ OPCs) in the INJ group compared to SHAM group. Furthermore, there was also a significant increase in the percent of apoptotic MBP+ OL and NG2+ OPC populations as evidenced by TUNEL assay. In addition, there was a significant reduction in the proliferation rate among NG2+ OPCs, which was confirmed by BrdU immunostaining. On the other hand, treatment with SCH58261 significantly enhanced survival, evidenced by the reduction in apoptotic indices for both cell types, and it is preserved the NG2+ OPC proliferation. Activation of adenosine A2A receptors may contribute to OL lineage cell loss in association with decreased mitotic behavior of OPCs in neonatal brains upon injury. Future investigations assessing ability of SCH58261 to regenerate myelin will provide insights into its wider clinical relevance.
Keywords: Adenosine A2A receptor, SCH58261, Neuroprotection, Oligodendrocyte, Apoptosis
1. Introduction
Brain injury often disrupts energy metabolism in neuronal cells, leading to dramatic increases in extracellular levels of adenosine (Sperlágh et al., 2000, Melani et al., 2003, Melani et al., 2009, Chen et al., 2014, Chen et al., 2019). An inhibitory neurotransmitter, adenosine modulates blood flow in the brain (Dunwiddie and Masino, 2001, Illes et al., 2020), and four distinct G-protein coupled receptors (A1, A2A, A2B and A3) are known to mediate its influence on brain tissues (Fredholm et al., 2001, Melani et al., 2009, Chen et al., 2014). Notably, the A2A receptor may play an important role in the damage incurred by strokes (Chen et al., 2007, Melani et al., 2009, Vincenzi et al., 2020) as one contemporary investigation revealed that transgenic mice lacking the functional version of this receptor experienced less brain damage than their healthy counterparts (Gui et al., 2009), and a related study found they were spared from harm to the striatum and cortex following transient occlusion of the middle cerebral artery (Melani et al., 2003).
The various cellular elements of developing brain are separately under ischaemic attack (Tekkök et al., 2007). The types of glia which are most vulnerable to injury are those of the OL lineage cells (Matute et al., 2007). OL express Ca2+-permeable GluRs and have low resistance to oxidative stress, two main factors that make them particularly vulnerable to injury (Back et al., 1998, Fern and Moller, 2000, Dewar et al., 2003, Wilke et al., 2004). Damage to OPCs and/or mature OLs results in a loss of appropriate myelination (Arai and Lo, 2009). However, myelinating OLs can repair myelin after injury-induced demyelination, and myelin repair derives from recruitment and differentiation of an endogenous population of OPCs (Baumann and Pham-Dinh, 2001, Levine et al., 2001, Mctigue et al., 2001, Suyama et al., 2007, Rivers et al., 2008). The lineage of OLs traverses many distinct steps, from OPC expressing a characteristic set of markers including PDGFαR and the proteoglycan NG2, to mature OLs expressing the myelin genes: myelin associated glycoprotein (MAG), myelin oligodendrocyte glycoprotein (MOG), myelin basic protein (MBP), 2′-3′-cyclic nucleotide 3′ phosphohydrolase (CNP), and proteolipid protein (PLP) and their product proteins (Levine et al., 2001, Mctigue et al., 2001, Rivers et al., 2008). Modulation of the OL pathway is multifactorial, and transcription factors such as bHLH Olig1 and Olig2, and homeodomain Nkx2.2 play crucial roles during OPC differentiation and remyelination (Fancy et al., 2004, Lin et al., 2006, Ligon et al., 2006). Therefore, analysis of pharmacological agents promoting myelin regeneration would need to study their effects on proliferation of OPCs, myelin gene expression, and transcription factors that modulate OL development and myelination (Nicolay et al., 2007).
In recent years, antagonists of the A2A receptor have helped to minimise the severity or side effects of brain injuries by reducing release of excitatory amino acids that accelerate ischaemic and post-ischaemic cell death (Xu et al., 2016). For example, SCH58261 has consistently succeeded in attenuating the outflow of glutamate from the striatum in rats (Corsi et al., 2000, Chiodi et al., 2016), selective A2A receptor agonists have been shown to stimulate its release from the cortex under ischaemic conditions (Calabresi et al., 2000, Beggiato et al., 2016), and treatment with DMPX has preserved neurons in the hippocampus (Latini and Pedata, 2001, Yu-Liang et al., 2016). For this study, we investigated how inhibition of the A2A receptor, using SCH58261, might impact the viability of OLs in rats after a transient deprivation of oxygen and glucose.
Among the adenosine receptors cloned so far, A1, A2A and A3 receptors are shown to mediate neuroprotective effects of adenosine (Fredholm et al., 2001, Melani et al., 2009, Chen et al., 2014). The protective effects of A1 adenosine receptor against cerebral hypoxia–ischaemia is well established, while those of A2A and A3 remains controversial (Latini and Pedata, 2001, Pedata et al., 2005, Pedata et al., 2007, Yu-Liang et al., 2016, Kratimenos et al., 2017, Zhou et al., 2019). Recently it was shown that striatal and cortical damage, and neurological deficit induced by transient middle cerebral artery occlusion were absent in A2A receptor knock-out mice demonstrating the pivotal role of A2A receptors in the incidence of ischemic and hypoxic damage (Melani et al., 2003).
2. Materials and methods
2.1. Animals and treatment details
A total of 24 immature Wistar rats, aged 6.5 days and weighing 34.2 ± 2.6 g, were used in this study. They were housed in a ventilated facility with an ambient temperature (26 ± 2 °C) and 12-hour light/dark cycles. All rats had free access to a standard diet and water.
Prior to the experiments, animals were randomly divided into equal groups of four. The sham (SHAM) group received no treatment, the vehicle (VEHICLE) group received 0.1% dimethylsufoxide (DMSO) (Sigma, Germany), the injury (INJ) group was exposed to 20 min of oxygen-glucose deprivation (OGD) and the injury + SCH58261 (INJ + SCH58261) group was exposed to 20 min of OGD and received 1 μM SCH58261 (7-(2-phenylethyl)-5-amino-2-(2-furyl)-pyrazole-[4,3-e]-1,2,4-triazolo[1,5-c]pyrimidine, Tocris Bioscience, Germany). All research was performed according to regulations by the Research Ethics Committee at the Biotechnology Research Center of the University of Tripoli, Libya (approval number: BEC-BTRC 8–2019).
2.2. Induction of brain tissue injury
Brain tissues for the INJ and INJ+SCH58261 groups were injured via OGD (Al-griw and Solter, 2014, Raffa et al., 2020). In brief, they were subjected to a sterilised, deoxygenated, glucose-free 50% Eagle’s minimum essential medium (Sigma, Germany) in a 37 °C ± 0.5 °C anaerobic chamber with 95% N2/5% CO2 for 20 min. The tissues were then washed at least three times with fresh, oxygenated medium containing 5 mg/mL D-glucose with 2% B27 and returned to their culture conditions under normoxic atmosphere (5% CO2) at 37 °C. Control cultures were maintained under identical conditions. They were further incubated for 72 h at 5% CO2 and 37 °C to mimic in vivo conditions of reperfusion before being fixed for analysis.
2.3. Viability assay
A Live/Dead Viability/Cytotoxicity kit (Molecular Probes, Invitrogen, Germany) was used to evaluate cell survival and death, as previously described (Al-Griw et al., 2014). In brief, tissues were incubated in a solution containing 4 µM ethidium homodimer-AM and 2 µM calcein-AM at 37 °C for 35 min, and then were fixed in phosphate-buffered saline (PBS) (Oxoid, Germany) with 4% paraformaldehyde (PFA) (Sigma, Germany) for 30 min.
2.4. Nuclear staining and cell scoring
Tissue sections were stained with the fluorescent dye, DAPI (4,6-Diamidino-2-Phenylindole, Vector Laboratories, UK), to examine nuclear alterations in cells undergoing apoptosis, as previously described (Bossenmeyer-Pourié et al., 2000, Toriuchi et al., 2020)
2.5. Terminal deoxynucleotidyl transferase dUTP nick end labelling (TUNEL) assay
A TUNEL assay was utilised to evaluate DNA fragmentation (Chemicon, ApopTag Fluorescein in Situ Apoptosis Detection Kit, Germany). Briefly, tissue sections were treated in the dark with a TDT enzyme at 37 °C for one hour. They were then washed with a buffer to terminate the reaction and stained with DAPI. To identify the TUNEL cells, both TUNEL staining and MBP+ or NG2+ immunostaining were performed.
2.6. Immunocytochemistry
Tissue sections from all groups were fixed in 4% PFA at room temperature for one hour. They were then washed with PBS, mounted on glass and incubated in a solution of 10% normal goat serum in PBS (MP Biomedical, UK) and 0.25% Triton X-100 (Sigma, Germany) for one hour. Next, the tissues were incubated with 100 µL of a primary antibody (anti-MBP, MBL, anti-NG2 and anti-OX-42; Millipore, Germany) at room temperature for 90 min or in the dark at 4 °C overnight. They were washed three times with PBS and stained with secondary antibodies (Alexa Fluor® 633 and 488, Invitrogen, Germany) that were diluted in PBS (1:100). Of note, a few tissue sections were processed without primary antibodies in order to examine the specificity of immunolabelling with the antibodies, and this resulted in no immunostaining.
2.7. Proliferation assay
A BrdU (5-bromo-2‘-deoxyuridine) Assay Kit (Aldrich-Sigma) was utilised to assess the mitotic behaviour of neuronal cells. In brief, tissue sections were incubated in a medium containing 20 µM BrdU for 24 h. Next, tissues were fixed and incubated with 1 N HCl on ice for 10 min, incubated with 2 N HCl at room temperature for 10 min, and then placed in an incubator at 37 °C for 20 min. To neutralise the acid, the tissues were incubated in 0.1 M borate buffer (pH of 8.5) at room temperature for 12 min, and then washed three times in a solution of PBS and 1% Triton 100-X. Following this, the tissues were permeabilised in a solution containing 1 M glycine, 5% normal goat serum, PBS, and 1% Triton 100-X for an hour, and then incubated overnight with anti-BrdU mono-antibody (eBioscience) in PBS (1:50). Finally, they were stained with DAPI and double immunostained to examine the phenotype of the new cells.
2.8. Microscopy and cell scoring
For each tissue section, three areas of interest were scanned and imaged using confocal microscopy (Leica, Germany). In z-axis, one µm thickness of 1024 × 1024 pixel sized 30 sections were collected in a single microscopic field with 63X objective lens under pinhole, fixed gain, PMT, and laser power, settings.
To maintain accuracy and compare differences detected by immunostaining, tissue sections were processed together. Flattened image stacks were opened in ImageJ software (NIH, USA), thresholded with constant pixel intensity level settings to a white background and then analysed. All quantifications were performed by an individual blinded to the study.
Five visual fields from each tissue section were analysed, yielding a total of 30 measurements per condition. Cells containing DAPI-stained nuclei were considered immunoreactive and included in the assessment. To avoid the possibility of miscounting the number of cells in each group, the number nuclei in each group was tabulated and compared between groups. Immunoreactive cells and DAPI-labelled pyknotic nuclei were counted in the same fields, and the percentage of immunoreactive cells against the number of nuclei present were indicated as cell count.
2.9. Statistical analyses
Analysis of variance was employed to compare differences in cell viability and death between the groups, followed by Dunnett’s post hoc test to determine which differences were significant. The Kolmogorov-Smirnov test evaluated normality. Values are presented as mean ± standard error of the mean (SEM), derived from at least five independent experiments. All data were analysed using SPSS software (Chicago, IL, USA) and statistical significance was set at p < 0.05.
3. Results
3.1. SCH58261 attenuates neural cell survival post-injury
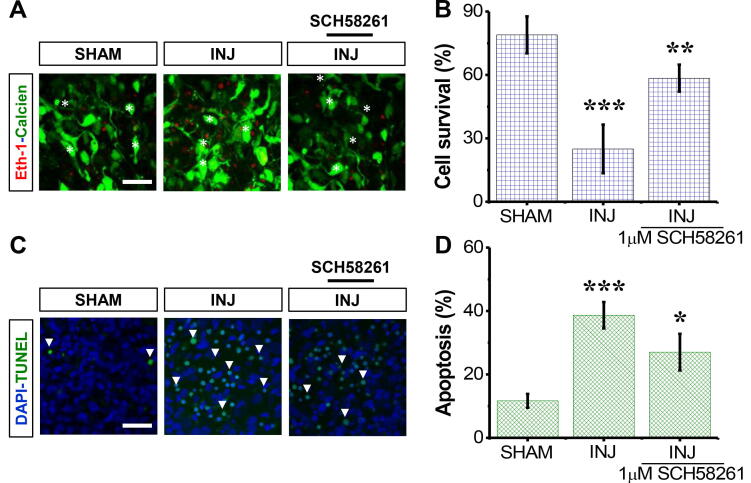
The survival rate for neural cells in the INJ group was 4-fold lower than that of the SHAM group (p < 0.001; Fig. 1A and B); no difference was detected between the SHAM and VEHICLE groups. Treatment with SCH58261 increased the survival of cells in the INJ+SCH58261 group by 71.94 ± 3.47% when compared against the INJ group. Of note, administration of SCH58261 and DMSO (0.1%) had no effect on the survival of cells not exposed to any insult.
Fig. 1.
SCH58261 enhances neural cell survival post-injury. The SHAM group received no treatment, the INJ group was exposed to 20 min of oxygen-glucose deprivation (OGD), and the INJ+SCH58261 group was exposed to 20 min of OGD and received 1 μM SCH58261. (A) Immunofluorescent images of dead (red areas) and living (green areas) cells. (B) Quantification of neural cell survival. (C) Immunofluorescent images of pyknotic nuclei (refer to the white arrows pointing to the dense, brighter nuclei). (D) Quantification of apoptosis. Scale bar: 20 µm. Data are shown as mean ± SEM (n = 6 per group). (*) indicates p < 0.05, (**) indicates p < 0.01, and (***) indicates p < 0.001.
Regarding apoptosis, there was an increase in the percentage of pyknotic nuclei for the INJ group in contrast to the SHAM group (Fig. 1C). Moreover, pyknotic nuclei percent was 2.9-fold higher in the INJ group than the SHAM group (37.99 ± 5.91% vs. 13.91 ± 2.36%, p < 0.001) (Fig. 1D). On the other hand, SCH58261 treatment minimised an increase in pyknotic nuclei counts, which were comparable to those in the SHAM group (Fig. 1D).
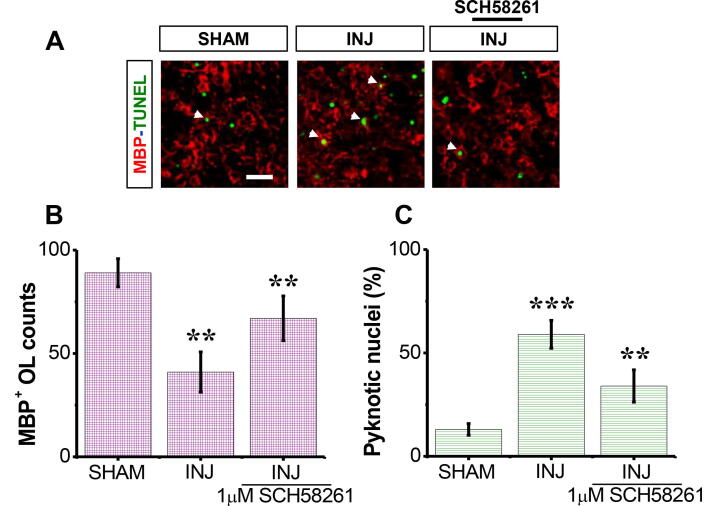
3.2. SCH58261 restores MBP+ OL loss post-injury
Under SHAM condition, there was a high number of mature MBP+ OLs (Fig. 2A). In contrast, there was a roughly 60% (33.2 ± 8.13 cell per filed) reduction (P < 0.01) in the mean number of the MBP+ OLs in INJ group compared to SHAM group (Fig. 2A), but VEHICLE alone had no significant effect when administrated under control condition. There was also a significant elevation (p < 0.001) in the counts of pyknotic nuclei among MBP+ OL population. SCH58261 application showed higher MBP+ OLs 1.9-fold (63.17 ± 6.48 cell per filed) post-insult (Fig. 2B). There was also decrease in the counts of pyknotic nuclei among MBP+ OL population (p < 0.001; Fig. 2C).
Fig. 2.
SCH58261 protects against loss of MBP+ OLs post-injury. The SHAM group received no treatment, the INJ group was exposed to 20 min of OGD, and the INJ+SCH58261 group was exposed to 20 min of OGD and received 1 μM SCH58261. (A) Immunofluorescent images of MBP+ OLs (red areas) and pyknotic nuclei (refer to the white arrows pointing to the dense, brighter green nuclei). (B) Quantification of viable MBP+ OLs. (C) Quantification of apoptotic MBP+ OLs. Scale bar: 10 µm. Data are shown as mean ± SEM (n = 6 per group). (**) indicates p < 0.01 and (***) indicates p < 0.001.
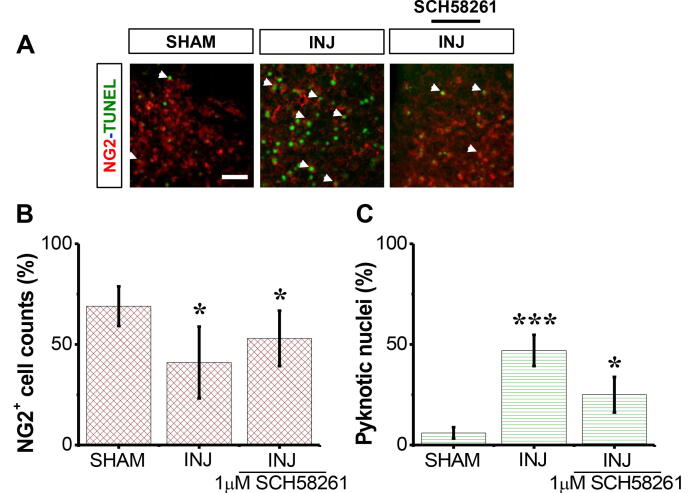
3.3. SCH58261 protects NG2+ OPCs post-injury
The results showed that there was a high number of NG2+ OPCs in the SHAM group compared to INJ group (Fig. 3A). SCH58261 application after injury showed higher NG2+ OPCs 1.9-fold (63.17 ± 6.48 cell per filed) compared to INJ group (Fig. 3B). In addition, there was also a reduction in the percent of pyknotic nuclei among NG2+OPC population from 73.8 ± 5.2% to 3.3 ± 0.7% (Fig. 3C).
Fig. 3.
SCH58261 protects against loss of NG2+ OPCs post-injury. The SHAM group received no treatment, the INJ group was exposed to 20 min of OGD, and the INJ + SCH58261 group was exposed to 20 min of OGD and received 1 μM SCH58261. (A) Immunofluorescent images of NG2+OPCs (red areas) and pyknotic nuclei (refer to the white arrows pointing to the dense, brighter green nuclei). (B) Quantification of viable NG2+ OPCs. (C) Quantification of apoptotic NG2+ OPCs. Scale bar: 10 µm. Data are shown as mean ± SEM (n = 6 per group). (*) indicates p < 0.05 and (***) indicates p < 0.001.
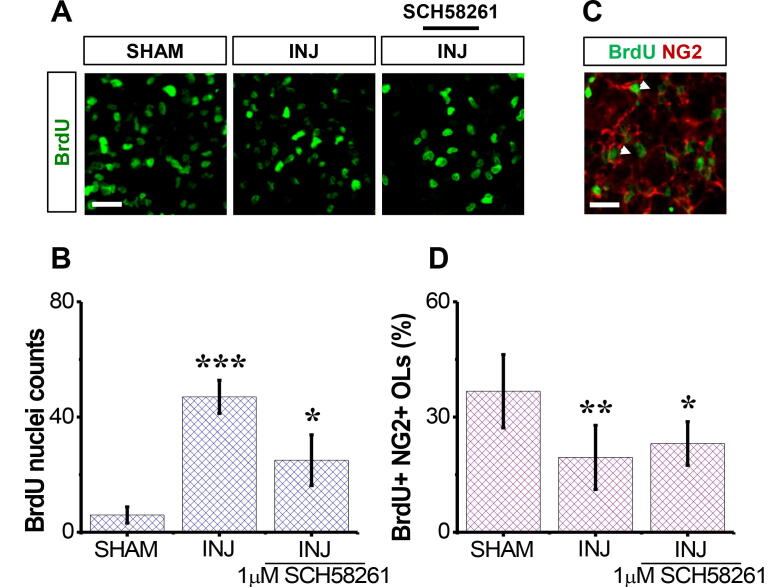
3.4. SCH58261 preserves the mitotic behaviour of NG2+ OPCs post-injury
We next evaluated the SCH58261 effect on neural cell mitotic behavior in response to injury. To this end, BrdU assay was carried out (Fig. 4A). We found that there was a significant (p< 0.001; Fig. 4B) reduction in the population of BrdU+ nuclei/cells in the INJ group compared to SHAM group, and that SCH58261 treatment significantly preserved their density (p< 0.05, Fig. 4B). Because inhibition of adenosine A2A receptor activity with SCH58261 enhanced OPC survival in our model, we postulated that SCH58261 treatment might also affect OPC proliferation. To this end, BrdU-NG2 double staining was carried out. A significant difference in the percentage of proliferating NG2+ OPCs was observed between the INJ and INJ+SCH58261 groups (p = 0.03; Fig. 4C and D), suggesting that SCH58261 preserving the mitotic behaviour of OPCs, and may involve in enhancing remyelination after injury.
Fig. 4.
. SCH58261 preserves the mitotic behaviour in NG2+ cells post-injury. The SHAM group received no treatment, the INJ group was exposed to 20 min of OGD, and the INJ+SCH58261 group was exposed to 20 min of OGD and received 1 μM SCH58261. (A) Immunofluorescent images of NG2+ cells (green areas). (B) Quantification of BrdU+nuclei/cells . (C) Co-stained cultures for BrdU (green areas) and NG2+ OPCs (refer to the white arrows pointing to the red areas). Scale bar: 50 µm. (D) Quantification of proliferating NG2+ cells. Data are shown as mean ± SEM (n = 6 per group). (*) indicates p < 0.05, (**) indicates p < 0.01, and (***) indicates p < 0.001.
4. Discussion
The novel finding from this study was that treatment with the selective A2A receptor inhibitor, SCH58261, following a brain injury helped defend against apoptosis in OLs and enhanced proliferation of their precursors in white matter. To our knowledge, this is the first investigation to show that A2A receptor inhibition helps preserve neural development and viability after injury.
Damage to OLs, evidenced by the emergence of axolemma or occurrence of demyelination, has been observed three hours after induction of middle cerebral artery occlusion in mice and rats (Hase et al., 2018). Consistently, myelinated fibres and OLs seem to be extremely vulnerable to stress and injury, leading them to experience early changes in white matter (Hase et al., 2018). In specific, maturing OLs will extend myelin sheaths around their axons (Naruse, 2019) and participate in stroke-related white matter destruction and axonal conduction (Sasaki et al., 2007, Hwang et al., 2019). Within the demyelination region, surviving OLs will not assist in remyelination since they are not stimulated to divide (Levine et al., 2001, Zhang et al., 2020). Precursors to mature OLs, on the other hand, will respond to insults by increasing their cell division (Skaper et al., 2018). In this study, immunohistochemistry revealed a definitive staining of MBP+ and NG2+ cells in white matter, and the antibodies for their immunolabels were easily detectable (Kuhlmann et al., 2008, Espitia Pinzón et al., 2019). Additionally, a large decrease in the number of pyknotic nuclei was observed, indicating a higher reactivity of OLs to injury. Here we report that under the hypoxic conditions, A2A receptor inhibition led to the apoptosis of OLs. Overactivation of the A2A receptor has been identified on both neurons and microglia in the same mouse model (Trincavelli et al., 2008, Chen et al., 2014), and since glia and neurons are differentially affected by stress or injury, it can be postulated that different regions of the brain will be activated simultaneously in response to ischaemia.
Increased expression of JNK and NF-kB has been reported in OLs, which are major targets in multiple sclerosis (Licht-Mayer et al., 2015). Accordingly, inhibition of JNK by selective inhibitors helps prevent cell death induced by OGD in vitro and in vivo (Melani et al., 2009, Chen et al., 2014). OLs are extremely sensitive to the presence of adenosine and inflammatory cytokines (Back, 2006, Carloni et al., 2020); therefore, JNK activation in response to ischaemia could be attributed to toxicity and oxidative stress. In one study using cultured OLs from the cortex, application of the pan-JNK inhibitor, SP600125, was found to significantly reduce glutamate toxicity (Rosin et al., 2004, Chen et al., 2018). Moreover, it was previously shown that release of glutamate was reduced by an adenosine A2A receptor antagonist within the first hour of ischaemia (Melani et al., 2009). By reducing release of this excitatory amino acid, such antagonists may reduce JNK activation and allow for better functionality and/or survival of pre- and myelinating OLs (Melani et al., 2009, Chen et al., 2014).
Therefore, better functionality and/or survival of mature myelinating OLs, could be attributed to reduced glutamate due to inhibition of JNK activation by A2A receptor antagonists as evident from the normalisation of MAG staining in the ischaemic striatum. Also, protection of developing OL precursors from damage might be a resultant of reduced JNK activation.
5. Conclusions
This study demonstrates that selective A2A receptor inhibitor SCH58261 has significant protective roles upon brain injury in young rats. However, we were incapable of finding the precise mechanism/s by which SCH58261exerts its actions. Nevertheless, our findings imply that some effects may be mediated by preserving survival and mitotic behavior of OPCs.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial or not-for profit sectors.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
Acknowledgments
The authors would like to express their deepest appreciation to the University of Tripoli for supporting this work.
Authors' contributions
M.A.G. conceived, designed and organized the study. M.A.G., R.O.A., N.A., A.A.E., W.S.A. and A.M.A. contributed to the conduct of the study. M.A.G., R.O.A., N.A., A.M.A., N.A.B. and G.S. performed the experiments, M.A.G. analyzed the data. M.A.G., R.O.A., N.A., A.A.E., W.S.A., N.A.B., G.S. and A.M.A. drafted the manuscript and critiqued the output for intellectual content. All authors discussed the results and commented on the manuscript.
Footnotes
Peer review under responsibility of King Saud University.
Supplementary data to this article can be found online at https://doi.org/10.1016/j.sjbs.2020.09.063.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
References
- Al-griw M.A., Elnfati A.H.S., Ali M.M. Gene delivery using adenoviral vector into the organotypic culture system of the chick retinae. J. Basic Appl. Sci. 2014;20(1):28–36. [Google Scholar]
- Al-griw M.A., Solter M. Apoptotic mechanisms increased by GluT Inhibitor DL-TBOA following brain cellular damage. J. Basic Appl. Sci. 2014;20(1):11–20. [Google Scholar]
- Arai K., Lo E. Experimental models for analysis of oligodendrocyte pathophysiology in stroke. Exp. Transl. Stroke Med. 2009;1(1):1–6. doi: 10.1186/2040-7378-1-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Back S.A. Perinatal white matter injury: the changing spectrum of pathology and emerging insights into pathogenetic mechanisms. Dev. Disabil. Res. Rev. 2006;12(2):129–140. doi: 10.1002/mrdd.20107. [DOI] [PubMed] [Google Scholar]
- Back S.A., Gan X., Rosenberg P.A., Volpe J.J. Maturation-dependent vulnerability of oligodendrocytes to oxidative stress-induced apoptosis caused by glutathione. J. Neurosci. 1998;18(16):6241–6253. doi: 10.1523/JNEUROSCI.18-16-06241.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baumann N., Pham-Dinh D. Biology of oligodendrocyte and myelin in the mammalian central nervous system. Physiol. Rev. 2001 doi: 10.1152/physrev.2001.81.2.871. [DOI] [PubMed] [Google Scholar]
- Beggiato S., Tomasini M.C., Borelli A.C., Borroto-Escuela D.O., Fuxe K., Antonelli T., Tanganelli S., Ferraro L. Functional role of striatal A2A, D2, and mG lu5 receptor interactions in regulating striatopallidal GABA neuronal transmission. J. Neurochem. 2016;138(2):254–264. doi: 10.1111/jnc.13652. [DOI] [PubMed] [Google Scholar]
- Bossenmeyer-Pourié C., Koziel V., Daval J.-L. Effects of hypothermia on hypoxia-induced apoptosis in cultured neurons from developing rat forebrain: comparison with preconditioning. Pediatr. Res. 2000;47(3):385–391. doi: 10.1203/00006450-200003000-00017. [DOI] [PubMed] [Google Scholar]
- Calabresi P., Picconi B., Saulle E., Centonze D., Hainsworth A.H., Bernardi G. Is pharmacological neuroprotection dependent on reduced glutamate release? Stroke. 2000;31(3):766–772. doi: 10.1161/01.str.31.3.766. [DOI] [PubMed] [Google Scholar]
- Carloni S., Crinelli R., Palma L., Alvarez F.J., Piomelli D., Duranti A., Balduini W., Alonso-alconada D. The synthetic cannabinoid URB447 reduces brain injury and the associated white matter demyelination after hypoxia-ischemia in neonatal rats. ACS Chem. Neurosci. 2020;11(9):1291–1299. doi: 10.1021/acschemneuro.0c00047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen B.H., Park J.H., Lee Y.L., Kang I.J., Kim D.W., Hwang I.K., Lee C.-H., Yan B.C., Kim Y.-M., Lee T.-K. Melatonin improves vascular cognitive impairment induced by ischemic stroke by remyelination via activation of ERK1/2 signaling and restoration of glutamatergic synapses in the gerbil hippocampus. Biomed. Pharmacother. 2018;108:687–697. doi: 10.1016/j.biopha.2018.09.077. [DOI] [PubMed] [Google Scholar]
- Chen G.-H., Li X.-L., Deng Y.-Q., Zhou F.-M., Zou W.-Q., Jiang W.-X., Shangguan S.-Q., Lu Z.-N. The molecular mechanism of EPO regulates the angiogenesis after cerebral ischemia through AMPK-KLF2 signaling pathway. Crit. Rev. Eukaryot. Gene Expr. 2019;29:2. doi: 10.1615/CritRevEukaryotGeneExpr.2019029018. [DOI] [PubMed] [Google Scholar]
- Chen J.-F., Sonsalla P.K., Pedata F., Melani A., Domenici M.R., Popoli P., Geiger J., Lopes L.V., De Mendonca A. Adenosine A2A receptors and brain injury: broad spectrum of neuroprotection, multifaceted actions and “fine tuning” modulation. Prog. Neurobiol. 2007;83(5):310–331. doi: 10.1016/j.pneurobio.2007.09.002. [DOI] [PubMed] [Google Scholar]
- Chen Z., Xiong C., Pancyr C., Stockwell J., Walz W., Cayabyab F.S. Prolonged adenosine A1 receptor activation in hypoxia and pial vessel disruption focal cortical ischemia facilitates clathrin-mediated AMPA receptor endocytosis and long-lasting synaptic inhibition in rat hippocampal CA3-CA1 synapses: differential regulation of GluA2 and GluA1 subunits by p38 MAPK and JNK. J. Neurosci. 2014;34(29):9621–9643. doi: 10.1523/JNEUROSCI.3991-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiodi V., Ferrante A., Ferraro L., Potenza R.L., Armida M., Beggiato S., Pèzzola A., Bader M., Fuxe K., Popoli P. Striatal adenosine–cannabinoid receptor interactions in rats over-expressing adenosine A2A receptors. J. Neurochem. 2016;136(5):907–917. doi: 10.1111/jnc.13421. [DOI] [PubMed] [Google Scholar]
- Corsi C., Melani A., Bianchi L., Pedata F. Striatal A2A adenosine receptor antagonism differentially modifies striatal glutamate outflow in vivo in young and aged rats. NeuroReport. 2000;11(11):2591–2595. doi: 10.1097/00001756-200008030-00048. [DOI] [PubMed] [Google Scholar]
- Dewar D., Underhill S.M., Goldberg M.P. Oligodendrocytes and ischemic brain injury. J. Cereb. Blood Flow Metab. 2003;23(3):263–274. doi: 10.1097/01.WCB.0000053472.41007.F9. [DOI] [PubMed] [Google Scholar]
- Dunwiddie T.V., Masino S.A. The role and regulation of adenosine in the central nervous system. Annu. Rev. Neurosci. 2001;24(1):31–55. doi: 10.1146/annurev.neuro.24.1.31. [DOI] [PubMed] [Google Scholar]
- Espitia Pinzón N., Van Mierlo H., De Jonge J.C., Brevé J.J., Bol J.G., Drukarch B., Van Dam A.-M., Baron W. Tissue transglutaminase promotes early differentiation of oligodendrocyte progenitor cells. Front. Cell. Neurosci. 2019;13:281. doi: 10.3389/fncel.2019.00281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fancy S.P., Zhao C., Franklin R.J. Increased expression of Nkx2.2 and Olig2 identifies reactive oligodendrocyte progenitor cells responding to demyelination in the adult CNS. Mol. Cell. Neurosci. 2004;27(3):247–254. doi: 10.1016/j.mcn.2004.06.015. [DOI] [PubMed] [Google Scholar]
- Fern R., Moller T. Rapid ischemic cell death in immature oligodendrocytes: a fatal glutamate release feedback loop. J. Neurosci. 2000;20(1):34–42. doi: 10.1523/JNEUROSCI.20-01-00034.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fredholm B.B., Ijzerman A.P., Jacobson K.A., Klotz K.-N., Linden J. International union of pharmacology. XXV. Nomenclature and classification of adenosine receptors. Pharmacol. Rev. 2001;53(4):527–552. [PMC free article] [PubMed] [Google Scholar]
- Gui L., Duan W., Tian H., Li C., Zhu J., Chen J.-F., Zheng J. Adenosine A2A receptor deficiency reduces striatal glutamate outflow and attenuates brain injury induced by transient focal cerebral ischemia in mice. Brain Res. 2009;1297:185–193. doi: 10.1016/j.brainres.2009.08.050. [DOI] [PubMed] [Google Scholar]
- Hase Y., Chen A., Bates L.L., Craggs L.J., Yamamoto Y., Gemmell E., Oakley A.E., Korolchuk V.I., Kalaria R.N. Severe white matter astrocytopathy in CADASIL. Brain Patholo. 2018;28(6):832–843. doi: 10.1111/bpa.12621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hwang K., Jung K., Kim I.-S., Kim M., Han J., Lim J., Shin J.E., Jang J.-H., Park K.I. Glial cell line-derived neurotrophic factor-overexpressing human neural stem/progenitor cells enhance therapeutic efficiency in rat with traumatic spinal cord injury. Exp. Neurobiol. 2019;28(6):679. doi: 10.5607/en.2019.28.6.679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Illes P., Rubini P., Ulrich H., Zhao Y., Tang Y. Regulation of microglial functions by purinergic mechanisms in the healthy and diseased CNS. Cells. 2020;9(5):1108. doi: 10.3390/cells9051108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kratimenos P., Koutroulis I., Agarwal B., Theocharis S., Delivoria-Papadopoulos M. Effect of Src kinase inhibition on cytochrome c, Smac/DIABLO and apoptosis inducing factor (AIF) following cerebral hypoxia-ischemia in newborn piglets. Sci. Rep. 2017;7(1):1–10. doi: 10.1038/s41598-017-16983-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuhlmann T., Miron V., Cuo Q., Wegner C., Antel J., Brück W. Differentiation block of oligodendroglial progenitor cells as a cause for remyelination failure in chronic multiple sclerosis. Brain. 2008;131(7):1749–1758. doi: 10.1093/brain/awn096. [DOI] [PubMed] [Google Scholar]
- Latini S., Pedata F. Adenosine in the central nervous system: release mechanisms and extracellular concentrations. J. Neurochem. 2001;79(3):463–484. doi: 10.1046/j.1471-4159.2001.00607.x. [DOI] [PubMed] [Google Scholar]
- Levine J.M., Reynolds R., Fawcett J.W. The oligodendrocyte precursor cell in health and disease. Trends Neurosci. 2001;24(1):39–47. doi: 10.1016/s0166-2236(00)01691-x. [DOI] [PubMed] [Google Scholar]
- Licht-mayer S., Wimmer I., Traffehn S., Metz I., Brück W., Bauer J., Bradl M., Lassmann H. Cell type-specific Nrf2 expression in multiple sclerosis lesions. Acta Neuropathol. 2015;130(2):263–277. doi: 10.1007/s00401-015-1452-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ligon K.L., Fancy S.P., Franklin R.J., Rowitch D.H. Olig gene function in CNS development and disease. Glia. 2006;54(1):1–10. doi: 10.1002/glia.20273. [DOI] [PubMed] [Google Scholar]
- Lin T., Xiang Z., Cui L., Stallcup W., Reeves S.A. New mouse oligodendrocyte precursor (mOP) cells for studies on oligodendrocyte maturation and function. J. Neurosci. Methods. 2006;157(2):187–194. doi: 10.1016/j.jneumeth.2006.04.014. [DOI] [PubMed] [Google Scholar]
- Matute C., Alberdi E., Domercq M., Sanchez-gomez M., Perez-samartin A., Rodriguez-antiguedad A., Perez-cerda F. Excitotoxic damage to white matter. J. Anat. 2007;210(6):693–702. doi: 10.1111/j.1469-7580.2007.00733.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mctigue D.M., Wei P., Stokes B.T. Proliferation of NG2-positive cells and altered oligodendrocyte numbers in the contused rat spinal cord. J. Neurosci. 2001;21(10):3392–3400. doi: 10.1523/JNEUROSCI.21-10-03392.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melani A., Cipriani S., Vannucchi M.G., Nosi D., Donati C., Bruni P., Giovannini M.G., Pedata F. Selective adenosine A2a receptor antagonism reduces JNK activation in oligodendrocytes after cerebral ischaemia. Brain. 2009;132(6):1480–1495. doi: 10.1093/brain/awp076. [DOI] [PubMed] [Google Scholar]
- Melani A., Pantoni L., Bordoni F., Gianfriddo M., Bianchi L., Vannucchi M.G., Bertorelli R., Monopoli A., Pedata F. The selective A2A receptor antagonist SCH 58261 reduces striatal transmitter outflow, turning behavior and ischemic brain damage induced by permanent focal ischemia in the rat. Brain Res. 2003;959(2):243–250. doi: 10.1016/s0006-8993(02)03753-8. [DOI] [PubMed] [Google Scholar]
- Naruse K. Springer; Myelin: 2019. Schwann Cells as Crucial Players in Diabetic Neuropathy. [DOI] [PubMed] [Google Scholar]
- Nicolay D.J., Doucette J.R., Nazarali A.J. Transcriptional control of oligodendrogenesis. Glia. 2007;55(13):1287–1299. doi: 10.1002/glia.20540. [DOI] [PubMed] [Google Scholar]
- Pedata F., Gianfriddo M., Turchi D., Melani A. The protective effect of adenosine A2A receptor antagonism in cerebral ischemia. Neurol. Res. 2005;27(2):169–174. doi: 10.1179/016164105X21913. [DOI] [PubMed] [Google Scholar]
- Pedata F., Melani A., Pugliese A., Coppi E., Cipriani S., Traini C. The role of ATP and adenosine in the brain under normoxic and ischemic conditions. Purinergic Signal. 2007;3(4):299–310. doi: 10.1007/s11302-007-9085-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raffa P., Scattolini V., Gerli M.F.M., Perin S., Cui M., De Coppi P., Elvassore N., Caccin P., Luni C., Urciuolo A. Decellularized skeletal muscles display neurotrophic effects in three-dimensional organotypic cultures. Stem Cells Transl. Med. 2020 doi: 10.1002/sctm.20-0090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rivers L.E., Young K.M., Rizzi M., Jamen F., Psachoulia K., Wade A., Kessaris N., Richardson W.D. PDGFRA/NG2 glia generate myelinating oligodendrocytes and piriform projection neurons in adult mice. Nat. Neurosci. 2008;11(12):1392–1401. doi: 10.1038/nn.2220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosin C., Bates T.E., Skaper S.D. Excitatory amino acid induced oligodendrocyte cell death in vitro: receptor-dependent and-independent mechanisms. J. Neurochem.. 2004;90(5):1173–1185. doi: 10.1111/j.1471-4159.2004.02584.x. [DOI] [PubMed] [Google Scholar]
- Sasaki M., Li B., Lankford K.L., Radtke C., Kocsis J.D. Remyelination of the injured spinal cord. Prog. Brain Res. 2007;161:419–433. doi: 10.1016/S0079-6123(06)61030-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skaper S.D., Barbierato M., Facci L., Borri M., Contarini G., Zusso M., Giusti P. Co-ultramicronized palmitoylethanolamide/luteolin facilitates the development of differentiating and undifferentiated rat oligodendrocyte progenitor cells. Mol. Neurobiol. 2018;55(1):103–114. doi: 10.1007/s12035-017-0722-0. [DOI] [PubMed] [Google Scholar]
- Sperlágh B., Dóda M., Baranyi M., Haskó G. Ischemic-like condition releases norepinephrine and purines from different sources in superfused rat spleen strips. J. Neuroimmunol. 2000;111(1–2):45–54. doi: 10.1016/s0165-5728(00)00365-9. [DOI] [PubMed] [Google Scholar]
- Suyama K., Watanabe M., Sakai D., Osada T., Imai M., Mochida J. Nkx2.2 expression in differentiation of oligodendrocyte precursor cells and inhibitory factors for differentiation of oligodendrocytes after traumatic spinal cord injury. J. Neurotrauma. 2007;24(6):1013–1025. doi: 10.1089/neu.2006.0151. [DOI] [PubMed] [Google Scholar]
- Tekkök S.B., Ye Z., Ransom B.R. Excitotoxic mechanisms of ischemic injury in myelinated white matter. J. Cereb. Blood Flow Metab. 2007;27(9):1540–1552. doi: 10.1038/sj.jcbfm.9600455. [DOI] [PubMed] [Google Scholar]
- Toriuchi K., Kakita H., Tamura T., Takeshita S., Yamada Y., Aoyama M. Prolonged astrocyte-derived erythropoietin expression attenuates neuronal damage under hypothermic conditions. J. Neuroinflammation. 2020;17:1–11. doi: 10.1186/s12974-020-01831-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trincavelli M.L., Melani A., Guidi S., Cuboni S., Cipriani S., Pedata F., Martini C. Regulation of A2A adenosine receptor expression and functioning following permanent focal ischemia in rat brain. J. Neurochem. 2008;104(2):479–490. doi: 10.1111/j.1471-4159.2007.04990.x. [DOI] [PubMed] [Google Scholar]
- Vincenzi F., Pasquini S., Gessi S., Merighi S., Romagnoli R., Borea P.A., Varani K. The detrimental action of adenosine on glutamate-induced cytotoxicity in PC12 cells can be shifted towards a neuroprotective role through A1AR positive allosteric modulation. Cells. 2020;9(5):1242. doi: 10.3390/cells9051242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilke S., Salter M., Thomas P., Allcock N., Fern R. Mechanism of acute ischemic injury of oligodendroglia in early myelinating white matter: The importance of astrocyte injury and glutamate release. J. Neuropathol. Exp. Neurol. 2004;63(8):872–881. doi: 10.1093/jnen/63.8.872. [DOI] [PubMed] [Google Scholar]
- Xu B., Xiao A.-J., Chen W., Turlova E., Liu R., Barszczyk A., Sun C.L., Liu L., Tymianski M., Feng Z.P. Neuroprotective effects of a PSD-95 inhibitor in neonatal hypoxic-ischemic brain injury. Mol. Neurobiol. 2016;53(9):5962–5970. doi: 10.1007/s12035-015-9488-4. [DOI] [PubMed] [Google Scholar]
- Yu-Liang C., Ya-nan Z., Zhong-zhuang W., Wei-gang X., Run-ping L., Jun-dong Z. Effects of adenosine metabolism in astrocytes on central nervous system oxygen toxicity. Brain Res. 2016;1635:180–189. doi: 10.1016/j.brainres.2016.01.026. [DOI] [PubMed] [Google Scholar]
- Zhang N., Liu C., Zhang R., Jin L., Yin X., Zheng X., Siebert H.-C., Li Y., Wang Z., Loers G. Amelioration of clinical course and demyelination in the cuprizone mouse model in relation to ketogenic diet. Food Funct. 2020 doi: 10.1039/c9fo02944c. [DOI] [PubMed] [Google Scholar]
- Zhou Y., Zeng X., Li G., Yang Q., Xu J., Zhang M., Mao X., Cao Y., Wang L., Xu Y. Inactivation of endothelial adenosine A2A receptors protects mice from cerebral ischaemia-induced brain injury. Br. J. Pharmacol. 2019;176(13):2250–2263. doi: 10.1111/bph.14673. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.