Abstract
This study examined the impact of different cooking methods on fatty acid (FAs) composition of shrimp meat and the ability of these foods to protect against high cholesterol (HC) diet-induced non-alcoholic fatty liver disease (NAFLD) in rats. Shrimp were cooked for 10 min boiled, grilled, or fried in sunflower oil. Rats (n = 6/group) were fed a normal diet (ND)or high-cholesterol diet (HC) each containing boiled, grilled or fried shrimp powder (15% w/w) (NDBS, NDFS, NDGS for ND or HCBS, HCFS, HCDGS for HC diet). Frying alone significantly reduced total levels of saturated FAs (SFA) and increased total mono- and polyunsaturated FAs (MSFA, and PUFAs, respectively) in shrimp meat. It also increased levels of n-6 PUFAs and linoleic acid (LA) and decreased levels of n-3 PUFAs including eicosapentaenoic FAs (EPA) and docosahexaenoic fatty acid (DHA). When fed to HC rats, only diets containing the grilled and boiled shrimp powders significantly prevented the weight loss, lowered fasting and glucose levels, improved glucose and insulin tolerance, and prevented the increase in serum liver markers, ALT and AST. They also reduced hepatic fat accumulation, reduced serum levels and hepatic levels of cholesterol and triglycerides (TGs), reduced hepatic levels of MDA, tumor necrosis factor-alpha (TNF-α), and IL-6, and increased those of glutathione (GSH) and superoxide dismutase (SOD). No alterations in all these parameters were observed in HC-fed rats which fed fried shrimp. In conclusion, boiling and grilling but not frying are the best method to cook shrimp to preserve their fatty acid content and its nutritional value in ameliorating NAFLD.
Keywords: Shrimp, Cooked, High-cholesterol, Fatty liver, Rats
1. Introduction
Non-alcoholic fatty liver disease (NAFLD) is the most dominant liver disorder worldwide and clinically identified by intrahepatic accumulation of triglycerides (TGs) (steatosis) with or without inflammation and fibrosis (steatohepatitis, NASH) (Li et al., 2016, Polyzos and Mantzoros, 2016, Polyzos et al., 2019). According to the multiple parallel-hit model environmental (i.e. high-fat diet, lack of exercise, obesity, etc) and genetic factors (i.e. polymorphisms) initiates the first hit (hepatic TGs accumulation) while oxidative stress and inflammation, and cholesterol lipotoxicity (second hit) lead to the progression of the disease non-alcoholic steatohepatitis (Polyzos et al., 2019). However, clinical and experimental studies have shown that NAFLD occurs in obese and non-obese individuals and can be induced using diets rich in high-fat (HFD) or high cholesterol (Kim et al., 2014, Hirsch et al., 2016, Tirosh, 2018).
The interaction between the quality of our diet, liver metabolism, and NAFLD is well established (Kim et al., 2010, Parker et al., 2012, Nseir et al., 2014, Mirmiran et al., 2017). Currently, diets rich in refined carbohydrates, processed meat, cholesterol, saturated fatty acids (SFAs), sugar-sweetened beverages are associated with NAFLD (Simopoulos, 2016; Tirosh, 2018, Asgari-Taee et al., 2019, Polyzos et al., 2019). Also, several studies have confirmed that diet rich in omega-6 polyunsaturated fatty acids (n-6 PUFAs) and poor in omega-3 (n-3 PUFA) (high n-6/n-3 ratio) induces obesity, insulin resistance (IR), NAFLD, and numerous kinds of cardiovascular disease (CVDs) (Simopoulos, 2008; Pachikian et al., 2008, Spadaro et al., 2008, Shapiro et al., 2011, Blasbalg et al., 2011, Parker et al., 2012, Mirmiran et al., 2012, Scorletti and Byrne, 2013; Simopoulos et al., 2016). Due to the well-established hypolipidemic, anti-inflammatory, and antioxidant potential, the current dietary recommendations suggest increasing the intake of n-3 PUFAs such as: eicosapentaenoic fatty acid (EPA) and docosahexaenoic fatty acid (DHA) to prevent metabolic, liver, and CVDs disorders (Scorletti and Byrne, 2013; Simopoulos, 2016; Yang et al., 2019).
The recommended ratio of n-6/n-3 PUFAs in our diet should be, at least, 1:1– 4 (Simopoulos, 2016). Fish (i.e. salmon and sardines) and crustaceans (i.e., shrimps) are major consumable resources of n-3 PUFAs (Scorletti and Byrne, 2013). However, people rarely consume raw seafood and prefer to eat them cooked. Major methods of seafood cooking include boiling, heating, grilling, and frying. However, several experimental studies have demonstrated that method of food processing and preparation (i.e. the time, temperature, and method of cooking) can adversely affect the quality and the valuable biological health benefits of the seafood diet by altering the structure and composition of their fatty acid content and the ratio of n-6/n-3 PUFAs (mainly by oxidation) (Bastías et al., 2017,García-Arias et al., 2003 Gladyshev et al., 2006, Zotos et al., 2013). Within this context, cooking food by boiling, steaming, grilling, and baking but not frying (i.e. in sunflower or corn oil) are among the best cooking methods to preserved the source levels of n-3 PUFAs (Bastías et al., 2017, Bordin et al., 2013, Gladyshev et al., 2006, Naseri et al., 2013, Stephen et al., 2010, Zotos et al., 2013; Choo et al., 2020).
The shrimp represent a major source of seafood diet worldwide. In the Arabian area, the rate of consumption of shrimp, as a dietary food or natural additive (in salad and soup), is relatively high. From a nutritional perspective, the shrimps are characterized by neutral flavor and low-calorie value due to its high water and protein content and low SFAs (Dayal et al., 2013). The shrimp can be eaten usually cooked, grilled, or fried with or without the shell (Dayal et al., 2013). Shrimps are also rich in n-3PUFAs, minerals, vitamins, and antioxidants (Venugopal, 2009, Dayal et al., 2013). However, global awareness is increased concerning the consumption of shrimp due to their high cholesterol content (Dayal et al., 2013). Nonetheless, the anti-obesity, hypolipidemic, and antioxidant effects of different shrimp extracts have been reported in several experimental and animal studies. Indeed, extract from pink shrimps reduced weight gain and serum levels of TGs and cholesterol, and improved glucose homeostasis in HFD-fed mice (Mezzomo et al., 2015). In the same line, dietary intake of shrimps lowered serum and hepatic TGs and cholesterol, as well as LDL-c in normal subjects and animals fed high cholesterol diet (De Oliveira e Silva et al., 1996, Hossain et al., 2007). Furthermore, shrimp oil extracted from the processing wastes (head and tails) improved insulin homeostasis and prevented the development of IR in HFD-rats (Nair et al., 2017).
If Fatty acids (FAs) composition of shrimp is affected by different cooking methods still not investigated yet. Besides, the protective effect of processed (cooked) shrimp on NAFLD in rats was never investigated before. Therefore, this study was designed to achieve two aims. First, to examine the effect of different cooking methods (i.e. boiling, grilling, and frying in sun flower oil) on the content of FAs composition (SFAs, MUFAs, and PUFAs) in shrimp meat. Secondly, to study the metabolic protective effect of all these preparations of shrimp diets in high cholesterol diet-induced NAFLD.
2. Materials and methods
2.1. Shrimp processing cooking yield, and powder preparation
Penaeus semislcats is the most common shrimp strain available to the red sea and the most commonly consumed type in Saudi Arabia. These shrimps (20 kg) were provided freshly from a certified supplier from the wet market of Riyadh, KSA, and was used directly. After fishing and purchase, all shrimp samples were always kept in an icebox filled with ice packs. The average length of shrimp was 8 cm. The legs, shells, and the tails were carefully removed. The resulted meat was processed in the following ways: 1) Grilled shrimp: 500 g of shrimp and grilled on a gas-operated oven (covered with foil) for 10 min (5 min of each side) at 180℃. 2) Fried shrimp: was done using the conventional frying method. In this regard, 500 g of the shrimp meats were added to 1 L of commercially available boiled sunflower oil for 10 min in 2 L stainless steel cooking pan. After frying, the cocked shrimps were soaked by special cooking filter papers. 3) Boiled shrimp: 500 g of shrimp meat was boiled in 1 L boiling water for 10 min in 4 L stainless steel pan. Two kg of shrimp were used for each processing method (500 g/process) (Czech et al., 2015; Choo et al., 2020).
2.2. Preparation of shrimp powder
Shrimps from each group were pooled together, dried at 50° C for 72 h, cooled, and then crushed manually to prepare the powders. These powders were stored in the fridge until further use (Czech et al., 2015).
2.3. Lipid extraction
Extraction of lipids from shrimp (Bligh and Dyer, 1959). In brief, 50 g of shrimp meat was homogenized in methanol/chloroform reagent (2:1, v/v) (60/30 ml) for 2 min followed by the addition of another 30 ml of chloroform. The mixture was allowed to separate and the lower phase containing the lipids were separated and underwent rotatory evaporation. The extracted lipids were preserved in the fridge and used directly for FAs analysis. A similar procedure was done for liver samples obtained from treated rats but the lipids were reconstituted in 500 µl isopropanol and stored at −80℃ until lipid profile analysis (Bligh and Dyer, 1959).
2.4. Determination of fatty acid composition
Initially, the fatty acid methyl esters (FAME) were prepared using by transesterification of extracted oils with methanol (MeOH) as previously shown by (Choo et al., 2020). In brief, 50 mg of the extracted fats from all treatments were soaked in 5 ml of 0.5 M NaOH (in MeOH solution) in a plastic centrifuge tube and boiled for 5 min. The mixture was then cooled and 4 ml of 12% Boron trifluoride diethyl etherate (BF3) (in MeOH) was added to every tube. The tube was then boiled again for 25 min followed by the addition of 2 ml of isocantane to allow separation. For FMAE analysis, a capillary gas chromatography (Model number 7890A, Agilent Technologies, CA, USA) equipped with a 5975C inert MSD mass detector was used. A special column for separation of FAME (Model Number Supelco SPTM 2560; 0.2 μm film thickness, 0.25 mm inner diameter, 100 m length) (Germany) was used. The ready solutions for injection were prepared in dichloromethane (CH2Cl2). Analysis conditions were 1 µl injection volume, 140℃ to 240℃ (initial to final temperature), and heating 4℃/min). All FAs were identified by comparing their retention time with using a standard mixture of 37 FAS FAME mix 47 885-U, Supelco, Germany). Levels of total Fatty acid, SFAs, PUFAs, MUFAs, DHA, and PEA were quantified and presented as mg/100 g or as % of total fat based on the peak area of that FAs in relation to all eluted FAs in the sample or using the calibration curve of the standard mixture.
2.5. Diet preparation
The preparation of the high-cholesterol (HC) diet was adopted from the study of Harb et al. (2019). In brief, cholesterol powder, cholic acid, sheep fat, and corn oil (2%, 1%, 20%, and 2%, respectively) were crushed with the normal diet (AIN-39 M) (Dyets, Bethlehem, PA) and reconstituted in distilled water. Then the resulted mixture was allowed to dry at 30℃. This diet was prepared every week. Besides, the diet of shrimp-treated groups was prepared in the same manner by mixing the desired ratios of the shrimp powders with the designated diet.
2.6. Animal experiments
Forty-eight adult healthy male Wistar albino rats (age 6 week/120 ± 10 g) were provided from the animal facility unit at King Saud University, Riyadh, KSA. The housing condition were humidity of 50–55%, temperature of 22℃, and 12/12 h dark/light. The experimental protocol was approved by the official Review Board at Princess Nourah University, Riyadh, KSA (IRB Number 19–0015). The animals were randomly divided into 8 groups as 1) control rats: fed normal diet (ND), 2) NDBS-fed rats: control rats which received ND containing 15% boiled shrimp powder, 3) NDFS: control rats which were fed ND containing 15% fried shrimp powder, 4) NDGS: control rats that received ND containing 15% grilled shrimp, 5) HC-fed rats: fed HC diet, 6) HCBS: fed HC diet containing 15% boiled shrimp, 7) HCBS: fed HC diet 15% fried shrimp, and 8) HCGS: fed HC diet containing 15% grilled shrimp. All treatments were given to rats for 8 weeks. This period was shown in our preliminary data to induce IR and NAFLD in this rat’s strain under these experimental conditions. Changes in food intake and body weights were recorded weekly.
2.7. Glucose and insulin tolerance
By the end of week 8, oral glucose tolerance test (OGTT) or intraperitoneal insulin tolerant test (IIPTT) were conducted as described by Wong et al. (2015) using single administration of oral glucose solution (2 g/kg) and i.p. administration of insulin (0.75 units/kg) conducting on two different days on 12-h fasted rats. In both tests, blood samples (350 µl) were collected form the tail at different time intervals (0.0, 15, 30, 60, and 120 min) in EDTA containing tubes to collect plasma (1000 × g for 5 min). Plasma samples were stored at −20℃ and used to later to measure glucose and insulin levels using an assay and ELISA kits for rats (Cat. No. 81,693 and Cat. No. 90010, respectively, Crystal Chem, IL, USA). Plasma insulin and glucose levels measured at the 0.0 min were considered fasting levels.
2.8. Serum and tissue collection and processing
Then, the rats were anesthetized (55 mg/kg sodium pentobarbital) (Mohamed et al., 2020). Once anesthesia was confirmed, blood samples (1 ml) were collected by cardiac puncture and centrifuged at 1000 × g to collect serum. This serum was stored at −20℃ until further biochemical analysis. Then, all rats were killed by cervical dislocation and livers samples were directly collected on ice. All liver samples were snap-frozen and stored at −80℃ and used later for lipid extraction and the biochemical analysis (as shown above). For the biochemical evaluation, total liver homogenates were prepared by homogenizing the tissue in ice-cold phosphate buffer saline (PBS) (pH 7.4) and isolating the supernatant (centrifugation 1200 × g, 4℃, 10 min). Other parts were directly fixed in 10% buffered formalin and forwarded for the histology lab for the routine hematoxylin and eosin staining.
2.9. Biochemical determination in the serum and liver
Serum and hepatic levels of total cholesterol and HDL-C were measured by an assay fluorometric kit (Cat. No. STA-384, Cellbiolabs, CA, USA). Hepatic and serum levels of TGs were measured by an enzymatically colorimetric assay kit (Cat. NO. STA-396, Cellbiolabs, CA, USA). Hepatic and serum concentrations of LDL-C were measured using an enzymatic assay kit (Cat. No. 80069, Crystal Chem, CA, USA). Serum levels of Alanine aminotransferase (ALT) and aspartate aminotransferase (AST) were determined using MyBioSource assay kits, (Cat. No. MBS2540581 and Cat. No. MBS2540582, respectively). Hepatic levels of malondialdehyde (MDA) and glutathione (GSH) were measured using a rat’s ELISA kit (Cat. No. MBS738685 and Cat No. MBS265966, MyBioSource, CA, USA). The activity of GPx was measured using rat’s specific double antibody sandwich ELISA kit (Cat. No. MBS774703, Cat. No. MBS738685, MyBioSource, CA, USA). The hepatic activity of SOD was measured in all homogenates using a rat’s specific competitive ELISA kit (Cat. No: MBS036924, MyBioSource, CA, USA). Hepatic levels of CAT were calculated using a rat’s ELISA kit (Cat. No. MBS9712526, MyBioSource, CA, USA). All procedures were done per the manufacture’s instruction. Hepatic levels of tumor necrosis factor-alpha (TNF-α) and interleukin-6 (IL-6) were measured using rats’ ELISA kit (Cat. No. MBS2507393, Cat. No. MBS175908, MyBioSource, CA, USA).
2.10. Liver histology
Initially, all liver samples were fixed in 10% buffered formalin solution for 18 h. Next day, the liver was dehydrated in ethyl alcohol (ascending concentrations70-100%), cleared in xylene, embedded in paraffin wax, cut in a microtome (5 μm), and then stained with hematoxylin and eosin (HE) (Ankle and Joshi, 2011). All photographs were collected under a light microscope and photographed by an independent pathologist who was unaware of the experimental groups.
2.11. Statistical analysis
All data were analyzed using GraphPad Prism (Version 8, Australia). The analysis was performed using 1 way and 2-way ANOVA followed by Tukey’s t-test. Data were considered significantly different at P < 0.05.
3. Results
3.1. Levels of FAs in the raw shrimp
Analysis of total FAs revealed that PUFA is the most dominant FA in raw shrimp meat (354 ± 22.5 mg/100 g), followed by SFA (234 ± 14.3 mg/100 g) and MUFAs (112 ± 9.4 mg/ 100 g). (Table 1). The ratio of total PUFAs/SFA was 1.52 ± 0.44% (Table 1). Also, and as measured per 100 g dry weight, the raw shrimp meat contained 224 ± 13.7 mg total n-3 PUFAs in (85.6 ± 7.4 mg DHA & 132 ± 12.1 mg EPA) and contained 112 ± 14.3 mg n-6 PUFA in which 18.9 ± 3.60 mg are LA (Table 2 and Table 3). This resulted in a ratio of n6/n-3 PUFAs ratio of 0.51 ± 0.07% (Table 3).
Table 1.
Average analysis of total fatty acid content (mg/100 g) of shrimp meat with all various cooking methods.
| Processing | ∑ SFAs | ∑ MUFAs | ∑ PUFAs | PUFA/SFA (%) |
|
|---|---|---|---|---|---|
| Raw | 234 ± 14.3 | 112 ± 9.4 | 354 ± 22.5 | 1.52 ± 0.44 | |
| Boiled | 226 ± 11.5 | 104 ± 12.6 | 338 ± 27.6 | 1.49 ± 0.36 | |
| % of raw | 96.3 ± 8.6 | 92.6 ± 6.7 | 95.1 ± 8.2 | 98.4 ± 4.3 | |
| Fried | 113 ± 15.7ab | 134 ± 8.9ab | 487 ± 33.2ab | 4.33 ± 0.93ab | |
| % of raw | 48.2 ± 4.4ab | 119.3 ± 9.3ab | 138.7 ± 11.5ab | 284.7 ± 12.2ab | |
| Grilled | 202 ± 12.4abc | 100 ± 11.3ac | 331 ± 18.9c | 1.58 ± 0.63c | |
| % of raw | 86. 6 ± 8.4abc | 89.2 ± 7.2ac | 90.3 ± 6.9c | 103.5 ± 9.3c |
Data are presented as mean ± SD. Values were analyzed by 1-way ANOVA and considered significantly different at p < 0.05. (a): significantly different as compared to raw shrimp meat. (b): significantly different as compared to shrimped cooked by boiling. (c): significantly different as compared to shrimp meat cooked by frying. SFAs: Saturated fatty acids. MUFAs: monounsaturated fatty acids. PUFAs: polyunsaturated fatty acids.
Table 2.
Average analysis of omega-3 (n-3) and omega-6 (n-6) polyunsaturated fatty acids total fatty acid (PUFAs) content (mg/100 g) of shrimp meat with all various cooking methods.
| Processing | ∑n-3 PUFAs | ∑ n-6 PUFAs | n-6/n-3 PUFAs(%) | |
|---|---|---|---|---|
| Non | 224 ± 13.7 | 112 ± 14.3 | 0.51 ± 0.07 | |
| Boiled | 216 ± 14.6 | 106.2 ± 9.3 | 0.49 ± 0.05 | |
| % of raw | 96.3 ± 6.7 | 94.6 ± 7.8 | 96.4 ± 8.1 | |
| Fried | 134 ± 8.6ab | 212.4 ± 8.7ab | 1.59 ± 0.32ab | |
| % of raw | 59.6 ± 7.4ab | 189.5 ± 13.3ab | 311.3 ± 16.7ab | |
| Grilled | 196 ± 8.6ac | 104.4 ± 7.9c | 0.53 ± 0.05c | |
| % of raw | 87.6 ± 4.9ac | 92.8 ± 6.9c | 99.5 ± 7.4c |
Data are presented as mean ± SD. Values were analyzed by 1-way ANOVA and considered significantly different at p < 0.05. (a): significantly different as compared to raw shrimp meat. (b): significantly different as compared to shrimped cooked by boiling. (c): significantly different as compared to shrimp meat cooked by frying.
Table 3.
Average analysis of Linoleic acid, docosahexaenoic fatty acid (DHA) and eicosapentaenoic fatty acid (EPA) content (mg/100 g) of shrimp meat with all various cooking methods.
| processing | Linoleic acid (18:2) | DHA (22:6) |
EPA (20:5) |
|
|---|---|---|---|---|
| Non | 18.9 ± 3.6 | 85.6 ± 7.4 | 132 ± 12.1 | |
| Boiled | 17.8 ± 4.3 | 87.3 ± 8.1 | 127.7 ± 7.9 | |
| % of raw | 93.7 ± 5.4 | 102.3 ± 6.4 | 96.1 ± 7.8 | |
| Fried | 57.8 ± 6.7ab | 45.6 ± 6.4ab | 88.5 ± 7.3ab | |
| % of raw | 306 ± 13.2ab | 54.3 ± 5.6ab | 66.7 ± 6.9ab | |
| Grilled | 16.9 ± 3.2c | 87.6 ± 7.4c | 124.4 ± 13.4c | |
| % of raw | 89.4 ± 6.1c | 102.3 ± 4.8c | 94.5 ± 6.9c |
Data are presented as mean ± SD. Values were analyzed by 1-way ANOVA and considered significantly different at p < 0.05. (a): significantly different as compared to raw shrimp meat. (b): significantly different as compared to shrimped cooked by boiling. (c): significantly different as compared to shrimp meat cooked by frying.
3.2. Alteration in FAs composition with different cooking methods
Levels of total SFAs, MUFAs, PUFAs, n-6 PUFAs, n-3 PUFAs, DHA, EPA, and LA, as well as the ratios of PUFAs/SFAs and n-6/n-3 PUFAs, were not significantly different between the raw shrimp meat and boiled shrimp meat (Table 1, Table 2, Table 3). However, grilling only and significantly reduced total levels of SFA (14%), MUFAs (11%), n-3 PUFAs (13%), and LA (11%) but didn’t affect levels of total PUFAs, n-6 PUFAs, DHA, EPA in the processed shrimp meat as compared to raw shrimp meat (Table 1, Table 2, Table 3). Despite these alterations, normal ratios of total PUFAs/SFAs and n-6/n-3 PUFAs, like that of the raw shrimp meat, were seen in the grilled shrimp meat (Table1 and Table 2). On the other hand, frying in sunflower oil significantly reduced SFAs (52%) and increased total MUFAs (19%), total PUFAs (37.5%), and the ratio of total SFAs/PUFAs (2.8 folds) as compared to raw shrimp meat (Table 1). Besides frying decreased the levels of n-3 PUFA (41%), DHA (44%), and the levels of EPA (35%) and increased the levels of n-6 PUFAs (90%) and LA (3 folds) in cooked shrimps as compared to raw shrimp (Table 2 and Table 3). The calculated ratio of n-6 /n-3 PUFAs in fried shrimp was increased by 3.1 when compared to the corresponding ratio in the raw shrimp meat (Table 2).
3.3. Effect of shrimp frying, grilling, and boiling on rats’ bodyweight
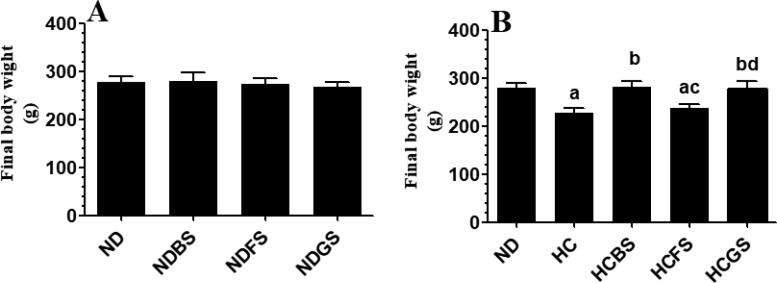
No significant alterations in final body weights were observed in rats fed NDBS, NDFS, and NDGS as compared to rats fed ND after 8 weeks feeding (Fig. 1A). However, the administration of the HC diet for 8 weeks to rats significantly increased rats’ body weights as compared to rats fed ND (Fig. 1B). On the other hand, while HCFS diet didn’t affect rat's body weights, HCBS and HCGS diets significantly increased rats’ body weights as compared to rats fed HCFS diet; where the weights of these rats were not statistically different as compared to ND-fed rats (Fig. 1B).
Fig. 1.
Changes in final body weights among all experimental groups of rats (n = 6/group). Data are presented as mean ± SD. Values were analysed by 2-way ANOVA and considered significantly different at p < 0.05. (a): significantly different as compared to normal diet-fed rats (ND). (b): significantly different as compared to high-cholesterol (HC) fed rats (HCD), (c): significantly different as compared to HC diet containing boiled shrimp meat (HCBS). (d): significantly different as compared to HC diet containing fried shrimp meat (HCFS) HCGS: HC diet containing grilled shrimp meat. NDBS, NDFS, and NDGS correspond to rats fed a normal diet containing boiled, fried, or grilled shrimp meat powder, respectively. For both ND and HC diets, shrimp was mixed at 15% w/w.
3.4. Effect of shrimp frying, grilling, and boiling on the levels of glucose and insulin in the normal and HC-fed rats
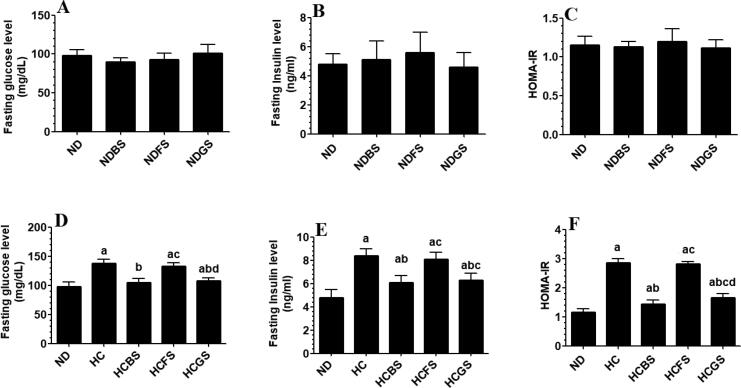
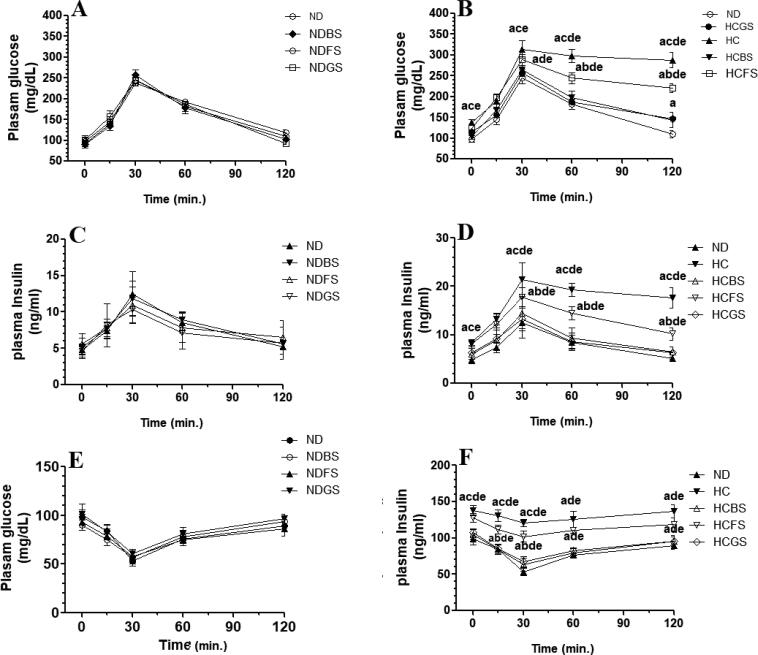
Fasting blood glucose and insulin levels, as well as calculated MOHA-IR index didn’t significantly vary between rats fed NDBS, NDFS, or NDBS but significantly increased in rats fed HC and HCFS diets as compared to ND-fed rats (Fig. 2 A-F). There was no significant difference in the levels of fasting glucose and insulin, as well as levels of HOMA-IR index when rats fed-HC and HCFS diets rats were compared to each other (Fig. 2D–F). However, levels of all these biochemical endpoints were significantly decreased in rats fed the HCBS and HCGS diets as compared to HC diet-fed rats. Also, there were no significant variations in the glucose and insulin levels between the ND, NDFS, NDBS, NDGS-fed rats as measured at 15, 30, 60, 120 min during the OGTT and IPITT (Fig. 3 A, C, E). On the other hand, the HC diet significantly increased the levels of glucose levels in rats during the OGTT and increased both glucose and insulin levels during the IPITT at all measured time intervals as compared to ND-fed rats (Fig. 3B, D, F). Of interest, levels of glucose and insulin levels measured at 15, 30, 60, 120 min during the OGTT and IPITT were significantly decreased in rats fed HCBS and HCGS as compared to rats fed HC diet and their levels at (all these time intervals) were not significantly different as compared to those measured in ND-fed rats (Fig. 3B, D, F). Nonetheless, rats fed HCFS diet showed a slight but a significant decrease in glucose and insulin levels at 30, 60, 120 min during the OGTT and IPITT as compared to rats fed HC diet but their levels remained significantly higher than those measured in ND, NDBS, NDGS-fed rats (Fig. 3B, D, F).
Fig. 2.
Changes in glucose levels after the oral glucose tolerance test (OGTT) (A&B) and in glucose and insulin levels after the intraperitoneal insulin tolerance test (IPITT) (C-F) in all experimental groups of rats (n = 6/group). Data are presented as mean ± SD. Values were analyzed by 2-way ANOVA and considered significantly different at p < 0.05. (a): significantly different as compared to normal diet-fed rats (ND). (b): significantly different as compared to high-cholesterol (HC) fed rats (HCD), (c): significantly different as compared to HC diet containing boiled shrimp meat (HCBS). (d): significantly different as compared to HC diet containing fried shrimp meat (HCFS) HCGS: HC diet containing grilled shrimp meat. NDBS, NDFS, and NDGS correspond to rats fed a normal diet containing boiled, fried, or grilled shrimp meat powder, respectively. For both ND and HC diets, shrimp was mixed at 15% w/w.
Fig. 3.
Fasting levels of glucose and insulin, as well as ration of calculated homeostatic Model Assessment of Insulin Resistance (glucose X insulin/405) in both rats fed-standard diet (A-C) or high-cholesterol diet (HC) (D-F) containing processed shrimps powder. Changes in glucose levels after the oral glucose tolerance test (OGTT) (A&B) and in glucose and insulin levels after the intraperitoneal insulin tolerance test (IPITT) (C-F) in all experimental groups of rats (n = 6/group). Data are presented as mean ± SD. Values were analyzed by 2-way ANOVA and considered significantly different at p < 0.05. (a): significantly different as compared to normal diet-fed rats (ND). (b): significantly different as compared to high-cholesterol (HC) fed rats (HCD), (c): significantly different as compared to HC diet containing boiled shrimp meat (HCBS). (d): significantly different as compared to HC diet containing fried shrimp meat (HCFS) HCGS: HC diet containing grilled shrimp meat. NDBS, NDFS, and NDGS correspond to rats fed a normal diet containing boiled, fried, or grilled shrimp meat powder, respectively. For both ND and HC diets, shrimp was mixed at 15% w/w.
3.5. Effect of shrimp frying, grilling, and boiling on liver architectures in the normal and HC-fed rats
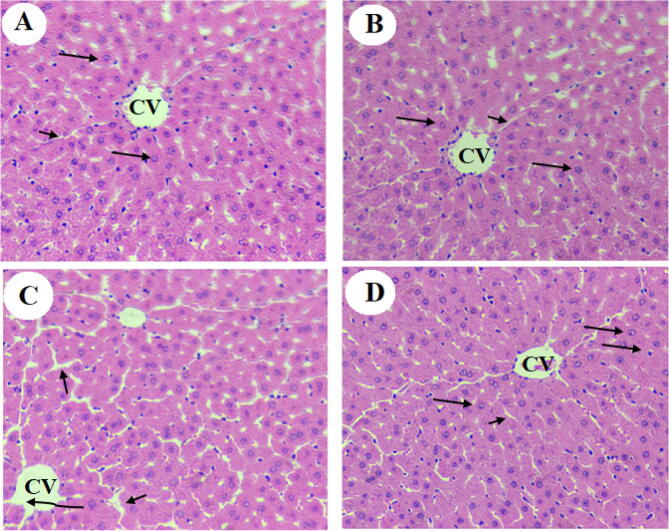
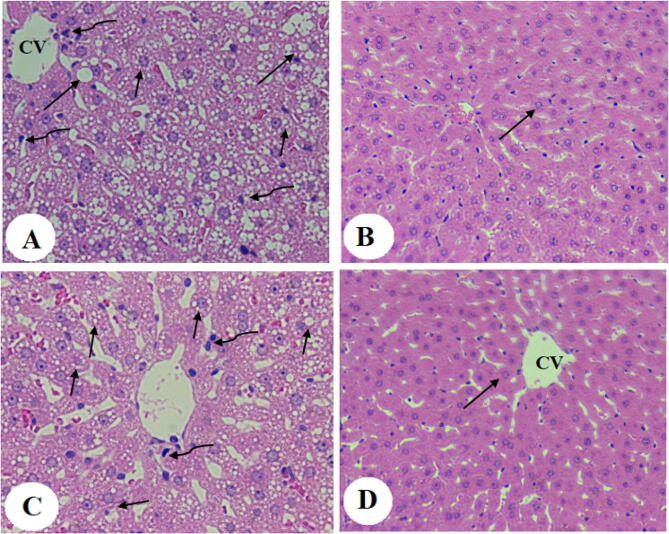
Liver architectures with an intact central vein normally sized rounded euchromatic nuclei radiating from the central vein, and sinusoids were observed in rats fed ND, NDBS, and NDGS. Although they had intact hepatocytes and almost normal hepatocytes organization, livers obtained from NDFS showed slight damage in the central vein with dilated sinusoids (Fig. 4A–D). On the other hand, disorganized swollen hepatocytes with increased accumulation of fat droplet of all sizes (small, medium, and large), and infiltration of leukocytes were observed in the liver of both HC and HCFS-fed rats but were not seen in the livers of HCBS and HCGS-fed rats (Fig. 5A–D). In fact, the liver of both HCBS and HCGS-fed rats had a normal structure similar to ND-fed rats (Fig. 5A–D).
Fig. 4.
Photomicrographs from livers obtained from rats fed a normal diet (ND) (A), normal diet containing boiled shrimp powder (NDBS) (B), normal diet containing fried shrimp powder (NDFS), and a normal diet containing grilled shrimp powder (NDGS). Photos from ND, NDBS, and NDGS showed normal liver architectures with intact normal size central vein (CV) and sinusoids (short arrow) with normally sized rounded euchromatic nuclei hepatocytes (long arrow) radiating from the CV. In C (NDFS-fed rats), the liver showed slight damage in the CV (Curved arrow) with slightly dilated sinusoids (short arrow) and intact hepatocytes. 200X.
Fig. 5.
Photomicrographs from liver obtained from rats fed high-cholesterol (HC) diet (HCD) (A), HC containing boiled shrimp powder (HCBS) (B), HC diet containing fried shrimp powder (HCFS), and HC diet containing grilled shrimp powder (HCGS). Livers obtained from rats fed HC diet showed accumulation of fat granules of small, medium, and large sizes (long arrow) with an abundancy of welled hepatocytes (short arrow). The liver from rats fed HCFS diet showed little improvement with the abundancy of fat droplets (Long size) of all sizes and swelled hepatocytes (short arrow). Also, they showed abnormally dilated sinusoids. The liver of rats fed either HCBS or HCGS showed almost normal architecture like the rats fed a normal diet. Fat droplets in both groups barely seen. 200X.
3.6. Effect of shrimp frying, grilling, and boiling on serum and hepatic lipid profile in normal and HC-fed rats
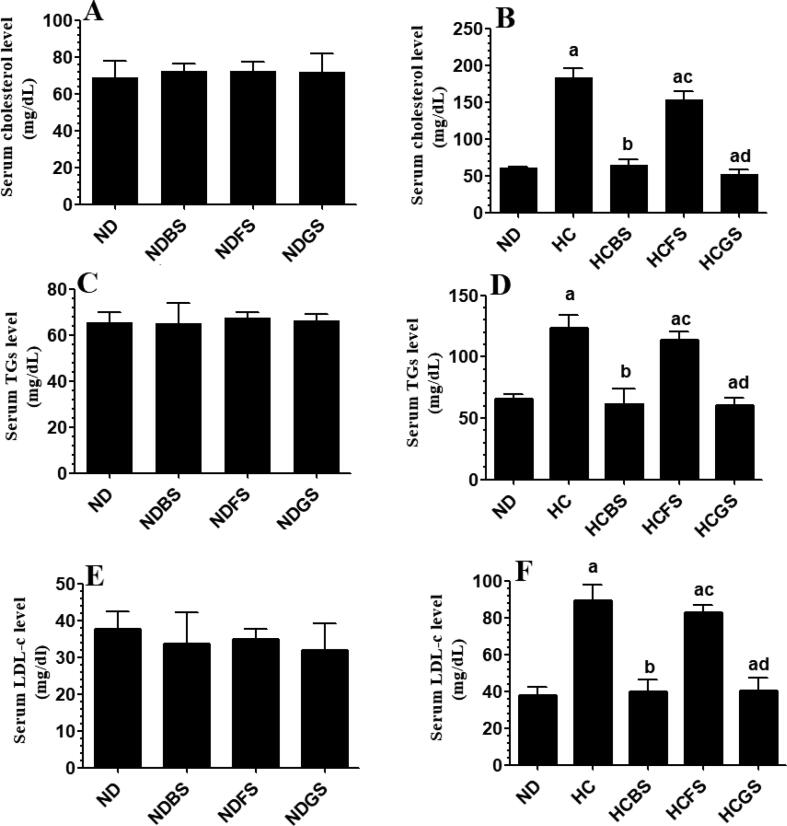
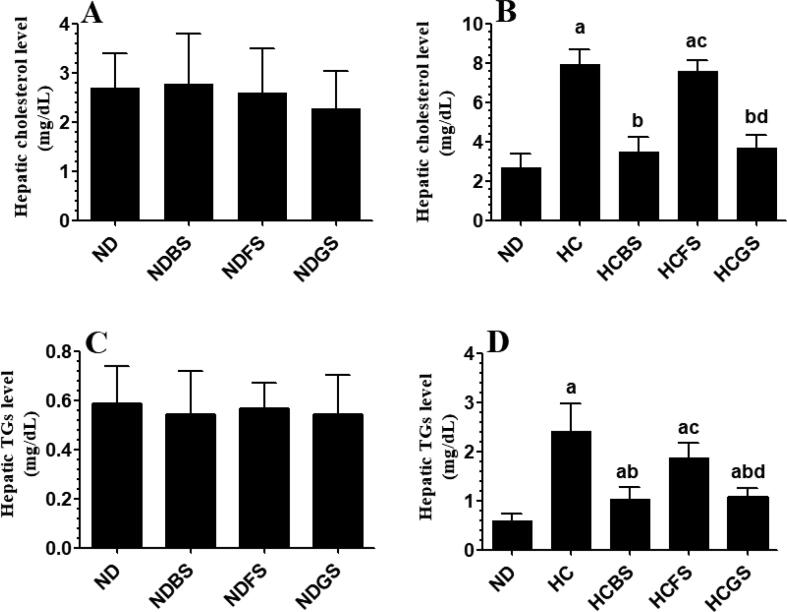
Serum levels of total cholesterol, TGs, and LDL-c, as well as hepatic levels of total cholesterol and TGs, were not significantly different between ND, NDBS, NDFS, and NDGS-fed rats when compared with each other (Fig. 6A, C, E, and Fig. 7-A&C). On the other hand, levels of all these biochemical markers were significantly increased in the serum and liver of HC diet-fed rats and HCFS-fed rats as compared to ND-fed rats (Fig. 6B, D, F, and Fig. 7Band D). However, the levels of all these markers were not significantly different between HC and HCFS-fed rats (Fig. 6B, D, F, and Fig. 7B and D). Nevertheless, the serum levels of total cholesterol, TGs, and LDL-c, as well hepatic levels of total cholesterol and TGs were significantly decreased to in HCBS and HCGS-fed rats as compared to HC diet-fed rats (Fig. 6B, D, F and Fig. 7B and D). The serum and hepatic levels of all these markers in HCBS and HCGS-fed rats were not significantly different from their corresponding levels measured in ND-fed rats (Fig. 6B, D, F, and Fig. 7B and D).
Fig. 6.
Serum levels of total cholesterol (A&B), triglycerides (TGs) (C&D), and low-density lipoprotein-cholesterol (LDL-c) in all experimental groups of rats. Data are presented as mean ± SD for n = 6 rats/group. Values were analyzed by 2-way ANOVA and considered significantly different at p < 0.05. (a): significantly different as compared to normal diet-fed rats (ND). (b): significantly different as compared to high-cholesterol (HC) fed rats (HCD), (c): significantly different as compared to HC diet containing boiled shrimp meat (HCBS). (d): significantly different as compared to HC diet containing fried shrimp meat (HCFS) HCGS: HC diet containing grilled shrimp meat. NDBS, NDFS, and NDGS correspond to rats fed a normal diet containing boiled, fried, or grilled shrimp meat powder, respectively. For both ND and HC diets, shrimp was mixed at 15% w/w.
Fig. 7.
Hepatic levels of total cholesterol (A&B) and triglycerides (TGs) (C&D) in all experimental groups of rats. Data are presented as mean ± SD for n = 6 rats/group. Values were analyzed by 2-way ANOVA and considered significantly different at p < 0.05. (a): significantly different as compared to normal diet-fed rats (ND). (b): significantly different as compared to high-cholesterol (HC) fed rats (HCD), (c): significantly different as compared to HC diet containing boiled shrimp meat (HCBS). (d): significantly different as compared to HC diet containing fried shrimp meat (HCFS) HCGS: HC diet containing grilled shrimp meat. NDBS, NDFS, and NDGS correspond to rats fed a normal diet containing boiled, fried, or grilled shrimp meat powder, respectively. For both ND and HC diets, shrimp was mixed at 15% w/w.
3.7. Effect of shrimp frying, grilling, and boiling on serum liver enzymes and hepatic markers of oxidative stress and inflammation in normal and HC-fed rats
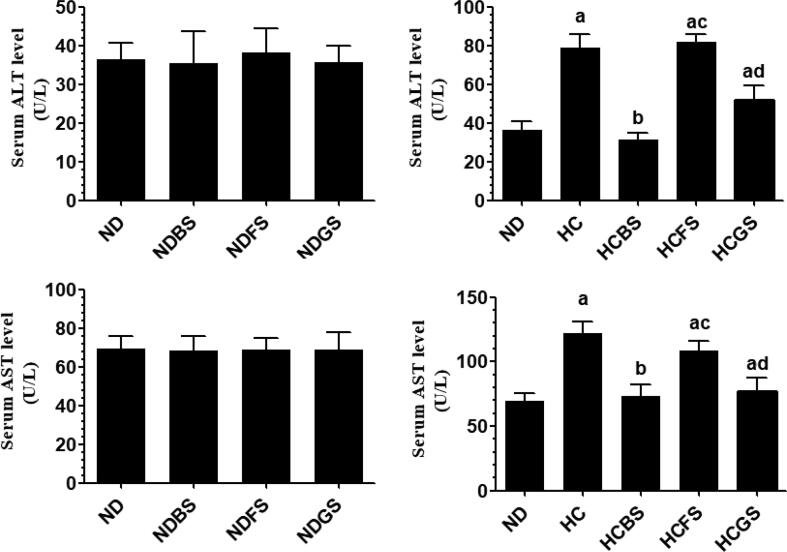
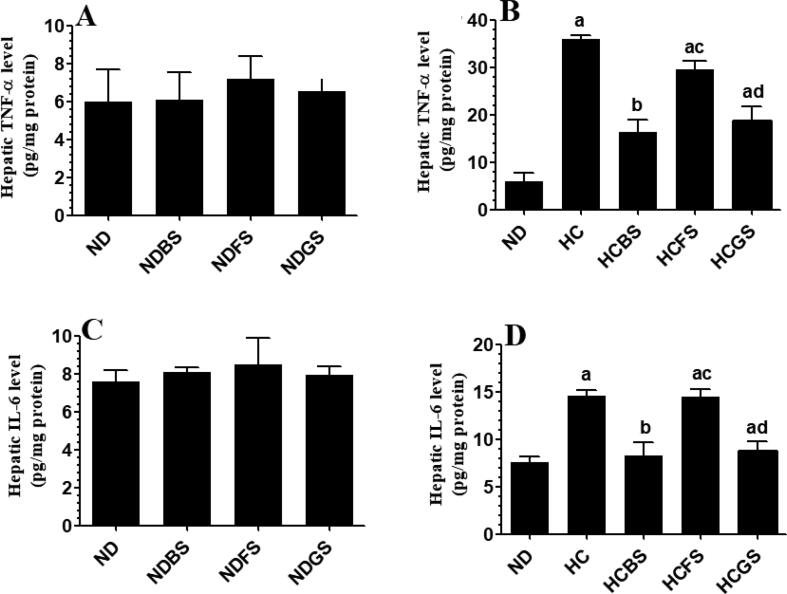
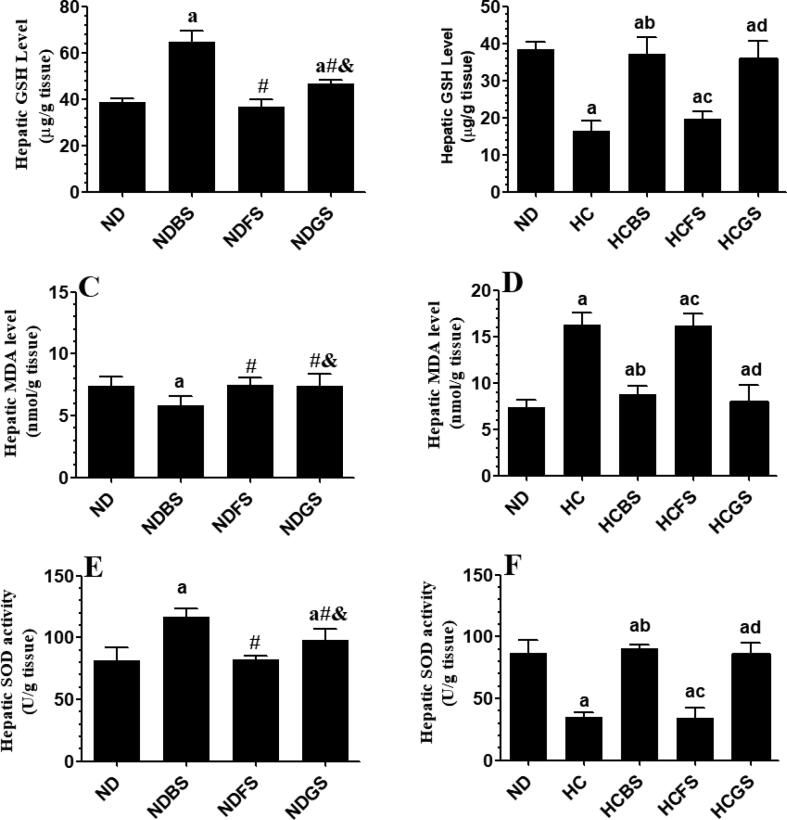
Serum levels of ALT, AST, as well as hepatic levels of TNF-α and IL-6 were not significantly different between rats fed ND, NDBS, NDFS, and NDGS (Fig. 8A&C and Fig. 9A&C). However, feeding the rats NDBS and NDGS significantly increased levels of GSH and activity of SOD and suppressed levels of MDA as compared to ND-fed rats (Fig. 10 A, C, E). However, levels of GSH and MDA, as well as the activity of SOD were not significantly varied between ND and NDFS-fed rats (Fig. 10A, C, E). However, the liver obtained from HC and HCFS-fed rats showed a significant increase in the serum levels of ALT and AST and hepatic levels of TNF-α and IL-6, and MDA with a concomitant decrease in the hepatic levels of GSH and levels of SOD, without any significant difference in the levels of these markers between the two groups (Fig. 8B&D, Fig. 9B&D, and Fig. 10B, D, F). The levels of all these parameters were returned to normal levels like those measured in the ND-fed rats in HCBS and HCGS-fed rats (Fig. 8B&D, Fig. 9B&D, and Fig. 10B, D, F).
Fig. 8.
Levels of alanine aminotransferase (ALT) (A&B) and aspartate aminotransferase (AST) (C&D) in the serum of all experimental groups of rats. Data are presented as mean ± SD for n = 6 rats/group. Values were analyzed by 2-way ANOVA and considered significantly different at p < 0.05. (a): significantly different as compared to normal diet-fed rats (ND). (b): significantly different as compared to high-cholesterol (HC) fed rats (HCD), (c): significantly different as compared to HC diet containing boiled shrimp meat (HCBS). (d): significantly different as compared to HC diet containing fried shrimp meat (HCFS) HCGS: HC diet containing grilled shrimp meat. NDBS, NDFS, and NDGS correspond to rats fed a normal diet containing boiled, fried, or grilled shrimp meat powder, respectively. For both ND and HC diets, shrimp was mixed at 15% w/w.
Fig. 9.
Levels of tumor necrosis factor-α (TNF-α) (A&B) and interlukin-6 (IL-6) (C&D) in the livers of all experimental groups of rats. Data are presented as mean ± SD for n = 6 rats/group. Values were analyzed by 2-way ANOVA and considered significantly different at p < 0.05. (a): significantly different as compared to normal diet-fed rats (ND). (b): significantly different as compared to high-cholesterol (HC) fed rats (HCD), (c): significantly different as compared to HC diet containing boiled shrimp meat (HCBS). (d): significantly different as compared to HC diet containing fried shrimp meat (HCFS) HCGS: HC diet containing grilled shrimp meat. NDBS, NDFS, and NDGS correspond to rats fed a normal diet containing boiled, fried, or grilled shrimp meat powder, respectively. For both ND and HC diets, shrimp was mixed at 15% w/w.
Fig. 10.
Levels of reduced glutathione (A&B) and malondialdehyde (MDA) (C&D), as well as the activity of superoxide dismutase (SOD) (E&F) in the livers of all experimental groups of rats. Data are presented as mean ± SD for n = 6 rats/group. Values were analyzed by 2-way ANOVA and considered significantly different at p < 0.05. (a): significantly different as compared to normal diet-fed rats (ND). (b): significantly different as compared to high-cholesterol (HC) fed rats (HCD), (c): significantly different as compared to HC diet containing boiled shrimp meat (HCBS). (d): significantly different as compared to HC diet containing fried shrimp meat (HCFS) HCGS: HC diet containing grilled shrimp meat. NDBS, NDFS, and NDGS correspond to rats fed a normal diet containing boiled, fried, or grilled shrimp meat powder, respectively. For both ND and HC diets, shrimp was mixed at 15% w/w.
4. Discussion
In this study, we evaluated the impact of different cooking methods (i.e. boiling, frying, & grilling) on FAs content of the meat of the pacific white shrimp and examined its metabolic effect in control and high cholesterol (HC) diet-fed rats. The major findings reported in this study show that only frying reduces the total levels of SFAs, MUFAs, and PUFAs, lowers levels of n-3 PUFA (EPA & DHA), and increase the ratio of n-3/n-6 PUFAs (3 folds). However, only boiled and grilled shrimp, but not the fried shrimp, and possibly due to their high content of n-3 PUFAs, were able to suppress hepatic and serum lipid levels, improves glucose and insulin tolerance, and suppress hepatic oxidative stress and inflammation in HC diet-fed rats and to stimulated levels of endogenous antioxidants (i.e. GSH, SOD, and CAT) in the liver of control rats fed the standard diet.
Previous studies have demonstrated that stable ratios of SFAs, n-3 PUFAs, and n-6 PUFAs are determinant to our health (Simopoulos, 2008, Simopoulos, 2016, Peter et al., 2013, Khandelwal et al., 2013, Briggs et al., 2017). Generally, diets rich in SFA and n-6 PUFAs are associated with inflammation, adiposity, IR, and CVDs (Simopoulos, 2008, Simopoulos, 2016). On the other hand, n-3 PUFAs are associated with contradictory effects and exert many metabolic health benefits due to their antioxidant, hypolipidemic, and anti-inflammatory effects (Zanetti et al., 2015). A healthy diet should contain PUFAs/SFAs ratio of at least 0.54 and above and n-3/n-6 PUFAs ratio no <0.2 with a daily uptake of EPA and DHA of 1 g/day (Arts et al., 2001, Dayal et al., 2013, Gladyshev et al., 2006, Simopoulos, 2008: Naseri et al., 2013). Therefore, consuming seafood was shown to protect against metabolic and chronic disorders due to its high levels of n-3 PUFAs (Simopoulos, 2008, Simopoulos, 2016).
Unlike the fish, the shrimp diet was always considered a healthy functional food due to its high low-calorie intake (89 Kcal/100 g), high water and protein content (76 and 19.4 mg/100 g) and very low lipid content (1.15 mg/100 mg) (Dayal et al., 2013). The phospholipids represent 65–70% of raw shrimp- where the relative average levels of SFAs, MUFAs, and PUFAs are 275, 163, and 321 mg/100 g, respectively (Dayal et al., 2013). Besides, 100 g of raw shrimp contains106 mg n-6 PUFA and 204 mg n-3 PUFAs (EPA 112 mg and DHA 75 mg), thus giving a ratio of n6/n-3 of 0.5. (Dayal et al., 2013). Also, the ratio of PUFAs/SFA in raw shrimp is 1.3 (Dayal et al., 2013). In the same line with this evidence, we have also analyzed the content of major fatty acid in the raw shrimp meat and found that PUFAs were the abundant FAs. The ratio of PUFAs/SFA in the raw shrimp of this study was 1.5. Besides, we have found that the n-3 PUFAs were 2 folds higher n-6 PUFA, thus yielding a ratio of 0.51. Besides, we have found that EPA levels were higher than those of DHA and LA (132, 85, and 19 mg/100 g, respectively). These findings are the same page to many other authors who showed that shrimps is true functional food with a high content of n-3 PUFAs (De Oliveira e Silva et al., 1996, Seok et al., 2004, Dayal et al., 2013).
However, several studies have shown that cooking of various methods can induce physical and chemical interactions that improve or impair the nutritional value of the seafood by altering the quantities and structure of FAs, vitamins, and other essential minerals and components (Czech et al., 2015, Gladyshev et al., 2006, Larsen et al., 2010, Naseri et al., 2013; Choo et al., 2020). However, this was shown to depend on fish (seafood) type, cooking method, cooking duration, and food processing (Naseri et al., 2013). Nowadays, frying becomes the most common method for preparing kinds of seafood due to the high fast rate, distinctive modifications it produces on aroma, flavor, color, and texture, and the consumers believe that it kills most microorganisms (Bordin et al., 2013). Of note, most of these studies have investigated the effect of various cooking methods on FAs composition in craps and various fish species, which makes our study unique to be first done on shrimp. The data of this study confirm that boiling and grilling are the safest methods to preserve the FAs composition in shrimps. Herein, we are showing stable levels of SFAs, MUFAS, n-3/n-6 PUFAs, LA, DHA, and EPA in shrimp after grilling or boiling. On the other hand, a significant reduction in the total levels of SFAs, n-3 PUFAs (i.e. EPA & DHA) with a parallel increase in the total levels of MUFA, PUFAs, and LA were observed in fried shrimp. These alterations resulted in a significant increase in PUFA/SFA and n-6/n-3 PUFAs, both to almost three folds, thus confirming that frying methods alter the chemical composition of FAs in shrimp.
Although poorly investigated in shrimp, many other studies in other marine species support our findings. Indeed, deep frying rose-shrimp croquettes in corn oil decreased SFAs and increased levels of LA (Cankiriligil and Berik, 2017). Also, frying sardines and salmons in sunflower oil but not boiling, grilling, baking, roasting, and microwave heating significantly decreases n-3 PUFAs and increased those of n-6 PUFAs (García-Arias et al., 2003; Gladyshev et al., 2006, Larsen et al., 2010; Naseri et al., 2013). Besides, frying the silver carp with sunflower, soybean, or corn oil increased total PUFAs and n-6 PUFAs levels, decrease total levels SFAs and MUFAs, and increased n-6/n-3 PUFAs (Zakipour-Rahimabadi and Dad, 2012, Naseri et al., 2013). In the same line, frying the New Zealand king salmon in sunflower oil significantly reduced n-3 PUFAs and increased n-6 PUFAs, as well as the content of LA (Larsen et al., 2010). Moreover, deep frying significantly decreased SFAs and n-3 PUFAs and increased total PUFAs and n-6 PUFAs in frozen shrimp, squid, and octopus (Czech et al., 2015). Furthermore, frying significantly reduced EPA, DHA, SFAs, and increased n-6 PUFAs in selected Fish fillets (Choo et al., 2020). Similar results were also observed in fried sardines (Sanchez-Muniz et al., 1992).
Such increase in PUFAs and LA content, as well as the slight increase in MUFAs after frying in sunflower oil, is expected and can be explained by the absorption of n-6 PUFAs (LA) from the culinary sunflower oil during the frying process which has been suggested and confirmed by many authors (Czech et al., 2015, García-Arias et al., 2003, Naseri et al., 2013, Sioen et al., 2006, Varela and Ruiz-Roso, 1992, Zakipour-Rahimabadi and Dad, 2012). On the other hand, some other studies contradict our findings and have shown reduced levels of n-3 PUFAs after streaming, boiling, and grilling of salmon (Al-Saghir et al., 2004; Choo et al., 2020). Such a contradiction with our results could be explained by the difference in the meat texture, cooking period, cooking methods procedure, and FAs contents between fish, salmon, and shrimp.
On the other hand, NAFLD can occur in obese and non-obese individuals (Kim and Kim, 2017, Kumar and Mohan, 2017). Although it is mainly induced by high TGs rich diet, IR, and obesity, the currently available data suggest that high HC diet associated bile toxicity are independent risk factors to induce NAFLD in non-obese individuals and animals (Hirsch et al., 2016, Tirosh, 2018). In rodents, high cholesterol diet results in similar NAFLD and hepatic features like those seen in non-obese individuals with visceral adiposity, and dyslipidemia but with a slight loss in body weight and adipose tissue mass and higher mortality rate (Hirsch et al., 2016, Kim and Kim, 2017, Kumar and Mohan, 2017, Tirosh, 2018). However, HC diet-induced NAFLD in rodents is associated with minimum IR but is always associated with impairing glucose homeostasis and severe hepatic IR by altering caveolin-1 expression and insulin receptors localization (Schattenberg and Galle, 2010, Hahn-Obercyger et al., 2009, Tirosh, 2018).
Hepatic inflammation and oxidative stress are the key mechanisms by which HC diet induces NAFLD (Tirosh, 2018). In this regard, free cholesterol can impair the mitochondria membrane fluidity, depletes its endogenous GSH levels, and induces ROS and mitochondria damage (Marí et al., 2006, Tirosh, 2018). Besides, the hydrolysis of cholesterol to cholesteryl ester in the kupffer cells activates oxidative stress and inflammation and induces endoplasmic reticulum (ER) stress and apoptosis (Tirosh, 2018). Moreover, the free cholesterol can upregulate several transcription factors that induces cholesterol synthesis and NAFLD (i.e. the sterol regulatory element-binding protein 2 (SREBP-2) (Van Rooyen and Farrell, 2011). Accumulated hepatic cholesterol can also supresses the conversion of cholesterol to bile leading to lipotoxicity which stimulates oxidative stress and inflammation (Van Rooyen and Farrell, 2011). Furthermore, HC diet stimulates hepatocytes oxidative stress, apoptosis, and the progression to NASH by mechanisms including induction of endoplasmic reticulum stress, mitochondria dysfunction, and activation of apoptotic pathways (i.e. Cyps) (Tirosh, 2018).
Similar to other findings (Tous et al., 2005; Ma et al., 2008, Kim et al., 2014, Arguello et al., 2015, Lee et al., 2019), HC diet induces features to NAFLD as evidenced by the hepatic accumulation of fat droplet, dyslipidemia, increased hepatic TGs, and cholesterol synthesis. Also, HC diet exaggerated the oxidative stress response by decreasing the levels of endogenous antioxidants (SOD, CAT, & GSH) induced lipid peroxidation, and increased hepatic inflammatory cytokine levels (TNF-α & IL-6) in the livers of rats. Besides, HC diet used in this study didn’t alter rat’s weights but significantly induces hyperglycemia and hyperinsulinemia. HOMA-IR is the most widely accepted model to measure central (hepatic) IR (Qureshi et al., 2010). HC diet of this study impaired OGTT and IPITT and significantly increased the HOMA-IR index. All these data validate our animal model and suggest that HC diet in rats is associated with NAFLD and hepatic IR. Many other authors reported similar effects (Tous et al., 2005; Ma et al., 2008; Kim et al., 2014, Arguello et al., 2015, Lee et al., 2019).
On the other hand, a novel finding in this study is that only boiled and grilled shrimp powders were able to completely prevent all the above-mentioned adverse effects of HC diet in rats. However, feeding the rats the HC diet containing the fried shrimp failed to ameliorate the loss on body weights, hyperglycemia, hyperinsulinemia, the impairment in glucose and insulin tolerance, dyslipidemia, hepatic oxidative stress and inflammation and the liver of these rats showed high levels of TGs and cholesterol, as well as fat accumulation. Such variation between the effects of these diets could be explained by the alterations in n-3/n-6 PUFAs due to the cooking method as discussed above. Indeed, the lower levels of n-3 PUFAs (i.e. EPA and DHA), the higher levels of n-6 PUFA, and the lower ratio of n-3/n-6 PUFAs in the fried shrimp may explain the inability of this cooked shrimp to ameliorate NAFLD features in these HC diet-fed rats.
Indeed, the antioxidant, hypolipidemic, and anti-inflammatory effects of n-3 PUFA and their underlying mechanisms are well reported in both humans and animals and discussed in more detail in excellent reviews (Scorletti and Byrne, 2013; Zanetti et al., 2015). Herein, n-3 PUFAs was shown to a potent ability to supress hepatic FAs and cholesterol synthesis and stimulate FAs oxidiation by downregulating of SREBP1/2 and activation of PPRAα (Scorletti and Byrne, 2013, Zanetti et al., 2015). Besides, n-3 PUFAs can inhibit lipid synthesis and glucose production by suppressing the activity of ChREBP (Dentin et al., 2005). Besides, omega-3 fatty acids can inhibit macrophage infiltration and downregulate the levels of inflammatory cytokines chemoattractant, and adhesive molecules by several mechanisms including suppression of nuclear factor kappa beta (NF-kB) (Calder, 2013, Oh et al., 2010, Oh and Walenta, 2014, Raphael and Sordillo, 2013, Scorletti and Byrne, 2013, Zanetti et al., 2015). Furthermore, n-3 PUFAs can ameliorate oxidative stress and increases the expression of serval antioxidant genes with or without increasing the activity of the nuclear factor-2 (Nrf-2) transcription factor (Xi and Chen, 2000, Romieu et al., 2008, Patten et al., 2013, Tatsumi et al., 2019). This could explain why the liver of control rats fed the standard diet which contained the boiled and grilled shrimp showed higher basal levels of GSH, SOD, and CAT which were absent in the liver of control rats fed a standard diet containing the fried shrimp.
However, in interesting observation noticed here is that although the frying the shrimp in sunflower oil increased the ratio of n-6/n-3 PUFA which is expected to induced obesity, IR, and possibly NAFLD in rats, we didn’t observe significant alterations in these parameters in control rats fed the standard diet containing the fried shrimp. In fact, as happened with the western diet, studies have shown that higher ratios of n-6/n-3 ratios (18–20:1) induce obesity, IR, and NAFLD (Simopoulos, 2008, Simopoulos, 2016). However, the ratio of n-3/n-6 in the fried shrimp diet was 1:3 which much lower than the ratio needed to induce obesity and NAFLD. Such a ratio seems to be safe and may explain these results. However, further studies using longer treatment period is needed to establish awareness about this.
5. Conclusion
Frying the shrimp in sunflower oil reduces its content of EPA and DHA. Such an effect could lead to losing its functional properties in ameliorating NAFLD. However, cooking the shrimp by boiling and grilling seems to be the most suitable methods to preserve the FAs composition and the nutritional value of the shrimp.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
The authors wish to thank The Ministry of Environment, Water and Agriculture, Kingdom of Saudi Arabia, for the financial assistance provided (T-A-6) to conduct this research. This research was funded by the Deanship of Scientific Research at Princess Nourah bint Abdulrahman University through the Fast-track Research Funding Program.
Footnotes
Peer review under responsibility of King Saud University.
References
- Al-Saghir S., Thurner K., Wagner K.H., Frisch G., Luf W., Razzazi-Fazeli E., Elmadfa I. Effects of different cooking procedures on lipid quality and cholesterol oxidation of farmed salmon fish (Salmo salar) J. Agric. Food Chem. 2004;52(16):5290–5296. doi: 10.1021/jf0495946. [DOI] [PubMed] [Google Scholar]
- Ankle M.R., Joshi P.S. A study to evaluate the efficacy of xylene-free hematoxylin and eosin staining procedure as compared to the conventional hematoxylin and eosin staining: An experimental study. J. Oral Maxillofac. Pathol. 2011;15(2):161–167. doi: 10.4103/0973-029X.84482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arguello G., Balboa E., Arrese M., Zanlungo S. Recent insights on the role of cholesterol in non-alcoholic fatty liver disease. Bioch Biophys Acta. 2015;1852(9):1765–1778. doi: 10.1016/j.bbadis.2015.05.015. [DOI] [PubMed] [Google Scholar]
- Arts M.T., Ackman R.G., Holub B.J. Essential fatty Acids. in aquatic ecosystems: a crucial link between diet and human health and evolution. Can. J. Fish. Aquat. Sci. 2001;58:122–137. [Google Scholar]
- Asgari-Taee F., Zerafati-Shoae N., Dehghani M., Sadeghi M., Baradaran H.R., Jazayeri S. Association of consumption with non-alcoholic fatty liver disease: A systematic review and meta-analysis. Eur. J. Nutr. 2019;58:1759–1769. doi: 10.1007/s00394-018-1711-4. [DOI] [PubMed] [Google Scholar]
- Bastías J.M., Balladares P., Acuña S., Quevedo R., Muñoz O. Determining the effect of different cooking methods on the nutritional composition of salmon (Salmo salar) and chilean jack mackerel (Trachurus murphyi) fillets. PLoS One. 2017;12(7) doi: 10.1371/journal.pone.0180993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blasbalg T.L., Hibbeln J.R., Ramsden C.E., Majchrzak S.F., Rawlings R.R. Changes in consumption of omega-3 and omega-6 fatty acids in the United States during the 20th century. Am. J. Clin. Nutr. 2011;93(5):950–962. doi: 10.3945/ajcn.110.006643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bligh E.G., Dyer W.J. A rapid method of total lipid extraction and purification. Can. J. Biochem. Physiol. 1959;37:911–917. doi: 10.1139/o59-099. [DOI] [PubMed] [Google Scholar]
- Bordin K., Kunitake M.T., Aracava K.K., Trindade C.S. Changes in food caused by deep fat frying–a review. Arch. Latinoam. Nutr. 2013;63(1):5–13. [PubMed] [Google Scholar]
- Briggs M.A., Petersen K.S., Kris-Etherton P.M. Saturated Fatty Acids and Cardiovascular Disease: Replacements for Saturated Fat to Reduce Cardiovascular Risk. Healthcare (Basel). 2017;5(2):29. doi: 10.3390/healthcare5020029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calder P.C. Omega-3 polyunsaturated fatty acids and inflammatory processes: nutrition or pharmacology? Br. J. Clin. Pharmacol. 2013;75(3):645–662. doi: 10.1111/j.1365-2125.2012.04374.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cankiriligil E.C., Berik N. Changes in fatty acid and mineral compositions of rose-shrimp croquettes during production process. Am. J. Food Technol. 2017;12:254–261. [Google Scholar]
- Choo P.Y., Azlan A., Khoo H.E. Cooking methods affect total fatty acid composition and retention of DHA and EPA in selected fish fillets. ScienceAsia. 2020;44(2):92–101. [Google Scholar]
- Czech A., Grela E.R., Ognik K. Effect of Frying on Nutrients Content and Fatty Acid Composition of Muscles of Selected Freezing Seafoods. J. Food Nutr. Res-Slov. 2015;3(1):9–14. [Google Scholar]
- Dayal J.S., Ponniah A.G., Imran Khan H., Madhu Babu E.P., Ambasankar K., Kumarguru Vasagam K.P. Shrimps – a nutritional perspective. Curr. Sci. 2013;104(11):1487–1491. [Google Scholar]
- De Oliveira e Silva E.R., Seidman C.E., Tian J.J., Hudgins L.C., Sacks F.M., Breslow J.L. Effects of shrimp consumption on plasma lipoproteins. Am. J. Clin. Nutr. 1996;64:712–717. doi: 10.1093/ajcn/64.5.712. [DOI] [PubMed] [Google Scholar]
- Dentin R., Benhamed F., Pégorier J.P., Foufelle F., Viollet B., Vaulont S., Girard J., Postic C. Polyunsaturated fatty acids suppress glycolytic and lipogenic genes through the inhibition of ChREBP nuclear protein translocation. J. Clin. Invest. 2005;115(10):2843–2854. doi: 10.1172/JCI25256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- García-Arias M.T., Pontes E.A., García-Linares M.C., García-Fernández M.C., Sánchez-Muniz F.J. Cooking-freezing-reheating (CFR) of sardine (Sardina pilchardus) fillets. Effect of different cooking and reheating procedures on the proximate and fatty acid compositions. Food Chem. 2003;83(3):349–356. doi: 10.1016/S0308-8146(03)00095-5. [DOI] [Google Scholar]
- Gladyshev M.I., Sushchik N.N., Gubanenko G.A., Demirchieva S.M., Kalachova G.S. Effect of way of cooking on content of essential polyunsaturated fatty acids in muscle tissue of humpback salmon (Oncorhynchus gorbuscha) Food Chem. 2006;96(3):446–451. [Google Scholar]
- Hahn-Obercyger M., Graeve L., Madar Z.A. high-cholesterol diet increases the association between caveolae and insulin receptors in rat liver. J. Lipid Res. 2009;50(1):98–107. doi: 10.1194/jlr.M800441-JLR200. [DOI] [PubMed] [Google Scholar]
- Harb A.A., Bustanji Y.K., Almasri I.M., Abdalla S.S. Eugenol Reduces LDL Cholesterol and Hepatic Steatosis in Hypercholesterolemic Rats by Modulating TRPV1 Receptor. Sci. Rep. 2019;9(1):14003. doi: 10.1038/s41598-019-50352-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirsch N., Konstantinov A., Anavi S., Aronis A., Hagay Z., Madar Z., Tirosh O. Prolonged feeding with green tea polyphenols exacerbates cholesterol-induced fatty liver disease in mice. Mol. Nutr. Food Res. 2016;60(12):2542–2553. doi: 10.1002/mnfr.201600221. [DOI] [PubMed] [Google Scholar]
- Hossain S., Rahman A., Kabir Y., Shams A.A., Afros F., Hashimoto M. Effects of shrimp (Macrobracium rosenbergii)-derived chitosan on plasma lipid profile and liver lipid peroxide levels in normo- and hypercholesterolaemic rats. Clin. Exp. Pharmacol. Physiol. 2007;34(3):170–176. doi: 10.1111/j.1440-1681.2007.04568.x. [DOI] [PubMed] [Google Scholar]
- Khandelwal S., Kelly L., Malik R., Prabhakaran D., Reddy S. Impact of omega-6 fatty acids on cardiovascular outcomes: A review. J. Preventive Cardiol. 2013;2(3):325–336. [PMC free article] [PubMed] [Google Scholar]
- Kim C.H., Kallman J.B., Bai C., Pawloski L., Gewa C., Arsalla A., Sabatella M.E., Younossi Z.M. Nutritional assessments of patients with non-alcoholic fatty liver disease. Obes. Surg. 2010;20(2):154–160. doi: 10.1007/s11695-008-9549-0. [DOI] [PubMed] [Google Scholar]
- Kim D., Kim W.R. Nonobese Fatty Liver Disease. Clin. Gastroenterol. Hepatol. 2017;15(4):474–485. doi: 10.1016/j.cgh.2016.08.028. [DOI] [PubMed] [Google Scholar]
- Kim E.J., Kim B.H., Seo H.S., Lee Y.J., Kim H.H., Son H.H., Choi M.H. Cholesterol-induced non-alcoholic fatty liver disease and atherosclerosis aggravated by systemic inflammation. PloS one. 2014;9(6) doi: 10.1371/journal.pone.0097841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar R., Mohan S. Non-alcoholic Fatty Liver Disease in Lean Subjects: Characteristics and Implications. J. Clin. Transl. Hepatol. 2017;5(3):216–223. doi: 10.14218/JCTH.2016.00068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larsen D., Quek S.Y., Eyres L. Effect of cooking method on the fatty acid profile of New Zealand King Salmon (Oncorhynchus tshawytscha) Food Chem. 2010;119:785–790. [Google Scholar]
- Lee Y.H., Cho Y., Lee B.W., Park C.Y., Lee D.H., Cha B.S., Rhee E.J. Nonalcoholic Fatty Liver Disease in Diabetes. Part I: Epidemiology and Diagnosis. Diabet. Metab. J. 2019;43(1):31–45. doi: 10.4093/dmj.2019.0011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li L., Liu D.W., Yan H.Y., Wang Z.Y., Zhao S.H., Wang B. Obesity is an independent risk factor for non-alcoholic fatty liver disease: evidence from a meta-analysis of 21 cohort studies. Obesity Rev. Off. J. Int. Assoc. Study Obes. 2016;17(6):510–519. doi: 10.1111/obr.12407. [DOI] [PubMed] [Google Scholar]
- Ma K.L., Ruan X.Z., Powis S.H., Chen Y., Moorhead J.F., Varghese Z. Inflammatory stress exacerbates lipid accumulation in hepatic cells and fatty livers of apolipoprotein E knockout mice. Hepatology. 2008;48(3):770–781. doi: 10.1002/hep.22423. [DOI] [PubMed] [Google Scholar]
- Marí M., Caballero F., Colell A., Morales A., Caballeria J., Fernandez A., Enrich C., Fernandez-Checa J.C., García-Ruiz C. Mitochondrial free cholesterol loading sensitizes to TNF- and Fas-mediated steatohepatitis. CellMetab. 2006;4(3):185–198. doi: 10.1016/j.cmet.2006.07.006. [DOI] [PubMed] [Google Scholar]
- Mezzomo N., Laura T., Mirelle S., Fariasb M., Tereza F., Rozangela C. Evidence of anti-obesity and mixed hypolipidemic effects of extracts from pink shrimp (Penaeus brasiliensis and Penaeus paulensis) processing residue. J. Supercrit. Fluids. 2015;96:252–261. [Google Scholar]
- Mirmiran P., Amirhamidi Z., Ejtahed H.S., Bahadoran Z., Azizi F. Relationship between Diet and Non-alcoholic Fatty Liver Disease: A Review Article. Iran J. Public Health. 2017;46(8):1007–1017. [PMC free article] [PubMed] [Google Scholar]
- Mirmiran P., Hosseinpour-Niazi S., Naderi Z., Bahadoran Z., Sadeghi M., Azizi F. Association between interaction and ratio of ω-3 and ω-6 polyunsaturated fatty acid and the metabolic syndrome in adults. Nutrition. 2012;28(9):856–863. doi: 10.1016/j.nut.2011.11.031. [DOI] [PubMed] [Google Scholar]
- Mohamed A.S., Hosney M., Bassiony H., Hassanein S.S., Soliman A.M., Fahmy S.R., Gaafar K. Sodium pentobarbital dosages for exsanguination affect biochemical, molecular and histological measurements in rats. Sci Rep. 2020;10(1):378. doi: 10.1038/s41598-019-57252-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nair S., Gagnon J., Pelletier C., Tchoukanova N., Zhang J., Ewart H.S., Ewart K.V., Jiao G., Wang Y. Shrimp oil extracted from the shrimp processing waste reduces the development of insulin resistance and metabolic phenotypes in diet-induced obese rats. Appl. Physiol. Nutr. Metab. 2017;42(8):841–849. doi: 10.1139/apnm-2016-0644. [DOI] [PubMed] [Google Scholar]
- Naseri M., Abedi E., Mohammadzadeh B., Afsharnaderi A. Effect of frying in different culinary fats on the fatty acid composition of silver carp. Food Sci. Nutr. 2013;1(4):292–297. doi: 10.1002/fsn3.40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nseir W., Hellou E., Assy N. Role of diet and lifestyle changes in nonalcoholic fatty liver disease. World J. Gastroenterol. 2014;20(28):9338–9344. doi: 10.3748/wjg.v20.i28.9338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oh D.Y., Walenta E. The role of omega-3 fatty acid receptor GPR120 in insulin resistance. Int. J. Obes. 2014;4(Suppl 1):S14–S16. doi: 10.1038/ijosup.2014.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oh D.Y., Talukdar S., Bae E.J., Imamura T., Morinaga H., Fan W., Li P., Lu W.J., Watkins S.M., Olefsky J.M. GPR120 is an omega-3 fatty acid receptor mediating potent anti-inflammatory and insulin-sensitizing effects. Cell. 2010;142(5):687–698. doi: 10.1016/j.cell.2010.07.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pachikian B.D., Neyrinck A.M., Cani P.D., Portois L., Deldicque L., De Backer F.C., Bindels L.B., Sohet F.M., Malaisse W.J., Francaux M., Carpentier Y.A., Delzenne N.M. Hepatic steatosis in n-3 fatty acid depleted mice: focus on metabolic alterations related to tissue fatty acid composition. BMC Physiol. 2008;8:21. doi: 10.1186/1472-6793-8-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parker H.M., Johnson N.A., Burdon C.A., Cohn J.S., O'Connor H.T., George J. Omega-3 supplementation and non-alcoholic fatty liver disease: a systematic review and meta-analysis. J. Hepatol. 2012;56(4):944–951. doi: 10.1016/j.jhep.2011.08.018. [DOI] [PubMed] [Google Scholar]
- Patten A.R., Brocardo P.S., Christie B.R. Omega-3 supplementation can restore glutathione levels and prevent oxidative damage caused by prenatal ethanol exposure. J. Nutr. Biochem. 2013;24(5):760–769. doi: 10.1016/j.jnutbio.2012.04.003. [DOI] [PubMed] [Google Scholar]
- Peter S., Chopra S., Jacob J.J. A fish a day, keeps the cardiologist away! - A review of the effect of omega-3 fatty acids in the cardiovascular system. Indian. Endocrinol. Metab. 2013;17(3):422–429. doi: 10.4103/2230-8210.111630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polyzos S.A., Mantzoros C.S. Nonalcoholic fatty future disease. Metabolism. 2016;65(8):1007–1016. doi: 10.1016/j.metabol.2015.12.009. [DOI] [PubMed] [Google Scholar]
- Polyzos S.A., Kountouras J., Mantzoros C.S. Obesity and nonalcoholic fatty liver disease: From pathophysiology to therapeutics. Metabolism. 2019;92:82–97. doi: 10.1016/j.metabol.2018.11.014. [DOI] [PubMed] [Google Scholar]
- Qureshi K., Clements R.H., Saeed F., Abrams G.A. Comparative evaluation of whole body and hepatic insulin resistance using indices from oral glucose tolerance test in morbidly obese subjects with nonalcoholic Fatty liver disease. J. Obes. 2010;2010 doi: 10.1155/2010/741521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raphael W., Sordillo L.M. Dietary polyunsaturated fatty acids and inflammation: the role of phospholipid byosynthesis. Int. J. Mol. Sci. 2013;14:21167–21188. doi: 10.3390/ijms141021167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romieu I., Garcia-Esteban R., Sunyer J., Rios C., Alcaraz-Zubeldia M., Velasco S.R., Holguin F. The effect of supplementation with omega-3 polyunsaturated fatty acids on markers of oxidative stress in elderly exposed to PM (2.5) Environ. Health Perspect. 2008;116(9):1237–1242. doi: 10.1289/ehp.10578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanchez-Muniz F.J., Viejo J.M., Medina R. Deep frying of sardines in different culinary fats. Changes in the fatty acid composition of sardines and frying fats. J. Agric Food Chem. 1992;40(11):2252–2256. [Google Scholar]
- Schattenberg J.M., Galle P.R. Animal models of non-alcoholic steatohepatitis: of mice and man. Dig. Dis. 2010;8(1):247–254. doi: 10.1159/000282097. [DOI] [PubMed] [Google Scholar]
- Scorletti E., Byrne C.D. Omega-3 fatty acids, hepatic lipid metabolism, and nonalcoholic fatty liver disease. Annu. Rev. Nutr. 2013;33:231–248. doi: 10.1146/annurev-nutr-071812-161230. [DOI] [PubMed] [Google Scholar]
- Seok S.H., Park J.H., Choa S.A., Choi S.A., Park J.H. Cholesterol lowering effect of SG-GN3, the extract of salted and fermented small shrimps, Acetes japonicus, in Triton WR-1339 or high cholesterol-diet induced hypercholesterolemic rats. J. Ethnopharmacol. 2004;91:231–235. doi: 10.1016/j.jep.2003.12.032. [DOI] [PubMed] [Google Scholar]
- Shapiro H., Tehilla M., Attal-Singer J., Bruck R., Luzzatti R., Singer P. The therapeutic potential of long-chain omega-3 fatty acids in nonalcoholic fatty liver disease. Clin. Nutr. 2011;30(1):6–19. doi: 10.1016/j.clnu.2010.06.001. [DOI] [PubMed] [Google Scholar]
- Simopoulos A.P. The importance of the omega-6/omega-3 fatty acid ratio in cardiovascular disease and other chronic diseases. Exp. Biol. Med (Maywood) 2008;233(6):674–688. doi: 10.3181/0711-MR-311. [DOI] [PubMed] [Google Scholar]
- Simopoulos A.P. An increase in the Omega-6/Omega-3 fatty acid ratio increases the risk for obesity. Nutrients. 2016;8(3):128. doi: 10.3390/nu8030128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sioen I., Haak L., Raes K., Hermans C., De Henauw S., De Smet S., Camp J.V. Effects of panfrying in margarine and olive oil on the fatty acid composition of cod and salmon. Food Chem. 2006;98:609–617. [Google Scholar]
- Spadaro L., Magliocco O., Spampinato D., Piro S., Oliveri C., Alagona C., Papa G., Rabuazzo A.M., Purrello F. Effects of n-3 polyunsaturated fatty acids in subjects with nonalcoholic fatty liver disease. Dig. Liver Dis. 2008;40(3):194–199. doi: 10.1016/j.dld.2007.10.003. [DOI] [PubMed] [Google Scholar]
- Stephen N.M., Jeya Shakila R., Jeyasekaran G., Sukumar D. Effect of different types of heat processing on chemical changes in tuna. J. Food Sci. Technol. 2010;47(2):174–181. doi: 10.1007/s13197-010-0024-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tatsumi Y., Kato A., Sango K., Himeno T., Kondo M., Kato Y., Kamiya H., Nakamura J., Kato K. Omega-3 polyunsaturated fatty acids exert anti-oxidant effects through the nuclear factor (erythroid-derived 2)-related factor 2 pathway in immortalized mouse Schwann cells. J. DiabetesInvestig. 2019;10(3):602–612. doi: 10.1111/jdi.12931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tirosh O. Hypoxic Signaling and Cholesterol Lipotoxicity in Fatty Liver Disease Progression. Oxid. Med. Cell Longev. 2018;2018:2548154. doi: 10.1155/2018/2548154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tous M., Ferré N., Camps J., Riu F., Joven J. Feeding apolipoprotein E-knockout mice with cholesterol and fat enriched diets may be a model of non-alcoholic steatohepatitis. Mol. CellBiochem. 2005;268(1–2):53–58. doi: 10.1007/s11010-005-2997-0. [DOI] [PubMed] [Google Scholar]
- Van Rooyen D.M., Farrell G.C. SREBP-2: a link between insulin resistance, hepatic cholesterol, and inflammation in NASH. J. Gastroenterol Hepatol. 2011;26(5):789–792. doi: 10.1111/j.1440-1746.2011.06704.x. [DOI] [PubMed] [Google Scholar]
- Varela G., Ruiz-Roso B. Some effects of deep frying on the dietary fat intake. Nutr Reviews. 1992;50:256–262. doi: 10.1111/j.1753-4887.1992.tb01342.x. [DOI] [PubMed] [Google Scholar]
- Venugopal V. Marine Products for Healthcare: Functional and Bioactive Nutraceutical Compounds from the Ocean. CRC Press; London: 2009. ed; pp. 221–239. [Google Scholar]
- Wong C.K., Botta A., Pither J., Dai C., Gibson W.T., Ghosh S.A. high-fat diet rich in corn oil reduces spontaneous locomotor activity and induces insulin resistance in mice. J. Nutr. Biochem. 2015;26(4):319–326. doi: 10.1016/j.jnutbio.2014.11.004. [DOI] [PubMed] [Google Scholar]
- Xi S., Chen L.H. Effects of dietary fish oil on tissue glutathione and antioxidant defense enzymes in mice with murine aids. Nutr. Rese. 2000;20(9):1287–1299. [Google Scholar]
- Yang J., Fernández-Galilea M., Martínez-Fernández L., González-Muniesa P., Pérez-Chávez A., Martínez J.A., Moreno-Aliaga M.J. Oxidative Stress and Non-Alcoholic Fatty Liver Disease: Effects of Omega-3 Fatty Acid Supplementation. Nutrients. 2019;11(4):872. doi: 10.3390/nu11040872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zakipour-Rahimabadi E., Dad S. Effects of frying by different frying oils on fatty acid profile of silver carp (Hypophthalmichthys molitrix) Iran. J. Fish. Sci. 2012;11(3):704–712. [Google Scholar]
- Zanetti M., Grillo A., Losurdo P., Panizon E., Mearelli F., Cattin L., Barazzoni R., Carretta R. Omega-3 Polyunsaturated Fatty Acids: Structural and Functional Effects on the Vascular Wall. Biomed Res. Int. 2015;2015 doi: 10.1155/2015/791978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zotos A., Kotaras A., Mikras E. Effect of baking of sardine (Sardina pilchardus) and frying of anchovy (Engraulis encrasicholus) in olive and sunflower oil on their quality. Food Sci. Technol. Int. 2013;19(1):11–23. doi: 10.1177/1082013212442179. [DOI] [PubMed] [Google Scholar]