Abstract
Deoxynivalenol (DON) is Fusarium mycotoxin that is frequently found in many cereal-based foods, and its ingestion has a deleterious impact on human health. In this investigation, we studied the mechanism of DON-induced neurotoxicity and followed by cytoprotective efficacy of quercetin (QUE) in contradiction of DON-induced neurotoxicity through assessing the oxidative stress and apoptotic demise in the human neuronal model, i.e. SH-SY5Y cells. DON diminished the proliferation of cells in the manner of dose and time-dependent as revealed by cell viability investigations, i.e. MTT and lactate dehydrogenase assays. Additional studies, such as intracellular reactive oxygen species (ROS), lipid peroxidation (LPO), mitochondrial membrane potential (MMP), DNA damage, cell cycle, and neuronal biomarkers (amino acid decarboxylase, tyrosine hydroxylase, and brain-derived neurotrophic factor) demonstrated that DON induces apoptotic demise in neuronal cells through oxidative stress intermediaries. On another hand, pre-treatment of neuronal cells with 1 mM of quercetin (QUE) showed decent viability upon exposure to 100 µM of DON. In detailed studies demonstrated that QUE (1 mM) pre-treated cells show strong attenuation efficiency against DON-induced ROS generation, LPO, MMP loss, DNA impairment, cell cycle arrest, and down-regulation of neuronal biomarkers. The consequences of the investigation concluded that QUE mitigates the DON-induced stress viz., decreased ROS production and LPO generation, upholding MMP and DNA integrity and regulation of neuronal biomarker gene expression in SH-SY5Y cells.
Keywords: Deoxynivalenol, Quercetin, Oxidative stress, Apoptosis, Neurotoxicity
1. Introduction
Fusarium toxins are the major cluster among the identified 400 mycotoxins (Berthiller et al., 2007). Human contact to these Fusarium toxins occurs through direct ingestion of contaminated food or indirect through the food chain by ingestion of meat foodstuffs of the livestock that fed with mycotoxin contaminated food (Alshannaq and Yu, 2017). These mycotoxins fetch mild to lethal toxic effects in humans as well as livestock (Fraeyman et al., 2017). Among the Fusarium toxins, deoxynivalenol (DON) causes the highest health risk in humans and livestock (Pestka, 2007, Sobrova et al., 2010). DON, belong to the trichothecene group of mycotoxin and generally known as vomitoxin, which is produced in various food sources (cereals and cereal products, fruits and vegetables, spices, etc.) by diverse fungal species, i.e. Stachybotrys, Myrothecium, and Fusarium (Ramana et al., 2011).
DON primarily disturbs the immune and gastrointestinal systems, and it drives its noxious effects by quashing the humoral and cell-mediated immunity and thereby, compromise the immune system and make vulnerable to other diseases (Pestka and Bondy, 1990). DON at a low rate of consumption is known to cause irritations in the gastrointestinal tract and reduce food intake, and whereas, at a high rate of consumption fetches para oesophageal stomach ulcers and vomiting (Rotter, 1996, Hsia et al., 2004). On the other hand, DON inhibits protein synthesis and decreases the uptake of tryptophan by the brain, and adversely affects serotonin levels. In this scenario, much research is essential to comprehend the toxic possessions of DON on the neuronal system, and their plausible mechanisms are not well documented. The consequences of DON on the nerve cells apprehended confined consideration, and just a few descriptions are present on the neurotoxic effects (Wang et al., 2018). Razafimanjato et al. detailed the neurotoxicity of DON in microglial cells and proposed that toxicity was through the mediation of cytokine secretion. Moreover, reported that DON expressed its toxic possessions on astrocytes via directing the glutamate uptake plasma membrane reporters (Razafimanjato et al., 2011). Some other investigations have established that other mycotoxins (T-2, aflatoxin B1, fumonisin B1, and ochratoxin A) cross the barrier of the blood–brain and accumulate in the brain, which fetches a variety of neurological ailments (Weidner et al., 2013, Alonso-Garrido et al., 2020).
Generally, neurotoxicological evaluations are performed in laboratory animals. However, since the last decade, several neuron cell line models were introduced to evaluate the toxicity, which delivers ease of use and has no ethical issues (Weidner et al., 2013). Nowadays, human cell line models gain much attraction over animal cells. Regrettably, the toxic effects of DON in the neuronal system at the cell and molecular level were not yet fully assessed and need in detail research.
Till the date, attempts to alleviate DON-mediated cytotoxicity is limited. Dietary intake of antioxidants has come to the attention of oxidants scavenging, contradictory to apoptosis and inflammatory, and anti-carcinogenic assets (Vina et al., 2006). Among dietary antioxidants, quercetin (QUE) is one of the noticeable bioflavonoids and that usually found in certain fruits and vegetables, including apples, passionflower, buckwheat, black and green tea, figs, citrus fruits, grapes, etc. (Formica and Regelson, 1995). As a protective agent, QUE has demonstrated several pharmacological activities, including cardioprotective, vasoprotective, neuroprotective, and antitumor pursuits (Gupta et al., 2016). QUE is a powerful antioxidant compound and in the clinical settings has been implicated in maintaining the cell health through upholding the oxidative balance of the cell (Cheserek et al., 2016, Lee et al., 2016). So far, there is no report on the use of QUE as a cytoprotective agent against DON mediated oxidative imbalance, inflammatory responses, and apoptosis in animal systems either in-vivo or in-vitro.
The objective of this contemporary investigation was to evaluate the molecular mechanism underlying the cytotoxic potential of DON using SH-SY5Y human neuronal cell line with special reference to oxidative stress and apoptosis mechanisms. Furthermore, we studied the cytoprotective role of QUE on DON in reversing the cytotoxicity in human neuronal cells.
2. Materials and methods
2.1. Chemicals and reagents
Deoxynivalenol (DON), 4′,6-diamidino-2-phenylindole (DAPI), penicillin, rhodamine 123, (3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide) (MTT), BCA and malondialdehyde (MDA) assay kits, streptomycin, 2′,7′-dichloro-dihydro-fluorescein diacetate (DCFH-DA), thiobarbituric acid, DMEM/F-12 medium, O-phthaldialdehyde, propidium iodide, quercetin (QUE), RNA-isolation and cDNA synthesis kits, 2X Syber green master mix, fetal bovine serum (FBS), and oligonucleotide primers were attained from Sigma Aldrich (Bengaluru, India). The other chemicals and reagents were obtained from Merck (Bengaluru, India).
2.2. Cell culture and maintenance
The neuronal cell line model of Homo sapiens, SH-SY5Y neuronal cancer cells was acquired from the National Centre for Cell Sciences, India. Neuronal cells were cultured in DMEM/F-12 (1:1) medium accompanied with 10% FBS, penicillin (100 U/mL), and streptomycin (100 μg/mL) in a moist incubator chamber of 5% CO2 at 37 °C. Neuronal cells were typically grown in 75 cm2 flask, and media were renewed at every substitute day. Succeeding, confluent cells were collected and used in investigations throughout the report. Besides, stock solution of DON and QUE made in DMSO (0.05%) and further diluted with DMEM/F-12 media devoid of FBS at required concentrations and measured as test samples.
2.3. Effect of QUE and DON on cell viability
2.3.1. MTT assay
The effects of DON and QUE on cell growth of SH-SY5Y cells were revealed by MTT evaluation (Maroli et al., 2019). Briefly, 50,000 cells were plated into 96-well plates and permitted to stick for 6 h. Following, cells were exposed distinctly with distinctive concentrations of DON (up to 200 µM) and QUE (up to 10 mM) for two-time intervals, i.e. 6 and 24 h. The cells not exposed either with DON or QUE was considered as control. After, MTT (5 mg/mL) solution of 10 µL was supplemented into the culture medium and allowed to react for 4 h. Subsequently, medium containing MTT was swapped by DMSO for 30 min and transmission density was calculated using the plate reader at 570 nm (Tecan, Switzerland). The cell proliferation of test samples was concluded concerning control (100%).
2.3.2. LDH assay
The effect of QUE and DON on plasma membrane impairment was measured by the quantity of extracellular LDH released (Visconti et al., 1991, Maroli et al., 2019). Briefly, 5 lakh cells/well were plated in 24-well cell culture plates and permitted to stick for 6 h. Ensuing, cells were exposed distinctly with various doses of DON (up to 200 µM) and QUE (up to 10 mM) in DMEM/F-12 media devoid of FBS for two-time intervals, i.e. 6 and 24 h. Following, cells were scrapped out and the supernatant was collected at 3,250 rpm at 4 °C for 5 min and culture supernatant was used for quantification of LDH using kit as per instructions of the manufacturer. The untreated cells were lysed with 2% Triton X-100 and used as a reference to denote the LDH content in test samples.
2.4. Cell viability restoration efficacy of QUE on DON-induced toxicity
2.4.1. Cell viability assays
Cell viability restoration of QUE pre-treatment cells was tested against DON-induced toxicity by cell viable assays, i.e. MTT and LDH leakage assays (Visconti et al., 1991, Maroli et al., 2019). Approximately, 50 thousand cells (MTT assay) and 5 lakh cells (LDH assay) were correspondingly plated in 96 and 24-well cell culture plate and treated with QUE (up to 10 mM) for 6 h. Following, QUE pre-treated cells were exposed for 24 h with 100 µM of DON, and a superlative attenuating dose of QUE was determined by MTT and LDH leakage assays.
2.4.2. Effect of DON on cell morphological changes and its restoration by QUE
Approximately, 10 lakh cells were plated in 6-well cell culture plates and enabled to attach for 6 h. Next, cells were exposed to distinctive doses of DON (up to 100 µM) for 24 h with or without pre-treatment of superlative protective concentration of QUE (1 mM) for 12 h. Succeeding, the morphology of cells was perceived under the microscope, and images were captured (Olympus, Japan).
2.5. Cytoprotective role of QUE on DON-induced oxidative stress
2.5.1. Effects of DON on ROS generation and its attenuation by QUE
ROS content was measured spectrophotometrically using 2, 7- DCFH-DA probe (Ali et al., 1992, Kalagatur et al., 2017, Ramachandrappa et al., 2019, Swaminathan et al., 2019). Approximately, cells were plated at the compactness of 5 lakh cells/well in 24-well cell culture plates and allowed to stick for 6 h. Succeeding, treated with the different doses of DON (up to 100 µM) for the period of 6 and 12 h. In another set of studies, to reveal the cytoprotective role QUE, cells were pre-treated with superlative protective doses of QUE (up to 1 mM) for 6 h and treated to 100 µM of DON for 24 h. Upon incubation, test samples (cells) were stained for 45 min in DMEM/F-12 media devoid of FBS with 5 µM of DCFH-DA. Succeeding, cells were washed off for two times with PBS pH 7.4 and DON-induced ROS generation and its reversal by QUE was observed by fluorescence microscopy and fluorometric quantification was accomplished at an excitation/emission of 485 and 522 nm (Tecan, Switzerland).
2.5.2. Effects of DON on lipid peroxidation and its attenuation by QUE (TBRS assay)
The cells were exposed to DON and QUE as mentioned in the earlier section “Effects of DON on ROS generation and its attenuation by QUE”. Following, cells were disrupted using cell lysis buffer under ice-cold and protein quantified by BCA protein assay kit (Ohkawa et al., 1979, Maroli et al., 2019). MDA analysis was done using a kit as per directives as of the manufacturer and used for quantifying the malondialdehyde (MDA).
2.5.3. Effects of DON on antioxidant enzyme levels and its restoration by QUE
The impact of DON on the level of antioxidant enzymes (GSH, CAT, and SOD) and its restoration by QUE were determined by Maroli et al. (2019). The cells were exposed to DON and QUE as mentioned in the earlier section “Effects of DON on ROS generation and its attenuation by QUE”. Succeeding, cells were exposed to wash with cold DPBS for two times and cells were rigorously disrupted with cell lysis buffer and cellular supernatant was collected by centrifugation at 12,500 rpm for 8 min at 4 °C. The determination of GSH (nM), CAT (U/mg of protein), and SOD (U/mg of protein) from the culture supernatant was completed as per directives of the kit’s manufacturer (Sigma-Aldrich, India).
2.6. Effects of DON on MMP and its restoration by QUE
MMP was quantified with selective probe Rhodamine 123 dye (Scaduto and Grotyohann, 1999, Kalagatur et al., 2018, Gunti et al., 2019). About, 5 lakh cells/well were plated in 24 well plates and exposed with DON and QUE as mentioned in the earlier section “Effects of DON on ROS generation and its attenuation by QUE”. Next, 10 µg/mL of Rhodamine 123 was used to stain for 60 min at 37 °C in DMEM/F-12 media devoid of FBS. Succeeding, cells were subjected to wash off for twice with PBS pH 7.4, and DON-induced MMP loss and its restoration by QUE were observed by fluorescence microscopy and quantified the fluorescence at an excitation/emission of 485/535 nm using the plate reader (Tecan, Switzerland).
2.7. Effects of DON on nuclear DNA damage and apoptosis and its reversal by QUE
2.7.1. Comet assay
Approximately, 5 lakh cells/well were plated in 24-well cell culture plates and exposed with DON and QUE as mentioned in the earlier section “Effects of DON on ROS generation and its attenuation by QUE”. Following, Comet assay was completed to evaluate the DNA impairment induced by DON and its reversal by QUE followed by Singh et al. (1988). The obtained outcomes were communicated as a percent of tail length.
2.7.2. DAPI staining
DAPI assessment was undertaken to perceive the apoptosis triggered by DON in SH-SY5Y cells and its reversal by QUE (Kalagatur et al., 2018). Briefly, 5 lakh cells/well were plated in 24-well cell culture plates and exposed with DON and QUE as mentioned in the earlier section “Effects of DON on ROS generation and its attenuation by QUE”. Following, treated cells were stained with 100 ng/mL of DAPI in DMEM/F-12 media devoid of FBS, and the reaction was allowed for 10 min at 37 °C. Succeeding, cells were washed off two times with PBS pH 7.4 and apoptotic nuclei were perceived under the fluorescent microscope with DAPI filter (ZEISS microscope).
2.7.3. Propidium iodide staining and flow cytometry
A flow cytometry study was carried out to reveal the DON-induced nuclear apoptosis and its reversal by QUE (Agrawal et al., 2012). Briefly, 5 lakh cells/well were plated in 24-well cell culture plates and exposed with DON and QUE as mentioned in the earlier section “Effects of DON on ROS generation and its attenuation by QUE”. Following, cells were stained for 1 hr with 10 µg/mL of propidium iodide and washed with PBS pH 7.4 for twice. Next, distinctive nuclei were measured using a BD Accuri flow cytometer (BD, New Jersey, US) and data was processed using MODFIT software.
2.8. DON-induced regulation of neuronal biomarkers and its reversal by QUE pre-treatment
Briefly, 1 × 102 lakh cells were grown in 75 cm2 flasks and allowed to stick for 6 h. Following, cells were exposed to DON and QUE as mentioned in the earlier section “Effects of DON on ROS generation and its attenuation by QUE”. Next, cells were scrapped and total RNA was isolated and cDNA was synthesized using a kit conferring to the manufacturer’s directions.
The transcription expression of neuronal biomarkers, i.e. aromatic L-amino acid decarboxylase (AADC), brain-derived neurotrophic factor (BDNF), and tyrosine hydroxylase (TH) was assessed by quantitative real-time PCR. The sequences of target genes primers were shown in Table 1. The analysis was done in Roche Light Cycler 480 as per the instructions of the manufacturer. The relative fold level of transcript expression was conquered from the 2nd derivative maximum investigation over the crossing points of the transcript. The crossing point of the genes was normalized to the respective crossing point values for the housekeeping gene (β-2 myoglobulin). Obtained gene expression results processed by Roche Applied Science E-Method and results were shown as normalized ratios with standard error (Tellmann and Geulen, 2006).
Table 1.
Primer sequences for genes used in study.
| Gene target | Gene | Primer sequence | Tm (°C) |
|---|---|---|---|
| β-2 myoglobulin | B2m-F | ACAGGTTGCTCCACAGGTA | 57 |
| B2m-R | GAGTGCAAGAGATTGAAGAG | ||
| Brain derived neurotrophic factor | BDNF-F | ATGACCATCCTTTTCCTTACT | 56 |
| BDNF-R | GCCACCTTGTCCTCGGAT | ||
| Tyrosine hydroxylase | TH-F | GAGGAGAAGGAGGGGAAG | 58 |
| TH-R | ACTCAAACACCTTCACAGCT | ||
| Aromatic L-amino acid decarboxylase | AADC-F | AACAAAGTGAATGAAGCTCTTC | 58 |
| AADC-R | GCTCTTTGATGTGTTCCCAG |
2.9. Statistical analysis
Experiments were executed independently in six repeats (n = 6), and outcomes were communicated as mean ± standard deviation. The statistical differences between control and target experiment groups were decided following Dunnett’s test, and significance was determined at p ≤ 0.05.
3. Results
3.1. Toxic effect of DON on cell viability and its restoration by QUE
3.1.1. Effects of DON and QUE on cell viability
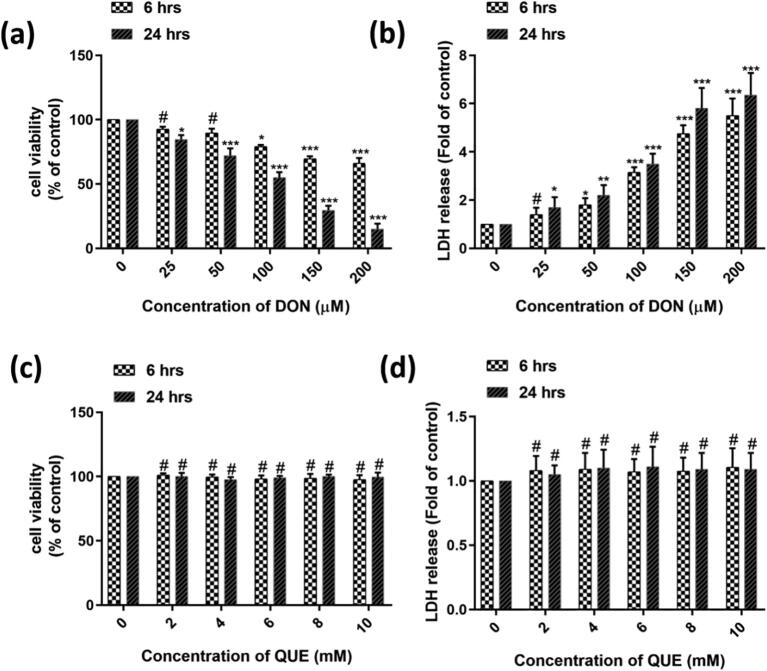
Dose and time-dependent influence of DON and QUE on the viability of cells was completed by cell viable assays, i.e. MTT and LDH assays (Fig. 1). MTT evaluation was executed with different doses of DON (up to 200 µM) and QUE (up to 10 mM). As shown in Fig. 1a, DON has not shown a significant difference in cell viability at a subsidiary dose of 50 µM, and further cell viability was significantly decreased with increasing DON concentration (100, 150, and 200 µM) related to control during the exposure period of 6 h. At 24 h of post-treatment of DON, cells demonstrated significantly lowered cell viability at the lower concentration of DON (25 µM). The cell viability of about 15% was noticed at 200 µM of DON related to control cells for 24 h. The MTT study revealed that DON affected the cell viability in the mode of dose and time-dependent.
Fig. 1.
Dose and time-dependent effect of deoxynivalenol (DON) and quercetin (QUE) on the viability of SH-SY5Y cells. (a) Dose and time-dependent effect of DON on cell viability by MTT assay. (b) Dose and time-dependent effect of DON on cellular membrane damage and LDH leakage. (c) Dose and time-dependent effect of QUE on cell viability by MTT assay. (d) Dose and time-dependent effect of QUE on cellular membrane damage and LDH leakage. The studies were executed in six replicates (n = 6) and results were expressed as mean ± SD. The statistical difference between control and test groups was determined following Dunnett’s test and significance was determined at p ≤ 0.05 (* ≤ 0.05, ** < 0.01, and *** < 0.001). The p-value was considered as not significant at > 0.05 and denoted as ‘#’.
Further, cytotoxicity of DON was confirmed by another parameter, i.e. LDH release (Fig. 1b). The level of extracellular LDH is reliant on dead cells, and it is directly proportionate. In this study, DON has dose and time-dependently increased the release of LDH and the highest LDH outflow was ascertained at 24 h of post-exposure at 200 µM of DON. The result of the study was in agreement with the MTT assay.
On the contrary, no significant cell fatality was noticed in QUE exposed cells (up to 10 mM) contrasted to control as perceived in both the cell viability studies, i.e. MTT and LDH assays (Fig. 1c and d).
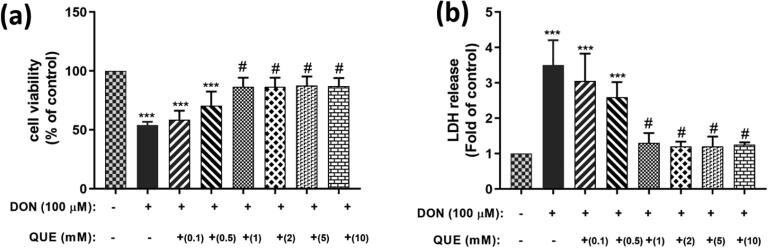
3.1.2. Effect of QUE pre-treatment on DON-induced cell viability and membrane damage
In this study evaluated the protective efficacy of antioxidant QUE pre-treatment against DON induced cytotoxic effects in SH-SY5Y cells. Different concentrations of QUE were tested (0.1, 0.5, 1, 2, 5, and 10 mM) as pre-treatment for 6 h followed by the challenge with a higher dose of 100 µM DON for 24 h. Cell viability was completed by MTT appraisal, and the results showed that 1 mM QUE concentration was effective in reversing the decrease in viability owed to DON (Fig. 2a). QUE concentration above 1 mM did not give any additional protection against DON. Similarly, QUE at 1 mM protected the DON (100 µM) induced cell killing by restoring the plasma membrane integrity and LDH leakage (Fig. 2b).
Fig. 2.
Dose-dependent protective efficacy of QUE (0.1 – 10 mM) against DON (100 µM) induced cell death by (a) MTT and (b) LDH assay for 24 h. The studies were executed in six replicates (n = 6) and results were expressed as mean ± SD. The statistical difference between control and test groups was determined following Dunnett’s test and significance was determined at p ≤ 0.05 (* ≤ 0.05, ** < 0.01, and *** < 0.001). The p-value was considered as not significant at > 0.05 and denoted as ‘#’.
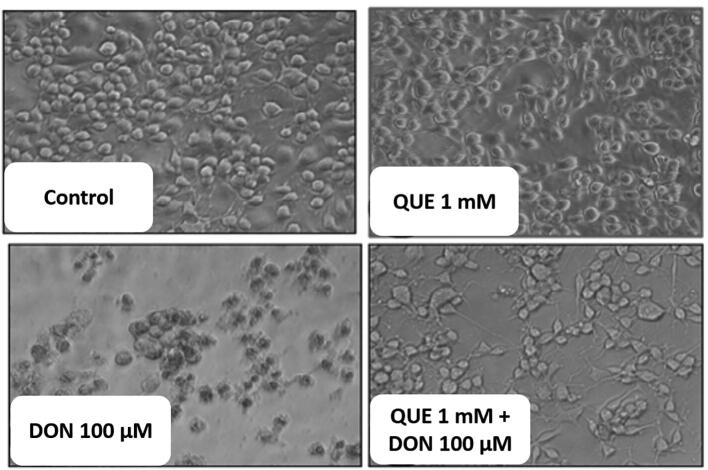
3.1.3. Effects of DON on cell morphology and its restoration by QUE
Morphological changes in SH-SY5Y cells after DON treatment for 24 h were shown in Fig. 3. The cytotoxic effects include the decrease in cell density, disruption of monolayers, and rounding of cells with increasing the concentration of DON. On the contrary, cells pre-treated with QUE (1 mM) and followed by DON exposure (100 µM) have shown healthy morphology and characteristic of control cells compared to cells treated with 100 µM of DON (Fig. 4).
Fig. 3.
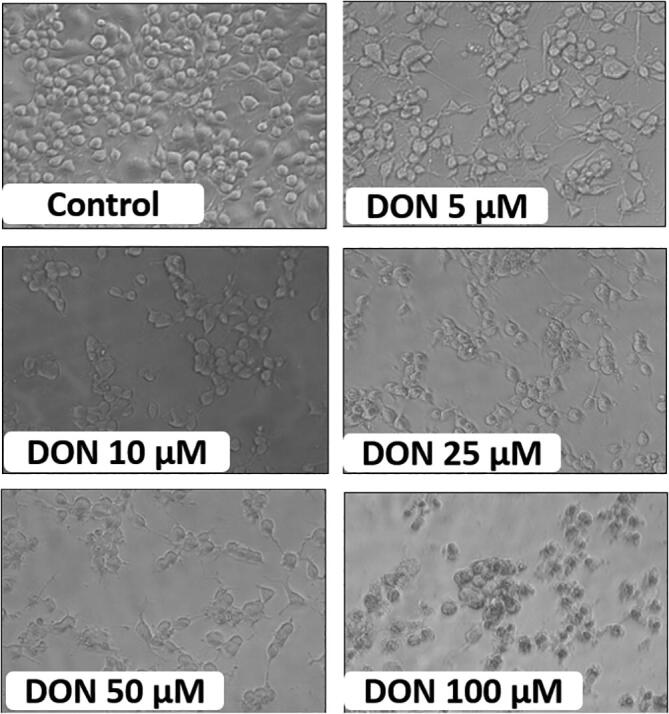
Dose-dependent detrimental effect of DON on micro-morphology of SH-SY5Y cells at 0 (control), 5, 10, 25, 50, and 100 µM for 24 h. Control cells display monolayer and healthy in micro-morphology. Whereas, cells treated with DON (dose-dependent) exhibit altered micro-morphology, i.e. loss of cell density and integrity, and formation of apoptotic bodies. The images were captured at a magnification of 400x.
Fig. 4.
Protective efficacy of QUE against DON induced detrimental changes in the micro-morphology of SH-SY5Y cells for 24 h. Control cells depict healthy morphology. The cells treated with 1 mM of QUE exhibit healthy morphology. Whereas, cells treated with DON (100 µM) exhibit altered morphology, i.e. loss of cell integrity and density, and formation of apoptotic bodies. Remarkably, cells pre-treated with QUE (1 mM) and followed by exposed to DON (100 µM) present restored healthy cell morphology and density. The images were captured at a magnification of 400x.
3.2. Attenuation role of QUE on DON-induced oxidative stress
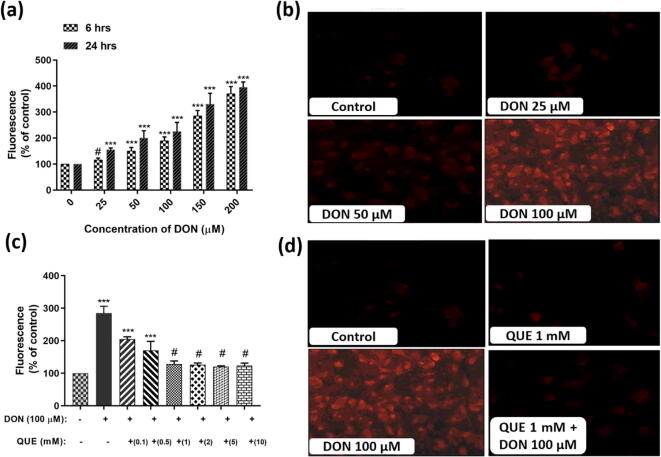
3.2.1. DON-induced ROS generation and its attenuation by QUE
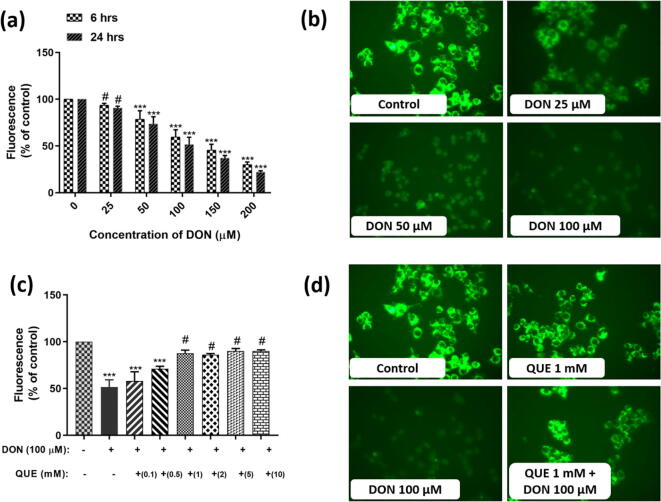
The influence of DON on intracellular ROS formation and its restoration by pre-exposure with QUE was determined by DCFH-DA staining. The intracellular ROS content was perceived qualitatively by fluorescence microscopy and quantitatively by fluorimetry (Fig. 5). In Fig. 5a and b, fluorescent microscopic images and fluorimetry data of control and DON treated cells were depicted. The strength of fluorescence (intracellular ROS levels) was quite elevated in cells exposed to DON related to control cells and the intracellular ROS level was found dependent upon the dose of DON. The study conveyed that DON induces the oxidative stress through intracellular ROS generation.
Fig. 5.
DON-induced ROS generation and its attenuation by pre-treatment with QUE in SH-SY5Y cells. (a) Dose-dependent effect of DON on intracellular ROS generation determined by fluorometry quantification for 6 and 24 h. (b) Fluorescent microscopic images depict the dose-dependent effect of DON on intracellular ROS generation (strength of fluorescence) for 24 h. (c) Dose-dependent attenuation efficiency of QUE (0.1 – 10 mM) against DON (100 µM) induced intracellular ROS generation for 24 h. (d) Fluorescent microscopic images depict the protective efficacy of QUE (1 mM) against DON (100 µM) induced intracellular ROS generation (strength of fluorescence). The images were captured at a magnification of 400x. The studies were executed in six replicates (n = 6) and results were expressed as mean ± SD. The statistical difference between control and test groups was determined following Dunnett’s test and significance was determined at p ≤ 0.05 (* ≤ 0.05, ** < 0.01, and *** <0.001). The p-value was considered as not significant at > 0.05 and denoted as ‘#’.
The attenuation effect of antioxidant QUE against DON induced intracellular ROS generation was shown in Fig. 5c and d. Interestingly, lowered level of intracellular ROS was noticed in cells pre-treated with 1 mM of QUE and followed by exposed to DON (100 µM) related to cells treated with DON (100 µM) (Fig. 5c). The fluorescent microscopic images depict that cells pre-treated with 1 mM of QUE for 6 h and followed by exposed to DON (100 µM) shown lower strength of fluorescence (intracellular ROS levels) related to DON treated cells (100 µM) for 24 h (Fig. 5d). The study reveals that antioxidant QUE effectively scavenges the DON induced intracellular ROS molecules and protects the cells.
3.2.2. DON-induced LPO and depletion of antioxidant enzymes and its attenuation by QUE
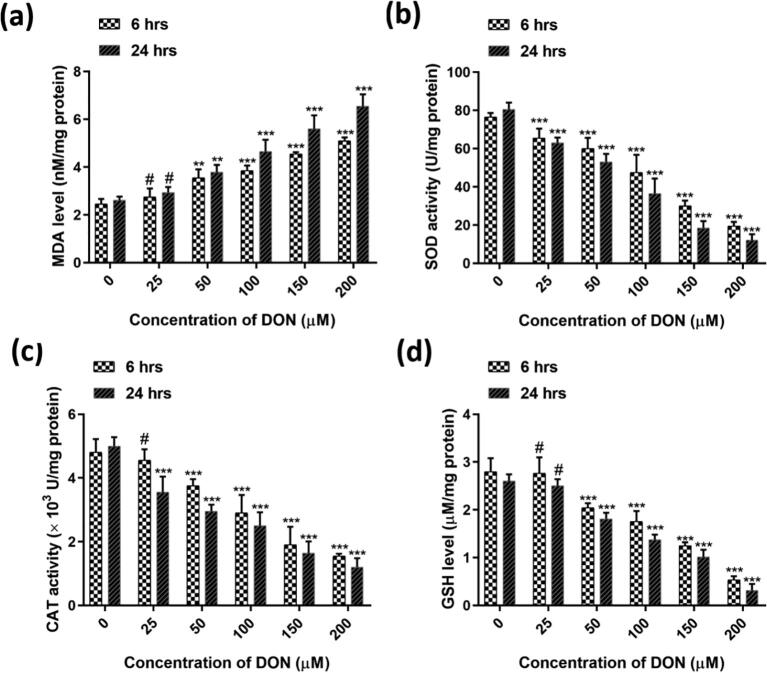
The dose-dependent influence of DON (up to 200 µM) on LPO and antioxidant enzyme levels of cells were studied for 6 h and 24 h (Fig. 6). The impact of DON on LPO was calculated in rates of MDA. The DON treatment raised the LPO in the mode of dose-dependent (Fig. 6a). On the other hand, DON depleted the antioxidant enzyme levels (SOD, CAT, and GSH) in cells, and it was established in dose-dependent style (Fig. 6b, c, and d). The study reveals that DON induces oxidative stress over the induction of LPO and exhaustion of antioxidant enzymes.
Fig. 6.
Dose-dependent effect of DON on (a) escalation of lipid peroxidation (MDA level) and depletion of (b) SOD, (c) CAT, and (d) GSH antioxidant enzymes for 6 h and 24 h. The studies were executed in six replicates (n = 6) and results were expressed as mean ± SD. The statistical difference between control and test groups was determined following Dunnett’s test and significance was determined at p ≤ 0.05 (* ≤ 0.05, ** < 0.01, and *** < 0.001). The p-value was considered as not significant at > 0.05 and denoted as ‘#’.
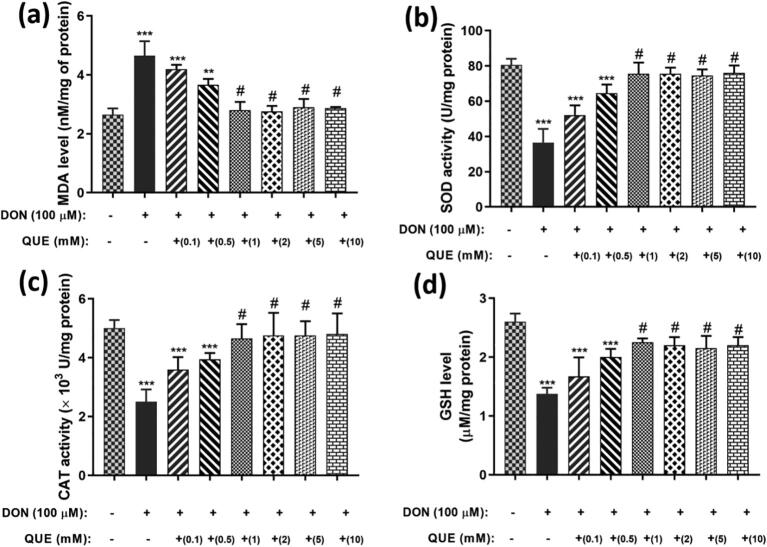
In another investigation, cells pre-exposed with QUE (1 mM) for 6 h and chased by exposed to DON (100 µM) for 24 h exhibited a significant reduction in lipid peroxidation and upheld antioxidant enzyme levels related to DON (100 µM) treated cells (Fig. 7). The results revealed that antioxidant QUE protects the cells from DON-induced LPO and depletion of antioxidant enzyme levels (GSH, CAT, and SOD).
Fig. 7.
Dose-dependent attenuation efficiency of pre-treated QUE (0.1 – 10 mM) against DON (100 µM) induced (a) lipid peroxidation (MDA level) and depletion of (b) SOD, (c) CAT, and (d) GSH antioxidant enzymes in SH-SY5Y cells for 24 h. The studies were executed in six replicates (n = 6) and results were expressed as mean ± SD. The statistical difference between control and test groups was determined following Dunnett’s test and significance was determined at p ≤ 0.05 (* ≤ 0.05, ** < 0.01, and *** < 0.001). The p-value was considered as not significant at > 0.05 and denoted as ‘#’.
3.3. Attenuation effect of QUE on DON-induced MMP loss
MMP is vital for the functionality of mitochondria. The loss of MMP causes a decline in ATP synthesis and endorses the apoptotic death of cells. In the contemporary investigation, the influence of DON on MMP loss and its reversal by QUE was appraised by rhodamine 123 staining. The MMP level was assessed qualitatively by fluorescence microscopy and quantitatively by fluorimetry (Fig. 8).
Fig. 8.
DON-induced MMP loss and its attenuation by pre-treatment with QUE in SH-SY5Y cells. (a) Dose-dependent effect of DON on depletion on MMP levels determined by fluorometry quantification for 6 and 24 h. (b) Fluorescent microscopic images depict the dose-dependent effect of DON on MMP levels (strength of fluorescence) for 24 h. (c) Dose-dependent attenuation efficiency of QUE (0.1–10 mM) against DON (100 µM) induced MMP loss for 24 h. (d) Fluorescent microscopic images depict the protective efficiency of QUE (1 mM) against DON (100 µM) induced MMP loss (strength of fluorescence). The images were captured at a magnification of 400x. The studies were executed in six replicates (n = 6) and results were expressed as mean ± SD. The statistical difference between control and test groups was determined following Dunnett’s test and significance was determined at p ≤ 0.05 (* ≤ 0.05, ** < 0.01, and *** < 0.001). The p-value was considered as not significant at > 0.05 and denoted as ‘#’.
In the study, DON has detrimentally affected the health of the cells by declining the MMP in the fashion of the time and dose-dependent manner (Fig. 8a). In Fig. 8b, control cells exhibited high MPP levels (intense fluorescence). Whereas, cells treated with different concentrations of DON exhibited weak fluorescence (low MMP levels) related to control cells. The cells exposed to the high dose of 100 µM DON exhibited much low fluorescence related to control. The study determined that DON declined MMP levels of cells and could halt ATP synthesis.
In another study, cells pre-treated with 1 mM of QUE for 6 h and followed by exposed to 100 µM of DON has shown confrontation against DON (100 µM) induced MMP loss, and their MMP levels were sustained (Fig. 8c and d). These results suggest that DON-induced MMP loss could be reversed QUE pre-treatment and thereby, could protect the DON-induced cell death.
3.4. DON induced nuclear DNA damage and apoptosis and its reversal by QUE
3.4.1. QUE prevents the DON-induced DNA breakage
The influence of DON on DNA detriment and its attenuation by pre-exposed with QUE in SH-SY5Y cells was ascertained by comet analysis (Fig. 9, Fig. 10). The comet appraisal is established as the criterion that intact DNA restrains in a secured systematized matrix with protein in the nucleus and upon DNA damage, DNA-protein organization will be disrupted and the disorganized and shorter portions of broken DNA move quicker and were visualized in the line of a comet under electrophoresis. Whereas undamaged DNA strands in healthy cells maintain DNA-protein organization and do not withdrawal the cavity, and stretch of the comet will be minimal under electrophoresis. In the comet assay, the tail stretch of the comet is often calculated as a directory of DNA detriment of the cell.
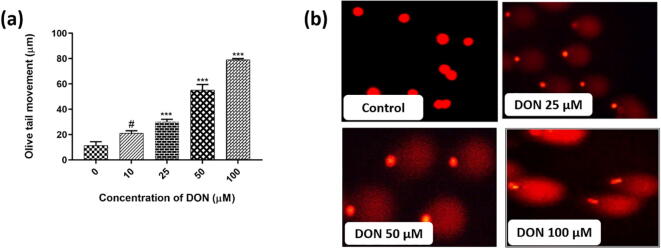
Fig. 9.
Effect of DON on DNA damage of SH-SY5Y cells by comet assay for 24 h. (a) Dose-dependent effect of DON on DNA damage (Olive tail movement) of cells. (b) Depict the Olive tail movement (DNA damage) of cells exposed with different concentrations of DON at 0 (control), 25, 50, and 100 µM. The images were captured at a magnification of 400x. The studies were executed in six replicates (n = 6) and results were expressed as mean ± SD. The statistical difference between control and test groups was determined following Dunnett’s test and significance was determined at p ≤ 0.05 (* ≤ 0.05, ** < 0.01, and *** < 0.001). The p-value was considered as not significant at > 0.05 and denoted as ‘#’.
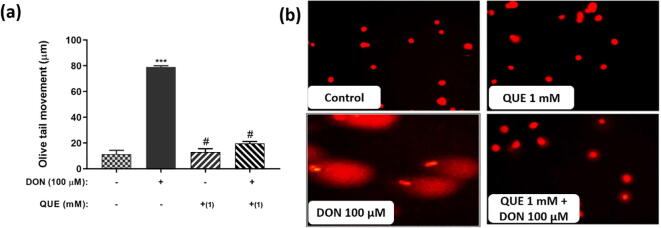
Fig. 10.
DON-induced DNA damage (Olive tail movement) and its attenuation by pre-treatment with QUE in SH-SY5Y cells. (a) Protective efficacy of QUE (pre-treated for 6 h) against DON induced DNA damage for 24 h. (b) Depict the protective efficacy of QUE pre-treatment (1 mM for 6 h) against DON (100 µM) induced DNA damage. The images were captured at a magnification of 400x. The studies were executed in six replicates (n = 6) and results were expressed as mean ± SD. The statistical difference between control and test groups was determined following Dunnett’s test and significance was determined at p ≤ 0.05 (* ≤ 0.05, ** < 0.01, and *** < 0.001). The p-value was considered as not significant at > 0.05 and denoted as ‘#’.
In the present study, stretch of the Olive tail indicated the trend of the dose-dependent intensification with the rise in the dose of DON (Fig. 9). The comet tail stretch of control cells was perceived as 11.0 ± 1.2 µm. Whereas, comet tail length was measured as 28 ± 2.1 µm at 25 µM of DON and, which was further amplified to 78 ± 5.3 µm at 100 µM of DON (Fig. 9a). At higher a concentration, most of the cells shown long Olive tails with no or not much DNA left in the head domain (Fig. 9b). Results display that DON-induced DNA damage may be the climax foremost event to the death of neuronal cells.
On the contrary, cells pre-exposed with QUE (1 mM) for 6 h and chased by DON exposed (100 µM) have reduced the Olive tail length compared to cells treated with 100 µM of DON (Fig. 10a and b). The results evidenced that QUE plays a substantial antioxidant character in contrast to DON-induced oxidative damage and protect the DNA of SH-SY5Y cells.
3.4.2. QUE attenuates the DON-induced apoptotic nuclei formation
In the contemporary investigation, effect of DON on nuclear apoptosis and its reverse by QUE in cells was ascertained by DAPI staining and flow cytometry. DAPI staining was completed to review the attendance of apoptotic and dispersion of nuclei after 24 h of DON exposure with or deprived of QUE pre-treatment (Fig. 11a). In the contemporary investigation, 24 h of DON exposure disclosed the significant upsurge in apoptotic nuclei formation contrasted to control cells. On the contrary, cells pre-treated with QUE (1 mM) and followed by DON (100 µM) exposure have shown the decrease in the number of apoptotic nuclei formation related to cells treated with 100 µM of DON (Fig. 11b). These effects are in line with the achieved outcomes of the comet assay.
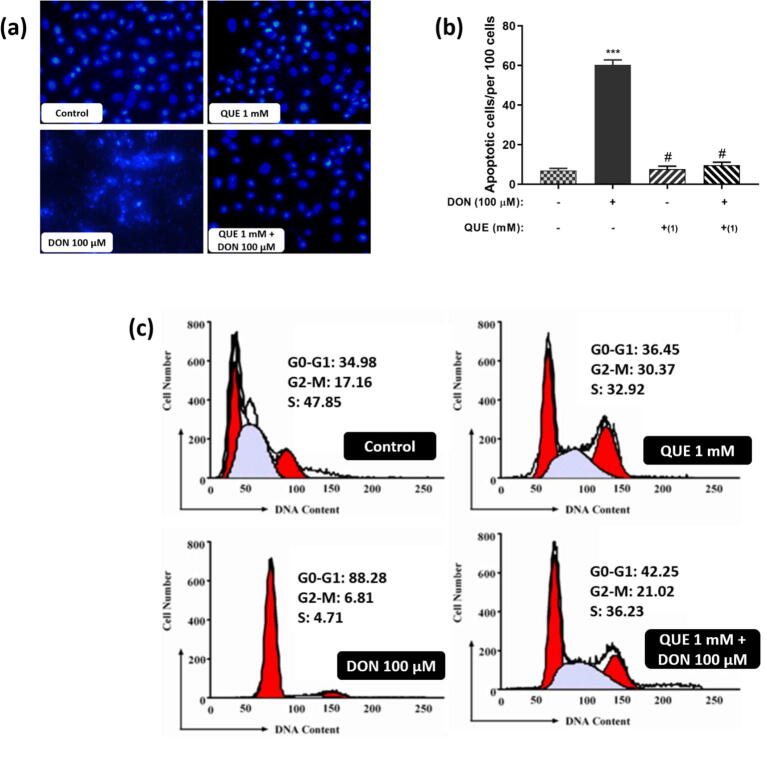
Fig. 11.
DON-induced apoptotic nuclei formation and its reversal by pre-treatment with QUE in SH-SY5Y cells. (a) Depict the attenuated efficacy of QUE against DON induced DNA damage for 24 h. The images were captured at a magnification of 400x. (b) Effect of DON (100 µM for 24 h) on apoptotic bodies formation and its reversal by pre-treatment with QUE (1 mM for 6 h). A minimum of 1000 cells was counted in 10 different microscopic fields. (c) Depict the protective efficacy of QUE (1 mM pre-treated for 6 h) against DON (100 µM for 24 h) induced cell cycle arrest for 24 h by flow cytometry analysis. The studies were executed in six replicates (n = 6) and results were expressed as mean ± SD. The statistical difference between control and test groups was determined following Dunnett’s test and significance was determined at p ≤ 0.05 (* ≤ 0.05, ** < 0.01, and *** < 0.001). The p-value was considered as not significant at > 0.05 and denoted as ‘#’.
To quantify the impact of DON-induced nuclear apoptosis and its reverse by QUE pre-treatment in SH-SY5Y cells, a flow cytometric investigation was attempted by probe propidium iodide (Fig. 11c). Cells exposed to DON solitary presented a significantly high count of cells in the sub-G1 phase contrasted to control. Conversely, cells pre-treated with QUE (1 mM) and chased by DON (100 µM) exposure declined the sub-G1 phase cells contrasted to DON-treated cells.
3.5. DON-induced regulation of target gene expression and its reversal by QUE pre-treatment
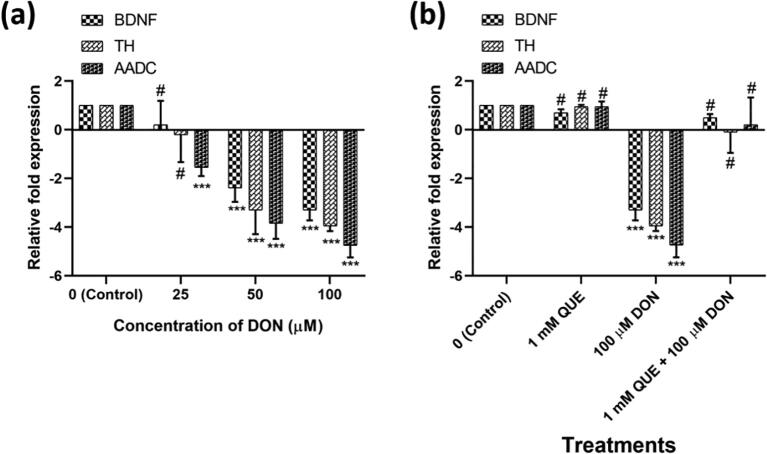
Impact of DON on neuronal markers (BDNF, TH, and AADC) expression and its setback by pre-treated with QUE and followed by DON treatment in SH-SY5Y cells was assessed by QRT-PCR (Fig. 12). BDNF and TH mRNA expression were not altered at 25 µM DON exposure, but significantly down-regulated at 50 and 100 µM. AADC gene expression was down-regulated at all three DON concentrations studied (Fig. 12a).
Fig. 12.
DON-induced transcription expression of neuronal biomarkers (BDNF, TH, and AADC) and its reversal by pre-treatment with QUE in SH-SY5Y cells assessed by quantitative real-time-PCR analysis. (a) Dose-dependent effect of DON on neuronal biomarkers (BDNF, TH, and AADC) for 24 h. (b) Attenuation efficiency of QUE (pre-treated with 1 mM for 6 h) against DON (treated with 100 µM for 24 h) induced transcription expression of neuronal biomarkers. The relative fold expression of targeted genes over control was calculated with β-2 myoglobulin as a house-keeping gene adopting the procedure of Roche Applied Science E-method. The studies were executed in six replicates (n = 6) and results were expressed as mean ± SD. The statistical difference between control and test groups was determined following Dunnett’s test and significance was determined at p ≤ 0.05 (* ≤ 0.05, ** < 0.01, and *** < 0.001). The p-value was considered as not significant at > 0.05 and denoted as ‘#’.
The gene expression levels of neuronal markers were not significantly changed on exposure to QUE and their levels were noticed as 0.7 ± 0.14, 0.95 ± 0.07, and 0.95 ± 0.21 for BDNF, TH, and AADC, respectively. Whereas, fold level of BDNF, TH, and AADC in 100 µM of DON treated cells were correspondingly down-regulated to −4.4 ± 0.42, −4.95 ± 0.21, and −5.75 ± 0.49 when compared to control cells. Whereas, cells pre-treated with QUE (1 mM for 6 h) and followed by treated with 100 µM of DON have shown up-regulation of neuronal markers (0.5 ± 0.14-fold for BDNF, −0.1 ± 0.84-fold for TH, and 0.2 ± 1.13-fold for AADC) related to cells treated with 100 µM of DON (Fig. 12b). The outcomes of the present investigation demonstrate that QUE offers the attenuation against DON-induced stress through the regulation of neuronal marker gene expression.
4. Discussion
Deoxynivalenol is ribotoxin and radically harmful to promptly proliferating cells, a resultant in immunocompromise in the humans and other farm animals. DON upsets human and animal well-being, resulting in transient fever with severe vomiting, nausea, giddiness, and looseness of the bowels (Pestka, 2007). DON was detected in cereals and its food products like flour, bakery foods, malt, and beer. For example, concentration of DON in fermented beer samples in Holland showed 26000–41000 µg/L and in German beers above 0.2 µg/mL (Sobrova et al., 2010). These levels were surpassed the provisional maximum tolerable daily intake as recommended by the European Commission, (2006).
In the present study, as a first objective, we investigated the mechanism of neurotoxicity of DON using SH-SY5Y cells. We selected a concentration range of 0–200 µM DON based on previously published in-vivo and in-vitro reports. DON-induced cytotoxicity in terms of loss of viability by MTT assay and LDH leakage in the fashion of dose and time-dependent. Loss of viability was observed as early as 6 h at higher concentrations and 24 h at a lower concentration of DON.
The toxic act of DON in mammal organization will be conceivably explicated using cellular and subcellular organizations (Bennett and Klich, 2003). The cytotoxic activity of DON on cells was well correlative with LDH outflow to the culture medium and ascertained morphologic transformations.
Dopamine nerve cells come across higher content of ROS than other nerve cells. Oxidative stress-induced by mitochondrial hindrance endorses the cell's decease precise to dopamine nerve cells escorted by Lewy body establishment (Murase and McKay, 2006). The aptitude to overcome oxidative stress is necessary for the survival of nerve cells, and the generation of ROS is implicated in several neurodegenerative diseases like Parkinson's and Alzheimer's (Yu et al., 2011). DON treatment in the contemporary investigation directed to upraised ROS levels at 6 h with a significant increase by 24 h in SH-SY5Y cells. DON-induced oxidative stress is well demonstrated through the build-up of MDA, which is resulted in when pro-oxidants collude with the lipid content of the cellular membrane. An in-vivo investigation by Frankic et al. disclosed DON-induced impairment in the liver, spleen, and lymphocytes of intoxicated laboratory animals as a result of free radical formation (Frankič et al., 2008). In thymocytes of Mus musculus, DON (0.5–8.0 mg/kg) has dose-dependently caused a considerable rise in apoptosis counts contrasted to controls under in-vivo (Schmeits et al., 2014). Also, in-vitro studies have revealed DON-induced apoptosis in the colon and Hep G2 cells of humans (Bensassi et al., 2009, Zhang et al., 2009).
The concurrence among oxidative stress and MMP has been concurrently considered using different mammalian cells and mycotoxins in human cervical cancer cells, HT-29 cells, murine embryonic stem cells (Chaudhari et al., 2009, Fang et al., 2012, Chen et al., 2013). Employing flow cytometric and immunofluorescence assays, Bensassi et al. (2012) revealed that DON induces apoptotic demise in colon cells HCT 116 cells through impairment of mitochondria, which include the onset of the mitochondrial permeability transition pore, MMP loss, and release of apoptotic messenger cytochrome-c. The earlier report of Chaudhari et al. (2009) demonstrated that mycotoxin T-2 prompts MMP depletion and extricates of apoptogenic factors, i.e. Bax, Bcl-2, cytochrome-c, which will be engaged in by activation of caspases. These studies support our findings that DON-induced ROS generation leading to MMP loss in neuronal cells is an influential cellular event leading to the decease of the cells.
The results of alkaline comet assay in our investigation revealed DON-induced dose and time-dependent DNA damage in SH-SY5Y cells by 24 h of post-treatment and DNA damage was observed at even lower concentrations of 5 and 10 µM of DON. However, a greater stretch of the Olive tail moment was perceived in a higher concentration of DON (100 µM). In support of our inference, Hsia et al. (2004) has extracted the DON from contaminated food matrices and demonstrated the chromosome aberrations feature of DON in Chinese hamster V79 cells and revealed that chromosome aberrations are as a result of the detrimental interaction of hydroxyl radical with nuclear material of the cell. The conclusions made in our study indicated that DNA damage induced by DON was precisely due to the generation of intracellular ROS and LPO. Further, Zhang et al. (2009) reported an upsurge in DNA damage at a higher dose of DON in HepG2 cells. In our study, a greater stretch of Olive tail moment was perceived at 100 µM of DON and, which was well following the report of Zhang et al. (2009).
The neuronal biomolecules viz., TH, BDNF, and AADC play the pivotal representation in the cell endurance as well as in the divergence of dopaminergic nerve cells (Chen et al., 2013). In this study, expression level of BDNF, TH, and AADC genes was assessed by QRT-PCR. DON treatment ensued in the down-regulation of mRNA expression of neuronal biomarkers in the fashion of dose-dependent. DON transformed the gene expression of neuronal biomarkers to confirm its neurotoxic perspective. BDNF has been exhibited to encourage the proliferation of dopaminergic nerve cells and subsidence in the formation of free radicals (Gerecke et al., 2012). BDNF is well recognized for its role of antioxidative and has evidenced its role in the enhancement of the activity of antioxidant enzymes. Amyloid β-peptide /BDNF relation could also be elucidated by Aβ interference with signaling pathways used by BDNF to drive its defensive effects. The process of signaling pathway comprises of BDNF- induced Arc protein expression, CREB phosphorylation, or its nuclear translocation (Arancibia et al., 2008). Only a few reports are present on the noxious impact of Fusarium toxins on neurotransmitter metabolism (Pestka, 2007, Venkataramana et al., 2014, Wang et al., 2018). The gene expression results of our study show that DON is mediating neurotoxicity through suppression of specific neuronal and antioxidant biomarkers, which perform a vital play in safeguarding the neuronal cells in contrast to oxidative stress.
As a second objective of the present investigation, we studied the cytoprotective efficacy of QUE pre-treatment on the reversal of DON-induced toxicity in SH-SY5Y cells. QUE is an effective scavenger of free radicals and is considered as a potent antioxidant. These properties of quercetin enable it to interfere with lipid peroxidation and mitigate oxidative stress-mediated cell death (Boots et al., 2008). In our experimental conditions, QUE exerted defensive property in contrast to the DON-induced stress by counterbalancing the ROS production, reversing the ROS stepped in lipid peroxidation, up-holding intracellular GSH levels, and sustaining the MMP and DNA integrity. QUE pre-treatment also leads to the up-regulation of neuronal biomarker gene expressions, which leads to effective survival and controlled neuronal transmitter metabolism of SH-SY5Y cells under DON-induced stress. Previous reports have revealed that QUE is an effective scavenger of free radicals and plays a role as an inhibitory characteristic against oxidative damage induced by DON in murine lymphoma cells (Strasser et al., 2013). Furthermore, Soundararajan et al. (2008) unveiled that QUE protected the SH-SY5Y cells resistingly oxidative damage through biosynthesis of SREBP-2 cholesterol. Moreover, flavonoid QUE protected human neuroblastoma cells from oxidative stress by inhibiting KLF4 (Xi et al., 2012). These reports combined with our details propose a promising mechanism of attenuation by which QUE could be a protector for SH-SY5Y cells from DON-induced oxidative stress.
5. Conclusion
The outcomes of our investigation established that DON-induced dose and time-dependent cytotoxicity patterns in terms of ROS generation, LPO, intracellular GSH release, loss of MMP, and DNA damage in SH-SY5Y cells. Also, down-regulation of gene countenance of neurotrophic factors viz. BDNF, TH, and AADC showed that DON-induced cell death leads to impaired regulation of neurotransmitter release as well as the decrease in neuronal function. Due to observed antioxidant and free radical scavenging properties put together, signify that QUE has a possible antidote for DON-mediated toxicosis. QUE pre-treatment attenuated the DON-induced toxicity viz., mitigation of ROS production, restoration of MMP, upholding the antioxidant enzyme levels, and conserving nuclear DNA integrity. QUE treatment also resulted in the up-regulation of brain neuronal marker gene expressions, thereby controlling the apoptosis responses in SH-SY5Y cells. The investigation shows a newfangled insight on the oxidative, apoptotic, as well as neuromodulator assets of QUE. However, it should be validated in in-vivo animal models before going to human administration.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
The first author acknowledges the Department of Science and Technology (DST), Govt of India for providing National Post-Doctoral Fellowship (NPDF). The authors would also like to acknowledges DST-PURSE program, DST, New Delhi to the University of Mysore, Mysuru-570 005, India. The authors would like to extend their sincere appreciation to the Researchers Supporting Project Number (RSP-2020/134), King Saud University, Riyadh, Saudi Arabia.
Footnotes
Peer review under responsibility of King Saud University.
Contributor Information
Naveen Kumar Kalagatur, Email: knaveenkumardfrl@gmail.com.
Venkataramana Mudili, Email: ramana.micro@gmail.com.
Chandranayaka Siddaiah, Email: moonnayak@gmail.com.
References
- Agrawal M., Yadav P., Lomash V., Bhaskar A.S.B., Rao P.L. T-2 toxin induced skin inflammation and cutaneous injury in mice. Toxicology. 2012;302(2–3):255–265. doi: 10.1016/j.tox.2012.08.007. [DOI] [PubMed] [Google Scholar]
- Ali S.F., LeBel C.P., Bondy S.C. Reactive oxygen species formation as a biomarker of methylmercury and trimethyltin neurotoxicity. Neurotoxicology. 1992;13(3):637–648. [PubMed] [Google Scholar]
- Alonso-Garrido M., Tedeschi P., Maietti A., Font G., Marchetti N., Manyes L. Mitochondrial transcriptional study of the effect of aflatoxins, enniatins and carotenoids in vitro in a blood brain barrier model. Food Chem. Toxicol. 2020:111077. doi: 10.1016/j.fct.2019.111077. [DOI] [PubMed] [Google Scholar]
- Alshannaq A., Yu J.H. Occurrence, toxicity, and analysis of major mycotoxins in food. Int. J. Environ. Res. Public Health. 2017;14(6):632. doi: 10.3390/ijerph14060632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arancibia S., Silhol M., Mouliere F., Meffre J., Höllinger I., Maurice T., Tapia-Arancibia L. Protective effect of BDNF against beta-amyloid induced neurotoxicity in vitro and in vivo in rats. Neurobiol. Dis. 2008;31(3):316–326. doi: 10.1016/j.nbd.2008.05.012. [DOI] [PubMed] [Google Scholar]
- Bennett J., Klich M. Mycotoxins. Clin. Microbiol. Rev. 2003;16:497–516. doi: 10.1128/CMR.16.3.497-516.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bensassi F., El Golli-Bennour E., Abid-Essefi S., Bouaziz C., Hajlaoui M.R., Bacha H. Pathway of deoxynivalenol-induced apoptosis in human colon carcinoma cells. Toxicology. 2009;264(1–2):104–109. doi: 10.1016/j.tox.2009.07.020. [DOI] [PubMed] [Google Scholar]
- Bensassi F., Gallerne C., El Dein O.S., Lemaire C., Hajlaoui M.R., Bacha H. Involvement of mitochondria-mediated apoptosis in deoxynivalenol cytotoxicity. Food Chem. Toxicol. 2012;50(5):1680–1689. doi: 10.1016/j.fct.2012.01.015. [DOI] [PubMed] [Google Scholar]
- Berthiller F., Sulyok M., Krska R., Schuhmacher R. Chromatographic methods for the simultaneous determination of mycotoxins and their conjugates in cereals. Int. J. Food Microbiol. 2007;119(1–2):33–37. doi: 10.1016/j.ijfoodmicro.2007.07.022. [DOI] [PubMed] [Google Scholar]
- Boots A.W., Haenen G.R., Bast A. Health effects of quercetin: from antioxidant to nutraceutical. Eur. J. Pharmacol. 2008;585(2–3):325–337. doi: 10.1016/j.ejphar.2008.03.008. [DOI] [PubMed] [Google Scholar]
- Chaudhari M., Jayaraj R., Bhaskar A.S.B., Rao P.L. Oxidative stress induction by T-2 toxin causes DNA damage and triggers apoptosis via caspase pathway in human cervical cancer cells. Toxicology. 2009;262(2):153–161. doi: 10.1016/j.tox.2009.06.002. [DOI] [PubMed] [Google Scholar]
- Chen B.Y., Wang X., Wang Z.Y., Wang Y.Z., Chen L.W., Luo Z.J. Brain-derived neurotrophic factor stimulates proliferation and differentiation of neural stem cells, possibly by triggering the Wnt/β-catenin signaling pathway. J. Neurosci. Res. 2013;91(1):30–41. doi: 10.1002/jnr.23138. [DOI] [PubMed] [Google Scholar]
- Cheserek M.J., Wu G., Li L., Li L., Karangwa E., Shi Y., Le G. Cardioprotective effects of lipoic acid, quercetin and resveratrol on oxidative stress related to thyroid hormone alterations in long-term obesity. J. Nutr. Biochem. 2016;33:36–44. doi: 10.1016/j.jnutbio.2016.02.008. [DOI] [PubMed] [Google Scholar]
- Commission E. Commission Regulation (EC) No 1881/2006 of 19 December 2006 setting maximum levels for certain contaminants in foodstuffs. Off. J. Eur. Union. 2006;364:5–24. [Google Scholar]
- Fang H., Wu Y., Guo J., Rong J., Ma L., Zhao Z., Zuo, Peng S. T-2 toxin induces apoptosis in differentiated murine embryonic stem cells through reactive oxygen species-mediated mitochondrial pathway. Apoptosis. 2012;17(8):895–907. doi: 10.1007/s10495-012-0724-3. [DOI] [PubMed] [Google Scholar]
- Formica J.V., Regelson W. Review of the biology of quercetin and related bioflavonoids. Food Chem. Toxicol. 1995;33(12):1061–1080. doi: 10.1016/0278-6915(95)00077-1. [DOI] [PubMed] [Google Scholar]
- Fraeyman S., Croubels S., Devreese M., Antonissen G. Emerging fusarium and alternaria mycotoxins: Occurrence, toxicity and toxicokinetics. Toxins. 2017;9(7):228. doi: 10.3390/toxins9070228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frankič T., Salobir J., Rezar V. The effect of vitamin E supplementation on reduction of lymphocyte DNA damage induced by T-2 toxin and deoxynivalenol in weaned pigs. Anim. Feed Sci. Tech. 2008;141(3–4):274–286. [Google Scholar]
- Gerecke K.M., Jiao Y., Pagala V., Smeyne R.J. Exercise does not protect against MPTP-induced neurotoxicity in BDNF happloinsufficent mice. PLoS ONE. 2012;7(8) doi: 10.1371/journal.pone.0043250. e43250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gunti L., Dass R.S., Kalagatur N.K. Phytofabrication of selenium nanoparticles from Emblica officinalis fruit extract and exploring its biopotential applications: antioxidant, antimicrobial, and biocompatibility. Front. Microbiol. 2019;10:931. doi: 10.3389/fmicb.2019.00931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupta A., Birhman K., Raheja I., Sharma S.K., Kar H.K. Quercetin: A wonder bioflavonoid with therapeutic potential in disease management. Asian Pac. J. Trop. Dis. 2016;6(3):248–252. [Google Scholar]
- Hsia C.C., Wu Z.Y., Li Y.S., Zhang F., Sun Z.T. Nivalenol, a main Fusarium toxin in dietary foods from high-risk areas of cancer of esophagus and gastric cardia in China, induced benign and malignant tumors in mice. Oncol. Rep. 2004;12(2):449–456. [PubMed] [Google Scholar]
- Kalagatur N.K., Kamasani J.R., Mudili V. Assessment of detoxification efficacy of irradiation on zearalenone mycotoxin in various fruit juices by response surface methodology and elucidation of its in-vitro toxicity. Front Microbiol. 2018;9:2937. doi: 10.3389/fmicb.2018.02937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalagatur N.K., Karthick K., Allen J.A., Nirmal Ghosh O.S., Chandranayaka S., Gupta V.K., Krishna K., Mudili V. Application of activated carbon derived from seed shells of Jatropha curcas for decontamination of zearalenone mycotoxin. Front. Microbiol. 2017;8:760. doi: 10.3389/fphar.2017.00760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee M., McGeer E.G., McGeer P.L. Quercetin, not caffeine, is a major neuroprotective component in coffee. Neurobiol. Aging. 2016;46:113–123. doi: 10.1016/j.neurobiolaging.2016.06.015. [DOI] [PubMed] [Google Scholar]
- Maroli N., Kalagatur N.K., Bhasuran B., Jayakrishnan A., Manoharan R.R., Kolandaivel P., Natarajan J., Kadirvelu K. Molecular Mechanism of T-2 Toxin-Induced Cerebral Edema by Aquaporin-4 Blocking and Permeation. J. Chem. Inf. Model. 2019;59(11):4942–4958. doi: 10.1021/acs.jcim.9b00711. [DOI] [PubMed] [Google Scholar]
- Murase S., McKay R.D. A specific survival response in dopamine neurons at most risk in Parkinson's disease. J. Neurosci. 2006;26(38):9750–9760. doi: 10.1523/JNEUROSCI.2745-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohkawa H., Ohishi N., Yagi K. Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Anal. Biochem. 1979;95(2):351–358. doi: 10.1016/0003-2697(79)90738-3. [DOI] [PubMed] [Google Scholar]
- Pestka J.J. Deoxynivalenol: Toxicity, mechanisms and animal health risks. Anim. Feed Sci. Technol. 2007;137(3–4):283–298. [Google Scholar]
- Pestka J.J., Bondy G.S. Alteration of immune function following dietary mycotoxin exposure. Can. J. Physiol. Pharmacol. 1990;68(7):1009–1016. doi: 10.1139/y90-154. [DOI] [PubMed] [Google Scholar]
- Ramachandrappa L.T., Kalagatur N.K., Mohan C.D., Rangappa S., Prasad D., Hashem A., Alqarawi A.A., Malik J.A., Abd Allah E.F., Gupta V.K., Siddaiah C.N., Niranjana S.R. Biofabrication of zinc oxide nanoparticles with Syzygium aromaticum flower buds extract and finding its novel application in controlling the growth and mycotoxins of Fusarium graminearum. Front. Microbiol. 2019;10:1244. doi: 10.3389/fmicb.2019.01244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramana M.V., Balakrishna K., Murali H.C.S., Batra H.V. Multiplex PCR-based strategy to detect contamination with mycotoxigenic Fusarium species in rice and fingermillet collected from southern India. J. Sci. Food Agric. 2011;91(9):1666–1673. doi: 10.1002/jsfa.4365. [DOI] [PubMed] [Google Scholar]
- Razafimanjato H., Benzaria A., Taïeb N., Guo X.J., Vidal N., Di Scala C., Varini K., Maresca M. The ribotoxin deoxynivalenol affects the viability and functions of glial cells. Glia. 2011;59(11):1672–1683. doi: 10.1002/glia.21214. [DOI] [PubMed] [Google Scholar]
- Rotter B.A. Invited review: Toxicology of deoxynivalenol (vomitoxin) J. Toxicol. Environ. Health Part A. 1996;48(1):1–34. doi: 10.1080/009841096161447. [DOI] [PubMed] [Google Scholar]
- Scaduto R.C., Jr, Grotyohann L.W. Measurement of mitochondrial membrane potential using fluorescent rhodamine derivatives. Biophys. J. 1999;76(1):469–477. doi: 10.1016/S0006-3495(99)77214-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmeits P.C., van Kol S., van Loveren H., Peijnenburg A.A., Hendriksen P.J. The effects of tributyltin oxide and deoxynivalenol on the transcriptome of the mouse thymoma cell line EL-4. Toxicol. Res. 2014;3(4):254–265. [Google Scholar]
- Singh N.P., McCoy M.T., Tice R.R., Schneider E.L. A simple technique for quantitation of low levels of DNA damage in individual cells. Exp. Cell Res. 1988;175(1):184–191. doi: 10.1016/0014-4827(88)90265-0. [DOI] [PubMed] [Google Scholar]
- Sobrova P., Adam V., Vasatkova A., Beklova M., Zeman L., Kizek R. Deoxynivalenol and its toxicity. Interdiscip. Toxicol. 2010;3(3):94–99. doi: 10.2478/v10102-010-0019-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soundararajan R., Wishart A.D., Rupasinghe H.V., Arcellana-Panlilio M., Nelson C.M., Mayne M., Robertson G.S. Quercetin 3-glucoside protects neuroblastoma (SH-SY5Y) cells in vitro against oxidative damage by inducing sterol regulatory element-binding protein-2-mediated cholesterol biosynthesis. J. Biol. Chem. 2008;283(4):2231–2245. doi: 10.1074/jbc.M703583200. [DOI] [PubMed] [Google Scholar]
- Strasser A., Carra M., Ghareeb K., Awad W., Böhm J. Protective effects of antioxidants on deoxynivalenol-induced damage in murine lymphoma cells. Mycotoxin Res. 2013;29(3):203–208. doi: 10.1007/s12550-013-0170-2. [DOI] [PubMed] [Google Scholar]
- Swaminathan S., Haribabu J., Kalagatur N.K., Konakanchi R., Balakrishnan N., Bhuvanesh N., Karvembu R. Synthesis and anticancer activity of [RuCl2 (η6-arene)(aroylthiourea)] complexes—high activity against the human neuroblastoma (IMR-32) cancer cell line. ACS Omega. 2019;4(4):6245–6256. doi: 10.1021/acsomega.9b00349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tellmann G., Geulen O. LightCycler® 480 Real-Time PCR system: Innovative solutions for relative quantification. Biochemica. 2006;4:16. [Google Scholar]
- Venkataramana M., Nayaka S.C., Anand T., Rajesh R., Aiyaz M., Divakara S.T., Murali H.S., Prakash H.S., Rao P.L. Zearalenone induced toxicity in SHSY-5Y cells: the role of oxidative stress evidenced by N-acetyl cysteine. Food Chem. Toxicol. 2014;65:335–342. doi: 10.1016/j.fct.2013.12.042. [DOI] [PubMed] [Google Scholar]
- Vina J., Borras C., Gomez-Cabrera M.C., Orr W.C. Part of the Series: From Dietary Antioxidants to Regulators in Cellular Signalling and Gene ExpressionRole of reactive oxygen species and (phyto) oestrogens in the modulation of adaptive response to stress. Free Radic. Res. 2006;40(2):111–119. doi: 10.1080/10715760500405778. [DOI] [PubMed] [Google Scholar]
- Visconti A., Minervini F., Lucivero G., Gambatesa V. Cytotoxic and immunotoxic effects of Fusarium mycotoxins using a rapid colorimetric bioassay. Mycopathologia. 1991;113(3):181–186. doi: 10.1007/BF00436128. [DOI] [PubMed] [Google Scholar]
- Wang X., Fan M., Chu X., Zhang Y., ur Rahman S., Jiang Y., Chen X., Zhu D., Feng S., Li Y., Wu J. Deoxynivalenol induces toxicity and apoptosis in piglet hippocampal nerve cells via the MAPK signaling pathway. Toxicon. 2018;155:1–8. doi: 10.1016/j.toxicon.2018.09.006. [DOI] [PubMed] [Google Scholar]
- Weidner M., Hüwel S., Ebert F., Schwerdtle T., Galla H.J., Humpf H.U. Influence of T-2 and HT-2 toxin on the blood-brain barrier in vitro: new experimental hints for neurotoxic effects. PLoS ONE. 2013;8(3) doi: 10.1371/journal.pone.0060484. e60484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xi J., Zhang B., Luo F., Liu J., Yang T. Quercetin protects neuroblastoma SH-SY5Y cells against oxidative stress by inhibiting expression of Krüppel-like factor 4. Neurosci. Lett. 2012;527(2):115–120. doi: 10.1016/j.neulet.2012.08.082. [DOI] [PubMed] [Google Scholar]
- Yu J.Y., Zheng Z.H., Son Y.O., Shi X., Jang Y.O., Lee J.C. Mycotoxin zearalenone induces AIF-and ROS-mediated cell death through p53-and MAPK-dependent signaling pathways in RAW264. 7 macrophages. Toxicol. Vitro. 2011;25(8):1654–1663. doi: 10.1016/j.tiv.2011.07.002. [DOI] [PubMed] [Google Scholar]
- Zhang X., Jiang L., Geng C., Cao J., Zhong L. The role of oxidative stress in deoxynivalenol-induced DNA damage in HepG2 cells. Toxicon. 2009;54(4):513–518. doi: 10.1016/j.toxicon.2009.05.021. [DOI] [PubMed] [Google Scholar]