Abstract
The main objective of this work is to analyze the perinatal protective effects of curcumin (Cur) on the toxicity of inorganic mercury (mercuric chloride – HgCl2) in the developing mice offspring on their behavioral and biochemical changes. Six groups of pregnant mice (consisting of ten animals in each) were allocated in a way that Group I consuming tap water was used as control. Groups II to VI were the experimentally treated groups in which Group II and III received 150 and 300 ppm of curcumin, respectively; Group IV was given 10 ppm of HgCl2; and Group V and VI were also exposed to 10 ppm of HgCl2 but concurrently they were also treated with 150 and 300 ppm of curcumin, respectively. Appearance of vaginal plug was considered as the first day of pregnancy and all treatment started from day one of pregnancy until post-natal day 15 (PD 15) and the mothers were switched to plain tap water thereafter. At the age of PD 40, the male pups were subjected to measuring the depression in the light-dark chambers, forced swimming and tail suspension tests and to measuring their anxiety in plus-maze and open-field tests. Subsequently, after behavioral tests, the levels of corticosterone and cortisol hormones were estimated in the plasma of the experimental offspring. Behavioral tests were measured in the HgCl2 treated offspring for the light-dark chambers; forced swimming test; tail suspension test; plus-maze test; and open –field test showed significant alterations in their depression, anxiety and locomotory activities. Biochemical estimation of corticosterone and cortisol hormones in the plasma of these offspring showed significant depletion in their levels. Treatment of these offspring with curcumin significantly and dose dependently ameliorated all the behavioral and biochemical disruptive effects in the offspring due to HgCl2 toxicity. In conclusion, curcumin ameliorates the toxic effects of HgCl2 in the offspring during gestation and lactation periods. Thus, exposure to HgCl2 to mothers during pregnancy needs careful monitoring for minimizing its toxicity. Curcumin appears to be a promising ameliorating agent for such HgCl2 toxicity; however, further studies are needed for establishing these preliminary findings.
Keywords: Perinatal exposure, Mercuric chloride, Curcumin, Mice offspring, Anxiety, Depression, Corticosterone, Cortisol
1. Introduction
Mercury is a heavy, silvery-white liquid metal. Compared to other metals, it is a poor conductor of heat, but a fair conductor of electricity. It is a chemical element with the symbol Hg and atomic number 80. Hg is a highly toxic heavy metal pollutant inducing severe alterations in the body tissues of both humans and animals. Mercury exists in soil and water pollutant globally and is known to pollute by natural phenomenon like volcanic eruptions and other industrial activities. Marine food resources are also known to be polluted by mercury. Preservative compounds used in vaccines and even amalgam fillings used for teeth can also be the possible causes of mercury intoxication (Obrist et al., 2018). Mercury has also been used as components in beauty creams, laxative drugs and contrast materials (Ho et al., 2017). The toxicity of mercury metal depends on the occurrence of its form; however, all forms of mercury are considered toxic. The inorganic form of mercury such as mercuric chloride (HgCl2) is known to be toxic in humans and animals due to its binding ability to thiol-containing molecules (Zalups, 2000).
Placenta plays important roles in supplying the fetus with nutrients and oxygen and freeing it from waste and carbon dioxide (CO2). Furthermore, placenta also protects the fetus from heavy metals like mercury through the placental- blood barrier (Abu-Taweel, 2019). Exposure to heavy metals including mercury during pregnancy and/or lactation periods is extremely dangerous to fetus and/or infants (Oliveira et al., 2016). Although a placental-blood barrier exists between the developing fetus and mother, some heavy elements like mercury can reach the fetus and accumulate in their tissues (Abu-Taweel, 2018). HgCl2 accumulation in human and animal tissues can lead to many physiological, histological, behavioral alterations, neurotoxic effects, sensory deficits, memory loss, depression, and sleeping disturbances. Recently, Abu Bakar et al. (2016) investigated the effects of HgCl2 in zebra fish and showed implications on their locomotor and biochemical defects affecting motor performance and anxiety-like responses.
Curcumin (Cur), belonging to the family Zingiberaceae, is a naturally occurring bioactive phytochemical compound rich in phenol (diferuloylmethane) and is found in the rhizome of turmeric plant in abundance (Altintas et al., 2016). Cur is highly effective in treating various diseases and has potential therapeutic and prophylactic use as antiinfectious, antifungal, antiparasitic, antiviral, anticarcinogenic, antimutagen, antioxidant, and anti-inflammatory compound (Ciftci et al., 2010). Furthermore, Cur has been reported for being neuroprotective effect to treat age-related neurodegenerative diseases, as an anticonvulsant against epilepsy (Ahmad, 2013), and also as an antioxidant in experimental animals. The present study investigated the effects of perinatal exposure to HgCl2 on anxiety, behaviors and related hormones such as, corticosterone and cortisol hormones in plasma of the mice offspring. Furthermore, the protective effects of Cur on these HgCl2 induced toxic effects have also been studied.
2. Materials and methods
2.1. Animals
One male to three females of Swiss–Webster strain mice were allowed to breed in opaque plastic cages measuring 30 × 12 × 11 cm, in Zoology Department animal facility at King Saud University, Riyadh, Saudi Arabia, using a reversed 12 h lighting conditions and ambient temperature (18–22 °C). On finding the vaginal plug (considered as day one of pregnancy), the males were removed from the cages and the pregnant females were subjected to experimental treatments. All protocols and procedures for the care and use of laboratory animals were followed according to the ethical guidelines approved by the local Ethics and Care of Experimental Animals Committee.
2.2. HgCl2 and Cur administration and experimental protocol
The pregnant mice were allocated to six groups of ten animals in each. Group I consuming tap water was used as control. Groups II, III and VI were the experimentally treated groups in which Group II and III received 150 and 300 ppm of Cur only; Group IV was given 10 ppm of HgCl2; and Group V and VI were also exposed to 10 ppm of HgCl2 but were also concurrently treated with 150 and 300 ppm of Cur respectively. All animals had free access to food and water except during the experimental handlings. All groups of animals were further sub-divided into four subgroups on the day of pregnancy as follows:
-
1.
Sub-Group Ι was subjected to the light–dark transition testing anxiety as well as
-
2.
anxiety in plus-maze test.
-
3.
Sub-Group ΙΙ was subjected to enforced swimming test.
-
4.
Sub-Group Ш was subjected to depression tail suspension test and for anxiety in open-field test.
-
5.
Sub-Group IV was subjected to hormones assay biochemically.
All treatment started from day one of pregnancy until post-natal day 15 (PD 15) and the mothers were switched to plain tap water thereafter. The pups at birth on PD1 were culled to eight per dam in each group and were left with their mothers until PD 22. All offspring at the age of PD40 were subjected to various studies as described below.
2.3. Behavioral studies
2.3.1. Depression tests
2.3.1.1. The light-dark chamber test
At the age of PD 40, male mice offspring were subjected to the light-dark test including various parameters for this test using two light/dark boxes made of plywood for 5 min (Bourin and Hascoet, 2003). After 5 min, mice were removed from the box by the base of their tails and returned to their home cage. To clean the maze 70% ethyl alcohol was used for m inimizing the possible nonspecific odors between the tests. Behaviors scored included: latency to enter the dark chamber, number of transitions, locomotion, numbers of rear and wall rear, stretchattend postures, grooming, duration spent in dark and light chambers.
2.3.1.2. Forced swimming test
At the age of PD40 a sub-group of male mice offspring were subjected to Forced Swimming Test (FST) which is also known as the behavioral despair test. It is a test, centered on a rodent's response to the threat of drowning into a cylindrical tank containing clean water, whose result has been interpreted as measuring susceptibility to negative mood and using various indices to measure despair in the animals (Petit-Demouliere et al., 2005).
2.3.1.3. Tail suspension test
The tail suspension test (TST) is more sensitive to anti-depressant agents than the FST because the animal remains immobile for a longer period in the TST than the FST (Cryan et al., 2005). TST is used to measure stress affecting depression related behaviors in rodents and assessing other manipulations that are expected to affect depression related behaviors (Can et al., 2012).
2.3.2. Anxiety tests
2.3.2.1. Plus-maze test
For evaluation of the risk assessment and anxiety behaviors in ethologically derived animal models, the elevated plus-maze (with 2 opened and 2 enclosed arms) is frequently used (Wall and Messier, 2001).
2.3.2.2. Open – field test
The open field test is yet another useful experiment used to assay general locomotor activity levels, anxiety, and willingness to explore in rodents (Stanford, 2007). In this test, the male offspring are isolated and kept in groups of two or three for 14 days. Subsequently, 10 males from each treated group are subjected to locomotor activity tests. Various behavioral elements were observed as described by Belovicova et al., 2017, Abu-Taweel, 2020.
2.4. Corticosterone and cortisol estimations
The blood samples were collected in tubes and stored overnight at 4 °C and centrifuged to remove blood cells and obtain plasma that was stored at −20 °C until analysis. The plasma concentration of corticosterone and cortisol were quantified with enzyme-linked immunosorbent assay (ELISA), using a commercial ELISA kit (Correlate-EIA; Assay-Designs Inc., Ann Arbor, MI, USA) following the manufacturer's instructions (Sundbom et al., 2011).
2.5. Statistical analysis
All data were analyzed by one-way analysis of variance (ANOVA), between the experimental groups followed by Student-Newman-Keuls multiple comparison test. The levels of significance were defined at P ≤ 0.05, P ≤ 0.01, and P ≤ 0.001 (Khan et al., 2019, Khan et al., 2015).
3. Results
3.1. Behavioral studies
3.1.1. Depression tests
3.1.1.1. The Light-Dark experiment
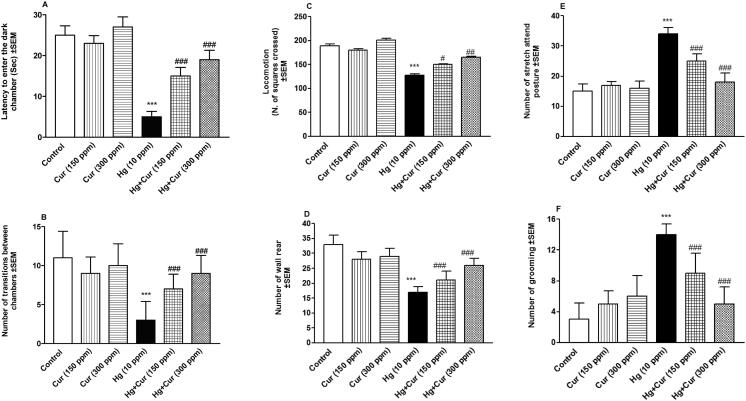
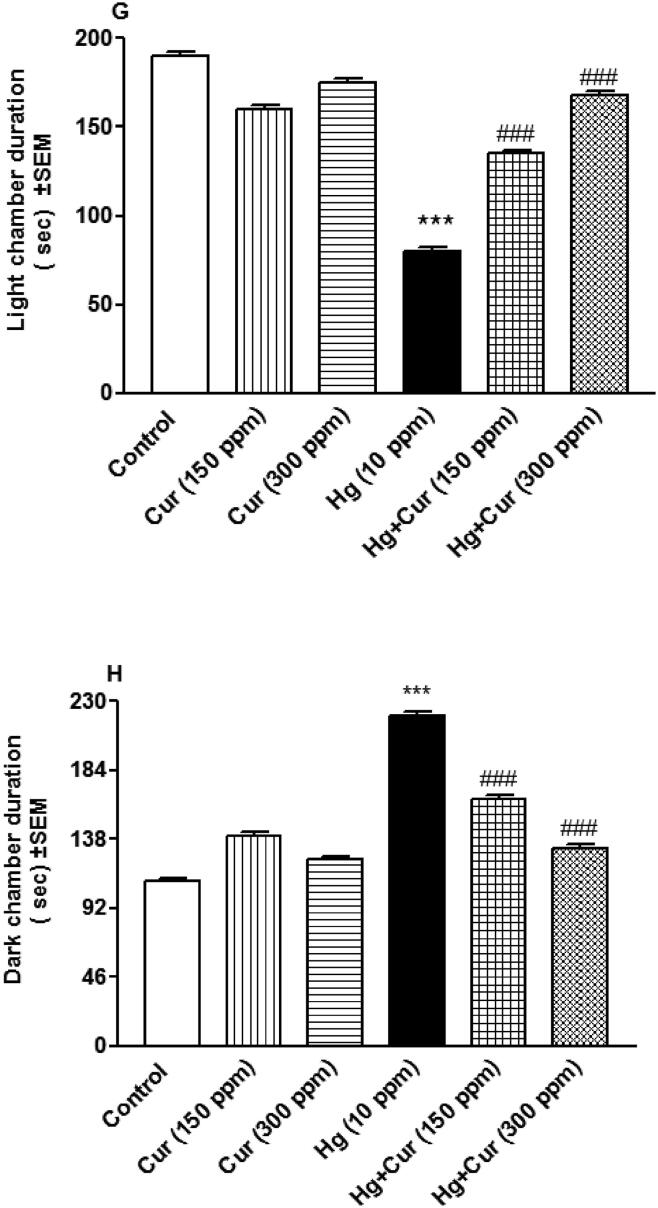
Fig. 1(A–J) showed that perinatal exposure to HgCl2 increased depression-like behavior in treated animals compared to control. Stretch attend posture, grooming and dark chamber duration were increased significantly (p < 0.001), while latency to enter the dark chamber, transitions (number of light and dark entries), locomotion (number of squares crossed), wall rear, rear and light chamber duration were significantly decreased (p < 0.001) compared to control. Treatment with Cur (150 and 300 ppm) attenuated these effects significantly (p < 0.05p < 0.01 and p < 0.001).
Fig. 1.
A–H. Effect of perinatal exposure to mercury (Hg) and curcumin (Cur) on depression of mice offspring inlight-dark test that included (A) latency to enter the dark chamber, (B) transitions, (C) locomotion, (D) wall rear, (E) rear, (F) grooming, (G) light duration, and (H) dark duration. *** shows statistically significant at P < 0.001, from the control and #, ## and ### show statistically significant at P < 0.05, P < 0.01 and P < 0.00, respectively, from Hg group by ANOVA and student's t-test.
3.1.1.2. Forced swimming test
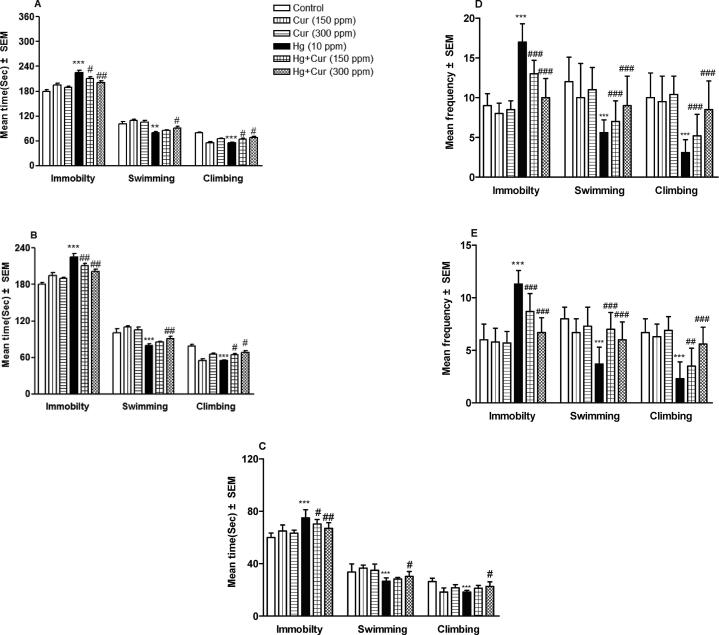
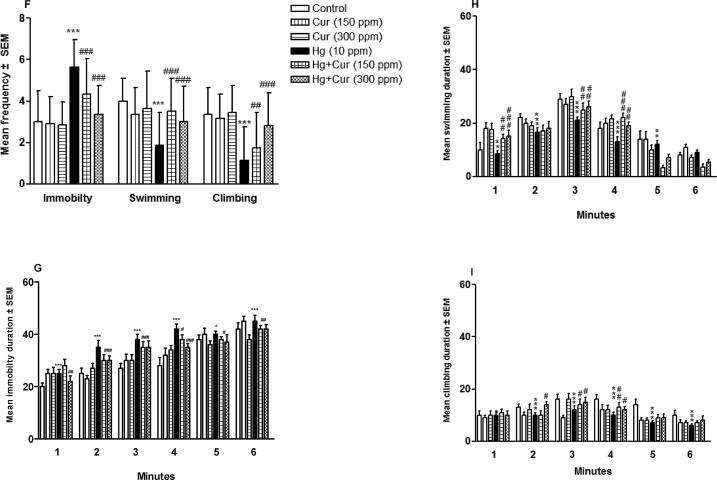
Perinatal exposure to HgCl2 led to increase depression in offspring significantly. FST elements showed depression behavior in HgCl2 -induced disruptions. Immobility was increased (p < 0.001) within 2, 4 and 6 min of test compared to control (Fig. 2A–C). Swimming and climbing were reduced significantly (p < 0.01and p < 0.001) compared to the control. Frequency of swimming and climbing were reduced significantly (p < 0.001) while immobility was increased significantly (p < 0.001) compared to control (Fig. 2D–F). The duration of immobility was increased (p < 0.001) whereas, duration of swimming and climbing were reduced generally compared to their control (Fig. 2H and I). Perinatal exposure to Cur attenuated all the above observed HgCl2 – induced toxicity (Fig. 2A–I).
Fig. 2.
A–I. Perinatal exposure to mercury (Hg) and curcumin (Cur) on depression of mice offspring in (A, B and C) forced swimming test, showing immobility, swimming and climbing behavioral results after 6 min (A), 4 min (B) and 2 min (C) respectively; (D, E and F) shows frequency of immobility, swimming and climbing behavioral results after 6 min (D), 4 min (E) and 2 min (F) respectively; and (G, H and I) showing duration of immobility (G), swimming (H) and climbing (I) in total 6 min respectively.** and *** show statistically significant at P < 0.01 and P < 0.001 respectively compared to control, and #, ## and ### show statistically significant at P < 0.05, P < 0.01 and P < 0.001 respectively compared to Hg group by ANOVA and student's t-test.
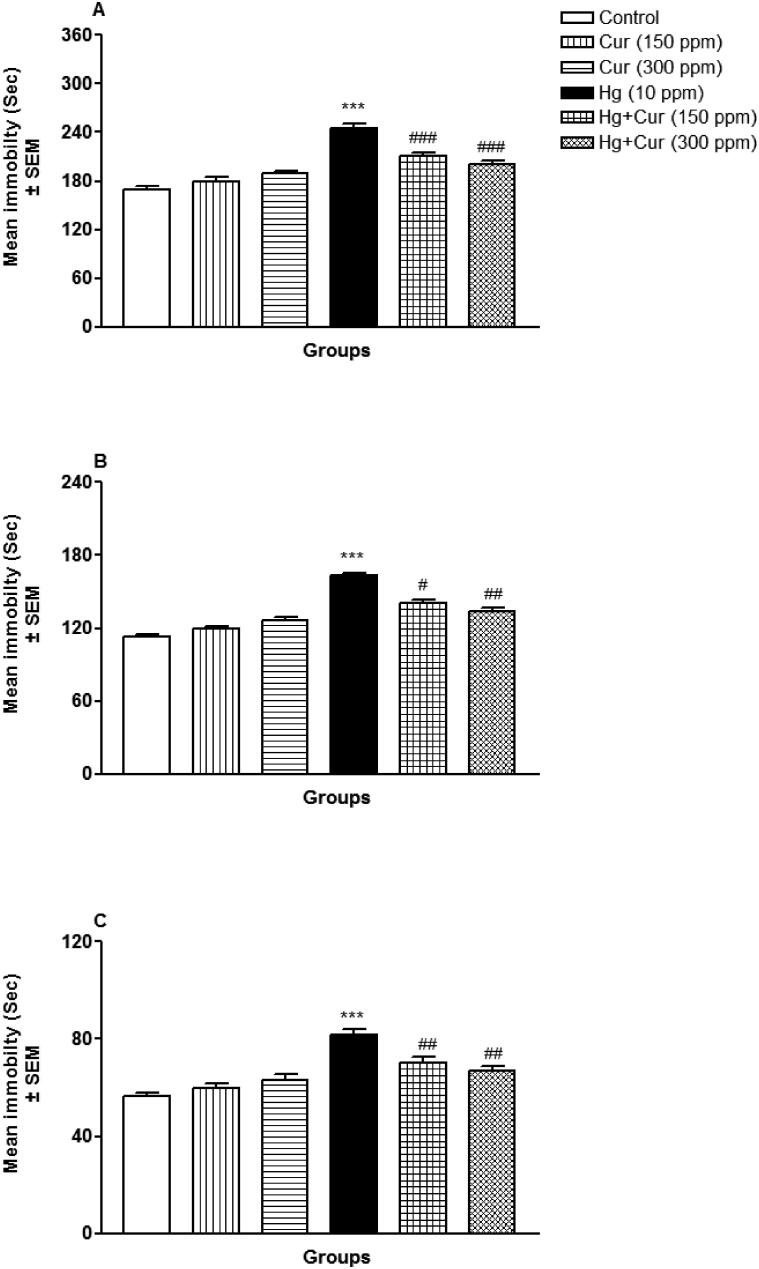
3.1.1.3. Tail suspension test
Perinatal exposure to HgCl2 induced depression-like behavior and perinatal Cur exposure ameliorated significantly (p < 0.05 and p < 0.01, at 4 min; p < 0.01, at 2 min and p < 0.001, at 6 min) the HgCl2-induced effects. Immobility increased significantly (p < 0.001) throughout (Fig. 3A, at 6 min; 3B, at 4 min; and 3C, at 2 min).
Fig. 3.
A–C. Effect of perinatal mercury (Hg) and curcumin (Cur) exposure on depression- like behavior of mice offspring in tail suspension test. Immobility duration is shown after 6 min (A), 4 min (B) and 2 min (C).*** shows statistically significant at P < 0.001 from the control group; #, ## and ### show statistically significant at P < 0.05, P < 0.01 and P < 0.001 respectively from Hg group by ANOVA and student's t-test.
3.1.2. Anxiety tests
3.1.2.1. Plus-Maze test
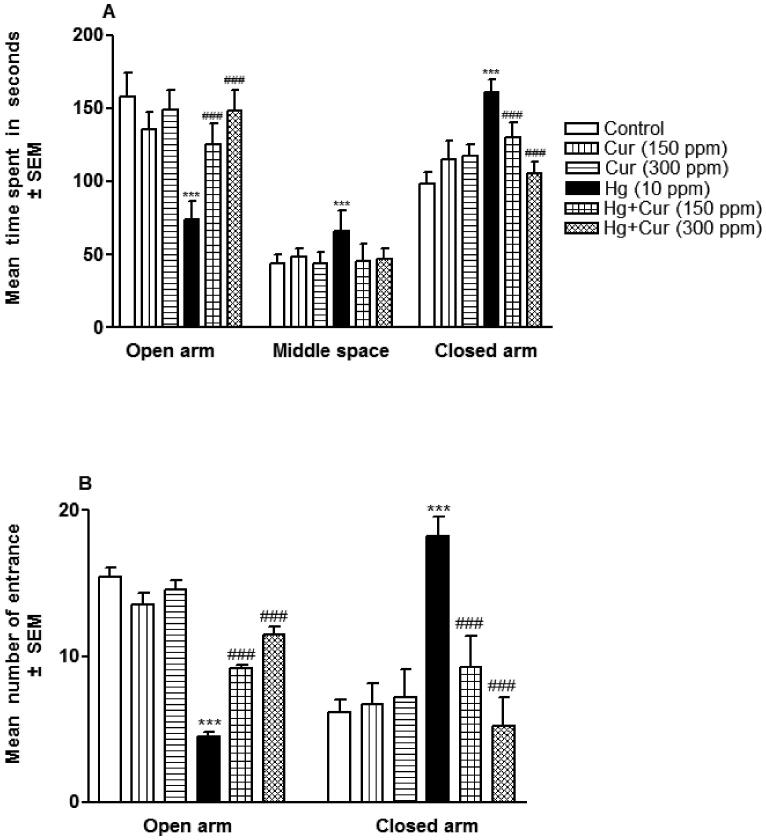
Perinatal HgCl2 exposure induced changes in the anxiety behavior of the offspring. Fig. 4A and B showed that HgCl2 treated animals spent more time (P < 0.001) into the closed arm and less time (P < 0.001) into open arm as compared to control groups. The number of entries into the closed arm was increased significantly (P 0.001) whereas in open arm it was decreased as compared to control (Fig. 4B). Cur ameliorated the HgCl2-induced effects significantly (P < 0.001) as compared to HgCl2 group (Fig. 4A and B).
Fig. 4.
A and B. Perinatal mercury (Hg) (10 ppm) and curcumin (Cur) (150 and 300 ppm) exposure affects on the time spent in open and closed arms (A), and the number of entries in open and closed arms (B). *** represents statistically significant (P < 0.001) from the control and ### represents statistically significant (P < 0.001) from Hg group.
3.1.2.2. Open – field test
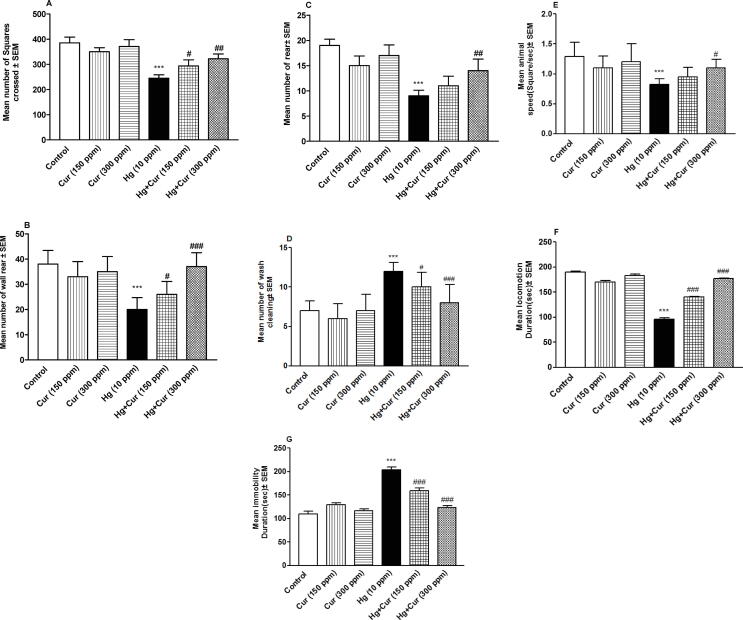
Fig. 5(A–G) showed that perinatal effects of HgCl2 had a significant effect on the locomotor behavior of mice offspring in open-field test. Number of squares crossed, wall rear, rear, animal speed (square/sec) and locomotion duration were decreased (P < 0.001) whereas number of wash cleaning and immobility duration were increased significantly (P < 0.05 and P < 0.001 respectively) compared to control. Exposure to Cur ameliorated the effects of HgCl2 significantly (P < 0.05, P < 0.01 and P < 0.001) comparing to HgCl2 group (Figure A–G).
Fig. 5.
A–G. Protective effect of curcumin (Cur) on locomotor activity disturbance induced by mercury (Hg) in mice offspring. The parameters measured were (A) number of squares crossed, (B) wall rear, (C) rear, (D) wash cleaning, (E) animal speed, (F) locomotion duration, and (G) immobilityduration.*** represents statistically significant (P < 0.001) from the control and #, ## and ### represent statistically significant (P < 0.05, P < 0.01 and P < 0.001 respectively) from Hg group.
3.2. Corticosterone and cortisol
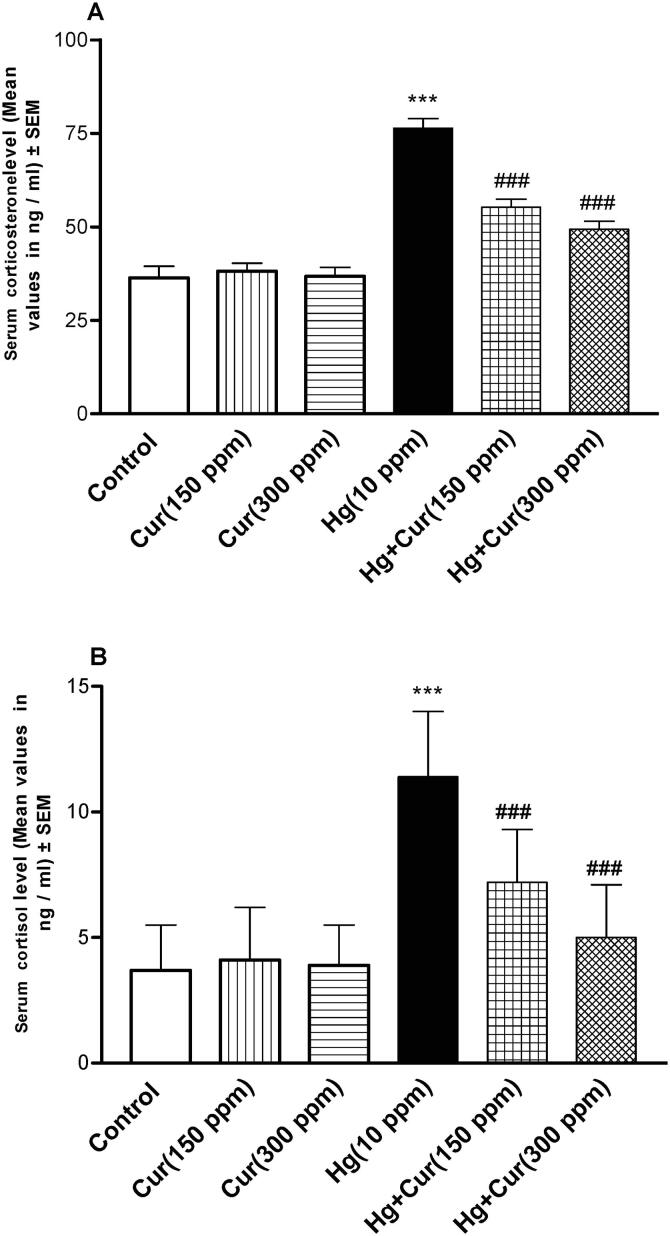
Perinatal exposure to HgCl2 caused a significant increase (P < 0.001) in corticosterone and cortisol hormone levels in the offspring plasma as compared to control. Cur attenuated the levels of corticosterone and cortisol hormones significantly (P < 0.001) comparing to HgCl2 group (Fig. 6A and B).
Fig. 6.
A and B. Effect of mercury (Hg) and curcumin (Cur) on corticosterone (A) and cortisol (B) hormones levels in mice offspring. *** represents statistically significant (P < 0.001) from the control group, and ### represents statistically significant (P < 0.001) from Hg group.
4. Discussion
Mercuric chloride penetrates into the organism upon inhalation, food and percutaneous contacts. Studies have shown that such phenomenon are the outcome of environmental and food pollutions with heavy metals and certain medical procedures such as vaccination and dental filling with amalgam. Relation of depression with mercury has gained researcher’s attention and has been supported by some epidemiology studies recently (Sundbom, 2012). The present results have indicated significant reduction in anxiety and depression-like behaviours due to exposure to HgCl2 during gestation and lactation period. The results also showed protective effects of Cur from HgCl2 – induced effects on its concurrent exposure to the offspring during the same duration of HgCl2 exposure. Other reported studies were also in agreement with the present findings (Chehimi et al., 2012).
The brain controls the depression and anxiety (Lago et al., 2017). A number of macro- and microscopic structural alterations in the hippocampus, cortical and subcortical brain regions of the patients are possibly involved in the general developmental disorders resulting in an increased risk for depression and anxiety. Hippocampal volume reduction has been found in both early-onset and late-life depression. An increased prevalence of white-matter lesions was observed only in late-life depression (Janssen et al., 2004). The present results suggest for a direct action of HgCl2 on the developing pups in utero as well as during the lactation period, because HgCl2 has been shown to be transferred to offspring through placenta and/or milk respectively. Furthermore, it is important to be noted that a major portion of brain cells of the closely related rats form after birth (Patel, 1983). Recently, studies using radio isotopic Hg have shown that considerable amounts of Hg administered to pregnant and/or lactating rats, cross the blood brain barrier (BBB) and are deposited into the brain of fetuses and suckling through trans placental passage and/or maternal milk and remain persistent throughout their lifetime (Sherin et al., 2016). However, Chehimi et al. (2012) reported high mercury contents immediately after birth in the brain, blood and milk in rat mothers. Ultimately, exposure to HgCl2 and other drugs to the pups during fetal and suckling life have been reported to retard motor development and physical maturation (Abu-Taweel et al., 2012). Another study led to the conclusion that neuromotor reflexes in pups are not improved with cross-fostering the mothers and that the maternal behavior towards HgCl2 treated pups was altered without any influence which proves the absence of interaction between cross-fostering mothers on the treated pups showing the direct toxic effects of HgCl2 (Abu – Taweel, 2016).
Over the past four decades, the focus on the brain monoaminergic systems, which contain serotonin (5-hydroxytryptamine, 5-HT), norepinephrine, and dopamine (DA), has contributed significantly to our knowledge of the pathophysiology and treatment of depression (Zmudzka et al., 2018). The levels of dopamine (DA), serotonin (5-HT) and acetylcholinesterase (AChE) have been reported to significantly reduce in forebrain of perinatal HgCl2 treated pups. Also described that perinatal exposure to Cur attenuated HgCl2 toxicity. In the present study it is shown that the newborn mice exposed to HgCl2 spent more time in the closed arms suggesting for their anxiety behavior. As a consequence, this may be attributed to the mercuric accumulation into the developing brain during fetal life and also during lactation in the offspring.
Depression and anxiety were also affected by hormones such as corticosterone and cortisol (Ahn et al., 2018). An endocrine disruptor is broadly defined as an exogenous agent, either synthetic or natural, that interferes with the synthesis, storage, release, transport, metabolism, binding, action, or elimination of natural hormones in the body. Zhu et al. (2000) reported that heavy metals such as lead, cadmium, and mercury have an endocrine disruptive potential. Accumulation of mercury in the hypothalamus, pituitary, thyroid, adrenal glands, and gonads (testis and ovary) have been shown in laboratory animals as well as in humans (Björkman et al., 2007). The broad enzyme, Na+K+-ATPase, inhibition and the influence of combining of hormones with their receptors like Na + K + -ATPase may account for the primary mechanism of mercury toxicity. Interference with intracellular calcium metabolism, and peroxidation may also be involved in the primary mechanism of mercury toxicity.
Furthermore, the present study demonstrates that Cur has a significant ameliorating effect on the HgCl2-induced depression, anxiety and biochemical disorders. Also, it is shown that CUR alone is non-toxic and has no adverse effects on the experimental animals and it has an ameliorating effect on the behavioral and biochemical toxicity induced by Hg. Experimentally, it has been proven in earlier studies that Cur has potent antioxidant activity (Stajn et al., 1997), anti-inflammatory effect (Motterlini et al., 2000) and chemo protective properties. Thus, the biochemical damages induced due to HgCl2 in the present study and the ameliorating effect of Cur may be due to the fact that Cur has multiple possible reported benefits, however, complete mechanisms are not yet fully elucidated and more work is needed in this field.
5. Conclusions
Perinatal exposure to HgCl2 led to disruption of anxiety and depression behavior and related hormones like corticosterone and cortisol levels in mice offspring. These changes caused by exposure to HgCl2 confirmed that Hg can reach the fetus through the placenta and/or to the offspring during the lactation period. The present results further indicate that CUR can have a protective effect if administered to the offspring during perinatal period by ameliorating HgCl2 intoxication.
Footnotes
Peer review under responsibility of King Saud University.
References
- Abu Bakar N., Sata A.N.S., Ramlan N.F., Ibrahim W.N.W., Zulkifli S.Z., Abdullah C.A.C., Ahmad S., Amal N.M.A. Evaluation of the neurotoxic effects of chronic embryonic exposure with inorganic mercury on motor and anxiety-like responses in zebrafish (Danio rerio) larvae. Neurotoxicol. Teratol. 2016;59:53–61. doi: 10.1016/j.ntt.2016.11.008. [DOI] [PubMed] [Google Scholar]
- Abu-Taweel G.M. Effect of administration of mercuric chloride onthe social behavior, neuromuscular coordination, motor activity, blood parameters and liverstructure alterations in mice offspring. Pakistan J. Zool. 2020;52(3):957–969. [Google Scholar]
- Abu-Taweel G.M. Neurobehavioral protective properties of curcumin against the mercury chloride treated mice offspring. Saudi J. Biol. Sci. 2019;26:736–743. doi: 10.1016/j.sjbs.2018.10.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abu-Taweel G.M. Curcumin attenuates lead (Pb)–Induced neurobehavioral and neurochemical dysfunction: a review. Int. J. Pharm. Pharm. Sci. 2018;10(8):23–28. [Google Scholar]
- Abu – Taweel, G.M., 2016. Effects of curcumin on the social behavior, blood composition, reproductive hormones in plasma and brain acetylcholinesterase in cadmium intoxicated mice. Saudi J. Biol. Sci. 2016; 23, 219–228. [DOI] [PMC free article] [PubMed]
- Abu-Taweel G.M., Ajarem J.S., Ahmad M. Neurobehavioral toxic effects of perinatal oral exposure to aluminum on the developmental motor reflexes, learning, memory and brain neurotransmitters of mice offspring. Pharmacol. Biochem. Behavior. 2012;101:49–56. doi: 10.1016/j.pbb.2011.11.003. [DOI] [PubMed] [Google Scholar]
- Ahmad M. Effects of curcumin against lithium-pilocarpine induced status epilepticus, cognitive dysfunction and oxidative stress in young rats. Saudi J. Biol. Sci. 2013;20:155–162. doi: 10.1016/j.sjbs.2013.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahn T., Bae C.S., Yun C.H. Acute stress-induced changes in hormone and lipid levels in mouse plasma. Veterinarni Medicina. 2018;61(2):57–64. [Google Scholar]
- Altintas O., Ozgen A.M., Kumas M., Asil T. Neuroprotective effect of ischemic preconditioning via modulating the expression of cerebral miRNAs against transient cerebral ischemia in diabetic rats. Neurol. Res. 2016;38(11):1003. doi: 10.1080/01616412.2016.1232013. [DOI] [PubMed] [Google Scholar]
- Belovicova, K., Bogi, E., Csatlosova, K., Dubovicky, M., 2017. Animal tests for anxiety-like anddepression-like behavior in rats. Interdiscip Toxicol 10(1) (2017) 40–43. [DOI] [PMC free article] [PubMed]
- Björkman L., Lundekvam B.F., Lægreid T. Mercury in human brain, blood, muscle and toenails in relation to exposure: an autopsy study. Environmen Health. 2007;2007(6):30–44. doi: 10.1186/1476-069X-6-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bourin M., Hascoet M. The mouse light/dark box test. Eur J Pharmacol. 2003;463:55–65. doi: 10.1016/s0014-2999(03)01274-3. [DOI] [PubMed] [Google Scholar]
- Can A., Dao D.T., Terrillion C.E., Piantadosi S.C., Bhat S., Gould T.D. The tail suspension test. J Vis Exp. 2012;59:1–5. doi: 10.3791/3769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chehimi L., Roy V., Jeljeli M., Sakly M. Chronic exposure to mercuric chloride during gestation affects sensorimotor development and later behaviour in rats. Behavioural Brain Res. 2012;234:43–50. doi: 10.1016/j.bbr.2012.06.005. [DOI] [PubMed] [Google Scholar]
- Ciftci O., Tanyildizi S., Godekmerdan A. Protective effect of curcumin on immune system and body weight gain on rats intoxicated with 2, 3, 7, 8-Tetrachlorodibenzo-p-dioxin (TCDD) Immunopharmacol Immunotoxicol. 2010;32(1):99–104. doi: 10.3109/08923970903164318. [DOI] [PubMed] [Google Scholar]
- Cryan John F, Cedric M., Annick V. The tail suspension test as a model for assessing antidepressant activity: review of pharmacological and genetic studies in mice. Neurosci Biobehav Rev. 2005;29:571–625. doi: 10.1016/j.neubiorev.2005.03.009. [DOI] [PubMed] [Google Scholar]
- Ho Y.B., Abdullah N.H., Hamsan H., Tan E.S.S. Mercury contamination in facial skin lightening creams and its health risks to user. Regulatory Toxicol Pharmacol. 2017;88:72–76. doi: 10.1016/j.yrtph.2017.05.018. [DOI] [PubMed] [Google Scholar]
- Janssen J., Hulshoff Pol H.E., Lampe I.K. Hippocampal changes and white matter lesions in early-onset depression. Biol Psychiatry. 2004;56:825–831. doi: 10.1016/j.biopsych.2004.09.011. [DOI] [PubMed] [Google Scholar]
- Khan I.A., Jahan P., Hasan Q., Rao P. Genetic confirmation of T2DM meta-analysis variants studied in gestational diabetes mellitus in an Indian population. Diabetes Metabol. Syndrom: Clin. Res. Rev. 2019;13:688–694. doi: 10.1016/j.dsx.2018.11.035. [DOI] [PubMed] [Google Scholar]
- Khan I.A., Jahan P., Hasan Q., Rao P. Relationship between PTEN and gestational diabetes in Asian Indians womens. J. Health Specialties. 2015;3:184. [Google Scholar]
- Lago T., Davis A., Grillon C., Ernst M. Striatum on the anxiety map: small detours into adolescence. Brain Res. 2017;1654:177–184. doi: 10.1016/j.brainres.2016.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Motterlini R., Foresti R., Bassi R., Green C.J. Curcumin, an antioxidant and anti-inflammatory agent, induces heme oxygenase-1and protects endothelial cells against oxidative stress. Free Radical. Biol. Med. 2000;28:1303–1312. doi: 10.1016/s0891-5849(00)00294-x. [DOI] [PubMed] [Google Scholar]
- Obrist D., Kirk J.L., Zhang L., Sunderland E.M., Jiskra M., Selin N.E. A review of global environmental mercury processes in response to human and natural perturbations: changes of emissions, climate, and land use. Ambio. 2018;47:116–140. doi: 10.1007/s13280-017-1004-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oliveira C., OliveiraVA Costa LM, PedrosoTF Fonseca MM, Bernardi J.S., Fiuza T.L., Pereira M.E. Inorganic mercury exposure in drinking water alters essential metal homeostasis in pregnant rats without altering rat pup behavior. Reproduct. Toxicol. 2016;65:18–23. doi: 10.1016/j.reprotox.2016.06.013. [DOI] [PubMed] [Google Scholar]
- Patel A.J. Undernutrition and brain development. Trends Nat. Sci. 1983;6:151–154. [Google Scholar]
- Petit-Demouliere B., Chenu F., Bourin M. Forced swimming test in mice: a review of antidepressant activity. Psychopharmacol. 2005;177(3):245–255. doi: 10.1007/s00213-004-2048-7. [DOI] [PubMed] [Google Scholar]
- Sherin J., Sumathi T. Neurotoxic effects of gestational exposure of methyl mercury on different brain regions of F1 generation; neurobehavioural, biochemical and histological study during weaning period of rat. Int. J. Toxicol. Pharmacol. Res. 2016;8(2):83–93. [Google Scholar]
- Stajn A., Zikic R.V., Ognjanovic B., Saicic Z.S., Pavlovic S.Z., Kostic M.M., Petrovic V.M. Effect of cadmium and selenium on the antioxidant defense system in rat kidneys. Comp. Biochem. Physiol. 1997;117C:167–172. doi: 10.1016/s0742-8413(97)00063-7. [DOI] [PubMed] [Google Scholar]
- Stanford, S.C., 2007. The open field test: Reinventing the wheel. J. Psychopharmacol. 21(2), 134–134. [DOI] [PubMed]
- Sundbom R., Jacobsen K.R., Kalliokoski O., Hau Jabeison K.S.P. Post-operative corticosterone levels in plasma and feces of mice subjected to permanent catheterization and automated blood sampling. In vivo. 2011;25:335–342. [PubMed] [Google Scholar]
- Wall P.M., Messier G. Methodological and con- ceptual issues in the use of elevated plus-maze as a psychological measurement instrument of animal anxiety-like behavior. Neurosci. Biobehavior. Rev. 2001;25(3):275–286. doi: 10.1016/s0149-7634(01)00013-6. [DOI] [PubMed] [Google Scholar]
- Zalups R.K. Molecular interactions with mercury in the kidney. Pharmacol. Rev. 2000;52:113–143. [PubMed] [Google Scholar]
- Zhu X., Kusaka Y., Sato K., Zhang Q. The endocrine disruptive effects of mercury. Environmental health and preventive medicine. 2000;4:174–183. doi: 10.1007/BF02931255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zmudzka E., Sałaciak K., Sapa J., Pytka K. Serotonin receptors in depression and anxiety: Insights from animal studies. Life Sci. 2018;210:106–124. doi: 10.1016/j.lfs.2018.08.050. [DOI] [PubMed] [Google Scholar]
- Sundbom R, Jacobsen KR, Kalliokoski O, Hau Jabeison KSP. Post-operative Corticosterone Levels in Plasma and Feces of Mice Subjected to Permanent Catheterization and Automated Blood Sampling. In vivo. 2012;25:335–342. [PubMed] [Google Scholar]
Further Reading
- Bernhoft RA. Mercury toxicity and treatment: a review of the literature. J Environ Public Health 2012, Article ID 460508, 10 pages. [DOI] [PMC free article] [PubMed]
- Bernhoft R., A Mercury Toxicity and Treatment: A Review of the Literature. J Environ Public Health. 2012;2012(460508):1–6. doi: 10.1155/2012/460508. [DOI] [PMC free article] [PubMed] [Google Scholar]