Abstract
Antibacterial and cytotoxic activities of Euphorbia balsamifera, fractions and pure compounds were evaluated. The cytotoxic assays for HCT116, HePG2 and MCF7 showed a significant IC50: 54.7 and 76.2 µg/mL of non-polar fraction “n-hexane” against HCT116 and HePG2, respectively. Antibacterial results revealed that plant fractions exhibited significant potential against the tested pathogens than the total extract where n-butanol and ethyl acetate fractions showed significant antibacterial activity (P < 0.05) against tested bacterial strains. Isolation and structure determination of compounds from n-hexane and n-butanol fractions were performed. From n-hexane fraction, 29-nor-cycloartanol (1), lanost-8-en-3-ol (2a), cycloartanol (2b) and kampferol-3,4'-dimethyl ether (3) were isolated and structurally identified, along with 24 compounds were tentatively identified by GC–MS. From the polar n-butanol fraction, 4-O-β-D-glucopyranosyl-2-hydroxy-6-methoxyacetophenone (4), 4-O-α-L-rhamnosyl-(1 → 6)-β-D-glucopyranosyl-2-hydroxy-6methoxy-acetophenone (5), quercetin-3-O-glucopyranoside (6) and isoorientin (7) were assigned. Structures of the obtained compounds were determined by nuclear magnetic resonance (NMR) spectroscopy and mass spectrometry. Except compounds 1 and 5, all reported compounds announced antibacterial efficiency. Compound 2 showed selectively the highest activity against Enterococcus faecalis (22 ± 0.13 mm), meanwhile 4-O-β-D-glucopyranosyl-2-hydroxy-6-methoxyacetophenone (4) showed broadly the highest antibacterial activity with MIC of 1.15–1.88 mg/mL against the test Gram-positive and Gram-negative bacteria. Cytotoxic assays indicated that kampferol-3,4'-dimethyl ether (3) exhibited the highest activity with matching IC50 values to doxorubicin; 111.46, 42.67 and 44.90 µM against HCT116, HePG2 and MCF7, respectively, however, it is toxic on retina normal cell line RPE1.
Keywords: Isolation, GC–MS, Spectroscopy, Cytotoxicity, Antibacterial, Euphorbia balsamifera, Asir region
Abbreviations: d, Doublet; dd, Doublet of doublet; DEPT, Distortionless enhancement by polarization transfer; E. balsamifera, Euphorbia balsamifera; ESIMS, Electrospray Ionization Mass Spectrometry; GC–MS, Gas Chromatogrphy/ Mass Spectrometry; 1H–1H COSY, Proton Correlation Spectrometry; HMBC, Hetero-nuclear multiple bond correlation spectroscopy; HMQC, Hetero-nuclear multiple quantum correlation spectroscopy; HSQC, Heteronuclear single quantum coherence spectroscopy; HCT116, Colon cell line; HePG2, Human hepatocellular carcinoma cell line; J, Coupling Constant; MCF7, Human Caucasian breast adenocarcinoma; MTT, Colorimetric assay for measuring cell metabolic activity as an indicator of cell viability, proliferation, and cytotoxicity; TLC, Thin-layer Chromatography; δ, Chemical shift (in ppm)
1. Introduction
Plants and phytocompounds contribute the requirements for food, clothes and renewable sources for pharmaceutical agents with great potential of antimicrobial and anticancer agents. Plants extract including, roots, leaves and stems can be used as raw drugs with different medicinal properties (Mahesh and Satish, 2008) and phytocompounds showed a broad spectrum of anticancer with low side effects (Cragg and Newman, 2013, Newman and Cragg, 2016). Several strategies, including searching for new antimicrobials from natural products are highly recommended to combat antibiotic resistance (Enioutina et al., 2017, Ngezahayo et al., 2015), in addition to the high cost of synthetic antibiotics and their harmful side effects for infection treatment (Elizabeth, 2005). These imply searching for new alternative antibiotics from medicinal plants which can be used as a desirable tool to eradicate the population of pathogenic strains, particularly in the treatment of infectious and dreadful diseases (Enioutina et al., 2017). The antimicrobial drugs from natural products are getting greater interest in many research projects (Aliero and Afolayan, 2006, Eloff et al., 2005). Cancer diseases attributed to a human colorectal, liver and breast carcinoma are common in our zone (middle east and Arab countries), and hence finding compounds having potential activity against the referred cancer cell lines is highly recommended (Abu-Darwish and Efferth, 2018). Furthermore, plant extracts and phytocompounds have been reported to bear more than 60% of the common anticancer drugs (Seelinger et al., 2012). Euphorbia balsamifera belongs to family Euphorbiaceae, which is characterized by a broad spectrum of medicinal properties (Ben Jannet et al., 2017, Ernst et al., 2015). The latex of Euphorbia balsamifera is effective as a pulpal devitalizing agent (Yam, A. A., Gaye, F., Dieme, F. A., Bassene, E., & Ba, 1997). In addition, the plant’s leaves, stems and roots showed antimicrobial activities against some pathogenic microorganisms (Kamba and Hassan, 2010). Wound healing activity was observed for methanol extract of the plant which is identical with the standard drug Povidone-iodine (Ahmed et al., 2016), although its phytochemical screening revealed the presence of different classes of secondary metabolites. Isolation and structure assigning of these metabolites were not reported previously so far. Therefore, the aim of the present study is to evaluate the antibacterial and cytotoxic activity of the plant fractions and identify the chemical constituents for the most active fractions using different chromatographic and spectroscopic means, and then investigating their antibiotic and cytotoxic activities intensively.
2. Materials and methods
2.1. General material
NMR spectra (1H NMR, 13C NMR, DEPT, 1H,1H-COSY, HMQC, HSQC and HMBC) were recorded on Varian Unity 300 and Varian Inova 500 and Bruker 850. GC–MS analysis was carried out using gas chromatography-mass spectrometry instrument stands with the following specifications, Instrument: a TRACE GC Ultra Gas Chromatographs (THERMO Scientific Corp., USA), coupled with a thermo mass spectrometer detector (ISQ Single Quadrupole Mass Spectrometer). Direct electrospray ionization mass spectra (ESIMS) were measured with Advion compact mass spectrometer (CMS) NY-USA, acquisition speed of 10.000 m/z units/sec, flow rate of 10 μL/min to 1 mL/min, sensitivity 10 pg reserpine (FIA – 5 μL injection at 100 μL/min) 100:1 S/N (RMS) with SIM of m/z 609.3, mass range 100 to 1200. GC–MS system was equipped with a TR-5 MS column (30 m × 0.32 mm i.d., 0.25 μm film thickness). Analyses were carried out using helium as carrier gas at a flow rate of 1.0 mL/min and a split ratio of 1:10 using the following temperature program: 60 °C for 1 min; rising at 4.0 °C/min to 240 °C and held for 1 min. The injector and detector were held at 210 °C. Diluted samples (1:10 hexane, v/v) of 1 μL of the mixtures were always injected. Mass spectra were obtained by electron ionization (EI) at 70 eV, using a spectral range of m/z 40–450.
Column chromatography (CC): silica gel (40–0.063 ± 0.2 μm, Merck, Darmstadt, Germany). Thin layer chromatography (TLC): silica gel (0.25 and 1 mm precoated plates 60 F254, Merck. Sephadex LH-20 (Sigma-Aldrich, Germany), polyamide (Sigma-Aldrich, Germany), TLC silica gel 60 RP-18 (RP.PTLC; Aluminium sheets 20 × 20 cm, UV 254, CAMAG, Germany) , preparative TLC plates (PTLC; silica gel GF, 20 × 20 cm, UV254, Sigma-Aldrich, Germany).
2.2. Plant material
The aerial parts of Euphorbia balsamifera Aiton were collected from Asir region in the road of Khamis-Najran, Saudi Arabia, in February 2018 (GPS: 18 04 47.3 N, 43 14 24.9E). The plant was identified by Dr. Mahmoud Fawzy, biology department, college of science, King Khalid University. A voucher specimen of the plant was identified and authenticated in the Herbarium of Botany department, college of science, King Khalid University.
2.3. Extraction and isolation
Dry samples of the aerial parts of E. balsamifera Aiton (3.0 kg) was placed in a closed glass container with 85% Ethanol (5.0 L) at room temperature for 72 h. Then the total ethanol extract was collected by filtration using Buchner funnel, and the extraction process was repeated three times. The total extract was concentrated by rotary evaporator at 45 °C to obtain the crude ethanol extract (289.4 g). The crude extract was suspended in water (1.0 L) followed by subsequent fractionation using n-hexane, ethyl acetate and saturated n-butanol (1.0 L for each solvent).
2.3.1. n-Hexane fraction: Isolation and purification
n-Hexane fraction (21.308 g) when subjected to silica gel column chromatography eluted successively with stepwise gradients of n-hexane/ethyl acetate and chloroform/methanol afforded four main fractions (I-IV). n-Hexane and its sub-fractions (I and III) were analyzed using GC–MS analysis, detecting the presence of totally 24 compounds. Fraction II was purified by successive column chromatography on silica gel eluting with hexane/ethyl acetate (9:1, 8.5:1.5 and 8:2) resulting in 29-nor-cycloartanol (1, 3.8 mg) and lanost-8-en-3-ol (2a), cycloartanol (2b) (25.4 mg). Fraction IV was purified by two successive columns on silica gel using n-hexane/ethyl acetate (7:3) followed by Sephadex LH-20 using chloroform/methanol to give pure kampferol-3,4′-dimethyl ether (3, 4.0 mg).
2.3.2. n-Butanol fraction: Isolation and purification
The n-butanol fraction (36.7 g) was subjected to column chromatography on silica gel and eluted with a gradient system of chloroform and chloroform/methanol leading to three main fractions I, II and III. Fraction I (960 mg) was chromatographed on sephadex LH-20 column with a methanol/chloroform (6:4) to afford a sub fraction which was purified on silica gel column using chloroform/methanol (8:2) to give 4-O-β-D-glucopyranosyl-2-hydroxy-6-methoxyacetophenone (4,2.8 mg). Fraction II (1.5 g) was purified on sephadex LH-20 column, eluted with MeOH, and followed by silica gel using chloroform/methanol (7.5:2.5) giving the major compound 5, which was further purified by reversed phase-preparative thin layer chromatography (RP-PTLC) using water/methanol (1:1) system and visualized by UV lamp (245 and 366 nm) to give 4-O-α-L-rhamnosyl-(1 → 6)-β-D-glucopyranosyl-2-hydroxy-6-methoxy-acetophenone (5, 4.4 mg) in pure form.
An application of fraction III (1.0 g) to column chromatography on polyamide eluted with water/methanol afforded two sub-fractions. The first sub-fraction (350 mg) was subjected to sephadex LH-20 (MeOH), and the obtained semi pure compound 6 was re-purified by PTLC (preparative thin layer chromatography) using chloroform/methanol/water (7.5:2.5:0.25) system to give quercetin-3-O-glucopyranoside (6, 10.0 mg). The second sub-fraction (420 mg) was purified on silica gel column and elution with chloroform/methanol (7:3) and the obtained compound was re-purified using RP-PTLC eluted with water/methanol (4:6) and visualized by UV lamp to afford isoorientin (7, 12.7 mg).
2.4. Spectroscopic data of isolated compounds
2.4.1. 29-nor-cycloartanol (1)
1H NMR (500 MHz, CD3OD) δ = 3.10 (ddd, J = 15.5, 9.0, 4.5 Hz, H-3), 1.98 (H-11a) 1.88 (H-16a), 1.68 (H-2a), 1.63 (H-12), 1.61 (H-6a), 1.59 (H-17), 1.58 (H-1a), 1.52 (H-2b), 1.52 (H-25), 1.50 (H-8), 1.38 (H-22a), 1.31 (H-20), 1.31 (H-23a), 1.30 (H-7a), 1.29 (H-5), 1.28 (H-15), 1.26 (H-1b), 1.26 (H-16b), 1.18 (H-4), 1.14 (H-23b), 1.13 (H-24), 1.11 (H-11b), 1.09 (H-7b), 0.99 (H-22b), 0.95 (H-18), 0.89 (H-28), 0.87 (H-26), 0.86 (H-30), 0.85 (H-21), 0.85 (H-27), 0.78 (H-6b), 0.39 (d, J = 4. Hz, H-19a), 0.15 (d, J = 4. Hz, H-19b). 13C NMR (125 MHz, CD3OD) δ = 77.2 (C-3), 53.7 (C-7), 50.1 (C-14), 46.6 (C-8), 45.5 (C-5), 45.0 (C-13), 40.7 (C-4), 37.4 (C-24), 36.6 (C-22), 35.7 (C-20), 34.2 (C-15), 32.4 (C-12), 32.1 (C-1), 30.8 (C-2), 29.3 (C-19), 29.2 (C-16), 29.2 (C-25), 28.3 (C-11), 28.1 (C-10), 26.4 (C-7), 26.0 (C-23), 25.2 (C-27), 24.7 (C-26), 23.2 (C-6), 22.9 (C-9), 19.7 (C-30), 18.9 (C-21), 18.5 (C-18), 14.9 (C-28). (+)-ESIMS: m/z 415 ([M + H]+).
2.4.2. lanost-8-en-3-ol (2a) and cycloartanol (2b)
1H NMR (500 MHz, CDCl3) δ = 3.22 (dd, J = 11.6, 4.4 Hz, 2H-3 for 2a/2b), 0.54 (d, J = 4. Hz, H-19a for 2b), 0.32 (d, J = 4. Hz, H-19b for 2b). 13C NMR (125 MHz, CDCl3) δ = 134.51(C-8 for 2a), 134.48 (C-9 for 2a), 79.09, 79.05 (C-3 for 2a/2b). GCMS: Rt: 32.86 for 2a, Rt: 33.48 for 2b, m/z 428 ([M]+), 413 ([M−15]+), 395.
2.4.3. kampferol-3,4′-dimethyl ether (3)
1H NMR (500 MHz, CDCl3) δ = 12.75 (1H, s, 5-OH), 8.05 (2H, d, J = 9.0 Hz, H-2′, H-6′), 7.00 (2H, d, J = 9.0 Hz, H-3′, H-5′), 6.39 (1H, br. s, H-8), 6.26 (1H, br. s, H-6), 3.88 (3H, s, 4′-OMe), 3.84 (3H, s, 3-OMe). 13C NMR (125 MHz, CDCl3) (see Table 2). (+)-ESIMS m/z 337 ([M + Na]+).
Table 2.
13C NMR data of compounds 3–7.
| Nr. | 3 | 6 | 7 | Nr. | 4 | 5 |
|---|---|---|---|---|---|---|
| CDCl3 | DMSO‑d6 | DMSO‑d6 | CD3OD | CD3OD | ||
| 2 | 155.2 | 155.7 | 163.8 | 1 | 106.4 | 106.4 |
| 3 | 138.0 | 133.2 | 103.4 | 2 | 166.3 | 166.4 |
| 4 | 178.0 | 176.9 | 181.5 | 3 | 91.4 | 91.5 |
| 5 | 161.3 | 160.9 | 161.1 | 4 | 164.1 | 163.9 |
| 6 | 99.0 | 98.8 | 109.0 | 5 | 96.4 | 96.3 |
| 7 | 164.4 | 160.8 | 163.7 | 6 | 163.3 | 163.3 |
| 8 | 93.9 | 93.5 | 94.0 | 1′ | 99.9 | 100.7 |
| 9 | 156.5 | 156.2 | 156.4 | 2′ | 74.3 | 73.6 |
| 10 | 104.2 | 105.1 | 103.7 | 3′ | 77.1 | 76.6 |
| 1′ | 122.2 | 120.7 | 121.7 | 4′ | 69.9 | 70.9 |
| 2′ | 130.2 | 115.7 | 113.8 | 5′ | 76.5 | 76.4 |
| 3′ | 114.1 | 148.5 | 145.6 | 6′ | 61.1 | 68.4 |
| 4′ | 161.4 | 144.7 | 149.6 | 1′' | – | 101.7 |
| 5′ | 114.1 | 115.0 | 116.5 | 2′' | – | 70.6 |
| 6′ | 130.2 | 121.7 | 119.2 | 3′' | – | 70.9 |
| 1′' | – | 101.8 | 73.6 | 4′' | – | 73.3 |
| 2′' | – | 73.1 | 70.8 | 5′' | – | 69.9 |
| 3′' | – | 75.7 | 78.9 | 6′' | – | 16.5 |
| 4′' | – | 71.1 | 70.9 | OCH3 | 54.3 | 54.3 |
| 5′' | – | 75.8 | 81.5 | CO | 203.5 | 203.4 |
| 6′' | – | 60.0 | 61.5 | COCH3 | 31.8 | 31.8 |
| 3-OMe | 60.0 | – | – | – | – | – |
| 4′-OMe | 56.0 | – | – | – | – | – |
2.4.4. 4-O-β-D-glucopyranosyl-2-hydroxy-6-methoxyacetophenone (4)
1H NMR (850 MHz, CD3OD) δ = 6.29 (d, J = 2.6 Hz, H-5), 6.22 (d, J = 2.6 Hz, H-3), 5.0 (d, J = 9.4 Hz, H-1′), 3.9 (s, OCH3), 3.88\ 3.68 (2H, H-6′), 3.48 (H-3′), 3.45 (H-5′), 3.42 (H-2′), 3.35 (H-4′), 2.61 (CH3CO). 13C NMR (212.5 MHz, CD3OD) (see Table 2). (-)-ESIMS: m/z 343 ([M−H]-).
2.4.5. 4-O-α-L-rhamnosyl-(1 → 6)-β-D-glucopyranosyl-2-hydroxy-6 methoxy acetophenone (5)
1H NMR (850 MHz, CD3OD) δ = 6.24 (d, J = 2.5 Hz, H-5), 6.23 (d, J = 2.5 Hz, H-3), 4.96 (d, J = 7.7 Hz, H-1′), 4.71 (d, J = 1.7 Hz, H-1′'), 4.02 (H-6′a), 3.9 (s, OCH3), 3.85 (H- 2′'), 3.65 (H-3′'), 3.62 (H-3′), 3.62 (H-5′'), 3.57 ((H-6′b), 3.45 (H-5′), 3.43 (H-2′), 3.34 (H-4′), 3.34 (H-4′'), 2.61 (CH3CO), 1.2 (d, J = 6.0 Hz, H-6′'). 13C NMR (212.5 MHz, CD3OD) (see Table 2). (-)-ESIMS: m/z 489 ([M−H]-).
2.4.6. Quercetin-3-β-O-glucopyranoside (6)
1H NMR (300 MHz, DMSO‑d6) δ = 7.65 (dd, J = 8.5, 2.2 Hz, 1H), 7.52 (d, J = 2.2 Hz, 1H), 6.89 (s, 1H), 6.80 (d, J = 8.5 Hz, 1H), 6.35 (d, J = 2.0 Hz, 1H), 6.14 (d, J = 2.0 Hz, 1H), 5.34 (d, J = 7.6 Hz, 1H), 3.97 (t, J = 6.6 Hz, 1H), 3.65 (d, J = 3.3 Hz, 1H), 3.57 (dd, J = 9.4, 7.7 Hz, 1H), 3.52 – 3.42 (m, 1H), 3.40 – 3.24 (m, 3H). 13C NMR (75 MHz, DMSO‑d6) (see Table 2). (+)-ESIMS: m/z 465 ([M + H]+).
2.4.7. Isooorientin (7)
1H NMR (300 MHz, DMSO‑d6) δ = 7.37 (H-2′), 7.34 (H-6′), 6.86 (H-5′), 6.60 (H-3), 6.48 (H-8), 4.58 (d, J = 9.9 Hz, H-1′'), 4.0 (t, J = 7.2 Hz, H-2′'), 3.17 (H-3′'), 3.48 (H-4′'), 3.14 (H-5′'), 3.39/3.64 (2H, H-6′'). (+)-ESIMS: m/z, 449 [M + H]+, 471 [M + Na]+, 919 [2 M + Na]+. 13C NMR (75 MHz, DMSO‑d6) (Table 2).
2.5. Antimicrobial activity
The antibacterial activity of the plant’s extract, corresponding fractions and obtained compounds was performed against eight bacterial strains according to Murray et al (Murray, P.R., Baron, E.J., Pfaller, M.A., Tenover, F.C. & Yolken, 1995). The bacterial suspension was adjusted to a density of bacterial cells of 1.2 × 108 CFU/mL. A sterile swab immersed in this bacterial suspension was used to inoculate the entire surface of Mueller-Hinton Agar (MHA: M173, India) plates. Wells of 6-mm diameter were made on MHA plates using sterilized gel puncture. About 30 μL of each plant extract concentrations 0.0 up to 100 μg/mL) diluted in dimethyl sulfoxide (DMSO) were transferred onto each well of all plates then, the plates were then incubated for 24 h at 37 °C. The inhibition zones (mm) were measured (Villar et al., 1986). Chloramphenicol solution (30 μg/mL) was prepared as positive control, and all experiments were done in triplicate. The following eight bacterial strains were served during the present antibacterial activity study: Gram positive bacteria (Listeria monocytogenes Scott A, Staphylococcus aureus (ATCC 6538), Bacillus cereus and Enterococcus faecalis) and Gram negative bacteria (Salmonella enterica serotype Enteritidis, Pseudomonas aeruginosa, Klebsiella pneumonia and Escherichia coli (ATCC 8739) were obtained from Egyptian Microbial Culture Collection, Microbiological Resource Center (The Cairo MIRCEN: Ain Shams University, Cairo, Egypt). Each strain was aseptically sub-cultured in Tryptic Soy broth (TSB: Merck 1.05459, Darmstadt, Germany) and checked for purity onto Tryptic Soy agar plates (TSA: Merck 1.05458, Darmstadt, Germany), incubated for 24 h at 37 ◦C.
2.5.1. Minimum inhibitory concentration (MIC) and minimum bactericidal concentration (MBC)
Minimum inhibitory concentration (MIC) and minimum bactericidal concentration (MBC) were evaluated using standard inoculums of 1.0–1.5 × 105CFU/ ml from each strains (Rex et al., 2001). Serial dilutions of each plant extract, previously dissolved in DMSO, were prepared to final concentrations of 0.0 up to 40.0 mg/ml of TSB. To each tube, 0.1 mL of each strain was inoculated and incubated for 24 h at 37 °C. At the end of incubation time, MIC and MBC were visually identified as the lowest concentration of the test compound which inhibits the visible growth and confirmed by measuring the OD600 and plating onto MHA of all treatments. Tests using sterilized distilled water as negative control and Chloramphenicol as positive control were carried out in parallel. All tests were performed in triplicate.
2.6. Statistical analysis
The data represent mean of three replicates ± standard error (SE). Results were subjected to multiway analysis of variance, and the mean comparisons were performed by Tukey’s multiple range test using SPSS version 20.0 (Statistical Package for the Social Sciences, Inc., Chicago, IL, United States). Differences between means were considered significant at p-value < 0.05.
2.7. Cytotoxicity assays
The Cytotoxicity of the plant’s extract, fractions and pure compounds were evaluated in vitro against three human cancer cell lines: HCT116 [Colon cell line], HePG-2 [Human hepatocellular carcinoma cell line], MCF7 [Human Caucasian breast adenocarcinoma], alongside normal cell line RPE1[normal retina cell line] by using MTT assay. Cell viability was assessed by the mitochondrial-dependent reduction of yellow MTT (3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide) to purple formazan. The cultivation of the cell lines and the cytotoxicity assays were performed as described by (Mosmann, 1983). This cytotoxic activity test (In vitro bioassay on human tumor cell lines) was conducted and determined by the Bioassay-Cell Culture Laboratory, National Research Centre, El-Tahrir St., Dokki, Cairo 12622, Egypt.
3. Results
3.1. GC–MS and tentative identification
GC–MS analysis of n-hexane and two sub-fractions (I&III) tentatively identified the presence of 24 compounds (Table 1) where lupeol acetate (27.46%) and cycloartanol (17.74%) are the major components followed by α-amyrine (11.4%), lanostenol (11.2%) and germanicol (10.10%) in n-hexane (see Figures S1-S3 in Supplementary data). The main components of sub-fraction I were lupeol acetate (36.48%) followed by α-amyrine (20.70%) and germanicol (20.62%) while sub-fraction III composed mainly of β-sitosterol (48.87%) followed by nonanal (8.23%).
Table 1.
GC/MS of n-hexane, sub-fractions I and III.
| The percentage (%) for each compound |
|||||
|---|---|---|---|---|---|
| Compound* | RT | Molecular Formula | n-Hexane | Sub-FI | Sub-FIII |
| Limonene | 3.95 | C10H16 | 1.07 | 3.47 | |
| Eucalyptol | 4.00 | C10H18O | – | 2.78 | – |
| Nonanal | 5.15 | C9H18O | – | – | 8.23 |
| Octadecane | 15.65 | C18H38 | – | 1.18 | |
| 2,4-di-tert-butylphenol | 16.71 | C14H22O | 1.23 | 3.29 | |
| Caryophyllene oxide | 18.86 | C15H24O | 0.92 | 2.96 | – |
| Heneicosane | 21.61 | C21H44 | – | 1.92 | – |
| Hexacosane | 31.57 | C26H54 | – | – | 1.79 |
| Octacosane | 43.74 | C28H58 | – | – | 1.63 |
| Cholesterol | 51.37 | C27H46O | – | – | 1.99 |
| 9,19-Cyclocholestan-3-ol, 14-methyl | 52.49 | C28H48O | 1.66 | ||
| Campesterol | 53.05 | C28H48O | 3.03 | ||
| Cholest-7-en-3-ol, 2,2dimethyl | 53.44 | C29H50O | 8.32 | – | 3.07 |
| Lanost-8-en-3-ol | 54.04 | C30H52O | 11.2 | ||
| β- Sitosterol | 54.06 | C29H50O | – | 1.14 | 48.87 |
| Cycloartanol | 54.92 | C30H52O | 17.74 | – | – |
| 9,19-Cyclolanostan-3-ol, acetate | 54.99 | C32H54O2 | – | – | 1.91 |
| Lanost-8-en-3-ol, acetate | 55.22 | C32H54O2 | 1.33 | 3.67 | – |
| Lupeol | 55.76 | C30H50O | 4.24 | – | – |
| Lup20(29)-en-3ol, acetate | 55.88 | C32H52O2 | – | 1.78 | – |
| α-Amyrin | 56.14 | C30H50O | 11.4 | 20.70 | – |
| Germanicol | 56.27 | C30H50O | 10.10 | 20.62 | – |
| Lup-20(29)en-3-ol-acetate | 56.94 | C30H52O2 | 27.46 | 36.48 | – |
| Retinoic acid, 5,6-epoxy-5,6-dihydro | 57.09 | C20H28O3 | – | – | 3.65 |
RT = retention time, •MF = Molecular formula
3.2. Isolation
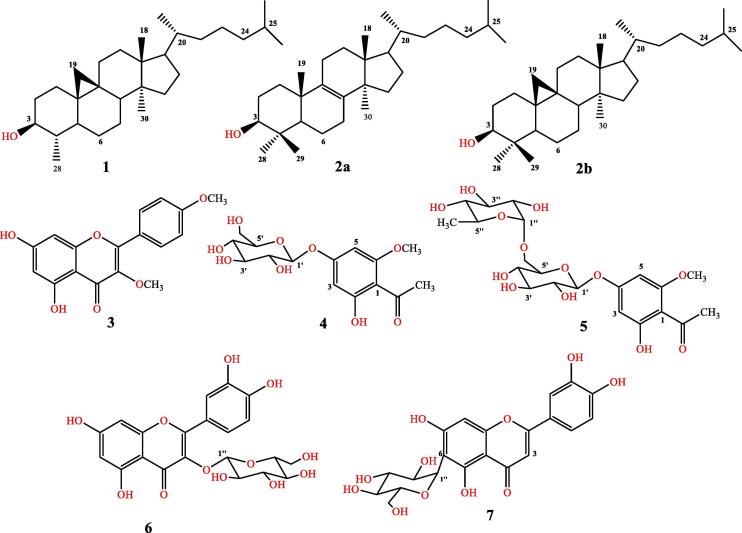
Seven bioactive compounds were obtained from the plant’s extract, three among them (1–3) were isolated from n-hexane fraction, while the other four compounds (4–7) were isolated from polar n-butanol fraction as depicted in (Fig. 1), and described in the experimental section.
Fig. 1.
Isolated Compounds 1–7 from E. balsamifera.
3.3. Structure determination
Compound 1 was isolated as amorphous powder, displaying a positive response to Liebermann-burchardt reagent, as indicative for a triterpene moiety. The molecular weight of 1 was determined as 414 Daltons according to GC–MS, which has been further confirmed by ESIMS, exhibiting a quasi-molecular ion peak at m/z 415 [M + H] with a corresponding molecular formula C29H50O. 1H NMR exhibited two doublets of high field shifted signals at δ 0.22 and 0.45 (J = 4.0 Hz) which characteristic for non-equivalent methylene protons of cyclopropane, being mostly for the cyclopropyl-9,19 methylene moiety, together with an-oxymethine proton at δ 3.17 (ddd, J = 15.5, 9.0, 4.5) attributed to H-3. The coupling constant value of the latter suggested its β-configuration, meanwhile the C-4 bounded doublet methyl (δ 1.02, CH3-28) is of α- orientation. Further three doublet methyl signals (J = 6.5) appeared at δ, 0.94 (6H, H3-26, H3-27) and 0.93 (H3-21) along with two singlet methyl at δ 1.06 (H3-18), 0.98 (H3-30) were assigned. According to 13C NMR, the oxymethine carbon (C-3) was shown at δ 77.2 with δΔ ~ −1.0 compared to those moieties containing two methyl groups at C-4 (Kamisako et al., 1987). Based on the revealed chromatographic properties and spectroscopic features and comparison with literature, compound 1 showed high similarity to 3β-hydroxy-29-nor-cycloart-24-one (Ma et al., 2001) except disappearance of carbonyl group at C-24, and hence it was finally assigned as 29-norcycloartanol (Agarwal et al., 2010, Chang et al., 2012). According to our search in literature and so for knowledge, this is the first time to assign 29-norcycloartanol on the bases of 1D and 2D NMR data (see Figures S4-S8 in Supplementary data).
According to GC–MS analysis, compound 2 was confirmed as mixture of two major peaks with retention times of 32.86 and 33.48 (see Figures S9-S10 in Supplementary data). Their molecular weight was identically confirmed as 414 Daltons, with corresponding molecular formula C30H52O. The unresolved mixture of compound 2 was further established by 1H- and 13C NMR spectral data: In compound 2a, two quaternary carbon signals were deduced at δ 134.51 and 134.48 which are being attributed to a Δ8/9 structure. On the other hand, the second compound (2b) showed two characteristic signals in 1H NMR at δ 0.31 and 0.54 (J = 4.0 Hz) indicating the presence of non-equivalent protons of a methylene cyclopropane. The oxymethine protons were shown at δ 3.21 for both compounds (dd, J = 4.4, 11.6, 2H, H-3), and their corresponding carbon signals were established at δ 79.05 and 79.09. Further study of the spectral data of compound 2 and comparison with literature confirmed its mixture nature of lanost-8-en-3-ol (2a) and cycloartanol (2b) (see Figures S11-S12 in Supplementary data).
As yellow solid, compound 3 was obtained showing an UV absorbance during TLC. The molecular weight (m/z 314) and corresponding molecular formula (C17H14O6) of 3 were established on the bases of ESIMS. According to 1H NMR spectrum, structure 3 showed two aromatic spin systems: Two m-coupled protons of AB spin system for H-6 and H-8 were shown at δ 6.26 and 6.39; and two doublet signals (each with 2H integration, J = 9.0 Hz) being for AA'XX' spin system (i.e. 1,4-disubstituted aromatic residue) were shown at δ 8.05, 7.0 corresponding to H-2′/H-6′ and H-3′/H-5′ in ring B, respectively. In the aliphatic region, two methoxy signals appeared at δ 3.84 and 3.88 with corresponding carbon signals at δ 56.0 and 60.0 through HMQC experiment (Table 2). Complete analysis of 1D (1H,13C) and 2D NMR (HMQC and HMBC) and comparison with literature established the structure of compound 3 as kampferol −3,4′ dimethyl ether. So far as we know, compound 3 was isolated herein to first time from E. balsamifera.
As polar colourless solid, with an UV absorbance (254 nm) during TLC, compound 4 was obtained. According to the negative mode of ESIMS, a pseudo ion peak was shown at m/z 343 [M−H], and the corresponding molecular formula as C15H20O9, indicating the presence of six degrees of unsaturation (DBE). The 1H NMR spectrum of compound 4 showed two m-coupled (d, J = 2.6 Hz) aromatic signals of AB spin system at δ 6.22 and 6.29 and anomeric proton at δ 5.0 (d, J = 9.4 Hz) establishing the presence of β-configured sugar moiety. The sugar unit was identified as β-glucose by the signals in the range of 3.38 – 3.82 with a coupling constant ≈ 9.0 Hz as well as comparison the 13C NMR data with the reported literature (Atta et al., 1982). Two singlet signals, corresponding to methyl ether (δ 3.93) and aromatic bounded acetyl group (2.61) were assigned, respectively. Based on the 13C NMR spectrum, 15 carbon signals were deduced, which classified into six sugar carbons, six aromatic signals, acetophenone carbonyl (δ 203.5), one methoxy (δ 54.9) and a sp3 acetyl carbon (δ 31.8) (Table 2). HMBC showed a 3J -linkage between the anomeric proton H-1′ and the quaternary aromatic carbon C-4 (δ 163.3), meanwhile H-3 (δ 6.22) showed HMBC connectivities with C-2 (δ 166.3), C-4 (164.1), C-5 (91.4) and C-1 (106.4). Based on the chromatographic and intensive spectroscopic data, the structure of compound 4 was confirmed as 4-O-β-D-glucopyranosyl-2-hydroxy-6-methoxyacetophenone. The latter was isolated previously from the roots of Prunus armeniaca and Euphorbia fischeriana (Huang et al., 2017, Prasad, 1999), meanwhile it is reported herein to first time from E. balsamifera.
With higher polarity and closely related chromatographic and spectroscopic features to 4-O-β-D-glucopyranosyl-2-hydroxy-6-methoxyacetophenone (4), compound 5 was obtained. According to the negative mode of ESIMS, a pseudo molecular ion peak was exhibited at m/z = 489 [M−H], revealing the presence of rhamnose as additional sugar moiety. In accordance, the 1H NMR and 13C NMR spectra of compound 5 showed the same aromatic pattern of 4, however, with additional sugar unit of α-configuration (J = 1.7 Hz) due to the presence of a doublet anomeric proton (H-1′') at δ 4.71 and corresponding anomeric carbon C-1′' at δ 101.7, reflecting the α-configuration nature of this sugar moiety having a doublet (J = 6.0 Hz) methyl signal at δ 1.20 (H3-6′', δC: 16.5).
Based on the COSY and HSQC experiments, the last sugar unit was identified as α-L-rhamnose, and hence the structure of 5 was finally determined as 4-O-α-L-rhamnosyl-(1 → 6)-β-D-glucopyranosyl-2-hydroxy-6 methoxy acetophenone on the bases of long range correlation (HMBC). Compound 5 was previously reported from Erythroxylum cambodianum (Kanchanapoom et al., 2005), however, and as far as we know, it has never been reported from E. balsamifera.
As yellow solid of UV absorbance and visible yellow appearance during TLC, compound 6 was afforded. Under long UV light (366 nm), the compound showed fluorescence colour, which turned deep yellow on exposing to flavonoid spraying reagent (Aluminium chloride), suggesting its flavonoid nature. The molecular weigh (m/z 464) and corresponding molecular formula (C21H20O12) of compound 6 were determined by (+)-ESIMS, bearing 12 DBE. 1H NMR spectrum exhibited two spins aromatic signals, the first of them was of m-coupled AB spin system at δ 6.35 and 6.14, (d, J = 2.0 Hz, 1H) for H-6 and H-8 of ring-A, respectively. The second spin system is corresponding to 1,2,4-trisubstiuted aromatic residue, showing three proton signals at δ 7.65 (dd, J = 8.5, 2.2 Hz), 7.52 (d, J = 2.2 Hz), 6.80 (d, J = 8.5 Hz) being for H-6′, H-2′ and H-5′ of ring-B, respectively. In accordance, the quercetin aglycone skeleton was established. In the aliphatic region, a doublet signal (J = 7.6 Hz) of an anomeric proton was exhibited at δ 5.34, indicating the presence of one sugar moiety with a corresponding anomeric carbon at δ 101.8 according to HSQC experiment. According to the 13C NMR spectrum, 15 carbon signals corresponding to quercetin aglycone were assigned. This was together with 6 oxygenated sp3 methine/methylene carbons being for the mentioned before sugar unit (Table 1). Based on the shown multiplicity and coupling constant of anomeric proton, the sugar moiety was assigned as β-D-glucose. Complete analysis of COSY, HSQC and HMBC experiments confirmed the structure of 6 as quercetin 3-β-D-glucoside, which was isolated herein for first time from E. balsamifera.
As closely related to 6, compound 7 was obtained exhibiting similar chromatographic properties with tinny less polarity, as indicative for a further glycosidic flavonoid system. Based on (+) ESIMS, compound 7 displayed three quasi molecular ion peaks at m/z 449, 471 and 919 corresponding to [M + H]+, [M + Na]+ and [2 M + Na]+, respectively. Accordingly, the corresponding molecular formula of 7 was confirmed as C21H20O11, having same unsaturation degree of 6 (12 DBE). The 1H- and 13C NMR spectra revealed the presence of same pattern of ring-B in compound 6, meanwhile the O-glycoside C-3 in ring C of 6 showed a singlet signal at δ 6.0 along with a sole singlet proton in ring-A at δ 6.48 (H-8). In the glucoside region, an oxygenated methine carbon was visible at δ 73.6, instead of the O-glycosidic carbon (101.8) shown in compound 6, suggesting C-glycoside unit at C-6 in compound 7 (Table 2) according to HMBC experiment. Based on the long range correlations (HMBC) H-1′' (δ 4.57) of the glucoside unit exhibited a 3J cross sections with C-7 (δ: 163.7) and C-5 (δ:161.1), confirmed its attachment to C-6 of ring A. Consequently, compound 7 was identified as isooreintin (homoorientin), which was isolated to herein to first time from E. balsamifera, exhibiting a complete structural agreement with the previously isolated one from Antidesma ghaesembilla (Canh et al., 2015).
3.4. Antibacterial activity
The antibacterial activity of the total ethanolic extract, n-hexane, ethyl acetate and n-butanol fractions of E. balsamifera against LM, Listeria monocytogenes Scott A; SA, Staphylococcus aureus (ATCC 6538), BC, Bacillus cereus and EF, Enterococcus faecalis; SE; Salmonella enterica serotype Enteritidis; PA; Pseudomonas aeruginosa; EC, Escherichia coli (ATCC 8739); KP, Klebsiella pneumoniae have been assessed (Table, 3). The results revealed that the ethanolic extract is efficiently suppressing the growth of the tested pathogenic bacteria and had the maximum zone of inhibition against EC (25 ± 0.32 mm), whereas Chloramphenicol showed a maximum zone of inhibition against EC (26 ± 0.21 mm). The ethanolic extract exhibited inhibitory effect against eight of the pathogenic bacteria (LM, SA, BC, EF, SE, PA, EC and KP) at the concentration 30 μg/mL, while at 20 μg/mL was effective against five strains only (KP, PA, EC, BC, and SE). In the n-hexane, ethyl acetate and n-butanol fractions, just ethyl acetate and n-butanol had more valuable results (p < 0.05) against the tested pathogenic bacteria than n-hexane fraction at low concentration 10 or 20 μg/mL (with inhibition zone against SA (28 ± 0.15 and 29 ± 0.19 mm at 10 μg/mL compared to 11 ± 0.12 mm for the hexane fraction), respectively. The minimum inhibitory concentration (MIC) was varied between the extract and fractions (see Table S33 in Supplementary data). The MIC of ethanolic extract was 30 µg/ml against both of Gram positive and Gram-negative bacteria, the MIC of hexane extract was between 20 and 30 µg/ml against all the tested strains. The MIC of ethyl acetate and n-butanol were 10 to 20 µg/ml against all the tested strains where the lowest MIC was observed for n-butanol fraction with the highest antibacterial activity.
The zone of inhibition (mm) for pure compounds are shown in Table 4. All compounds showed antibacterial efficiency against all the tested bacteria except compounds 1 and 5. These compounds inhibited the growth of bacteria and showed inhibiting zone ranged from 9 ± 0.02 to 22 ± 0.13 mm. The largest of inhibition zone by compound 2 was observed for EF (22 ± 0.13 mm). The minimum inhibitory concentration (MIC) and MBC of five compounds are shown in Table 5. The MIC or Minimum bactericidal concentration (MBC) was ranged between 1.15 and 2.48 mg/mL and 2.05 to 3.00 mg/mL, respectively against the six bacteria indicating that the antibacterial action of the substances begins the highest concentration of compound 7 against BC while the lowest concentration of compound 4 against SA. Thus, the compound 4 showed lowest MIC and MBC (1.15 and 2.05 mg/mL), respectively indicating its higher antibacterial activity than all tested compounds. While compound 7 recorded the highest MIC and MBC (2.48 and 3.00 mg/mL) while no significant antimicrobial activity for compound 7 is reported (Kumarasamy et al., 2004). The antimicrobial activity of compound 6 showed agreement with its previously published data (Mohammed et al., 2015).
Table 4.
In vitro antibacterial activity of Compounds 1–7 (Inhibition zone, mm).
| Compounds (μg/mL) | Minimum Inhibitory zone (mm) |
|||||||
|---|---|---|---|---|---|---|---|---|
| LM | SA | BC | EF | EC | SE | PA | KP | |
| Control | 0.0 ± 0.0 | 0.0 ± 0.0 | 0.0 ± 0.0 | 0.0 ± 0.0 | 0.0 ± 0.0 | 0.0 ± 0.0 | 0.0 ± 0.0 | 0.0 ± 0.0 |
| 1 | ND | ND | ND | ND | ND | ND | ND | ND |
| 2 | 12 ± 0.11 | 11 ± 0.13 | 10 ± 0.09 | 22 ± 0.13 | 9 ± 0.17 | 9 ± 0.05 | 9 ± 0.02 | 9 ± 0.17 |
| 3 | 11 ± 0.09 | 13 ± 0.07 | 12 ± 0.11 | 14 ± 0.16 | 15 ± 0.15 | 10 ± 0.06 | 13 ± 0.11 | 13 ± 0.14 |
| 4 | 13 ± 0.23 | 12 ± 0.11 | 10 ± 0.13 | 10 ± 0.09 | 10 ± 0.08 | 10 ± 0.11 | 10 ± 0.16 | 10 ± 0.17 |
| 5 | ND | ND | ND | ND | ND | ND | ND | ND |
| 6 | 11 ± 0.12 | 12 ± 0.07 | 11 ± 0.18 | 13 ± 0.04 | 10 ± 0.09 | 9 ± 0.11 | 10 ± 0.23 | 13 ± 0.21 |
| 7 | 12 ± 0.22 | 13 ± 0.26 | 11 ± 0.16 | 13 ± 0.14 | 11 ± 0.12 | 10 ± 0.13 | 13 ± 0.14 | 12 ± 0.06 |
Values are means of three replicates ± standard error. LM, Listeria monocytogenes Scott A; SA, Staphylococcus aureus (ATCC 6538), BC, Bacillus cereus and EF, Enterococcus faecalis; SE; Salmonella enterica serotype Enteritidis; PA; Pseudomonas aeruginosa ; EC, Escherichia coli (ATCC 8739); KP, Klebsiella pneumonia, ND; Not Determined.
Table 5.
In vitro Minimum Inhibitory concentration (MIC) and Minimum bactericidal concentration (MBC) of compounds 2–4, 6–7 (mg/mL).
| Compounds (μg/mL) | MIC (mg/ml) |
MBC (mg/ml) |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| LM | SA | BC | SE | EC | PA | LM | SA | BC | SE | EC | PA | |
| 2 | 2.35 | 2.30 | 2.45 | 2.34 | 2.08 | 2.19 | 2.93 | 2.55 | 2.87 | 2.98 | 2.88 | 2.69 |
| 3 | 2.15 | 2.09 | 2.05 | 2.12 | 2.22 | 2.16 | 2.59 | 2.84 | 2.82 | 2.72 | 2.86 | 2.83 |
| 4 | 1.60 | 1.70 | 1.15 | 1.24 | 1.88 | 1.79 | 2.13 | 2.05 | 2.17 | 2.28 | 2.23 | 2.21 |
| 6 | 2.30 | 2.12 | 2.12 | 2.22 | 2.34 | 2.19 | 2.68 | 2.45 | 2.86 | 2.69 | 2.40 | 2.81 |
| 7 | 2.54 | 2.08 | 2.08 | 2.26 | 2.39 | 2.48 | 2.74 | 2.74 | 3.00 | 2.89 | 2.91 | 2.53 |
LM, Listeria monocytogenes Scott A; SA, Staphylococcus aureus (ATCC 6538); BC, Bacillus cereus; SE, Salmonella enterica; EC, Escherichia coli (ATCC 8739); PA; Pseudomonas aeruginosa.
3.5. Cytotoxic activity
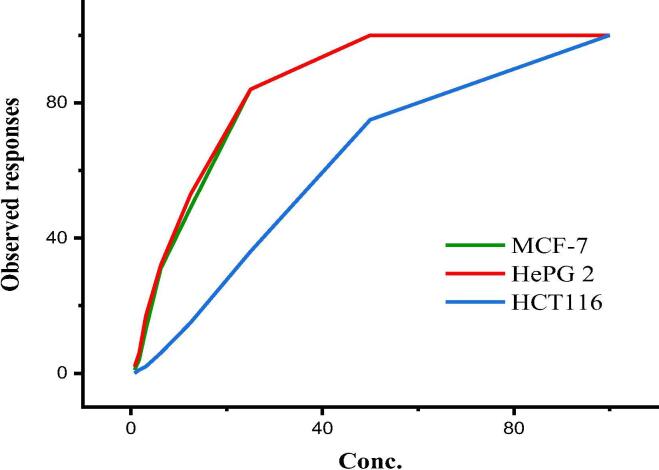
The plant fractions, n-hexane, ethyl acetate, n-butanol were evaluated in vitro for their cytotoxic activity against three human cancer cell lines HepG2, HCT116 and MCF7. Interesting result was observed for n- hexane fraction against HCT116 and HepG2 with IC50 of 76.2 µg/ml and 54.7 µg/ml respectively with low cytotoxicity against normal retina cell line RPE1 of IC50: 79.2 µg/ml (see Figure S32 in Supplementary data). A significant activity was observed for crude compound 3 while the pure compound 3 displayed higher activity than the reference drug against HCT116, HepG2 and MCF7 (Fig. 2) with IC50 of 111.46 µM, 42.67 µM and 44.90 µM, respectively. Unfortunately, the cytotoxicity of compound 3 on normal cell was high with IC50 of 16.6 µg/ml. Therefore, the crude n-hexane fraction could be applied in vivo as it is much safe on the normal cell compared to pure compound 3. No significant activities observed for other compounds and sub-fractions (see Table S34 in Supplementary data). The literature showed complete agreement for inactivity of compounds 4, 6 against human cancer cell lines (Huang et al., 2017, Yang et al., 2014). In contrast to our results, isooreintin (7) showed a moderate activity against four cancer cell lines including HepG2 and MC7 (Farid et al., 2015).
Fig. 2.
Observed responses of compound 3 against MCF7, HePG2 and HCT116 cell lines.
4. Discussion
Euphorbia balsamifera (family Euphorbiaceae) has a broad spectrum of medicinal properties: The latex of Euphorbia balsamifera is effective as a pulpal devitalizing agent (Yam, A. A., Gaye, F., Dieme, F. A., Bassene, E., & Ba, 1997), the plant’s leaves, stems and roots showed antimicrobial activities against some pathogenic microorganisms (Kamba and Hassan, 2010), and the methanol extract of the plant showed wound healing activity (Ahmed et al., 2016).
In our ongoing for search of bioactive compounds from plants and study their broad biological activities, we report herein the isolation and structure assigning of Euphorbia balsamifera produced metabolites and investigate their antibacterial and cytotoxic activity to first time so far. In accordance, an application of the plant’s extract into fraction using n-hexane and n-butanol, followed by GC–MS analysis for the un polar fractions (n-hexane fraction and sub-fractions I&III) tentatively revealed the presence of 24 compounds, at where lupeol acetate, cycloartenol, α-amyrine, lanostenol and germanicol, represent the most abundant metabolites, respectively.
Chromatographic purification of the n-hexane fraction using different techniques afforded three compounds namely 29-norcycloartanol (1), mixture of lanost-8-en-3-ol (2a) and cycloartanol (2b) and the flavonoid kampferol −3,4′-dimethyl ether (3). Alternatively, purification of the n-butanol fraction using diverse chromatographic techniques (see experimental section) resulted in four glycosidal compounds namely, 4-O-β-D-glucopyranosyl-2-hydroxy-6-methoxyacetophenone (4), 4-O-α-L-rhamnosyl-(1 → 6)-β-D-glucopyranosyl-2-hydroxy-6 methoxy acetophenone (5), the flavonoid glycoside quercetin 3-β-D-glucoside (6) and isooreintin (homoorientin) (7). Structures of the obtained compounds have been identified intensively on the bases of their chromatographic properties, 1D and 2D NMR and MS spectroscopy (Table 2, see Supplementary Materials S4-31) and comparison with the corresponding literatures.
29-Norcycloartanol (1) was reported before from other species, however, without full assignment (Agarwal et al., 2010, Chang et al., 2012), and it is reported herein to first time from E. balsamifera, and its full assignment on the bases of 1D and 2D NMR data is recoded to first time as well. So far as we know as well, compound 3 was isolated herein to first time from E. balsamifera. Compound 4 was isolated previously from the roots of Prunus armeniaca and Euphorbia fischeriana (Huang et al., 2017, Prasad, 1999), meanwhile it is reported herein to first time from E. balsamifera. Compound 5 was as well reported from Erythroxylum cambodianum (Kanchanapoom et al., 2005), however, and as far as we know, it has never been reported from E. balsamifera. Likely, compounds 6 and 7 (Canh et al., 2015) were isolated to herein to first time from E. balsamifera.
The antibacterial activity of Euphorbia balsamifera, fractions and pure compounds were evaluated against eight pathogenic bacterial strains (Table 3). According to this study, the ethanolic extract showed high activity against the Gram-negative bacteria E. coli (25 mm) in comparison with chloramphenicol (26 ± 0.21 mm). Particularly, the ethanol extract was only effective against five strains: KP, PA, EC, BC, and SE at concentration of of 30 μg/mL. The obtained fractions, ethyl acetate and n-butanol fractions displayed higher antibacterial activity at low concentration 10 ~ 20 μg/mL (28 ± 0.15, 29 ± 0.19 mm at 10 μg/mL against SA) than those shown for n-hexane fraction (11 ± 0.12 mm). This might be mostly attributed to their abundance with flavonoid and glycosidic compounds rather than those shown in n-hexane fraction.
Table 3.
In vitro antibacterial activity of total ethanolic extracts, hexane fraction, ethyl acetate fraction and n-butanol fraction of E. balsamifera (Inhibition zone, mm).
| Plant extracts/fractions (μg/mL) |
Minimum Inhibitory zone (mm) |
||||||||
|---|---|---|---|---|---|---|---|---|---|
| LM* | SA | BC | EF | EC | SE | PA | KP | ||
| Control | 0 | 0.0 ± 0.0 | 0.0 ± 0.0 | 0.0 ± 0.0 | 0.0 ± 0.0 | 0.0 ± 0.0 | 0.0 ± 0.0 | 0.0 ± 0.0 | 0.0 ± 0.0 |
| Total extract | 10 | 7 ± 0.11 | 9 ± 0.21 | 11 ± 0.23 | 8 ± 0.12 | 17 ± 0.02 | 18 ± 0.16 | 15 ± 0.18 | 19 ± 0.20 |
| 20 | 8 ± 0.21 | 10 ± 0.25 | 15 ± 0.24 | 9 ± 0.23 | 19 ± 0.22 | 20 ± 0.28 | 19 ± 0.26 | 21 ± 0.27 | |
| 30 | 14 ± 0.22 | 17 ± 0.12 | 21 ± 0.28 | 18 ± 0.21 | 25 ± 0.32 | 21 ± 0.42 | 23 ± 0.12 | 22 ± 0.02 | |
| Hexane fraction | 10 | 9 ± 0.20 | 11 ± 0.12 | 13 ± 0.16 | 13 ± 0.31 | 14 ± 0.32 | 15 ± 0.25 | 13 ± 0.26 | 15 ± 0.22 |
| 20 | 11 ± 0.24 | 16 ± 0.22 | 14 ± 0.17 | 14 ± 0.27 | 15 ± 0.28 | 17 ± 0.15 | 16 ± 0.20 | 17 ± 0.12 | |
| 30 | 15 ± 0.22 | 18 ± 0.25 | 17 ± 0.12 | 15 ± 0.26 | 18 ± 0.29 | 19 ± 0.19 | 18 ± 0.28 | 19 ± 0.29 | |
| Ethyl acetate fraction | 10 | 26 ± 0.17 | 28 ± 0.15 | 26 ± 0.18 | 23 ± 0.19 | 18 ± 0.12 | 21 ± 0.19 | 17 ± 0.28 | 16 ± 0.16 |
| 20 | 30 ± 0.29 | 34 ± 0.27 | 35 ± 0.20 | 31 ± 0.31 | 22 ± 0.17 | 24 ± 0.22 | 21 ± 0.15 | 19 ± 0.12 | |
| 30 | 36 ± 0.23 | 38 ± 0.24 | 38 ± 0.12 | 36 ± 0.20 | 25 ± 0.18 | 26 ± 0.20 | 27 ± 0.19 | 25 ± 0.18 | |
| n-butanol fraction | 10 | 21 ± 0.11 | 29 ± 0.19 | 19 ± 0.22 | 19 ± 0.23 | 13 ± 0.21 | 15 ± 0.16 | 13 ± 0.17 | 12 ± 0.27 |
| 20 | 28 ± 0.25 | 34 ± 0.15 | 27 ± 0.27 | 30 ± 0.23 | 13 ± 0.26 | 17 ± 0.15 | 15 ± 0.19 | 13 ± 0.23 | |
| 30 | 35 ± 0.17 | 36 ± 0.13 | 33 ± 0.23 | 34 ± 0.21 | 15 ± 0.25 | 17 ± 0.11 | 18 ± 0.21 | 16 ± 0.25 | |
| Chloramphenicol | 100 | 30 ± 0.17 | 33 ± 0.22 | 32 ± 0.27 | 31 ± 0.32 | 26 ± 0.21 | 23 ± 0.24 | 21 ± 0.20 | 20 ± 0.12 |
Values are means of three replicates ± standard error. *LM, Listeria monocytogenes Scott A; SA, Staphylococcus aureus (ATCC 6538), BC, Bacillus cereus and EF, Enterococcus faecalis; SE; Salmonella enterica serotype Enteritidis; PA; Pseudomonas aeruginosa; EC, Escherichia coli (ATCC 8739); KP, Klebsiella pneumoniae
According to MIC studies of the plant’s extract (ethanolic extract) and its fractions, n-hexane, ethyl acetate showed MIC of 30 µg/mL, 20 ~ 30 µg/mL, and 10 ~ 20 µg/mL, respectively, meanwhile n-butanol fraction displayed the lowest MIC and highest antibacterial activity. It is worthy to refer herein that, several plants such as oregano, cumin, cinnamon, sage, and other spices possessed significant (P < 0.05) antibacterial activities against wide range of Gram-positive and Gram-negative (Liu et al., 2017, Nassan et al., 2015).
Alternatively, the antibacterial activity of the isolated compounds (1–7) was reported (Table 4). Except compounds 1 and 5, all compounds were active against the whole test bacterial strains (9 ~ 22 mm). Compound 2 was the most active one against Enterococcus faecalis (22 ± 0.13 mm). According to our MIC and MBC studies (Table 5), it has been investigated that compound 4 showed the lowest MIC and MBC (1.15 and 2.05 mg/mL), indicating its highest antibacterial activity, while compound 7 recorded the highest MIC and MBC (2.48 and 3.00 mg/mL), i.e. lowest activity, although Kumarasamy et al (Kumarasamy et al., 2004) reported no significant antimicrobial activity for the latter. Finally, compound 6 showed a consistent activity with previously reported data (Kumarasamy et al., 2004).
The cytotoxic activity of the plant’s extracts, and afforded fractions and corresponding isolated compounds were investigated against three human cell lines, MCF7, HePG2 and HCT116. Accordingly, the n-hexane fraction among the three fractions showed a significant IC50: 54.7 and 76.2 µg/ml against HCT116 and HePG2, respectively, and low cytotoxicity against normal retina cell line RPE1 (IC50: 79.2 µg/ml). Kampferol-3,4′-dimethyl ether (3) exhibited the highest cytotoxic activity (IC50 111.46, 42.67 and 44.90 µM) against HCT116, HePG2 and MCF7, respectively (Fig. 2) than those reported by doxorubicin. However, the cytotoxicity of compound 3 on normal cell was high with IC50 of 16.6 µg/ml, excluding it as anticancer agent or it needs further modification into other safe drugs to decrease such cytotoxicity. Therefore, the crude n-hexane fraction could be applied in vivo as it is much safe on the normal cell compared to pure compound 3. In contrast to our negative cytotoxic results, isooreintin (7) showed a moderate activity against four cancer cell lines including HepG2 and MCF7 (Farid et al., 2015).
5. Conclusions
Euphorbia balsamifera is a valuable natural source for antibacterial agents which could be useful in vivo study. Antibacterial assays showed that n-butanol fraction is the most active against tested strains while cytotoxic assays revealed that n-hexane fraction is the most active against HCT116 and HePG2 cancer cell lines. The antibacterial activities of glycoside compounds are lower than non-glycosides which were observed for a significant antibacterial activity of compound 4 while no activity for compound 5 in addition to higher activity of compound 3 than compounds 6,7 confirmed the antibacterial activity decrease with glycosylation. Kampferol-3,4′-dimethyl ether is the most cytotoxic compound against three human cancer cell lines in crude and pure form while other tested compounds isolated from n-hexane and n-butanol fractions are not active, but it is toxic against normal cell line. All compounds were isolated for the first time from E. balsamifera and it is first full assignment of 1H and 13C NMR of 29-nor-cycloartanol compound based on 1D and 2D NMR.
Footnotes
Peer review under responsibility of King Saud University.
Supplementary data to this article can be found online at https://doi.org/10.1016/j.sjbs.2020.10.025.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
References
- Abu-Darwish M.S., Efferth T. Medicinal plants from near east for cancer therapy. Front. Pharmacol. 2018;9:1–17. doi: 10.3389/fphar.2018.00056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Agarwal O.P., Saxena U., Jain S.C., Jain R. A new biscoumarin and other constituents from Guazuma tomentosa roots. Chem. Nat. Compd. 2010;46:713–715. doi: 10.1007/s10600-010-9722-2. [DOI] [Google Scholar]
- Ahmed S., Yousaf M., Mothana R.A., Al-Rehaily A.J. Studies on wound healing activity of some Euphorbia species on experimental rats. African J. Tradit. Complement. Altern. Med. 2016;13:145–152. doi: 10.21010/ajtcam.v13i5.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aliero A.A., Afolayan A.J. Antimicrobial activity of Solanum aculeastrum. African J. Biotechnol. 2006;5:369–372. [Google Scholar]
- Atta U.R. hma, Ansari A.A., Drexler S.A., Clardy J. The isolation and structure of nahagenin. Heterocycles. 1982;19(2), 217–220 19:217–220. [Google Scholar]
- Ben Jannet S., Hymery N., Bourgou S., Jdey A., Lachaal M., Magné C., Ksouri R. Antioxidant and selective anticancer activities of two Euphorbia species in human acute myeloid leukemia. Biomed. Pharmacother. 2017;90:375–385. doi: 10.1016/j.biopha.2017.03.072. [DOI] [PubMed] [Google Scholar]
- Canh L., Cuong V., Trang D.T., Cuc N.T., Nhiem N.X., Yen P.H., Le H., Anh T., Huong L.M., Minh C. Van, Kiem P. Van. FLAVONOID GLYCOSIDES FROM Antidesma ghaesembilla. Vietnam J. Chem. 2015;53:94–97. doi: 10.15625/0866-7144.2015-2e-022. [DOI] [Google Scholar]
- Chang F.R., Yen C.T., Ei-Shazly M., Lin W.H., Yen M.H., Lin K.H., Wu Y.C. Anti-human coronavirus (anti-HCoV) triterpenoids from the leaves of Euphorbia neriifolia. Nat. Prod. Commun. 2012;7:1415–1417. doi: 10.1177/1934578x1200701103. [DOI] [PubMed] [Google Scholar]
- Cragg G.M., Newman D.J. Natural products: A continuing source of novel drug leads. Biochim. Biophys. Acta - Gen. Subj. 2013;1830:3670–3695. doi: 10.1016/j.bbagen.2013.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elizabeth K.M. Antimicrobial activity of Terminalia bellerica. Indian J. Clin. Biochem. 2005;20:150–153. doi: 10.1007/BF02867416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eloff J.N., Famakin J.O., Katerere D.R.P. Isolation of an antibacterial stilbene from Combretum woodii (Combretaceae) leaves. African J. Biotechnol. 2005;4:1167–1171. doi: 10.5897/AJB2005.000-3232. [DOI] [Google Scholar]
- Enioutina E.Y., Teng L., Fateeva T.V., Brown J.C.S., Job K.M., Bortnikova V.V., Krepkova L.V., Gubarev M.I., Sherwin C.M.T. Phytotherapy as an alternative to conventional antimicrobials: combating microbial resistance. Expert Rev. Clin. Pharmacol. 2017;10:1203–1214. doi: 10.1080/17512433.2017.1371591. [DOI] [PubMed] [Google Scholar]
- Ernst M., Grace O.M., Saslis-Lagoudakis C.H., Nilsson N., Simonsen H.T., Rønsted N. Global medicinal uses of Euphorbia L. (Euphorbiaceae) J. Ethnopharmacol. 2015;176:90–101. doi: 10.1016/j.jep.2015.10.025. [DOI] [PubMed] [Google Scholar]
- Farid M.M., Hussein S.R., Ibrahim L.F., El Desouky M.A., Elsayed A.M., El Oqlah A.A., Saker M.M. Cytotoxic activity and phytochemical analysis of Arum palaestinum Boiss. Asian Pac. J. Trop. Biomed. 2015;5:944–947. doi: 10.1016/j.apjtb.2015.07.019. [DOI] [Google Scholar]
- Huang S.S., Li P., Zhang B.J., Deng S., Zhang H.L., Sun C.P., Huo X.K., Tian X.G., Ma X.C., Wang C. Acetophenone glycosides from the roots of Euphorbia fischeriana and their inhibitory effects against Mycobacterium smegmatis. Phytochem. Lett. 2017;19:151–155. doi: 10.1016/j.phytol.2016.12.032. [DOI] [Google Scholar]
- Kamba A.S., Hassan L.G. Phytochemical screening and antimicrobial activities of Euphorbia balsamifera leaves, stems and root against some pathogenic microorganisms. African J. Pharm. Pharmacol. 2010;4:645–652. [Google Scholar]
- Kamisako W., Honda C., Suwa K., Isoi K. Studies of 13C NMR Spectra of 13C-Enriched Cycloartenol Biosynthesized from [1-’3C]-, [2-13C]- Assignments of Cycloartenol and Cycloartanol and and [1,2-’3C2]-Acetate. Revised 13C NMR Spectral Accepted Skeleton Formation Mechanism of 13C NMR Spectral S. Magn. Reson. Chem. 1987;25:683–687. [Google Scholar]
- Kanchanapoom T., Noiarsa P., Tiengtham P., Otsuka H., Ruchirawat S. Acetophenone diglycosides from erythroxylum cambodianum. Chem. Pharm. Bull. 2005;53:579–581. doi: 10.1248/cpb.53.579. [DOI] [PubMed] [Google Scholar]
- Kumarasamy Y., Byres M., Cox P.J., Delazar A., Jaspars M., Nahar L., Shoeb M., Sarker S.D. Isolation, structure elucidation, and biological activity of flavone 6-C-glycosides from Alliaria petiolata. Chem. Nat. Compd. 2004;40:122–128. doi: 10.1023/B:CONC.0000033926.72396.41. [DOI] [Google Scholar]
- Liu Q., Meng X., Li Y., Zhao C.N., Tang G.Y., Li H. Bin. Antibacterial and antifungal activities of spices. Int. J. Mol. Sci. 2017;18:1–62. doi: 10.3390/ijms18061283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma C.M., Nakamura N., Min Byung Sun, Hattori M. Triterpenes and lignans from Artemisia caruifolia and their cytotoxic effects on Meth-A and LLC tumor cell lines. Chem. Pharm. Bull. 2001;49:183–187. doi: 10.1248/cpb.49.183. [DOI] [PubMed] [Google Scholar]
- Mahesh B., Satish S. Antimicrobial Activity of Some Important Medicinal Plant Against Plant and Human Pathogens. World J. Agric. Sci. 2008;4:839–843. [Google Scholar]
- Mohammed R.S., El Souda S.S., Taie H.A.A., Moharam M.E., Shaker K.H. Antioxidant, antimicrobial activities of flavonoids glycoside from Leucaena leucocephala leaves. J. Appl. Pharm. Sci. 2015;5:138–147. doi: 10.7324/JAPS.2015.50623. [DOI] [Google Scholar]
- Mosmann T. Rapid colorimetric assay for cellular growth and survival: Application to proliferation and cytotoxicity assays. J. Immunol. Methods. 1983;65:55–63. doi: 10.1016/0022-1759(83)90303-4. [DOI] [PubMed] [Google Scholar]
- Murray, P.R., Baron, E.J., Pfaller, M.A., Tenover, F.C. & Yolken, R.., 1995. Manual of clinical microbiology, 6th ed. Washington, D.C. : ASM Press, c1995., United States.
- Nassan M.A., Mohamed E.H., Abdelhafez S., Ismail T.A. Effect of clove and cinnamon extracts on experimental model of acute hematogenous pyelonephritis in albino rats: Immunopathological and antimicrobial study. Int. J. Immunopathol. Pharmacol. 2015;28:60–68. doi: 10.1177/0394632015572075. [DOI] [PubMed] [Google Scholar]
- Newman D.J., Cragg G.M. Natural Products as Sources of New Drugs from 1981 to 2014. J. Nat. Prod. 2016;79:629–661. doi: 10.1021/acs.jnatprod.5b01055. [DOI] [PubMed] [Google Scholar]
- Ngezahayo J., Havyarimana F., Hari L., Stévigny C., Duez P. Medicinal plants used by Burundian traditional healers for the treatment of microbial diseases. J. Ethnopharmacol. 2015;173:338–351. doi: 10.1016/j.jep.2015.07.028. [DOI] [PubMed] [Google Scholar]
- Prasad D. A new aromatic glycoside from the roots of Prunus armeniaca. Fitoterapia. 1999;70:266–268. doi: 10.1016/S0367-326X(99)00032-5. [DOI] [Google Scholar]
- Rex J.H., Pfaller M.A., Walsh T.J., Chaturvedi V., Espinel-Ingroff A., Ghannoum M.A., Gosey L.L., Odds F.C., Rinaldi M.G., Sheehan D.J., Warnock D.W. Antifungal susceptibility testing: Practical aspects and current challenges. Clin. Microbiol. Rev. 2001;14:643–658. doi: 10.1128/CMR.14.4.643-658.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seelinger M., Popescu R., Giessrigl B., Jarukamjorn K., Unger C., Wallnöfer B., Fritzer-Szekeres M., Szekeres T., Diaz R., Jäger W., Frisch R., Kopp B., Krupitza G. Methanol extract of the ethnopharmaceutical remedy Smilax spinosa exhibits anti-neoplastic activity. Int. J. Oncol. 2012;41:1164–1172. doi: 10.3892/ijo.2012.1538. [DOI] [PubMed] [Google Scholar]
- Villar A., Rios J.L., Reciot M.C., Cortes D., Cave A. Antimicrobial Activity of Benzylisoquinoline Al- kaloids, II. Relation Between Chemical Composi- tion and Antimicrobial Activity. Planta Med. 1986;52:556–557. doi: 10.1055/s-2007-969371. [DOI] [PubMed] [Google Scholar]
- Yam A.A., Gaye F., Dieme F.A., Bassene E., Ba I. Application of phytotherapy in odontology: the case of Euphorbia balsamifera. Endodontic clinical trial. Dakar Med. 1997;42:169–171. [PubMed] [Google Scholar]
- Yang D.S., Peng W.B., Li Z.L., Wang X., Wei J.G., He Q.X., Yang Y.P., Liu K.C., Li X.L. Chemical constituents from euphorbia stracheyi and their biological activities. Fitoterapia. 2014;97:211–218. doi: 10.1016/j.fitote.2014.06.013. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.