Abstract
Brucellosis is considered a prevailing endemic infectious disease in the Kingdom of Saudi Arabia and represents a health problem with socio-economic burden. There are two main Brucella species that cause human brucellosis; Brucella abortus, and Brucella melitensis. The clinical features range from asymptomatic to the acute symptoms of fever, joint pain, muscle pain, headache, nausea/vomiting, anorexia and malaise in addition to the subsequent complications that might occur. The endemicity of brucellosis might be explained due to obstacles in controlling the importation of animals for slaughtering during Hajj periods and for several other predisposing factors. The distribution of the disease is all over the country and the most prevalent part is the south followed by north and then the east and central parts. However, in the complexity of brucellosis control measures, there are several activities which have been implemented to tackle the disease such as mass vaccination of animals, regulating importation of slaughter animals, and improving public awareness. This review provides a detailed description of the status of brucellosis in Saudi Arabia, which includes epidemiology, clinical characteristics, virulence and pathophysiology, and prevention of the disease.
Keywords: Brucellosis, Prevalence, Epidemiology, Virulence, Saudi Arabia
1. Introduction
The Kingdom of Saudi Arabia (KSA) absorbs a broad range of workforce from various countries, which in turn raises the prevalence of different infectious diseases (Alkharsah et al., 2018). One among such diseases is brucellosis, an infectious disease caused by species of genus Brucella, belonging to class Alphaproteobacteria of phylum Proteobacteria. These are Gram negative facultative intracellular pleomorphic bacteria. Previously, there were number of synonyms for this infection: Malta fever, Mediterranean fever, Gibraltar fever, Cyprus fever and undulant fever, later referred as ‘Brucellosis’. It was first isolated by Sir David Bruce in 1887 from a British soldier, who was dying of a malta fever. In 1920, Mayer and Shaw discovered the genus ‘Brucella’ to accommodate all these bacteria (M.J., C., 2006). There are four species of Brucella: B. melitensis (goats, sheep and camels), B. abortus (cows), B. suis (pigs) and B. Canis (dogs). B. melitensis is the most invasive species producing more severe disease and is considered as the principal cause of human brucellosis worldwide. It has been eradicated in many developed countries like Europe, Australia, Canada, Israel, Japan and New Zealand, but still remains a health concern in Africa, Mediterranean, Middle East, parts of Asia and Latin America, due to high endemicity in such regions (Gul and Khan, 2007). In some areas like China, there was no report of this disease historically, however, since 2010 it has been reported from almost all provinces (Li et al., 2020).
Human brucellosis is a neglected disease among rural population and in highly agrarian livestock dependent societies (M.J., C., 2006). KSA is one of the highest animal import countries as it has to meet the need of millions of immigrants as well as for slaughter during the Hajj and Umrah period. KSA is a vast reservoir of human brucellosis. The transmission could happen either directly or indirectly, through the contact with contaminated animals or dairy products. The high occurrence rate of brucellosis within the country is attributed to other factors also like insufficient quarantine measures and deficiency in health literacy (Bilal et al., 1991, Elbeltagy, 2001, Memish, 2001). Although the Saudi Ministry of Agriculture has now made it compulsory to do a brucellosis inoculation test for livestock and organizing general public wellness programs, still it is considered endemic in KSA, where the national sero-prevalence is 15% (Jokhdar, 2009, Bilir Goksugur et al., 2015). In this review, we aim to summarize the epidemiology and pathogenesis of human brucellosis in KSA.
2. Epidemiology of human brucellosis in KSA
2.1. Historical outlook
Evaluating the epidemiological condition of brucellosis in KSA is the first step in its prevention and control. Brucellosis, a common zoonotic disease caused by gram-negative bacteria under genus Brucella, is an old disease which was known and even mentioned by Hippocrates in his publication “Epidemics”. However, it was only in late 1880’s that the causative agent, “Micrococcus melitensis” was discovered from the milk product of goats and identified as the source of infection which created havoc in the military troops of Malta. Even almost one and a half centuries has passed since its discovery, the disease is still prevalent especially in developing countries including Middle East causing considerable human losses in terms of economy, food security and public health (McDermott et al., 2013, Rubach et al., 2013). Brucellosis, because of its endemic nature; complexity of its effect on multiple livestock species like cattle, sheep, goats, camels etc; and its ability to infect humans; makes it a considerable food safety and public health threat in many developing countries including middle-east (McElwain and Thumbi, 2017).
In KSA, although only occasional cases of brucellosis occurred between 1956 and 1982 (Arrighi, 1986), the highest incidence was reported during Hajj in 1977 (Norton, 1984). In late 1970′s, the economic condition of KSA was on boom and the government encouraged the establishment of dairy farms and livestock breeding projects. These subsidized schemes led to import of various livestock animals from the underdeveloped neighboring countries with poor screening leading to subsequent introduction of brucellosis in the Kingdom. In 1977, the first sero-epidemiological survey for brucellosis antibodies was carried out and it was shown that higher incidence of infection was found in non-local breeds of livestock (Arrighi, 1986, Radwan et al., 1983). During 1980s, the disease was considered as a critical health problem, after increased rates of annual admission for brucellosis at tertiary care hospitals (Kambal et al., 1983). At this stage, the surge in brucellosis cases in animals and humans became endemic and was primarily linked with uncontrolled importation of potentially infectious animals as well as habit of ingesting raw milk or its products among the nomadic population of KSA. In 1999, World Health Organization (WHO) and Food and Agriculture Organization (FAO) of the United Nations reported KSA with the highest incidence of human brucellosis in the Middle East (Morelli, 1998).
2.2. Prevalence and distribution of human Brucella in KSA
Brucellosis, worldwide, is known to be the commonest zoonotic disease; however, many of the endemic countries have controlled the incidence of the disease over the years. Some other developing countries including KSA still has a high incidence compared to the world average with an average incidence per 100,000 of 15.34 annually from 2003 to 2018 (Statistical Year Book. Ministry of Health, Kingdom of Saudi Arabia, 2008, Statistical Year book. Ministry of Health, Kingdom of Saudi Arabia, 2018, Ministry of Health) (Fig. 1).
Fig. 1.
Incidence of Brucellosis in KSA (2003–2018); Source: Ministry of Health, KSA (Statistical Year Book. Ministry of Health, Kingdom of Saudi Arabia, 2008, Statistical Year book. Ministry of Health, Kingdom of Saudi Arabia, 2018, Ministry of Health).
In comparison, the average incidence e.g. in China in 2009, Greece in 2007–2012, Italy in 2005, Mexico in 2000, and Iran in 2008 was 2.7, 1.43, 1.40, 25.69, and 43.2 per 100,000 (Zhong, et al., 2013, Fouskis, et al., 2018, De Massis, et al., 2005, Doyle and Bryan, 2000, Mostafavi and Asmand, 1991). Even though the incidence is decreasing worldwide over the years, it increased in Saudi Arabia from 10.11/100,000 in 2014 to 16.33/100,000 in 2018.
Genus Brucella is a group of an aerobic gram negative coccobacilli, having an ability to enter both phagocytic and non-phagocytic cells, where they survive by finding ways to escape immune system (Moreno et al., 2002, Ficht, 2010). Historically, the classification of Brucella species is based on the preferred host and phenotypic traits including requirement of CO2, production of H2S, oxidative metabolic patterns etc. (Morgan and Corbel, 1976, Al Dahouk et al., 2003). Based on this classification, the genus Brucella (B.) comprises of seven main species (Table 1), four of which are known to cause disease in humans (B abortus, B melitensis, B Canis, B Suis) (Ficht, 2010).
Table 1.
Currently recognized Brucella species.
| Organism | Animal reservoir |
|---|---|
| Brucella abortus | Cows, buffalo, camels, yaks |
| Brucella melitensis | Goats, sheep, camels |
| Brucella suis | Pigs |
| Brucella canis | Canines |
| Brucella ovis | Sheep |
| Brucella neotomae | Rodents |
| Brucella pinnipediae & Brucella cetaceae | Marine animals, minke whales, dolphins, seals |
B. melitensis is considered as the most virulent and the most prevalent strain worldwide, including KSA. The infection is acquired by exposure to animals and animal products. On the other hand, B abortus is most widely distributed, but less pathogenic for both animals and humans. B. Suis is a serious pathogen in humans while B.Canis causes mild disease. Some variants of species of Brucella, known as biovar, differ physiologically and biochemically. Three species which are divided into biovar are B. abortus, B. melitensis, and B. Suis with 7, 3, and 5 biovars respectively (Franco, 2007). In the desert climate of Saudi Arabia, the species and types of Brucella that have been reported in various human studies include:
-
▪
Jokhdor et al. (2009) (Jokhdar, 2009) in their case report found that the investigation of blood sample was positive for B. Melitensis and B. Abortus.
-
▪
Nemenqani et al. (2009) (Nemenqani et al., 2009) in one breast brucellosis infection study conducted in Taif, KSA reported that B. melitensis was the cause.
-
▪
Kamal et al. (2013) (Kamal et al., 2013) in a study in Tabuk and Jeddah on 101 patients with acute febrile illness (AFI) of unknown cause reported that most of them had both B. abortus and B. melitensis positive.
-
▪
Alshaalan et al. (2014) (Alshaalan et al., 2014) in a study conducted among children in Saudi Arabia reported that the most prevalent species of brucellosis causing pathogen among children was Brucella melitensis followed by Brucella abortus.
-
▪
Alrheam et al. (2015) (Alrheam et al., 2015) reported that in Dawadmi Governate (central region of Saudi Arabia), B. melitensis was the prevalent causative organism of brucellosis in humans.
-
▪
Alnemri et al. (2017) (Alnemri et al., 2017) in a case report on neonatal brucellosis of a vertical transmission from mother to baby during pregnancy found that B. abortus was isolated from baby’s blood while both B. abortus and B. melitensis were isolated from the mother’s blood.
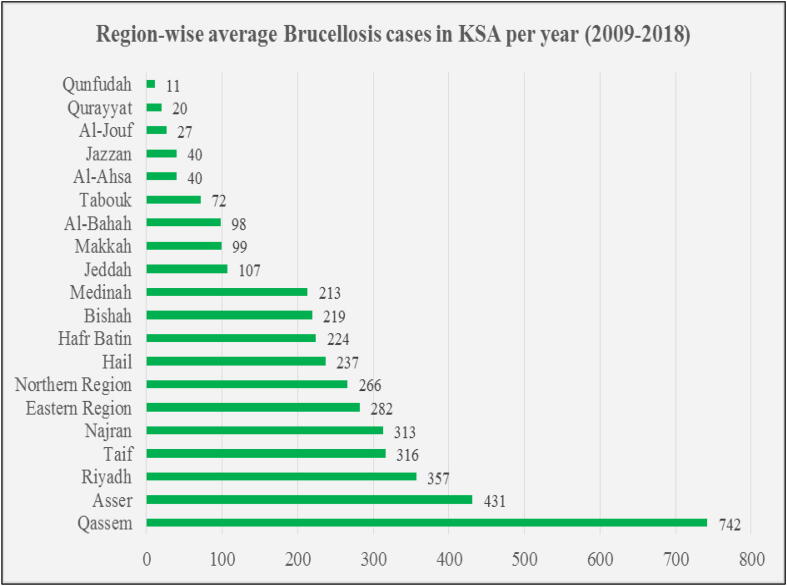
Brucellosis has a wide distribution over all geographical areas of Saudi Arabia, however, high incidence is found in the central part, where the population has nomadic heritage and are at increased risk of contracting the infection. The study of Aloufi (2016) showed that the incidence rate of brucellosis is still high in Al-Qassem, Aseer, Hail, northern areas and Najran regions of KSA and are comparatively low in Al-Jouf, Jazan, Tabouk, Makkah, and Al-Riyadh (Aloufi, et al., 2016). According to the statistical yearbook provided by the Ministry of Health (MOH) (Statistical Year book. Ministry of Health, Kingdom of Saudi Arabia, 2018, Ministry of Health), Al-Qassem had the highest average yearly brucellosis cases of 742 for a period of ten years from 2009 to 2018 followed by Aseer (431), Riyadh (357), while Al-Jouf (27), Jazan (40) and Makkah (99) had comparatively much lower cases (Fig. 2).
Fig. 2.
Region-wise yearly average Brucellosis cases in KSA (2009–2018). Source: Ministry of Health, KSA (Ministry of Health).
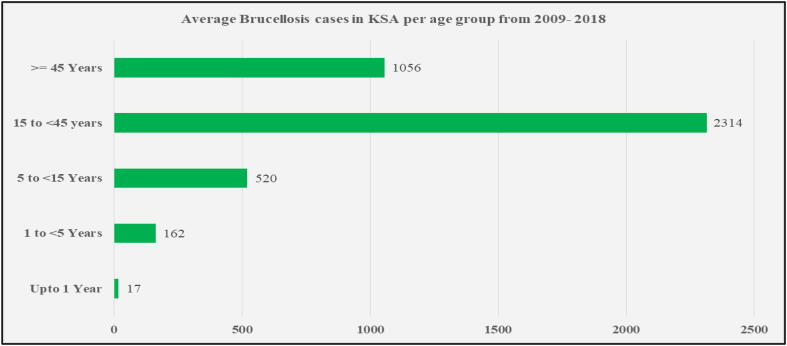
The morbidity rate was also high among the agrarian people with the range of infection between 1.6 and 2.6%. As brucellosis is an occupational disease, therefore the effected age group lies in the range of 15–50 years and males are more affected than females. For a period of 10 years (2009–2018), the average yearly brucellosis cases were highest in the age group of 15–44 years (2314) and were lowest in infants under one year of age (Fig. 3) (Statistical Year book. Ministry of Health, Kingdom of Saudi Arabia, 2018, Ministry of Health).
Fig. 3.
Average yearly Brucellosis cases in KSA as per age group (2009–2018); Source: Ministry of Health, KSA (Ministry of Health)
Also, the average percentage distribution of Brucellosis cases for this period (2009–2018) was highest in Saudi males (41% of the total of 40,682 cases during this period) and was lowest in non-Saudi females (only 2%) (Fig. 4) (Statistical Year Book. Ministry of Health, Kingdom of Saudi Arabia, 2008, Statistical Year book. Ministry of Health, Kingdom of Saudi Arabia, 2018). The reason could be that males are being more involved in activities such as slaughter and handling of livestock and hence are at higher risk of infection than females. (Refai, 2002, Elfaki et al., 2015).
Fig. 4.
Average share of Brucellosis cases in KSA as per gender and residency status (from total of 40,682 cases during 2009–2018); Source: Ministry of Health, KSA (Ministry of Health)
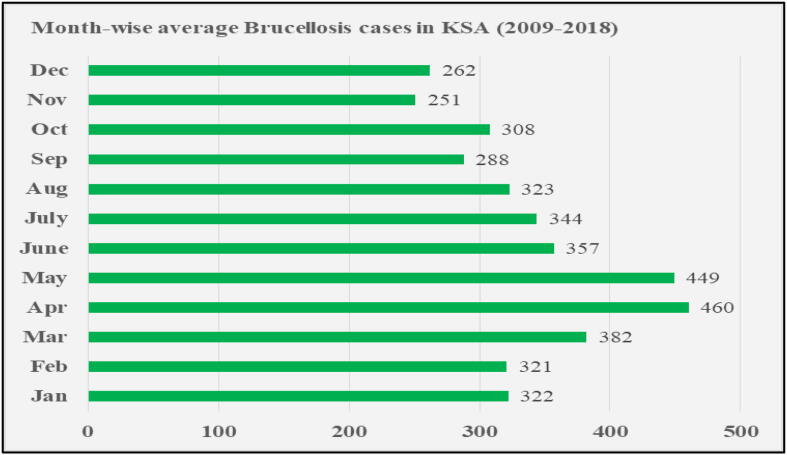
Additionally, the seasonal patterns are found related to the onset of symptoms of infection. During the spring season, sheep and goats are in their parturition phase, making a higher possibility of contact with vaginal discharge, fetuses and placentas, thus increasing the risk of exposure to infection (Robinson and Production, 2003, Fthenakis et al., 2012). The month-wise average yearly Brucellosis cases in KSA for the period of 10 years from 2008 to 2019 (Statistical Year book. Ministry of Health, Kingdom of Saudi Arabia, 2018, Ministry of Health) is shown in Fig. 5.
Fig. 5.
Average month-wise Brucellosis cases in KSA yearly from 2009 to 2018; Source: Ministry of Health, KSA (Ministry of Health)
The occupational pattern was also found proportional to the prevalence of brucellosis. Infection was more common among abattoir workers, butchers, veterinarians and administrative personnel. Greater risk was found in those people who were involved with consumption of unpasteurized dairy products. Other factors like illiteracy, and the old age traditional customs and dietary habits of nomadic heritage build the barriers in control and prevention of brucellosis in KSA (Almuneef et al., 2004, Alsubaie et al., 2005, Abu-Rabia, 2015).
3. Virulence and pathophysiology
Brucella transmission can occur in humans via inhalation, ingestion, or by wounds and mucosa. After the infection, bacteria travels to the lymph nodes, and then can spread by macrophages to other organs such as spleen, liver, bone marrow and even reproductive organs. The bacteria have the ability to resist the immune response of the human body, as their replication occurs mainly inside the macrophages and non-phagocytic cells. Inside the bloodstream, bacteria quickly become intracellular pathogens and makes the use of numerous mechanisms to suppress bactericidal response (Janeway et al., 1999). This type of proliferation can cause chronic diseases of brucellosis, which can lead to spondylitis, arthritis, meningitis and endocarditis in infected humans (Boschiroli et al., 2001). Brucella infects macrophages, where they undergo phagocytosis, acidification and phagosome-lysosome fusion where some internalized Brucella survive in close contact with phagosomal wall (Rittig et al., 2001). Interaction is controlled by compound host-pathogen virulence genes. The most important of them are lipopolysaccharides (LPS) in outer surface membrane, periplasmic enzymes, cyclic glucans and Heat shock proteins (HSP). As an important virulence factor of Brucella, LPS consists of lipid A with aminoglycose; oligosaccharide core with mannose and glucose; and O-antigen comprising of 4-foramido-4,6-dideoxymannose; which attaches to the lipid rafts on the surface of macrophage leading to its infection (Lapaque et al., 2005, Rossetti et al., 2012). The bacterium resides in a special vacuole called Brucella containing vacuole (BCV) inside the mononuclear cells. This helps the bacteria to sustain, modify the intracellular traffic and interact with endoplasmic reticulum (ER) forming a Brucella-multiplication compartment (Köhler et al., 2002). To survive the environment inside this compartment, a multiple enzyme system forms the main line of defense counteracting reactive oxygen intermediates (ROIs) like superoxide (O2–) and hydrogen peroxide (H2O2), produced by the macrophages for destruction of bacteria inside the cell. Important ones among them are superoxide dismutase (SOD) and catalase (Gopal and Elumalai, 2017). Also present are cyclic beta-1-2-glucans (CβG) which helps in control of phagosome-lysosome fusion forming phagolysosome (Arellano-Reynoso et al., 2005). To enable survival in an acid environment, Brucella produces urease enzyme which changes urea to ammonium form and helps in raising the pH (Seleem et al., 2008). To counter the low oxygen conditions inside macrophages, the bacterium produces cytochrome oxidase and nitric oxide reductase (NOR) (Loisel-Meyer, et al., 2005, Stevanin, et al., 2005). The LPS binds to lipid and membrane receptors of macrophages and activates phagocytosis in these cells by cyclic-AMP/pkA pathway, followed by phosphorylation of transcription factors. The two-component regulatory system BvrR/BvrS controls the expression of outer membrane proteins (Omp): Omp3a (Omp25a) or Omp3b (Omp22) and influences additional Brucella properties involved in virulence (Manterola et al., 2007).
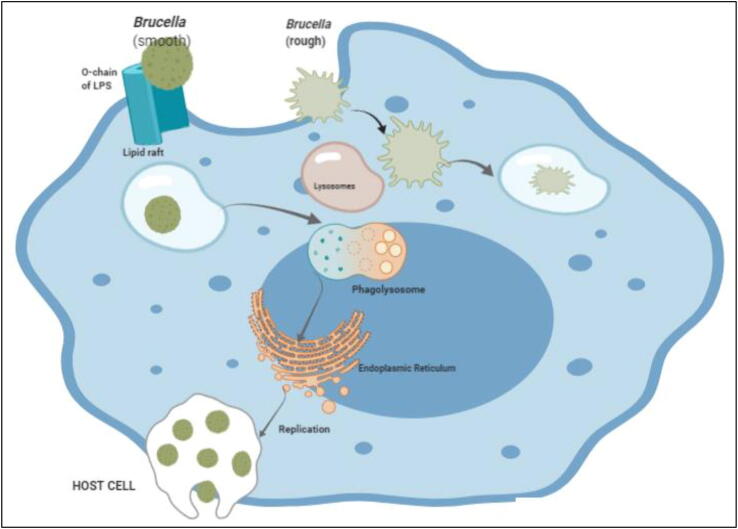
The bacteria also recruit actin filaments in epithelial cells and gets accumulated inside. These accumulated bacteria spread to other host cells. Internalization of bacteria may also be done with the help of lipid rafts, present on the membrane of macrophages (Xavier et al., 2010). LPS coat protein helps in intracellular survival because of adenine and guanine monophosphate production, which helps in phagosomal fusion and oxidative burst activity. When they are entrapped in phagosome only 15–30% of Brucella survives (Kim and Jinju, 2015). Bacteria that survive are transported into a lymphatic system and relocated in cells and various organs like kidneys, liver, spleen, breast tissue or joints causing localized and systemic infection. With the help of hemolysis and induced cell necrosis, the Brucella are released in endoplasmic reticulum. The infection is controlled by cell mediated immunity where initially IgM level rises followed by IgG titers indicating the chronic and relapsed infection (Giambartolomei et al., 2019, Mohamed et al., 2019). The cellular invasion, intracellular trafficking, and replication by Brucella is shown in Fig. 6.
Fig. 6.
Cellular Invasion, Intracellular trafficking, and replication by Brucella; Smooth Brucella invasion into a cell by lipid rafts, modification of lipid rafts, interaction with lysosomes, regulation of intracellular trafficking from autophagosome to ER, and the replication of Brucella. Rough Brucella gets exterminated by lysosomes as they do not penetrate by lipid rafts.
4. Clinical manifestations
The clinical features of brucellosis in humans, according to earlier studies in KSA, are not specific and could range from asymptomatic to severe form of disease and complications. Brucellosis is characterized by fever, joint pain, headache, muscle pain, anorexia malaise and nausea or vomiting as reported in several studies and case reports conducted in KSA. Fever is the most common persistent symptom of brucellosis. Table 2 summarizes the clinical features of brucellosis with reference to the studies in which signs and symptoms were reported.
Table 2.
Clinical features of Brucellosis.
In addition to the symptoms listed in Table 2, some specific complications have also been reported such as:
-
▪
Nemenqani et al. 2009 (Nemenqani et al., 2009) documented breast lump (abscess like background) in breast brucellosis
-
▪
Alyousef et al. 2015 (Alyousef et al., 2015) reported the associated symptoms as hypochondrial pain and splenic infarction.
-
▪
Elzien et al. 2014 (Elzein, 2014) reported Guillain–Barre syndrome.
-
▪
Elzien et al. 2016 (Elzein and Sherbeeni, 2016) has reported septic arthritis as symptoms..
-
▪
Al-Tawfiq et al. 2013 (Al-Tawfiq et al., 2013) reported a case of abortion in female patient.
5. Control and preventive measures
From the above findings, we can say that the magnitude of brucellosis in KSA is so high that it can serve as a barometer of ubiquity of the disease. The foremost step in controlling brucellosis in humans is eradicating it from animals. This has been successfully done in many developed countries by maintaining their brucellosis free herds, which undergo certain serological tests and other precautionary measures before importation or exportation from/to other countries. However, the control measures in KSA are challenging, because of huge need of slaughter of animals every year for Hajj and also throughout the year for consumption. Other hurdles are poor animal quarantine procedures and lack of legislation to control marketing and movement of animals. One other issue among many developed countries including KSA is about food safety and handling. In one recent meta-analysis about prevalence of Brucella spp. in raw milk or milk products from several countries in Middle East region including KSA, the overall prevalence was estimated to be 29% (Abedi et al., 2020). Although in KSA, much has been done so far to eradicate the disease especially from those areas with high infection rate, but still organized national brucellosis control programs are lacking. There should be legislation, which will strictly emphasize on pasteurization of milk and milk products. Public education as listed below can help in preventing the transmission of brucellosis from animals to humans:
-
▪
Educating the public about the nature and routes of disease by which it gets transmitted.
-
▪
Educating the public about the symptoms associated with the disease and about the complications, if left untreated.
-
▪
Knowledge about the administered medications against the disease.
-
▪
Design of systematic plan including diet, therapy, or medication to improve and maintain the health of patients.
-
▪
Factors associated with reassurance concerning recurrent symptoms in case of acute brucellosis disease.
-
▪
Using some standard operating protocols while handling infectious potentials or avoiding potentially contaminated foods.
Additionally, effective strategies are needed to control the circulation of Brucella throughout KSA, such as effective surveillance and institutional prophylactic measures, which requires understanding of the molecular epidemiology of the circulating strains/alleles by different techniques like multilocus sequence typing (MLST), pulsed field gel electrophoresis (PFGE), etc (Alkharsah et al., 2019). Last and most important, developing a safe and effective vaccine should be the priority as there are no licensed vaccines available yet for human anti-Brucella. Although some candidate vaccines like S19 vaccine strain have been proposed and tested (Lalsiamthara and Lee, 2017), the deployment has been limited as these live, attenuated vaccines still present residual virulence. Researchers are still working on developing safe, effective, cross-protective and human exclusive vaccines which needs to be expedited as there are always concerns of re-emergence of brucellosis (Suárez-Esquivel, 2017, Tasiame, et al., 2016, Tukana, 2017).
6. Conclusion
Unlike other countries, the control of brucellosis in KSA is a complicated and difficult task, due to huge influx of pilgrims during Hajj/Umrah as well as due to country’s huge population of expatriates. This requires slaughter of millions of animals. Also, increasing demand for dairy products, accompanied with changing and intensified farming practices raises the concern for further spread and perpetuation of the disease. As we discussed about the epidemiological complexity of brucellosis and its distribution among the people from various walks of life, effective control with a continuous and careful efforts by health authorities is required. A national brucellosis control program is therefore needed which largely depends on good administrative arrangement, ensuring the maximum cooperation of the community, active cooperation between health services and veterinary services. Timely reporting of brucellosis to the relevant health authorities, livestock mass vaccination and improved farm hygiene can effectively control brucellosis in the animal population and thereby reduce the transmission to the human population. Establishment of ministerial co-ordination with formulation of inter-governmental veterinary agreements between the Saudi government and the relevant authorities of animal exporting countries can be used to prioritize a disease control policy for brucellosis. The epidemiological data mentioned in this review is still incomplete or partly explained due to lack of proper laboratory facilities in some remote areas as well as poor exchange of information between veterinary and health services. Some other measures which include control of the disease in animal hosts, improved food hygiene including the pasteurization of milk and proper handling of other animal produce by using protective clothing/barriers can prevent the occupational exposure to the disease. Brucellosis cases are sometimes misdiagnosed due to their misleading nature and the varied clinical manifestations, thus making diagnosis quite difficult. Symptoms and signs are usually confused with those of other diseases, such as typhoid fever or rheumatic fever. Thus, in the endemic region of KSA, the clinicians should be in collaboration with microbiologist in order to correctly diagnose and treat the patients. Screening of shepherds, abattoir workers, veterinarians, dairy industry professionals and personnel in microbiology laboratories for the disease should be implemented. These are some important steps which will inevitably result in lower morbidity and mortality rates of Brucellosis in KSA.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Footnotes
Peer review under responsibility of King Saud University.
References
- Abedi A.-S. The prevalence of Brucella spp. in dairy products in the Middle East region: A systematic review and meta-analysis. Acta Trop. 2020;202 doi: 10.1016/j.actatropica.2019.105241. 105241. [DOI] [PubMed] [Google Scholar]
- Suárez-Esquivel M. Brucella neotomae infection in humans. Costa Rica. Emerging Infectious Diseases, 2017;23(6):997. doi: 10.3201/eid2306.162018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abu-Rabia, A., 2015. Indigenous medicine among the Bedouin in the Middle East. Berghahn Books.
- Al Dahouk S. Laboratory-based diagnosis of brucellosis–a review of the literature. Part I: Techniques for direct detection and identification of Brucella spp. Clin. Lab. 2003;49(9–10):487–505. [PubMed] [Google Scholar]
- Algahtani, H., et al., 2017. Occupational neurobrucellosis mimicking a brain tumor: a case report and review of the literature, 2017. [DOI] [PMC free article] [PubMed]
- Alkharsah K.R. Comparative and molecular analysis of MRSA isolates from infection sites and carrier colonization sites. Ann. Clin. Microbiol. Antimicrobials. 2018;17(1):7. doi: 10.1186/s12941-018-0260-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alkharsah K.R. Molecular typing of MRSA isolates by spa and PFGE. J. King Saud Univ.-Sci. 2019;31(4):999–1004. [Google Scholar]
- Almuneef, M., et al., 2004. Importance of screening household members of acute brucellosis cases in endemic areas, 132(3), pp. 533–540 [DOI] [PMC free article] [PubMed]
- Alnemri, A.R.M., et al., 2017. Neonatal brucellosis: A case report. 11(02), pp. 199–202 [DOI] [PubMed]
- Aloufi, A.D., et al., 2016. Trends of reported human cases of brucellosis, Kingdom of Saudi Arabia, 2004–2012, 6(1), pp. 11–18. [DOI] [PMC free article] [PubMed]
- Alrheam, A., et al., 2015. Human Brucellosis incidence trends in central Saudi Arabia (DawadmiGovernate), 3(5), pp. 1580–1586.
- Alshaalan, M.A., et al., 2014. Brucellosis in children: Prevention, diagnosis and management guidelines for general pediatricians endorsed by the Saudi Pediatric Infectious Diseases Society (SPIDS), 1(1), pp. 40–46.
- Alsoghair, M.I.J.I.J.o.C.M., Health, P., 2017. Epidemiological characteristics of human brucellosis in Al-Qassim region, Saudi Arabia, between 2010 and 2014. 3(2), pp. 397–402.
- Alsubaie, S., et al., 2005. Acute brucellosis in Saudi families: relationship between brucella serology and clinical symptoms, 9(4), pp. 218–224 [DOI] [PubMed]
- Al-Tawfiq, J.A.J.E.r.o.a.-i.t., 2008. Therapeutic options for human brucellosis, 6(1), pp. 109–120. [DOI] [PubMed]
- A Al-Tawfiq, J., Z.J.R.p.o.a.-i.d.d. A Memish, 2013. Pregnancy associated brucellosis. 8(1), pp. 47–50. [DOI] [PubMed]
- Alyousef, M., Enani, M., Elkhatim, M.J.C.r.i.i.d., 2015. Acute brucellosis with splenic infarcts: a case report from a tertiary care hospital in Saudi Arabia, 2015.Case reports in infectious diseases. [DOI] [PMC free article] [PubMed]
- Arellano-Reynoso, B., et al., 2005. Cyclic β-1, 2-glucan is a Brucella virulence factor required for intracellular survival, 6(6), pp. 618–625 [DOI] [PubMed]
- Arrighi, H.J.A.o.S.M., 1986. Brucellosis surveillance in Saudi-Arabia eastern province. 6(4), p. S5-S10.
- Asaad A.M., Alqahtani J.M. Serological and molecular diagnosis of human brucellosis in Najran, Southwestern Saudi Arabia. J. Infect. Public Health. 2012;5(2):189–194. doi: 10.1016/j.jiph.2012.02.001. [DOI] [PubMed] [Google Scholar]
- Bilal, N., et al., 1991. A study of the knowledge, attitude and practice (KAP) of a Saudi Arabian community towards the problem of brucellosis, 66(1-2), pp. 227–238 [PubMed]
- Bilir Goksugur, S., et al., 2015. An interesting case of childhood brucellosis with unusual features, 54(1), pp. 107–110 [PubMed]
- Boschiroli, M.-L., Foulongne, V., O'Callaghan, D.J.C.o.i.m., 2001. Brucellosis: a worldwide zoonosis, 4(1), pp. 58–64. [DOI] [PubMed]
- De Massis, F., et al., 2005. Correlation between animal and human brucellosis in Italy during the period 1997–2002, 11(8), pp. 632–636. [DOI] [PubMed]
- Doyle, T.J., Bryan, R.T.J.T.J.o.i.d., 2000. Infectious disease morbidity in the US region bordering Mexico, 1990–1998, 182(5), pp. 1503–1510. [DOI] [PubMed]
- Elbeltagy, K., 2001. An epidemiological profile of brucellosis in Tabuk Province, Saudi Arabia. [PubMed]
- Elfaki, M.G., Alaidan, A.A., Al-Hokail, A.A.J.T.J.o.I.i.D.C., 2015. Host response to Brucella infection: review and future perspective, 9(07), pp. 697–701. [DOI] [PubMed]
- Elzein, F.E., Sherbeeni, N.J.C.r.i.i.d., 2016. Brucella septic arthritis: case reports and review of the literature. 2016. [DOI] [PMC free article] [PubMed]
- Elzein, F.E., M.J.T.A.j.o.t.m. Mursi, and hygiene, Brucella Induced Guillain–Barré Syndrome, 2014, 91(6), pp. 1179–1180. [DOI] [PMC free article] [PubMed]
- Ficht, T.J.F.m., 2010. Brucella taxonomy and evolution, 5(6), pp. 859–866. [DOI] [PMC free article] [PubMed]
- Fouskis, I., et al., 2018. The epidemiology of Brucellosis in Greece, 2007–2012: a ‘One Health’approach, 112(3), pp. 124–135. [DOI] [PubMed]
- Fthenakis, G., et al., 2012. Health management of ewes during pregnancy, 130(3-4), pp. 198–212 [DOI] [PubMed]
- Giambartolomei, G.H., Delpino, M.V.J.F.i.C., Microbiology, I., 2019. Immunopathogenesis of Hepatic Brucellosis, 9. [DOI] [PMC free article] [PubMed]
- Gopal, R., Elumalai, S.J.J.P.H., 2017. Industrial production of superoxide dismutase (SOD): A mini review, 5
- Gul, S., Khan, A.J.P.v.j., 2007. Epidemiology and epizootology of brucellosis: A review, 27(3), p. 145.
- Janeway, C.A., Capra, J.D., Travers, P., Walport, M., 1999. Immunobiology: the immune system in health and disease. (No. 577.27 JAN).
- Jokhdar, H.J.M.J.C.U., 2009. Brucellosis in Saudi Arabia: review of literature and an alarming case report in a hospital in Jeddah, 77(3), pp. 47–55
- Kamal, I.H., et al., 2013. Two-stage PCR assay for detection of human brucellosis in endemic areas, 13(1), pp. 145 [DOI] [PMC free article] [PubMed]
- Kambal, A., et al., 1983. Brucellosis in Riyadh, Saudi Arabia. A microbiological and clinical study, 77(6), pp. 820–824 [DOI] [PubMed]
- Franco M.P. Human brucellosis. The Lancet infectious diseases. 2007;7(12):775–786. doi: 10.1016/S1473-3099(07)70286-4. [DOI] [PubMed] [Google Scholar]
- Kiel, F.W., Khan, M.Y.J.S.S., 1989. Medicine. Brucellosis in Saudi Arabia, 29(8), pp. 999–1001. [DOI] [PubMed]
- Kim, S.J.U.o.B.I., Jinju, 2015. The interaction between Brucella and the host cell in phagocytosis, pp. 45–60.
- Köhler, S., et al., 2002. The analysis of the intramacrophagic virulome of Brucella suis deciphers the environment encountered by the pathogen inside the macrophage host cell, 99(24), pp. 15711–15716 [DOI] [PMC free article] [PubMed]
- Lalsiamthara, J., Lee, J.H.J.J.o.v.s., 2017. Development and trial of vaccines against Brucella. 18(S1), pp. 281–290. [DOI] [PMC free article] [PubMed]
- Lapaque, N., et al., 2005. Brucella lipopolysaccharide acts as a virulence factor, 8(1), pp. 60–66 [DOI] [PubMed]
- Khalaf O.H. Interaction of Brucella abortus with osteoclasts: a step towards understanding osteoarticular brucellosis and vaccine safety. Infect. Immun. 2020;23;88(4). doi: 10.1128/IAI.00822-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Z. Molecular characteristics of Brucella isolates collected from humans in Hainan Province, China. Front. Microbiol. 2020;11:452. doi: 10.3389/fmicb.2020.00452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loisel-Meyer, S., et al., 2005. Differential use of the two high-oxygen-affinity terminal oxidases of Brucella suis for in vitro and intramacrophagic multiplication, 73(11), pp. 7768–7771. [DOI] [PMC free article] [PubMed]
- M.J., C., Brucellosis in humans and animals. World Health Organization, 2006.
- Manterola, L., et al., 2007. BvrR/BvrS-controlled outer membrane proteins Omp3a and Omp3b are not essential for Brucella abortus virulence, 75(10), pp. 4867–4874. [DOI] [PMC free article] [PubMed]
- McDermott J., Grace D., Zinsstag J. Economics of brucellosis impact and control in low-income countries. Rev. Sci. Tech. 2013;32(1):249–261. doi: 10.20506/rst.32.1.2197. [DOI] [PubMed] [Google Scholar]
- McElwain T.F., Thumbi S.M. Animal pathogens and their impact on animal health, the economy, food security, food safety and public health. Rev. Sci. Tech. 2017;36(2):423–433. doi: 10.20506/rst.36.2.2663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Memish, Z., 2001. Brucellosis control in Saudi Arabia: prospects and challenges. Taylor & Francis. [DOI] [PubMed]
- Ministry of Health, S.A., Statistical Yearbook.
- Mohamed G., Elfaki Diagnosis of human brucellosis by immunoblotting of serum IgG antibodies. EC Microbiology. 2019;15:198–205. [Google Scholar]
- Morelli, D., 1998. General considerations on the epidemiological surveillance and control of brucellosis. In The MZCP report on the third workshop on human and animal Brucellosis Epidemiological Surveillance in the MZCP countries. Damascus, Syrian Arab Republic.
- Moreno E., Cloeckaert A., Moriyón I.J.V.m. Brucella Evolution and Taxonomy. 2002;90(1–4):209–227. doi: 10.1016/s0378-1135(02)00210-9. [DOI] [PubMed] [Google Scholar]
- Morgan W.J., Corbel M.J. Recommendations for the description of species and biotypes of the genus Brucella. Dev. Biol. Stand. 1976;31:27–37. [PubMed] [Google Scholar]
- Mostafavi, E., Asmand, M.J.I.J.o.E., 2012. Trend of brucellosis in Iran from 1991 to 2008, 8(1).
- Nemenqani, D., Yaqoob, N., Khoja, H.J.T.J.o.I.i.D.C., 2009. Breast Brucellosis in Taif, Saudi Arabia: cluster of six cases with emphasis on FNA evaluation, 3(04), pp. 255–259. [DOI] [PubMed]
- Norton W.L. Brucellosis and rheumatic syndromes in Saudi Arabia. An.n Rheum. Dis. 1984;43(6):810–815. doi: 10.1136/ard.43.6.810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radwan, A.I., et al., 1983. Incidence of brucellosis in domestic livestock in Saudi Arabia. 15(3), pp. 139–143 [DOI] [PubMed]
- Refai, M.J.V.m., 2002. Incidence and control of brucellosis in the Near East region, 90(1-4), pp. 81–110. [DOI] [PubMed]
- Rittig, M.G., et al., 2001. Intracellular survival of Brucellaspp. In human monocytes involves conventional uptake but special Phagosomes, 69(6), pp. 3995–4006 [DOI] [PMC free article] [PubMed]
- Robinson, A., Production, A.,2003. Guidelines for coordinated human and animal brucellosis surveillance. FAO Rome, Italy.
- Rossetti, C.A., et al., 2012. Transcriptome analysis of HeLa cells response to Brucella melitensis infection: a molecular approach to understand the role of the mucosal epithelium in the onset of the Brucella pathogenesis, 14(9), pp. 756–767 [DOI] [PMC free article] [PubMed]
- Rubach M.P. Brucellosis in low-income and middle-income countries. Curr. Opin. Infect. Dis. 2013;26(5):404–412. doi: 10.1097/QCO.0b013e3283638104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seleem, M.N., Boyle, S.M., Sriranganathan, N.J.V.m., 2008. Brucella: a pathogen without classic virulence genes, 129(1-2), pp. 1–14. [DOI] [PubMed]
- Statistical Year Book. Ministry of Health, Kingdom of Saudi Arabia, 2008.
- Statistical Year book. Ministry of Health, Kingdom of Saudi Arabia, 2018.
- Stevanin, T.M., et al., 2005. Nitric oxide detoxification systems enhance survival of Neisseria meningitidis in human macrophages and in nasopharyngeal mucosa, 73(6), pp. 3322–3329. [DOI] [PMC free article] [PubMed]
- Tasiame, W., et al., 2016. The prevalence of brucellosis in cattle and their handlers in North Tongu District of Volta Region, Ghana. 10(2), pp. 111–117. [DOI] [PMC free article] [PubMed]
- Tukana, A., Gummow, B.J.T.a.h., and production, Dairy farm demographics and management factors that played a role in the re-emergence of brucellosis on dairy cattle farms in Fiji. 2017, 49(6), pp. 1171–1178. [DOI] [PubMed]
- Xavier, M.N., et al., 2010. Pathogenesis of Brucella spp., 4(1).
- Zhong, Z., et al., 2013. Human brucellosis in the People's Republic of China during 2005–2010. 17(5), pp. e289-e292. [DOI] [PubMed]