Abstract
The study was devised to prepare berberine nanoparticles by anti-solvent precipitation method and were assessed for their hepatoprotective effect in Male Sprague-Dawley rats against carbon tetrachloride. The pharmacokinetic parameters of the prepared nanoparticles and berberine were evaluated in rabbits. Histopathological studies and blood biochemical analyses were carried out to evaluate the role of both forms of berberine in the experimental animals. Substantial improvement in the liver function test enzymes levels and liver histopathology were achieved in the animals treated with berberine nanoparticles in comparison to the unprocessed berberine whereas, pharmacokinetic parameters for nanoform of berberine were about 3.97 and 3.88 folds higher than that of the unprocessed berberine. The study revealed that the reduction of berberine particle size to nano range improved pharmacokinetic parameters in rabbits. The nano berberine provided better liver protection in experimental rats and high berberine blood concentration. Thus, better hepatoprotective and pharmacokinetics effects were observed for the nano form in comparison to unprocessed form.
Keywords: Berberine nanoparticles, APSP, Liver, Histopathology, Pharmacokinetic parameters
1. Introduction
Prevalence of liver disorders throughout the world is very common, irrespective of patient’s age, gender or racial background. Certain pathological conditions result in liver cirrhosis in which fibrosis and architectural damage occurs, affecting the liver physiology characterized by various clinical symptoms and complications (Bedogni et al., 2005, Williams et al., 2011, Younossi et al., 2016). In the current drug market, a wide variety of allopathic formulations as well as herbal medicines are available for the treatment of liver diseases (Janbaz and Gilani, 2000). However, the allopathic medicines used in the treatment and management of liver diseases are not suitable due to their side effects (Gurusamy et al., 2019). Herbal medicines, on the other hand, produce better results and have less side effects as compared to the modern allopathic drugs (Dixit et al., 2007). Due to this reason, herbal medicines have attracted the attention of clinicians. One of the important medicines used for the treatment of liver diseases is berberine. Berberine commonly known as “Daru haldi”, is an isoquinoline alkaloid, extracted from the stem-bark and roots of a plant called Berberis aristata (family Berberidaceae) (Janbaz and Gilani, 2000). Berberine is an important drug of the Ayurveda and traditional Chinese medicine (Taylor et al., 1999) that is used extensively in the treatment of various diseases like hypertension (Pan et al., 2003), inflammatory conditions (Küpeli et al., 2002, Yeşilada and Küpeli, 2002), as antimicrobial (Birdsall and Kelly, 1997, Hayashi et al., 2007, Sahibzada et al., 2018) and also as hepato-protective agent (Teodoro et al., 2013). Problem with the berberine is its poor water-solubility, which results in poor dissolution rate and oral bioavailability (Cheng et al., 2010, Hua et al., 2007, Mullauer et al., 2011, Wang et al., 2000, Zuo et al., 2006, Arasu et al., 2017, Arasu et al., 2019). Many efforts have been made to enhance the bioavailability of berberine including complexation of the drug with different excipients like cyclodextrin for example and preparation of solid dispersions (Arcari et al., 1992, Barzaghi et al., 1990, Chen et al., 2005, Morazzoni et al., 1992, Valsalam et al., 2019). With the development of nanotechnology, attempts have been made to manipulate the bioavailability of berberine by reducing its particle size thereby improving its surface area (Javed et al., 2011, Sahibzada et al., 2020).
An attempt was successfully made to improve the limited solubility and hence bioavailability of berberine by reducing the particle size to nano range, presented in the paper published by Sahibzada et al (2018). In connection to our previous study, the antisolvent precipitation method was used to prepare berberine nanoparticles using a syringe pump (APSP). This method involves precipitation technique, which is simple, robust and the nanoparticles attained have larger surface area and have adequate particle size. The prepared nanoparticles were evaluated for their pharmacokinetics and hepatoprotective effects in in vivo studies in experimental animals (rats and rabbits).
2. Material and methods
Berberine was acquired from PCSIR laboratories, Peshawar, Khyber Pakhtunkhwa, Pakistan. Silymarin was purchased from Sigma-Aldrich, St. Louis, USA. All other chemicals were obtained from standard supplier. The chemicals used in the preparation of slides in histopathology studies were prepared according to the standard laboratory protocols (Prophet et al., 1992). Male Sprague Dawley rats (150–200 g) were purchased from the National Institute of Health, Islamabad and used in hepatoprotective studies. For bioavailability studies, healthy rabbits (2–3 kg) were acquired, while the chromatography system used was Perkin Elmer Series 200 HPLC system. Prior to the analysis, the selected animals were acclimatized for 7 days with free access to food and water ad libitum. In-vivo experiments were performed according to ARRIVE guidelines. The protocol laid down by the ethical committee of the University of Malakand adopted in bye-Laws 2008 (Scientific Procedure Issue-I) were also strictly followed.
2.1. Nanoparticles fabrication
APSP technique was utilized to fabricate nanoparticles from the unprocessed berberine (Sahibzada et al., 2018). The drug solution of the unprocessed berberine was injected to the stabilizer solution, keeping the rate and stirring speed constant. Water containing propylene glycol was used as antisolvent phase. Berberine nanoparticles were obtained by evaporating the solvents through rotary evaporator under vacuum.
A number of analytical tools were used to characterize the prepared nanoparticles and to confirm the size reduction (Sahibzada et al., 2018) where, surface morphology was assessed through scanning electron microscopy while the compatibility of the nanoparticles and solvents used in the preparation of nanoparticles was assessed using Fourier transform infrared spectroscopy. Crystalline structure of the fabricated nanoparticle was confirmed using X-ray diffraction and diffraction scanning calorimetry analysis as described in our previously published article (Sahibzada et al., 2018).
2.2. Bioavailability studies
About 12 healthy rabbits, divided into two groups were used in this study. Animals were abstained from food for about 12 h while, access was given to water. Unprocessed berberine was administered to one group whereas the other group was administered with berberine nanoparticles. About 50 mg/kg body weight of berberine and prepared nanoparticles were administered orally to these groups. Blood samples were taken at predetermined time after the dose administration (0.0, 0.5, 1, 1.5, 2, 4, 6, 12 and 24 h). The collected blood samples were centrifuged immediately at 3,000 rpm for 20 min to obtain plasma which was then stored in refrigerator. A method previously reported was used to analyze the samples using HPLC (Hu et al., 2013).
2.3. Hepatoprotective studies
2.3.1. Animal groups
The experimental rats were divided into 6 groups as given in Table 1 along with dosing details. To negative control group, saline solution containing 0.5% carboxy methyl cellulose (CMC) was administered. Carbon tetrachloride (CCl4) was used as toxicant that normally causes liver damage and the group was designated as toxic control. To positive control group, silymarin 200 mg/kg body weight suspended in 1% CMC was given. To 4th group suspensions of berberine at dose 160 mg/kg body weight as studied previously (Feng et al., 2010) was given . To 5th and 6th groups berberine nanoparticles at doses of 40 and 80 mg/kg body weight suspended in saline, were administered orally by oral gavage tube for successive 21 days followed by a single 2 mL/kg body weight dose of CCl4 in olive oil was administered intraperitoneally on 21st day.
Table 1.
Group allocation for the hepatoprotective study.
| Groups | Drug Administered |
|---|---|
| 1 | Negative Control |
| 2 | Toxic Control; CCl4(2 mL/kg, Intraperitonially, in Olive Oil) |
| 3 | Positive Control; Silymarin (Orally) |
| 4 | BB-160 (Orally) |
| 5 | BB-APSP-40 (Orally) |
| 6 | BB-APSP-80 (Orally) |
BB = Berberine, BB-APSP = Berberine nanoparticles prepared by antisolvent method.
2.3.2. Biochemical investigations of the blood samples
From the experimental rats, blood sampling was made 24 h after the CCl4 treatment. The samples were centrifuged at 3000 rpm for 15 min (K240R, Centurion scientific, UK), to separate serum, which was kept refrigerated till further analysis. Alanine aminotransferase (ALT), alkaline phosphatase (ALP), and aspartate aminotransferase (AST) levels were determined using standard tool (GO F400 CH, Chema Diagnostica, Italy).
2.3.3. Liver histology assessment
During the histological assessment, liver from every animal was removed and kept instantly in 10% neutral buffer; formalin for 48 h. Ethanol solutions (50, 70, 80, 90, and 100%) were used for dehydration of the liver tissues followed by 100% xylene treatment, then permeated and fixed in paraffin wax. Tissue slides of 4 μm were prepared using rotary microtome. Hematoxylin and eosin (H&E) were used to stain the slides for the microscopic analysis.
2.4. Statistical analysis
One-way ANOVA followed by Tukey’s multiple comparison post hoc test was used to determine the difference amongst the groups.
3. Results and discussion
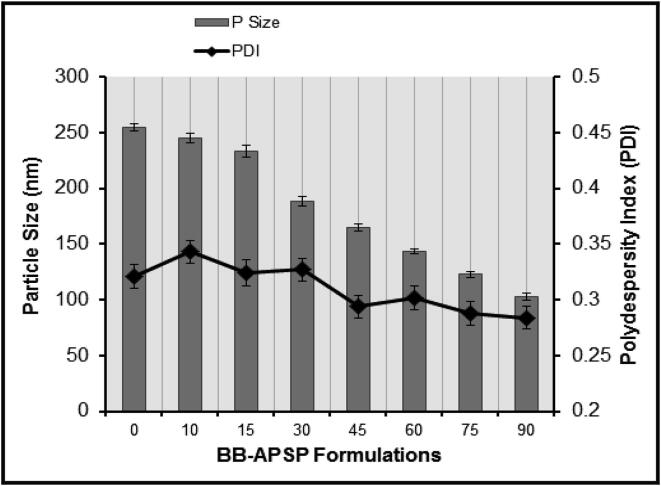
The characterization of the fabricated nanoparticles as reported previously by Sahibzada et al (2018). Parameters optimized for nanoparticles preparation revealed that optimal PDI and particle size for BB-APSP were attained at 3,000 rpm stirring speed, and 1:10 solvent-antisolvent ratio. The stabilizer solution used in this study was 1.0% w/v propylene glycol. The optimized experimental parameters yield BB-APSP nanoparticles having a particle size of 102.62 ± 2.8 nm having PDI 0.284 ± 0.03 whereas the zeta potential recorded was −35.27 mV as can be seen in Fig. 1. The reduced particle size offer greater surface area which thus increase solubility, dissolution and ultimately bioavailability (Sahibzada et al., 2018).
Fig. 1.
Optimization of Particle size and PDI results for APSP method; BB-APSP.
3.1. Bioavailability studies
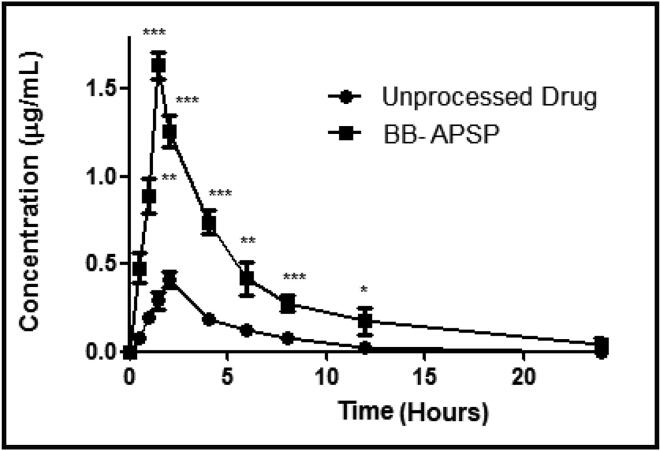
In-vivo bioavailability study was carried out in rabbits to evaluate the impact of size reduction on berberine bioavailability. Certain bioavailability parameters like Cmax, Tmax and area under curve were evaluated for nanoparticles and compared with those of unprocessed berberine. The results clearly indicates that BB-APSP has enhanced bioavailability (Fig. 2) in comparison to that of BB. The pharmacokinetic parameters of the BB-APSP were 3.97 and 3.88 folds higher than BB, as presented in Table 2.
Fig. 2.
Bioavailability studies.
Table 2.
Pharmacokinetics parameters summary.
| Material | Pharmacokinetic parameter |
||
|---|---|---|---|
| Tmax(h) | Cmax(µg/mL) | AUC0-t (µg-h/mL) | |
| BB | 2.0 ± 0.21 | 0.411 ± 0.01 | 1.922 ± 0.16 |
| BB-APSP | 1.5 ± 0.28 | 1.633 ± 0.11 | 7.458 ± 0.18 |
n = 6 rabbits per sample, values are expressed as mean ± SD.
The subsequent increase in the bioavailability of BB-APSP upon oral administration could be ascribed to quicker absorption of nanoparticles when compared with BB same doses. Besides, the enhanced bioavailability brought about by particle size reduction they offer quicker cell membrane adhesion that help in saturation of drug inside cells.
3.2. Hepatoprotective studies
3.2.1. Effect of berberine nanoparticles on serum ALT, AST and ALP
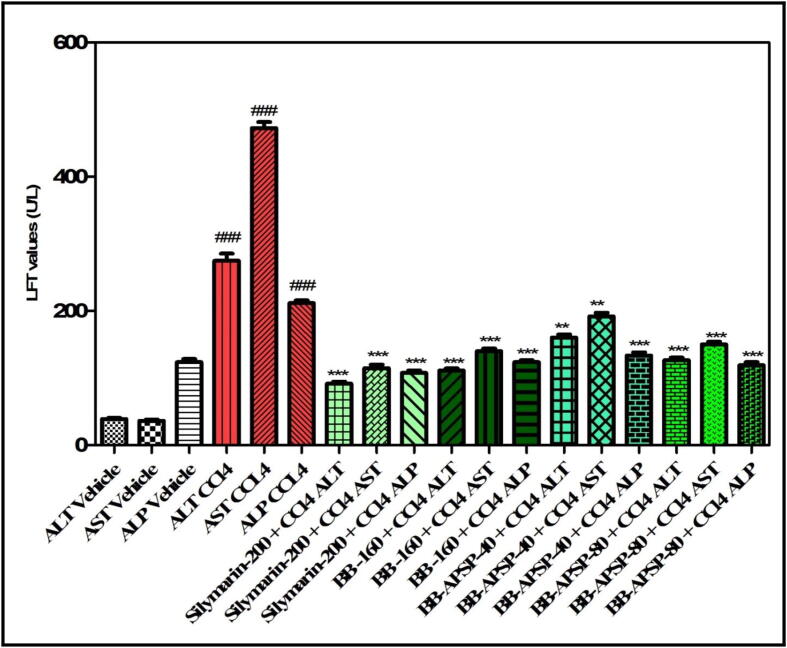
Berberine nanoparticles were evaluated for their effectiveness as hepato-protectant in laboratory animals against the carbon tetrachloride toxicity by assessing the liver function test enzymes (LFT) levels and histopathological studies. Substantial changes in AST [F(7,40) = 20.18, P < 0.0001], ALT [F(7,40) = 29.64, P < 0.0001] and ALP [F(7,40) = 6.592, P < 0.0001] were observed in the animals treated with BB, BB-APSP, silymarin and CCl4 respectively as presented in Fig. 3. It was revealed in the post hoc test that all LFT enzymes serum level was increased (P < 0.001) as a result of treatment with CCl4 in comparison to the serum levels of the animals treated with control saline. The effects were further elevated in the serum when pretreatment with the 40 and 80 mg/kg body weight of BB-APSP was applied that restored high serum level of ALT (P < 0.001), ALP (P < 0.01) and AST (P < 0.05). The silymarin (200 mg/kg) and BB (160 mg/kg) also protected the animal against the CCl4 toxicity.
Fig. 3.
Effect of berberine and its nanoparticles on liver function test enzymes (Values represented as Mean ± SEM. ###P < 0.001 compared to negative control group, *P < 0.05, **P < 0.01, ***P < 0.001 compared to toxic control group. One-way ANOVA followed by post hoc Tukey’s multiple (n = 7 rats per group) comparison test).
3.2.2. Histopathological evaluation
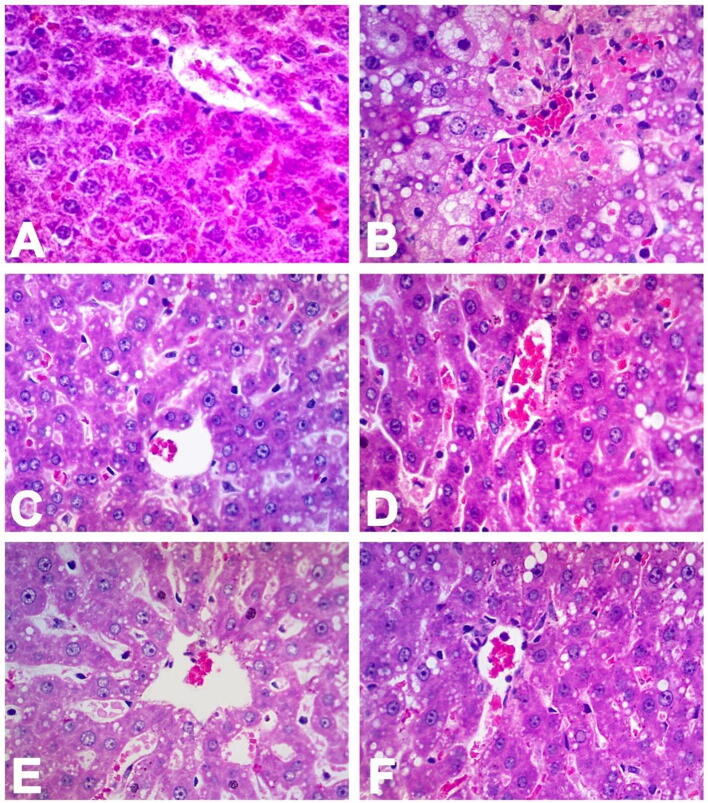
The images in Fig. 4 of the liver samples reveals extensive hemorrhages and hepatic lobular necrosis. The central vein was hard to envision which was collapsed due to the necrosis induced by CCl4 and was covered in the red blood cells, necrotic fragments and mononuclear lymphocytes. The same was observed for the dilated sinusoidal space and was difficult to visualize. Severe ballooning/deterioration of both macro vesicular and micro vesicular steatosis, were observed in the perivenular hepatocytes. Centrilobular hepatocytes exhibited intense and broad coagulative necrosis (Fig. 4B).
Fig. 4.
Histopathological evaluation of toxic liver damage by CCl4 pretreated with unprocessed berberine and its nanoparticles. (H & E; x400 original magnification).
Fig. 4 shows the photomicrographs of a liver cross sections from the animals included in the study. Photomicrograph 4A, represents the animals included in the negative control group (saline); here, the normal physiology of the central vein can be seen with clear and defined margins, plates of hepatocytes and sinusoidal spaces, both appear normal as well. Liver cross section of the toxic control group is shown in photomicrograph 4B where the central vein appears congested. Severe necrosis in the hepatocytes are visible and also steatosis as a result of lipid peroxidation due to CCl4 (both macro vesicular and micro vesicular steatosis) can be seen. The photomicrograph 4C represent the liver cross section of animals included in the positive control group (silymarin 200 mg) where the normal liver histoarchitecture can be observed due to the protection provided by the silymarin against the CCl4 induced histopathological changes, although some mild histopathological changes are there. The photomicrograph 4D represent the liver cross section of animals received BB, 160 mg/kg body weight dose plus CCl4, the liver histoarchitecture appears normal although some micro vesicular steatosis can be seen there. The photomicrograph 4E represent the liver cross section of animals received BB-APSP 40 mg/kg body weight dose plus CCl4, which shows that the liver has been preserved well by the nanoparticles against the toxicity of CCl4 as the sinusoidal dilatation and some micro vesicular steatosis can be seen. The photomicrograph 4F represent the liver cross section of animals that received BB-APSP 80 mg/kg body weight dose plus CCl4 and it is observed that liver histoarchitecture is not much disturbed and only micro-vesicular steatosis can be seen due to CCl4; likewise, similar but profound effects are visible in the photomicrograph 4E.
It is suggested that the onset of hepatotoxicity resulted in the loss of metabolic enzymes due to changes in the intracellular endoplasmic reticulum (Jain et al., 2008, Recknagel, 1983). The ingestion of CCL4 produce a toxic radical due to metabolism i.e., carbon trichloride (CCl3). CCl3 upon reaction with oxygen in the presence of enzyme cytochrome P450 2E1 produces trichloromethyl per-oxy radical that causes per-oxidative degradation of adipose tissue and lipid membranes after covalently binding to the macromolecules. One of the important effects of berberine is to reduce the serum levels of liver function test enzymes like AST, ALP and AST which is an indication of plasma membrane stabilization and thus recovery of damaged liver tissues (Thabrew et al., 1987). Our studies show that the serum level of the mentioned enzymes were further reduced upon changing the particle size of berberine to the nano scale.
4. Conclusion
Medicinal properties and benefits of berberine are diverse, noteworthy, and established. It is commonly taken through the oral route for treatment of hyperglycemia, hyperlipidemia hypertension, and many other miscellaneous pathological conditions. However, due to its limited solubility in an aqueous medium, the therapeutic effect of berberine when taken orally could be sufficiently compromised. In the view, nanoparticles of berberine were synthesized to increase its solubility with subsequent increase in efficacy of berberine. The berberine nanoparticles were effectively synthesized through the APSP method. The unprocessed berberine and the synthesized nanoparticles were assessed for their potential effects on hematology, serum biochemistry, and liver histology. The results revealed that reduction in size to nano range improve and enhance pharmacokinetic parameters and also protection against CCl4. Moreover, the nanoparticles have produced better results on the liver function test enzymes and histopathology clearly indicating the hepatoprotective properties of berberine. The synthesized nanoparticles also exhibited an enhanced positive effect on the hematology and serum biochemistry in the animal model used.
Author Contributions
M.U.K.S., M.Z. and A.S. conceived the idea and designed the experiments; M.U.K.S. and M.Z. performed the experiments. S.N., F.U., MS., and AMAM analyzed the data; All the authors contributed equally in paper write up and approved the final draft.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
The authors extend their appreciation to the Researchers supporting project number (RSP-2020/247) King Saud University, Riyadh, Saudi Arabia.
References
- Arcari M., Brambilla A., Brandt A., Caponi R., Corsi G., Di M.R., Wachter W. A new inclusion complex of silibinin and beta-cyclodextrins: in vitro dissolution kinetics and in vivo absorption in comparison with traditional formulations. Boll. Chim. Farm. 1992;131(5):205–209. [PubMed] [Google Scholar]
- Arasu M.V., Thirumamagal R., Srinivasan M.P., Al-Dhabi N.A., Ayeshamariam A., Saravana Kumar D., Punithavelan N., Jayachandran M. Green chemical approach towards the synthesis of CeO 2 doped with seashell and its bacterial applications intermediated with fruit extracts. J. Photochem. Photobiol., B. 2017;173:50–60. doi: 10.1016/j.jphotobiol.2017.05.032. [DOI] [PubMed] [Google Scholar]
- Arasu M.V., Arokiyaraj S., Viayaraghavan P., Kumar T.S.J., Duraipandiyan V., Al-Dhabi N.A., Kaviyarasu K. One step green synthesis of larvicidal, and azo dye degrading antibacterial nanoparticles by response surface methodology. J. Photochem. Photobiol., B. 2019;190:154–162. doi: 10.1016/j.jphotobiol.2018.11.020. [DOI] [PubMed] [Google Scholar]
- Barzaghi N., Crema F., Gatti G., Pifferi G., Perucca E. Pharmacokinetic studies on IdB 1016, a silybin-phosphatidylcholine complex, in healthy human subjects. Eur. J. Drug Metab. Pharmacokinet. 1990;15(4):333–338. doi: 10.1007/BF03190223. [DOI] [PubMed] [Google Scholar]
- Bedogni G., Miglioli L., Masutti F., Tiribelli C., Marchesini G., Bellentani S. Prevalence of and risk factors for nonalcoholic fatty liver disease: the Dionysos nutrition and liver study. Hepatology. 2005;42(1):44–52. doi: 10.1002/hep.20734. [DOI] [PubMed] [Google Scholar]
- Birdsall T.C., Kelly G.S. Berberine therapeutic potential of an alkaloid found in several medicinal plants. Alternative Med. Rev. 1997;2(2) [Google Scholar]
- Chen W., Xia H., Wu W. Optimized preparation of silymarin dripping pills by a central composite design response surface method. Chin. Trad. Herb. Drug. 2005;36:679–683. [Google Scholar]
- Cheng Z., Chen A.-F., Wu F., Sheng L.i., Zhang H.-K., Gu M., Li Y.-Y., Zhang L.-N., Hu L.-H., Li J.-Y., Li J. 8,8-Dimethyldihydroberberine with improved bioavailability and oral efficacy on obese and diabetic mouse models. Bioorg. Med. Chem. 2010;18(16):5915–5924. doi: 10.1016/j.bmc.2010.06.085. [DOI] [PubMed] [Google Scholar]
- Dixit N., Baboota S., Kohli K., Ahmad S., Ali J. Silymarin: a review of pharmacological aspects and bioavailability enhancement approaches. Indian J. Pharmacol. 2007;39(4):172. doi: 10.4103/0253-7613.36534. [DOI] [Google Scholar]
- Feng Y., Siu K.-Y., Ye X., Wang N., Yuen M.-F., Leung C.-H., Tong Y., Kobayashi S. Hepatoprotective effects of berberine on carbon tetrachloride-induced acute hepatotoxicity in rats. Chin. Med. 2010;5(1):33. doi: 10.1186/1749-8546-5-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gurusamy S., Kulanthaisamy M.R., Hari D.G., Veleeswaran A., Thulasinathan B., Muthuramalingam J.B., Balasubramani R., Chang S.W., Arasu M.V., Al-Dhabi N.A., Selvaraj A., Alagarsamy A. Environmental friendly synthesis of TiO2-ZnO nanocomposite catalyst and silver nanomaterials for the enhanced production of biodiesel from Ulva lactuca seaweed and potential antimicrobial properties against the microbial pathogens. J. Photochem. Photobiol., B. 2019;193:118–130. doi: 10.1016/j.jphotobiol.2019.02.011. [DOI] [PubMed] [Google Scholar]
- Hayashi K., Minoda K., Nagaoka Y., Hayashi T., Uesato S. Antiviral activity of berberine and related compounds against human cytomegalovirus. Bioorg. Med. Chem. Lett. 2007;17(6):1562–1564. doi: 10.1016/j.bmcl.2006.12.085. [DOI] [PubMed] [Google Scholar]
- Hu X., Li Y., Zhang E., Wang X., Xing M., Wang Q., Lei J., Huang H. Preparation and evaluation of orally disintegrating tablets containing taste-masked microcapsules of berberine hydrochloride. AAPS PharmSciTech. 2013;14(1):29–37. doi: 10.1208/s12249-012-9880-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hua W., Ding L.i., Chen Y., Gong B., He J., Xu G. Determination of berberine in human plasma by liquid chromatography–electrospray ionization–mass spectrometry. J. Pharm. Biomed. Anal. 2007;44(4):931–937. doi: 10.1016/j.jpba.2007.03.022. [DOI] [PubMed] [Google Scholar]
- Jain A., Soni M., Deb L., Jain A., Rout S.P., Gupta V.B., Krishna K.L. Antioxidant and hepatoprotective activity of ethanolic and aqueous extracts of Momordica dioica Roxb. leaves. J. Ethnopharmacol. 2008;115(1):61–66. doi: 10.1016/j.jep.2007.09.009. [DOI] [PubMed] [Google Scholar]
- Janbaz K.H., Gilani A.H. Studies on preventive and curative effects of berberine on chemical-induced hepatotoxicity in rodents. Fitoterapia. 2000;71(1):25–33. doi: 10.1016/s0367-326x(99)00098-2. [DOI] [PubMed] [Google Scholar]
- Javed S., Kohli K., Ali M. Reassessing bioavailability of silymarin. Alternative Med. Rev. 2011;16(3):239. [PubMed] [Google Scholar]
- Küpeli E., Koşar M., Yeşilada E., Başer K.H.C. A comparative study on the anti-inflammatory, antinociceptive and antipyretic effects of isoquinoline alkaloids from the roots of Turkish Berberis species. Life Sci. 2002;72(6):645–657. doi: 10.1016/s0024-3205(02)02200-2. [DOI] [PubMed] [Google Scholar]
- Morazzoni P., Magistretti M.J., Giachetti C., Zanolo G. Comparative bioavailability of Silipide, a new flavanolignan complex, in rats. Eur. J. Drug Metab. Pharmacokinet. 1992;17(1):39–44. doi: 10.1007/BF03189986. [DOI] [PubMed] [Google Scholar]
- Mullauer F.B., van Bloois L., Daalhuisen J.B., Ten Brink M.S., Storm G., Medema J.P., Schiffelers R.M., Kessler J.H. Betulinic acid delivered in liposomes reduces growth of human lung and colon cancers in mice without causing systemic toxicity. Anticancer Drugs. 2011;22(3):223–233. doi: 10.1097/CAD.0b013e3283421035. [DOI] [PubMed] [Google Scholar]
- Pan G.-Y., Huang Z.-J., Wang G.-J., Fawcett J.P., Liu X.-D., Zhao X.-C., Xie Y.-Y. The antihyperglycaemic activity of berberine arises from a decrease of glucose absorption. Planta Med. 2003;69(07):632–636. doi: 10.1055/s-2003-41121. [DOI] [PubMed] [Google Scholar]
- Prophet E.B., Mills B., Arrington J.B., Sobin L.H. American Registry of Pathology; Washington DC: 1992. Laboratory Methods in Histotechnology. [Google Scholar]
- Recknagel R.O. A new direction in the study of carbon tetrachloride hepatotoxicity. Life Sci. 1983;33(5):401–408. doi: 10.1016/0024-3205(83)90787-7. [DOI] [PubMed] [Google Scholar]
- Sahibzada M.U.K., Sadiq A., Faidah H.S., Khurram M., Amin M.U., Haseeb A., Kakar M. Berberine nanoparticles with enhanced in vitro bioavailability: characterization and antimicrobial activity. Drug Des., Develop. Therap. 2018;12:303. doi: 10.2147/DDDT.S156123. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Sahibzada M.U.K., Sadiq A., Zahoor M., Naz S., Shahid M., Qureshi N.A. Enhancement of bioavailability and hepatoprotection by silibinin through conversion to nanoparticles prepared by liquid antisolvent method. Arabian J. Chem. 2020;13(2):3682–3689. [Google Scholar]
- Taylor C.T., Winter D.C., Skelly M.M., O'Donoghue D.P., O'Sullivan G.C., Harvey B.J., Baird A.W. Berberine inhibits ion transport in human colonic epithelia. Eur. J. Pharmacol. 1999;368(1):111–118. doi: 10.1016/s0014-2999(99)00023-0. [DOI] [PubMed] [Google Scholar]
- Teodoro J.S., Duarte F.V., Gomes A.P., Varela A.T., Peixoto F.M., Rolo A.P., Palmeira C.M. Berberine reverts hepatic mitochondrial dysfunction in high-fat fed rats: a possible role for SirT3 activation. Mitochondrion. 2013;13(6):637–646. doi: 10.1016/j.mito.2013.09.002. [DOI] [PubMed] [Google Scholar]
- Thabrew M., Joice P., Rajatissa W. A comparative study of the efficacy of Pavetta indica and Osbeckia octandra in the treatment of liver dysfunction. Planta Med. 1987;53(03):239–241. doi: 10.1055/s-2006-962691. [DOI] [PubMed] [Google Scholar]
- Valsalam S., Agastian P., Arasu M.V., Al-Dhabi N.A., Ghilan A.-K., Kaviyarasu K., Ravindran B., Chang S.W., Arokiyaraj S. Rapid biosynthesis and characterization of silver nanoparticles from the leaf extract of Tropaeolum majus L. and its enhanced in-vitro antibacterial, antifungal, antioxidant and anticancer properties. J. Photochem. Photobiol., B. 2019;191:65–74. doi: 10.1016/j.jphotobiol.2018.12.010. [DOI] [PubMed] [Google Scholar]
- Wang S., Song B., Li K. Determination of berberine in decocted liquid from shenshu granules with water by reversed-phase liquid chromatography. Se pu= Chinese J. Chromatogr./Zhongguo hua xue hui. 2000;18(3):261–262. [PubMed] [Google Scholar]
- Williams C.D., Stengel J., Asike M.I., Torres D.M., Shaw J., Contreras M., Landt C.L., Harrison S.A. Prevalence of nonalcoholic fatty liver disease and nonalcoholic steatohepatitis among a largely middle-aged population utilizing ultrasound and liver biopsy: a prospective study. Gastroenterology. 2011;140(1):124–131. doi: 10.1053/j.gastro.2010.09.038. [DOI] [PubMed] [Google Scholar]
- Yeşilada E., Küpeli E. Berberis crataegina DC. root exhibits potent anti-inflammatory, analgesic and febrifuge effects in mice and rats. J. Ethnopharmacol. 2002;79(2):237–248. doi: 10.1016/s0378-8741(01)00387-7. [DOI] [PubMed] [Google Scholar]
- Younossi Z.M., Koenig A.B., Abdelatif D., Fazel Y., Henry L., Wymer M. Global epidemiology of nonalcoholic fatty liver disease-Meta-analytic assessment of prevalence, incidence, and outcomes. Hepatology. 2016;64(1):73–84. doi: 10.1002/hep.28431. [DOI] [PubMed] [Google Scholar]
- Zuo F., Nakamura N., Akao T., Hattori M. Pharmacokinetics of berberine and its main metabolites in conventional and pseudo germ-free rats determined by liquid chromatography/ion trap mass spectrometry. Drug Metab. Dispos. 2006;34(12):2064–2072. doi: 10.1124/dmd.106.011361. [DOI] [PubMed] [Google Scholar]
Further Reading
- Sahibzada M.U.K., Sadiq A., Khan S., Faidah H.S. Fabrication, characterization and in vitro evaluation of silibinin nanoparticles: an attempt to enhance its oral bioavailability. Drug Des., Develop. Therapy. 2017;11:1453. doi: 10.2147/DDDT.S133806. [DOI] [PMC free article] [PubMed] [Google Scholar]