Abstract
Surgical prostate cancer (PCa) treatment delay (TD) may increase the likelihood of recurrence of disease, and influence quality of life as well as survival disparities between Black and White men. We used latent class analysis (LCA) to identify risk profiles in localized, malignant PCa surgical treatment delays while assessing co-occurring social determinants of health. Profiles were identified by age, marital status, race, county of residence (non-Appalachian or Appalachian), and health insurance type (none/self-pay, public, or private) reported in the Tennessee Department of Health cancer registry from 2005 to 2015 for adults ≥18 years (N = 18,088). We identified three risk profiles. The highest surgical delay profile (11% of the sample) with a 30% likelihood of delaying surgery >90 days were young Black men, <55 years old, living in a non-Appalachian county, and single/never married, with a high probability of having private health insurance. The medium surgical delay profile (46% of the sample) with a 21% likelihood of delay were 55–69 years old, White, married, and having private health insurance. The lowest surgical delay profile (42% of the sample) with a 14% likelihood of delay were ≥70 years with public health insurance as well as had a high probability of being White and married. We identified that even with health insurance coverage, Blacks living in non-Appalachian counties had the highest surgical delay, which was almost double that of Whites in the lowest delay profile. These disparities in PCa surgical delay may explain differences in health outcomes in Blacks who are most at-risk.
Keywords: prostate cancer, surgical treatment delay, latent class analysis, health disparities
Prostate cancer (PCa) is the most commonly diagnosed cancer and the second leading cause of cancer death among men in the United States (Siegel et al., 2020); compared to Whites, Black men are more likely to develop PCa. Furthermore, Black men are also more likely to have advanced prostate cancer, and 2.4 times more likely to die from it (Howlader et al., 2012; Stokes et al., 2013). Although survival outcomes have improved in general population, the difference in PCa incidence, survival, and mortality rates in Black and White men represents the largest health disparities of any malignancy (Gilligan, 2005).
The underlying causes for these PCa disparities are unclear but involve complex multiple determinants of health including biological, cultural, access to health care, and sociodemographic differences. Regional and nationally representative data from the United States suggest that race and ethnicity strongly correlate with survival following a PCa diagnosis (Adams et al., 2017; Benjamins et al., 2016; Chornokur et al., 2011; Dess et al., 2019; Krimphove et al., 2019). In a systematic review, Chornokur et al. (2011) found survival and diagnostic differences in surgical intervention, radiation therapy, and nonaggressive treatments between Black men and White men with PCa. Black men were also more likely to experience a longer wait times between diagnosis and treatment (incidence rate ratio [IRR] = 1.19) compared to White men, while sociodemographic, clinical, and psychosocial factors were not able to explain these racial differences (Kinlock et al., 2016). Similarly, using the National Center for Health Statistics death files, Benjamins et al. (2016) analyzed racial disparities in PCa in the 50 largest U.S. cities from 1994 to 2004. Results revealed that even after controlling for differences in the stage at diagnosis and treatment, there were still significant disparities between Black and White men in PCa mortality (Benjamins et al., 2016).
The Institute of Medicine has identified the timely delivery of health-care services as one of six primary goals for improving the quality of health care in the United States (Corrigan, 2005). Treatment delay (TD) was defined as the time between PCa diagnosis and treatment initiation. TD is associated with increased risk for biochemical recurrence and survival (Freedland et al., 2006; Nguyen et al., 2005; O’Brien et al., 2011), especially for patients with aggressive, high-risk neoplasms. TD is also a potentially modifiable factor that may also contribute to racial disparities in PCa diagnosis and treatment initiation (Stokes et al., 2013). Schmid et al. (2016) examined differences in quality of life and survival outcomes among recipients of radical prostatectomy using the Surveillance, Epidemiology, and End Results (SEER)–Medicare linked database. Schmid et al. (2016) found that Black men undergoing radical prostatectomy treatment tended to have longer wait time between diagnosis and surgery (average of 79 days), were less likely to undergo lymph node dissection, more likely to experience postoperative complications, have subsequent emergency department visits as well as readmissions to hospital compared to non-Hispanic Whites (i.e., 71 days).
PCa disparities are also based on geography. States in the United States may experience issues pertaining to health-care access and screening in specific regions like Appalachia when compared to non-Appalachian regions (Yao et al., 2017), in addition to racial disparities. PCa disparity studies in the Appalachian region, while predominantly White with low racial/ethnic diversity, have reported lower incidence but higher mortality (Myint et al., 2018, 2019; Yao et al., 2017). Moreover, TD may present differently in Appalachian compared to non-Appalachian areas. A study by McDonald et al. (2020) revealed that the highest incidence rate and aggressive type of PCa was found in non-Appalachian urban Pennsylvania compared to rural and urban Appalachian areas. Differential PCa outcomes within and between the Tennessee Appalachian and non-Appalachian regions while accounting for race are, however, limited. Furthermore, geography and residence in Tennessee present a unique opportunity to understand PCa disparities as they present in Appalachian and non-Appalachian counties.
Given the racial/ethnic differences in PCa incidence as well as how TD impacts quality of life and survival outcomes, it is necessary to identify profiles of risk through factors that may influence the delay of surgical treatment of PCa while accounting for Appalachian and non-Appalachian areas in Tennessee. To fill this gap in the literature, this study used latent class analysis (LCA)—a person-centered approach—to examine the constellation of health determinants in PCa surgical TD on the Tennessee Cancer Registry (TCR) data. We identified risk profiles of patients who experienced TD by co-occurring sociodemographic determinants of health reported in TCR data from 2005–2015. Profiles identified may help explain health disparities to be intervened upon. Moreover, the purpose of this study was not to make a clinical recommendation as we recognize the current debate on overtreatment of PCa (Yang et al., 2017). This study intended to identify factors in TD and whether there were differences in TD between Black and White men in Tennessee.
Materials and Methods
Study Population and Data Source
The study population included all male Tennessee residents aged ≥18 years diagnosed with histologically confirmed PCa as the primary site of diagnosis as coded by the International Classification of Diseases for Oncology, Third Edition (ICD-O-3) and reported to the TCR from January 1, 2005 to December 31, 2015. The TCR is a population-based, central cancer registry serving the citizens of Tennessee and was established by state law to collect and monitor cancer incidence (https://www.tn.gov/health/health-program-areas/tcr/tennessee-cancer-registry-data.html). The research protocol and access to the data for this study went to full review by the Tennessee Department of Health Institutional Review Board (IRB) and was approved on 1 February 2018 (TDH-IRB 1057486). The National Institutes of Health – Intramural Research Program IRB – Human Research Protections Program – Office of Human Subjects Research Protections determined that our protocol did not involve human subjects and was excluded from IRB review (18-NIMHD-00722). Analysis was performed on a total of 18,088 localized malignant/invasive cases. Data used for this analysis are restricted but available by request to the Tennessee Department of Health TCR (https://www.tn.gov/education/data/data-downloads/request-data.html). All analytical files are available by reasonable request.
Latent Variable Indicators
The latent outcome was surgical TD of localized malignant PCa. We used the following sociodemographic variables for our LCA model: race, age at diagnosis, marital status, county of residence, health insurance type, and surgical TD. Race was categorized into White, Black, or other. The other race category included participants that self-identified as American Indian/Alaska Native and Asian or Pacific Islander. Age was categorized into three groups based on the U.S. Preventive Services Task Force screening recommendations for PCa (i.e., <55; 55–69; and ≥70; US Preventive Services Task Force, 2018). Marital status was grouped into four categories as (a) single, never married; (b) married, which includes common law; (c) divorced/separated; and (d) widow/widower. Area of residence was based on county of diagnosis/residence and was coded as either Appalachian or non-Appalachian based on Appalachian Regional Commission demarcations (Pollard et al., 2020). Type of health insurance was categorized as: (a) not insured and/or self-pay; (b) public that included Medicaid, Medicare, Military/TRICARE, Veterans Affairs, and Indian/Public Health Service; and (c) private that included managed care, health maintenance option (HMO), preferred provider organization (PPO), or fee-for-service. The distal outcome of surgical TD—based on the extant literature (Ginsburg et al., 2020; Graefen et al., 2005; Korets et al., 2012; Reichard et al., 2019)—was coded as (a) ≤90 days, (b) >90–180 days, (c) >180–365 days, and (d) >365 days from the date patients were diagnosed to when surgical treatment was provided and recorded in the TCR.
Covariates
Tumor grade (i.e., I–IV) was accounted for in our final model as a covariate to assess PCa grade differences between identified TD profiles.
Statistical Analysis
We first conducted a descriptive analysis to report the characteristics of the TCR localized malignant PCa study sample. Second, we performed LCA, a person-centered analytical method, to identify and characterize PCa surgical TD profiles associated with conditional probabilities of observed sociodemographic and geographic indicators using delay in surgical treatment as a distal outcome. To select the best fitting LCA model, we employed a comparative approach that compares multiple models (i.e., 1-class to 6-class solution) by Bayesian information criterion (BIC), sample size adjusted-BIC (SSA-BIC), Lo-Mendell-Rubin (LMR) adjusted likelihood-ratio-test (LRT), parametric bootstrapped (PB) LRT, and entropy. Entropy provides an index of reliability for separation of classes (Nylund et al., 2007). Once the final model was selected, a multinomial logistic regression was used to assess the role of tumor grade between profiles. All analyses were performed in Mplus 8.4 (Muthén & Muthén).
Results
Patients’ Sociodemographic Characteristics
Localized malignant PCa cases were primarily White (84.4%) followed by Black (14.9%) and predominantly of non-Hispanic origin (99.5%). The sample was mostly 55–69 years of age (64.1%) and married (83.0%) having either public (45.0%) or private (54.0%) health insurance. The sample was almost evenly distributed between Appalachian and non-Appalachian residence. The majority of patients (54.6%) were recorded as having grade II tumor. Nearly 80% of the sample reported a ≤90-day delay in surgical treatment (Table 1).
Table 1.
Localized Malignant Prostate Cancer Patient Sample Descriptives (N = 18,088).
| Race | ||
| White | 15,254 | 84.4% |
| Black | 2,699 | 14.9% |
| Other | 128 | 0.7% |
| Hispanic origin | ||
| No | 16,104 | 99.5% |
| Yes | 80 | 0.5% |
| Age at diagnosis | ||
| 18–54 | 2,652 | 14.7% |
| 55–69 | 11,587 | 64.1% |
| ≥70 | 3,849 | 21.3% |
| Marital status | ||
| Single | 1,220 | 7.5% |
| Married | 13,535 | 83.0% |
| Divorced/Separated | 1,042 | 6.4% |
| Widow/Widower | 511 | 3.1% |
| County of residence | ||
| Non-Appalachian | 9,300 | 51.4% |
| Appalachian | 8,785 | 48.6% |
| Health insurance type | ||
| None | 181 | 1.0% |
| Public | 7,803 | 45.0% |
| Private | 9,373 | 54.0% |
| Tumor grade | ||
| I | 1,472 | 8.3% |
| II | 9,200 | 54.6% |
| III | 7,095 | 39.8% |
| IV | 50 | 0.3% |
| Delay in surgical treatment | ||
| 90 days or less | 14,511 | 80.3% |
| 90–180 days | 3,380 | 18.7% |
| 180–365 days | 500 | 2.8% |
| More than 365 days | 183 | 1.0% |
Latent Class Risk Profiles Delay
The best fitting LCA model was a three class-solution with an entropy of 0.69 as seen in Table 2. The three classes were named lowest, medium, and highest delay profile based on the probabilities for delaying PCa surgical treatment. Class 1 or the lowest surgical delay profile represents 42% of sample, had high conditional likelihoods of being primarily White (92.3%), ≥70 years at diagnosis (51.6%), married (83.7%%), and residing in Appalachian county (54.3%) compared to all delay profiles. Class 1 had the highest conditional probability of utilizing public health insurance (89.3%).
Table 2.
Latent Class Analysis Model Fits for Comparison (N = 18,088).
| BIC | SSA-BIC | Entropy | LMR-LRT | p value | PB-LRT | p value | |
|---|---|---|---|---|---|---|---|
| 1-Class Solution | 142,863.9 | 142,822.6 | - | - | - | - | - |
| 2-Class Solution | 137,660.7 | 137,574.9 | 0.662 | 5,301.8 | .000 | 5,340.4 | .000 |
| 3-Class Solution | 136,502.5 | 136,372.2 | 0.693 | 1,286.1 | .000 | 1,295.4 | .000 |
| 4-Class Solution | 136,324.3 | 136,149.5 | 0.731 | 313.2 | .138 | 315.5 | .000 |
Note. BIC = Bayesian information criterion; SSA-BIC = sample size adjusted-Bayesian information criterion; LMR = Lo-Mendell-Rubin; LRT = adjusted likelihood-ratio-test; PB = parametric bootstrapped.
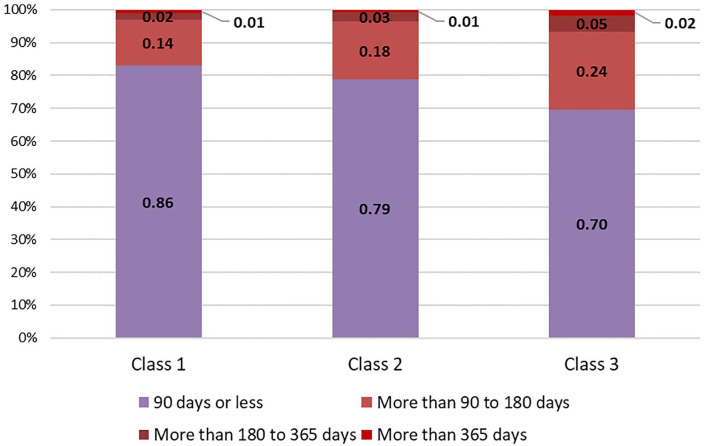
Class 2, or the medium delay profile (46% of sample), had the highest likelihoods of being predominantly White (96.0%), between ages 55 and 69 years (76.4%), married (85.8%), with private health insurance (87.6%). Class 3, the highest delay profile, constitutes 12% of sample, had the highest likelihood of being Black (80.9%) and living in non-Appalachian county (90.0%). This class/group was also younger, as they had the highest conditional probability of being <55 years of age (28.4%). The highest delay group also had the highest conditional probabilities of being single (17.2%) or divorced/separated (10.2%). This delay group had a 30% and 69% conditional probability of having public and private health insurance, respectively. See Table 3 for all conditional probabilities. Using multinomial logistic regression, we found that the highest delay profile had 22% lower odds (95% CI [.67, .90]) of having a grade II tumor compared to patients with grade I in the lowest delay profile (Table 4). Figure 1 provides a comparison of surgical TD categories by profile.
Table 3.
Latent Class Analysis of Localized Malignant Prostate Cancer Surgical Delay Groups With Conditional Probabilities (N = 18,088).
| Class 1 | Class 2 | Class 3 | |
|---|---|---|---|
| Lowest | Medium | Highest | |
| Delay | Delay | Delay | |
| N = 7,620 | N = 8,391 | N = 2,077 | |
| 42% | 46% | 12% | |
| Race | |||
| White | 0.923 | 0.960 | 0.179 |
| Black | 0.070 | 0.034 | 0.809 |
| Other | 0.007 | 0.006 | 0.011 |
| Age at diagnosis | |||
| Under 55 | 0.000 | 0.236 | 0.284 |
| 55–69 | 0.484 | 0.764 | 0.686 |
| 70 and over | 0.516 | 0.000 | 0.030 |
| Marital status | |||
| Single, never married | 0.051 | 0.068 | 0.172 |
| Married/Common law | 0.837 | 0.858 | 0.707 |
| Divorced/Separated | 0.049 | 0.066 | 0.102 |
| Widow/Widower | 0.063 | 0.009 | 0.020 |
| County of residence | |||
| Non-Appalachian | 0.457 | 0.456 | 0.900 |
| Appalachian | 0.543 | 0.544 | 0.100 |
| Health insurance type | |||
| No insurance/Self-pay | 0.001 | 0.013 | 0.032 |
| Public insurance | 0.893 | 0.112 | 0.289 |
| Private Insurance | 0.107 | 0.875 | 0.678 |
| Delay in surgical treatment | |||
| 90 days or less | 0.855 | 0.787 | 0.697 |
| 90–180 days | 0.144 | 0.177 | 0.237 |
| 180–365 days | 0.021 | 0.027 | 0.050 |
| More than 365 days | 0.010 | 0.008 | 0.017 |
Table 4.
Multinomial Logistic Regression of Tumor Grade Covariates Using (Class 1) Lowest Delay as Reference (N = 17,817).
| Class 2 | Class 3 | |||||||
|---|---|---|---|---|---|---|---|---|
| Medium Delay | Highest Delay | |||||||
| 95% CI | 95% CI | |||||||
| OR | Lower | Upper | p value | OR | Lower | Upper | p value | |
| Grade I | ref. | - | - | - | ref. | - | - | - |
| Grade II | 0.96 | 0.76 | 1.21 | .723 | 0.78 | 0.67 | 0.90 | .001 |
| Grade III | 1.06 | 0.84 | 1.35 | .623 | 0.99 | 0.86 | 1.14 | .891 |
| Grade IV | 1.77 | 0.58 | 5.43 | .316 | 1.51 | 0.70 | 3.29 | .296 |
Figure 1.
Surgical delay profiles by class.
Discussion
Evidence suggests that, overall, Black men experience worse health outcomes from PCa compared to White men. Factors contributing to these disparities include unequal access to health-care services leading to delay in diagnosis and treatment (Adams et al., 2017; Benjamins et al., 2016; Chornokur et al., 2011; Krimphove et al., 2019), but limited research exists on the profiles or the group of populations most likely to delay treatment. In this study, we used a person-centered approach to determine profiles of patients at-risk of surgical TD for localized malignant PCa from a statewide cancer registry. Our findings make two major contributions to the literature on health disparities in the surgical treatment of PCa. First, we found three profiles of patients who delayed PCa surgical treatment. These groups were based on sociodemographic factors, health insurance type, and geographic region of residence. Second, we found that the profiles that were most likely to delay surgical treatment were Black, younger adults with private insurance, single or separated/divorced, and residing in a non-Appalachian county. Moreover, tumor grades did not differentiate between profile differences, with the exception of the highest TD profile; that is, the highest TD profile had lower odds of being treated for grade II tumor when compared to the lowest TD profile.
The Appalachian region of the United States faces health disparities in cancer burden. Disparities in PCa survival among Appalachian residents are well documented in Kentucky, though the difference was related to high comorbidity score, high poverty rate, and low education (Myint et al., 2018, 2019). In contrast to current findings, our results based on TCR data show that the group with highest delay (i.e., >90 days from diagnosis) in PCa surgical treatment did not reside in Appalachian counties. The lowest and medium PCa surgical delay profile groups had slightly higher conditional probabilities to reside in Appalachian counties and married compared to the highest delay profile. Nonetheless, the lowest risk profile of delay was comparatively older at time of diagnosis and had publicly provided health insurance. Among the medium and highest delay profiles, both had a high conditional probability to have private health insurance. These findings are consistent with Al Rowas et al. (2017), who reported that private health insurance is associated with delay in seeking care and treatment due to high cost-related co-pays and deductibles compared to publicly insured individuals (15.6% vs. 8.1%, respectively).
Our results suggest that despite being insured, Black men, more specifically middle-age adults, were less likely to undergo surgery to treat PCa. This is alarming as the higher likelihood among Black men to have advanced PCa is evidenced (Howlader et al., 2012; Stokes et al., 2013). It is also shown that Black patients with PCa are more likely to die due to the disease. Our study adds to the evidence on the underlying factors associated with higher mortality among Black patients with PCa. Factors at the patient level that may be driving excess mortality are mistrust, health-seeking behaviors, rurality, and clinical or system-level factors (Kan et al., 2018). Although access to health care has been known to be a main driver of health disparities and is also an important predictor of treatment behavior (Burt et al., 2018; Gordon et al., 2019; Noonan et al., 2016), our study shows that patients delayed treatment despite having health insurance coverage. A recent study by Gordon et al. (2019) analyzed the North Carolina Prostate Cancer Comparative Effectiveness and Survivorship Study and reported that Black patients were more likely to consider cost (69.2%), treatment (69.2%), and recovery time (73.3%) as important therapeutic decision-making factors compared to White patients (34.9%, 33.8%, and 46.9%, respectively). Another study used SEER data to assess trends in PCa treatment from 2004 to 2014 concluded significant differences exist by patient age, insurance status, socioeconomic status (SES), and geographical residence (Burt et al., 2018).
Our findings indicated that having private health insurance may not be a sufficient factor to determine early treatment initiation for PCa, and that treatment delays may manifest as constellations of social determinants. Getting timely treatment and reducing delays for Black adults with aggressive PCa is crucial for improving survival outcomes and reducing cancer disparities. It is, therefore, critical that these social determinants are given a major consideration in the development of effective interventions. Moreover, the role of Appalachian and non-Appalachian regions in context of TD and the social determinants must be further explored to understand the complex cancer health disparities affecting men. Multilevel intervention studies are needed to focus on patient, clinician, and health-care system-level factors to address these PCa disparities.
Nevertheless, to address PCa health disparities and inequities, we must go beyond census bureau categories of race/ethnicity. Attention needs to be given to data collection in cancer registries to gather more detail on racial/ethnic categorizations to include biological ancestry and biomarkers (e.g., prostate-specific antigen; tumor necrosis factor alpha) for more personalized risk assessments, interventions, and medical treatment. To further improve risk categorizations, cancer registry data will also need to collect geographic context of socioeconomic and ecological factors. The granular level of geography must go beyond the county and zip code level to include neighborhood as well as housing history. SES like family income would considerably improve the person-centered models as well as socioecological factors to better target risk mitigation efforts and health promotion. Socioecological factors would include environmental exposure from early adverse events (e.g., violence, crime) to toxicants and xenobiotics (e.g., total tobacco exposure, air pollution, radon, pesticides). Behavioral and health risk factors such as alcohol consumption, binge drinking, physical activity from work or exercise, and diet would have improved our risk profiles further. Therefore, future policies regarding cancer registry data collection should include more contextual data to help understand risk in context to prevent disparate health outcomes in an effective and efficacious manner to intervene upon the most salient factors given a specific at-risk PCa treatment profile. Through the collection of contextual cancer data risk assessments can we move beyond linear categorizations of risk by race and ethnicity to targeted person-centered care to meaningfully address cancer health disparities and move toward health equity.
Strength and Limitations
This study contributes to the limited knowledge on PCa surgical TD by identifying risk profiles of patients in Tennessee, a unique geographical region currently not covered in the SEER program (National Cancer Institute Surveillance Epidemiology and End Result Program, 2020). Although the study used standard and validated measures involving a large population of PCa patients not in the SEER program, there were few limitations. First, we were limited by the retrospective administrative variables available to us. Cancer registries are not mandated to collect SES data such as income and education, and quality of treatment received by patients. Additionally, demographic variables are only collected at the time of diagnosis and hence may not be up-to-date. Despite the large sample size, the results may not be generalizable to the entire U.S. population because the TCR is tasked with collecting cancer data covering the state of Tennessee as well as patients diagnosed and/or treated for cancer in state although they are residents of neighboring states. Nonetheless, the findings are significant because they provide a better understanding of subgroups/population most at-risk to delay PCa treatment in Tennessee.
Conclusion
This study identified three risk subgroups—lowest, medium, and highest PCa surgical TD profiles/subpopulations—based on patient sociodemographic and geographic residence as well health insurance characteristics. In particular, we identified that even with health insurance, young Blacks aged <55 years, living in non-Appalachian counties, had the highest surgical delay, which was almost double that of the whites in the lowest delay class. These findings have important policy implications to inform utilization of private health insurance for required surgical treatment cases of treatable PCa. Furthermore, the disparities in PCa surgical delay may explain differences in health outcomes in Blacks who are most at-risk; however, structural factors, clinical care, and treatment outcomes could play a role in these delays but were not available for analysis. Lastly, the use of person-centered approaches can help health researchers better identify cancer risk profiles or subpopulations, and the underlying determinants that may be overlooked, but needed for tailored interventional programs to address PCa disparities.
Supplemental Material
Supplemental material, sj-pdf-1-jmh-10.1177_1557988320984282 for Identifying Risk Profiles of Malignant Prostate Cancer Surgical Delay Using a Person-Centered Approach to Understand Prostate Cancer Disparities: The Constellation of Health Determinants Using Latent Class Analysis on Cancer Registry Data by Francisco A. Montiel Ishino, Claire Rowan, Rina Das, Janani Thapa, Ewan Cobran, Martin Whiteside and Faustine Williams in American Journal of Men's Health
Acknowledgments
We would like to thank Kevin Villalobos for his time and support. Drs. Montiel Ishino and Williams’ efforts were supported by the Division of Intramural Research, National Institute on Minority Health and Health Disparities, National Institutes of Health. Dr. Rina Das’s effort was supported by the Division of Extramural Scientific Programs, National Institute on Minority Health and Health Disparities, National Institutes of Health. The content of this work is solely the responsibility of the authors and does not necessarily reflect the views of the National Institutes of Health. The National Institutes of Health, Emory University, University of Georgia, and Tennessee Cancer Registry did not have a role in the design of the study; collection, management, analysis, and interpretation of the data; preparation, review, [or approval of the manuscript]; and decision to submit the manuscript for publication. Tennessee Department of Health data used in this study was obtained from the Tennessee Cancer Registry, Tennessee Department of Health (TDH). Use of these data does not imply TDH agrees or disagrees with any presentations, analyses, interpretations or conclusions herein.
Footnotes
Credits: FW and FAMI conceived and designed the study. FW and MW acquired the data. FAMI and CR cleaned, managed analyzed and interpreted the results. RD and JT drafted the first manuscript and modified by EC. MW and RD substantially modified the submitted version of the manuscript. All authors read and approved the final version of the manuscript.
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
ORCID iDs: Francisco A. Montiel Ishino  https://orcid.org/0000-0002-2837-726X
https://orcid.org/0000-0002-2837-726X
Faustine Williams  https://orcid.org/0000-0002-7960-2463
https://orcid.org/0000-0002-7960-2463
Supplemental Material: Supplemental material for this article is available online.
References
- Adams S. V., Bansal A., Burnett-Hartman A. N., Cohen S. A., Karnopp A., Warren-Mears V., Ramsey S. D. (2017). Cancer treatment delays in American Indians and Alaska natives enrolled in medicare. J Health Care Poor Underserved, 28(1), 350–361. doi: 10.1353/hpu.2017.0027 [DOI] [PubMed] [Google Scholar]
- Al Rowas S., Rothberg M. B., Johnson B., Miller J., Al Mahmoud M., Friderici J., Goff S. L., Lagu T. (2017). The association between insurance type and cost-related delay in care: A survey. The American Journal of Managed Care, 23(7), 435. [PMC free article] [PubMed] [Google Scholar]
- Benjamins M. R., Hunt B. R., Raleigh S. M., Hirschtick J. L., Hughes M. M. (2016). Racial disparities in prostate cancer mortality in the 50 largest US cities. Cancer Epidemiology, 44, 125–131. 10.1016/j.canep.2016.07.019 [DOI] [PubMed] [Google Scholar]
- Burt L. M., Shrieve D. C., Tward J. D. (2018). Factors influencing prostate cancer patterns of care: An analysis of treatment variation using the SEER database. Advances in Radiation Oncology, 3(2), 170–180. doi: 10.1016/j.adro.2017.12.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chornokur G., Dalton K., Borysova M. E., Kumar N. B. (2011). Disparities at presentation, diagnosis, treatment, and survival in African American men, affected by prostate cancer. Prostate, 71(9), 985–997. doi: 10.1002/pros.21314 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corrigan J. (2005). Crossing the quality chasm. In National Academy of Engineering and Institute of Medicine, Committee on Engineering and the Health Care System. In Reid P. P., Compton W. D., Grossman J. H., Fanjiang G. (Eds.), Building a better delivery system: A new engineering/health care partnership. National Academies Press. [Google Scholar]
- Dess R. T., Hartman H. E., Mahal B. A., Soni P. D., Jackson W. C., Cooperberg M. R., Amling C. L., Aronson W. J., Kane C. J., Terris M. K., Zumsteg Z. S., Butler S., Osborne J. R., Morgan T. M., Mehra R., Salami S. S., Kishan A. U., Wang C., Schaeffer E. M., , . . . Spratt D. E. (2019). Association of Black race with prostate cancer-specific and other-cause mortality. JAMA Oncology, 5(7), 975–983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freedland S. J., Kane C. J., Amling C. L., Aronson W. J., Presti J. C., Jr, Terris M. K., Group S. D. S. (2006). Delay of radical prostatectomy and risk of biochemical progression in men with low risk prostate cancer. The Journal of Urology, 175(4), 1298–1303. [DOI] [PubMed] [Google Scholar]
- Gilligan T. (2005). Social disparities and prostate cancer: Mapping the gaps in our knowledge. Cancer Causes and Control, 16(1), 45–53. doi: 10.1007/s10552-004-1291-x [DOI] [PubMed] [Google Scholar]
- Ginsburg K. B., Curtis G. L., Timar R. E., George A. K., Cher M. L. (2020). Delayed radical prostatectomy is not associated with adverse oncologic outcomes: Implications for men experiencing surgical delay due to the COVID-19 pandemic. Journal of Urology, 204(4), 720–725. doi: 10.1097/JU.0000000000001089 [DOI] [PubMed] [Google Scholar]
- Gordon B.-B. E., Basak R., Carpenter W. R., Usinger D., Godley P. A., Chen R. C. (2019). Factors influencing prostate cancer treatment decisions for African American and white men. Cancer, 125(10), 1693–1700. doi: 10.1002/cncr.31932 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graefen M., Walz J., Chun K.-H. F., Schlomm T., Haese A., Huland H. (2005). Reasonable delay of surgical treatment in men with localized prostate cancer–impact on prognosis? European Urology, 47(6), 756–760. doi: 10.1016/j.eururo.2005.02.004 [DOI] [PubMed] [Google Scholar]
- Howlader N., Noone A., Krapcho M., Neyman N., Aminou R., Altekruse S., Kosary C. L., Ruhl J., Tatalovich Z., Cho H. (2012). SEER cancer statistics review, 1975–2009 (vintage 2009 populations). National Cancer Institute. [Google Scholar]
- Kan C. K., Qureshi M. M., Gupta A., Agarwal A., Gignac G. A., Bloch B. N., Thoreson N., Hirsch A. E. (2018). Risk factors involved in treatment delays and differences in treatment type for patients with prostate cancer by risk category in an academic safety net hospital. Advances in Radiation Oncology, 3(2), 181–189. 10.1016/j.adro.2017.12.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kinlock B. L., Thorpe R. J., Howard D. L., Bowie J. V., Ross L. E., Fakunle D. O., Laveist T. A. (2016). Racial disparity in time between first diagnosis and initial treatment of prostate cancer. Cancer Control, 23(1), 47–51. doi: 10.1177/107327481602300108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korets R., Seager C. M., Pitman M. S., Hruby G. W., Benson M. C., McKiernan J. M. (2012). Effect of delaying surgery on radical prostatectomy outcomes: A contemporary analysis. BJU International, 110(2), 211–216. doi: 10.1111/j.1464-410X.2011.10666.x [DOI] [PubMed] [Google Scholar]
- Krimphove M. J., Fletcher S. A., Cole A. P., Berg S., Sun M., Lipsitz S. R., Mahal B. A., Nguyen P. L., Choueiri T. K., Kibel A. S., Kluth L. A., Weissman J. S., Trinh Q. D. (2019). Quality of care in the treatment of localized intermediate and high risk prostate cancer at minority serving hospitals. Journal of Urology, 201(4), 735–741. doi: 10.1016/j.juro.2018.10.024 [DOI] [PubMed] [Google Scholar]
- McDonald A. C., Wasserman E., Lengerich E. J., Raman J. D., Geyer N. R., Hohl R. J., Wang M. (2020). Prostate cancer incidence and aggressiveness in Appalachia versus non-Appalachia populations in Pennsylvania by urban-rural regions, 2004–2014. Cancer Epidemiology, Biomarkers and Prevention, 29(7), 1365–1373. doi: 10.1158/1055-9965.Epi-19-1232 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Myint Z., Huang B., Chen Q., Wang P. (2018). Epidemiology and survival disparities of prostate cancer in Appalachian Kentucky. Journal of Clinical Oncology, 36(6_suppl), 292. doi: 10.1200/JCO.2018.36.6_suppl.292 [DOI] [Google Scholar]
- Myint Z. W., O’Neal R., Chen Q., Huang B., Vanderpool R., Wang P. (2019). Disparities in prostate cancer survival in Appalachian Kentucky: A population-based study. Rural and Remote Health, 19(2), 4989. doi: 10.22605/RRH4989 [DOI] [PubMed] [Google Scholar]
- National Cancer Institute Surveillance Epidemiology and End Result Program. (2020). National cancer institute surveillance epidemiology and end result. https://seer.cancer.gov/archive/csr/1975_2011/browse_csr.php?sectionSEL=1&pageSEL=sect_01_zfig.01.html
- Nguyen P. L., Whittington R., Koo S., Schultz D., Cote K. B., Loffredo M., McMahon E., Renshaw A. A., Tomaszewski J. E., D’Amico A. V. (2005). The impact of a delay in initiating radiation therapy on prostate-specific antigen outcome for patients with clinically localized prostate carcinoma. Cancer: Interdisciplinary International Journal of the American Cancer Society, 103(10), 2053–2059. [DOI] [PubMed] [Google Scholar]
- Noonan A. S., Velasco-Mondragon H. E., Wagner F. A. (2016). Improving the health of African Americans in the USA: An overdue opportunity for social justice. Public Health Reviews, 37(1), 12. doi: 10.1186/s40985-016-0025-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nylund K. L., Asparouhov T., Muthén B. O. (2007). Deciding on the number of classes in latent class analysis and growth mixture modeling: A Monte Carlo simulation study. Structural Equation Modeling: A Multidisciplinary Journal, 14(4), 535–569. [Google Scholar]
- O’Brien D., Loeb S., Carvalhal G. F., McGuire B. B., Kan D., Hofer M. D., Casey J. T., Helfand B. T., Catalona W. J. (2011). Delay of surgery in men with low risk prostate cancer. The Journal of Urology, 185(6), 2143–2147. [DOI] [PubMed] [Google Scholar]
- Pollard K., Jacobsen L. A., & Population Reference Bureau. (2020). The Appalachian region: A data overview from the 2014–2018 American Community Survey. https://www.arc.gov/report/the-appalachian-region-a-data-overview-from-the-2014-2018-american-community-survey/
- Reichard C. A., Nyame Y. A., Sundi D., Tosoian J., Wilkins L., Alam R., Achim M. F., Wang X., Stephenson A. J., Klein E. A., Ross A. E., Davis J. W., Chapin B. F. (2019). Does time from diagnosis to treatment of high- or very-high-risk prostate cancer affect outcome? BJU International, 124(2), 282–289. doi: 10.1111/bju.14671 [DOI] [PubMed] [Google Scholar]
- Schmid M., Meyer C. P., Reznor G., Choueiri T. K., Hanske J., Sammon J. D., Abdollah F., Chun F. K. H., Kibel A. S., Tucker-Seeley R. D., Kantoff P. W., Lipsitz S. R., Menon M., Nguyen P. L., Trinh Q. D. (2016). Racial differences in the surgical care of medicare beneficiaries with localized prostate cancer. JAMA Oncol, 2(1), 85–93. doi: 10.1001/jamaoncol.2015.3384 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siegel R. L., Miller K. D., Jemal A. (2020). Cancer statistics, 2020. CA: A Cancer Journal for Clinicians, 70(1), 7–30. doi: 10.3322/caac.21590 [DOI] [PubMed] [Google Scholar]
- Stokes W. A., Hendrix L. H., Royce T. J., Allen I. M., Godley P. A., Wang A. Z., Chen R. C. (2013). Racial differences in time from prostate cancer diagnosis to treatment initiation: A population-based study. Cancer, 119(13), 2486–2493. doi: 10.1002/cncr.27975; 10.1002/cncr.27975 [DOI] [PubMed] [Google Scholar]
- US Preventive Services Task Force. (2018). Prostate cancer screening final recommendation. [Google Scholar]
- Yang W., Lin P., Cheng D., Zhang L., Wu Y., Liu Y., Pei X., Zhou F. (2017). Contribution of charges in polyvinyl alcohol networks to marine antifouling. ACS Applied Materials & Interfaces, 9(21), 18295–18304. doi: 10.1021/acsami.7b04079 [DOI] [PubMed] [Google Scholar]
- Yao N., Alcalá H. E., Anderson R., Balkrishnan R. (2017). Cancer disparities in rural Appalachia: Incidence, early detection, and survivorship. The Journal of Rural Health, 33(4), 375–381. doi: 10.1111/jrh.12213 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, sj-pdf-1-jmh-10.1177_1557988320984282 for Identifying Risk Profiles of Malignant Prostate Cancer Surgical Delay Using a Person-Centered Approach to Understand Prostate Cancer Disparities: The Constellation of Health Determinants Using Latent Class Analysis on Cancer Registry Data by Francisco A. Montiel Ishino, Claire Rowan, Rina Das, Janani Thapa, Ewan Cobran, Martin Whiteside and Faustine Williams in American Journal of Men's Health