Abstract
The coupling of transition-metal to photoredox catalytic cycles through single-electron transfer steps has become a powerful tool in the development of catalytic processes. In this work, we demonstrated that transition-metal catalysis can be coupled to alternating current (AC) through electron transfer steps that occur periodically at the same electrode. AC-assisted Ni-catalyzed amination, etherification, and esterification of aromatic bromides showed higher yields and selectivity compared to that observed in the control experiments with direct current. Our mechanistic studies suggested the importance of both reduction and oxidation processes in the maintenance of the AC-assisted catalytic reactions. As described in presented examples, the AC assistance should be well-suited for catalytic cycles involving reductive elimination or oxidative addition as a limiting step.
Introduction1
Ni-based catalysis has attracted much attention as an effective tool for forming the C(aryl)–heteroatom bond,2 which is frequently found in drug-like molecules, dyes, and conjugated polymers. However, Ni-catalytic systems are often inferior to more prevalent but expensive Pd-catalytic systems. Unfavorable reductive elimination from Ni(II) species and the instability of the Ni(I) species are among the factors causing this inferiority. Nevertheless, Ni catalysis is susceptible to facilitation by energy inputs from light or electricity because of the availability of redox states of nickel ranging from 0 to +4 (Figure 1A,B).
Figure 1.
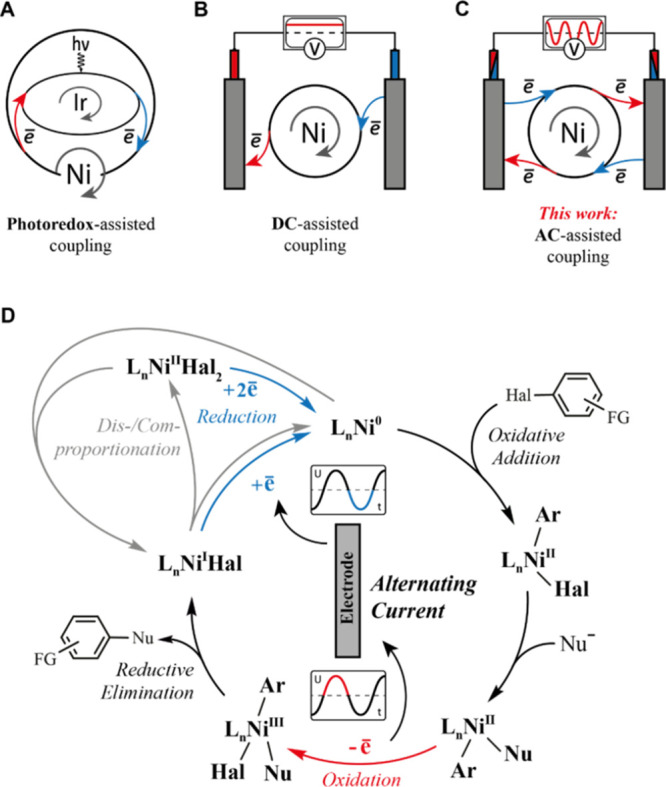
Some methods for activating Ni-based catalytic cycles by sequential oxidation and reduction: (A) photoredox-assisted; (B) DC-assisted; and (C) AC-assisted couplings. (D) Working hypothesis of the mechanism underlying the coupling of AC to the nickel catalytic cycle for cross-coupling reactions.
By merging photoredox and nickel catalysis, MacMillan and others enabled previously elusive Ni-catalyzed C–C, C–N, and C–O coupling reactions under batch and flow conditions.3 In most situations, the coupling between photoredox and nickel catalysis involves single-electron oxidation and reduction of nickel catalytic species by a photoredox catalyst (Figure 1A).4 Thus, oxidation to Ni(III) species enables reductive elimination, whereas reduction to Ni(0) species accelerates oxidative addition. Later, Baran’s group, regarding the example of C–N coupling, showed that the photocatalytic approach might be successfully replaced with assistance by direct current (DC) electrolysis.5 Here, nickel species are oxidized on one electrode, enabling reductive elimination, and reduced on another electrode, accelerating oxidative addition (Figure 1B); the current-assisted catalytic cycle involving nickel species in oxidation states +1, +2, and +3 was suggested.6 This approach was effective for the C–S,7 C–P,8 Heck,9 and C–O coupling reactions.10
Although some disagreements exist regarding key reaction intermediates,6,11 mechanistic studies of both electrochemically and photochemically enabled nickel cross-coupling suggest that both oxidation and reduction processes are essential for maintaining the sustainable catalytic pathway.3a,3d,4a,4b These mechanistic considerations led us to the idea of merging alternating current (AC) with Ni-catalyzed cross-coupling reactions (Figure 1C). AC authentically fits the idea of a catalytic cycle assisted by a pair of redox processes. Because of the periodical polarity switch, both redox steps can successfully occur at the same electrode with a tunable delay between them (Figure 1D). In this work, we performed AC-assisted Ni-catalyzed amination, etherification, and esterification of aromatic bromides. For all reactions, AC displayed advantages over DC in our electrochemical cell.
Currently, AC has a significantly more modest application in organic synthesis compared with DC—generally, it is used for preventing electrode fouling. Notable works showed that AC can be successfully applied in the electrosynthesis of phenol,12 trifluoromethylated arenes,13 or in accelerating S–S bond metathesis through reversible redox steps.14
Results and Discussion
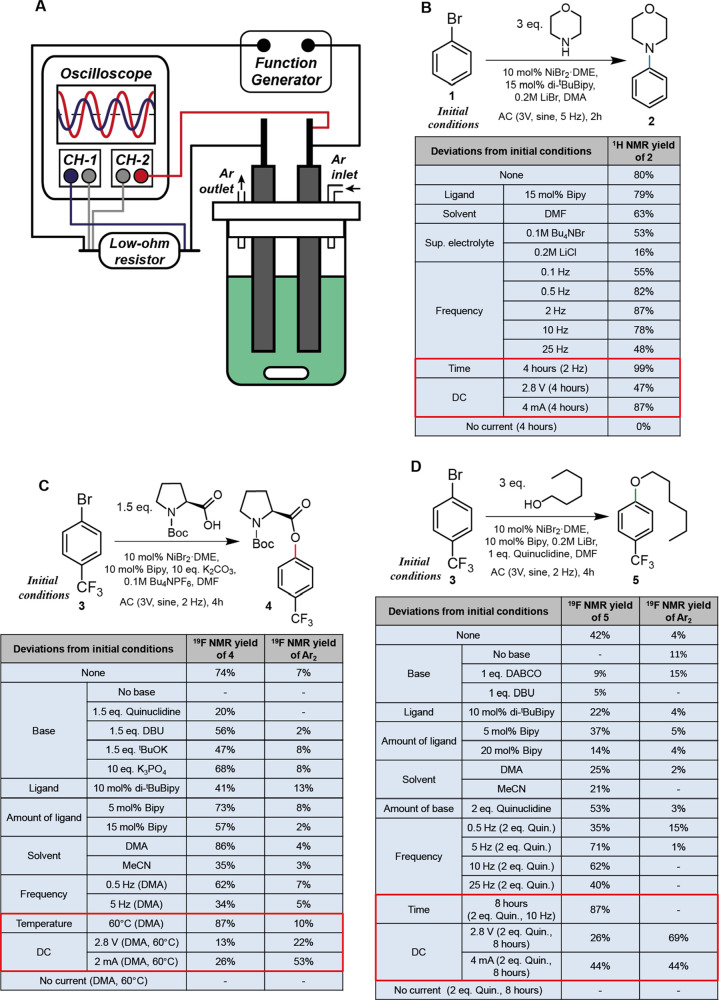
We performed reactions in an electrochemical cell equipped with two glassy carbon (GC) rod electrodes. A commercial waveform generator supplied sinusoidal voltage to the electrodes; we used an oscilloscope to measure both the voltage and current in our experiments (Figures 2A and S3). The combinations of NiBr2·DME (DME—dimethoxyethane) with 2,2′-dipyridyl (Bipy) or 4,4′-Di-tert-butyl-2,2′-dipyridyl (di-tBuBipy) were chosen as catalysts because they were successfully employed in various catalytic systems.3d,6
Figure 2.
(A) Experimental setup for the AC-assisted cross-coupling reactions. (B–D) Optimization of conditions for AC-assisted amination, esterification, and etherification reactions, respectively. The comparison of the results of the experiments with AC assistance (using optimized conditions) and DC-assistance (using potentiostatic and galvanostatic conditions) is given in red boxes.
To perform the catalytic cycle based on both reduction and oxidation of the catalyst, the voltage should be sufficient to perform the reduction on one of the electrodes, whereas the oxidation occurs on another electrode. We proposed that reduction of [Ni(Bipy)x]2+ to [Ni(Bipy)x]0 [for x = 3, −1.25 V vs saturated calomel electrode (SCE) in CH3CN] is most likely to be a process that would favor oxidative addition.15 Similarly, oxidation of [NiL(Ar)(Nu)] to [NiL(Ar)(Nu)]+ (L—ligand, Ar—aryl, Nu—nucleophile) would favor the reductive elimination of Ar–Nu.16 Considering that the potential for oxidation of [Ni(C6H3(CF3)2)(OCH2CF3)] to [Ni(C6H3(CF3)2)(OCH2CF3)]+ is 0.83 V versus SCE in CH3CN, which is at the high end of the expected oxidation potentials for the [NiL(Ar)(Nu)] species,3d a voltage higher than ∼2.1 V is required to simultaneously perform the desired oxidation and reduction of nickel species. We found a peak voltage of 3 V to be effective in most of our experiments; in such conditions the peak value of current varied from 10 to 20 mA in different experiments.
In the initial screening, we aimed to optimize the conditions for amination, etherification, and esterification of aryl bromides (Figure 2B–D and Tables S1–S3). We used the reaction between bromobenzene (1) (50 mM) and morpholine (150 mM) for optimization of the amination conditions because 1 being a less reactive substrate than 4-bromobenzotrifluoride (3) offered more room for the optimization (Figure 2B). Different combinations of ligands (di-tBuBipy and Bipy) and solvents [dimethylformamide (DMF) and dimethylacetamide (DMA)] worked, but combining DMA with di-tBuBipy provided better stability to the nickel complex in the solution and more reproducible results than the other systems did. The frequency of the applied voltage is a unique parameter for AC-assisted catalysis. Interestingly, the dependency of yield on frequency has a maximum of around 2 Hz (87%). The increase in frequency to 25 Hz caused a steep drop in yield, most likely because of the increased contribution of charging and discharging of the electrical double layer (see Figure S6 for quantitative analysis) and of nonproductive reversible Ni(II)/Ni(I) and Ni(II)/Ni(0) oxidation/reduction cycles to the current at high frequencies. At the same time, the decrease in frequency as well as the use of DC also led to diminished yields. We can speculate at this point that in case of the use of AC assistance, some optimum “resonance” frequency exists which is defined by the complex combination of the parameters such as rates of chemical and electron-transfer steps, rate of diffusion of the catalytic and non-catalytic species from and to the surface of the electrodes, and stirring rate. However, we expect that under specific conditions, the value of “resonance” frequency may provide valuable data related to the kinetics of the discussed reactions.
In contrast to amination, the base is an essential component in esterification (Figure 2C). We achieved the best results using a suspension of potassium carbonate unlike in photo-activated esterification;17 organic bases were found to be less effective. The increase of the ligand concentration or the use of more sterically hindered ligands than bipyridine resulted in diminished yields. The choice of NBu4PF6 as a supporting electrolyte was instrumental for getting good yields of esters; we suggest two possible reasons for that. First, carboxylate is a relatively weak ligand; thus, eliminating the competition of carboxylate with bromide at the nickel center increases the efficiency of a catalytic cycle. Second, carboxylate is a more electronegative group than amine or alcoholate; thus, the oxidation potential of the [NiL(Ar)(RCOO)] species (e.g., 0.9 V vs SCE for [Ni(C6H3(CF3)2)(OAc)]) is expected to be higher than that of [NiL(Ar)(OR)] and the [NiL(Ar)(NHR2)] species.3d,16 Avoiding excessive anodic oxidation of Br– allows one to achieve higher oxidation potentials in experiments with NBu4PF6 than with LiBr (Figure S19), which might be necessary for the oxidation of the [NiL(Ar)(RCOO)] species. The optimum frequency for the esterification reaction was 2 Hz. The control DC experiments at potentiostatic (2.8 V) and galvanostatic (2, 4, and 6 mA) conditions demonstrated low yields (13–26%) and high amounts (22–53%) of the biaryl side product (see Table S2).
As esterification, etherification also requires the addition of a base (Figure 2D); we tested 1,8-diazabicyclo[5.4.0]undec-7-ene (DBU) and 1,4-diazabicyclo[2.2.2]octane (DABCO) as bases; however, the best results were achieved with quinuclidine, analogously to photoredox-assisted etherification.3d Interestingly, the use of non-cyclic tertiary amines led to the formation of the homocoupling product in considerable amounts (Table S3)—presumably, they serve as sacrificial reductants in this case. Comparing the results of AC- and DC-assisted (both in the potentiostatic and galvanostatic modes) etherification further highlights that AC assistance demonstrates higher selectivity toward the formation of cross-coupling products versus homocoupling products than DC assistance. Moreover, the decrease in the amount of the biaryl product occurs gradually with an increase in the frequency. Two hypotheses could explain these observations: (i) the shorter lifetime of [NiL(Ar)(Nu)] species in the AC than in the DC experiments and (ii) fewer chances for the second oxidative addition in the AC than in the DC experiments. With a short lifetime, [NiL(Ar)(Nu)] does not have time to disproportionate appreciably to the [NiL(Ar)2] intermediate with two aryl groups at one nickel center, which affords diaryl coupling products by reductive elimination.18 In the DC experiments, a continuous strongly reducing environment near the cathode increases chances that the oxidative addition product [NiL(Ar)Br] will undergo reduction to Ni(I) species and subsequently a second oxidative addition, resulting in [NiL(Ar)2Br] intermediates that eliminate diaryl products.18,19
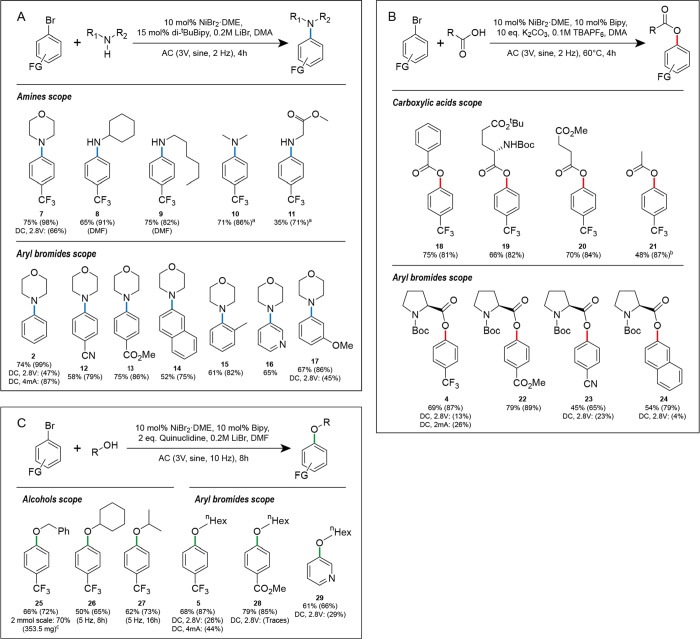
Next, we studied the substrate scope of the discussed coupling reactions (Figure 3). Amination appears to be the most robust reaction in relation to the activity of the substrates—couplings with non-activated (1), sterically hindered aryl halides (o-bromotoluene), and halides bearing electron-donor groups (m-bromoanisole) exhibited good yields (61–74%) (Figure 3A). The amine scope is not limited only to secondary and primary amines—the hydrochlorides of amines successfully reacted in the presence of the suspension of K2CO3.
Figure 3.
Scope of AC-assisted nickel-catalyzed amination (A), esterification (B), and etherification (C). Isolated yields are shown. NMR yields are shown in parentheses. DC-assisted control experiments were performed under potentiostatic or galvanostatic conditions. For details of the experimental protocols, see the Supporting Information. Boc—tert-butyloxycarbonyl. aA mixture of the corresponding amine hydrochloride (3 equiv) and K2CO3 (5 equiv) was used instead of amine. bSodium acetate (5 equiv) was used instead of a mixture of carboxylic acid and K2CO3. c2 mmol scale reaction was performed using a setup with four GC rod electrodes (see the Experimental Section and Supporting Information for details).
Esterification of 3 resulted in 48–75% yields for aliphatic-, aromatic-, and Boc (tert-butyloxycarbonyl)-protected amino acids (Figure 3B). Other electron-deficient aryl bromides (i.e., 4-bromobenzonitrile and methyl 4-bromobenzoate) and 2-bromonaphthalene also reacted smoothly with Boc-l-proline with 45–79% yields. As expected, the highest yields (66–79%) in etherification were achieved in reactions with electron-deficient aryl bromides and primary alcohols (Figure 3C). The reactions with secondary alcohols—cyclohexanol and isopropanol—gave 50 and 62% yield, respectively, but required a 6-fold instead of a 3-fold excess of alcohol and a prolonged reaction time. Larger-scale etherification (2 mmol) showed 70% (353.5 mg) of yield of 25, demonstrating the scalability of AC-assisted catalytic reactions.
It is worth mentioning that additional control DC-assisted experiments of amination, esterification, and etherification, performed using the potentiostatic mode(for substrates 7, 17, 23, 24, 28, and 29), demonstrated lower NMR yields and higher quantities of side products compared to the results achieved in identical AC-assisted reactions (Figure 3 and Experimental Section). These observations highlight the crucial impact of AC assistance on the efficiency of the discussed reactions.
Overall, the advantage of AC over DC assistance is the most pronounced for esterification and etherification and, to a less extent, for amination. This observation is in agreement with the literature data: while a very broad scope was demonstrated for DC-assisted amination,5,6 only a humble scope with 32–43% yields was shown for etherification.6 DC-assisted esterification was enabled only using a specific microfluidic flow cell with a narrow (≤0.5 mm) gap between the electrodes.10 This close distance between the electrodes allowed researchers to perform reactions that involve short-living intermediates existing between reduction and oxidation steps. AC provides an alternative way to conduct reactions that require a short interval between reduction and oxidation.
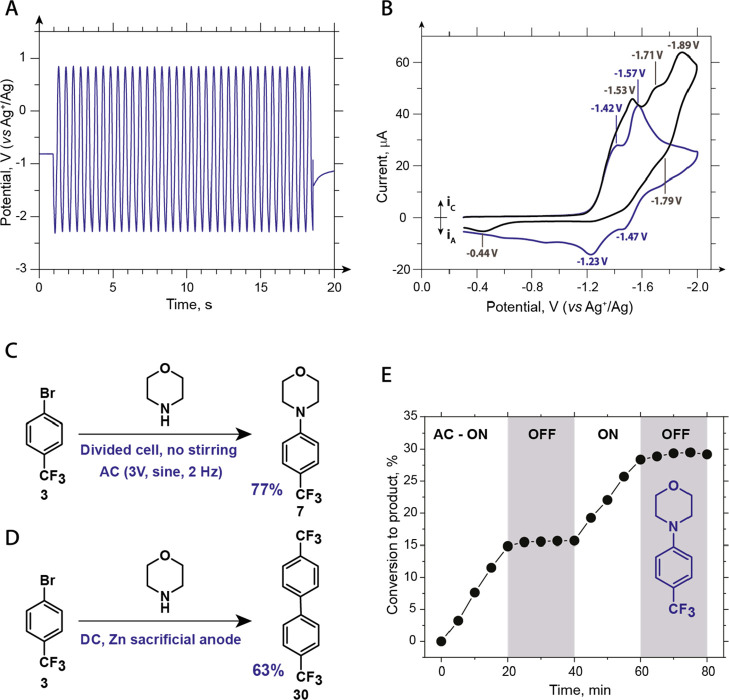
To better understand the basic mechanistic features of AC-assisted nickel-catalyzed cross-coupling, we performed electrochemical studies of the discussed reactions. First, we measured the potentials of the electrodes (GC rods), relatively standard nonaqueous silver electrode (Ag+/Ag), in typical amination, etherification, and esterification experiments. As Figure 4A shows, the potential changes from 0.8 to −2.2 V (vs Ag+/Ag) in the amination reaction. This interval remains the same for the etherification (Figure S20); however, for esterification, it is shifted to slightly higher potentials because of the absence of Br– in the electrolyte (Figure S21). To better understand what electrochemical processes occur within these potentials in the reactions under investigation, we performed cyclic voltammetry (CV) studies (Figure 4B and Supporting Information). The voltammogram of Ni(DME)Br2 (2 mM) and Bipy (2 mM) showed two partially separated quasi-reversible reduction waves with peaks at −1.42 and −1.57 V (Figure 4B). The coulometric studies20 and the combination of the voltammograms for [Ni(Bipy)3]2+ and Ni(Bipy)Br218,20b,21 strongly indicate that these waves represent the sequential reduction of Ni(II) to Ni(0) through a Ni(I) intermediate. When 3 (2 mM) was added to a solution of Ni(Bipy)Br2, the original reduction waves could still be detected, but they became less resolved and fully irreversible; two new reduction waves appeared with peak potentials of −1.71 and −1.89 V. We hypothesized that oxidative addition occurs after the reduction of nickel to a zero oxidation state, making the reduction waves irreversible, and that these additional waves (−1.71 and −1.89 V) correspond to the sequential reduction of the [NiL(Ar)Br] species.22 Nevertheless, the voltammetry under conditions as close as possible to the synthetic experiments (GC rods as working and counter electrodes, a scan rate of 24 V/s, and the concentrations of reagents as in synthesis) indicated that in synthetic experiments, the reduction of the [NiL(Ar)Br] species might be less significant than in analytical CV because of the higher surface area of the electrode, the higher concentration of nickel ions, and the higher scan rate (Figures S8, S12, and S16). These data imply that NiL(Ar)Br is the most probable species to undergo further ligand exchange and oxidation to afford the product of cross-coupling (Figure 1D). The anodic area of the CV curves of the amination and etherification reaction mixtures is dominated by the oxidation of Br– (Figure S18); therefore, Br3– and Br2 are probably mediators of the electrochemical oxidation of nickel species in these experiments.
Figure 4.
Mechanistic studies of the cross-coupling reactions. (A) GC electrode potential versus Ag+/Ag in a typical amination experiment [3 (50 mM), morpholine (150 mM), NiBr2·DME (5 mM), and di-tBuBipy (7.5 mM) in LiBr (0.2M) DMA solution]. (B) CV curves of a NiBr2·DME (2mM) and a Bipy (2mM) solution before (blue) and after (gray) the addition of 3 (2mM) (0.1M TBAPF6, DMA). (C) Amination in the divided cell without stirring. The concentrations of the reagents are identical to A. (D) Control experiment of amination with the DC and Zn sacrificial anode. The concentrations of the reagents are identical to A. (E) Kinetics of the amination reaction between 3 (50 mM) and morpholine (150 mM), NiBr2·DME (5 mM), and Bipy (7.5 mM) in LiBr (0.2M) DMA solution) with ON/OFF cycles of AC. Conversion of 3 to 7 was determined by HPLC. See the Supporting Information for details.
To confirm that processes at one electrode are sufficient to perform the reactions, we conducted the AC-assisted reaction between 3 and morpholine in a divided cell without stirring (Figure 4C). This experiment resulted in a 77% yield, confirming that the transfer of intermediates between electrodes is unnecessary for this reaction to proceed. To probe the possibility that only the reduction phase of the AC cycle is essential for the coupling, we performed DC amination and etherification of 3 with a Zn sacrificial anode and set the potential of the GC cathode equal to the peak negative potential in AC experiments (−2.2 V vs Ag+/Ag) (Figures 4D and S23). Both experiments resulted in the formation of the biaryl product (30) with 60–70% yields and only minor quantities (2–8%) of the coupling products 7 and 5, thus indicating the essential role of the oxidation phase of the AC cycle in forming the coupling products. To probe the role of self-sustainable catalysis by Ni(I) species in bulk solution (Figure 5A),11 we performed kinetic experiments with the ON/OFF cycles of AC (Figures 4E, S24 and S25). The kinetics of the amination reaction between 3 and morpholine as well as the data for similar esterification and etherification experiments indicate that the reactions stop almost immediately when AC is OFF and start again when AC is ON, demonstrating that the contribution of the long-living (minutes timescale) self-sustainable Ni(III)/Ni(I) cycles to the formation of the products (7, 5, and 18) is insignificant; short-living Ni(III)/Ni(I) cycles that require constant regeneration of Ni(I) remain possible.
Figure 5.
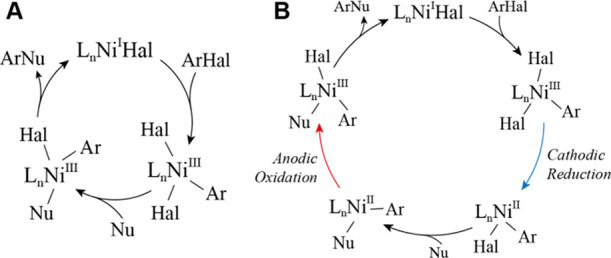
Possible catalytic cycles involving oxidative addition to Ni(I) species. (A) Self-sustainable Ni(III)/Ni(I) cycle.11 (B) Current-assisted Ni(III)/Ni(I) cycle.6
Overall, our mechanistic studies are consistent with the proposal shown in Figure 1D. Likely, the catalytic cycle is initiated by the reduction of Ni(II), which is abundant in the solution, to the Ni(0) species, which undergo oxidative addition. During the low-voltage phase, when neither oxidation nor reduction processes are expected, the oxidative addition product undergoes ligand exchange. The further oxidation of [NiL(Ar)Nu] species to a Ni(III) state favors reductive elimination, which would furnish the desired coupling product and release Ni(I) species. There are at least two ways by which Ni(I) could be reduced to Ni(0): (i) direct reduction at the electrode and (ii) reversible disproportionation to Ni(II) and Ni(0) with the subsequent reduction of Ni(II) to Ni(0) at the electrode. At the same time, current-assisted catalytic cycles involving oxidative addition to Ni(I) species (Figure 5B)6 may coexist with the proposed cycle (Figure 1D).
Conclusions
To summarize, we demonstrated the coupling of AC to transition-metal catalysis using three examples of related nickel-catalyzed coupling reactions, but the idea of enabling new catalytic cycles by periodically oxidizing and reducing catalytic intermediates by AC could be applied to various catalytic systems, especially to ones that involve oxidative addition or reductive elimination as a rate-limiting step. The use of AC provides two important advantages: (i) the absence of the need to transfer reactive intermediates between electrodes, which prevents their dilution and allows working with short-living intermediates; (ii) the frequency and the waveform of AC are easily tunable experimental parameters that can be used to achieve the selectivity of reactions. Moreover, side electrochemical reactions might be tolerated in experiments with AC if they are fully reversible. We hope that further development of synthetic methods based on the coupling of transition-metal catalysis to AC will enable new efficient transformations.
In addition, this work demonstrates well how external oscillations (not necessarily electrochemical ones) could couple to catalytic cycles to perform otherwise unfavorable chemical transformations. The oscillator pumps energy into the catalytic cycle by periodically modifying its intermediates. This point of view might be relevant for development of catalytic processes enabled by various oscillatory fields.
Experimental Section
Materials and Methods
All reactions were performed under an argon atmosphere according to the methods indicated in the general procedures. Anhydrous solvents (Extra Dry over Molecular Sieve, AcroSeal)—DMF, DMA, and acetonitrile (MeCN)—were purchased from Acros Organics and stored under a positive pressure of argon. CDCl3 was purchased from Cambridge Isotope Laboratories. All other commercially available chemicals and reagents were used as received from Sigma-Aldrich, Alfa Aesar, Acros Organics, and Fisher chemicals unless otherwise noted. Flash column chromatography was performed using Merck silica gel (60 mesh, particle size 0.043–0.063 mm).
GC rods (100 mm × 6 mm diameter) were purchased from Alfa Aesar. Platinum wire (1 mm diameter) was purchased from Holland-Moran. Ltd. (Israel). A GC disk (1 mm diameter) working electrode, a platinum wire counter-electrode, and an Ag+/Ag reference electrode were purchased from CH Instruments, Inc.
NMR spectra were measured on a Bruker AVANCE III-300 spectrometer at 300 MHz for 1H and 282.4 MHz for 19F, on a Bruker AVANCE III-400 spectrometer at 400 MHz for 1H and 100.6 MHz for 13C{1H}, and on a Bruker AVANCE III HD-500 spectrometer at 500 MHz for 1H, 125.8 MHz for 13C{1H}, and 470.6 MHz for 19F. Chemical shifts for 1H and 13C are given in ppm relative to tetramethylsilane and those for 19F relative to CFCl3. 1H and 13C spectra were calibrated using a residual solvent peak as an internal reference (1H NMR: δ = 7.26 ppm, 13C NMR: δ = 77.16 ppm). The following abbreviations were used to explain NMR peak multiplicities: s = singlet, d = doublet, t = triplet, q = quartet, p = pentet, m = multiplet, and br = broad.
A B&K Precision 4053B dual channel function/arbitrary waveform generator served as a tunable source for the AC. The measurements and tracking of voltage drops over the electrochemical cell and the resistor were performed using a Global Specialties DSC-5300 50 MHz Digital Storage Oscilloscope. DC electrolysis, CV, and open-circuit potential measurements were performed using a CH Instruments 600E potentiostat/galvanostat.
High-resolution mass spectra were recorded on a Waters Xevo G2-XS QTof mass spectrometer (Manchester, UK) with an electrospray ionization (ESI) source.
Analytical chromatographic separation was performed on a Waters Acquity liquid chromatography system equipped with a 2998 PDA Detector and a Waters QDa mass detector (a mass range of 85–1250 m/z) with ESI.
Synthesis of Compounds
General Procedure for Amination Experiments
With Amines as Substrates
To an Ar-flushed mixture containing NiBr2·DME (6.2 mg, 0.02 mmol, and 0.1 equiv), di-tBuBipy (8.1 mg, 0.03 mmol, and 0.15 equiv), and LiBr (69.5 mg) in a screw-capped vial, 4 mL of DMA (unless otherwise noted) was added. The solution was stirred until the reagents were completely dissolved, and then amine(0.6 mmol, 3 equiv) was added. Next, the mixture was transferred to a glass cell as much as possible with the addition of aryl bromide (0.2 mmol, 1 equiv). The Teflon cap equipped with GC rod electrodes was placed on top of the cell; then the cap was sealed tightly with parafilm; the argon inlet and outlet (optional) were inserted into the corresponding holes in the cap. Thereafter, an argon inlet was immersed into the solution, and argon was bubbled through the solution for at least 5 min with moderate stirring. Then, the Ar inlet was set above the solution, and the position of the electrodes was adjusted to be as deep in the solution as possible (ca. 1 cm). The electric circuit was assembled, as described in the “Setup preparation” section of the Supporting Information. The electrolysis was conducted for 4 h with the following parameters of AC: sine waveform, 2 Hz frequency, 3 V peak voltage, and a stirring rate of 1400 rpm.
After the electrolysis, 0.5 mL of the solution was transferred to a screw-capped vial, mixed with a small amount of Na2H2EDTA (EDTA—ethylenediaminetetraacetate) water solution (for Ni complexation), diluted with an aqueous solution of K2CO3 (0.1 M), and extracted with a pentane/Et2O (1/1 ratio) mixture. The extract was dried over Na2SO4, and the solvent was evaporated carefully under vacuum (since some of the aryl bromides and products are quite volatile, soft conditions are required). Then, the residue was used to determine the NMR yield of the experiment. The remaining 3.5 mL of the reaction mixture was diluted with an aqueous solution of K2CO3 (0.1 M) and extracted four times with a pentane/Et2O (1/1 ratio) mixture. The combined organic fractions were washed with a small amount of 0.1 M NaCl water solution and dried over Na2SO4. Next, the solution was dried carefully under vacuum, and the residue was dissolved in a minimum amount of hexane (with the addition of dichloromethane if needed) and subjected to column chromatography on SiO2 to afford the desired product. The isolated yield in each case was calculated while considering the use of only 3.5 mL of solution for the isolation.
With Amine Hydrochlorides as Substrates
To an Ar-flushed mixture containing NiBr2·DME (6.2 mg, 0.02 mmol, 0.1 equiv), di-tBuBipy (8.1 mg, 0.03 mmol, 0.15 equiv), amine hydrochloride (0.6 mmol, 3 equiv), and LiBr (69.5 mg) in a screw-capped vial, 4 mL of DMA (unless otherwise noted) was added. The solution was stirred until the reagents were completely dissolved; the mixture was transferred to a glass cell as much as possible, with the addition of aryl bromide (0.2 mmol, 1 equiv) and K2CO3 (138 mg, 1 mmol, 5 equiv). The following procedure is identical to the one described above.
General Procedure for Esterification Experiments
To an Ar-flushed mixture containing NiBr2·DME (6.2 mg, 0.02 mmol, and 0.1 equiv), Bipy (3.1 mg, 0.02 mmol, and 0.1 equiv), carboxylic acid (0.3 mmol, 1.5 equiv) (unless otherwise noted), and Bu4NPF6 (155 mg) in a screw-capped vial, 4 mL of DMA was added. The solution was stirred until the reagents were completely dissolved; thereafter, the mixture was transferred to a glass cell as much as possible with the addition of aryl bromide (0.2 mmol, 1 equiv) and K2CO3 (276 mg, 10 equiv) (unless otherwise noted). The Teflon cap equipped with GC rod electrodes was placed on top of the cell; then, the cap was sealed tightly with parafilm; and the argon inlet and outlet (optional) were inserted into the corresponding holes in the cap. The temperature of the hotplate was set to 60 °C. Next, the argon inlet was immersed into the solution, and argon was bubbled through the solution for at least 5 min with moderate stirring. Then, the Ar inlet was set above the solution, and the position of the electrodes was adjusted to be as deep in the solution as possible (ca. 1 cm). The electric circuit was assembled, as described in the “Setup preparation” section of the Supporting Information. The electrolysis was conducted for 4 h (unless otherwise noted) with the following parameters of AC: sine waveform, 2 Hz frequency, 3 V peak voltage, and a stirring rate of 200 rpm.
After the electrolysis, 0.5 mL of the solution was transferred to a screw-capped vial, mixed with a small amount of Na2H2EDTA water solution (for Ni complexation), diluted with deionized water (DI) water, and extracted with a pentane/Et2O (1/1 ratio) mixture. The extract was dried over Na2SO4, and the solvent was evaporated carefully under vacuum. Then, the residue was used to determine the NMR yield of the experiment. The remaining 3.5 mL of the reaction mixture was carefully diluted with an aqueous solution of KH2PO4 (1 M) and extracted four times with a pentane/Et2O (1/1 ratio) mixture. The combined organic fractions were washed with a small amount of 0.1 M NaCl water solution and dried over Na2SO4. Next, the solution was dried carefully under vacuum, and the residue was dissolved in a minimum amount of hexane (with the addition of dichloromethane if needed) and subjected to column chromatography on SiO2 to afford the desired product. The isolated yield in each case was calculated while considering the use of only 3.5 mL of solution for the isolation.
General Procedure for Etherification Experiments
To an Ar-flushed mixture containing NiBr2·DME (6.2 mg, 0.02 mmol, and 0.1 equiv), Bipy (3.1 mg, 0.02 mmol, and 0.1 equiv), and LiBr (69.5 mg) in a screw-capped vial, 4 mL of DMF was added. The solution was stirred until the reagents were completely dissolved, and then quinuclidine (44.5 mg, 0.4 mmol, and 2 equiv) and alcohol (0.6 mmol, 3 equiv—unless otherwise noted) were added. Next, the mixture was transferred to a glass cell as much as possible with the addition of aryl bromide (0.2 mmol, 1 equiv). The Teflon cap equipped with GC rod electrodes was placed on top of the cell; then, the cap was sealed tightly with parafilm; and the argon inlet and outlet (optional) were inserted into the corresponding holes in the cap. Thereafter, an argon inlet was immersed into the solution, and argon was bubbled through the solution for at least 5 min with moderate stirring. Then, the Ar inlet was set above the solution, and the position of the electrodes was adjusted to be as deep in the solution as possible (ca. 1 cm). The electric circuit was assembled, as described in the “Setup preparation” section of the Supporting Information. The electrolysis was conducted for 8 h (unless otherwise noted) with the following parameters of AC: a sine waveform, 10 Hz frequency (unless otherwise noted), 3 V peak voltage, and a stirring rate of 200 rpm.
After the electrolysis, 0.5 mL of the solution was transferred to a screw-capped vial, mixed with a small amount of Na2H2EDTA water solution (for Ni complexation), diluted with DI water, and extracted with a pentane/Et2O (1/1 ratio) mixture. The extract was dried over Na2SO4, and the solvent was evaporated carefully under vacuum. Then, the residue was used to determine the NMR yield of the experiment. The remaining 3.5 mL of the reaction mixture was diluted with DI water and extracted four times with a pentane/Et2O (1/1 ratio) mixture. The combined organic fractions were washed with a small amount of 0.1 M NaCl water solution and dried over Na2SO4. Next, the solution was dried carefully under vacuum, and the residue was dissolved in a minimum amount of hexane or pentane and subjected to column chromatography on SiO2 to afford the desired product. The isolated yield in each case was calculated while considering the use of only 3.5 mL of solution for the isolation.
Synthetic Procedures and Compound Characterization Data
Amination
4-(4-(Trifluoromethyl)phenyl)morpholine (Figure 3, 7)
Prepared according to the procedure described in the “General procedure for amination experiments” section with morpholine (52.5 μL, 0.6 mmol, 3 equiv) as amine and 4-bromobenzotrifluoride (28.0 μL, 0.2 mmol, 1 equiv) as aryl bromide. Hexane–dichloromethane mixture with gradient (from pure hexane to 1:1 ratio, respectively) was used as an eluent. NMR yield—98%, isolated yield—75% (30.3 mg, white solid). Control DC-assisted experiment (potentiostatic conditions—2.8 V) demonstrated 66% NMR yield (9% of the diaryl product was also observed). The spectra data matched with values reported in the literature.5 Rf (dichloromethane)—0.59. 1H NMR (500 MHz, CDCl3): δ 7.50 (d, J = 8.0 Hz, 2H), 6.92 (d, J = 8.1 Hz, 2H), 3.87 (m, 4H), 3.24 (m, 4H). 13C{1H} NMR (125.8 MHz, CDCl3): δ 153.5, 126.6 (q, C–F, 3JC–F = 3.8 Hz), 124.8 (q, C–F, 1JC–F = 269.9 Hz), 121.1 (q, C–F, 2JC–F = 33.4 Hz), 114.5, 66.8, 48.3. 19F NMR (282.4 MHz, CDCl3): δ −62.39.
N-Cyclohexyl-4-(trifluoromethyl)aniline (Figure 3, 8)
Prepared according to the procedure described in the “General procedure for amination experiments” section with cyclohexylamine (68.6 μL, 0.6 mmol, 3 equiv) as amine and 4-bromobenzotrifluoride (28.0 μL, 0.2 mmol, 1 equiv) as aryl bromide in DMF. Hexane was used as an eluent. NMR yield—91%, isolated yield—65% (27.7 mg, colorless liquid). The spectra data matched with values reported in the literature.5 Rf (hexane)—0.17. 1H NMR (500 MHz, CDCl3): δ 7.37 (d, J = 8.5 Hz, 2H), 6.57 (d, J = 8.7 Hz, 2H), 3.88 (br, s, 1H), 3.29 (t, J = 9.6, 1H), 2.05 (dd, J = 12.8, 3.2 Hz, 2H), 1.78 (dt, J = 13.6, 3.9 Hz, 2H), 1.69–1.65 (m, 1H), 1.43–1.34 (m, 2H), 1.28–1.14 (m, 3H). 13C{1H} NMR (100.6 MHz, CDCl3): δ 149.9, 126.6 (q, C–F, 3JC–F = 3.9 Hz), 125.2 (q, C–F, 1JC–F = 270.1 Hz), 118.2 (q, C–F, 2JC–F = 32.6 Hz), 112.1, 51.5, 33.3, 25.9, 25.0. 19F NMR (470.6 MHz, CDCl3): δ −61.88.
N-Hexyl-4-(trifluoromethyl)aniline (Figure 3, 9)
Prepared according to the procedure described in the “General procedure for amination experiments” section with 1-hexylamine (79.3 μL, 0.6 mmol, 3 equiv) as amine and 4-bromobenzotrifluoride (28.0 μL, 0.2 mmol, 1 equiv) as aryl bromide in DMF. Hexane was used as an eluent. NMR yield—82%, isolated yield—75% (32.2 mg, colorless liquid). The spectra data matched with values reported in the literature.3a Rf (hexane)—0.17. 1H NMR (500 MHz, CDCl3): δ 7.39 (d, J = 8.5 Hz, 2H), 6.58 (d, J = 8.7 Hz, 2H), 3.94 (br, s, 1H), 3.14 (m, 2H), 1.63 (p, J = 7.4, 2H), 1.43–1.30 (m, 6H), 0.92–0.89 (m, 3H). 13C{1H} NMR (125.8 MHz, CDCl3): δ 151.0, 126.7 (q, C–F, 3JC–F = 3.8 Hz), 125.2 (q, C–F, 1JC–F = 269.9 Hz), 118.5 (q, C–F, 2JC–F = 32.4 Hz), 111.8, 43.7, 31.7, 29.4, 26.9, 22.8, 14.2. 19F NMR (282.4 MHz, CDCl3): δ −61.90.
N,N-Dimethyl-4-(trifluoromethyl)aniline (Figure 3, 10)
Prepared according to the procedure described in the “General procedure for amination experiments” section with dimethylamine hydrochloride (48.9 mg, 0.6 mmol, 3 equiv) as amine hydrochloride and 4-bromobenzotrifluoride (28.0 μL, 0.2 mmol, 1 equiv) as aryl bromide. Hexane was used as an eluent. NMR yield—86%, isolated yield—71% (23.5 mg, white solid). The spectra data matched with values reported in the literature.23 Rf (hexane)—0.24. 1H NMR (500 MHz, CDCl3): δ 7.46 (d, J = 8.8 Hz, 2H), 6.70 (d, J = 8.8 Hz, 2H), 3.01 (s, 6H). 13C{1H} NMR (100.6 MHz, CDCl3): δ 152.4, 126.5 (q, C–F, 3JC–F = 3.9 Hz), 125.3 (q, C–F, 1JC–F = 270.1 Hz), 117.7 (q, C–F, 2JC–F = 32.6 Hz), 111.3, 40.3. 19F NMR (282.4 MHz, CDCl3): δ −61.81.
Methyl (4-(trifluoromethyl)phenyl)glycinate (Figure 3, 11)
Prepared according to the procedure described in the “General procedure for amination experiments” section with glycine methyl ester hydrochloride (75.3 mg, 0.6 mmol, 3 equiv) as amine hydrochloride and 4-bromobenzotrifluoride (28.0 μL, 0.2 mmol, 1 equiv) as aryl bromide. Hexane–dichloromethane mixture with gradient (from pure hexane to 1:1 ratio, respectively) was used as an eluent. NMR yield—71%, isolated yield—35% (14.3 mg, white solid). The spectra data matched with values reported in the literature.6 Rf (dichloromethane)—0.61. 1H NMR (500 MHz, CDCl3): δ 7.43 (d, J = 8.4 Hz, 2H), 6.61 (d, J = 8.4 Hz, 2H), 4.61 (s, 1H), 3.95 (d, J = 5.2 Hz, 2H), 3.81 (s, 3H). 13C{1H} NMR (100.6 MHz, CDCl3): δ 171.1, 149.5, 126.9 (q, C–F, 3JC–F = 3.9 Hz), 125.0 (q, C–F, 1JC–F = 270.5 Hz), 120.0 (q, C–F, 2JC–F = 32.8 Hz), 112.3, 52.6, 45.2. 19F NMR (270.6 MHz, CDCl3): δ −62.18.
4-Phenylmorpholine (Figure 3, 2)
Prepared according to the procedure described in the “General procedure for amination experiments” section with morpholine (52.5 μL, 0.6 mmol, 3 equiv) as amine and bromobenzene (21.3 μL, 0.2 mmol, 1 equiv) as aryl bromide. Hexane–dichloromethane mixture with gradient (from pure hexane to 1:1 ratio, respectively) was used as an eluent. NMR yield—99%, isolated yield—74% (21.1 mg, white solid). The spectra data matched with values reported in the literature.3f Rf (dichloromethane)—0.65. 1H NMR (500 MHz, CDCl3): δ 7.33–7.26 (m, 2H), 6.95–6.86 (m, 3H), 3.87 (t, J = 4.0 Hz, 4H), 3.17 (t, J = 4.0 Hz, 4H). 13C{1H} NMR (100.6 MHz, CDCl3): δ 151.4, 129.3, 120.2, 115.9, 67.1, 49.5.
4-Morpholinobenzonitrile (Figure 3, 12)
Prepared according to the procedure described in the “General procedure for amination experiments” section with morpholine (52.5 μL, 0.6 mmol, 3 equiv) as amine and 4-bromobenzonitrile (36.4 mg, 0.2 mmol, 1 equiv) as aryl bromide. Hexane–dichloromethane mixture with gradient (from pure hexane to 1:1 ratio, respectively) was used as an eluent. NMR yield—79%, isolated yield—58% (19.1 mg, white solid). The spectra data matched with values reported in the literature.3f Rf (dichloromethane)—0.68. 1H NMR (400 MHz, CDCl3): δ 7.54–7.47 (m, 2H), 6.89–6.82 (m, 2H), 3.85 (t, J = 4.9 Hz, 4H), 3.27 (t, J = 4.9 Hz, 4H). 13C{1H} NMR (100.6 MHz, CDCl3): δ 153.6, 133.6, 120.0, 114.2, 101.0, 66.6, 47.4.
Methyl 4-morpholinobenzoate (Figure 3, 13)
Prepared according to the procedure described in the “General procedure for amination experiments” section with morpholine (52.5 μL, 0.6 mmol, 3 equiv) as amine and methyl 4-bromobenzoate (43.0 mg, 0.2 mmol, 1 equiv) as aryl bromide. Hexane–dichloromethane mixture with gradient (from pure hexane to 1:1 ratio, respectively) was used as an eluent. NMR yield—86%, isolated yield—75% (29.0 mg, white solid). The spectra data matched with values reported in the literature.3f Rf (dichloromethane)—0.4. 1H NMR (500 MHz, CDCl3): δ 7.94 (d, J = 8.3 Hz, 2H), 6.86 (d, J = 8.3 Hz, 2H), 3.90–3.82 (m, 7H), 3.28 (m, 4H). 13C{1H} NMR (125.8 MHz, CDCl3): δ 167.2, 154.4, 131.3, 120.5, 113.6, 66.7, 51.8, 47.9.
4-(Naphthalen-2-yl)morpholine (Figure 3, 14)
Prepared according to the procedure described in the “General procedure for amination experiments” section with morpholine (52.5 μL, 0.6 mmol, 3 equiv) as amine and 2-bromonaphthalene (41.4 mg, 0.2 mmol, 1 equiv) as aryl bromide. Hexane–dichloromethane mixture with gradient (from pure hexane to 1:1 ratio, respectively) was used as an eluent. NMR yield—75%, isolated yield—52% (19.4 mg, white solid). The spectra data matched with values reported in the literature.24 Rf (dichloromethane)—0.52. 1H NMR (500 MHz, CDCl3): δ 7.77–7.68 (m, 3H), 7.45–7.40 (m, 1H), 7.34–7.39 (m, 1H), 7.27 (dd, J = 8.9 Hz, J = 2.5 Hz, 1H), 7.13 (d, J = 2.2 Hz, 1H), 3.92 (t, J = 4.8 Hz, 4H), 3.27 (t, J = 4.8 Hz, 4H). 13C{1H} NMR (125.8 MHz, CDCl3): δ 149.2, 134.7, 129.0, 128.8, 127.6, 126.9, 126.5, 123.7, 119.0, 110.2, 67.1, 49.9.
4-(o-Tolyl)morpholine (Figure 3, 15)
Prepared according to the procedure described in the “General procedure for amination experiments” section with morpholine (52.5 μL, 0.6 mmol, 3 equiv) as amine and 2-bromotoluene (24.1 μL, 0.2 mmol, 1 equiv) as aryl bromide. Hexane–dichloromethane mixture with gradient (from pure hexane to 1:1 ratio, respectively) was used as an eluent. NMR yield—82%, isolated yield—61% (18.9 mg, colorless liquid). The spectra data matched with values reported in the literature.25 Rf (dichloromethane)—0.57. 1H NMR (400 MHz, CDCl3): δ 7.23–7.17 (m, 2H), 7.07–6.98 (m, 2H), 3.87 (t, J = 4.5 Hz, 4H), 2.93 (t, J = 4.5 Hz, 4H), 2.34 (s, 3H). 13C{1H} NMR (100.6 MHz, CDCl3): δ 151.4, 132.7, 131.3, 126.8, 123.5, 119.1, 67.6, 52.4, 87.0.
4-(Pyridin-3-yl)morpholine (Figure 3, 16)
Prepared according to the procedure described in the “General procedure for amination experiments” section with morpholine (52.5 μL, 0.6 mmol, 3 equiv) as amine and 3-bromopyridine (19.3 μL, 0.2 mmol, 1 equiv) as aryl bromide. The whole mixture was extracted with ethyl acetate four times. Combined organic fractions were washed with small amount of 0.1 M NaCl water solution and dried over Na2SO4. Next, the solution was dried carefully under vacuum, the residue dissolved in the minimum amount of dichloromethane and subjected to column chromatography on SiO2 (dichloromethane/diethyl ether mixture with gradient—from pure dichloromethane to pure diethyl ether) to furnish the product. Isolated yield—65% (from the whole reaction mixture, 21.3 mg, yellowish liquid). The spectra data matched with values reported in the literature.3f Rf (diethyl ether)—0.22. 1H NMR (500 MHz, CDCl3): δ 8.29 (s, 1H), 8.14–8.10 (m, 1H), 7.19–7.14 (m, 2H), 3.90–3.83 (m, 4H), 3.22–3.15 (m, 4H). 13C{1H} NMR (125.8 MHz, CDCl3): δ 147.0, 141.2, 138.4, 123.7, 122.3, 66.8, 48.7.
4-(3-Methoxyphenyl)morpholine (Figure 3, 17)
Prepared according to the procedure described in the “General procedure for amination experiments” section with morpholine (52.5 μL, 0.6 mmol, 3 equiv) as amine and 3-bromoanisole (25.3 μL, 0.2 mmol, 1 equiv) as aryl bromide. Hexane–dichloromethane mixture with gradient (from pure hexane to 1:1 ratio, respectively) was used as an eluent. NMR yield—86%, isolated yield—67% (22.7 mg, colorless liquid). Control DC-assisted experiment (potentiostatic conditions—2.8 V) demonstrated 45% NMR yield. The spectra data matched with values reported in the literature.3f Rf (dichloromethane)—0.43. 1H NMR (500 MHz, CDCl3): δ 7.24–7.16 (m, 1H), 6.56–6.50 (m, 1H), 6.48–6.42 (m, 2H), 3.85 (t, J = 4.8 Hz, 4H), 3.80 (s, 3H), 3.15 (t, J = 4.8 Hz, 4H). 13C{1H} NMR (125.8 MHz, CDCl3): δ 160.8, 152.9, 130.0, 108.6, 104.9, 102.4, 67.0, 55.4, 49.4.
Esterification
4-(Trifluoromethyl)phenyl benzoate (Figure 3, 18)
Prepared according to the procedure described in the “General procedure for esterification experiments” section with benzoic acid (36.6 mg, 0.3 mmol, 1.5 equiv) as carboxylic acid and 4-bromobenzotrifluoride (28.0 μL, 0.2 mmol, 1 equiv) as aryl bromide. Hexane–dichloromethane mixture with gradient (from pure hexane to 10:1 ratio, respectively) was used as an eluent. NMR yield—81%, isolated yield—75% (34.9 mg, white solid). The spectra data matched with values reported in the literature.17 Rf (hexane)—0.11. 1H NMR (500 MHz, CDCl3): δ 8.25–8.18 (m, 2H), 7.72 (d, J = 7.3 Hz, 2H), 7.69–7.64 (m, 1H), 7.57–7.51 (m, 2H), 7.37 (d, J = 7.2 Hz, 2H). 13C{1H} NMR (125.8 MHz, CDCl3): δ 164.8, 153.6, 134.1, 130.4, 129.1, 128.9, 128.3 (q, C–F, 2JC–F = 33.4 Hz), 127.0 (q, C–F, 3JC–F = 3.8 Hz), 124.0 (q, C–F, 1JC–F = 271.8 Hz), 122.4. 19F NMR (282.4 MHz, CDCl3): δ −63.18.
5-(tert-butyl) 1-(4-(trifluoromethyl)phenyl) (tert-butoxycarbonyl)-l-glutamate (Figure 3, 19)
Prepared according to the procedure described in the “General procedure for esterification experiments” section with Boc-l-glutamic acid 5-tert-butyl ester (61.0 mg, 0.3 mmol, 1.5 equiv) as carboxylic acid and 4-bromobenzotrifluoride (28.0 μL, 0.2 mmol, 1 equiv) as aryl bromide. Hexane–dichloromethane mixture with gradient (from pure hexane to 1:1 ratio, respectively) was used as an eluent. NMR yield—82%, isolated yield—66% (51.7 mg, white solid). Rf (dichloromethane)—0.45. 1H NMR (500 MHz, CDCl3): δ 7.66 (d, J = 8.5 Hz, 2H), 7.25 (d, J = 8.4 Hz, 2H), 5.20 (d, J = 7.6 Hz, 1H), 4.53 (m, 1H), 2.50–2.37 (m, 2H), 2.34–2.25 (m, 1H), 2.15–2.05 (m, 1H), 1.46 (m, 18H). 13C{1H} NMR (125.8 MHz, CDCl3): δ 172.1, 170.9, 155.6, 153.1, 128.6 (q, C–F, 2JC–F = 33.4 Hz), 127.0 (q, C–F, 3JC–F = 3.8 Hz), 123.9 (q, C–F, 1JC–F = 272.8 Hz), 122.1, 81.3, 80.5, 53.6, 31.7, 28.4, 28.2, 27.3. 19F NMR (282.4 MHz, CDCl3): δ −63.27. HRMS (ESI) m/z: [M + Na]+ calcd for C21H28NO6F3Na 470.1766; found 470.1769.
Methyl (4-(trifluoromethyl)phenyl) succinate (Figure 3, 20)
Prepared according to the procedure described in the “General procedure for esterification experiments” section with succinic acid methyl ester (39.6 mg, 0.3 mmol, 1.5 equiv) as carboxylic acid and 4-bromobenzotrifluoride (28.0 μL, 0.2 mmol, 1 equiv) as aryl bromide. Hexane–dichloromethane mixture with gradient (from pure hexane to 1:1 ratio, respectively) was used as an eluent. NMR yield—84%, isolated yield—70% (33.8 mg, colorless liquid). Rf (dichloromethane)—0.67. 1H NMR (500 MHz, CDCl3): δ 7.65 (d, J = 7.8 Hz, 2H), 7.23 (d, J = 8.0 Hz, 2H), 3.73 (s, 3H), 2.93–2.86 (m, 2H), 2.78–2.75 (m, 2H). 13C{1H} NMR (125.8 MHz, CDCl3): δ 172.6, 170.6, 153.2, 128.3 (q, C–F, 2JC–F = 33.4 Hz), 126.9 (q, C–F, 3JC–F = 3.8 Hz), 124.0 (q, C–F, 1JC–F = 271.8 Hz), 122.16, 52.17, 29.4, 28.9. 19F NMR (470.6 MHz, CDCl3): δ −63.26. HRMS (ESI) m/z: [M + Na]+ calcd for C12H11O4F3Na 299.0507; found 299.0507.
4-(Trifluoromethyl)phenyl acetate (Figure 3, 21)
Prepared according to the procedure described in the “General procedure for esterification experiments” section with the sodium acetate suspension (82.0 mg, 1 mmol, 5 equiv) instead of the carboxylic acid and K2CO3 mixture, and 4-bromobenzotrifluoride (28.0 μL, 0.2 mmol, 1 equiv) as aryl bromide. Hexane–dichloromethane mixture with gradient (from pure hexane to 1/1 ratio, respectively) was used as an eluent. NMR yield—87%, isolated yield—48% (17.1 mg, colorless liquid). The spectra data matched with values reported in the literature.26 Rf (dichloromethane)—0.65. 1H NMR (500 MHz, CDCl3): δ 7.65 (d, J = 8.5 Hz, 2H), 7.22 (d, J = 8.5 Hz, 2H), 2.33 (s, 3H). 13C{1H} NMR (125.8 MHz, CDCl3): δ 169.0, 153.3, 128.3 (q, C–F, 2JC–F = 32.4 Hz), 126.9 (q, C–F, 3JC–F = 3.8 Hz), 124.0 (q, C–F, 1JC–F = 271.8 Hz), 122.2, 21.2. 19F NMR (282.4 MHz, CDCl3): δ −63.25.
1-(tert-Butyl) 2-(4-(trifluoromethyl)phenyl) (S)-pyrrolidine-1,2-dicarboxylate (Figure 3, 4)
Prepared according to the procedure described in the “General procedure for esterification experiments” section with Boc-l-proline (64.6 mg, 0.3 mmol, 1.5 equiv) as carboxylic acid and 4-bromobenzotrifluoride (28.0 μL, 0.2 mmol, 1 equiv) as aryl bromide. Hexane–dichloromethane mixture with gradient (from pure hexane to 1/1 ratio, respectively) was used as an eluent. NMR yield—87%, isolated yield—69% (43.4 mg, colorless liquid). The spectra data matched with values reported in the literature.27 Rf (dichloromethane)—0.37. 1H NMR (500 MHz, CDCl3): (rotameric mixture, resonances for minor rotamer are enclosed in parenthesis) δ 7.67 (7.64) (d, J = 8.4 Hz, 2H), 7.25 (7.23) (d, J = 8.7 Hz, 2H), 4.53 (4.47) (dd, J = 8.7, 4.4 Hz, 1H), 3.67–3.40 (m, 2H), 2.45–2.29 (m, 1H), 2.22–2.10 (m, 1H), 2.09–1.90 (m, 2H), (1.48) 1.46 (s, 9H). 13C{1H} NMR (125.8 MHz, CDCl3): (rotameric mixture, resonances for minor rotamer are enclosed in parenthesis) δ (171.4) 171.3, (154.6) 153.8, (153.5) 153.2, 128.4 (128.2) (q, C–F, 2JC–F = 33.4 Hz), 127.0 (126.8) (q, C–F, 3JC–F = 3.8 Hz), (123.8) 123.9 (d, C–F, 1JC–F = 271.8 Hz), (122.2) 121.8, 80.5 (80.4), 59.3 (59.2), (46.8) 46.6, 31.2 (30.1), 28.6, (24.7) 23.9. 19F NMR (282.4 MHz, CDCl3): (rotameric mixture, resonances for minor rotamer are enclosed in parenthesis) δ −63.22 (−63.27).
1-(tert-Butyl) 2-(4-(methoxycarbonyl)phenyl) (S)-pyrrolidine-1,2-dicarboxylate (Figure 3, 22)
Prepared according to the procedure described in the “General procedure for esterification experiments” section with Boc-l-proline (64.6 mg, 0.3 mmol, 1.5 equiv) as carboxylic acid and methyl 4-bromobenzoate (43.0 mg, 0.2 mmol, 1 equiv) as aryl bromide. Hexane–dichloromethane mixture with gradient (from pure hexane to pure dichloromethane) was used as an eluent. NMR yield—89%, isolated yield—79% (48.3 mg, white solid). The spectra data matched with values reported in the literature.27 Rf (dichloromethane)—0.2. 1H NMR (500 MHz, CDCl3): (rotameric mixture, resonances for minor rotamer are enclosed in parenthesis) δ 8.08 (8.05) (d, J = 8.4 Hz, 2H), 7.2 (7.18) (d, J = 8.5 Hz, 2H), (4.53) 4.46 (dd, J = 8.5, 4.3 Hz, 1H), 3.91 (3.90) (s, 3H), 3.69–3.40 (m, 2H), 2.47–2.28 (m, 1H), 2.23–2.11 (m, 1H), 2.10–1.90 (m, 2H), (1.48) 1.45 (s, 9H). 13C{1H} NMR (125.8 MHz, CDCl3): δ (rotameric mixture, resonances for minor rotamer are enclosed in parenthesis) (171.3) 171.25, (166.5) 166.4, (154.6) 154.3, 153.8, 131.4 (131.3), 128.0 (127.8), (121.7) 121.3, 80.5 (80.3), 59.3 (59.2), 52.4 (52.3), (46.8), 46.6, 31.2 (30.1), 28.6, (24.7) 23.9.
1-(tert-Butyl) 2-(4-cyanophenyl) (S)-pyrrolidine-1,2-dicarboxylate (Figure 3, 23)
Prepared according to the procedure described in the “General procedure for esterification experiments” section with Boc-l-proline (64.6 mg, 0.3 mmol, 1.5 equiv) as carboxylic acid and 4-bromobenzonitrile (36.4 mg, 0.2 mmol, 1 equiv) as aryl bromide. Hexane–dichloromethane mixture with gradient (from pure hexane to pure dichloromethane) was used as an eluent. NMR yield—65%, isolated yield—45% (24.9 mg, colorless liquid). Control DC-assisted experiment (potentiostatic conditions—2.8 V) demonstrated 23% NMR yield (37% of the diaryl product was also observed). The spectra data matched with values reported in the literature. The spectra data matched with values reported in the literature.27 Rf (dichloromethane)—0.2. 1H NMR (500 MHz, CDCl3): (rotameric mixture, resonances for one of the rotamers are enclosed in parenthesis) δ 7.70 (7.67) (d, J = 8.5 Hz, 2H), 7.26 (7.24) (d, J = 8.7 Hz, 2H), 4.51 (4.46) (dd, J = 8.5, 4.5 Hz, 1 H), 3.67–3.41 (m, 2H), 2.45–2.30 (m, 1H), 2.20–1.90 (m, 3H), 1.47 (1.44) (s, 9H). 13C{1H} NMR (125.8 MHz, CDCl3): (rotameric mixture, resonances for one of the rotamers are enclosed in parenthesis) δ (171.1) 171.0, (154.6) 154.3, 154.0 (153.7) 133.9 (133.8), (122.8) 122.4, (118.4) 118.2, (110.1) 109.9, 80.6 (80.4), 59.3 (59.2), (46.8) 46.6, (31.2) 30.1, 28.5, (24.7) 23.9.
1-(tert-Butyl) 2-(naphthalen-2-yl) (S)-pyrrolidine-1,2-dicarboxylate (Figure 3, 24)
Prepared according to the procedure described in the “General procedure for esterification experiments” section with Boc-l-proline (64.6 mg, 0.3 mmol, 1.5 equiv) as carboxylic acid and 2-bromonaphthalene (41.4 mg, 0.2 mmol, 1 equiv) as aryl bromide. Hexane–dichloromethane mixture with gradient (from pure hexane to 1/1 ratio, respectively) was used as an eluent. NMR yield—79%, isolated yield—54% (32.3 mg, yellowish liquid). Control DC-assisted experiment (potentiostatic conditions—2.8 V) demonstrated 4% NMR yield (9% of the diaryl product was also observed). Rf (dichloromethane)—0.36. 1H NMR (300 MHz, CDCl3): (rotameric mixture, resonances for minor rotamer are enclosed in parenthesis) δ 7.92–7.75 (m, 3H), 7.62–7.4 (m, 3H), 7.32–7.18 (m, 1H), 4.59 (4.51) (dd, J = 8.4, 4.0 Hz, 1H), 3.75–3.40 (m, 2H), 2.51–1.9 (m, 4H), 1.50 (m, 9H). 13C{1H} NMR (100.6 MHz, CDCl3): (rotameric mixture, resonances for minor rotamer are enclosed in parenthesis) (171.93) 171.89, (154.7) 153.9, (148.6) 148.4, 133.8, 131.6, 129.6 (129.5), 127.91 (127.87), 127.75, 126.8 (126.6), 125.9 (125.8), (121.2) 120.7, (118.6) 118.2, 80.4 (80.2), 59.4 (59.3), 46.8 (46.6), 31.2 (30.2), 28.6, (24.7) 23.9. HRMS (ESI) m/z: [M + Na]+ calcd for C20H23NO4Na 364.1525; found 364.1524.
Etherification
1-(Benzyloxy)-4-(trifluoromethyl)benzene (Figure 3, 25)
Prepared according to the procedure described in the “General procedure for etherification experiments” section with benzyl alcohol (62.1 μL, 0.6 mmol, 3 equiv) as alcohol and 4-bromobenzotrifluoride (28.0 μL, 0.2 mmol, 1 equiv) as aryl bromide. Hexane was used as an eluent. NMR yield—72%, isolated yield—66% (29.1 mg, white solid). A 2 mmol scale experiment was performed using a setup with four GC rod electrodes and 20 ml vial as an electrochemical cell (see the Supporting Information) with 4-bromobenzotrifluoride (280.0 μL, 2 mmol, 1 equiv), benzyl alcohol (620.9 μL, 6 mmol, 3 equiv), NiBr2·DME (61.7 mg, 0.2 mmol, 0.1 equiv), Bipy (31.2 mg, 0.2 mmol, 0.1 equiv), and quinuclidine (444.7 mg, 4 mmol, 2 equiv) in 10 mL of 0.2M LiBr (174 mg) DMF solution. The electrolysis was conducted for 20 h with the following parameters of AC: a sine waveform, 10 Hz frequency, 3 V peak voltage, and a stirring rate of 300 rpm. The preparation of the initial reaction mixture and the purification of the product are identical to the procedure described in the “General procedure for etherification experiments” section. The isolated yield of 2 mmol scale experiment—70% (353.5 mg). The spectra data matched with values reported in the literature.28 Rf (hexane)—0.2. 1H NMR (400 MHz, CDCl3): δ 7.55 (d, J = 8.6 Hz, 2H), 7.47–7.33 (m, 5H), 7.04 (d, J = 8.6 Hz, 2H), 5.12 (s, 2H). 13C{1H} NMR (100.6 MHz, CDCl3): δ 161.3, 136.4, 128.9, 128.4, 127.6, 127.1 (q, C–F, 3JC–F = 3.7 Hz), 124.7 (q, C–F, 1JC–F = 271.1 Hz), 123.3 (q, C–F, 2JC–F = 32.6 Hz), 115.0, 70.3. 19F NMR (282.4 MHz, CDCl3): δ −62.48.
1-(Cyclohexyloxy)-4-(trifluoromethyl)benzene (Figure 3, 26)
Prepared according to the procedure described in the “General procedure for etherification experiments” section with cyclohexanol (126.8 μL, 1.2 mmol, 6 equiv) as alcohol and 4-bromobenzotrifluoride (28.0 μL, 0.2 mmol, 1 equiv) as aryl bromide. The electrolysis was conducted for 8 h with the frequency of 5 Hz. Hexane was used as an eluent. NMR yield—65%, isolated yield—50% (21.4 mg, colorless liquid). The spectra data matched with values reported in the literature.29 Rf (hexane)—0.48. 1H NMR (500 MHz, CDCl3): δ 7.51 (d, J = 8.5 Hz, 2 H), 6.94 (d, J = 8.5 Hz, 2 H), 4.34–4.27 (m, 1H), 2.03–1.94 (m, 2H), 1.85–1.76 (m, 2H), 1.63–1.50 (m, 3H), 1.45–1.28 (m, 3H). 13C{1H} NMR (125.8 MHz, CDCl3): δ 160.5, 127.0 (q, C–F, 3JC–F = 3.8 Hz), 124.7 (q, C–F, 1JC–F = 271.8 Hz), 122.5 (q, C–F, 2JC–F = 33.4 Hz), 115.7, 75.6, 31.7, 25.7, 23.8. 19F NMR (470.6 MHz, CDCl3): δ −62.42.
1-Isopropoxy-4-(trifluoromethyl)benzene (Figure 3, 27)
Prepared according to the procedure described in the “General procedure for etherification experiments” section with 2-propanol (91.9 μL, 1.2 mmol, 6 equiv) as alcohol and 4-bromobenzotrifluoride (28.0 μL, 0.2 mmol, 1 equiv) as aryl bromide. The electrolysis was conducted for 16 h with the frequency of 5 Hz. Pentane was used as an eluent. NMR yield—73%, isolated yield—62% (22.2 mg, colorless liquid). The spectra data matched with values reported in the literature.29 Rf (hexane)—0.45. 1H NMR (500 MHz, CDCl3): δ 7.52 (d, J = 8.7 Hz, 2H), 6.93 (d, J = 8.7 Hz, 2H), 4.61 (sept, J = 6.1 Hz, 1H), 1.36 (d, J = 6.1 Hz, 6H). 13C{1H} NMR (125.8 MHz, CDCl3): δ 160.6, 127.03 (q, C–F, 3JC–F = 3.8 Hz), 124.7 (q, C–F, 1JC–F = 270.8 Hz), 122.6 (q, C–F, 2JC–F = 32.4 Hz), 115.6, 70.3, 22.0. 19F NMR (282.4 MHz, CDCl3): δ −62.44.
1-(Hexyloxy)-4-(trifluoromethyl)benzene (Figure 3, 5)
Prepared according to the procedure described in the “General procedure for etherification experiments” section with 1-hexanol (75.3 μL, 0.6 mmol, 3 equiv) as alcohol and 4-bromobenzotrifluoride (28.0 μL, 0.2 mmol, 1 equiv) as aryl bromide. Hexane was used as an eluent. NMR yield—87%, isolated yield—68% (29.3 mg, colorless liquid). The spectra data matched with values reported in the literature.3d Rf (hexane)—0.56. 1H NMR (500 MHz, CDCl3): δ 7.53 (d, J = 8.4 Hz, 2H), 6.95 (d, J = 8.4 Hz, 2H), 3.99 (t, J = 6.5 Hz, 2H), 1.80 (tt, J = 7.3, 6.7 Hz, 2H), 1.51–1.45 (m, 2H), 1.39–1.32 (m, 4H), 0.95–0.87 (m, 3H). 13C{1H} NMR (125.8 MHz, CDCl3): δ 161.7, 127.0 (q, C–F, 3JC–F = 3.8 Hz), 124.7 (q, C–F, 1JC–F = 270.8 Hz), 122.7 (q, C–F, 2JC–F = 32.4 Hz), 114.6, 68.4, 31.7, 29.2, 25.8, 22.7, 14.2. 19F NMR (282.4 MHz, CDCl3): δ −62.42.
Methyl 4-(hexyloxy)benzoate (Figure 3, 28)
Prepared according to the procedure described in the “General procedure for etherification experiments” section with 1-hexanol (75.3 μL, 0.6 mmol, 3 equiv) as alcohol and methyl 4-bromobenzoate (43.0 mg, 0.2 mmol, 1 equiv) as aryl bromide. Hexane–dichloromethane mixture with gradient (from pure hexane to 1/1 ratio, respectively) was used as an eluent. NMR yield—85%, isolated yield—79% (32.7 mg, colorless liquid). Control DC-assisted experiment (potentiostatic conditions—2.8 V) demonstrated only traces of product in NMR spectra. The spectra data matched with values reported in the literature.3d Rf (dichloromethane)—0.7. 1H NMR (500 MHz, CDCl3): δ 7.97 (d, J = 8.9 Hz, 2H), 6.90 (d, J = 8.9 Hz, 2H), 4.00 (t, J = 6.6 Hz, 2H), 3.88 (s, 3H), 1.79 (m, 2H), 1.51–1.41 (m, 2H), 1.38–1.30 (m, 4H), 0.95–0.86 (m, 3H). 13C{1H} NMR (125.8 MHz, CDCl3): δ 167.1, 163.1, 131.7, 122.5, 114.2, 68.4, 52.0, 31.7, 29.2, 25.8, 22.7, 14.2.
3-(Hexyloxy)pyridine (Figure 3, 29)
Prepared according to the procedure described in the “General procedure for etherification experiments” section with 1-hexanol (75.3 μL, 0.6 mmol, 3 equiv) as alcohol and 3-bromopyridine (19.3 μL, 0.2 mmol, 1 equiv) as aryl bromide. Hexane–dichloromethane mixture with gradient (from pure hexane to pure dichloromethane) was used as an eluent. NMR yield—66%, isolated yield—61% (19.1 mg, yellowish liquid). Control DC-assisted experiment (potentiostatic conditions—2.8 V) demonstrated 29% NMR yield. The spectra data matched with values reported in the literature.30 Rf (dichloromethane)—0.27. 1H NMR (500 MHz, CDCl3): δ 8.30 (d, J = 2.3, 1H), 8.20 (dd, J = 4.3, 1.5 Hz, 1H), 7.23–7.15 (m, 2H), 3.99 (t, J = 6.5 Hz, 2H), 1.83–1.75 (m, 2H), 1.51–1.42 (m, 2H), 1.40–1.29 (m, 4H), 0.91 (t, J = 7.0 Hz, 3H). 13C{1H} NMR (125.8 MHz, CDCl3): δ 155.4, 142.0, 138.2, 123.9, 121.2, 68.5, 31.7, 29.3, 25.8, 22.7, 14.2.
Side Products
4,4′-Bis(trifluoromethyl)-1,1′-biphenyl (Figure 4D, 30)
Separated as a minor product in the reactions of amination, etherification, and esterification with 4-bromobenzotrifluoride or as a major product in DC-assisted amination and etherification with a Zn sacrificial anode. Purified by column chromatography—hexane was used as an eluent. NMR yield—73%, isolated yield—66% (19.5 mg, white solid—from DC-assisted etherification with a Zn sacrificial anode). The spectra data matched with values reported in the literature.31 Rf (hexane)—0.53. 1H NMR (400 MHz, CDCl3): δ 7.74 (d, J = 8.6 Hz, 4H), 7.70 (d, J = 8.6 Hz, 4H). 13C{1H} NMR (100.6 MHz, CDCl3): δ 143.4, 130.4 (q, C–F, 2JC–F = 32.6 Hz), 127.8, 126.1 (q, C–F, 3JC–F = 3.9 Hz), 124.2 (q, C–F, 1JC–F = 272.2 Hz). 19F NMR (282.4 MHz, CDCl3): δ −63.55.
Acknowledgments
We thank E. Narevicius for the help with setting up equipment for AC experiments. We thank R. Neumann and M. Somekh for helpful discussions.
Supporting Information Available
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/acs.joc.0c02350.
Experimental procedures, electrochemical analysis data, and compound characterization data (PDF)
This work was funded by Weizmann Institute of Science.
The authors declare the following competing financial interest(s): Authors are inventors on the patent application (277384) based on the results presented in this manuscript (Israel patent office).
Supplementary Material
References
- Parts of this manuscript are adapted from ChemRxiv preprint: Bortnikov E.; Semenov S.. Coupling of Alternating Current to Transition-Metal Catalysis: Examples of Nickel-Catalyzed Cross-Coupling. 2020. ChemRxiv. Preprint. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tasker S. Z.; Standley E. A.; Jamison T. F. Recent advances in homogeneous nickel catalysis. Nature 2014, 509, 299–309. 10.1038/nature13274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- a Corcoran E. B.; Pirnot M. T.; Lin S.; Dreher S. D.; DiRocco D. A.; Davies I. W.; Buchwald S. L.; MacMillan D. W. C. Aryl amination using ligand-free Ni(II) salts and photoredox catalysis. Science 2016, 353, 279–283. 10.1126/science.aag0209. [DOI] [PMC free article] [PubMed] [Google Scholar]; b Zuo Z.; Ahneman D. T.; Chu L.; Terrett J. A.; Doyle A. G.; MacMillan D. W. C. Merging photoredox with nickel catalysis: Coupling of α-carboxyl sp3-carbons with aryl halides. Science 2014, 345, 437–440. 10.1126/science.1255525. [DOI] [PMC free article] [PubMed] [Google Scholar]; c Johnston C. P.; Smith R. T.; Allmendinger S.; MacMillan D. W. C. Metallaphotoredox-catalysed sp(3)-sp(3) cross-coupling of carboxylic acids with alkyl halides. Nature 2016, 536, 322–325. 10.1038/nature19056. [DOI] [PMC free article] [PubMed] [Google Scholar]; d Terrett J. A.; Cuthbertson J. D.; Shurtleff V. W.; MacMillan D. W. C. Switching on elusive organometallic mechanisms with photoredox catalysis. Nature 2015, 524, 330–334. 10.1038/nature14875. [DOI] [PMC free article] [PubMed] [Google Scholar]; e Twilton J.; Le C.; Zhang P.; Shaw M. H.; Evans R. W.; MacMillan D. W. C. The merger of transition metal and photocatalysis. Nat. Rev. Chem. 2017, 1, 0052. 10.1038/s41570-017-0052. [DOI] [Google Scholar]; f Lim C.-H.; Kudisch M.; Liu B.; Miyake G. M. C-N Cross-Coupling via Photoexcitation of Nickel-Amine Complexes. J. Am. Chem. Soc. 2018, 140, 7667–7673. 10.1021/jacs.8b03744. [DOI] [PMC free article] [PubMed] [Google Scholar]; g Park B. Y.; Pirnot M. T.; Buchwald S. L. Visible Light-Mediated (Hetero)aryl Amination Using Ni(II) Salts and Photoredox Catalysis in Flow: A Synthesis of Tetracaine. J. Org. Chem. 2020, 85, 3234–3244. 10.1021/acs.joc.9b03107. [DOI] [PubMed] [Google Scholar]; h Yang L.; Lu H.-H.; Lai C.-H.; Li G.; Zhang W.; Cao R.; Liu F.; Wang C.; Xiao J.; Xue D. Light-Promoted Nickel Catalysis: Etherification of Aryl Electrophiles with Alcohols Catalyzed by a NiII-Aryl Complex. Angew. Chem., Int. Ed. 2020, 59, 12714–12719. 10.1002/anie.202003359. [DOI] [PubMed] [Google Scholar]; i Zhu D.-L.; Li H.-X.; Xu Z.-M.; Li H.-Y.; Young D. J.; Lang J.-P. Visible light driven, nickel-catalyzed aryl esterification using a triplet photosensitiser thioxanthen-9-one. Org. Chem. Front. 2019, 6, 2353–2359. 10.1039/c9qo00536f. [DOI] [Google Scholar]
- a Zhu B.; Yan L.-K.; Geng Y.; Ren H.; Guan W.; Su Z.-M. Ir(III)/Ni(II)-Metallaphotoredox catalysis: the oxidation state modulation mechanism versus the radical mechanism. Chem. Commun. 2018, 54, 5968–5971. 10.1039/c8cc03550d. [DOI] [PubMed] [Google Scholar]; b Qi Z.-H.; Ma J. Dual Role of a Photocatalyst: Generation of Ni(0) Catalyst and Promotion of Catalytic C–N Bond Formation. ACS Catal. 2018, 8, 1456–1463. 10.1021/acscatal.7b03024. [DOI] [Google Scholar]; c Levin M. D.; Kim S.; Toste F. D. Photoredox Catalysis Unlocks Single-Electron Elementary Steps in Transition Metal Catalyzed Cross-Coupling. ACS Cent. Sci. 2016, 2, 293–301. 10.1021/acscentsci.6b00090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li C.; Kawamata Y.; Nakamura H.; Vantourout J. C.; Liu Z.; Hou Q.; Bao D.; Starr J. T.; Chen J.; Yan M.; Baran P. S. Electrochemically Enabled, Nickel-Catalyzed Amination. Angew. Chem., Int. Ed. 2017, 56, 13088–13093. 10.1002/anie.201707906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawamata Y.; Vantourout J. C.; Hickey D. P.; Bai P.; Chen L.; Hou Q.; Qiao W.; Barman K.; Edwards M. A.; Garrido-Castro A. F.; deGruyter J. N.; Nakamura H.; Knouse K.; Qin C.; Clay K. J.; Bao D.; Li C.; Starr J. T.; Garcia-Irizarry C.; Sach N.; White H. S.; Neurock M.; Minteer S. D.; Baran P. S. Electrochemically Driven, Ni-Catalyzed Aryl Amination: Scope, Mechanism, and Applications. J. Am. Chem. Soc. 2019, 141, 6392–6402. 10.1021/jacs.9b01886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y.; Deng L.; Wang X.; Wu Z.; Wang Y.; Pan Y. Electrochemically Promoted Nickel-Catalyzed Carbon–Sulfur Bond Formation. ACS Catal. 2019, 9, 1630–1634. 10.1021/acscatal.8b04633. [DOI] [Google Scholar]
- Sengmany S.; Ollivier A.; Le Gall E.; Léonel E. A mild electroassisted synthesis of (hetero)arylphosphonates. Org. Biomol. Chem. 2018, 16, 4495–4500. 10.1039/c8ob00500a. [DOI] [PubMed] [Google Scholar]
- Tian J.; Moeller K. D. Electrochemically Assisted Heck Reactions. Org. Lett. 2005, 7, 5381–5383. 10.1021/ol0519487. [DOI] [PubMed] [Google Scholar]
- a Mo Y.; Lu Z.; Rughoobur G.; Patil P.; Gershenfeld N.; Akinwande A. I.; Buchwald S. L.; Jensen K. F. Microfluidic electrochemistry for single-electron transfer redox-neutral reactions. Science 2020, 368, 1352–1357. 10.1126/science.aba3823. [DOI] [PubMed] [Google Scholar]
- a Sun R.; Qin Y.; Nocera D. G. General Paradigm in Photoredox Nickel-Catalyzed Cross-Coupling Allows for Light-Free Access to Reactivity. Angew. Chem., Int. Ed. 2020, 59, 9527–9533. 10.1002/anie.201916398. [DOI] [PubMed] [Google Scholar]; b Sun R.; Qin Y.; Ruccolo S.; Schnedermann C.; Costentin C.; Nocera D. G. Elucidation of a Redox-Mediated Reaction Cycle for Nickel-Catalyzed Cross Coupling. J. Am. Chem. Soc. 2019, 141, 89–93. 10.1021/jacs.8b11262. [DOI] [PubMed] [Google Scholar]
- Lee B.; Naito H.; Nagao M.; Hibino T. Alternating-current electrolysis for the production of phenol from benzene. Angew. Chem., Int. Ed. 2012, 51, 6961–6965. 10.1002/anie.201202159. [DOI] [PubMed] [Google Scholar]
- Rodrigo S.; Um C.; Mixdorf J. C.; Gunasekera D.; Nguyen H. M.; Luo L. Alternating Current Electrolysis for Organic Electrosynthesis: Trifluoromethylation of (Hetero)arenes. Org. Lett. 2020, 22, 6719–6723. 10.1021/acs.orglett.0c01906. [DOI] [PubMed] [Google Scholar]
- Sattler L. E.; Otten C. J.; Hilt G. Alternating Current Electrolysis for the Electrocatalytic Synthesis of Mixed Disulfide via Sulfur-Sulfur Bond Metathesis towards Dynamic Disulfide Libraries. Chem. - Eur. J. 2020, 26, 3129–3136. 10.1002/chem.201904948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartlett P. N.; Eastwick-Field V. A reinvestigation of the electrochemistry of [Ni(II)(bpy)3(ClO)4)2] in acetonitrile using rotating disc and rotating ring-disc electrodes. Electrochim. Acta 1993, 38, 2515–2523. 10.1016/0013-4686(93)80147-r. [DOI] [Google Scholar]
- Tian L.; Till N. A.; Kudisch B.; MacMillan D. W. C.; Scholes G. D. Transient Absorption Spectroscopy Offers Mechanistic Insights for an Iridium/Nickel-Catalyzed C-O Coupling. J. Am. Chem. Soc. 2020, 142, 4555–4559. 10.1021/jacs.9b12835. [DOI] [PubMed] [Google Scholar]
- Welin E. R.; Le C.; Arias-Rotondo D. M.; McCusker J. K.; MacMillan D. W. C. Photosensitized, energy transfer-mediated organometallic catalysis through electronically excited nickel(II). Science 2017, 355, 380–385. 10.1126/science.aal2490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Durandetti M.; Devaud M.; Perichon J. Investigation of the reductive coupling of aryl halides and/or ethylchloroacetate electrocatalyzed by the precursor NiX2(bpy) with X(-)=Cl-, Br- or MeSO3- and bpy equals 2,2’-dipyridyl. New J. Chem. 1996, 20, 659–667. [Google Scholar]
- Klein A.; Budnikova Y. H.; Sinyashin O. G. Electron transfer in organonickel complexes of α-diimines: Versatile redox catalysts for C–C or C–P coupling reactions—A review. J. Organomet. Chem. 2007, 692, 3156–3166. 10.1016/j.jorganchem.2007.01.021. [DOI] [Google Scholar]
- a Derien S.; Dunach E.; Perichon J. From stoichiometry to catalysis: electroreductive coupling of alkynes and carbon dioxide with nickel-bipyridine complexes. Magnesium ions as the key for catalysis. J. Am. Chem. Soc. 1991, 113, 8447–8454. 10.1021/ja00022a037. [DOI] [Google Scholar]; b Troupel M.; Rollin Y.; Sock O.; Meyer G.; Perichon J. Electrochemistry of Nickel-Complexes Associated with 2,2’-Bipyridine in the N-Methylpyrrolidone Solvent—Application to Activation of Carbon-Halogen Bonds. New J. Chem. 1986, 10, 593–599. [Google Scholar]
- Cannes C.; Labbé E.; Durandetti M.; Devaud M.; Nédélec J. Y. Nickel-catalyzed electrochemical homocoupling of alkenyl halides: rates and mechanisms. J. Electroanal. Chem. 1996, 412, 85–93. 10.1016/0022-0728(96)04605-0. [DOI] [Google Scholar]
- a Yakhvarov D. G.; Tazeev D. I.; Sinyashin O. G.; Giambastiani G.; Bianchini C.; Segarra A. M.; Lönnecke P.; Hey-Hawkins E. Electrochemical synthesis of the σ-aryl complex [NiBr(Mes)(bpy)] and its use as catalyst precursor for the oligomerization of ethylene (Mes=2,4,6-trimethylphenyl, bpy=2,2′-bipyridine). Polyhedron 2006, 25, 1607–1612. 10.1016/j.poly.2005.10.032. [DOI] [Google Scholar]; b Budnikova Y. H.; Perichon J.; Yakhvarov D. G.; Kargin Y. M.; Sinyashin O. G. Highly reactive σ-organonickel complexes in electrocatalytic processes. J. Organomet. Chem. 2001, 630, 185–192. 10.1016/s0022-328x(01)00813-0. [DOI] [Google Scholar]
- Yang C.; Zhang F.; Deng G.-J.; Gong H. Amination of Aromatic Halides and Exploration of the Reactivity Sequence of Aromatic Halides. J. Org. Chem. 2019, 84, 181–190. 10.1021/acs.joc.8b02588. [DOI] [PubMed] [Google Scholar]
- Tobisu M.; Shimasaki T.; Chatani N. Ni0-catalyzed Direct Amination of Anisoles Involving the Cleavage of Carbon–Oxygen Bonds. Chem. Lett. 2009, 38, 710–711. 10.1246/cl.2009.710. [DOI] [Google Scholar]
- Hartwig J. F.; Kawatsura M.; Hauck S. I.; Shaughnessy K. H.; Alcazar-Roman L. M. Room-Temperature Palladium-Catalyzed Amination of Aryl Bromides and Chlorides and Extended Scope of Aromatic C-N Bond Formation with a Commercial Ligand. J. Org. Chem. 1999, 64, 5575–5580. 10.1021/jo990408i. [DOI] [PubMed] [Google Scholar]
- Lin J.; Li Z.; Kan J.; Huang S.; Su W.; Li Y. Photo-driven redox-neutral decarboxylative carbon-hydrogen trifluoromethylation of (hetero)arenes with trifluoroacetic acid. Nat. Commun. 2017, 8, 14353. 10.1038/ncomms14353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pieber B.; Malik J. A.; Cavedon C.; Gisbertz S.; Savateev A.; Cruz D.; Heil T.; Zhang G.; Seeberger P. H. Semi-heterogeneous Dual Nickel/Photocatalysis using Carbon Nitrides: Esterification of Carboxylic Acids with Aryl Halides. Angew. Chem., Int. Ed. 2019, 58, 9575–9580. 10.1002/anie.201902785. [DOI] [PubMed] [Google Scholar]
- Morimoto H.; Tsubogo T.; Litvinas N. D.; Hartwig J. F. A broadly applicable copper reagent for trifluoromethylations and perfluoroalkylations of aryl iodides and bromides. Angew. Chem., Int. Ed. 2011, 50, 3793–3798. 10.1002/anie.201100633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Escobar R. A.; Johannes J. W. A Unified and Practical Method for Carbon-Heteroatom Cross-Coupling using Nickel/Photo Dual Catalysis. Chem.—Eur. J. 2020, 26, 5168–5173. 10.1002/chem.202000052. [DOI] [PubMed] [Google Scholar]
- Cavedon C.; Madani A.; Seeberger P. H.; Pieber B. Semiheterogeneous Dual Nickel/Photocatalytic (Thio)etherification Using Carbon Nitrides. Org. Lett. 2019, 21, 5331–5334. 10.1021/acs.orglett.9b01957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keaveney S. T.; Schoenebeck F. Palladium-Catalyzed Decarbonylative Trifluoromethylation of Acid Fluorides. Angew. Chem., Int. Ed. 2018, 57, 4073–4077. 10.1002/anie.201800644. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.