Abstract
Due to the refractory and partial sensitive treatments to malignant cancers, immunotherapy has increasingly become a hotspot in effective anti-tumor research. However, at present, existing animal models could not accurately describe the interaction between human tissue and tumor cells for preclinical trials. Furthermore, it is a tough obstacle to reconstitute the immune system and microenvironment in a mouse model identical to humans due to species differences. In the establishment of the humanized mouse model, the co-transplantation of human immunocytes with/without tissues and tumor cells is the key breakthrough to solve this problem. The compelling progress has been investigated in the preclinical drug test for diverse tumor types. This review mainly summarized the development of immunodeficient mice, and the construction and practicability of the humanized mouse model. Furthermore, the investigators also highlight the pros and cons, and recent progress in immunotherapy research for advanced utility of human cancer diseases.
Keywords: Immunodeficient mice, humanized mouse model, cancer, immunotherapy
Introduction
With the aging of the population, the morbidity of malignant tumor increases with the global increase in mortality [1]. Even though chemotherapy, radiotherapy and ectomy are the most effective methods for treatment, the postoperative five-year survival rate remains unsatisfactory. As a remarkable leap forward in the development of onco-immunology, the immunotherapy attacks tumor cells by potentiating functional lymphocytes, rather than direct killing, and this has become one of the hot position fields in cancer treatment [2-4]. However, the development of its preclinical therapeutic evaluation in animal experiments has always been plagued by the species diversity between mice and humans [5]. Although the mice experiment is advantageous for specific questions, the curative effect of immunotherapy in clinical trials could not be accurately predicted due to the discrepancy in immune system activation and responses. Therefore, there is an urgent demand for novel preclinical models that could help bridge this gap with an appropriate immune microenvironment.
The humanized mouse model, as a novel experimental animal in biomedical research, was established by the transplantation of human-derived peripheral blood mononuclear cells (PBMCs) or hematopoietic stem cells (HSCs), and this could be identified as foreign tissues by murine innate and adaptive immune systems [6-8]. In order to optimize this effect, the modification of specific genes in animals could make these immunodeficient, and completely lack of T, B and NK cells, leading to a highly efficient approach to solve the challenge of immune rejection. Furthermore, humanized mice could accept the xenograft steady growth with the mimic human immune system, and recent studies have investigated the comparability of attendant tumor biological reaction and those in cancer patients [9]. As a result, the establishment of cell-line-derived xenograft (CDX) and patient-derived xenograft (PDX) into humanized mouse models is a remarkable stride for facilitating diverse applications in the exploration of cancer pathogenesis, and the evaluation of therapeutic effects [10-12].
This study provides an overview of the recent advances in humanized mouse models, and highlights the pros and cons of different ways of modeling, in order to gain a promising perspective of different onco-immunology applications.
The brief history of immunodeficient mice strain
In order to establish humanized mice models, constant pursuits in specific genes deletion could become indispensable parts of engineering processes (Figure 1). That is, the aim of the purposeful mutation was to reduce murine cells, allowing severely immunodeficient mice to accept the transplanted human-derived immune cells or HSCs.
Figure 1.
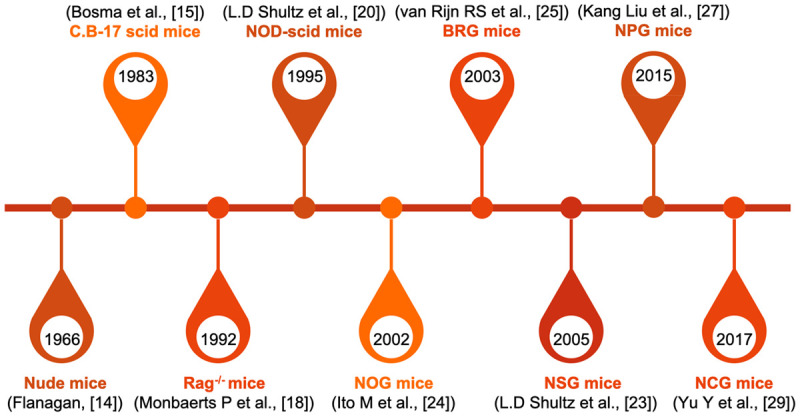
Advances in immunodeficient mice strain.
Nude mice, which lack of T lymphocytes, have been considered as the first models for malignant disease research, in which human tumor cells could steadily grow. However, the presence of B cells and innate immune cells led to severe rejection in the reconstruction of the human immune system [13,14]. As the root of the development of humanized mice, the C.B-17-Prkdcscid (C.B-17 scid) mice strain originated from the spontaneous subgene-mutation of catalytic polypeptide of DNA-activated protein kinase (Prkdc) [15]. The non-expression of Prkdc led to the lack of T and B lymphocytes, which is known as the symptom of severe combined immune deficiency (scid) syndrome [16]. Resulting in the avoidance of xenoreactivity, C.B-17 scid mice was made for the successful engraftments of human normal cells. Alternatively, the knockout of recombination activating gene (Rag) 1 or 2 (Rag1null or Rag2null) could cause the disruption of the V(D)J recombination. This might be a useful candidate for immunodeficiency [17-19]. Since these mice were developed and applied in scientific research, genetic engineering has become an important research field in human immune system reconstruction.
A significant breakthrough came with the introduction of scid mutation to the background of non-obese diabetic (NOD) mice, triggering a neoteric NOD-scid mice strain. These mice could remarkably improve the compatibility of the human immune system, which was driven by the reduction of innate immunity through the defective levels of NK and myeloid cell function [20-22]. However, mice with poor engraftment of human HSCs still remained to have a low utilization rate, which was probably due to the leakiness of murine T and B cells, and the remaining activity of NK cells.
In the early 2000, a major achievement in the history of humanized mice might be regarded as the knockout mutation that targeted the interleukin-2 receptor common γ-chain (IL2rgnull), and this played an essential role in the murine cytokine expression, including IL-2, 4, 7, 9, 15 and 21 [23-25]. The introduction of IL2rgnull mutation combined with scid mutation or Rag knockout generated three severely immunodeficient mice strains, namely, NSG (NOD.Cg-PrkdcscidIL2rgtm1Wjl), NOG (NOD.Cg-PrkdcscidIL2rgtm1Sug), and BRG (Balb/c Rag2-/-IL2rg-/-) [26]. Recently, some emerging strains have rapidly been developed, and have been internationally recognized as the family of the highest immunodeficient models, such as NPG (NOD-PrkdcscidIL2rgnull) and NCG (NOD-Prkdcem26IL2rgem26Nju) [27-29]. These new mice generations have exhibited a dramatic improvement in the rate of human engraftment, and have been recognized as the most frequently used models in the recapitulation of the human immune system.
Establishment of the humanized immune mouse model
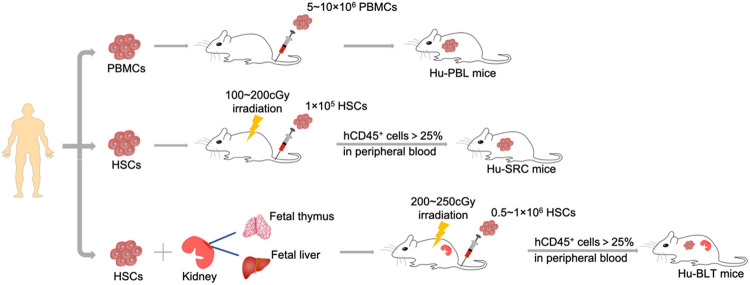
As one of the apparently different aspects between humans and mice, the innate immune system is the main reason for the mutual repulsion, which can be probably explained by the divergent evolution of these two species. The humanized immune mouse model, with severe combined immune deficiency as the background, has provided the possibility to overcome this problem. The principal standard approaches of humanization are summarized, as follows: (i) Hu-PBL, (ii) Hu-SRC, and (iii) Hu-BLT. The respective characteristics are described in detail below:
Hu-PBL mouse model
As widely recognized as the most cost-effective pattern, the hu-PBL mouse model has the simplest establishment process and a low expenditure. The transplantation of human PBMCs into immunodeficient mice by intravenous injection (I.V.) resulted in the majority of engraftment that comprised of T cells [7]. Researchers have argued that there is a lack of specific cytokines necessary for survival, which prevents B and NK cells from proliferating in vivo, but this could barely affect the microenvironment for the growth of T cells [30]. Compared with transplantation of CD34+ HSCs (explained below), the hu-PBL mouse model could reconstruct higher levels of human mature T cells, as well as the acquisition of CD3+, at approximately four weeks in advance [6,31]. The administration of IL-18 enhanced the grafting of human CD4+ and CD8+ T cells, as well as the observation of immunoglobulin A (IgA) deposits, impelling the development of an ideal humanized mouse model for IgA nephropathy [32]. In another combination with donor-matched dendritic cells (DCs), Harui A et al. reported that this represents a remarkable improvement in antigen responsiveness, breaking through the constraint of the absence of antigen-presenting cells [33]. Furthermore, these findings revealed that recombinant human prolactin (rhPRL) stimulation could dramatically promote the amount of human T cells engrafted into the thymus, lymph nodes and spleens, which is prone to the reconstitution of the human immune system in hu-PBL mice [34].
Although this made hu-PBL mice the best model for T-cell-related immune research, the invariability of the development of severe graft-versus-host disease (GVHD) has always led to limited experimental windows (usually a couple of weeks after PBMC injection, and depends on the quantity of the cell implantation) [35]. Recent reports have investigated that the pre-depletion of CD4+ T cells could yield to the alleviation of GVHD symptoms, even with the reduction in application scope [36]. For the alleviation of interracial immune rejection, a more common approach is the introduction of the genetic knock-out of the murine major histocompatibility complex (MHC) to prolong the survival period [37].
Hu-SRC mouse model
With a more complete immune reconstitution, the Hu-SRC mouse model, which is desired by the better recapitulation of human diseases, has also been commonly used. The approach of its establishment is to transfer the CD34+ HSCs obtained from the human bone marrow, umbilical cord blood (UCB), fetal liver (FL), or granulocyte colony-stimulating factor (G-CSF)-mobilized peripheral blood (MPB), presenting a multilineage development of hematopoietic cells [38]. In general, the criterion for its successful reconstruction is the existence of more than 25% of human CD45+ T cells in peripheral blood [8]. With the re-education of immunocytes generated from human HSCs in mice, the host rarely produces immunological rejection, thereby preserving the stabilization for up to 12 weeks [39]. The factors that affect the differentiation and maturation of immunocytes are mainly summarized as the HSC source, route of injection, and age of the recipient. Previously, Lepus et al. reported that FL or UCB-derived HSCs are superior to be colonized in vivo, when compared to the source of bone marrow and MPB, in terms of the significant proliferation of human CD45+ cells [40]. Newborn immunodeficient mice implanted with HSCs through intrahepatic injection within 72 hours of birth exhibited an accelerated T-cell maturation and better transplantation effect, when compared to adult mice [41]. However, this mouse model is hampered by the maturation of human T cells in the murine thymus with murine MHC, leading to the development of H2-restricted T cells, but these were not human leukocyte antigen (HLA)-restricted [38]. In addition, pre-experimental sub-lethal γ-irradiation is an indispensable step to emphasize the facilitation of human HSC engraftment due to the consequence of murine HSC depletion [42].
Hu-BLT mouse model
With regard to the distinction of MHCs, the advancement of the hu-BLT (bone marrow, liver and thymus) mouse model has strongly promoted the T-cell maturation in an autologous human thymus, getting rid of the murine H2 restriction. This provides T-cell differentiation a condition with human HLA restriction, and circumvents the critical unfeasibility of antigen-specific responses [43]. The latest protocol was described as the simultaneous implantation of human fetal thymus (FT) and FL pieces into the sub-renal capsule, and the injection of autologous CD34+ HSCs (from the same FL) [44]. Compared to the group without hepatocytes, Jinglong Guo et al. reported that the autologous hepatocyte engraftment was able to better reconstitute the immunocytes and chemokines in the liver, which is a potential for studies on immunopathogenesis and hepatotropic virus infections [45]. Using this strategy, this not only presented with the highest level of immune reconstitution with the complete immune components of B and T lymphocytes, macrophages, and dendritic cells, but also triggered a revolutionary leap to the repopulation of the human mucosal system for further studying mucosa-associated diseases, such as human immunodeficiency virus (HIV) and Ebola virus infection [46]. For this reason, the hu-BLT mouse model was applied to many aspects in HIV, including epidemic prevention, viral latency, and innate and adaptive immunity [47]. Importantly, the long-term maintenance of fully functional immunocytes in vivo drove its potential for an ideal model in immunology research, playing a specialty in tumor immunotherapy, chemotherapeutic drug response, and the prediction of cytokine release [48-50]. However, even though its mature human T cells are HLA-restricted, the experiment window was still greatly limited by the vulnerability to GVHD, and the symptoms revealed that this was significantly lighter than that of hu-PBL mice [51]. This might be attributed to the participation of murine DCs in the negative and positive selection process of human T-cell development. Kerry J Lavender et al. further revealed another strain with the genetic inactivation of CD47 on the C57BL/6 Rag2-/- γc-/- background, providing additional 15-20 weeks of healthy longevity [52]. Nonetheless, the existence of the mismatched HLA between immunocytes and tumor tissues is prone to immunologic rejection, suggesting that the engraftments for generation should preferably be derived from the same donor or carry out the HLA-matching detection. Thus, these complex techniques and ethical problems have become the most serious obstacles for its extensive usage due to the sophisticated surgical skills and required abortion material.
An overview of characteristics in these mice strains is outlined in Table 1 and Figure 2.
Table 1.
Comparison of establishments of the humanized immune mouse model
| Hu-PBL-SCID | Hu-SRC-SCID | Hu-BLT-SCID | |
|---|---|---|---|
| Source of immunocytes | PBMCs | Bone marrow; UCB; FL; MPB | FT; FL |
| Immune reconstitution | Mainly T cells (with activated phenotype) | Multiple of human hematopoietic lineages | Most completely functional immune system |
| Advantages | Easy and quick establishment; mature functional T cells | Injection to newborns increase reconstitution; long experimental window without GVHD | HLA-restricted T cells; development of mucosal human immune system |
| Limitations | Lack B or myeloid cells; development of GVHD within 4-6 weeks | H2-restricted T cells; Long period of cell differentiation; indispensable sub-lethal γ-irradiation | Long period of cell differentiation; indispensable sub-lethal γ-irradiation; increased possibility of GVHD; sophisticated technique and required material |
Figure 2.
Establishment of the humanized immune mouse model.
Next-generation of humanized mouse model
Although the technology of humanization is increasingly maturing, several key limitations have been amplified, powerfully impelling the development of genetic modification.
For example, the decisive ablation before HSC transplantation should be performed for supplying sufficient space for HSC cultivation in the bone marrow. Since the maintenance of HSCs is relevant to the expression of c-Kit (CD117), NBSGW mice, which carried the mutation of w41 in c-Kit, could overcome the inconvenient irradiation, and convert to a better HSC engraftment [53,54]. Meanwhile, the support of 5- to 12-fold higher rates of erythropoiesis and platelet development in NBSGW mice, compared with irradiated NSG mice, created bright potentials for studying human HSC differentiation and pathophysiology [55,56].
In addition, the lack of cross-reactivity of cytokines caused the minimal production of functional lymphoid cell differentiation [57]. This highlights that limitations are mainly due to the lack of IL-3, IL-4, IL-7, IL-15, stem cell factor (SCF), and thrombopoietin (TPO) [58]. In order to circumvent such limitations, replacing the coding genes of mice with human genes could induce a targeted expression. For example, it was reported that increased frequency of human NK cells were confirmed in human IL-7 and IL-15 double knock-in NSG mice engrafted with human HSCs [59]. Furthermore, the encoding-gene knock-ins of different cytokines and growth factors, including IL-3, SCF, macrophage colony-stimulating factor (M-CSF), granulocyte macrophage colony-stimulating factor (GM-CSF), TPO, or signal-regulatory protein alpha (SIRPα), into conventional humanized mouse models, produces NSG-SGM3, NOG-EXL and MISTRG mice for increasing the proportion of lymphoid cells (the details are shown in Table 2) [60-62]. Moreover, the immature T and B cells in the humanized mice engrafted with HSCs hardly resulted in the IgG antibody response to antigen stimulation. Qingfeng Chen et al. investigated that GM-CSF and IL-4 treatment could correct the defect by stimulating effective Ag-specific CD4+ T cell priming and inducting a significant level of B cells response, generating a novel mouse model for studying antibodies against clinically relevant targets [63]. The differentiation of IL-17-producing T-helper 17 (Th17) cells is generally stimulated by appropriate cytokines, including IL-6, IL-1β, TGF-β, and IL-23. Compared with non-expressive mice, it was observed that there was an aggressive expansion of IL-17- and interferon-gamma (IFN-γ)-producing pathogenic Th17 cells in the skin of the mouse models expressing human IL-1β and IL-23 [64]. In a pathogenesis study of airway inflammation, the administration of human IL-33 could activate Th2 and mast cells in the humanized mouse model, thereby driving the occurrence of asthmatic, as reported by Ryoji Ito et al. [65]. From these interesting observations, it could be inferred that among the various obstacles in the development and differentiation of immunocytes, the conservation of cytokines, which play one of the most important roles, could avoid the bias in the immunocyte maturation process.
Table 2.
Transgenic platforms for the humanized immune mouse model
| Name | Transgenic molecules | Advantages | Limitations | Reference |
|---|---|---|---|---|
| NBSGW | c-Kit | Without irradiation; development of erythropoiesis and platelet | Not reported | [56] |
| NSG-SGM3 | SCF, GM-CSF, IL-3 | Stable HSCs engraftment; increased monocytes, macrophages and DCs | Impaired stem cell function; short-term reconstitution | [72] |
| NOG-EXL | GM-CSF, IL-3 | Stable HSCs engraftment; increased monocytes, macrophages and DCs | Increased anemia | [73] |
| MISTRG | GM-CSF, IL-3, M-CSF, TPO, SIRPα | Stable HSCs engraftment; increased monocytes, macrophages and DCs; improved NK cells development | Increased anemia; shorter lifespan | [62] |
| NOG-dKO | β2mnull, IAβnull | Decreased GVHD | Less T-cell differentiation | [37] |
| NSG-HLA | HLA class I and II | HLA-restricted B- and T-cell functions; antigen-specific IgG responses | Pre-selection of HLA in donor cells | [74,75] |
A limitation in the present study was the mismatch of MHC among the species. In the case of hu-PBL mice, this might be charged to severe GVHD and the defect of T cell function. Thus, the administration of knock-out for murine class I and class II MHCs (β2mnull and IAβnull), which is also known as NOG-MHC double knockout (NOG-dKO) mice, was generated for milder xenograft rejection and GVHD, enabling a long-term window for experiments [66]. A more precise evaluation of anti-tumor T-cell response in immune checkpoint therapies was produced in NOG-dKO mice, when compared to NOG mice, due to the absence of the GVHD-induced nonspecific T-cell activation [67]. The further development of the transgenic engineered humanized mouse model was elucidated through the introduction of HLA class I and/or HLA class II molecules, which greatly promoted human HLA-restricted B- and T-cell functions [68,69], and antigen-specific IgG responses, making it a suitable model for investigating candidate vaccines and immunotherapies [70,71].
The comparisons of genetic modification among these mice strains are summarized in Table 2.
Preclinical applications for cancer immunotherapies in the humanized mouse model
Reproduction of the TME in hu-PDX mouse model
Tumor growth and therapeutic efficacy has been considered to be extremely associated to the tumor microenvironment (TME), which comprised of blood vessels, lymph vessels, fibroblast, stromal cells, immunocytes and the extracellular matrix (ECM) [76]. The topics and demands about this complex milieu are increasingly dominant in oncobiology, which is of great significance not only for the tumor development and metastasis, but also for the diagnosis, prevention and prognosis. Furthermore, the utility of humanized mice with cancer cell lines has been identified for immunotherapeutic evaluation, and the absence of individual tumor heredity directly limits the potential for personalized cancer treatment. Since the PDX model maintains the gene expression profiles and drug responses of patient-derived tumors, this could successfully recapitulate the TME for drug targets with genomic diversity, which is powerfully impelled to be the most reliable human tumor model [77].
Despite the rough persistence of tumor heterogeneity in PDX into immunosuppressed mice, the accurate assessment of immunotherapies is hurdled without the stable existence of human tumor-infiltrating lymphocytes (TILs), proceeding to the forward step of humanized PDX mouse models. Since the humanized PDX mouse model partially reproduces a TME similar to humans, the cytokines and chemokines released by tumor cells, stromal cells and TILs could regulate angiogenesis, metastasis and immune responses [78]. For example, myeloid-derived suppressor cells (MDSCs), which is a heterogeneity group with significant immunosuppressive activity in TME, emerges as a relative T cell inactivation, and this might due to the secretion of MDSC-induced reactive nitrogen species (RNS), and the induction of regulatory T cells (Tregs) in the presence of IFN-γ and IL-2 [79,80]. With the most lethal effect on cancer cells, CD8+ cytotoxic T lymphocytes (CTLs) also have multiple cross-talks with immunocytes, MDSCs, tumor-associated macrophages (TAMs) and cancer-associated fibroblasts (CAFs) through the promotion of cytokine secretion in TME [81]. A recent evidence from murine and clinical trials suggested that the dysfunctional states of intratumoral T cells might contribute to the multifaceted suppressive signals in TME, such as soluble mediators, metabolic factors and hypoxia [82].
Thus, understanding the role of TME in tumorigenesis and development might provide new strategies for tumor therapies. As the culmination of model technology, the simultaneous implantation of PDX and humanization recapitulates the interplay between the tumor and immunocytes, which especially brings about the increasing capacity for individual hypothesis outcomes [83]. The application examples are detailed in Table 3.
Table 3.
Application of the humanized mouse model for immunotherapy
| Disease | Tumor origin | Immune reconstitution | Therapy | Reference |
|---|---|---|---|---|
| Leukemia | CDX | HSCs | WT-1 TCR-T therapy | [86] |
| Melanoma | CDX | HSCs, FT, FL | F5 TCR-T therapy | [88] |
| B-ALL | PDX | HSCs, FT, FL | Anti-CD19 CAR-T therapy | [90] |
| Pancreatic cancer | CDX | PBMCs | PSCA CAR-T therapy | [92] |
| Gastric cancer | CDX | PBMCs | chA21-4-1BBz CAR-T therapy | [93] |
| NSCLC | CDX | PBMCs | EGFR CAR-T therapy | [94] |
| TNBC | PDX | HSCs | Anti-PD-1 therapy | [101] |
| NSCLC | CDX&PDX | PBMCs, HSCs | Anti-PD-1/PD-L1 therapy | [102] |
| Colorectal cancer | PDX | HSCs | Anti-PD-1 therapy | [103] |
| Osteosarcoma | CDX | PBMCs | Anti-PD-1 therapy | [100] |
| Lymphoma | CDX | HSCs, FT, FL | Anti-PD-1/CTLA-4 therapy | [104] |
| Ovarian cancer | PDX | TILs | Anti-PD-1 therapy | [105] |
| HCC | PDX | HSCs | Anti-PD-1/CTLA-4 therapy | [9] |
| Mesothelioma | CDX | PBMCs | CAR-T + anti-PD-1 therapy | [106] |
Humanized mouse model for genetic-modified T cells
Adoptive cellular therapy (ACT) refers to the expansion of the immunocompetent cells in vitro, and the reinjection back to the patients themselves, which stimulate the immune response for targeted killing tumor cells [84]. ACT infusion is mainly based on engineered T cells that express transgenic T cell receptors (TCRs) or chimeric antigen receptors (CARs), improving the affinity with tumor-associated antigens (TAAs) [85]. Although ACT has achieved ideal benefits for cancer patients, with the constant improvement of experimental recipients in vivo, the mechanism and safety of TCR-T/CAR-T cell therapy in exerting an explicitly potent antitumor effect was explored in the humanized mouse model, rather than in immunodeficient mice.
TCRs are natural antigen receptors that occur on the surface of T cells, which bind its cognate tumor antigen through the MHC-peptide complex. This is a strategic fulcrum for TCR-T cell therapy, which introduces the selected TCR gene sequence encoding the recognizing specific TAAs into T cells, enabling T cells without tumor recognition ability to effectively kill tumor cells. The related optimization research of transgenic TCRs has been carried out in humanized mice. Yuho Najima et al. recently conformed the development of transgenic Wilm’s Tumor-1 (WT-1) specific TCRs in HLA-I transgenic NSG mice transplanted with HSCs. WT-1 specific CTLs retained the capacity for proliferation, and exerts an antigen-specific cytokine response, amplifying the anti-tumor function [86]. The analogic results were supported by Francesca Giannoni et al. and Dimitrios N Vatakis et al. in the hu-BLT mouse model, in which transgenic CTLs specific for MART-1 (melanoma antigen recognized by T cells) were optimized for long-term function and the generation of tumor-targeted mature T cells in melanoma immunotherapy [87,88].
CARs serve as a “plug” for T cells to identify the targeted proteins on the surface of cancer cells, without the restriction of MHC. At present, CAR-T cell therapy has produced remarkable results in the treatment of B-cell lymphoma, leukemia, and other hematological malignancies, but these were less in solid tumors [89]. Chun-Hui Jin et al. generated a genetically modified CAR targeting CD19 in the hu-BLT mouse model with primary acute B-lymphoblastic leukemia (B-ALL), providing a proof-of-principle for the potential utility to characterize the host immunological changes associated with CAR-T cell therapy [90]. In addition, the introduction of a chimeric costimulatory motif, such as the most commonly used CD28 and CD137 (4-1BB), would trigger optimal signaling to induce CAR-T cell response, when compared to a CAR without co-stimulation. For example, Pratiksha Gulati et al. used the hu-SRC mouse model, and indicated the distinct capability of Δ-CD28/CD3ζ CAR-T cells to eradicate tumor growth and persist a durable antineoplastic activity [91]. Although CAR-T therapy has not achieved ideal clinical efficacy in solid tumors, this has made promising prospects in immunodeficient mice in various tumor CARs specific for prostate stem cell antigen (PSCA), chA21-4-1BBz, epidermal growth factor receptor (EGFR), etc. [92-94]. However, the safety of CAR-T cell therapy is frequently associated with treatment-related adverse reactions, including cytokine-release syndrome (CRS) and neurotoxicity. Marco L Davila et al. elucidated that these are primarily determined by human monocyte/macrophage-derived IL-1 and IL-6 [95]. Even though conventional hu-NSG mice could serve as an ideal model for preclinical efficacy studies, the identification of CRS (including cell function and cytokine production) could be tested in the hu-SGM3 strain, but not in hu-NSG mice, which might be due to the dependence of GM-CSF [96].
Humanized mouse model for immune checkpoint blockade therapy
The recognition and lethal effectiveness of immunocytes on target cells play a key role in immuno-oncology, which could be evaded by tumor cells through multi-approaches. Inhibitory receptors and signaling pathways, which are officially known as immune checkpoints, mainly include programmed cell death protein-1 (PD-1), cytotoxic T-lymphocyte-associated protein-4 (CTLA-4), T cell immunoglobulin-3 (TIM-3) and lymphocyte activation gene-3 (LAG-3), and are regulatory molecules that possess the suppression of excessively activated T cells from attacking self-tissues, thereby preventing the occurrence of autoimmune effects. The monoclonal antibodies of these immune checkpoints could cut the brakes from the self-recognition on T cells for activated function [97]. PD-1 and CTLA-4 inhibitors have won the Nobel Prize in physiology or medicine in 2018, changing the setup of cancer treatment. However, there are typical differences between mouse and human DNA in the expression fragment of immune checkpoints. The remarkably unique superiority of the humanized mouse model in the study of immune checkpoint inhibitors has been confirmed, in order to evaluate the effectiveness of individual clinical consultation [98]. Simultaneously, based on homologous recombination by CRISPR-Cas9, a new generation of human PD-1xLAG-3 knock-in mice, which replaced the mouse Pdcd1 gene with human PDCD1 to express human PD-1 protein, also accounted as a great place [99]. The potential outcome from these models was to permit the assessment of human immune response, including the analysis of tumor volume, lymphocyte proportion, cytokines, and etc. (the details are shown in Table 4). In the administration of antibodies against PD-1 and CTLA-4, the mono- or combination therapy revealed the significant tumor inhibition in humanized mice bearing CDX or PDX triple-negative breast cancer (TNBC), non-small cell lung cancer (NSCLC), colorectal cancer (CRC), osteosarcoma, lymphoma, ovarian cancer, and hepatocellular carcinoma (HCC) [100-105]. Furthermore, Leonid Cherkassky et al. elucidated that the simultaneous induction of the PD-1 blockade with CAR-T cell therapy could enhance the efficacy of monotherapy, promoting the onco-immunotherapy into the advanced era of comprehensive treatment [106].
Table 4.
Pharmacodynamic assessment of immune checkpoint therapies in the humanized mouse model
| Drug name | Target | Mouse model | Tumor character | Assessment | Reference |
|---|---|---|---|---|---|
| Nivolumab | PD-1 | BRG-SIRPα, engrafted with HSCs | TNBC, CRC | Inhibition of tumor growth curve; increased expression of IFN-γ+ and HLADR+ on hCD8+T cells | [103] |
| Pembrolizumab | PD-1 | NSG, engrafted with HSCs | Dedifferentiated liposarcoma | Inhibition of tumor growth curve; increased number of hCD3+hCD8+, hCD8+IFNγ+ T cells and hCD56+Ki-67+ NK cells | [107] |
| Sintilimab | PD-1 | NOG, engrafted with PBMCs | NCI-H292 | Inhibition of tumor growth curve and tumor weight; upregulation of IL-2; increased number of hCD3+, hCD8+, hCD8+IFNγ+ T cells; an increase in hCD8+T/hTregs ratio | [108] |
| Atezolizumab | PD-L1 | NSG, engrafted with HSCs | A375, A549, Caki-1, H1299, H1975, etc. | Inhibition of tumor growth curve; upregulation of hCD3+, hCD4+, and hCD8+ T cells; variable expression of PD-1 on T cells and PD-L1 on tumor cells and macrophages | [109] |
| Nivolumab Ipilimumab | PD-1 CTLA-4 | NSG, engrafted with HSCs | Nasopharyngeal carcinoma | Inhibition of tumor growth curve; upregulation of IFN-γ, IL-6; increased expression of HLA-DR+ on hCD8+ T cells; a decrease in hCD4+/hCD8+ ratio | [110] |
Humanized mouse model for NK cells therapy
NK cells are identified as one of the most principal segments of tumor immune surveillance. The activation of NK cells mainly through cytokines and other ex-stimulation is associated with more powerful antitumor function. Thus, efforts on the onco-immune research have increasingly focused on the NK cells activity and related cytokines therapy [111].
In HCS-engrafted NSG mice transplanted with human breast cancer cells, Anja K Wege et al. reported the further expansion of NK cell accumulation in all lymphoid and extra lymphoid tissues, which are dependent on the IL-15 treatment [5]. In addition, hIL-7 and hIL-15 double knock-in improved the account of NK cells engraftment in NSG mice injected with HSCs [59]. In contrast to humanized NSG mice, human IL-15 and SIRPα knock-in mouse models injected with HSCs based on the Rag2-/- IL2rg-/- background, which is known as the humanized SRG-15 mice strain, demonstrated the highly identical expression of inhibitory receptors on NK cells to humans [112]. Using the humanized SRG-15 mouse model, it was observed that the dramatic maturation and tissue residence of NK cells aimed to boost the preclinical research of NK-cell targeted therapies.
Future perspectives
In recent years, cancer treatment has made revolutionary progress, along with the striking increase of curative and survival ratio in cancer patients. As an effective tool for immunotherapeutic evaluation, the humanized mouse model could similarly reproduce the human immune microenvironment for simulating the process of tumor occurrence, development and metastasis. Particularly, the introduction of human immunocytes in the PDX mouse model improved the predictability of potential preclinical therapies in individuals. However, its establishment and application still face many challenges, including the MHC incompatibility between immunocytes and tumors, the residual murine innate immunocytes, and the lack of specific-specific cytokines. In order to address these gaps, several modifications with transgenic MHC or tissue implantation resulted in the increase in functionality level and complete subsets of the human immune system, but with more sophisticated technology. In addition, the application evaluation of targeted immunotherapy benefited from humanized mice. However, the effect of immunomodulatory therapy needs to be further explored. Thus, the long-term revolution for optimization of humanized mouse model in onco-immunology would be bound to provide an unprecedented platform for cancer immunotherapy.
Acknowledgements
This study was supported by National Science and Technology Major Project of China, No. 2018ZX10303502-003; Beijing Municipal Natural Science Foundation, No. 7192084; The Capital Health Research and Development of Special, No. 2020-2-1152; Beijing Municipal Institute of Public Medical Research Development and Reform Pilot Project (Jingyiyan 2019-6).
Disclosure of conflict of interest
None.
References
- 1.Ghouri YA, Mian I, Rowe JH. Review of hepatocellular carcinoma: epidemiology, etiology, and carcinogenesis. J Carcinog. 2017;16:1. doi: 10.4103/jcar.JCar_9_16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Aravalli RN, Steer CJ. Immune-mediated therapies for liver cancer. Genes (Basel) 2017;8:76. doi: 10.3390/genes8020076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Yang Y. Cancer immunotherapy: harnessing the immune system to battle cancer. J Clin Invest. 2015;125:3335–3337. doi: 10.1172/JCI83871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kakimi K, Karasaki T, Matsushita H, Sugie T. Advances in personalized cancer immunotherapy. Breast Cancer. 2017;24:16–24. doi: 10.1007/s12282-016-0688-1. [DOI] [PubMed] [Google Scholar]
- 5.Wege AK, Ernst W, Eckl J, Frankenberger B, Vollmann-Zwerenz A, Mannel DN, Ortmann O, Kroemer A, Brockhoff G. Humanized tumor mice--a new model to study and manipulate the immune response in advanced cancer therapy. Int J Cancer. 2011;129:2194–2206. doi: 10.1002/ijc.26159. [DOI] [PubMed] [Google Scholar]
- 6.De La Rochere P, Guil-Luna S, Decaudin D, Azar G, Sidhu SS, Piaggio E. Humanized mice for the study of immuno-oncology. Trends Immunol. 2018;39:748–763. doi: 10.1016/j.it.2018.07.001. [DOI] [PubMed] [Google Scholar]
- 7.Flerin NC, Bardhi A, Zheng JH, Korom M, Folkvord J, Kovacs C, Benko E, Truong R, Mota T, Connick E, Jones RB, Lynch RM, Goldstein H. Establishment of a novel humanized mouse model to investigate in vivo activation and depletion of patient-derived HIV latent reservoirs. J Virol. 2019;93:e02051–18. doi: 10.1128/JVI.02051-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Meraz IM, Majidi M, Meng F, Shao R, Ha MJ, Neri S, Fang B, Lin SH, Tinkey PT, Shpall EJ, Morris J, Roth JA. An improved patient-derived xenograft humanized mouse model for evaluation of lung cancer immune responses. Cancer Immunol Res. 2019;7:1267–1279. doi: 10.1158/2326-6066.CIR-18-0874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zhao Y, Shuen TWH, Toh TB, Chan XY, Liu M, Tan SY, Fan Y, Yang H, Lyer SG, Bonney GK, Loh E, Chang KTE, Tan TC, Zhai W, Chan JKY, Chow EK, Chee CE, Lee GH, Dan YY, Chow PK, Toh HC, Lim SG, Chen Q. Development of a new patient-derived xenograft humanised mouse model to study human-specific tumour microenvironment and immunotherapy. Gut. 2018;67:1845–1854. doi: 10.1136/gutjnl-2017-315201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ny L, Rizzo LY, Belgrano V, Karlsson J, Jespersen H, Carstam L, Bagge RO, Nilsson LM, Nilsson JA. Supporting clinical decision making in advanced melanoma by preclinical testing in personalized immune-humanized xenograft mouse models. Ann Oncol. 2020;31:266–273. doi: 10.1016/j.annonc.2019.11.002. [DOI] [PubMed] [Google Scholar]
- 11.Wang M, Yao LC, Cheng M, Cai D, Martinek J, Pan CX, Shi W, Ma AH, De Vere White RW, Airhart S, Liu ET, Banchereau J, Brehm MA, Greiner DL, Shultz LD, Palucka K, Keck JG. Humanized mice in studying efficacy and mechanisms of PD-1-targeted cancer immunotherapy. FASEB J. 2018;32:1537–1549. doi: 10.1096/fj.201700740R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Buque A, Galluzzi L. Modeling tumor immunology and immunotherapy in mice. Trends Cancer. 2018;4:599–601. doi: 10.1016/j.trecan.2018.07.003. [DOI] [PubMed] [Google Scholar]
- 13.Fujiwara S. Humanized mice: a brief overview on their diverse applications in biomedical research. J Cell Physiol. 2018;233:2889–2901. doi: 10.1002/jcp.26022. [DOI] [PubMed] [Google Scholar]
- 14.Flanagan SP. ‘Nude’, a new hairless gene with pleiotropic effects in the mouse. Genet Res. 1966;8:295–309. doi: 10.1017/s0016672300010168. [DOI] [PubMed] [Google Scholar]
- 15.Bosma GC, Custer RP, Bosma MJ. A severe combined immunodeficiency mutation in the mouse. Nature. 1983;301:527–530. doi: 10.1038/301527a0. [DOI] [PubMed] [Google Scholar]
- 16.Bosma MJ, Carroll AM. The SCID mouse mutant: definition, characterization, and potential uses. Annu Rev Immunol. 1991;9:323–350. doi: 10.1146/annurev.iy.09.040191.001543. [DOI] [PubMed] [Google Scholar]
- 17.Harris DT, Badowski M. Long term human reconstitution and immune aging in NOD-Rag (-)-gamma chain (-) mice. Immunobiology. 2014;219:131–137. doi: 10.1016/j.imbio.2013.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mombaerts P, Iacomini J, Johnson RS, Herrup K, Tonegawa S, Papaioannou VE. RAG-1-deficient mice have no mature B and T lymphocytes. Cell. 1992;68:869–877. doi: 10.1016/0092-8674(92)90030-g. [DOI] [PubMed] [Google Scholar]
- 19.Sanmamed MF, Rodriguez I, Schalper KA, Onate C, Azpilikueta A, Rodriguez-Ruiz ME, Morales-Kastresana A, Labiano S, Perez-Gracia JL, Martin-Algarra S, Alfaro C, Mazzolini G, Sarno F, Hidalgo M, Korman AJ, Jure-Kunkel M, Melero I. Nivolumab and urelumab enhance antitumor activity of human T lymphocytes engrafted in Rag2-/-IL2Rgammanull immunodeficient mice. Cancer Res. 2015;75:3466–3478. doi: 10.1158/0008-5472.CAN-14-3510. [DOI] [PubMed] [Google Scholar]
- 20.Shultz LD, Schweitzer PA, Christianson SW, Gott B, Schweitzer IB, Tennent B, McKenna S, Mobraaten L, Rajan TV, Greiner DL, et al. Multiple defects in innate and adaptive immunologic function in NOD/LtSz-scid mice. J Immunol. 1995;154:180–191. [PubMed] [Google Scholar]
- 21.Pflumio F, Izac B, Katz A, Shultz LD, Vainchenker W, Coulombel L. Phenotype and function of human hematopoietic cells engrafting immune-deficient CB17-severe combined immunodeficiency mice and nonobese diabetic-severe combined immunodeficiency mice after transplantation of human cord blood mononuclear cells. Blood. 1996;88:3731–3740. [PubMed] [Google Scholar]
- 22.Greiner DL, Shultz LD, Yates J, Appel MC, Perdrizet G, Hesselton RM, Schweitzer I, Beamer WG, Shultz KL, Pelsue SC, et al. Improved engraftment of human spleen cells in NOD/LtSz-scid/scid mice as compared with C. B-17-scid/scid mice. Am J Pathol. 1995;146:888–902. [PMC free article] [PubMed] [Google Scholar]
- 23.Shultz LD, Lyons BL, Burzenski LM, Gott B, Chen X, Chaleff S, Kotb M, Gillies SD, King M, Mangada J, Greiner DL, Handgretinger R. Human lymphoid and myeloid cell development in NOD/LtSz-scid IL2R gamma null mice engrafted with mobilized human hemopoietic stem cells. J Immunol. 2005;174:6477–6489. doi: 10.4049/jimmunol.174.10.6477. [DOI] [PubMed] [Google Scholar]
- 24.Ito M, Hiramatsu H, Kobayashi K, Suzue K, Kawahata M, Hioki K, Ueyama Y, Koyanagi Y, Sugamura K, Tsuji K, Heike T, Nakahata T. NOD/SCID/gamma(c)(null) mouse: an excellent recipient mouse model for engraftment of human cells. Blood. 2002;100:3175–3182. doi: 10.1182/blood-2001-12-0207. [DOI] [PubMed] [Google Scholar]
- 25.van Rijn RS, Simonetti ER, Hagenbeek A, Hogenes MC, de Weger RA, Canninga-van Dijk MR, Weijer K, Spits H, Storm G, van Bloois L, Rijkers G, Martens AC, Ebeling SB. A new xenograft model for graft-versus-host disease by intravenous transfer of human peripheral blood mononuclear cells in RAG2-/- gammac-/- double-mutant mice. Blood. 2003;102:2522–2531. doi: 10.1182/blood-2002-10-3241. [DOI] [PubMed] [Google Scholar]
- 26.Wege AK. Humanized mouse models for the preclinical assessment of cancer immunotherapy. BioDrugs. 2018;32:245–266. doi: 10.1007/s40259-018-0275-4. [DOI] [PubMed] [Google Scholar]
- 27.Liu K, Fang R, Li H, Yang W, Miao Z, Wen J, Deng H. Efficient derivation of embryonic stem cells from NOD-scid Il2rg (-/-) mice. Protein Cell. 2015;6:916–918. doi: 10.1007/s13238-015-0209-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bai Y, Kan S, Zhou S, Wang Y, Xu J, Cooke JP, Wen J, Deng H. Enhancement of the in vivo persistence and antitumor efficacy of CD19 chimeric antigen receptor T cells through the delivery of modified TERT mRNA. Cell Discov. 2015;1:15040. doi: 10.1038/celldisc.2015.40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yu Y, Li J, Zhu X, Tang X, Bao Y, Sun X, Huang Y, Tian F, Liu X, Yang L. Humanized CD7 nanobody-based immunotoxins exhibit promising anti-T-cell acute lymphoblastic leukemia potential. Int J Nanomedicine. 2017;12:1969–1983. doi: 10.2147/IJN.S127575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Shultz LD, Pearson T, King M, Giassi L, Carney L, Gott B, Lyons B, Rossini AA, Greiner DL. Humanized NOD/LtSz-scid IL2 receptor common gamma chain knockout mice in diabetes research. Ann N Y Acad Sci. 2007;1103:77–89. doi: 10.1196/annals.1394.002. [DOI] [PubMed] [Google Scholar]
- 31.Walsh NC, Kenney LL, Jangalwe S, Aryee KE, Greiner DL, Brehm MA, Shultz LD. Humanized mouse models of clinical disease. Annu Rev Pathol. 2017;12:187–215. doi: 10.1146/annurev-pathol-052016-100332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Senpuku H, Asano T, Matin K, Salam MA, Tsuha Y, Horibata S, Shimazu Y, Soeno Y, Aoba T, Sata T, Hanada N, Honda M. Effects of human interleukin-18 and interleukin-12 treatment on human lymphocyte engraftment in NOD-scid mouse. Immunology. 2002;107:232–242. doi: 10.1046/j.1365-2567.2002.01484.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Harui A, Kiertscher SM, Roth MD. Reconstitution of huPBL-NSG mice with donor-matched dendritic cells enables antigen-specific T-cell activation. J Neuroimmune Pharmacol. 2011;6:148–157. doi: 10.1007/s11481-010-9223-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sun R, Zhang J, Zhang C, Zhang J, Liang S, Sun A, Wang J, Tian Z. Human prolactin improves engraftment and reconstitution of human peripheral blood lymphocytes in SCID mice. Cell Mol Immunol. 2004;1:129–136. [PubMed] [Google Scholar]
- 35.Pyo KH, Kim JH, Lee JM, Kim SE, Cho JS, Lim SM, Cho BC. Promising preclinical platform for evaluation of immuno-oncology drugs using Hu-PBL-NSG lung cancer models. Lung Cancer. 2019;127:112–121. doi: 10.1016/j.lungcan.2018.11.035. [DOI] [PubMed] [Google Scholar]
- 36.Li J, Li X, Liang C, Ling L, Chen Z, Wong CK, Waldmann H, Lui KO. Coreceptor blockade targeting CD4 and CD8 allows acceptance of allogeneic human pluripotent stem cell grafts in humanized mice. Biomaterials. 2020;248:120013. doi: 10.1016/j.biomaterials.2020.120013. [DOI] [PubMed] [Google Scholar]
- 37.Brehm MA, Kenney LL, Wiles MV, Low BE, Tisch RM, Burzenski L, Mueller C, Greiner DL, Shultz LD. Lack of acute xenogeneic graft- versus-host disease, but retention of T-cell function following engraftment of human peripheral blood mononuclear cells in NSG mice deficient in MHC class I and II expression. FASEB J. 2019;33:3137–3151. doi: 10.1096/fj.201800636R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Watanabe Y, Takahashi T, Okajima A, Shiokawa M, Ishii N, Katano I, Ito R, Ito M, Minegishi M, Minegishi N, Tsuchiya S, Sugamura K. The analysis of the functions of human B and T cells in humanized NOD/shi-scid/gammac (null) (NOG) mice (hu-HSC NOG mice) Int Immunol. 2009;21:843–858. doi: 10.1093/intimm/dxp050. [DOI] [PubMed] [Google Scholar]
- 39.Danisch S, Slabik C, Cornelius A, Albanese M, Tagawa T, Chen YA, Kronke N, Eiz-Vesper B, Lienenklaus S, Bleich A, Theobald SJ, Schneider A, Ganser A, von Kaisenberg C, Zeidler R, Hammerschmidt W, Feuerhake F, Stripecke R. Spatiotemporally skewed activation of programmed cell death receptor 1-positive T cells after epstein-barr virus infection and tumor development in long-term fully humanized mice. Am J Pathol. 2019;189:521–539. doi: 10.1016/j.ajpath.2018.11.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lepus CM, Gibson TF, Gerber SA, Kawikova I, Szczepanik M, Hossain J, Ablamunits V, Kirkiles-Smith N, Herold KC, Donis RO, Bothwell AL, Pober JS, Harding MJ. Comparison of human fetal liver, umbilical cord blood, and adult blood hematopoietic stem cell engraftment in NOD-scid/gammac-/-, Balb/c-Rag1-/-gammac-/-, and C. B-17-scid/bg immunodeficient mice. Hum Immunol. 2009;70:790–802. doi: 10.1016/j.humimm.2009.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Katano I, Ito R, Kamisako T, Eto T, Ogura T, Kawai K, Suemizu H, Takahashi T, Kawakami Y, Ito M. NOD-Rag2null IL-2Rgammanull mice: an alternative to NOG mice for generation of humanized mice. Exp Anim. 2014;63:321–330. doi: 10.1538/expanim.63.321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Audige A, Rochat MA, Li D, Ivic S, Fahrny A, Muller CKS, Gers-Huber G, Myburgh R, Bredl S, Schlaepfer E, Scherrer AU, Kuster SP, Speck RF. Long-term leukocyte reconstitution in NSG mice transplanted with human cord blood hematopoietic stem and progenitor cells. BMC Immunol. 2017;18:28. doi: 10.1186/s12865-017-0209-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lan P, Tonomura N, Shimizu A, Wang S, Yang YG. Reconstitution of a functional human immune system in immunodeficient mice through combined human fetal thymus/liver and CD34+ cell transplantation. Blood. 2006;108:487–492. doi: 10.1182/blood-2005-11-4388. [DOI] [PubMed] [Google Scholar]
- 44.Brainard DM, Seung E, Frahm N, Cariappa A, Bailey CC, Hart WK, Shin HS, Brooks SF, Knight HL, Eichbaum Q, Yang YG, Sykes M, Walker BD, Freeman GJ, Pillai S, Westmoreland SV, Brander C, Luster AD, Tager AM. Induction of robust cellular and humoral virus-specific adaptive immune responses in human immunodeficiency virus-infected humanized BLT mice. J Virol. 2009;83:7305–7321. doi: 10.1128/JVI.02207-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Guo J, Li Y, Shan Y, Shu C, Wang F, Wang X, Zheng G, He J, Hu Z, Yang YG. Humanized mice reveal an essential role for human hepatocytes in the development of the liver immune system. Cell Death Dis. 2018;9:667. doi: 10.1038/s41419-018-0720-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Escudero-Perez B, Ruibal P, Rottstegge M, Ludtke A, Port JR, Hartmann K, Gomez-Medina S, Muller-Guhl J, Nelson EV, Krasemann S, Rodriguez E, Munoz-Fontela C. Comparative pathogenesis of Ebola virus and Reston virus infection in humanized mice. JCI Insight. 2019;4:e126070. doi: 10.1172/jci.insight.126070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Karpel ME, Boutwell CL, Allen TM. BLT humanized mice as a small animal model of HIV infection. Curr Opin Virol. 2015;13:75–80. doi: 10.1016/j.coviro.2015.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Yan H, Semple KM, Gonzalez CM, Howard KE. Bone marrow-liver-thymus (BLT) immune humanized mice as a model to predict cytokine release syndrome. Transl Res. 2019;210:43–56. doi: 10.1016/j.trsl.2019.04.007. [DOI] [PubMed] [Google Scholar]
- 49.Akkina R. Human immune responses and potential for vaccine assessment in humanized mice. Curr Opin Immunol. 2013;25:403–409. doi: 10.1016/j.coi.2013.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kaur K, Topchyan P, Kozlowska AK, Ohanian N, Chiang J, Maung PO, Park SH, Ko MW, Fang C, Nishimura I, Jewett A. Super-charged NK cells inhibit growth and progression of stem-like/poorly differentiated oral tumors in vivo in humanized BLT mice; effect on tumor differentiation and response to chemotherapeutic drugs. Oncoimmunology. 2018;7:e1426518. doi: 10.1080/2162402X.2018.1426518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Shultz LD, Brehm MA, Garcia-Martinez JV, Greiner DL. Humanized mice for immune system investigation: progress, promise and challenges. Nat Rev Immunol. 2012;12:786–798. doi: 10.1038/nri3311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Lavender KJ, Pang WW, Messer RJ, Duley AK, Race B, Phillips K, Scott D, Peterson KE, Chan CK, Dittmer U, Dudek T, Allen TM, Weissman IL, Hasenkrug KJ. BLT-humanized C57BL/6 Rag2-/-gammac-/-CD47-/- mice are resistant to GVHD and develop B- and T-cell immunity to HIV infection. Blood. 2013;122:4013–4020. doi: 10.1182/blood-2013-06-506949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kwon HS, Logan AC, Chhabra A, Pang WW, Czechowicz A, Tate K, Le A, Poyser J, Hollis R, Kelly BV, Kohn DB, Weissman IL, Prohaska SS, Shizuru JA. Anti-human CD117 antibody-mediated bone marrow niche clearance in nonhuman primates and humanized NSG mice. Blood. 2019;133:2104–2108. doi: 10.1182/blood-2018-06-853879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.McIntosh BE, Brown ME, Duffin BM, Maufort JP, Vereide DT, Slukvin II, Thomson JA. Nonirradiated NOD,B6. SCID Il2rgamma-/- Kit (W41/W41) (NBSGW) mice support multilineage engraftment of human hematopoietic cells. Stem Cell Rep. 2015;4:171–180. doi: 10.1016/j.stemcr.2014.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Rahmig S, Kronstein-Wiedemann R, Fohgrub J, Kronstein N, Nevmerzhitskaya A, Bornhauser M, Gassmann M, Platz A, Ordemann R, Tonn T, Waskow C. Improved human erythropoiesis and platelet formation in humanized NSGW41 mice. Stem Cell Rep. 2016;7:591–601. doi: 10.1016/j.stemcr.2016.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Leonard A, Yapundich M, Nassehi T, Gamer J, Drysdale CM, Haro-Mora JJ, Demirci S, Hsieh MM, Uchida N, Tisdale JF. Low-dose busulfan reduces human CD34(+) cell doses required for engraftment in c-kit mutant immunodeficient mice. Mol Ther Methods Clin Dev. 2019;15:430–437. doi: 10.1016/j.omtm.2019.10.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Rongvaux A, Takizawa H, Strowig T, Willinger T, Eynon EE, Flavell RA, Manz MG. Human hemato-lymphoid system mice: current use and future potential for medicine. Annu Rev Immunol. 2013;31:635–674. doi: 10.1146/annurev-immunol-032712-095921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Theocharides AP, Rongvaux A, Fritsch K, Flavell RA, Manz MG. Humanized hemato-lymphoid system mice. Haematologica. 2016;101:5–19. doi: 10.3324/haematol.2014.115212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Matsuda M, Ono R, Iyoda T, Endo T, Iwasaki M, Tomizawa-Murasawa M, Saito Y, Kaneko A, Shimizu K, Yamada D, Ogonuki N, Watanabe T, Nakayama M, Koseki Y, Kezuka-Shiotani F, Hasegawa T, Yabe H, Kato S, Ogura A, Shultz LD, Ohara O, Taniguchi M, Koseki H, Fujii SI, Ishikawa F. Human NK cell development in hIL-7 and hIL-15 knockin NOD/SCID/IL2rgKO mice. Life Sci Alliance. 2019;2:e201800195. doi: 10.26508/lsa.201800195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Perdomo-Celis F, Medina-Moreno S, Davis H, Bryant J, Taborda NA, Rugeles MT, Kottilil S, Zapata JC. High activation and skewed T cell differentiation are associated with low IL-17A levels in a hu-PBL-NSG-SGM3 mouse model of HIV infection. Clin Exp Immunol. 2020;200:185–198. doi: 10.1111/cei.13416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Weaver JL, Zadrozny LM, Gabrielson K, Semple KM, Shea KI, Howard KE. BLT-immune humanized mice as a model for nivolumab-induced immune-mediated adverse events: comparison of the NOG and NOG-EXL strains. Toxicol Sci. 2019;169:194–208. doi: 10.1093/toxsci/kfz045. [DOI] [PubMed] [Google Scholar]
- 62.Song Y, Rongvaux A, Taylor A, Jiang T, Tebaldi T, Balasubramanian K, Bagale A, Terzi YK, Gbyli R, Wang X, Fu X, Gao Y, Zhao J, Podoltsev N, Xu M, Neparidze N, Wong E, Torres R, Bruscia EM, Kluger Y, Manz MG, Flavell RA, Halene S. A highly efficient and faithful MDS patient-derived xenotransplantation model for pre-clinical studies. Nat Commun. 2019;10:366. doi: 10.1038/s41467-018-08166-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Chen Q, He F, Kwang J, Chan JK, Chen J. GM-CSF and IL-4 stimulate antibody responses in humanized mice by promoting T, B, and dendritic cell maturation. J Immunol. 2012;189:5223–5229. doi: 10.4049/jimmunol.1201789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Ito R, Katano I, Otsuka I, Hanazawa A, Takahashi T, Kawai K, Yagoto M, Goto M, Ogura T, Takahashi R, Ito M. Exacerbation of pathogenic Th17-cell-mediated cutaneous graft-versus-host-disease in human IL-1beta and IL-23 transgenic humanized mice. Biochem Biophys Res Commun. 2019;516:480–485. doi: 10.1016/j.bbrc.2019.06.094. [DOI] [PubMed] [Google Scholar]
- 65.Ito R, Maruoka S, Soda K, Katano I, Kawai K, Yagoto M, Hanazawa A, Takahashi T, Ogura T, Goto M, Takahashi R, Toyoshima S, Okayama Y, Izuhara K, Gon Y, Hashimoto S, Ito M, Nunomura S. A humanized mouse model to study asthmatic airway inflammation via the human IL-33/IL-13 axis. JCI Insight. 2018;3:e121580. doi: 10.1172/jci.insight.121580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Ashizawa T, Iizuka A, Nonomura C, Kondou R, Maeda C, Miyata H, Sugino T, Mitsuya K, Hayashi N, Nakasu Y, Maruyama K, Yamaguchi K, Katano I, Ito M, Akiyama Y. Antitumor effect of programmed death-1 (PD-1) blockade in humanized the NOG-MHC double knockout mouse. Clin Cancer Res. 2017;23:149–158. doi: 10.1158/1078-0432.CCR-16-0122. [DOI] [PubMed] [Google Scholar]
- 67.Yaguchi T, Kobayashi A, Inozume T, Morii K, Nagumo H, Nishio H, Iwata T, Ka Y, Katano I, Ito R, Ito M, Kawakami Y. Human PBMC-transferred murine MHC class I/II-deficient NOG mice enable long-term evaluation of human immune responses. Cell Mol Immunol. 2018;15:953–962. doi: 10.1038/cmi.2017.106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Shultz LD, Saito Y, Najima Y, Tanaka S, Ochi T, Tomizawa M, Doi T, Sone A, Suzuki N, Fujiwara H, Yasukawa M, Ishikawa F. Generation of functional human T-cell subsets with HLA-restricted immune responses in HLA class I expressing NOD/SCID/IL2r gamma(null) humanized mice. Proc Natl Acad Sci U S A. 2010;107:13022–13027. doi: 10.1073/pnas.1000475107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Billerbeck E, Horwitz JA, Labitt RN, Donovan BM, Vega K, Budell WC, Koo GC, Rice CM, Ploss A. Characterization of human antiviral adaptive immune responses during hepatotropic virus infection in HLA-transgenic human immune system mice. J Immunol. 2013;191:1753–1764. doi: 10.4049/jimmunol.1201518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Allam A, Majji S, Peachman K, Jagodzinski L, Kim J, Ratto-Kim S, Wijayalath W, Merbah M, Kim JH, Michael NL, Alving CR, Casares S, Rao M. TFH cells accumulate in mucosal tissues of humanized-DRAG mice and are highly permissive to HIV-1. Sci Rep. 2015;5:10443. doi: 10.1038/srep10443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Kim J, Peachman KK, Jobe O, Morrison EB, Allam A, Jagodzinski L, Casares SA, Rao M. Tracking human immunodeficiency virus-1 infection in the humanized DRAG mouse model. Front Immunol. 2017;8:1405. doi: 10.3389/fimmu.2017.01405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Billerbeck E, Barry WT, Mu K, Dorner M, Rice CM, Ploss A. Development of human CD4+FoxP3+ regulatory T cells in human stem cell factor-, granulocyte-macrophage colony-stimulating factor-, and interleukin-3-expressing NOD-SCID IL2Rgamma(null) humanized mice. Blood. 2011;117:3076–3086. doi: 10.1182/blood-2010-08-301507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Perdomo-Celis F, Medina-Moreno S, Davis H, Bryant J, Zapata JC. HIV Replication in humanized IL-3/GM-CSF-transgenic NOG mice. Pathogens. 2019;8:33. doi: 10.3390/pathogens8010033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Majji S, Wijayalath W, Shashikumar S, Pow-Sang L, Villasante E, Brumeanu TD, Casares S. Differential effect of HLA class-I versus class-II transgenes on human T and B cell reconstitution and function in NRG mice. Sci Rep. 2016;6:28093. doi: 10.1038/srep28093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Masse-Ranson G, Dusseaux M, Fiquet O, Darche S, Boussand M, Li Y, Lopez-Lastra S, Legrand N, Corcuff E, Toubert A, Centlivre M, Bruel T, Spits H, Schwartz O, Levy Y, Strick-Marchand H, Di Santo JP. Accelerated thymopoiesis and improved T-cell responses in HLA-A2/-DR2 transgenic BRGS-based human immune system mice. Eur J Immunol. 2019;49:954–965. doi: 10.1002/eji.201848001. [DOI] [PubMed] [Google Scholar]
- 76.Guo S, Deng CX. Effect of stromal cells in tumor microenvironment on metastasis initiation. Int J Biol Sci. 2018;14:2083–2093. doi: 10.7150/ijbs.25720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Chijiwa T, Kawai K, Noguchi A, Sato H, Hayashi A, Cho H, Shiozawa M, Kishida T, Morinaga S, Yokose T, Katayama M, Takenaka N, Suemizu H, Yamada R, Nakamura Y, Ohtsu T, Takano Y, Imai K, Miyagi Y, Nakamura M. Establishment of patient-derived cancer xenografts in immunodeficient NOG mice. Int J Oncol. 2015;47:61–70. doi: 10.3892/ijo.2015.2997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Morton JJ, Bird G, Keysar SB, Astling DP, Lyons TR, Anderson RT, Glogowska MJ, Estes P, Eagles JR, Le PN, Gan G, McGettigan B, Fernandez P, Padilla-Just N, Varella-Garcia M, Song JI, Bowles DW, Schedin P, Tan AC, Roop DR, Wang XJ, Refaeli Y, Jimeno A. XactMice: humanizing mouse bone marrow enables microenvironment reconstitution in a patient-derived xenograft model of head and neck cancer. Oncogene. 2016;35:290–300. doi: 10.1038/onc.2015.94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Kumar V, Patel S, Tcyganov E, Gabrilovich DI. The nature of myeloid-derived suppressor cells in the tumor microenvironment. Trends Immunol. 2016;37:208–220. doi: 10.1016/j.it.2016.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Feng S, Cheng X, Zhang L, Lu X, Chaudhary S, Teng R, Frederickson C, Champion MM, Zhao R, Cheng L, Gong Y, Deng H, Lu X. Myeloid-derived suppressor cells inhibit T cell activation through nitrating LCK in mouse cancers. Proc Natl Acad Sci U S A. 2018;115:10094–10099. doi: 10.1073/pnas.1800695115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Farhood B, Najafi M, Mortezaee K. CD8(+) cytotoxic T lymphocytes in cancer immunotherapy: a review. J Cell Physiol. 2019;234:8509–8521. doi: 10.1002/jcp.27782. [DOI] [PubMed] [Google Scholar]
- 82.Thommen DS, Schumacher TN. T cell dysfunction in cancer. Cancer Cell. 2018;33:547–562. doi: 10.1016/j.ccell.2018.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Roth MD, Harui A. Human tumor infiltrating lymphocytes cooperatively regulate prostate tumor growth in a humanized mouse model. J Immunother Cancer. 2015;3:12. doi: 10.1186/s40425-015-0056-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Rosenberg SA, Restifo NP, Yang JC, Morgan RA, Dudley ME. Adoptive cell transfer: a clinical path to effective cancer immunotherapy. Nat Rev Cancer. 2008;8:299–308. doi: 10.1038/nrc2355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Zhao L, Cao YJ. Engineered T cell therapy for cancer in the clinic. Front Immunol. 2019;10:2250. doi: 10.3389/fimmu.2019.02250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Najima Y, Tomizawa-Murasawa M, Saito Y, Watanabe T, Ono R, Ochi T, Suzuki N, Fujiwara H, Ohara O, Shultz LD, Yasukawa M, Ishikawa F. Induction of WT1-specific human CD8+ T cells from human HSCs in HLA class I Tg NOD/SCID/IL2rgKO mice. Blood. 2016;127:722–734. doi: 10.1182/blood-2014-10-604777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Giannoni F, Hardee CL, Wherley J, Gschweng E, Senadheera S, Kaufman ML, Chan R, Bahner I, Gersuk V, Wang X, Gjertson D, Baltimore D, Witte ON, Economou JS, Ribas A, Kohn DB. Allelic exclusion and peripheral reconstitution by TCR transgenic T cells arising from transduced human hematopoietic stem/progenitor cells. Mol Ther. 2013;21:1044–1054. doi: 10.1038/mt.2013.8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Vatakis DN, Koya RC, Nixon CC, Wei L, Kim SG, Avancena P, Bristol G, Baltimore D, Kohn DB, Ribas A, Radu CG, Galic Z, Zack JA. Antitumor activity from antigen-specific CD8 T cells generated in vivo from genetically engineered human hematopoietic stem cells. Proc Natl Acad Sci U S A. 2011;108:E1408–1416. doi: 10.1073/pnas.1115050108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Jacoby E, Shahani SA, Shah NN. Updates on CAR T-cell therapy in B-cell malignancies. Immunol Rev. 2019;290:39–59. doi: 10.1111/imr.12774. [DOI] [PubMed] [Google Scholar]
- 90.Jin CH, Xia J, Rafiq S, Huang X, Hu Z, Zhou X, Brentjens RJ, Yang YG. Modeling anti-CD19 CAR T cell therapy in humanized mice with human immunity and autologous leukemia. EBioMedicine. 2019;39:173–181. doi: 10.1016/j.ebiom.2018.12.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Gulati P, Ruhl J, Kannan A, Pircher M, Schuberth P, Nytko KJ, Pruschy M, Sulser S, Haefner M, Jensen S, Soltermann A, Jungraithmayr W, Eisenring M, Winder T, Samaras P, Tabor A, Stenger R, Stupp R, Weder W, Renner C, Munz C, Petrausch U. Aberrant lck signal via CD28 costimulation augments antigen-specific functionality and tumor control by redirected T cells with PD-1 blockade in humanized mice. Clin Cancer Res. 2018;24:3981–3993. doi: 10.1158/1078-0432.CCR-17-1788. [DOI] [PubMed] [Google Scholar]
- 92.Abate-Daga D, Lagisetty KH, Tran E, Zheng Z, Gattinoni L, Yu Z, Burns WR, Miermont AM, Teper Y, Rudloff U, Restifo NP, Feldman SA, Rosenberg SA, Morgan RA. A novel chimeric antigen receptor against prostate stem cell antigen mediates tumor destruction in a humanized mouse model of pancreatic cancer. Hum Gene Ther. 2014;25:1003–1012. doi: 10.1089/hum.2013.209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Han Y, Liu C, Li G, Li J, Lv X, Shi H, Liu J, Liu S, Yan P, Wang S, Sun Y, Sun M. Antitumor effects and persistence of a novel HER2 CAR T cells directed to gastric cancer in preclinical models. Am J Cancer Res. 2018;8:106–119. [PMC free article] [PubMed] [Google Scholar]
- 94.Li H, Huang Y, Jiang DQ, Cui LZ, He Z, Wang C, Zhang ZW, Zhu HL, Ding YM, Li LF, Li Q, Jin HJ, Qian QJ. Antitumor activity of EGFR-specific CAR T cells against non-small-cell lung cancer cells in vitro and in mice. Cell Death Dis. 2018;9:177. doi: 10.1038/s41419-017-0238-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Davila ML, Riviere I, Wang X, Bartido S, Park J, Curran K, Chung SS, Stefanski J, Borquez-Ojeda O, Olszewska M, Qu J, Wasielewska T, He Q, Fink M, Shinglot H, Youssif M, Satter M, Wang Y, Hosey J, Quintanilla H, Halton E, Bernal Y, Bouhassira DC, Arcila ME, Gonen M, Roboz GJ, Maslak P, Douer D, Frattini MG, Giralt S, Sadelain M, Brentjens R. Efficacy and toxicity management of 19-28z CAR T cell therapy in B cell acute lymphoblastic leukemia. Sci Transl Med. 2014;6:224ra225. doi: 10.1126/scitranslmed.3008226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Norelli M, Camisa B, Barbiera G, Falcone L, Purevdorj A, Genua M, Sanvito F, Ponzoni M, Doglioni C, Cristofori P, Traversari C, Bordignon C, Ciceri F, Ostuni R, Bonini C, Casucci M, Bondanza A. Monocyte-derived IL-1 and IL-6 are differentially required for cytokine-release syndrome and neurotoxicity due to CAR T cells. Nat Med. 2018;24:739–748. doi: 10.1038/s41591-018-0036-4. [DOI] [PubMed] [Google Scholar]
- 97.Morton JJ, Alzofon N, Jimeno A. The humanized mouse: emerging translational potential. Mol Carcinog. 2020;59:830–838. doi: 10.1002/mc.23195. [DOI] [PubMed] [Google Scholar]
- 98.Kametani Y, Ohno Y, Ohshima S, Tsuda B, Yasuda A, Seki T, Ito R, Tokuda Y. Humanized mice as an effective evaluation system for peptide vaccines and immune checkpoint inhibitors. Int J Mol Sci. 2019;20:6337. doi: 10.3390/ijms20246337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Burova E, Hermann A, Dai J, Ullman E, Halasz G, Potocky T, Hong S, Liu M, Allbritton O, Woodruff A, Pei J, Rafique A, Poueymirou W, Martin J, MacDonald D, Olson WC, Murphy A, Ioffe E, Thurston G, Mohrs M. Preclinical development of the anti-LAG-3 antibody regn3767: characterization and activity in combination with the anti-PD-1 antibody cemiplimab in human PD-1xLAG-3-knockin mice. Mol Cancer Ther. 2019;18:2051–2062. doi: 10.1158/1535-7163.MCT-18-1376. [DOI] [PubMed] [Google Scholar]
- 100.Zheng B, Ren T, Huang Y, Sun K, Wang S, Bao X, Liu K, Guo W. PD-1 axis expression in musculoskeletal tumors and antitumor effect of nivolumab in osteosarcoma model of humanized mouse. J Hematol Oncol. 2018;11:16. doi: 10.1186/s13045-018-0560-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Rosato RR, Davila-Gonzalez D, Choi DS, Qian W, Chen W, Kozielski AJ, Wong H, Dave B, Chang JC. Evaluation of anti-PD-1-based therapy against triple-negative breast cancer patient-derived xenograft tumors engrafted in humanized mouse models. Breast Cancer Res. 2018;20:108. doi: 10.1186/s13058-018-1037-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Lin S, Huang G, Cheng L, Li Z, Xiao Y, Deng Q, Jiang Y, Li B, Lin S, Wang S, Wu Q, Yao H, Cao S, Li Y, Liu P, Wei W, Pei D, Yao Y, Wen Z, Zhang X, Wu Y, Zhang Z, Cui S, Sun X, Qian X, Li P. Establishment of peripheral blood mononuclear cell-derived humanized lung cancer mouse models for studying efficacy of PD-L1/PD-1 targeted immunotherapy. MAbs. 2018;10:1301–1311. doi: 10.1080/19420862.2018.1518948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Capasso A, Lang J, Pitts TM, Jordan KR, Lieu CH, Davis SL, Diamond JR, Kopetz S, Barbee J, Peterson J, Freed BM, Yacob BW, Bagby SM, Messersmith WA, Slansky JE, Pelanda R, Eckhardt SG. Characterization of immune responses to anti-PD-1 mono and combination immunotherapy in hematopoietic humanized mice implanted with tumor xenografts. J Immunother Cancer. 2019;7:37. doi: 10.1186/s40425-019-0518-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Ma SD, Xu X, Jones R, Delecluse HJ, Zumwalde NA, Sharma A, Gumperz JE, Kenney SC. PD-1/CTLA-4 blockade inhibits epstein-barr virus-induced lymphoma growth in a cord blood humanized-mouse model. PLoS Pathog. 2016;12:e1005642. doi: 10.1371/journal.ppat.1005642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Gitto SB, Kim H, Rafail S, Omran DK, Medvedev S, Kinose Y, Rodriguez-Garcia A, Flowers AJ, Xu H, Schwartz LE, Powell DJ Jr, Simpkins F. An autologous humanized patient-derived-xenograft platform to evaluate immunotherapy in ovarian cancer. Gynecol Oncol. 2020;156:222–232. doi: 10.1016/j.ygyno.2019.10.011. [DOI] [PubMed] [Google Scholar]
- 106.Cherkassky L, Morello A, Villena-Vargas J, Feng Y, Dimitrov DS, Jones DR, Sadelain M, Adusumilli PS. Human CAR T cells with cell-intrinsic PD-1 checkpoint blockade resist tumor-mediated inhibition. J Clin Invest. 2016;126:3130–3144. doi: 10.1172/JCI83092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Choi B, Lee JS, Kim SJ, Hong D, Park JB, Lee KY. Anti-tumor effects of anti-PD-1 antibody, pembrolizumab, in humanized NSG PDX mice xenografted with dedifferentiated liposarcoma. Cancer Lett. 2020;478:56–69. doi: 10.1016/j.canlet.2020.02.042. [DOI] [PubMed] [Google Scholar]
- 108.Wang J, Fei K, Jing H, Wu Z, Wu W, Zhou S, Ni H, Chen B, Xiong Y, Liu Y, Peng B, Yu D, Jiang H, Liu J. Durable blockade of PD-1 signaling links preclinical efficacy of sintilimab to its clinical benefit. MAbs. 2019;11:1443–1451. doi: 10.1080/19420862.2019.1654303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Rios-Doria J, Stevens C, Maddage C, Lasky K, Koblish HK. Characterization of human cancer xenografts in humanized mice. J Immunother Cancer. 2020;8:e000416. doi: 10.1136/jitc-2019-000416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Liu WN, Fong SY, Tan WWS, Tan SY, Liu M, Cheng JY, Lim S, Suteja L, Huang EK, Chan JKY, Iyer NG, Yeong JPS, Lim DW, Chen Q. Establishment and characterization of humanized mouse NPC-PDX model for testing immunotherapy. Cancers (Basel) 2020;12:1025. doi: 10.3390/cancers12041025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Rezvani K, Rouce RH. The application of natural killer cell immunotherapy for the treatment of cancer. Front Immunol. 2015;6:578. doi: 10.3389/fimmu.2015.00578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Herndler-Brandstetter D, Shan L, Yao Y, Stecher C, Plajer V, Lietzenmayer M, Strowig T, de Zoete MR, Palm NW, Chen J, Blish CA, Frleta D, Gurer C, Macdonald LE, Murphy AJ, Yancopoulos GD, Montgomery RR, Flavell RA. Humanized mouse model supports development, function, and tissue residency of human natural killer cells. Proc Natl Acad Sci U S A. 2017;114:E9626–E9634. doi: 10.1073/pnas.1705301114. [DOI] [PMC free article] [PubMed] [Google Scholar]