Abstract
Omega-3 polyunsaturated fatty acids (PUFAs), such as those found in fish oil, are thought to have anti-tumorigenic effects and may help to treat and prevent cancer, including ovarian cancer. Thus, we aimed to evaluate the potential of docosahexaenoic acid (DHA), an omega-3 PUFA, as a therapeutic agent in ovarian cancer cell lines and a transgenic mouse model of ovarian cancer. DHA significantly inhibited cellular proliferation, induced cell cycle arrest and caused apoptosis in Hey and IGROV-1 cells. Pre-treatment with the anti-oxidant, N-acetylcysteine (NAC), reversed DHA-induced caspase 3 activity and prevented DHA-reduced cell proliferation. DHA also induced cellular reactive oxygen species (ROS) and inhibited adhesion and invasion in IGROV-1 and Hey cells. Furthermore, treatment with DHA demonstrated anti-tumorigenic and anti-invasive activity in a K18-gT121 +/-; p53fl/fl; Brca1fl/fl mouse model of ovarian cancer including downregulation of Ki67 and VEGF expression. The data provide a preclinical rationale for applying DHA for dietary intervention and therapeutic adjunct in patients with ovarian cancer.
Keywords: Docosahexaenoic acid, ovarian cancer, invasion, apoptosis, proliferation
Introduction
Ovarian cancer remains the highest mortality from gynecologic malignancies and ranks fifth in overall cancer deaths in women [1]. In 2020, nearly 21,750 new cases and 13,940 deaths of ovarian cancer are estimated in the US [2]. As the symptoms of ovarian cancer tend to be insidious, it is a disease most often diagnosed in later stages. The mainstay of treatment is chemotherapy with surgical debulking, although most patients will experience recurrence [3]. Platinum and taxane chemotherapy has remained the standard-of-care for primary ovarian cancer management. Recurrent and platinum resistant disease present additional management challenges, further contributing to the poor overall outcomes and survival [3-5]. With these challenges in mind, investigation into novel therapies including targeted therapy is needed.
Overweight and obesity have been linked with increased risk and unfavorable outcomes in many types of cancer including ovarian cancer [6,7]. Our recent study found that diet-induced obesity led to more aggressive tumor behavior in a K18-gT121 +/-; p53fl/fl; Brca1fl/fl mouse model of ovarian cancer and significantly increased tumor weight and volume. Metabolomic profiling revealed distinct metabolic differences between tumors in obese and non-obese mice. Major differences in ovarian tumors of obese vs non-obese mice included impairment of glycolysis and mitochondrial complex II and upregulation of omega fatty acid oxidation [8,9]. This suggests that nutrition has powerful biological effects on the simultaneous reprogramming of metabolic features and promotion of ovarian tumor growth.
Omega-3 fatty acids are essential polyunsaturated fat acids that play a crucial role in lipid and cell metabolism, membrane structure, cell signaling, gene expression and inflammation in the body. Epidemiological studies have demonstrated that diets rich in omega-3 polyunsaturated fatty acids (PUFAs) have a positive effect against many types of cancer including breast, colorectal, prostate, and ovarian cancer [10-14]. Docosahexaenoic acid (DHA), one of most important PUFAs from marine sources, has exhibited potent in vitro and in vivo anti-tumor activities via multiple mechanisms including modulating cell cycle distribution, triggering cell apoptosis and death, inducing cellular stress and inhibiting tumor angiogenesis and metastasis in leukemia, breast, endometrial, gastric, liver, prostate, and lung cancers [15-21]. Several studies have recently shown that DHA effectively inhibited ovarian cancer cell proliferation and invasion in a zebrafish model through modulation of the NK-KB, mTOR and MAPK pathways [22-24]. Additionally, treatment of ovarian cancer cells with DHA synergistically increases cisplatin-induced proliferation inhibition and apoptosis [25]. Long-term consumption of a flaxseed diet (containing rich DHA) significantly induces apoptosis and inhibits angiogenesis in the ovarian tumors of chickens but not in the normal chicken ovaries [26]. Multiple clinical trials have demonstrated that DHA is overall well tolerated and supplementation of DHA (fish oil) during chemotherapy is beneficial effects on drug-induced side effects, immune function, bone health, inflammation, tumor-induced cachexia and chemotherapeutic efficacy [27-33]. Collectively, these data support that DHA could be of value in the management of ovarian cancer. With established safety profile and promising evidence supporting the anti-tumorigenic effects of PUFAs, we investigated the anti-proliferative and anti-metastatic effects of DHA on ovarian cancer cells and in a transgenic mouse model of ovarian cancer.
Materials and methods
Cell culture and reagents
IGROV-1 and Hey cells were utilized in all experiments. The cell lines were cultured in RPMI supplemented with 5 or 10% fetal bovine serum, L-glutamine and 1% penicillin-streptomycin solution under 5% CO2. DHA of 98% purity was obtained from Cayman Chemical (Ann Arbor, Michigan), dissolved in sterile absolute ethyl alcohol and stored at -20°C. MTT (3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl-tetrazolium bromide) and NAC (N-acetyl-l-cysteine, Sigma, St. Louis, MO) were dissolved in PBS and stored at -20°C. All antibodies were obtained from Santa Cruz Biotechnology (Dallas, TX) and Cell Signaling Technology (Beverly, MA).
Assay of cytotoxicity
The IGROV-1 and Hey cells were seeded at 5 × 103 cells per well in a 96-well plate overnight. Cells were treated with DHA at indicated concentrations. After a 72 hours incubation, the cells were incubated with 5 µl MTT per well (5 mg/ml in PBS) for 1 hour. 100 ul dimethyl sulfoxide was added to wells to dissolve the MTT formazan crystals and mixed thoroughly by pipetting. Color intensity was measured at 570 nm. DHA-mediated cell inhibition was calculated as a percentage of control cell growth.
Annexin V assay
Annexin V assay (Biolegend, San Diego, CA) was performed to quantitate cell apoptosis using Cellometer (Nexcelom, Lawrence, MA). Following DHA treatment for 16 hours, cells were collected and gently resuspended in staining solution with Annexin V antibody and propidium iodide (PI). After incubation at 37°C for 15 minutes, Cellometer was utilized to quantify apoptosis in the samples. FCS Express (Pasadena, CA) was performed to interpret the data.
Cell cycle assay
After treatment with different concentrations of DHA for 36 hours, cells were typsinized and resuspended in 90% methanol overnight at -20°C. Cells were washed with PBS and then incubated with 50 µL propidium iodide solution for 40 minutes at 37°C. Cell cycle distributions were detected by the Cellometry Vision CBA system. FCS Express was used to analyze the results.
Adhesion assay
Corning® 96-well plates were coated for 3 hours at 37°C with Laminin-1. After the overlying fluid was aspirated, wells were blocked for 1-2 hours with 0.2% BSA in PBS, and then rinsed 2 times with PBS. 6000 cells along with DHA (1, 10 and 50 uM) were added to wells. After incubation for 90 minutes, 5% glutaraldehyde was added to wells to fix the cells for 30 minutes. Wells were gently washed twice with PBS before adding 0.1% crystal violet and incubating for 30 minutes. Bound dye was solubilized with acetic acid and quantified at 570 nm in the Tecan plate reader.
Transwell invasion assay
DHA-mediated cell invasion in OC cells were detected by transwell invasion assay. Transwell inserts were coated with 0.5-1 × BME. The lower compartment was filled with RPMI 1640 containing 10% FBS in a 96-well plate. The Hey and IGROV-1 cells were starved for 12 hours and then plated in the upper chambers of 96-wells plates with DHA (1, 10 and 50 uM). The plates were incubated for 3.5 hours before removing the inserts. After washing the lower compartment with PBS, 100 ul of calcein AM solution was added to each well and incubated in the dark for 30-50 minutes. Fluorescence Intensity (EX/EM 485/520 nm) was analyzed by a fluorescence reader (Tecan).
Measurement of intracellular ROS generation
Intracellular reactive oxygen species (ROS) levels were detected using 2’,7’-dichlorofluorescein diacetate (DCFH-DA). The Hey and IGROV-1 cells were cultured in a black 96-well plates at a seeding density of 1 × 104 cells/well and then incubated with DHA at doses of 1, 10 and 50 uM for 6 hours. Cells were exposed to DCFH-DA for 15 minutes at 37°C. Fluorescence depth (EX/EM 485/530 nm) was detected by a fluorescence reader (Tecan).
Western immunoblotting
After treating cells with DHA for 8 and 24 hours, cells were lysed with RIPA buffer supplemented with protease and phosphatase inhibitors. Protein concentrations were measured by BCA assay kit. Equal amounts of protein were separated by gel electrophoresis and transferred onto a PVDF membrane. After blocking with 5% BSA in PBS, the membrane was exposed to a primary antibody diluted 1:1,000 overnight in cold room. Blots were then treated with HRP-conjugated anti-mouse, anti-goat, or anti-rabbit antibodies for 1-2 hours. Antibody binding was detected using SuperSignal™ West Pico on the ChemiDoc™ Image System (Bio-Rad, Hercules, CA).
Measurement of VEGF levels in serum
Serum VEGF from DHA and control groups (7 samples/per group) was measured by VEGF ELISA Kit (R&D Systems, Minneapolis, MN) following the manufacturer’s instructions.
Wound healing assay
The cells were plated in 6-well plates at 3.5 × 105 cells/well for 24 hours and then replaced with media with 0.5% charcoal stripped FBS for 12 hours. A sterile 200 ul pipette tip was used to scratch a straight line across the plate in one direction. Cells were washed with fresh media to remove the detached cells and then treated with DHA. The images were acquired after 24 and 48 hours of treatment. Measurements of the width of the wound were performed at random intervals with the Adobe Photoshop CS6.
The K18-gT121 +/-; p53fl/fl; Brca1fl/fl mouse model of high grade serous ovarian cancer
The KpB (K18-gT121 +/-; p53fl/fl; Brca1fl/fl; KpB) transgenic mouse model of high grade serous epithelial OC has previously been described [34,35]. Animal protocol was approved by the UNC-CH Institutional Animal Care and Use Committee (IACUC). KpB mice were kept in a 12 h:12 h cycle with ad libitum access to food and water. 5 ul of 2.5 × 107 P.F.U of Ad5-CMV-Cre (Transfer Vector Core, University of Iowa) was injected into the left ovarian bursa of 6-8 week old KpB mice. When tumors had reached an average size of 0.1-0.2 cm in diameter by palpation, the mice (15 per group) were treated DHA (15 mg/kg, daily, IP) or vehicle (PBS, daily, IP) for 28 days. All mice were sacrificed in accordance with IACUC protocol and tumors were weighted. Volumes of ovarian tumor were calculated as following: (width2 × length)/2.
Immunohistochemical analysis
The preparation of ovarian tumor slides was performed by IHC Core Facility at UNC. IHC slides were stained with antibodies against Ki-67, VEGF, Bip, and phosphorylated-S6, respectively, in a humidified chamber overnight. After washing with PBS, the slides were treated with a HRP-conjugated secondary antibody for 1 hour. Color reaction and hematoxylin counterstaining was then performed on the sections using ABC-Staining Kits (Vector Labs, Burlingame, CA). Digital images were acquired by Motic scanner and analyzed by ImagePro software (Rockville, MD).
Statistical analysis
Data are formatted as the mean ± SD. Statistical significance was analyzed using the two-sided unpaired Student’s t-test from at least three replicates. Tumor growth between treatment arms was analyzed by one-way & Two-way ANOVA test. GraphPad Prism 5 (La Jolla, CA USA) was used for all graphs and significance tests. P values of <0.05 were considered to be statistically significant.
Results
Effect of DHA on ovarian cancer cell proliferation
In order to examine the cytotoxic effects of DHA on ovarian cancer cells, MTT assay was used in IGROV-1 and Hey cell lines to assess for DHA induced cytotoxicity. DHA, in a concentration range of 0.1-500 uM, decreased cell viability of both cells in a concentration dependent manner after 72 hours of treatment. The mean IC50 value for both cell lines was approximately 40 uM. DHA at a dose of 100 uM for 72 hours resulted in a loss of viability up to 80-90% (Figure 1A). Additionally, we treated IGROV-1 and Hey cells with 75 uM DHA for increasing time periods up to 72 hours. DHA also reduced cell viability of both cell lines in a time-dependent manner (Figure 1B and 1C, P<0.01). These results suggests that ovarian cancer cells are sensitive to DHA treatment.
Figure 1.
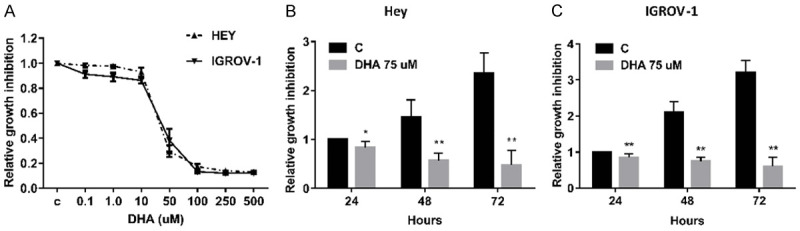
DHA induced dose-dependent growth inhibition in ovarian cancer cells. The Hey and IGROV-1 cells were treated with various doses of DHA for 72 hours. Cell proliferation was determined by MTT assay (A). Relative survival was determined by dividing the number of remaining DHA treated cells by the number of remaining viable DMSO. Treatment of the cells with DHA at 75 uM induced inhibition of cell proliferation in time dependent manner (B and C).
Effect of DHA on cell cycle in ovarian cancer cells
We characterized DHA-mediated suppression of cell growth in IGROV-1 and Hey cells by assessing cell cycle progression after DHA treatment using the Cellometer. Exposure of both cell lines to 1, 10 and 50 uM DHA for 36 hours caused G1 phase arrest in the IGROV-1 cells and G2 phase arrest in the Hey cells; however, this difference was only observed with treatment at a dose of 50 µM DHA (Figure 2A and 2B). The proportion of G1 accumulation increased from 52.8% in the control to 64.7% in IGROV-1 cells treated with 50 uM DHA. In Hey cells. 50 uM DHA increased G2 phase from 15.7% in control to 26.3%. Additionally, we measured DHA-mediated changes in associated cell-cycle regulatory proteins using Western immunoblotting. Treatment with DHA for 24 hours clearly down-regulated the expression of CDK4, CDK6 and Cyclin D1 in both cells (Figure 2C). These results indicate that DHA encouraged cell cycle arrest at different cell cycle checkpoints in ovarian cancer cells.
Figure 2.
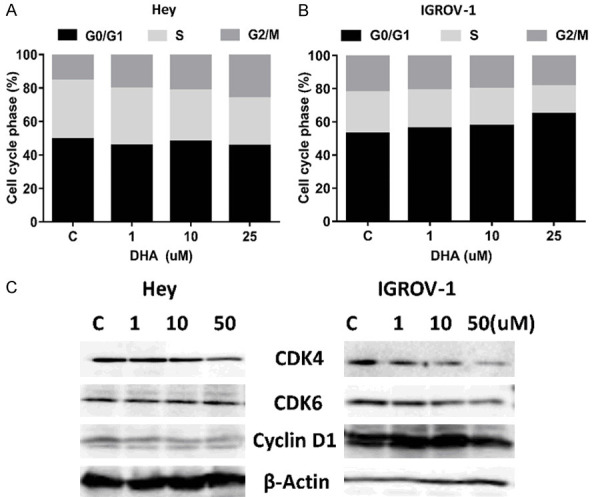
DHA induced cell cycle G1 arrest in ovarian cancer cells. The Hey and IGROV-1 cells were treated with indicated amount of DHA for 36 hours. DHA induced cell cycle arrest in G1 phase in the IGROV-1 cells and G2 phase in the Hey cells (A and B). Cell lysates from the Hey and IGROV-1 cells were subjected to Western blot analysis for CDK4, CDK6 and Cyclin D1 (C). Data are representative of one of three repeats. *P<0.05 and **P<0.01.
Effect of DHA on apoptosis in ovarian cancer cells
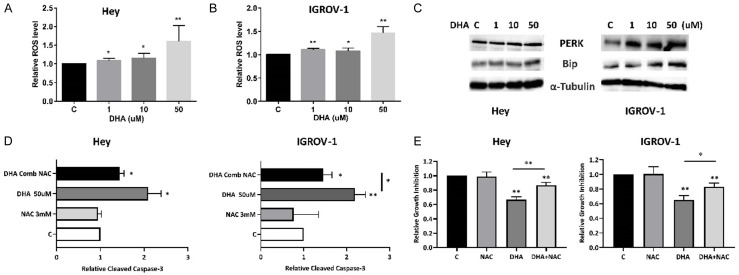
To determine whether the observed decrease in cell viability is attributable to apoptosis, we investigated the effects of DHA on apoptosis proteins using ELISA, western blotting and Annexin V assays. Exposure of IGROV-1 and Hey cells to DHA for 16 hours resulted in increased rates of apoptosis, measured by the percentage of Annexin V positive cells, with a threefold increase in both cells, 15.85% for 50 uM versus 5.5% for control in Hey cells and 14.6% for 50 uM versus 5.2% for control IGROV-1 cells (Figure 3A and 3B). Furthermore, expression of MCL-1 and BCL-2 was notably decreased in DHA treated IGROV-1 and Hey cells as detected by western blotting (Figure 3C).
Figure 3.
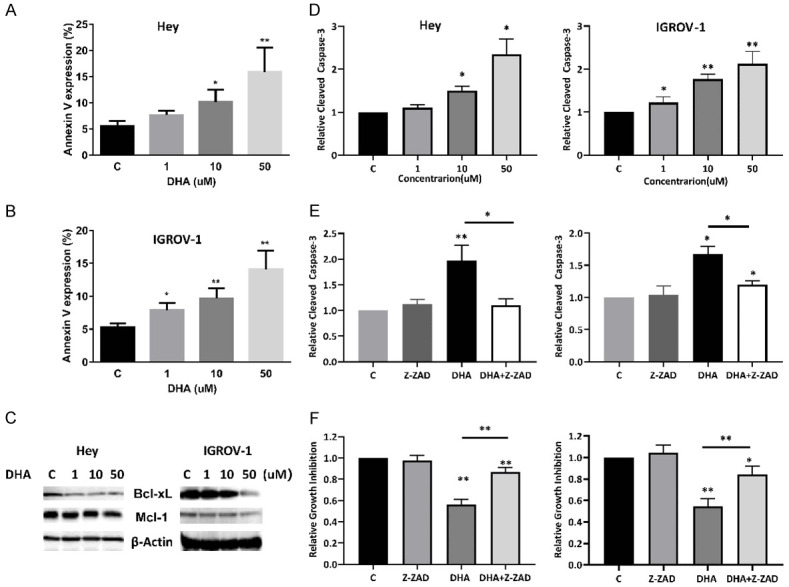
DHA induced apoptosis in ovarian cancer cells. The expression of annexin V in the Hey and IGROV-1 cells was determined by Annexin V/PI analyses after 16 hours of treatment with DHA (A and B). Western blotting results showed that DHA reduced BCL-2 and MCL-1 expression after 24 hours of treatment in both cells (C). DHA increased cleaved caspase 3 activity in both cell lines (D). Pretreatment of the cells with the pan-caspase inhibitor Z-VAD-FMK blocked cell growth inhibition and reduced caspase 3 activity induced by DHA (E and F). The results are shown as the mean ± SD and are representative of three independent experiments. *P<0.05 and **P<0.01.
To further delineate the role of DHA in apoptotic pathways, a cleaved caspase-3 activity assay was performed in the IGROV-1 and Hey cell lines. As shown in Figure 3D, the cells treated with DHA exhibited significantly higher cleaved caspase 3 activity. DHA at a dose of 50 uM increased cleaved caspase 3 production by 2.12 (P<0.01) in IGROV-1 cells and 2.35 (P<0.05) times in Hey cells compared with untreated groups.
To detect whether caspase activity is required for DHA induced apoptosis and cell viability, the broad-spectrum caspase inhibitor, Z-VAD-FMK, was used to block caspase activity in both cell lines. The effect of Z-VAD-FMK on DHA-mediated apoptosis and cell inhibition was determined by cleaved caspase-3 assay and MTT assay. DHA induced apoptosis and growth inhibition were partially blocked by Z-VAD-FMK in both cell lines (Figure 3E and 3F), which demonstrates that DHA induced inhibition of cell growth, to a certain extent, was dependent on mitochondrial pathway in ovarian cancer cells.
DHA increased cell stress
Given that DHA induces apoptosis via the endoplasmic reticulum (ER) stress pathway in breast and gastric cancer cells [36,37], we investigated whether DHA induced apoptosis was involved in ROS generation in ovarian cancer cells. DHA resulted in significantly increased production of ROS in a concentration dependent manner in IGROV-1 and Hey cells after 12 hours of treatment (Figure 4A and 4B, P<0.01). To examine whether ROS induced by DHA treatment were related to upregulated ER stress related proteins, we performed western blotting to detect expression of Bip and PERK in IGROV-1 and Hey cells, which are widely accepted markers of ER stress. Both cell lines displayed dose-dependent induction of Bip and PERK protein expression after being treated with DHA for 12 hours (Figure 4C). Pretreatment with the ROS scavenger NAC for 2 hours prior to DHA treatment at increasing doses for 12 hours showed that DHA-induced cleaved caspase-3 activity was almost completely blocked and Inhibition of DHA-mediated cell proliferation was partially rescued by NAC (Figure 4D, P<0.01). This suggests that DHA-induced apoptosis was dependent on cellular stress pathways in ovarian cancer cells.
Figure 4.
DHA induced ER stress in ovarian cancer cells. The Hey and IGROV-1 cells were treated with DHA at different concentrations for 12 hours. ROS products were measured by DCFH-DA assay. DHA significantly increased the levels of ROS in both cell lines compared to the control cells (A and B). Western blotting results showed that DHA increased the expression of cellular stress-related proteins including PERK and Bip in both cell lines after treatment for 24 hours (C). The cells were pre-treated with NAC for 3 hours, followed by treatment with DHA for 12 hours. NAC significantly reduced caspase 3 activity and cell growth inhibition induced by DHA (D and E). The results are shown as the mean ± SD and are representative of three independent experiments. *P<0.05, **P<0.01.
DHA suppressed tumor growth of ovarian cancer in transgenic mice
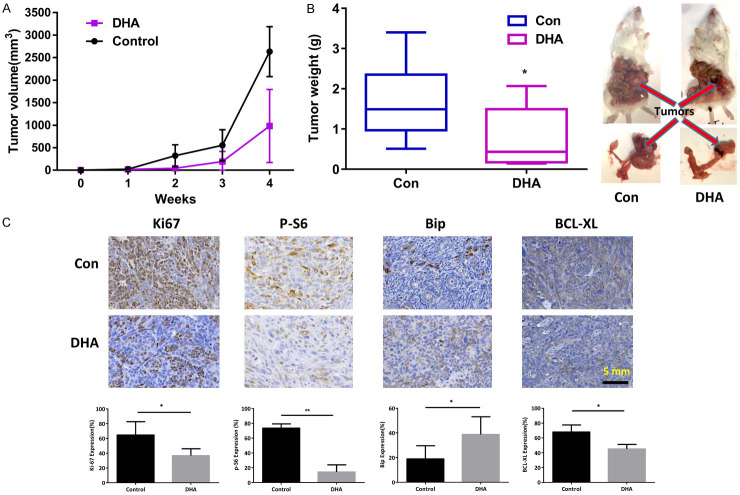
We investigated the potential effects of DHA on tumor growth using a K18-gT121 +/-; p53fl/fl; Brca1fl/fl; mouse model of ovarian cancer (KpB). DHA was injected intraperitoneally (i.p.) at 15 mg/kg per day for 4 weeks and had no toxic effect on KpB mice. The mean tumor volume in the control group was significantly higher than that of the DHA treatment group as seen in Figure 5A. DHA administration resulted in a substantial reduction in tumor weight by 44% as compared with control mice (1.36 versus 2.40 grams, P<0.05; Figure 5B). Histologic changes between the DHA-treated and control groups were examined using IHC-staining in paraffin-embedded ovarian tumors. Compared with the control group, expression of Ki-67 and BCL-XL was significantly reduced in the DHA-treated tumors (Figure 5C). In parallel with in vitro results, DHA treatment markedly increased Bip expression and decreased phosphorylated-S6 expression in the tumor tissues. These data confirm that DHA is highly effective in slowing ovarian tumor growth in vivo.
Figure 5.
DHA inhibited tumor growth in KpB mice. KpB mice were treated with vehicle (control) or DHA (15 mg/kg, daily) for 4 weeks when the tumors reached 0.1 cm in size. DHA significantly inhibited tumor volume and tumor weight (N=15 animals per group) as compared to the vehicle treated controls (A and B). Immunohistochemistry results showed that DHA decreased the expression of Ki-67, phos-S6 and BCL-XL and increased Bip expression in the ovarian tumor tissues (C). *P<0.05; **P<0.01.
Effect of DHA on cell adhesion and invasion
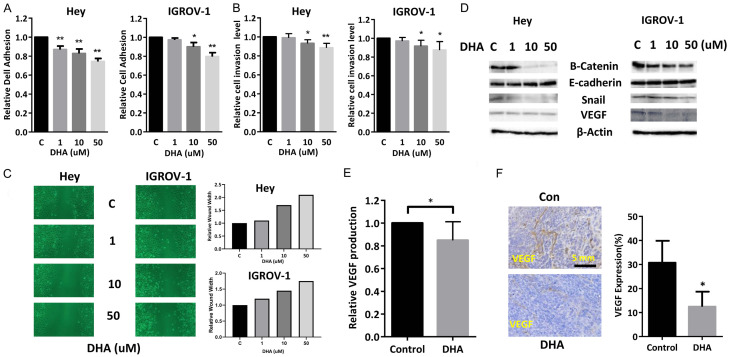
The role of DHA in cell adhesive and invasive potential were investigated using adhesion, wound healing and transwell migration assays. After treating IGROV-1 and Hey cells with DHA for 2 hours in 96-well plates coated with laminin-1, an obvious influence on cell adhesion was observed within the DHA treatment cells (Figure 6A). In addition, IGROV-1 and Hey cells exhibited a similar reduction in cell invasion with treatment of DHA, in a concentration-dependent manner as detected by transwell assay (Figure 6B). Wound healing assay demonstrated that a decrease in cell motility was also observed in both cells after 24 and 48 hours of treatment with DHA compared with untreated cells (Figure 6C). To explore changes in epithelial-to-mesenchymal transition (EMT) in DHA treated cells, several EMT markers including E-cadherin, B-Catenin and snail as well as VEGF were assessed by western blotting. The expression level of E-cadherin was increased, whereas that of B-Catenin and Snail were decreased in DHA-treated cells. Additionally, expression of VEGF protein was reduced in DHA treated tumors compared to untreated mice (Figure 6D). We next investigated whether DHA decreased serum VEGF levels in KpB mice. Serum levels of mouse VEGF were determined after complete of DHA treatment. DHA treated mice exhibited an 18% reduction in serum VEGF as compared to control (Figure 6E). IHC staining demonstrated reduced angiogenesis in DHA treated tumors as compared to control tumors (Figure 6F). Overall, these results confirm that DHA can antagonize EMT processes and angiogenesis, thus resulting in an inhibition of adhesion and invasion in vitro and in vivo.
Figure 6.
DHA inhibited adhesion and invasion in ovarian cancer cells. DHA inhibited cell adhesion and invasion in the Hey and IGROV-1 cells, assessed by Laminin and transwell assays (A and B). Migration was assessed by wound healing assay after treatment with DHA for 48 hours (C). Western blotting results showed that DHA increased the expression of E-cadherin and decreased the expression of B-catenin, snail and VEGF in both cell lines (D). DHA reduced serum VEGF production in KpB mice treated with DHA for 4 weeks (E). IHC results showed that DHA decreased the expression of VEGF in ovarian tumor tissues from KpB mice (F). *P<0.05, **P<0.01.
Discussion
Given that ovarian cancer has a low five year survival rate and is the leading cause of death from gynecologic cancer, it is crucial to investigate new therapeutic agents or combination regimens that efficiently target ovarian cancer, especially for late or recurrent stage disease. Accumulating evidence suggests that long-term consumption of omega-3 fatty acids is associated with a significantly reduced risk of some cancers [10,12,38]. The ability of DHA and its analogs to inhibit cell proliferation and tumor growth in preclinical models was previously confirmed by multiple studies [16,17,39]. Recent research has shown DHA’s potential to exert anti-tumorigenic activity through multiple cell signaling pathways involved in programmed cancer cell death in ovarian cancer cells [22,23,25]. In the current study, we performed experiments on ovarian cancer cells and a KpB mouse model where we showed that DHA exerts anti-tumor effects via several mechanisms including induction of cell cycle arrest, cellular stress and apoptosis, as well as anti-invasive activity via anti-angiogenesis and inhibition of EMT processes. Our results confirmed that DHA has anticancer effects in ovarian cancer in vitro and in vivo.
Molecular mechanisms involved in the anti-proliferative activities of DHA have been proposed to explain the inhibitory effect on tumor cell growth. Different cytotoxic effect of DHA on tumor cell proliferation may depend on the molecular biological background and degree of malignant potential of the cancer cells [40]. ROS production is often detected during cellular stress induced by therapeutic agents, which subsequently leads to the activation of apoptotic pathways and causes cancer cell death [41]. ROS have an impact on the activation of apoptotic signaling pathways and induction of cell cycle arrest in cancer cells [40]. DHA strongly induced caspase-8-dependent apoptosis in the breast cancer MCF-7 cells and corresponding MCF-7 xenograft model in athymic nude mice via ROS accumulation [36]. Pretreatment with the antioxidant NAC completely blocked the cell growth inhibition induced by DHA and reduced DHA-induced apoptotic activity in prostate cancer cells [42]. Recently, Tanaka et al. found that DHA inhibited proliferation of ovarian cancer cells via ROS-dependent MAP kinase activation [22]. Our studies confirmed that DHA exerted anti-tumorigenic activities through increased ROS accumulation and apoptotic signaling pathways. Conversely, blocking ROS production significantly rescued cell proliferation inhibition and reduced apoptosis caused by DHA in these cells. Overall, these data support that ROS mediates DHA-induced apoptosis in vitro and KpB mouse model.
Interrupting cell cycle progression is another mechanism by which DHA inhibits proliferation of cancer cells. Recent evidence shows that DHA induces G1 or G2 phase arrest in different types of cancer [43]. DHA-mediated differences in cell cycle distribution may be phenotype-specific and dependent on variability in experimental conditions [38,43]. The role of DHA in interference with cell cycle progression has not been adequately investigated in ovarian cancer. In our study, following exposure to DHA for 36 hours, the Hey and IGROV-1 cell lines demonstrated cell cycle arrest. Interestingly, the Hey cells demonstrated G2 arrest with DHA treatment while the IGROV-1 cells demonstrated G1 arrest, accompanied by downregulation of CDK4, CDK6 and cyclin D1. G2 arrest in Hey cells was only demonstrated following exposure to high doses of DHA; G2 arrest is often closely associated with enhanced apoptosis. Cell cycle arrest has limited effects on anti-proliferation in DHA-treated cells, and apoptosis may be a major pathway for inhibition of cell proliferation by DHA for Hey cells [44]. Collectively, these results suggest that DHA-induced cell cycle arrest is a cell type-specific event within the same type of cancer. However, the exact mechanism of cell cycle arrest induced by DHA in different types of cancer is worthy of further study.
Since DHA has the potential to inhibit angiogenesis through various mediators including VEGF, PDGF, PDECGF, COX-2, PGE2, nitric oxide, NFκB, matrix metalloproteinases and β-Catenin, anti-invasive and anti-metastatic efficacy of DHA has been investigated in breast, lung, pancreatic, prostate, colon, bladder and renal cancer [45-47]. DHA has been documented to have anti-invasive and anti-metastatic effects pre-clinical cancer models [48]. Using an osteolytic and visceral metastasis nude mouse model injected with MDA-MB-231-Luc cancer cells intra-cardially, DHA inhibited breast cancer metastasis specifically to bone and reduced the number of osteolytic lesions [49]. Epoxydocosapentaenoic acids (EDPs), one of DHA’s metabolites, inhibited VEGF and fibroblast growth factor 2-induced angiogenesis in vivo and decreased lung metastases in the Lewis mouse model of lung carcinoma [50]. One study by Ligo et al. in a metastatic colon carcinoma 26 (Co 26Lu) model found that a DHA rich diet exerted significant inhibitory effects on tumor growth at the implantation site and significantly decreased the numbers of lung metastatic nodules. Additionally, pre-treatment of Co 26 cells with DHA in this model also showed a low potential for lung colony formation when transferred to new hosts, suggesting that the effect of DHA on tumor metastasis directly affects tumor cells independent of the surrounding environment [47,51]. The anti-invasive activity of DHA has also been investigated in ovarian cancer cells and transgenic zebrafishes, and the results demonstrated that DHA exhibited inhibitory effects on angiogenesis, invasion, and metastasis via NF-KB signaling pathway in cancer cells and animal models [23]. Our study similarly confirmed that DHA inhibited cellular adhesion and invasion involving key EMT processes in ovarian cancer cells. Furthermore, DHA not only inhibited tumor growth but also decreased VEGF levels in serum and ovarian tumor tissues in the KpB mice.
In conclusion, omega-3 supplements have been previously found to have favorable impacts on quality of life, side effects, nutritional-inflammatory risk and immune function in cancer patients receiving chemotherapy and/or radiotherapy [27,29,33]. We clearly demonstrated that DHA effectively inhibited cell viability and decreased the invasive potential of ovarian cancer in vitro and in vivo. Similar to other studies, our data provides fundamental rationale for further investigation of DHA in clinical trials and highlights the potential of DHA as a novel dietary intervention and a promising and easily tolerable adjunct therapy for ovarian cancer, a malignancy that is in desperate need of novel interventions.
Acknowledgements
This work is supported by: (1) VLB: American Cancer Society (ACS) Research Scholar Grant-RSG CCE 128826. (2) VLB: NIH/NCI-R37CA226969.
Disclosure of conflict of interest
None.
References
- 1.Miller KD, Siegel RL, Lin CC, Mariotto AB, Kramer JL, Rowland JH, Stein KD, Alteri R, Jemal A. Cancer treatment and survivorship statistics, 2016. CA Cancer J Clin. 2016;66:271–289. doi: 10.3322/caac.21349. [DOI] [PubMed] [Google Scholar]
- 2.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2020. CA Cancer J Clin. 2020;70:7–30. doi: 10.3322/caac.21590. [DOI] [PubMed] [Google Scholar]
- 3.Narod S. Can advanced-stage ovarian cancer be cured? Nat Rev Clin Oncol. 2016;13:255–261. doi: 10.1038/nrclinonc.2015.224. [DOI] [PubMed] [Google Scholar]
- 4.Oronsky B, Ray CM, Spira AI, Trepel JB, Carter CA, Cottrill HM. A brief review of the management of platinum-resistant-platinum-refractory ovarian cancer. Med Oncol. 2017;34:103. doi: 10.1007/s12032-017-0960-z. [DOI] [PubMed] [Google Scholar]
- 5.Corrado G, Salutari V, Palluzzi E, Distefano MG, Scambia G, Ferrandina G. Optimizing treatment in recurrent epithelial ovarian cancer. Expert Rev Anticancer Ther. 2017;17:1147–1158. doi: 10.1080/14737140.2017.1398088. [DOI] [PubMed] [Google Scholar]
- 6.Allott EH, Hursting SD. Obesity and cancer: mechanistic insights from transdisciplinary studies. Endocr Relat Cancer. 2015;22:R365–386. doi: 10.1530/ERC-15-0400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yang HS, Yoon C, Myung SK, Park SM. Effect of obesity on survival of women with epithelial ovarian cancer: a systematic review and meta-analysis of observational studies. Int J Gynecol Cancer. 2011;21:1525–1532. doi: 10.1097/IGC.0b013e31822eb5f8. [DOI] [PubMed] [Google Scholar]
- 8.Han J, Wysham WZ, Zhong Y, Guo H, Zhang L, Malloy KM, Dickens HK, Huh G, Lee D, Makowski L, Zhou C, Bae-Jump VL. Increased efficacy of metformin corresponds to differential metabolic effects in the ovarian tumors from obese versus lean mice. Oncotarget. 2017;8:110965–110982. doi: 10.18632/oncotarget.20754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Makowski L, Zhou C, Zhong Y, Kuan PF, Fan C, Sampey BP, Difurio M, Bae-Jump VL. Obesity increases tumor aggressiveness in a genetically engineered mouse model of serous ovarian cancer. Gynecol Oncol. 2014;133:90–97. doi: 10.1016/j.ygyno.2013.12.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rose DP, Connolly JM, Coleman M. Effect of omega-3 fatty acids on the progression of metastases after the surgical excision of human breast cancer cell solid tumors growing in nude mice. Clin Cancer Res. 1996;2:1751–1756. [PubMed] [Google Scholar]
- 11.Rose DP, Connolly JM. Effects of dietary omega-3 fatty acids on human breast cancer growth and metastases in nude mice. J Natl Cancer Inst. 1993;85:1743–1747. doi: 10.1093/jnci/85.21.1743. [DOI] [PubMed] [Google Scholar]
- 12.Cockbain AJ, Toogood GJ, Hull MA. Omega-3 polyunsaturated fatty acids for the treatment and prevention of colorectal cancer. Gut. 2012;61:135–149. doi: 10.1136/gut.2010.233718. [DOI] [PubMed] [Google Scholar]
- 13.Rose DP, Connolly JM. Omega-3 fatty acids as cancer chemopreventive agents. Pharmacol Ther. 1999;83:217–244. doi: 10.1016/s0163-7258(99)00026-1. [DOI] [PubMed] [Google Scholar]
- 14.Khankari NK, Bradshaw PT, Steck SE, He K, Olshan AF, Shen J, Ahn J, Chen Y, Ahsan H, Terry MB, Teitelbaum SL, Neugut AI, Santella RM, Gammon MD. Polyunsaturated fatty acid interactions and breast cancer incidence: a population-based case-control study on Long Island, New York. Ann Epidemiol. 2015;25:929–935. doi: 10.1016/j.annepidem.2015.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Du J, Wang T, Li Y, Zhou Y, Wang X, Yu X, Ren X, An Y, Wu Y, Sun W, Fan W, Zhu Q, Wang Y, Tong X. DHA inhibits proliferation and induces ferroptosis of leukemia cells through autophagy dependent degradation of ferritin. Free Radic Biol Med. 2019;131:356–369. doi: 10.1016/j.freeradbiomed.2018.12.011. [DOI] [PubMed] [Google Scholar]
- 16.Bai X, Shao J, Zhou S, Zhao Z, Li F, Xiang R, Zhao AZ, Pan J. Inhibition of lung cancer growth and metastasis by DHA and its metabolite, RvD1, through miR-138-5p/FOXC1 pathway. J Exp Clin Cancer Res. 2019;38:479. doi: 10.1186/s13046-019-1478-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zheng H, Tang H, Liu M, He M, Lai P, Dong H, Lin J, Jia C, Zhong M, Dai Y, Bai X, Wang L. Inhibition of endometrial cancer by n-3 polyunsaturated fatty acids in preclinical models. Cancer Prev Res (Phila) 2014;7:824–834. doi: 10.1158/1940-6207.CAPR-13-0378-T. [DOI] [PubMed] [Google Scholar]
- 18.Shakeri S, Amoozyan N, Fekrat F, Maleki M. Antigastric cancer bioactive aurantiochytrium oil rich in docosahexaenoic acid: from media optimization to cancer cells cytotoxicity assessment. J Food Sci. 2017;82:2706–2718. doi: 10.1111/1750-3841.13925. [DOI] [PubMed] [Google Scholar]
- 19.Jump DB, Depner CM, Tripathy S, Lytle KA. Potential for dietary omega-3 fatty acids to prevent nonalcoholic fatty liver disease and reduce the risk of primary liver cancer. Adv Nutr. 2015;6:694–702. doi: 10.3945/an.115.009423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Oono K, Takahashi K, Sukehara S, Kurosawa H, Matsumura T, Taniguchi S, Ohta S. Inhibition of PC3 human prostate cancer cell proliferation, invasion and migration by eicosapentaenoic acid and docosahexaenoic acid. Mol Clin Oncol. 2017;7:217–220. doi: 10.3892/mco.2017.1287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mouradian M, Ma IV, Vicente ED, Kikawa KD, Pardini RS. Docosahexaenoic acid-mediated inhibition of heat shock protein 90-p23 chaperone complex and downstream client proteins in lung and breast cancer. Nutr Cancer. 2017;69:92–104. doi: 10.1080/01635581.2017.1247886. [DOI] [PubMed] [Google Scholar]
- 22.Tanaka A, Yamamoto A, Murota K, Tsujiuchi T, Iwamori M, Fukushima N. Polyunsaturated fatty acids induce ovarian cancer cell death through ROS-dependent MAP kinase activation. Biochem Biophys Res Commun. 2017;493:468–473. doi: 10.1016/j.bbrc.2017.08.168. [DOI] [PubMed] [Google Scholar]
- 23.Wang YC, Wu YN, Wang SL, Lin QH, He MF, Liu QL, Wang JH. Docosahexaenoic acid modulates invasion and metastasis of human ovarian cancer via multiple molecular pathways. Int J Gynecol Cancer. 2016;26:994–1003. doi: 10.1097/IGC.0000000000000746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wan XH, Fu X, Ababaikeli G. Docosahexaenoic acid induces growth suppression on epithelial ovarian cancer cells more effectively than eicosapentaenoic acid. Nutr Cancer. 2016;68:320–327. doi: 10.1080/01635581.2016.1142581. [DOI] [PubMed] [Google Scholar]
- 25.Zajdel A, Kalucka M, Chodurek E, Wilczok A. DHA but not AA enhances cisplatin cytotoxicity in ovarian cancer cells. Nutr Cancer. 2018;70:1118–1125. doi: 10.1080/01635581.2018.1497673. [DOI] [PubMed] [Google Scholar]
- 26.Pal P, Hales K, Petrik J, Hales DB. Pro-apoptotic and anti-angiogenic actions of 2-methoxyestradiol and docosahexaenoic acid, the biologically derived active compounds from flaxseed diet, in preventing ovarian cancer. J Ovarian Res. 2019;12:49. doi: 10.1186/s13048-019-0523-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.de Aguiar Pastore Silva J, Emilia de Souza Fabre M, Waitzberg DL. Omega-3 supplements for patients in chemotherapy and/or radiotherapy: a systematic review. Clin Nutr. 2015;34:359–366. doi: 10.1016/j.clnu.2014.11.005. [DOI] [PubMed] [Google Scholar]
- 28.Sharma T, Mandal CC. Omega-3 fatty acids in pathological calcification and bone health. J Food Biochem. 2020;44:e13333. doi: 10.1111/jfbc.13333. [DOI] [PubMed] [Google Scholar]
- 29.Paixao E, Oliveira ACM, Pizato N, Muniz-Junqueira MI, Magalhaes KG, Nakano EY, Ito MK. The effects of EPA and DHA enriched fish oil on nutritional and immunological markers of treatment naive breast cancer patients: a randomized double-blind controlled trial. Nutr J. 2017;16:71. doi: 10.1186/s12937-017-0295-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Trabal J, Leyes P, Forga M, Maurel J. Potential usefulness of an EPA-enriched nutritional supplement on chemotherapy tolerability in cancer patients without overt malnutrition. Nutr Hosp. 2010;25:736–740. [PubMed] [Google Scholar]
- 31.Camargo CQ, Mocellin MC, Brunetta HS, Chagas TR, Fabre MES, Trindade E, Silva ELD, Nunes EA. Fish oil decreases the severity of treatment-related adverse events in gastrointestinal cancer patients undergoing chemotherapy: a randomized, placebo-controlled, triple-blind clinical trial. Clin Nutr ESPEN. 2019;31:61–70. doi: 10.1016/j.clnesp.2019.02.015. [DOI] [PubMed] [Google Scholar]
- 32.Bougnoux P, Hajjaji N, Ferrasson MN, Giraudeau B, Couet C, Le Floch O. Improving outcome of chemotherapy of metastatic breast cancer by docosahexaenoic acid: a phase II trial. Br J Cancer. 2009;101:1978–1985. doi: 10.1038/sj.bjc.6605441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Eltweri AM, Thomas AL, Metcalfe M, Calder PC, Dennison AR, Bowrey DJ. Potential applications of fish oils rich in omega-3 polyunsaturated fatty acids in the management of gastrointestinal cancer. Clin Nutr. 2017;36:65–78. doi: 10.1016/j.clnu.2016.01.007. [DOI] [PubMed] [Google Scholar]
- 34.Suri A, Sheng X, Schuler KM, Zhong Y, Han X, Jones HM, Gehrig PA, Zhou C, Bae-Jump VL. The effect of celecoxib on tumor growth in ovarian cancer cells and a genetically engineered mouse model of serous ovarian cancer. Oncotarget. 2016;7:39582–39594. doi: 10.18632/oncotarget.8659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Szabova L, Yin C, Bupp S, Guerin TM, Schlomer JJ, Householder DB, Baran ML, Yi M, Song Y, Sun W, McDunn JE, Martin PL, Van Dyke T, Difilippantonio S. Perturbation of Rb, p53, and Brca1 or Brca2 cooperate in inducing metastatic serous epithelial ovarian cancer. Cancer Res. 2012;72:4141–4153. doi: 10.1158/0008-5472.CAN-11-3834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kang KS, Wang P, Yamabe N, Fukui M, Jay T, Zhu BT. Docosahexaenoic acid induces apoptosis in MCF-7 cells in vitro and in vivo via reactive oxygen species formation and caspase 8 activation. PLoS One. 2010;5:e10296. doi: 10.1371/journal.pone.0010296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Shin JI, Jeon YJ, Lee S, Lee YG, Kim JB, Lee K. G-protein-coupled receptor 120 mediates DHA-induced apoptosis by regulating IP3R, ROS and, ER stress levels in cisplatin-resistant cancer cells. Mol Cells. 2019;42:252–261. doi: 10.14348/molcells.2019.2440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Giordano C, Plastina P, Barone I, Catalano S, Bonofiglio D. n-3 polyunsaturated fatty acid amides: new avenues in the prevention and treatment of breast cancer. Int J Mol Sci. 2020;21:2279. doi: 10.3390/ijms21072279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.VanderSluis L, Mazurak VC, Damaraju S, Field CJ. Determination of the relative efficacy of eicosapentaenoic acid and docosahexaenoic acid for anti-cancer effects in human breast cancer models. Int J Mol Sci. 2017;18:2607. doi: 10.3390/ijms18122607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Song EA, Kim H. Docosahexaenoic acid induces oxidative DNA damage and apoptosis, and enhances the chemosensitivity of cancer cells. Int J Mol Sci. 2016;17:1257. doi: 10.3390/ijms17081257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Noh J, Kwon B, Han E, Park M, Yang W, Cho W, Yoo W, Khang G, Lee D. Amplification of oxidative stress by a dual stimuli-responsive hybrid drug enhances cancer cell death. Nat Commun. 2015;6:6907. doi: 10.1038/ncomms7907. [DOI] [PubMed] [Google Scholar]
- 42.Shin S, Jing K, Jeong S, Kim N, Song KS, Heo JY, Park JH, Seo KS, Han J, Park JI, Kweon GR, Park SK, Wu T, Hwang BD, Lim K. The omega-3 polyunsaturated fatty acid DHA induces simultaneous apoptosis and autophagy via mitochondrial ROS-mediated Akt-mTOR signaling in prostate cancer cells expressing mutant p53. Biomed Res Int. 2013;2013:568671. doi: 10.1155/2013/568671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Newell M, Baker K, Postovit LM, Field CJ. A critical review on the effect of docosahexaenoic acid (DHA) on cancer cell cycle progression. Int J Mol Sci. 2017;18:1784. doi: 10.3390/ijms18081784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.DiPaola RS. To arrest or not to G(2)-M Cell-cycle arrest: commentary re: A. K. Tyagi et al,Silibinin strongly synergizes human prostate carcinoma DU145 cells to doxorubicin-induced growth inhibition, G(2)-M arrest, and apoptosis. Clin Cancer Res. 2002;8:3311–3314. [PubMed] [Google Scholar]
- 45.Tasaki S, Horiguchi A, Asano T, Ito K, Asano T, Asakura H. Docosahexaenoic acid inhibits the phosphorylation of STAT3 and the growth and invasion of renal cancer cells. Exp Ther Med. 2017;14:1146–1152. doi: 10.3892/etm.2017.4616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Mandal CC, Ghosh-Choudhury T, Yoneda T, Choudhury GG, Ghosh-Choudhury N. Fish oil prevents breast cancer cell metastasis to bone. Biochem Biophys Res Commun. 2010;402:602–607. doi: 10.1016/j.bbrc.2010.10.063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Merendino N, Costantini L, Manzi L, Molinari R, D’Eliseo D, Velotti F. Dietary omega -3 polyunsaturated fatty acid DHA: a potential adjuvant in the treatment of cancer. Biomed Res Int. 2013;2013:310186. doi: 10.1155/2013/310186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Manzi L, Costantini L, Molinari R, Merendino N. Effect of dietary omega-3 polyunsaturated fatty acid DHA on glycolytic enzymes and warburg phenotypes in cancer. Biomed Res Int. 2015;2015:137097. doi: 10.1155/2015/137097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Rahman MM, Veigas JM, Williams PJ, Fernandes G. DHA is a more potent inhibitor of breast cancer metastasis to bone and related osteolysis than EPA. Breast Cancer Res Treat. 2013;141:341–352. doi: 10.1007/s10549-013-2703-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Zhang G, Panigrahy D, Mahakian LM, Yang J, Liu JY, Stephen Lee KS, Wettersten HI, Ulu A, Hu X, Tam S, Hwang SH, Ingham ES, Kieran MW, Weiss RH, Ferrara KW, Hammock BD. Epoxy metabolites of docosahexaenoic acid (DHA) inhibit angiogenesis, tumor growth, and metastasis. Proc Natl Acad Sci U S A. 2013;110:6530–6535. doi: 10.1073/pnas.1304321110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Iigo M, Nakagawa T, Ishikawa C, Iwahori Y, Asamoto M, Yazawa K, Araki E, Tsuda H. Inhibitory effects of docosahexaenoic acid on colon carcinoma 26 metastasis to the lung. Br J Cancer. 1997;75:650–655. doi: 10.1038/bjc.1997.116. [DOI] [PMC free article] [PubMed] [Google Scholar]