Abstract
Real-world predictors of the treatment efficacy of immune checkpoint inhibitors for hepatocellular carcinoma (HCC) are unknown. This retrospective study enrolled 87 consecutive patients with unresectable HCC from May 2017 to December 2019 at two hospitals. Of the 87 patients, 7, 9, 60, and 11 patients had Barcelona Clinic Liver Cancer stages A, B, C, and D, respectively, and 45, 30, and 10 patients were Child-Pugh class A, B, and C, respectively. The median injection numbers of nivolumab and treatment duration were 6 (3-8) and 2.53 (1.47-4.23) months, respectively, and 64.4% of patients received combination therapy. Radiological imaging was not assessed for 25 patients. Objective response (OR) and disease control rates were 19.5% and 39.1%, respectively. A single tumor (odds ratio: 9.542, P = .015) and ≥20% decline in serum α-fetoprotein protein (AFP) levels within the first 3 months of treatment (defined as AFP response, odds ratio: 5.997, P = .042) were predictors of OR. Lack of macrovascular invasion, combination therapy, and AFP response were predictors of progression-free survival. A Cancer of the Liver Italian Program (CLIP) score of 0-2 (hazard ratio [HR]: 3.717, P = .004) and grade 1-2 immune-related adverse events (irAEs, HR: 2.217, P = .049) were predictors of overall survival (OS) in the entire cohort, and a CLIP score of 0-2 (HR: 3.257, P = .009) was a predictor of OS in evaluable patients. IrAEs ≥ grade 3 were noted in 14 patients, and three died as a result. Having a single tumor and AFP response were predictors of OR, and CLIP score was a predictor of OS.
Keywords: AFP, CLIP score, hepatocellular carcinoma, immune-related adverse event, nivolumab, survival
Introduction
Hepatocellular carcinoma (HCC) is an important global health issue [1]. HCC is usually recognized at an advanced stage, despite the recommendation of routine HCC surveillance [2]. The standard of care for advanced HCC has been sorafenib, a multitargeted tyrosine kinase inhibitor (TKI), for a decade [3,4]. Recently, second-line therapies including regorafenib, cabozantinib, and ramucirumab have been approved in various circumstances [5-7]. Lenvatinib, another multitargeted TKI, was noninferior to sorafenib in the improvement of overall survival (OS) of advanced HCC patients without ≥50% liver occupation or main portal vein invasion [8]. However, the prolonged median survival time with these five drugs is short [3-8].
Immune checkpoint inhibitors (ICIs) are new therapeutic agents against HCC [9]. Two ICIs, antiprogrammed cell death-1 (PD-1) monoclonal antibodies (nivolumab and pembrolizumab), have been conditionally approved for patients with advanced HCC after sorafenib failure. The objective response (OR) rate (ORR) for advanced HCC has been shown to be 14%-20% [9,10], and the progression-free survival (PFS) and OS were long in a proportion of patients in the early phase I/II or phase II studies. However, further phase III trials of nivolumab as a first-line therapy (versus sorafenib) [11] and pembrolizumab [12] as a second-line therapy (versus placebo) after sorafenib failure did not demonstrate beneficial effects in prolonging survival.
Three recent real-world studies have reported the efficacy of second-line ICIs, nivolumab and pembrolizumab, in advanced HCC patients [13-15]. Real-world reports of using nivolumab for unresectable HCC after failure of TKI or loco-regional therapy remain limited. This retrospective study investigated the use of nivolumab therapy in patients with unresectable HCC and considered its clinical features, application in combination therapy, immune-related adverse events (irAEs), and factors associated with therapeutic outcomes, including OR, PFS, and OS.
Patients and methods
Patients
From May 2017 to December 2019, 92 consecutive patients with unresectable HCC from China Medical University Hospital and the affiliated Asia University Hospital in central Taiwan who had received at least one dose of nivolumab therapy were enrolled in this retrospective study. The exclusion criteria were as follows: having a malignancy other than HCC, undergone liver transplantation, and human immunodeficiency virus infection. Among the enrollees, five patients who had received nivolumab therapy but not yet reached the time point of first radiological assessment were excluded from analysis. Of the 87 patients included in the final analysis, 20 died and 5 were lost to follow-up before the first radiological assessment; in total, 62 had evaluable radiological imaging (Supplementary Figure 1). Demographic data and virological features were recorded at baseline. Complete blood count and biochemical data were recorded at baseline, 4, 8, and 12 weeks and then every 2-3 months after the initiation of nivolumab therapy. This study was carried out in accordance with the 1975 Declaration of Helsinki. The Research Ethics Committee of China Medical University Hospital, Taichung, Taiwan (CMUH108-REC3-140) approved the study. Each patient’s identification number was encrypted to protect their privacy; thus, the need for informed consent was waived.
Nivolumab dose, tumor assessment, and safety
The nivolumab dose was 2-3 mg/kg every 2 weeks as recommended. Tumor assessment was carried out using dynamic computerized tomography every 8-12 weeks according to the modified Response Evaluation Criteria in Solid Tumors (mRECIST) [16]. Safety assessment was performed in accordance with the National Cancer Institute Common Terminology Criteria for Adverse Events (version 4.03).
Laboratory tests
We performed complete blood count analyses (Sysmex HST-series, Sysmex, Kanagawa, Japan) and blood biochemistry tests (Beckman Coulter, Brea, CA, USA) in the central laboratory of the hospital. Hepatitis B virus (HBV) infection was determined by the presence of serum HBsAg for more than 6 months, and hepatitis C virus (HCV) infection was defined as the presence of serum anti-HCV antibody for more than 6 months and detectable HCV RNA (detection limit = 15 IU/mL; Roche Diagnostics, Branchburg, NJ, USA). Liver cirrhosis was diagnosed through explicit clinical, ultrasonographic, or pathological analysis.
Statistical analyses
Continuous variables are expressed as the median (first quartile-third quartile). Comparisons of continuous variables between two groups were performed using the Mann-Whitney U test. Logistic regression analysis was used to identify factors associated with OR, and Cox regression analysis was used to identify those associated with PFS or OS. Kaplan-Meier analysis with the log-rank test was used to compare the PFS and OS among patient subgroups. Total tumor volume (TTV) was defined as the sum of each tumor volume (formula = [4/3] × 3.14 × [radius of the tumor in cm]3) [17]. SPSS (IBM SPSS 25.0, NY, USA) was used to perform all statistical analyses. A two-sided P value of <.05 was considered statistically significant.
Results
Baseline characteristics
Of the 87 patients, 79 (90.8%) were male and 67 (77%) had liver cirrhosis. In total, 23 (26.4%), 51 (58.6%), 22 (25.3%), and 25 (28.7%) patients reported drinking alcohol, having HBV infection, having HCV infection, and having diabetes mellitus, respectively. The median age was 63.4 (55.4-68.6) years. The neutrophil-lymphocyte ratio (NLR), alanine aminotransferase (ALT) level, aspartate aminotransferase (AST) level, total bilirubin level, albumin level, international normalized ratio of prothrombin time (INR), and AFP level were 5.53 (3.26-10.23), 51 (29-69) U/L, 70 (43-120) U/L, 1.3 (0.8-2.0) mg/dL, 3.5 (3.0-4.0) g/dL, 1.12 (1.04-1.24), and 296.92 (15.36-7282.00) ng/mL, respectively. The median Child-Pugh score (CPS) was 6 (5-8). In total, 7 (8.0%), 9 (10.3%), 60 (69.0%), and 11 (12.6%) patients had Barcelona Clinic Liver Cancer (BCLC) stages A, B, C, and D, respectively, and the median Cancer of the Liver Italian Program (CLIP) score was 2 (1-4). Most patients (n = 50, 57.5%) had ≥4 hepatic tumors, and the maximum tumor size was 5.2 (2.7-8.3) cm. The TTV was 1032 (245-3844) cm3. Extrahepatic metastasis (EHM) and macrovascular invasion (MVI) were observed in 52 (59.8%) and 51 (58.6%) patients, respectively, and 32 patients had both EHM and MVI. Only 14 (16.1%) patients received nivolumab as the first-line systemic therapy. The most common prior therapy was transarterial chemoembolization (TACE, n = 49, 56.3%), with a median treatment length of 4 (2-6) sessions, followed by radiotherapy (n = 44, 50.6%). Most patients received combination therapy (n = 56, 64.4%). Patients receiving concurrent nivolumab and TKI therapy (n = 48) had received TKI for more than 7 days before the addition of nivolumab therapy. The most common concurrent TKI therapy was sorafenib (n = 24, 27.6%), followed by lenvatinib (n = 19, 21.8%) (Table 1).
Table 1.
Patient demographics, baseline characteristics, and therapeutic response
| Character (N = 87) | n (%) or median (IQR) |
|---|---|
| Age (years) | 63.4 (55.4-68.6) |
| Sex (male), n (%) | 79 (90.8) |
| Body mass index (kg/m2) | 23.26 (20.69-26.30) |
| NLR | 5.53 (3.26-10.23) |
| Platelet count (109/L) | 153 (95-231) |
| AST (U/L) | 70 (43-120) |
| ALT (U/L) | 51 (29-69) |
| Total bilirubin (mg/dL) | 1.3 (0.8-2.0) |
| Albumin (g/dL) | 3.5 (3.0-4.0) |
| INR | 1.12 (1.04-1.24) |
| Etiology | |
| Alcohol | 23 (26.4) |
| HBV | 51 (58.6) |
| HCV | 22 (25.3) |
| Diabetes mellitus | 25 (28.7) |
| Liver cirrhosis | 67 (77.0) |
| Child-Pugh score | 6 (5-8) |
| Class A/B/C | 45 (51.7)/30 (34.5)/10 (11.5) |
| ALBI grade 1/2/3 | 20 (23.8)/47 (56.0)/17 (20.2) |
| AFP (ng/mL) | 296.92 (15.36-7282.00) |
| AFP ≥400 ng/mL | 39 (44.8) |
| BCLC stage A/B/C/D | 7 (8.0)/9 (10.3)/60 (69.0)/11 (12.6) |
| CLIP score | 2 (1-4) |
| Max. tumor size (cm) | 5.2 (2.7-8.3) |
| Tumor number | |
| 1/2/3/≥4 | 20 (23.0)/8 (9.2)/9 (10.3)/50 (57.5) |
| Total tumor volume (cm3) | 1032 (245-3844) |
| MVIa | 51 (58.6) |
| VP3/VP4/hepatic vein | 18 (20.7)/31 (35.6)/2 (2.3) |
| EHMa | 52 (59.8) |
| Prior therapy | |
| Sorafenib | 43 (49.4) |
| Lenvatinib | 7 (8.0) |
| TACEc/TARE | 49 (56.3)/2 (2.3) |
| Radiotherapy | 44 (50.6) |
| Surgery | 16 (18.4) |
| RFA | 15 (17.2) |
| PEI | 4 (4.6) |
| Injection numbers of nivolumab/duration (months) | 6 (3-8)/2.53 (1.47-4.23) |
| Reduction >25% | 17 (19.5) |
| As 1st/2nd/3rd/4th-line systemic therapy | 14 (16.1)/55 (63.2)/12 (13.8)/6 (6.9) |
| Concurrent therapy | 56 (64.4) |
| Sorafenibb | 24 (27.6) |
| Regorafenibb | 5 (5.7) |
| Lenvatinibb | 19 (21.8) |
| Chemotherapy | 7 (8.0) |
| Radiotherapy | 13 (14.9) |
| TACE | 11 (12.6) |
| Therapeutic response | |
| Best Response | |
| Complete response | 9 (10.3) |
| Partial response | 8 (9.2) |
| Stable disease | 17 (19.5) |
| Progressive disease | 28 (32.2) |
| Not evaluable | |
| Death before evaluation | 20 (23.0) |
| Lost to follow-upd | 5 (5.7) |
| Objective response | 17 (19.5) |
| Disease control | 34 (39.1) |
| Progression-free survival (months) | 2.67 (1.87-6.90) |
| Overall survival (months) | 5.87 (2.43-15.93) |
Data presented as the median (first quartile-third quartile). AFP, α-fetoprotein; ALBI, albumin-bilirubin; ALT, alanine aminotransferase; AST, aspartate aminotransferase; BCLC, Barcelona Clinic Liver Cancer; EHM, extrahepatic metastasis; CLIP, Cancer of the Liver Italian Program; HBV, hepatitis B virus; HCV, hepatitis C virus; IQR, interquartile range; MVI, macrovascular invasion; NLR, neutrophil-lymphocyte ratio; TACE, transarterial chemoembolization; TARE, transarterial radioembolization; TKI, tyrosine kinase inhibitor; PEI, percutaneous ethanol injection; INR, international normalized ratio; RFA, radiofrequency ablation.
In total, 32 HCC patients had both macrovascular invasion and extrahepatic metastasis.
A total of 7 patients received sequential TKI therapy because of progressive disease: sorafenib→regorafenib (3), sorafenib→lenvatinib (3), and sorafenib→lenvatinib→regorafenib (1).
The median number of TACE sessions was 4 (2-6).
In total, 5 patients were lost to follow-up because of immune-related adverse events (n = 3), for economic reasons (n = 1), and because of rapidly deteriorated bone pain (n = 1).
Therapeutic response
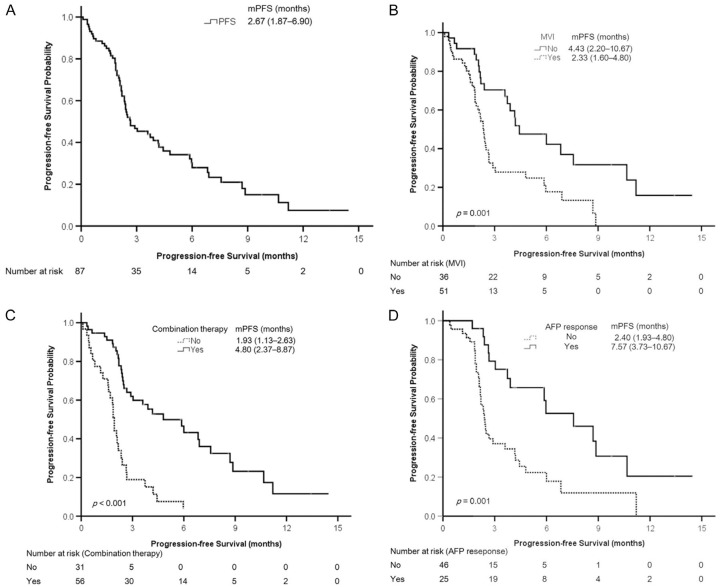
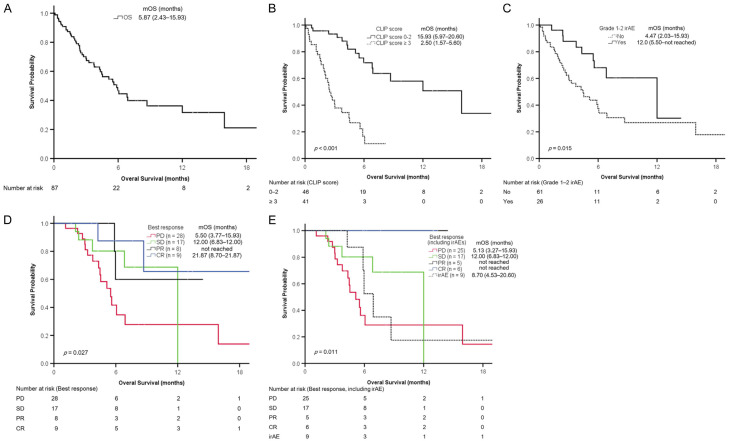
The median injection numbers of nivolumab and treatment duration were 6 (3-8) and 2.53 (1.47-4.23) months, respectively. A total of 25 patients were not assessed through radiological imaging; among them, 20 (23.0%) died before evaluation and 5 (5.7%) were lost to follow-up because of irAEs (n = 3), economic reasons (n = 1), and rapidly deteriorated bone pain (n = 1). The remaining 62 patients were assessed through radiological imaging. The number of patients with complete response (CR), partial response (PR), stable disease (SD), and progressive disease (PD) was 9 (10.3%), 8 (9.2%), 17 (19.5%), and 28 (32.2%), respectively. In comparison with patients who died before evaluation, the evaluable patients had higher body mass indices and albumin levels; lower levels of NLR, ALT, AST, total bilirubin, INR, and AFP; lower platelet count, CPS, and CLIP scores; smaller maximum tumor size and TTV; a lower proportion of EHM; a higher proportion of prior or combination therapy; and a longer nivolumab treatment duration, PFS, and OS (Supplementary Table 1). The ORR (CR + PR) and disease control rate (DCR, CR + PR + SD) were 19.5% (17/87) and 39.1% (34/87), respectively. The PFS and OS were 2.67 (1.87-6.90) and 5.87 (2.43-15.93) months, respectively (Figures 1A, 2A, and Table 1).
Figure 1.
Kaplan-Meier analyses of progression-free survival. A. All patients. B. Patients with or without macrovascular invasion (MVI). C. Patients with or without combination therapy. D. Patients with or without an AFP response. Continuous variables are presented as the median (first quartile-third quartile). AFP, α-fetoprotein protein; mPFS, median progression-free survival.
Figure 2.
Kaplan-Meier analyses of overall survival. A. All patients. B. Patients with higher (≥3) or lower (0-2) CLIP score. C. Patients with or without grade 1-2 irAEs. D. Patients with CR, PR, SD, or PD. E. Patients with CR, PR, SD, PD, or ≥ grade 3 irAEs. Continuous variables are presented as the median (first quartile-third quartile). CLIP, Cancer of the Liver Italian Program; CR, complete response; irAE, immune-related adverse event; mOS, median overall survival; PD, progressive disease; PR, partial response; SD, stable disease.
A total of 40 (46.0%) patients experienced at least one irAE of any grade (Supplementary Table 2). A total of 14 patients experienced ≥ grade 3 irAEs, including hepatitis (n = 5), dermatitis (n = 3), pneumonitis (n = 2), fatigue (n = 2), gastric necrosis (n = 1), and colitis (n = 1); 3 patients died from severe hepatic irAEs. The list of patients with ≥ grade 3 irAEs is presented in Supplementary Table 3. No factor was associated with ≥ grade 3 irAEs by multivariate logistic regression analysis (Supplementary Table 4).
Independent predictors of OR (CR + PR)
In 62 patients assessed using radiological imaging, univariate logistic regression analysis revealed that the significantly associated factors were TTV (≤1000 vs >1000 cm3), tumor number (single vs multiple), MVI (no vs yes), CLIP score (0-2 vs ≥3), CPS (A vs B/C), albumin-bilirubin (ALBI) grade (1 vs 2/3), NLR (≤3.0 vs >3.0), ≥ grade 3 irAEs, and a ≥20% decrease in serum AFP level within the initial 3 months of treatment (defined as AFP response herein). Multivariate logistic regression analysis indicated that tumor number (single vs multiple, odds ratio: 9.542, 95% confidence interval [CI]: 1.537-59.225, P = .015), and AFP response (odds ratio: 5.997, 95% CI: 1.070-33.600, P = .042) were independent predictors of OR (Supplementary Table 5).
Independent predictors of PFS
In the 87 enrolled patients, univariate Cox regression analysis revealed that the significantly associated factors were grade 1-2 irAEs, TTV (≤1000 vs >1000 cm3), tumor number (single vs multiple), MVI (no vs yes), CLIP score (0-2 vs ≥3), NLR (≤3.0 vs >3.0), ALT level (≤40 vs >40 U/L), AST level (≤40 vs >40 U/L), CPS (A vs B/C), combination therapy, concurrent TKI therapy, concurrent TACE, AFP response, and OR. Multivariate Cox regression analysis indicated that lack of MVI (hazard ratio [HR] = 4.266, 95% CI: 1.822-9.988, P = .001), nivolumab monotherapy (HR = 0.107, 95% CI: 0.046-0.248, P<.001), and AFP response (HR = 3.454, 95% CI 1.631-7.317, P = .002) were independent predictors of PFS (Table 2). Because most of the enrolled patients had BCLC stage C (n = 60, 69.0%), which is a confounding variable for MVI and EHM, BCLC stage was not analyzed as a variable.
Table 2.
Factors associated with progression-free survival in 87 patients with hepatocellular carcinoma
| Character | Univariate analysis | Multivariate analysis | |||
|---|---|---|---|---|---|
|
|
|
||||
| HR (95% CI) | P value | HR (95% CI) | P value | ||
| Age (year) | 0.991 (0.967-1.015) | .453 | |||
| Sex | Female vs male | 0.898 (0.407-1.982) | .790 | ||
| Alcohol | No vs yes | 1.174 (0.661-2.086) | .583 | ||
| HBV | No vs yes | 0.916 (0.552-1.518) | .733 | ||
| HCV | No vs yes | 0.830 (0.461-1.495) | .535 | ||
| Grade 1-2 irAEs | Yes vs no | 2.042 (1.138-3.664) | .017 | ||
| Grade ≥3 irAEs | Yes vs no | 1.032 (0.536-1.990) | .924 | ||
| TTV (cm3) | ≤1000 vs >1000 | 2.118 (1.274-3.523) | .004 | ||
| Tumor number | Single vs multiple | 2.995 (1.462-6.135) | .003 | ||
| MVI | No vs yes | 2.388 (1.3734.153) | .002 | 4.266 (1.822-9.988) | .001 |
| EHM | No vs yes | 1.497 (0.887-2.528) | .131 | ||
| CLIP score | 0-2 vs ≥3 | 3.967 (2.243-7.015) | <.001 | ||
| AFP (ng/mL) | <400 vs ≥400 | 0.959 (0.573-1.606) | .874 | ||
| NLR | ≤3.0 vs >3.0 | 2.530 (1.147-5.582) | .021 | ||
| AST (U/L) | ≤40 vs >40 | 2.274 (1.020-5.066) | .045 | ||
| ALT (U/L) | ≤40 vs >40 | 2.010 (1.155-3.500) | .014 | ||
| Child-Pugh class | A vs B/C | 1.905 (1.139-3.186) | .014 | ||
| ALBI grade | 1 vs 2/3 | 1.867 (0.966-3.607) | .063 | ||
| Prior therapy | |||||
| Sorafenib | No vs yes | 0.948 (0.573-1.567) | .835 | ||
| Lenvatinib | No vs yes | 1.100 (0.498-2.428) | .814 | ||
| Concurrent therapy | No vs yes | 0.261 (0.151-0.451) | <.001 | 0.107 (0.046-0.248) | <.001 |
| TKI | No vs yes | 0.472 (0.274-0.814) | .007 | ||
| TACE | No vs yes | 0.354 (0.141-0.888) | .027 | ||
| Radiotherapy | No vs yes | 1.685 (0.751-3.779) | .206 | ||
| AFP response | Yes vs No | 2.853 (1.502-5.422) | .001 | 3.454 (1.631-7.317) | .001 |
| Best response | CR + PR vs none | 4.166 (1.942-8.936) | <.001 | ||
AFP, α-fetoprotein; ALBI, albumin-bilirubin; ALT, alanine aminotransferase; AST, aspartate aminotransferase; CLIP, Cancer of the Liver Italian Program; CR + PR, complete response plus partial response; EHM, extrahepatic metastasis; HBV, hepatitis B virus; HCV, hepatitis C virus; irAEs, immune-related adverse events; MVI, macroscopic vascular invasion; NLR, neutrophil-lymphocyte ratio; TACE, transarterial chemoembolization; TKI, tyrosine kinase inhibitor; TTV, total tumor volume.
Kaplan-Meier analyses revealed that the probability of PFS differed significantly between patients with and without MVI (Figure 1B, P = .001), with and without combination therapy (Figure 1C, P<.001), and with and without AFP response (Figure 1D, P = .001).
Independent predictors of OS
Univariate Cox regression analysis identified the following significantly associated factors: grade 1-2 irAEs, TTV (≤1000 vs >1000 cm3), tumor number (single vs multiple), MVI (no vs yes), CLIP score (0-2 vs ≥3), CPS (A vs B/C), ALBI grade (1 vs 2/3), NLR (≤3.0 vs >3.0), ALT level (≤40 vs >40 U/L), combination therapy, concurrent TACE, and OR. Multivariate Cox regression analysis indicated that grade 1-2 irAEs (HR: 2.217, 95% CI: 1.005-4.892, P = .049) and CLIP score (0-2 vs ≥3, HR: 3.717, 95% CI: 1.537-8.988, P = .004) were independent predictors of OS in all patients (Table 3). Kaplan-Meier analyses revealed that the probabilities of survival were significantly different between patients with higher (≥3) or lower (0-2) CLIP score (Figure 2B, P<.001) and those with or without grade 1-2 irAEs (Figure 2C, P = .015).
Table 3.
Factors associated with overall survival in 87 patients with hepatocellular carcinoma
| Character | Univariate analysis | Multivariate analysis | |||
|---|---|---|---|---|---|
|
|
|
||||
| HR (95% CI) | P value | HR (95% CI) | P value | ||
| Age (year) | 0.998 (0.970-1.026) | .872 | |||
| Sex | Female vs male | 2.163 (0.659-7.105) | .203 | ||
| Alcohol | No vs yes | 1.347 (0.710-2.556) | .362 | ||
| HBV | No vs yes | 0.614 (0.342-1.105) | .104 | ||
| HCV | No vs yes | 1.053 (0.533-2.081) | .882 | ||
| Grade 1-2 irAEs | Yes vs no | 2.419 (1.157-5.055) | .019 | 2.217 (1.005-4.892) | .049 |
| Grade ≥3 irAEs | Yes vs no | 1.290 (0.573-2.905) | .539 | ||
| TTV (cm3) | ≤1000 vs >1000 | 3.511 (1.858-6.633) | <.001 | ||
| Tumor number | Single vs multiple | 3.144 (1.234-8.012) | .016 | ||
| MVI | No vs yes | 2.806 (1.443-5.458) | .002 | ||
| EHM | No vs yes | 1.614 (0.855-3.049) | .140 | ||
| CLIP score | 0-2 vs ≥3 | 6.146 (3.042-12.418) | <.001 | 3.717 (1.537-8.988) | .004 |
| AFP (ng/mL) | <400 vs ≥400 | 1.605 (0.878-2.935) | .124 | ||
| NLR | ≤3.0 vs >3.0 | 14.533 (1.993-105.976) | .008 | ||
| AST (U/L) | ≤40 vs >40 | 1.633 (0.687-3.885) | .267 | ||
| ALT (U/L) | ≤40 vs >40 | 2.080 (1.086-3.985) | .027 | ||
| Child-Pugh class | A vs B/C | 2.886 (1.556-5.355) | .001 | ||
| ALBI grade | 1 vs 2/3 | 4.202 (1.492-11.840) | .007 | ||
| Prior therapy | |||||
| Sorafenib | No vs yes | 0.852 (0.475-1.528) | .591 | ||
| Lenvatinib | No vs yes | 1.170 (0.491-2.788) | .723 | ||
| Concurrent therapy | No vs yes | 0.399 (0.222-0.716) | .002 | ||
| TKI | No vs yes | 0.635 (0.353-1.143) | .130 | ||
| TACE | No vs yes | 0.197 (0.047-0.818) | .025 | ||
| RT | No vs yes | 1.383 (0.543-3.518) | .496 | ||
| AFP response | Yes vs No | 1.872 (0.880-3.796) | .106 | ||
| Best response | CR + PR vs none | 4.935 (1.749-13.920) | .003 | ||
AFP, α-fetoprotein; ALT, alanine aminotransferase; AST, aspartate aminotransferase; CLIP, Cancer of the Liver Italian Program; CR + PR, complete response plus partial response; EHM, extrahepatic metastasis; HBV, hepatitis B virus; HCV, hepatitis C virus; irAEs, immune-related adverse events; MVI, macroscopic vascular invasion; NLR, neutrophil-lymphocyte ratio; RT, radiotherapy; TACE, transarterial chemoembolization; TKI, tyrosine kinase inhibitor; TTV, total tumor volume.
In 62 patients assessed using radiological imaging, the OS was 8.70 (4.53-20.60) months (Supplementary Figure 2A and Supplementary Table 1). Multivariate Cox regression analysis revealed that CLIP score (0-2 vs ≥3, HR: 3.257, 95% CI: 1.349-7.866, P = .009) was an independent predictor of OS (Table 4). Patients with lower (0-2) CLIP scores had longer OS than did those with higher (≥3) CLIP scores (Supplementary Figure 2B). The results of Kaplan-Meier analyses for the probability of survival in patients with CR, PR, SD, and PD are presented in Figure 2D. Furthermore, we classified patients with ≥ grade 3 irAEs related to nivolumab therapy into a fifth group; the results of Kaplan-Meier analyses for the probability of survival in these patients with CR, PR, SD, PD, and ≥ grade 3 irAEs are provided in Figure 2E. All patients with CR or PR were alive as of December 2019. Patients with OR but without ≥ grade 3 irAEs (n = 11) had significantly longer OS compared with those with OR and ≥ grade 3 irAEs (n = 7; 21.87 [8.7-21.87] vs 5.97 [5.87-8.70] months, P = .007).
Table 4.
Factors associated with overall survival in 62 patients with hepatocellular carcinoma who underwent evaluable radiological imaging
| Character | Univariate analysis | Multivariate analysis | |||
|---|---|---|---|---|---|
|
|
|
||||
| HR (95% CI) | P value | HR (95% CI) | P value | ||
| Age (year) | 0.985 (0.947-1.024) | .452 | |||
| Sex | Female vs male | 2.171 (0.497-9.479) | .303 | ||
| Alcohol | No vs yes | 1.237 (0.505-3.032) | .642 | ||
| HBV | No vs yes | 0.754 (0.338-1.679) | .489 | ||
| HCV | No vs yes | 0.891 (0.333-2.380) | .817 | ||
| Grade 1-2 irAEs | No vs yes | 1.786 (0.737-4.331) | .199 | ||
| Grade ≥3 irAEs | Yes vs no | 1.005 (0.373-2.710) | .992 | ||
| TTV (cm3) | ≤1000 vs >1000 | 3.500 (1.545-7.929) | .003 | ||
| Tumor number | Single vs multiple | 3.355 (0.996-11.302) | .051 | ||
| MVI | No vs yes | 3.222 (1.343-7.733) | .009 | ||
| EHM | No vs yes | 1.020 (0.451-2.304) | .962 | ||
| CLIP score | 0-2 vs ≥3 | 4.333 (1.811-10.367) | .001 | 3.257 (1.349-7.866) | .009 |
| AFP (ng/mL) | <400 vs ≥400 | 1.721 (0.779-3.800) | .179 | ||
| NLR | ≤3.0 vs >3.0 | 9.543 (1.282-71.038) | .028 | ||
| AST (U/L) | ≤40 vs >40 | 1.354 (0.456-4.014) | .585 | ||
| ALT (U/L) | ≤40 vs >40 | 2.571 (1.066-6.204) | .036 | ||
| Child-Pugh class | A vs B/C | 2.152 (0.960-4.826) | .063 | ||
| ALBI grade | 1 vs 2/3 | 11.028 (1.476-82.406) | .019 | ||
| Prior therapy | |||||
| Sorafenib | No vs yes | 1.476 (0.672-3.241) | .332 | ||
| Lenvatinib | No vs yes | 2.059 (0.808-5.2247) | .130 | ||
| Concurrent therapy | No vs yes | 0.611 (0.270-1.383) | .237 | ||
| TKI | No vs yes | 0.730 (0.336-1.585) | .426 | ||
| TACE | No vs yes | 0.302 (0.071-1.295) | .107 | ||
| RT | No vs yes | 1.515 (0.486-4.721) | .473 | ||
| AFP response | Yes vs no | 1.369 (0.600-3.124) | .455 | ||
| Best response | CR + PR vs none | 3.402 (1.160-9.982) | .026 | ||
AFP, α-fetoprotein; ALT, alanine aminotransferase; AST, aspartate aminotransferase; CLIP, Cancer of the Liver Italian Program; CR + PR, complete response plus partial response; EHM, extrahepatic metastasis; HBV, hepatitis B virus; HCV, hepatitis C virus; irAEs, immune-related adverse events; MVI, macroscopic vascular invasion; NLR, neutrophil-lymphocyte ratio; RT, radiotherapy; TACE, transarterial chemoembolization; TKI, tyrosine kinase inhibitor; TTV, total tumor volume.
Discussion
In this real-world study of nivolumab therapy for patients with unresectable HCC, we determined an ORR and DCR of 19.5% and 39.1%, respectively, and discovered that having a single tumor and AFP response were predictors of OR. Furthermore, the median OS was 5.87 months, and a CLIP score of 0-2 and grade 1-2 irAEs were predictors of OS in the entire cohort. The median OS was 8.70 months, and a CLIP score of 0-2 was a predictor of OS in the patients with evaluable radiological imaging (n = 62). Patients who achieved an OR without developing ≥ grade 3 irAEs had a median survival of 21.87 months.
ICIs are a newly approved class of agents for the treatment of cancer, including HCC. Pivotal clinical trials have reported ORRs of ICI monotherapy in advanced HCC patients in the range of 15%-20% [9,10,12]. Three recent real-world studies of patients with advanced or unresectable HCC reported ORRs of 11.8%, 12.3%, and 24.4% with nivolumab or pembrolizumab therapy [13-15]. Our result in an early cohort of patients with unresectable HCC is consistent with the results of the aforementioned studies. Because ICI therapy is costly and only effective in some patients, a baseline or early on-treatment biomarker of response is desirable to facilitate individualized therapy. Two recent studies revealed that early AFP response, defined as a >20% decrease in serum AFP level within the initial 4 weeks of treatment [18] or a >10% decrease in serum AFP level within the initial 4 weeks of treatment in patients with baseline AFP ≥10 ng/mL [15], could respectively predict PFS and OS [18] or OR and OS [15] in patients receiving ICI therapy. Our study demonstrated that a ≥20% decrease in serum AFP level within the initial 3 months of treatment is a predictor of PFS and OR. Because not all patients underwent monthly AFP measurements during treatment, we were unable to compare the predictive performance of AFP response at 1 or 2 months with that at 3 months. Together, these three studies highlighted the role of AFP measurement in predicting the response to ICI therapy for HCC. Furthermore, we demonstrated that having a single tumor was a predictor of response to nivolumab therapy. One possible explanation is that patients with a single tumor presented with a significantly smaller tumor volume (TTV 336.5 [28.2-3646.6] cm3 vs 1244.3 [279.3-4216.5] cm3, P = .049) and lower proportion of MVI (30% vs 67.2%, P = .003) than did those with multiple tumors. To determine whether tumor burden and vascular invasion negatively affect response to ICI therapy, further study is necessary.
Median OS was relatively short for the entire cohort and for the subgroup of patients with evaluable radiological imaging. This finding has several possible explanations. First, we enrolled a high proportion of patients with poor liver reserve or advanced HCC (11.5% Child-Pugh class C, median tumor size 5.2 cm, 58.6% MVI, 12.6% BCLC stage D, and 47.1% CLIP score ≥3). Only 18.4% of our patients received prior surgery, and 50.6% received prior palliative radiotherapy (Supplementary Table 6) [13-15,18]. This indicates that our patients initially presented with a less favorable liver reserve and a larger, more advanced, and unresectable HCC compared with previous study cohorts. Second, 23% of our patients died before the first radiological assessment, which accounts for the short OS of the entire cohort. These patients had significantly less favorable liver reserve (CPS and ALBI) and more advanced tumor stage (TTV, CLIP score, and BCLC stage) compared with patients who survived beyond the first radiological assessment (Supplementary Table 1). Hence, these patients might not be ideal candidates for nivolumab therapy in a clinical setting. Third, nivolumab became available to us in May 2017, and the median treatment duration was 2.53 months. A longer follow-up of the present cohort is required to demonstrate the real-world efficacy of nivolumab therapy for treating advanced HCC. Notably, all patients who achieved CR or PR without developing ≥ grade 3 irAEs during treatment were alive at the end of this study.
We identified a CLIP score of 0-2 as a predictor of OS in the entire cohort and in patients with evaluable radiological response. CLIP is a prognostic system originally used on a retrospective cohort from 16 Italian institutions (n = 435) [19]. Three parameters of the CLIP system represent tumor characteristics, namely tumor number and extent, portal vein invasion, and AFP level, and the fourth parameter is Child-Pugh class. This composite scoring system reflects both the tumor burden and the liver reserve and serves to prognosticate patients with HCC. The CLIP score is straightforward to use and is reportedly an excellent prognostic tool for patients with early to advanced HCC [20]. We demonstrated for the first time that CLIP score is an independent predictor of survival that can stratify the probability of survival in HCC patients receiving nivolumab therapy in real-world settings. Further large-scale research is needed to validate the role of CLIP score as a prognostic tool in HCC patients receiving nivolumab therapy.
Combination therapy was identified as a predictor of PFS in this study. One interpretation of this finding is that concurrent therapy in addition to nivolumab therapy acts synergistically to inhibit tumor growth and thereby enhance the patient’s response rate. Combination therapy could not be demonstrated to be a predictor of OS perhaps because of the limited number of enrolled patients (n = 87) with relatively short follow-up period. This appears to agree with the findings of several recent studies that reported an increased therapeutic efficacy of combination therapy such as pembrolizumab plus lenvatinib [21], nivolumab plus ipilimumab [22], or atezolizumab plus bevacizumab in patients with advanced HCC [23]. Notably, our patients received various modalities of concurrent therapy, including TKIs, radiation, and TACE. Whether all modalities provided synergistic effects could not be further evaluated because of the small number of patients receiving each combination therapy. The apparent beneficial effects of combination therapy could also be attributable to the possibility that patients with less advanced HCC, in the presence of better-preserved liver function, were preferentially selected for combination therapy. This may have biased the combination group toward a more favorable survival outcome. The observation that combination therapy was not a predictor of OR or OS in the evaluable patients (n = 62) in this study supports this hypothesis. Hence, the synergistic effect of concurrent therapy with ICIs in advanced HCC patients should be validated in future studies in real-world settings.
ICIs can trigger T cell immunity against cancer and self-antigens, resulting in the occurrence of various irAEs [24]. The incidence of ≥ grade 3 irAEs for nivolumab and pembrolizumab is in the range of 10%-20% in patients with HCC [25]. A tendency of a higher incidence of hepatic irAEs in HCC patients has also been reported [25]. In the current study, 14 patients (16.1%) developed hepatic irAEs of any grade; five patients (5.7%) developed ≥ grade 3 hepatitis, and three of them died. Moreover, patients who achieved OR but developed ≥ grade 3 irAEs (n = 7) had significantly shorter OS than did those who achieved OR without developing ≥ grade 3 irAEs (n = 11). This finding highlights the crucial role of ≥ grade 3 irAEs in determining the outcomes of patients with HCC receiving ICIs. However, we failed to identify any predictors of ≥ grade 3 irAEs. Previous studies revealed that melanoma or lung cancer patients receiving nivolumab therapy with irAEs (82% or more patients with grade 1-2 irAEs) had a better survival outcome [26,27]. Our finding that grade 1-2 irAEs was a predictor of OS in all enrolled patients (n = 87) was consistent with these results. Alternatively, patients with longer OS may have higher probability of developing irAEs. IrAEs of any grade were not a predictor of OR in this study (Supplementary Table 5). Further studies are needed to clarify the underlying mechanism for their association. Thus, vigilant surveillance, early recognition, and prompt management of irAEs to prevent its progression into ≥ grade 3 severity are imperative to securing favorable patient outcomes.
There are several limitations to this study. First, this retrospective study was performed at two hospitals, and only 87 patients were enrolled. Second, the follow-up period was short, and patients who achieved OR without developing ≥ grade 3 irAEs had not yet reached the median OS time by the end of the study period. The follow-up period should be extended to reveal the beneficial effect of ICIs in patients with HCC. Third, we used mRECIST [16] instead of RECIST version 1.1 for the assessment of radiological response [28] because 56.3% and 50.6% of our patients had received prior TACE and radiotherapy, respectively, for which mRECIST serves as a better assessment tool [29]. Furthermore, it has been demonstrated that mRECIST ORR is an independent predictor of survival in advanced HCC patients treated with molecular targeted therapy [14,29,30]. Fourth, we did not assess the association of PD-L1 expression in the tumor or immune cells with OR. Further study is warranted to explore the predictive role of PD-L1 expression for treatment response.
In conclusion, nivolumab was effective for treating some patients with unresectable HCC. Having a single tumor and AFP response were predictors of OR, and CLIP score was a predictor of OS in the evaluable patients. Patients who achieved OR without developing ≥ grade 3 irAEs exhibited the best survival. Appropriate selection of patients with less advanced disease status and high vigilance for irAEs during nivolumab therapy may help to improve survival in unresectable HCC patients.
Acknowledgements
This study was supported by a grant (DMR-109-252) from China Medical University Hospital, Taichung, Taiwan.
Disclosure of conflict of interest
None.
Supporting Information
References
- 1.GBD 2017 Causes of Death Collaborators. Global, regional, and national age-sex-specific mortality for 282 causes of death in 195 countries and territories, 1980-2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet. 2018;392:1736–1788. doi: 10.1016/S0140-6736(18)32203-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kanwal F, Singal AG. Surveillance for hepatocellular carcinoma: current best practice and future direction. Gastroenterology. 2019;157:54–64. doi: 10.1053/j.gastro.2019.02.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Llovet JM, Ricci S, Mazzaferro V, Hilgard P, Gane E, Blanc JF, de Oliveira AC, Santoro A, Raoul JL, Forner A, Schwartz M, Porta C, Zeuzem S, Bolondi L, Greten TF, Galle PR, Seitz JF, Borbath I, Häussinger D, Giannaris T, Shan M, Moscovici M, Voliotis D, Bruix J SHARP Investigators Study Group. Sorafenib in advanced hepatocellular carcinoma. N Engl J Med. 2008;359:378–390. doi: 10.1056/NEJMoa0708857. [DOI] [PubMed] [Google Scholar]
- 4.Cheng AL, Kang YK, Chen Z, Tsao CJ, Qin S, Kim JS, Luo R, Feng J, Ye S, Yang TS, Xu J, Sun Y, Liang H, Liu J, Wang J, Tak WY, Pan H, Burock K, Zou J, Voliotis D, Guan Z. Efficacy and safety of sorafenib in patients in the Asia-Pacific region with advanced hepatocellular carcinoma: a phase III randomised, double-blind, placebo-controlled trial. Lancet Oncol. 2009;10:25–34. doi: 10.1016/S1470-2045(08)70285-7. [DOI] [PubMed] [Google Scholar]
- 5.Bruix J, Qin S, Merle P, Granito A, Huang YH, Bodoky G, Pracht M, Yokosuka O, Rosmorduc O, Breder V, Gerolami R, Masi G, Ross PJ, Song T, Bronowicki JP, Ollivier-Hourmand I, Kudo M, Cheng AL, Llovet JM, Finn RS, LeBerre MA, Baumhauer A, Meinhardt G, Han G. Regorafenib for patients with hepatocellular carcinoma who progressed on sorafenib treatment (RESORCE): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet. 2017;389:56–66. doi: 10.1016/S0140-6736(16)32453-9. [DOI] [PubMed] [Google Scholar]
- 6.Abou-Alfa GK, Meyer T, Cheng AL, El-Khoueiry AB, Rimassa L, Ryoo BY, Cicin I, Merle P, Chen Y, Park JW, Blanc JF, Bolondi L, Klumpen HJ, Chan SL, Zagonel V, Pressiani T, Ryu MH, Venook AP, Hessel C, Borgman-Hagey AE, Schwab G, Kelley RK. Cabozantinib in patients with advanced and progressing hepatocellular carcinoma. N Engl J Med. 2018;379:54–63. doi: 10.1056/NEJMoa1717002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zhu AX, Kang YK, Yen CJ, Finn RS, Galle PR, Llovet JM, Assenat E, Brandi G, Pracht M, Lim HY, Rau KM, Motomura K, Ohno I, Merle P, Daniele B, Shin DB, Gerken G, Borg C, Hiriart JB, Okusaka T, Morimoto M, Hsu Y, Abada PB, Kudo M REACH-2 study investigators. Ramucirumab after sorafenib in patients with advanced hepatocellular carcinoma and increased alpha-fetoprotein concentrations (REACH-2): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Oncol. 2019;20:282–296. doi: 10.1016/S1470-2045(18)30937-9. [DOI] [PubMed] [Google Scholar]
- 8.Kudo M, Finn RS, Qin S, Han KH, Ikeda K, Piscaglia F, Baron A, Park JW, Han G, Jassem J, Blanc JF, Vogel A, Komov D, Evans TRJ, Lopez C, Dutcus C, Guo M, Saito K, Kraljevic S, Tamai T, Ren M, Cheng AL. Lenvatinib versus sorafenib in first-line treatment of patients with unresectable hepatocellular carcinoma: a randomised phase 3 non-inferiority trial. Lancet. 2018;391:1163–1173. doi: 10.1016/S0140-6736(18)30207-1. [DOI] [PubMed] [Google Scholar]
- 9.El-Khoueiry AB, Sangro B, Yau T, Crocenzi TS, Kudo M, Hsu C, Kim TY, Choo SP, Trojan J, Welling TH Rd, Meyer T, Kang YK, Yeo W, Chopra A, Anderson J, Dela Cruz C, Lang L, Neely J, Tang H, Dastani HB, Melero I. Nivolumab in patients with advanced hepatocellular carcinoma (CheckMate 040): an open-label, non-comparative, phase 1/2 dose escalation and expansion trial. Lancet. 2017;389:2492–2502. doi: 10.1016/S0140-6736(17)31046-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhu AX, Finn RS, Edeline J, Cattan S, Ogasawara S, Palmer D, Verslype C, Zagonel V, Fartoux L, Vogel A, Sarker D, Verset G, Chan SL, Knox J, Daniele B, Webber AL, Ebbinghaus SW, Ma J, Siegel AB, Cheng AL, Kudo M KEYNOTE-224 investigators. Pembrolizumab in patients with advanced hepatocellular carcinoma previously treated with sorafenib (KEYNOTE-224): a non-randomised, open-label phase 2 trial. Lancet Oncol. 2018;19:940–952. doi: 10.1016/S1470-2045(18)30351-6. [DOI] [PubMed] [Google Scholar]
- 11.Yau T, Park JW, Finn RS, Cheng AL, Mathurin P, Edeline J, Kudo M, Han KH, Harding JJ, Merle P, Rosmorduc O, Wyrwicz L, Schott E, Choo SP, Kelley RK, Begic D, Chen G, Neely J, Anderson J, Sangro B. CheckMate 459: a randomized, multi-center phase III study of nivolumab (NIVO) vs sorafenib (SOR) as first-line (1L) treatment in patients (pts) with advanced hepatocellular carcinoma (aHCC) Ann Oncol. 2019;30(Suppl 5):v874–v875. [Google Scholar]
- 12.Finn RS, Ryoo BY, Merle P, Kudo M, Bouattour M, Lim HY, Breder V, Edeline J, Chao Y, Ogasawara S, Yau T, Garrido M, Chan SL, Knox J, Daniele B, Ebbinghaus SW, Chen E, Siegel AB, Zhu AX, Cheng AL KEYNOTE-224 investigators. Pembrolizumab As second-line therapy in patients with advanced hepatocellular carcinoma in KEYNOTE-240: a randomized, double-blind, phase III Trial. J. Clin. Oncol. 2020;38:193–202. doi: 10.1200/JCO.19.01307. [DOI] [PubMed] [Google Scholar]
- 13.Finkelmeier F, Czauderna C, Perkhofer L, Ettrich TJ, Trojan J, Weinmann A, Marquardt JU, Vermehren J, Waidmann O. Feasibility and safety of nivolumab in advanced hepatocellular carcinoma: real-life experience from three German centers. J Cancer Res Clin Oncol. 2019;145:253–259. doi: 10.1007/s00432-018-2780-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Scheiner B, Kirstein MM, Hucke F, Finkelmeier F, Schulze K, von Felden J, Koch S, Schwabl P, Hinrichs JB, Waneck F, Waidmann O, Reiberger T, Muller C, Sieghart W, Trauner M, Weinmann A, Wege H, Trojan J, Peck-Radosavljevic M, Vogel A, Pinter M. Programmed cell death protein-1 (PD-1)-targeted immunotherapy in advanced hepatocellular carcinoma: efficacy and safety data from an international multicentre real-world cohort. Aliment Pharmacol Ther. 2019;49:1323–1333. doi: 10.1111/apt.15245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lee PC, Chao Y, Chen MH, Lan KH, Lee CJ, Lee IC, Chen SC, Hou MC, Huang YH. Predictors of response and survival in immune checkpoint inhibitor-treated unresectable hepatocellular carcinoma. Cancers (Basel) 2020;12:182. doi: 10.3390/cancers12010182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lencioni R, Llovet JM. Modified RECIST (mRECIST) assessment for hepatocellular carcinoma. Semin Liver Dis. 2010;30:52–60. doi: 10.1055/s-0030-1247132. [DOI] [PubMed] [Google Scholar]
- 17.Hsu CY, Huang YH, Hsia CY, Su CW, Lin HC, Loong CC, Chiou YY, Chiang JH, Lee PC, Huo TI, Lee SD. A new prognostic model for hepatocellular carcinoma based on total tumor volume: the Taipei Integrated Scoring System. J Hepatol. 2010;53:108–117. doi: 10.1016/j.jhep.2010.01.038. [DOI] [PubMed] [Google Scholar]
- 18.Shao YY, Liu TH, Hsu C, Lu LC, Shen YC, Lin ZZ, Cheng AL, Hsu CH. Early alpha-foetoprotein response associated with treatment efficacy of immune checkpoint inhibitors for advanced hepatocellular carcinoma. Liver Int. 2019;39:2184–2189. doi: 10.1111/liv.14210. [DOI] [PubMed] [Google Scholar]
- 19.A new prognostic system for hepatocellular carcinoma: a retrospective study of 435 patients: the cancer of the Liver Italian Program (CLIP) investigators. Hepatology. 1998;28:751–755. doi: 10.1002/hep.510280322. [DOI] [PubMed] [Google Scholar]
- 20.Hsu CY, Hsia CY, Huang YH, Su CW, Lin HC, Lee PC, Loong CC, Chiang JH, Huo TI, Lee SD. Selecting an optimal staging system for hepatocellular carcinoma: comparison of 5 currently used prognostic models. Cancer. 2010;116:3006–3014. doi: 10.1002/cncr.25044. [DOI] [PubMed] [Google Scholar]
- 21.Ikeda M, Sung MW, Kudo M, Kobayashi M, Baron AD, Finn RS, Kaneko S, Zhu AX, Kubota T, Kraljevic S, Ishikawa K, Siegel AB, Kumada H, Okusaka T. A phase 1b trial of lenvatinib (LEN) plus pembrolizumab (PEM) in patients (pts) with unresectable hepatocellular carcinoma (uHCC) J. Clin. Oncol. 2018;36:4076–4076. [Google Scholar]
- 22.Yau T, Kang YK, Kim TY, El-Khoueiry AB, Santoro A, Sangro B, Melero I, Kudo M, Hou MM, Matilla A, Tovoli F, Knox JJ, He AR, El-Rayes BF, Acosta-Rivera M, Neely J, Shen Y, Baccan C, Cruz CMD, Hsu C. Nivolumab (NIVO) + ipilimumab (IPI) combination therapy in patients (pts) with advanced hepatocellular carcinoma (aHCC): results from CheckMate 040. J. Clin. Oncol. 2019;37:4012–4012. [Google Scholar]
- 23.Finn RS, Qin S, Ikeda M, Galle PR, Ducreux M, Kim TY, Kudo M, Breder V, Merle P, Kaseb AO, Li D, Verret W, Xu DZ, Hernandez S, Liu J, Huang C, Mulla S, Wang Y, Lim HY, Zhu AX, Cheng AL, Investigators IM. Atezolizumab plus bevacizumab in unresectable hepatocellular carcinoma. N Engl J Med. 2020;382:1894–1905. doi: 10.1056/NEJMoa1915745. [DOI] [PubMed] [Google Scholar]
- 24.Baxi S, Yang A, Gennarelli RL, Khan N, Wang Z, Boyce L, Korenstein D. Immune-related adverse events for anti-PD-1 and anti-PD-L1 drugs: systematic review and meta-analysis. BMJ. 2018;360:k793. doi: 10.1136/bmj.k793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sangro B, Chan SL, Meyer T, Reig M, El-Khoueiry A, Galle PR. Diagnosis and management of toxicities of immune checkpoint inhibitors in hepatocellular carcinoma. J Hepatol. 2020;72:320–341. doi: 10.1016/j.jhep.2019.10.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Freeman-Keller M, Kim Y, Cronin H, Richards A, Gibney G, Weber JS. Nivolumab in resected and unresectable metastatic melanoma: characteristics of immune-related adverse events and association with outcomes. Clin Cancer Res. 2016;22:886–894. doi: 10.1158/1078-0432.CCR-15-1136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Haratani K, Hayashi H, Chiba Y, Kudo K, Yonesaka K, Kato R, Kaneda H, Hasegawa Y, Tanaka K, Takeda M, Nakagawa K. Association of immune-related adverse events with nivolumab efficacy in non-small-cell lung cancer. JAMA Oncol. 2018;4:374–378. doi: 10.1001/jamaoncol.2017.2925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Eisenhauer EA, Therasse P, Bogaerts J, Schwartz LH, Sargent D, Ford R, Dancey J, Arbuck S, Gwyther S, Mooney M, Rubinstein L, Shankar L, Dodd L, Kaplan R, Lacombe D, Verweij J. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1) Eur J Cancer. 2009;45:228–247. doi: 10.1016/j.ejca.2008.10.026. [DOI] [PubMed] [Google Scholar]
- 29.Choi MH, Park GE, Oh SN, Park MY, Rha SE, Lee YJ, Jung SE, Choi JI. Reproducibility of mRECIST in measurement and response assessment for hepatocellular carcinoma treated by transarterial chemoembolization. Acad Radiol. 2018;25:1363–1373. doi: 10.1016/j.acra.2018.02.013. [DOI] [PubMed] [Google Scholar]
- 30.Lencioni R, Montal R, Torres F, Park JW, Decaens T, Raoul JL, Kudo M, Chang C, Rios J, Boige V, Assenat E, Kang YK, Lim HY, Walters I, Llovet JM. Objective response by mRECIST as a predictor and potential surrogate end-point of overall survival in advanced HCC. J Hepatol. 2017;66:1166–1172. doi: 10.1016/j.jhep.2017.01.012. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.