Abstract
We recently demonstrated that silodosin, a selective α1-blocker often prescribed for the symptomatic treatment of benign prostatic hyperplasia (BPH), could inactivate a c-fos proto-oncogene regulator ELK1 in bladder cancer cells possessing a functional androgen receptor (AR). However, the clinical impact of α1-blockers on the development and progression of bladder cancer remained poorly understood. In the present study, we investigated if α1-blockers clinically used, including silodosin, tamsulosin, and naftopidil, could prevent the neoplastic/malignant transformation and cell growth, using non-neoplastic urothelial SVHUC sublines with carcinogen/MCA challenge and bladder cancer lines, respectively. Bladder cancers in men treated with silodosin, tamsulosin, or naftopidil for their BPH were then compared. Silodosin at 1-10 µM significantly inhibited the neoplastic transformation of MCA-SVHUC-AR cells, but not that of AR-negative MCA-SVHUC-control cells. In MCA-SVHUC-AR, silodosin significantly reduced the expression levels of oncogenes (c-fos/NF-κB1) and induced those of tumor suppressors (p27/PTEN). However, tamsulosin (up to 1 µM) or naftopidil (up to 10 µM) failed to significantly inhibit the neoplastic transformation of AR-positive or AR-negative urothelial cells. Similarly, cell proliferation/migration of AR-positive bladder cancer lines was considerably inhibited only by silodosin. Meanwhile, the incidence of bladder cancer in patients with silodosin [49/540 (9.1%)] was marginally lower, compared to those with tamsulosin [64/523 (12.2%); P=0.094] or tamsulosin or naftopidil [64+28/523+236 (12.1%); P=0.082]. There were no significant differences in tumor grade/stage among the 3 cohorts. Outcome analysis revealed lower risks for disease progression of non-muscle-invasive bladder tumors in the silodosin group than in the naftopidil group (P=0.011) or tamsulosin+naftopidil groups (P=0.035). Similarly, silodosin patients with muscle-invasive tumor had lower risks for disease progression, compared with tamsulosin (P=0.006) or tamsulosin+naftopidil (P=0.028) patients. Multivariate analysis further showed that silodosin treatment in those with non-muscle-invasive tumor was associated with improved progression-free survival, compared with naftopidil (hazard ratio=0.086; 95% confidence interval=0.008-0.905; P=0.041) or tamsulosin/naftopidil (hazard ratio=0.128; 95% confidence interval=0.016-1.036; P=0.054) treatment. Our in vitro studies thus indicate that both urothelial tumorigenesis and tumor growth are inhibited by silodosin, but not by tamsulosin or naftopidil. Clinical data further suggest that even pharmacological doses (e.g. 0.1 µM) of silodosin contribute to preventing bladder cancer progression.
Keywords: α1-blocker, androgen receptor, bladder cancer, naftopidil, silodosin, tamsulosin
Introduction
Urinary bladder cancer, mostly urothelial carcinoma, has been one of the commonly diagnosed malignancies predominantly affecting men throughout the world [1]. Non-muscle-invasive bladder tumors account for approximately three-fourth of all newly diagnosed cases and can typically be managed with relatively conservative approaches. However, these patients following transurethral surgery and currently available intravesical pharmacotherapy still carry a lifetime risk of tumor recurrence occasionally with an invasive disease for which aggressive treatment options are often required. Accordingly, new therapeutic disciplines that more effectively prevent the recurrence and/or progression of superficial bladder cancer need to be developed.
Developing evidence from preclinical studies indicates a crucial role of androgen-mediated androgen receptor (AR) signaling in the modulation of two distinct events, urothelial tumorigenesis and tumor progression, while precise mechanisms for the functions of AR and related signals in urothelial cells remain to be determined [2]. Specifically, we have demonstrated that AR antagonists, such as flutamide and enzalutamide, inhibit the development and growth of urothelial cancer [3-5]. We have also found that androgens induce the expression and/or activity of ELK1, a regulator of the c-fos proto-oncogene, in non-neoplastic urothelial cells or bladder cancer cells [6,7], suggesting an interplay between AR and ELK1 signals in urothelial cells.
A selective α1-adrenergic receptor antagonist silodosin has been prescribed primarily for the symptomatic management of benign prostatic hyperplasia (BPH) [8]. We have previously shown that silodosin could inactivate ELK1 signals in not only prostate cancer cells [9], but also non-neoplastic urothelial cells [7] and bladder cancer cells [10], expressing the AR, and thereby inhibited the growth of cancer cells and the neoplastic transformation of non-neoplastic cells. Meanwhile, in addition to silodosin, other α1-blockers possessing some differences particularly in true selectivity for the subtypes (i.e. α1A, α1D) of α1-adrenergic receptor [11,12] have been used in BPH patients. However, the clinical impact of these α1-blockers on urothelial cancer outgrowth remains uncertain. In the present study, we assessed whether α1-blockers clinically used, including silodosin as well as tamsulosin and naftopidil, could similarly suppress urothelial tumorigenesis and tumor progression.
Materials and methods
Cell culture and chemicals
Human bladder cancer lines, UMUC3 (AR-positive [3]) and 5637 (AR-negative [3]), and an immortalized human normal urothelial line, SVHUC (AR-negative [13]), were originally obtained from the American Type Culture Collection. Sublines stably expressing AR-short hairpin RNA (shRNA) (i.e. UMUC3-AR-shRNA) or human full-length wild-type AR (i.e. 5637-AR, SVHUC-AR) or vector only (i.e. SVHUC-control) were established in our previous studies [13,14]. UMUC3-, 5637-, and SVHUC-derived cells were maintained in DMEM (Gibco), RPMI1640 (Mediatech), and Ham’s F-12K (Mediatech), respectively, supplemented with 10% fetal bovine serum (FBS). These were then cultured in phenol red-free medium for the experimental treatment with silodosin/tamsulosin/naftopidil (all from Cayman Chemical).
In vitro transformation
An in vitro neoplastic/malignant transformation system, using SVHUC cells with exposure to 3-methylcholanthrene (MCA), was employed, as established in a previous study [15], with minor modifications. Briefly, cells (2 × 106/10-cm culture dish incubated for 48 hours) were cultured in serum-free Ham’s F-12K containing 5 μg/mL MCA (Sigma). After 24 hours of MCA exposure, 1% FBS was added to the medium. After additional 24 hours, the cells were cultured in medium containing 5% FBS without MCA until near confluence. Subcultured cells (1:3 split ratio) were again cultured in the presence of MCA for two 48-hour exposure periods, using the above protocol. These MCA-exposed cells were then subcultured for 6 weeks in the presence or absence of an α1-blocker and thereafter utilized for further assays.
Cell proliferation
The methylthiazolyldisphenyl-tetrazolium bromide (MTT) assay was used to assess cell viability/proliferation. Cells (3-5 × 103/well) seeded in 96-well tissue culture plates were incubated for 96 hours, and at the end of the culture 10 μL MTT stock solution (5 mg/mL; Sigma) was added to each well for 3 hours at 37°C. The medium was replaced with 100 μL dimethyl sulfoxide and incubated for 5 minutes at room temperature. The absorbance was then measured at a wavelength of 570 nm with background subtraction at 630 nm.
Cell migration
A scratch wound-healing assay was used to assess the ability of cell migration. Cells at a density of 90-100% confluence in 12-well tissue culture plates were scratched manually with a sterile 200 µL plastic pipette tip. After 24-hour culture in a serum-free condition, the cells were fixed with methanol and stained with 0.1% crystal violet. The width of the wound area was then quantitated, using ImageJ software (National Institutes of Health).
Colony formation
The plate colony formation assay was used to assess the clonogenic potential. Cells (5 × 102/well) seeded in 12-well tissue culture plates were allowed to grow until colonies in the control well were certainly visible. The cells/colonies were then fixed with methanol and stained with 0.1% crystal violet. The number of colonies was quantitated, using the ImageJ.
Real-time PCR
Total RNA isolated from cultured cells by TRIzol (Invitrogen) was reverse transcribed, using oligo (dT) primers and Omniscript reverse transcriptase (Qiagen). Real-time PCR was then performed, using iQ™ SYBR® Green Supermix (Bio-Rad), as we described previously [7,13]. The sequences of primer pairs are listed in Table 1.
Table 1.
Sequences of PCR primers
| Gene | Forward | Reverse |
|---|---|---|
| c-fos | 5’-CGAGATGGAGATCGGTATGGT-3’ | 5’-GGGTCTTCTTACCCGGCTTG-3’ |
| NF-κB1 | 5’-AACAGAGAGGATTTCGTTTCC-3’ | 5’-TTTGACCTGAGGGTAAGACTTCT-3’ |
| p27 | 5’-CGAGTGGCAAGAGGTGGAGA-3’ | 5’-GGAGCCCCAATTAAAGGCG-3’ |
| PTEN | 5’-GTTTACCGGCAGCATCAAAT-3’ | 5’-CCCCCACTTTAGTGCACAGT-3’ |
| GAPDH | 5’-AAGGTGAAGGTCGGAGTCAAC-3’ | 5’-GGGGTCATTGATGGCAACAATA-3’ |
Western blot
Equal amounts (30 µg) of proteins extracted from cell extracts were subjected to sodium dodecyl sulfate-polyacrylamide gel electrophoresis (10%). After transferring onto a polyvinylidene difluoride membrane electronically, the membrane was blocked with 1% milk and incubated with a primary antibody against ELK1 (clone I-20, Santa Cruz Biotechnology), NF-κB/p65 (clone F-6, Santa Cruz Biotechnology), or GAPDH (clone 6C5, Santa Cruz Biotechnology) at 4°C overnight, followed by 1-hour incubation with a HRP-conjugated secondary antibody (Cell Signaling Technology) at room temperature. Chemiluminescent signals were then detected using the ChemiDOC™ MP (Bio-Rad) imaging system.
Patient cohort
Upon approval by the Institutional Review Board including the request to waive the documentation of informed consent from the patients, we retrospectively collected data from those who had received α1-blocker (i.e. silodosin, tamsulosin, naftopidil) therapy for their BPH between 2006 (after all three drugs were available in Japan) and 2018 at Yokohama City University Medical Center. For those who were found to have bladder tumors, we searched the institution’s pathology database for their histopathological findings and also retrieved clinical/follow-up data. Cases transferred to our institution for the management of bladder cancer were included, but those who had two or more α1-blockers (both with and without bladder cancer), developed bladder cancer before α1-blocker therapy, or had a history of urothelial tumor were excluded from analysis. In patients with non-muscle-invasive bladder cancer, disease progression was defined as the development of high-grade tumor (from initial non-invasive low-grade), invasive tumor (from initial non-invasive), or muscle-invasive or metastatic tumor.
Statistical analysis
Chi-square test was used to evaluate the associations between categorized variables. Student’s t-test was used for a nonparametric two-group comparison. Survival rates were calculated by Kaplan-Meier method, and comparison was made by log-rank test. The Cox proportional hazards model was used to determine statistical significance of predictors in a multivariate setting. P values less than 0.05 were considered to be statistically significant.
Results
Efficacy of α1-blockers in urothelial tumorigenesis
We used an established in vitro model where non-neoplastic SVHUC cells could undergo neoplastic/malignant transformation induced by a chemical carcinogen MCA during the course of 6-week culture [15]. SVHUC-derived sublines (i.e. SVHUC-control, SVHUC-AR) exposed to MCA for 48 hours three times were subcultured with various concentrations of silodosin, tamsulosin, or naftopidil (i.e. pharmacologically reachable serum concentrations of respective drugs after oral administration and 10/100 times higher doses [16-18]) for 6 weeks during the process of transformation. Oncogenic activity in the transformed cells was then monitored by subsequent assays for cell viability (via MTT assay with 4-day culture; Figure 1A), cell migration (via wound-healing assay with 24-hour culture; Figure 1B), and colony formation (via clonogenic assay with 2-week culture; Figure 2) with no further α1-blocker treatment that could directly affect their results. We thus compared the degree of neoplastic transformation in urothelial cells with carcinogen challenge but did not intend to simply assess the effects of α1-blockers on the growth of SVHUC-derived cells. In these assays, silodosin treatment resulted in significant reduction in cell viability, cell migration, and colony formation of MCA-SVHUC-AR cells, but not cell viability/migration of AR-negative MCA-SVHUC-control cells, indicating, as consistent with our previous observations [7], the inhibition of neoplastic transformation of AR-positive urothelial cells by silodosin. However, tamsulosin and naftopidil failed to considerably prevent the neoplastic transformation of MCA-SVHUC-AR or MCA-SVHUC-control cells.
Figure 1.
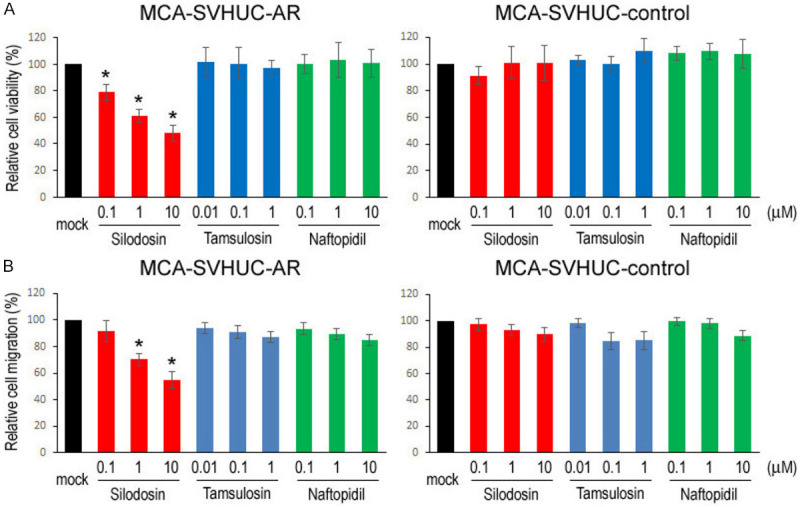
Effects of α1-blockers on neoplastic transformation of urothelial cells. A. MTT assay in SVHUC-AR or SVHUC-control cells exposed to MCA, subcultured for 6 weeks in the presence of ethanol (mock), silodosin (0.1-10 µM), tamsulosin (0.01-1 µM), or naftopidil (0.1-10 µM), and further incubated for 96 hours without α1-blocker treatment. The cell viability is presented relative to that in each subline with mock treatment. B. Wound-healing assay in SVHUC-AR or SVHUC-control cells exposed to MCA, subcultured for 6 weeks in the presence of ethanol (mock), silodosin (0.1-10 µM), tamsulosin (0.01-1 µM), or naftopidil (0.1-10 µM), and further incubated for 24 hours without α1-blocker treatment. The cell migration is presented relative to that in each subline with mock treatment. Each value represents the mean (± SD) from three independent experiments. *P<0.05 (vs. mock treatment).
Figure 2.
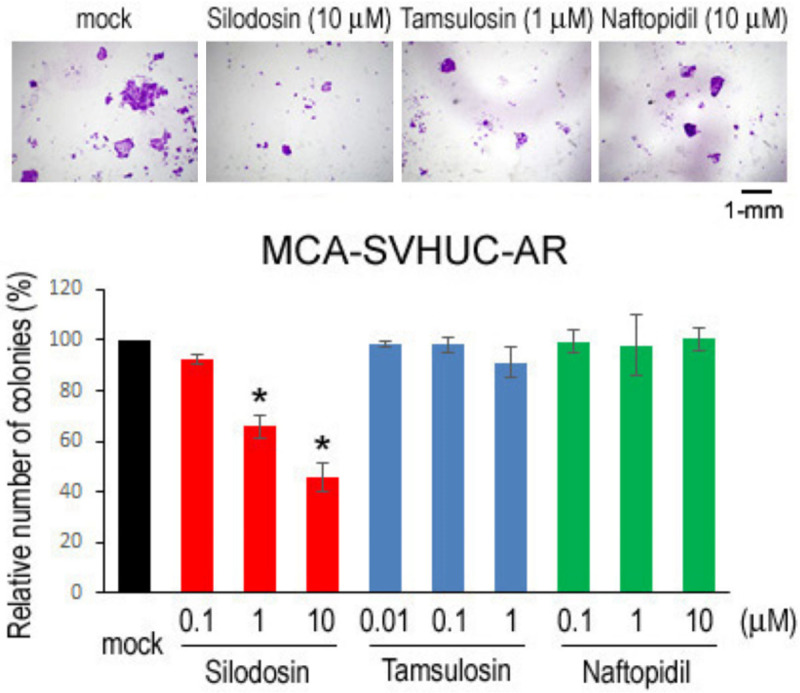
Effects of α1-blockers on neoplastic transformation of urothelial cells determined by colony-forming ability. SVHUC-AR cells first exposed to MCA and subsequently cultured for 6 weeks in the presence of ethanol (mock), silodosin (0.1-10 µM), tamsulosin (0.01-1 µM), or naftopidil (0.1-10 µM) were seeded for clonogenic assay (additional 2-week culture without α1-blocker treatment). The number of colony consisting of ≥20 cells is presented relative to that in each subline with mock treatment. Each value represents the mean (± SD) from three independent experiments. *P<0.05 (vs. mock treatment).
A quantitative PCR analysis was also employed to compare the expression levels of oncogenes and tumor suppressors for urothelial cancer in those undergoing neoplastic transformation while treating with α1-blockers. In MCA-SVHUC-AR cells, the expression of c-fos/NF-κB1 and p27/PTEN was significantly down- and up-regulated, respectively, by 6-week silodosin treatment (Figure 3), further supporting its preventive effect on urothelial tumorigenesis. By contrast, no considerable changes in their expression levels in tamsulosin- or naftopidil-treated cells were seen.
Figure 3.
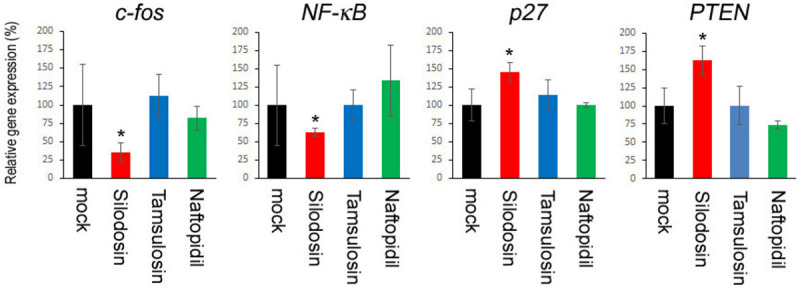
Effects of α1-blockers on neoplastic transformation of urothelial cells determined by the expression of oncogenes and tumor suppressor genes. SVHUC-AR cells first exposed to MCA and subsequently cultured for 6 weeks in the presence of ethanol (mock), silodosin (10 µM), tamsulosin (1 µM), or naftopidil (10 µM) were subjected to RNA extraction and subsequent real-time PCR for c-fos, NF-κB1, p27, and PTEN. Expression of each specific gene was normalized to that of GAPDH, and transcription amount is presented relative to that of mock treatment. Each value represents the mean (± SD) of three determinants. *P<0.05 (vs. mock treatment).
Efficacy of α1-blockers in urothelial tumor growth
We compared cell proliferation and migration of bladder cancer lines/sublines (i.e. AR-positive UMUC3, UMUC3-AR-shRNA, AR-negative 5637, 5637-AR) cultured with various concentrations of silodosin, tamsulosin, or naftopidil. In accordance with our previous findings [10], MTT assay showed significant decreases in the viability of AR-positive cells (Figure 4A, 4B), but not that of AR-negative cells (Figure 4C, 4D), by silodosin treatment. However, tamsulosin and naftopidil did not considerably affect the growth of AR-positive/AR-negative cells. Similarly, a wound-healing assay showed that the migration of AR-positive cells was significantly inhibited by silodosin, but not by tamsulosin or naftopidil (Figure 5A, 5B), while these 3 α1-blockers did not considerably affect that of AR-negative cells (Figure 5C, 5D).
Figure 4.
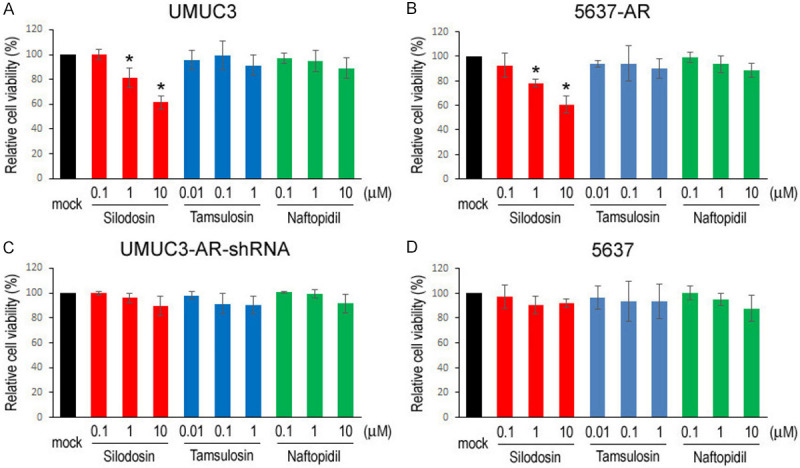
Effects of α1-blockers on the viability of bladder cancer cells. MTT assay in UMUC3 (A), 5637-AR (B), UMUC3-AR-shRNA (C), and 5637 (D) cells cultured in medium containing 5% FBS as well as ethanol (mock), silodosin (0.1-10 µM), tamsulosin (0.01-1 µM), or naftopidil (0.1-10 µM) for 96 hours. Cell viability is presented relative to that in each line with mock treatment. Each value represents the mean (± SD) from three independent experiments. *P<0.05 (vs. mock treatment).
Figure 5.
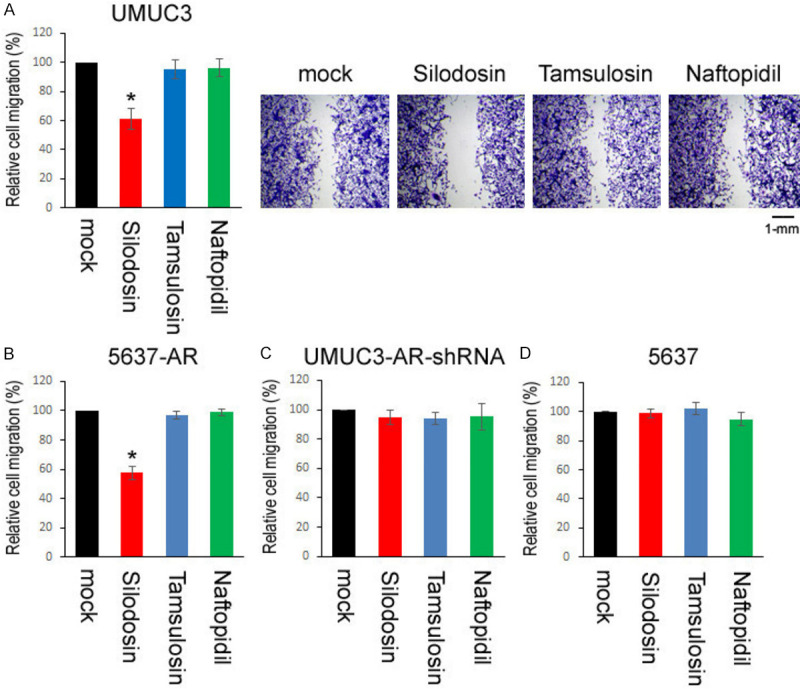
Effects of α1-blockers on the migration of bladder cancer cells. Wound-healing assay in UMUC3 (A), 5637-AR (B), UMUC3-AR-shRNA (C), and 5637 (D) cells. The cells grown to confluence were gently scratched, and the wound area was measured after 24-hour culture in serum-free medium containing ethanol (mock), silodosin (10 µM), tamsulosin (1 µM), or naftopidil (10 µM). The migration determined by the rate of cells filling the wound area is presented relative to that in each line with mock treatment. Each value represents the mean (± SD) from three independent experiments. *P<0.001 (vs. mock treatment).
Efficacy of α1-blockers in ELK1/NF-κB expression
We have previously demonstrated that silodosin inhibits the expression of not only ELK1 but also NF-κB in bladder cancer cells [10]. To further explore the differences in the effects of silodosin versus tamsulosin/naftopidil on urothelial tumorigenesis and tumor growth, we performed western blot for ELK1 and NF-κB/p65 in AR-positive SVHUC-AR (Figure 6A) and UMUC3 (Figure 6B). As expected, silodosin reduced the expression levels of ELK1 and NF-κB in both lines. However, no significant inhibitory effects of tamsulosin and naftopidil on ELK1/NF-κB expression were detected in these cells.
Figure 6.
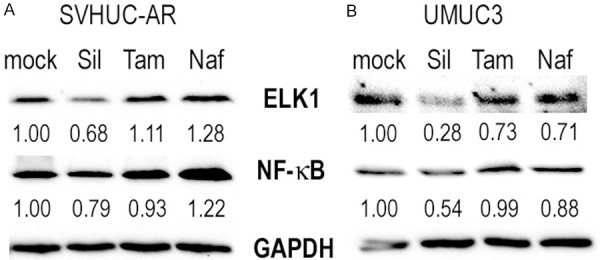
Effects of α1-blockers on the expression of ELK1/NF-κB in urothelial cells. Western blot of ELK1 and NF-κB in SVHUC-AR (A) and UMUC3 (B) cells treated with ethanol (mock), silodosin (Sil, 10 µM), tamsulosin (Tam, 1 µM), or naftopidil (Naf, 10 µM) for 48 hours. GAPDH served as an internal control. Densitometry values for ELK1/NF-κB standardized by GAPDH that are relative to those of mock treatment are included below the lanes.
Impact of α1-blocker therapy for BPH on bladder cancer development/progression
We next investigated the potential role of α1-blocker therapy in the development and progression of bladder cancer in BPH patients. We collected and analyzed clinical data from a total of 1,299 men who had received one of the α1-blockers for their BPH. Of these, 141 (10.9%) patients, including those with silodosin [49/540 (9.1%)], tamsulosin [64/523 (12.2%)], and naftopidil [28/236 (11.9%)], were found to develop bladder tumors histopathologically confirmed as urothelial carcinomas following α1-blocker therapy (Table 2). Thus, the incidence of bladder cancer was marginally lower in silodosin patients than in tamsulosin (P=0.094) or tamsulosin+naftopidil (P=0.082) patients, but not in naftopidil patients (P=0.232). Meanwhile, the naftopidil cohort was marginally (P=0.055) younger than the tamsulosin cohort. Bladder cancers included 83 low-grade and 58 high-grade urothelial carcinomas as well as 124 non-muscle-invasive and 17 muscle-invasive tumors, but significant differences in tumor grade or pT stage were not observed between any of two cohorts.
Table 2.
Clinicopathologic features of bladder tumors in men who received α1-blocker therapy
| Silodosin | Tamsulosin | Naftopidil | P value | ||||
|---|---|---|---|---|---|---|---|
|
| |||||||
| Sil vs. Tam | Sil vs. Naf | Sil vs. Tam+Naf | Tam vs. Naf | ||||
| Bladder tumors | 49/540 (9.1%) | 64/523 (12.2%) | 28/236 (11.9%) | 0.094 | 0.232 | 0.082 | 0.884 |
| Age (mean ± SD, yr) | 74.3 ± 7.9 | 75.6 ± 9.9 | 71.8 ± 8.0 | 0.426 | 0.196 | 0.904 | 0.055 |
| Tumor grade | 0.418 | 0.188 | 0.257 | 0.488 | |||
| Low grade | 32 (65.3%) | 37 (57.8%) | 14 (50.0%) | ||||
| High grade | 17 (34.7%) | 27 (42.2%) | 14 (50.0%) | ||||
| Tumor stage | 0.330 | 0.397 | 0.300 | 0.977 | |||
| NMI (≤pT1) | 45 (91.8%) | 55 (85.9%) | 24 (85.7%) | ||||
| MI (≥pT2) | 4 (8.2%) | 9 (14.1%) | 4 (14.3%) | ||||
| Recurrence of NMI | 16 (35.6%) | 27 (49.1%) | 12 (50.0%) | 0.174 | 0.245 | 0.137 | 0.941 |
| Progression of NMI | 1 (2.2%) | 6 (10.9%) | 5 (20.8%) | 0.090 | 0.009 | 0.034 | 0.241 |
| Progression of MI | 0 (0%) | 7 (77.8%) | 2 (50.0%) | 0.009 | 0.103 | 0.015 | 0.317 |
Sil: silodosin; Tam: tamsulosin; Naf: naftopidil; NMI: non-muscle-invasive; MI: muscle-invasive.
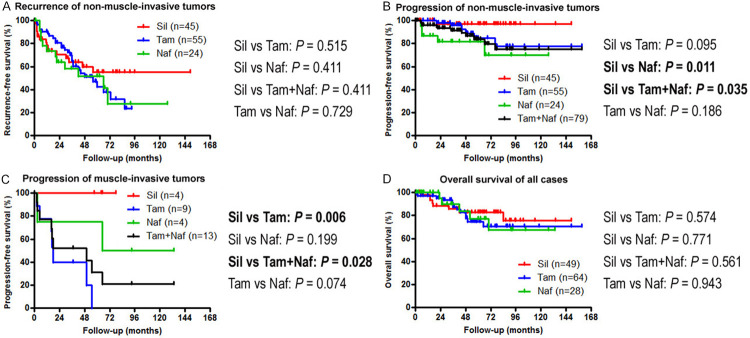
We then assessed possible associations between α1-blocker therapy and patient outcomes. There were no significant differences in the risk of intravesical recurrence of non-muscle-invasive bladder tumors between two cohorts (Figure 7A). However, in those with non-muscle-invasive bladder cancer, the silodosin cohort had a lower risk of disease progression, compared with the tamsulosin cohort (P=0.095), naftopidil cohort (P=0.011), or tamsulosin+naftopidil cohorts (P=0.035) (Figure 7B). Similarly, in those with muscle-invasive tumor, silodosin treatment was associated with a lower risk of disease progression, compared with tamsulosin treatment (P=0.006) or tamsulosin or naftopidil treatment (P=0.028) (Figure 7C). Naftopidil patients with muscle-invasive tumor also tended to show better prognosis, compared with tamsulosin patients (P=0.074). Additionally, in all cases, different α1-blocker treatments were not considerably associated with overall survival (Figure 7D), while only a small subset of patients in these cohorts died of bladder cancer during follow-up. To further determine if silodosin therapy was an independent predictor of disease progression of non-muscle-invasive tumors, multivariate analysis was performed with the Cox model, including dichotomized tumor grade, pT stage, and α1-blocker therapy (Table 3). In these subgroups, silodosin therapy showed significance and a trend toward significance for the progression, compared with naftopidil therapy [hazard ratio (HR)=0.086, 95% confidence interval (CI)=0.008-0.905, P=0.041] and tamsulosin (HR=0.137, 95% CI=0.016-1.191, P=0.072) or tamsulosin/naftopidil (HR=0.128, 95% CI=0.016-1.036, P=0.054) therapy, respectively.
Figure 7.
Kaplan-Meier curves for recurrence-free survival (A) or progression-free survival (B) in 124 patients with non-muscle-invasive bladder cancer, progression-free survival in 17 patients with muscle-invasive tumor (C), and overall survival in all 141 cases (D), according to the status of silodosin (Sil)/tamsulosin (Tam)/naftopidil (Naf) treatment. Comparisons between two groups were made by the log-rank test.
Table 3.
Multivariate analysis for progression-free survival in men with non-muscle-invasive bladder tumor
| Variable | HR | 95% CI | P value |
|---|---|---|---|
| Silodosin vs. Tamsulosin | |||
| Tumor grade (high vs. low) | 12.56 | 2.314-68.14 | 0.003 |
| Tumor stage (pT1 vs. non-invasive) | 9.722 | 1.064-88.81 | 0.044 |
| α1-Blocker | 0.137 | 0.016-1.191 | 0.072 |
| Silodosin vs. Naftopidil | |||
| Tumor grade (high vs. low) | 18.85 | 1.557-228.2 | 0.021 |
| Tumor stage (pT1 vs. non-invasive) | 2.009 | 0.296-13.64 | 0.476 |
| α1-Blocker | 0.086 | 0.008-0.905 | 0.041 |
| Silodosin vs. Tamsulosin or Naftopidil | |||
| Tumor grade (high vs. low) | 12.55 | 3.082-51.07 | <0.001 |
| Tumor stage (pT1 vs. non-invasive) | 3.176 | 0.855-11.80 | 0.084 |
| α1-Blocker | 0.128 | 0.016-1.036 | 0.054 |
HR: hazard ratio; CI: confidence interval.
Discussion
Using preclinical models for urothelial cancer, we previously demonstrated evidence indicating that AR activation was associated with the induction of the expression and/or activity of ELK1, a transcription factor whose phosphorylation via the MAPK/ERK pathway is known to lead to activation of downstream targets, including a proto-oncogene c-fos [6,7]. ELK1 is thus suggested to contribute to modulating urothelial tumorigenesis and tumor progression, presumably via the AR pathway. We also demonstrated that silodosin could inactivate ELK1 signals in both non-neoplastic urothelial cells and bladder cancer cells possessing a functional AR, and thereby exhibited an anti-tumor activity [7,10]. However, clinical significance of silodosin, as well as other selective α1-blockers prescribed in men with BPH, in bladder cancer remained unknown. The present study aimed to investigate the efficacy of silodosin, tamsulosin, and naftopidil in urothelial cancer outgrowth in cell line models and BPH patients.
In an in vitro system with non-neoplastic cells with carcinogen challenge, we compared oncogenic activities (e.g. cell viability/proliferation, cell migration, colony formation, expression of oncogenes and tumor suppressor genes) and found that tamsulosin or naftopidil did not considerably prevent the neoplastic/malignant transformation of urothelial cells. Similarly, in bladder cancer cells, tamsulosin and naftopidil showed no significant effects on their viability/proliferation and migration. As expected, however, silodosin strongly inhibited both the carcinogen-mediated neoplastic transformation of AR-positive urothelial cells and the growth of AR-positive bladder cancer cells. Correspondingly, in BPH patients treated with an α1-blocker, bladder cancer incidence was marginally lower in the silodosin cohort, compared with tamsulosin cohort or tamsulosin and naftopidil cohorts. Silodosin therapy was also associated with a significantly lower risk of the progression of non-muscle-invasive bladder cancer, compared with naftopidil therapy, as an independent prognosticator, or tamsulosin or naftopidil therapy in a univariate setting. In addition, the risk of disease progression of muscle-invasive tumors was significantly lower in silodosin patients than in tamsulosin patients or tamsulosin and naftopidil patients in a univariate setting. There were no significant differences in the risk of intravesical recurrence of non-muscle-invasive tumor or mortality of all cases between any of two treatment groups.
It remains uncertain why, of the three selective α1-blockers examined in this study, only silodosin has exhibited anti-tumor effects in in vitro models for urothelial cancer. Currently, silodosin appears to be the only α1-blocker which shows true selectivity for α1A, while naftopidil is selective for α1D with approximately 3-fold higher affinity than α1A [11,12]. Meanwhile, anti-proliferative effects of non-selective α1-blockers, such as terazosin and doxazosin, independent of α1-blockade, have been documented in non-urothelial cancer, including prostate cancer cells [19-21]. In particular, it was shown in prostate cancer that terazosin and doxazosin similarly inhibited the cell viability of AR-positive LNCaP versus AR-negative PC3 lines [21]. In addition, silodosin [22] or naftopidil [22,23] more strongly suppressed the growth of LNCaP cells than tamsulosin, primarily via inducing apoptosis. In one of the studies, the incidence of prostate cancer was shown to be significantly lower in men with ≥3-month naftopidil therapy than in those with tamsulosin therapy [23]. We here demonstrated down-regulation of the expression of c-fos, a downstream target of ELK1, by 6-week silodosin treatment in MCA-exposed AR-positive urothelial cells. More importantly, we found that the expression of ELK1 as well as NF-κB was inhibited by silodosin, but not by tamsulosin or naftopidil, in both non-neoplastic urothelial cells and bladder cancer cells possessing a functional AR, which may be an underlying reason for the differences in the anti-tumor activity of silodosin versus tamsulosin/naftopidil. Nonetheless, further studies are required to determine exactly how α1-blockers affect urothelial tumor outgrowth.
In prostate cancer where AR is well-known to play a critical role in its progression, the inhibitory effects of silodosin on the proliferation of AR-positive LNCaP and C4-2 cells were found to be more prominent than those in AR-negative PC3 and DU145 cells, although silodosin considerably reduced the levels of ELK1 expression even in these AR-negative cells [9]. Our previous [6,7,10] and current observations in urothelial cancer have also suggested that AR is most likely essential for mediating anti-tumor properties of silodosin. Of note, non-neoplastic urothelial cells usually express the AR [24], while its expression is often down-regulated in bladder cancers, especially advanced cases (e.g. 80% in non-neoplastic urothelial tissues, 55% in low-grade tumors, 36% in high-grade tumors, 51% in non-muscle-invasive tumors, 33% in muscle-invasive tumors [25]). Nonetheless, AR activation in bladder cancer has been linked to resistance to conventional non-surgical treatments, such as BCG immunotherapy [26], chemotherapy [27,28], and radiotherapy [29]. Thus, novel therapeutic modalities, apart from the application of androgen deprivation therapy [2], may especially be required for AR-positive urothelial cancers.
There are several limitations in our investigation, especially in clinical data. First, the present study is subject to potential selection bias due to the retrospective design. Second, we did not consider the duration of α1-blocker therapy, mainly because a history of α1-blocker therapy at outside institutions was unavailable in most of the patients. Additionally, although those who had developed bladder tumors prior to the treatment with an α1-blocker were excluded from analysis, cases where α1-blocker therapy had been discontinued for some time before the development of de novo (or recurrent) tumors were included. Third, because there were some cases that had been transferred to our institution primarily for the treatment of bladder cancer, its incidence in BPH patients might be relatively high. Fourth, we compared only cases receiving one of α1-blockers, but not those with versus without α1-blockers, and the benefit of tamsulosin or naftopidil over no α1-blocker therapy could not thus be precisely assessed. Moreover, most of our in vitro experiments have shown the significant effects of silodosin at 1-10 µM (that are indeed tolerable doses, as its plasma levels, without acute toxicity in rodents [7,30]), while plasma levels of silodosin in healthy men after receiving pharmacological doses are up to approximately 0.1 µM [16].
In conclusion, in vitro evidence indicates that both urothelial tumorigenesis and tumor growth can be inhibited by silodosin for which AR activation appears to be required, but not by tamsulosin or naftopidil. Available clinical data further support that even pharmacological doses of silodosin may contribute to preventing the development and progression of bladder cancer. These findings imply the potential application of silodosin to bladder cancer treatment as a drug repositioning opportunity and may also offer a preferable treatment with silodosin in men with BPH and concurrent bladder cancer or otherwise in BPH patients with a risk or history of bladder cancer.
Disclosure of conflict of interest
Hiroshi Miyamoto has received research funding from Astellas Scientific and Medical Affairs, Ferring Research Institute, and Bristol-Myers Squibb.
References
- 1.Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68:394–424. doi: 10.3322/caac.21492. [DOI] [PubMed] [Google Scholar]
- 2.Inoue S, Mizushima T, Miyamoto H. Role of the androgen receptor in urothelial cancer. Mol Cell Endocrinol. 2018;465:73–81. doi: 10.1016/j.mce.2017.06.021. [DOI] [PubMed] [Google Scholar]
- 3.Miyamoto H, Yang Z, Chen YT, Ishiguro H, Uemura H, Kubota Y, Nagashima Y, Chang YJ, Hu YC, Tsai MY, Yeh S, Messing EM, Chang C. Promotion of bladder cancer development and progression by androgen receptor signals. J Natl Cancer Inst. 2007;99:558–568. doi: 10.1093/jnci/djk113. [DOI] [PubMed] [Google Scholar]
- 4.Kawahara T, Ide H, Kashiwagi E, El-Shishtawy KA, Li Y, Reis LO, Zheng Y, Miyamoto H. Enzalutamide inhibits androgen receptor-positive bladder cancer cell growth. Urol Oncol. 2016;34:432, e15–23. doi: 10.1016/j.urolonc.2016.05.016. [DOI] [PubMed] [Google Scholar]
- 5.Kawahara T, Inoue S, Kashiwagi E, Chen J, Ide H, Mizushima T, Li Y, Zheng Y, Miyamoto H. Enzalutamide as an androgen receptor inhibitor prevents urothelial tumorigenesis. Am J Cancer Res. 2017;7:2041–2050. [PMC free article] [PubMed] [Google Scholar]
- 6.Kawahara T, Shareef HK, Aljarah AK, Ide H, Li Y, Kashiwagi E, Netto GJ, Zheng Y, Miyamoto H. ELK1 is up-regulated by androgen in bladder cancer cells and promotes tumor progression. Oncotarget. 2015;6:29860–29876. doi: 10.18632/oncotarget.5007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Inoue S, Ide H, Mizushima T, Jiang G, Kawahara T, Miyamoto H. ELK1 promotes urothelial tumorigenesis in the presence of an activated androgen receptor. Am J Cancer Res. 2018;8:2325–2336. [PMC free article] [PubMed] [Google Scholar]
- 8.McVary KT, Roehrborn CG, Avins AL, Barry MJ, Bruskewitz RC, Donnell RF, Foster HE Jr, Gonzalez CM, Kaplan SA, Penson DF, Ulchaker JC, Wei JT. Update on AUA guideline on the management of benign prostatic hyperplasia. J Urol. 2011;185:1793–1803. doi: 10.1016/j.juro.2011.01.074. [DOI] [PubMed] [Google Scholar]
- 9.Kawahara T, Aljarah AK, Shareef HK, Inoue S, Ide H, Patterson JD, Kashiwagi E, Han B, Li Y, Zheng Y, Miyamoto H. Silodosin inhibits prostate cancer cell growth via ELK1 inactivation and enhances the cytotoxic activity of gemcitabine. Prostate. 2016;76:744–756. doi: 10.1002/pros.23164. [DOI] [PubMed] [Google Scholar]
- 10.Kawahara T, Ide H, Kashiwagi E, Patterson JD, Inoue S, Shareef HK, Aljarah AK, Zheng Y, Baras AS, Miyamoto H. Silodosin inhibits the growth of bladder cancer cells and enhances the cytotoxic activity of cisplatin via ELK1 inactivation. Am J Cancer Res. 2015;5:2959–2968. [PMC free article] [PubMed] [Google Scholar]
- 11.Takei R, Ikegami I, Shibata K, Tsujimoto G, Asano T. Naftopidil, a novel alpha1-adrenoceptor antagonist, displays selective inhibition of canine prostatic pressure and high affinity binding to cloned human alpha1-adrenoceptors. Jpn J Pharmacol. 1999;79:447–454. doi: 10.1254/jjp.79.447. [DOI] [PubMed] [Google Scholar]
- 12.Lepor H, Kazzazi A, Diavan B. α-Blockers for benign prostatic hyperplasia: the new era. Curr Opin Urol. 2012;22:7–15. doi: 10.1097/MOU.0b013e32834d9bfd. [DOI] [PubMed] [Google Scholar]
- 13.Izumi K, Zheng Y, Hsu JW, Chang C, Miyamoto H. Androgen receptor signals regulate UDP-glucuronosyltransferases in the urinary bladder: a potential mechanism of androgen-induced bladder carcinogenesis. Mol Carcinogen. 2013;52:94–102. doi: 10.1002/mc.21833. [DOI] [PubMed] [Google Scholar]
- 14.Zheng Y, Izumi K, Yao JL, Miyamoto H. Dihydrotestosterone upregulates the expression of epidermal growth factor receptor and ERBB2 in androgen receptor-positive bladder cancer cells. Endocr-Relat Cancer. 2011;18:451–464. doi: 10.1530/ERC-11-0010. [DOI] [PubMed] [Google Scholar]
- 15.Reznikoff CA, Loretz LJ, Christian BJ, Wu SQ, Meisner LF. Neoplastic transformation of SV40-immortalized human urinary tract epithelial cells by in vitro exposure to 3-methylcholanthrene. Carcinogenesis. 1988;9:1427–1436. doi: 10.1093/carcin/9.8.1427. [DOI] [PubMed] [Google Scholar]
- 16.Zhou Y, Sun PH, Liu YW, Zhao X, Meng L, Cui YM. Safety and pharmacokinetic studies of silodosin, a new α1A-adrenoceptor selective antagonist, in healthy Chinese male subjects. Biol Pharm Bull. 2011;34:1240–1245. doi: 10.1248/bpb.34.1240. [DOI] [PubMed] [Google Scholar]
- 17.Franco-Salinas G, de la Rosette JJ, Michel MC. Pharmacokinetics and pharmacodynamics of tamsulosin in its modified-release and oral controlled absorption system formulations. Clin Pharmacokinet. 2010;49:177–188. doi: 10.2165/11317580-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 18.Nakashima M, Kanamaru M, Uematsu T. Phase I clinical study of naftopidil (KT-611), a new α1-adrenoceptor blocker. Rinsho Iyaku. 1992;8(Suppl 3):11–29. [Google Scholar]
- 19.Pan SL, Guh JH, Huang YW, Chern JW, Chou JY, Teng CM. Identification of apoptotic and antiangiogenic activities of terazosin in human prostate cancer and endothelial cells. J Urol. 2003;169:724–729. doi: 10.1097/01.ju.0000037731.83941.db. [DOI] [PubMed] [Google Scholar]
- 20.Walden PD, Globina Y, Nieder A. Induction of anoikis by doxazosin in prostate cancer cells is associated with activation of caspase-3 and a reduction of focal adhesion kinase. Urol Res. 2004;32:261–265. doi: 10.1007/s00240-003-0365-7. [DOI] [PubMed] [Google Scholar]
- 21.Forbes A, Anoopkumar-Dukie S, Chess-Williams R, McDermott C. Relative cytotoxic potencies and cell death mechanisms of α1-adrenoceptor antagonists in prostate cancer cell lines. Prostate. 2016;76:757–766. doi: 10.1002/pros.23167. [DOI] [PubMed] [Google Scholar]
- 22.Hori Y, Ishii K, Kanda H, Iwamoto Y, Nishikawa K, Soga N, Kise H, Arima K, Sugimura Y. Naftopidil, a selective α1-adrenoceptor antagonist, suppresses human prostate tumor growth by altering interactions between tumor cells and stroma. Cancer Prev Res. 2011;4:87–96. doi: 10.1158/1940-6207.CAPR-10-0189. [DOI] [PubMed] [Google Scholar]
- 23.Yamada D, Nishimatsu H, Kumano S, Hirano Y, Suzuki M, Fujimura T, Fukuhara H, Enomoto Y, Kume H, Homma Y. Reduction of prostate cancer incidence by naftopidil, an α1-adrenoceptor antagonist and transforming growth factor-β signaling inhibitor. Int J Urol. 2013;20:1220–1227. doi: 10.1111/iju.12156. [DOI] [PubMed] [Google Scholar]
- 24.Yasui M, Kawahara T, Takamoto D, Izumi K, Uemura H, Miyamoto H. Distribution of androgen receptor expression in the urinary bladder. Int J Urol. 2019;26:305–306. doi: 10.1111/iju.13841. [DOI] [PubMed] [Google Scholar]
- 25.Miyamoto H, Yao JL, Chaux A, Zheng Y, Hsu I, Izumi K, Chang C, Messing EM, Netto GJ, Yeh S. Expression of androgen and oestrogen receptors and its prognostic significance in urothelial neoplasm of the urinary bladder. BJU Int. 2012;109:1716–1726. doi: 10.1111/j.1464-410X.2011.10706.x. [DOI] [PubMed] [Google Scholar]
- 26.Mizushima T, Jiang G, Kawahara T, Li P, Han B, Inoue S, Ide H, Kato I, Jalalizadeh M, Miyagi E, Fukuda M, Reis LO, Miyamoto H. Androgen receptor signaling reduces the efficacy of bacillus Calmette-Guérin therapy for bladder cancer via modulating Rab27b-induced exocytosis. Mol Cancer Ther. 2020;19:1930–1942. doi: 10.1158/1535-7163.MCT-20-0050. [DOI] [PubMed] [Google Scholar]
- 27.Shiota M, Takeuchi A, Yokomizo A, Kashiwagi E, Tatsugami K, Kuroiwa K, Naito S. Androgen receptor signaling regulates cell growth and vulnerability to doxorubicin in bladder cancer. J Urol. 2012;188:276–286. doi: 10.1016/j.juro.2012.02.2554. [DOI] [PubMed] [Google Scholar]
- 28.Kashiwagi E, Ide H, Inoue S, Kawahara T, Zheng Y, Reis LO, Baras AS, Miyamoto H. Androgen receptor activity modulates responses to cisplatin treatment in bladder cancer. Oncotarget. 2016;7:49169–46179. doi: 10.18632/oncotarget.9994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ide H, Inoue S, Mizushima T, Jiang G, Chuang KH, Oya M, Miyamoto H. Androgen receptor signaling reduces radiosensitivity in bladder cancer. Mol Cancer Ther. 2018;17:1566–1574. doi: 10.1158/1535-7163.MCT-17-1061. [DOI] [PubMed] [Google Scholar]
- 30. U.S. Food and Drug Administration via https://www.accessdata.fda.gov/drugsatfda_docs/nda/2008/022206s000_MedR_P1.pdf. [DOI] [PubMed]