Abstract
There is a critical need for development of improved methods capable of accurately predicting the RAS (KRAS and NRAS) and BRAF gene mutation status in patients with advanced colorectal cancer (CRC). The purpose of this study was to investigate whether radiomics and/or semantic features could improve the detection accuracy of RAS/BRAF gene mutation status in patients with colorectal liver metastasis (CRLM). In this retrospective study, 159 patients who had been diagnosed with CRLM in two hospitals were enrolled. All patients received lung and abdominal contrast-enhanced CT (CECT) scans prior to radiation therapy and chemotherapy. Semantic features were independently assessed by two radiologists. Radiomics features were extracted from the portal venous phase (PVP) of the CT scan for each patient. Seven machine learning algorithms were used to establish three scores based on the semantic, radiomics and the combination of both features. Two semantic and 851 radiomics features were used to predict the mutation status of RAS and BRAF using an artificial neural network method (ANN). This approach performed best out of the seven tested algorithms. We constructed three scores which were based on radiomics, semantic features and the combined scores. The combined score could distinguish between wild-type and mutant patients with an AUC of 0.95 in the primary cohort and 0.79 in the validation cohort. This study proved that the application of radiomics together with semantic features can improve non-invasive assessment of the gene mutation status of RAS (KRAS and NRAS) and BRAF in CRLM.
Keywords: RAS, BRAF, colorectal cancer, radiomics, artificial neural network
Introduction
Colorectal cancer (CRC) is the third most common and deadly tumor in males and females worldwide [1]. According to the World Health Organization (WHO), 1.8 million new cases of CRC were diagnosed worldwide in 2018, with almost 861,000 deaths. In recent years, death rates from CRC have progressively declined in many countries due to improved early detection and more effective primary and adjuvant therapies [2,3]. However, the overall survival (OS) in patients with advanced CRC remains poor. Liver metastasis is the main cause of death in patients with CRC [4]. 15%-25% of patients are commonly diagnosed with synchronous liver metastases at the time of primary diagnosis [5].
Several studies have reported that patient outcomes can be improved through precise identification and stratification of patients harboring specific mutant genes [6-8]. Therefore, genomic characterization to optimize patient selection is of great research and clinical interest. With improvements in precision medicine, RAS and BRAF gene mutation status should be used to inform decision making in the clinic [9-11]. Gain-of-function mutations of RAS and BRAF genes resulting in continuous activation of the RAS-mitogen-activated protein kinase (MAPK) pathway have been characterized in most cases of CRC. KRAS mutations represent an early event in CRC tumorigenesis and occur in 35-45% of CRC cases. BRAF mutations, prevalently c.1799T > A (V600E) mutation have been found in 4.7% to 8.7% of CRC [12]. For surgical treatment of these patients, it has been suggested that a resection margin of at least 10 mm should be obtained [13,14]. However, there is no clear recommendation for the most optimal resection margin for CRLM patients harboring RAS and/or BRAF mutation as patients with both mutations have a higher risk of positive surgical margins [15]. Therefore, the selection of resection margin should be different for CRLM patients harboring RAS and/or BRAF mutation [16,17]. In addition, RAS and BRAF mutations are associated with shorter disease-free survival (DFS) and OS [18-20]. These data suggest that pre-treatment CT scans could potentially be used to predict the RAS and BRAF mutation status in patients with CRLM and to further guide treatments with surgery and/or chemotherapy.
The detection of driver gene mutation status requires the analysis of specimens obtained from surgery or biopsy, which are invasive and expensive procedures. Since Lambin and his colleagues, first proposed the concept of Radiomics in 2012, this high-throughput, non-invasive strategy has been shown to provide addition information that can benefit clinical decision making in multi-centric settings and many different cancers [21-32]. Radiomics holds great promises in representing the underlying differences in the molecular phenotype of tumors [33]. Most previous radiomics studies have focused on KRAS mutation status in the primary lesions of CRC [34,35]. The segmentation of primary lesions is greatly affected by bowel movement. Little is known regarding the radiomics features of metastatic liver lesions and their relationship to the genomic characteristics of RAS and BRAF.
In this study, we aimed to characterize the radiomics and semantic features in a multi-institutional cohort of patients with RAS- (KRAS and NRAS) and BRAF-mutated CRLM prior to any treatment. This study proved that the application of radiomics features together with semantic features, using an ANN method, can improve the non-invasive assessment of the mutation of RAS and BRAF in CRLM.
Materials and methods
Patient
The entire cohort was acquired from the January 2014 to October 2019 records of the institutional picture archiving and communication system (PACS, Philips), which was used to identify patients who had confirmed CRLM histologically. A total of 159 patients was confirmed to the criteria. The following are the inclusion criteria for this study: (1) the patient is older than 18 years; (2) the patient was confirmed as colorectal adenocarcinoma with hepatic metastasis by histopathological examination; (3) tissue samples of all primary tumors for the mutation analysis were acquired either through biopsy or surgical resection; (4) RAS and BRAF gene status were obtained by sequencing or hybridization methods; (5) abdominal contrast-enhanced CT (CECT) images before chemical or radical therapy were available; (6) the slice thickness of CT ≤ 2 mm (to avoid data inconsistency); (7) the intervals between lung and abdominal CT examination and histopathological diagnosis were less than 31 days (range 4-30 days). The following are the exclusion criteria for this study: (1) patients with more than one primary tumor location; (2) poor quality of CT images due to patients’ breathing or movement artifacts; (3) patients with too blurred edges to delineate. Baseline demographic and clinical characteristics were collected from the PACS medical records. Clinical-pathological factors included age, sex, RAS (KRAS and NRAS) and BRAF status. RAS and BRAF genes are both in the RAS-RAF-MAPK pathway and previous studies had classified patients with any mutant RAS or BRAF genes into one category [36,37]. Based on the status of RAS and BRAF genes, the patients were classified into two groups: the mutant group and the wild group. Patients with any mutant RAS or BRAF were classified into the mutant group (N = 93), others were classified into the wild group (N = 66).
This retrospective study was performed in accordance with the principles of the Helsinki Declaration and was approved by the Ethics Committee of the First Hospital of China Medical University. All of the written informed consent was obtained from all enrolled patients in this study.
CT protocol
The contrast administration of abdomen CT scans are patient specific and based on clinical guidelines [38]. All CT examinations performing with 64-row spiral CT scanners were acquired according to standardized scanning protocols [39]. CT manufacturers included Elscint, GE, Phillips, Siemens, and Toshiba. A 1.2-1.5 mL/kg body weight bolus of iohexol was injected intravenously at a flow rate of 2.5 mL/s, followed by a 20-30 mL saline flush. Patients were imaged in the supine position at full inspiration [40,41]. Portal venous phase (PVP) was obtained 60-70 s after intravenous injection of contrast [42,43]. The scanning parameters were as follows: tube voltage was 120 kVp (range 100-140 kVp), slice thickness was 2 mm, matrix was 512 × 512, tube current was 333 mA (range 100-752 mA), exposure time 751 ms (range 500-1782 ms), a standard reconstruction algorithm.
All the steps are compliant to Image Biomarker Standardisation Initiative (IBSI) standard [44-47]. All CT images are stored in the DICOM format. Visual inspection of each images was conducted in advance to sending these images for radiomics analysis in order to ensure that collected images are qualified for being used for analysis [48].
Segmentation
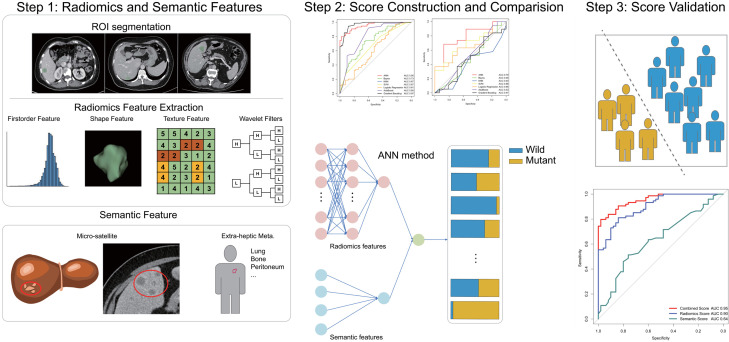
Radiomics extracts high-throughput quantitative imaging features to perform subsequent data analysis related to target clinical outcome. The workflow of a typical radiomics process consists of four steps: tumor segmentation, feature extraction, score construction and score evaluation.
Regions of interest (ROIs) in the portal venous phase (PVP) CT images were segmented with a three-dimensional semi-automatic segmentation method by two radiologists. Radiomics features were extracted from within the defined ROI to quantify tumor intensity, shape and texture. Two semantic features (“micro-satellite” and the presence of metastatic lesions other than the liver and regional lymph nodes) were assessed by two radiologists. We constructed the semantic, radiomics and combined scores using seven machine learning algorithms. Three scores using ANN method showed the best predictive performance based on semantic, radiomics and combined features. The performances of the three constructed scores were assessed by the area under a receiver operating characteristic (ROC) curve followed by decision curve analysis.
PVP CT images were anonymized for all individual and institutional data and were labelled with a random number. Three-dimensional semi-automatic segmentation was performed by 2 radiologists with work experience of 9 years, using the open-source 3D-Slicer software (www.slicer.org). The metastatic liver lesions with the largest cross-sectional area and clear boundary were selected. First, the chosen liver lesions were contoured using soft tissue windows (window width: 350 HU, window level: 40 HU) using a semi-automatic fast-marching segmentation algorithm on all PVP images slice-by-slice for each patient, slightly along the visible borders of the lesion to include the entire lesion’s volume approximated [49]. Then the radiologists manually modified slice-by-slice again to erase adjacent normal tissue or surrounding bile ducts. Thirdly, the final “ball” segmentation resulted as regions of interest (ROIs) were inspected by a senior radiologist with 17 years of work experience. CT images in the DICOM format were imported into the 3D-Slicer software, subsequently the ROIs were exported into NRRD and MRML format for storage and further analysis [50].
Radiomics features preprocessing and selection
Radiomics features of the ROIs were extracted using Pyradiomics package in 3D-Slicer software [51]. The extracted radiomics features could be divided into 3 kinds: first-order features, shape-based features and textural features. First-order features describe the distribution of voxel intensities within the ROIs. Shape-based features captured the direct-viewing characteristics of ROIs as two- and three-dimensional size and shape. These features are independent from the gray level intensity distribution in the ROIs. Textural features were extracted based on five textural matrixes: (1) Gray Level Co-occurrence Matrix (GLCM), (2) Gray Level Size Zone Matrix (GLSZM), (3) Gray Level Run Length Matrix (GLRLM), (4) Neighbouring Gray Tone Difference Matrix (NGTDM) and (5) Gray Level Dependence Matrix (GLDM). Besides, we used a wavelet filter onto the original image in order to suppress noise and to extract detailed, high-dimensional radiomics feature [52].
Features with an exact same value in all patients, which were not discriminative, were excluded [53]. In order to verify the robustness and stability of the radiomics features, the intraclass correlation coefficient (ICC) and concordance correlation coefficient (CCC) for every extracted radiomics features in 30 randomly selected ROIs were also calculated. To calculate ICC, 2 radiologists with work experience of 9 years performed the ROIs segmentation. Both of them knew about the diagnosis of CRLM but were blinded to the clinical or pathological information. To calculate CCC, one radiologist repeated the ROIs segmentation and radiomics features extraction twice in a two-week period. Features with an ICC or CCC lower than 0.75 were excluded for subsequent analysis.
Semantic features
Two representative semantic features were also included in this study. Most of the semantic features that have been reported in previous radomics-related studies on digestive tract cancers are the tumor size, whether the edges are clear, and whether there is a specific metastasis [54,55]. First, the tumor size has been included in the radiomics features that we have analyzed. Secondly, previous literature suggested that lesions with too blurred edges would affect the accuracy of segmentation [49,56]. Therefore, based on the exclusion criteria in the study, we excluded patients with too blurred edges to delineate to maximize the accuracy of the segmentation. Thirdly, several studies have documented that the treatment options and prognosis are different among liver metastases and other metastases in colorectal cancer [57-61]. Therefore, we summarized the first semantic feature as the presence of metastatic lesions other than the liver and regional lymph nodes.
Besides, the relationship between RAS/BRAF mutation and positive resection margins has been widely investigated [15,62]. Therefore, we summarized the second semantic feature, “micro-satellite” phenomenon, referred that a single large lesion is surrounded by multiple small lesions. Two radiologists with over 5 years of working experience independently reviewed all pre-treatment CT images and assessed the presence or absence of two semantic features.
Score construction and evaluation
Three scores were constructed to predict gene mutation status: a semantic score, a radiomics score and a combined score. The combined score integrated both of the radiomics and semantic features. In the training cohort, seven machine learning methods were used to construct three scores predicting the gene mutation status, including Artificial Neural Network (ANN), Gaussian Naive Bayes (GNB), K-Nearest Neighbors (KNN), Support Vector Machine (SVM), Logistic Regression, AdaBoost, Gradient Boost Classifier. The prediction performance of the three scores afforded by several methods was illustrated analyzing the area under the receiver operating characteristic (ROC) curve (AUC) in primary and validation datasets using “pROC” package in R. Delong validation was used to compare AUCs in different scores and determine whether they differed significantly. Besides, accuracy, sensitivity and specificity were calculated among different algorithms in primary and validation datasets. Decision curve analysis was performed to illustrate decision benefit using “Decision Curve” package in R. Among these seven algorithms, the algorithm with the best prediction performance was selected.
Subgroup analysis
The subgroup analysis was conducted to assess whether the score had a better predictive performance among a particular type of patient [63]. The evaluation of the algorithm with the best prediction performance in several subgroups was conducted. Considering that RAS and BRAF mutations are not identical, we performed a subgroup analysis of patients with different mutant statuses in RAS and BRAF genes.
Analysis and comparison between different institutions
Since the entire cohort was acquired in multiple institutions, there were some differences which might directly affect the analysis of radiomics features between the two institutions. Therefore, we compared the prediction performance among patients from the two institutions to evaluate the generality of the three scores.
Statistical analysis
We used Pytorch (version 3.6.10) and R (version 3.5.3) for all statistical analysis. The Fisher’s exact test was used to determine whether the clinical-pathological and semantic variables differed significantly between the primary and validation sets. Two-sided p values < 0.05 were considered statistically significant. Source code of statistical analysis can be accessed at: https://github.com/WissingChen/DeepRadiomics.
Results
Patient characteristics
One hundred and forty-eight patients (92 men and 56 women, median age of 60 years, age ranging between 35-79 years) from the First Hospital of China Medical University and 11 patients (5 men and 6 women, median age of 56 years, age ranging between 42-75 years) from the First Affiliated Hospital of Jinzhou Medical University were recruited to the study. Figure 1 shows the patient recruitment process in this study. Of the 159 total patients, 124 patients were randomly allocated to the primary cohort, and 35 patients allocated to the validation cohort. The characteristics of the primary and validation cohorts are summarized in Table 1. No significant differences in age, sex, mutation status of KRAS, NRAS and BRAF genes, micro-satellite or extra-hepatic metastasis were detected between the primary and validation cohorts (P = 0.12-0.99). No significant differences between the wild-type and mutant groups were found in the primary and validation datasets. Our study flow diagram is shown in Figure 2.
Figure 1.
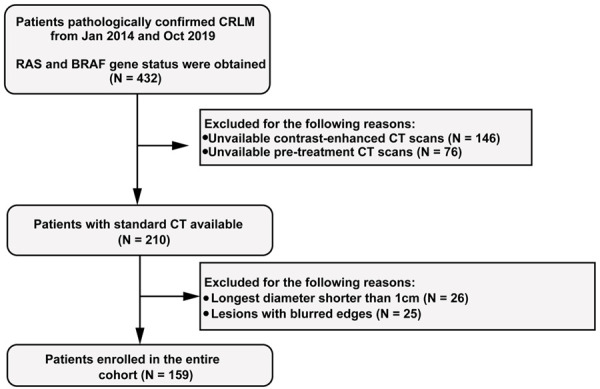
Flow chart of the enrolled patients in the study.
Table 1.
Characteristics of patients in the primary and validation cohorts
| Characteristics | Primary Cohort (N = 124) | Validation Cohort (N = 35) | P Value |
|---|---|---|---|
| Age | > .99 | ||
| ≤ 55 | 45 (36.29%) | 12 (34.29%) | |
| > 55 | 79 (63.71%) | 23 (65.71%) | |
| Sex | 0.85 | ||
| Woman | 49 (39.52%) | 13 (37.14%) | |
| Man | 75 (60.48%) | 22 (62.86%) | |
| KRAS status | > .99 | ||
| Mutant | 64 (51.61%) | 18 (51.43%) | |
| Wild | 60 (48.39%) | 17 (48.57%) | |
| NRAS status | 0.12 | ||
| Mutant | 10 (8.06%) | 0 | |
| Wild | 96 (77.42%) | 30 (85.71%) | |
| NA | 18 (14.52%) | 5 (14.29%) | |
| BRAF status | 0.69 | ||
| Mutant | 7 (5.65%) | 1 (2.86%) | |
| Wild | 110 (88.71%) | 32 (91.43%) | |
| NA | 7 (5.65%) | 2 (5.71%) | |
| Microsatellite | 0.15 | ||
| No | 83 (66.94%) | 28 (80%) | |
| Yes | 41 (33.06%) | 7 (20%) | |
| Extrahepatic Meta. | 0.84 | ||
| No | 82 (66.13%) | 24 (68.57%) | |
| Yes | 42 (33.87%) | 11 (31.43%) | |
| Gene Status | 0.57 | ||
| Mutant | 74 (59.68%) | 19 (54.29%) | |
| Wild | 50 (40.32%) | 16 (45.71%) |
Note: The p values derived from the Fisher’s exact test. “Micro-satellite” refers that a single large lesion is surrounded by multiple small lesions. “Extra-hepatic Meta”. refers to presence of metastatic lesions other than the liver and regional lymph nodes.
Figure 2.
Workflow of the necessary steps in this study. Regions of interest (ROIs) in the portal venous phase (PVP). CT images were segmented with a three-dimensional, semi-automatic segmentation method by two radiologists. Radiomics features were extracted from the defined ROI to quantify tumor intensity, shape and texture. Two semantic features (“micro-satellite” and the presence of metastatic lesions other than the liver and regional lymph nodes) were assessed by two radiologists. We constructed the semantic, radiomics and combined scores using seven machine learning algorithms. Three scores using the artificial neural network (ANN) method showed the best predictive performance based on semantic, radiomics and combined features. The performances of the three constructed scores were evaluated by the area under a receiver operating characteristic (ROC) curves followed by decision curve analysis.
Construction and validation of the three scores
Of the 888 extracted radiomics features, 37 features with ICC and CCC lower than 0.75 were excluded from the subsequent analysis. Eight hundred and fifty-one features were retained for subsequent analysis. These radiomics features were classified into four categories: first-order features (N = 18), textural features (N = 75), shape-based features (N = 14) and wavelet features (N = 744).
Amongst the seven machine learning methods, three scores (semantic, radiomics and combined scores) using the ANN method showed the best predictive performance based on semantic, radiomics and combined features (Figures 3, S1 and Table S1). ANN fixed parameters of batch normalization and dropout layer, to reduce the possibility of overfitting and to guarantee the robustness of the radiomics features.
Figure 3.
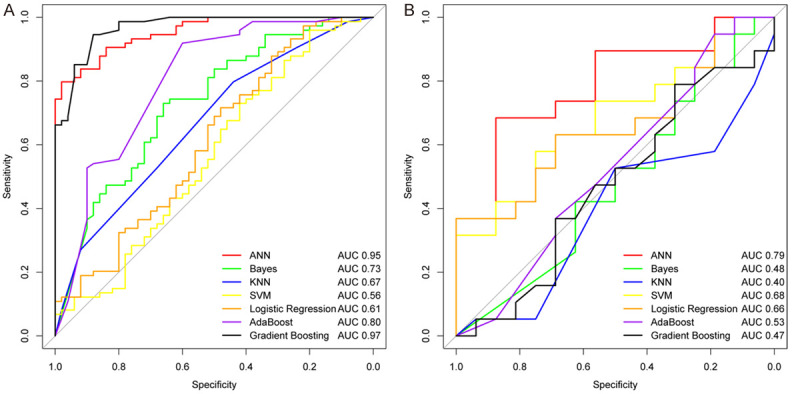
The ROC curves of the combined scores using seven methods in primary (A) and validation cohorts (B).
To evaluate the predictive performance, we applied the three scores based on the ANN algorithm in the primary and validation datasets. The AUC value of the radiomics score reached 0.90 in the training dataset. After combining the radiomics with the effective semantic score, the predictive performance of the combined score was shown to be significantly improved with an AUC of 0.95 in the primary dataset and 0.79 in the validation dataset, showing the best discriminative efficacy (Figure 4A and 4B).
Figure 4.
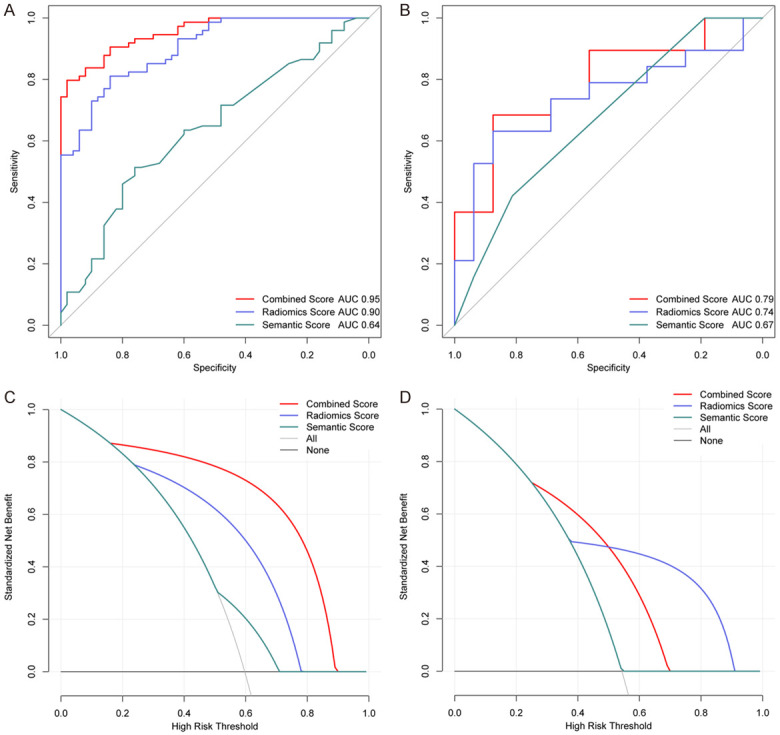
The ROC curves of the semantic, radiomics and combined scores using the ANN method in primary (A) and validation cohorts (B). Decision curve analysis for the three scores in the primary (C) and validation cohorts (D). The y-axis represents the net benefit and the x-axis denotes the threshold probability.
The best-performed combined score and both of the radiomics and semantic scores were compared. The DeLong’s test manifested a significant difference in the primary and validation datasets with p values < 0.05, separately. The accuracies of the combined scores in the primary and validation cohorts were 0.87 and 0.71, respectively. Decision curve analysis indicated that the combined score could improve the benefit compared with the measures that treat all patients and treat no patients (Figure 4C and 4D).
Subgroup analysis
The performance of the combined score in several subgroups was displayed in Table 2. The accuracy of the combined score was relatively high in these subgroups, especially in the patients with mutant status of RAS and BRAF genes.
Table 2.
Performance of the combined score using the ANN method
| Subset | Accuracy (%) | Sensitivity (%) | Specificity (%) | PPV (%) | NPV (%) |
|---|---|---|---|---|---|
| Training set | 87.10 | 89.19 | 84.00 | 89.19 | 84.00 |
| Validation set | 71.43 | 69.57 | 75.00 | 84.21 | 56.25 |
| Micro-satellite | 89.58 | 91.18 | 85.71 | 93.94 | 80.00 |
| No micro-satellite | 81.08 | 80.95 | 81.25 | 85.00 | 76.47 |
| Extra-heptic Meta. | 86.79 | 92.11 | 73.33 | 89.74 | 78.57 |
| No Extra-heptic Meta. | 82.08 | 79.66 | 85.11 | 87.04 | 76.92 |
| RAS Wild-type | 78.57 | 21.05 | 100 | 100 | 77.27 |
| RAS Mutant-type | 87.50 | 87.50 | * | 100 | * |
| BRAF Wild-type | 83.10 | 81.71 | 85.00 | 88.16 | 77.27 |
| BRAF Mutant-type | 100.00 | 100.00 | * | 100 | * |
Note: ANN: artificial neural network; PPV: positive predictive value; NPV: negative predictive value. *According to the combined score, all patients were predicted to be mutant patients.
Analysis and comparison between different institutions
The performances of the combined score constructed using the ANN method were also evaluated amongst patients in two hospitals. The predictive performances of the combined scores, in terms of AUC, accuracy, sensitivity and specificity, were similar in cohorts of patients from both hospitals (Figure S2).
Discussion
Our analysis indicated that the lesions of RAS and BRAF mutant liver metastases exhibit radiomics and semantic features that allow them to be distinguished from wild-type tumors. In this study, we mined and validated a combined score that tracks RAS (KRAS and NRAS) and BRAF mutant phenotypes in CRLM from CT image data.
The steps used in this study attempt to strictly follow the radiomics quality score (RQS) proposed by Lambin and colleagues [64]. We provided well-documented CT protocols, analysis of feature robustness, combination of semantic features, analysis of discrimination and calibration, and a comparison to the current gold standard. The process of semi-automatic segmentation with 3D slicer was proven as a better substitution for manual segmentation during quantification of radiomics features. This method of quantification could reduce uncertainty associated with tumor delineation and allowed more robust quantification of radiomics features with reduced processing time [39,65]. In recent studies, 3D image features have been shown to provide equivalent predictive performance and better quantification of tumor heterogeneity compared to 2D image features [66-68]. Of seven different machine learning methods, we selected a multi-layer perception, which constructed three scores with the best predictive performance. Multi-layer perception is a deep Artificial Neural Network (ANN) with modern techniques such as weight decay regularization, Leaky Rectified Linear Units (ReLU) with batch normalization, dropout layer, Adaptive Moment Estimation (Adam), gradient clipping and learning rate scheduling. In the training cohort, we used a validation mode in PyTorch with L2 regularization to reduce the possibility of overfitting, and to guarantee the robustness of the radiomics features. Due to the fast convergence speed of ANN training and the large number of model parameters, it was potentially easy to overfit the data. The parameters with the best effect of validation set were selected as the saved model during the training process. The proposed combined score incorporating the radiomics and semantic features exhibited favorable discrimination in both the primary and the validation cohorts which strongly supports the high reliability and repeatability of the data in supporting the conclusions from this study.
For patients with liver metastasis which may be resected after chemotherapy, the net benefit of prior chemotherapy versus prior resection remains uncertain, and these patients need to be treated individually. When balancing remaining liver function and R0 resection, it is also unclear whether patients with CRLM harboring RAS and/or BRAF mutations should undergo chemotherapy to minimize the resection field and increase the resection margin whilst ensuring acceptable remaining liver tissue function.
In clinical practice, abdominal CECT is a routine method of evaluation for patients with CRLM. Thus, the combined score can be applied in almost any clinical setting where abdominal CECT is performed. Given these data, we confirmed the potential role of a non-invasive radiomics based method in aiding the subtle genomic evaluation in CRLM, especially for patients unfit for surgery or biopsy. According to National Comprehensive Cancer Network (NCCN) guidelines, the evaluation of RAS and BRAF mutations can be conducted on primary colorectal cancers or metastatic sites [69]. Previous studies have observed a high concordance value (ranging between 96-100%) of RAS and BRAF mutations between primary colorectal tumors and corresponding liver metastases, as well as in multiple liver metastases [70-72]. The concordance of gene status was lower between primary lesion and lung metastases [73]. In the study, we selected a single liver metastasis as the ROI for analysis instead of the entire lesion, the primary CRC lesion or lung metastases. This approach could avoid deviation or poor repeatability caused by bowel movements and ensured the high consistency of gene status.
Despite the advantages of using a validation cohort, our study had some limitations. Firstly, the data in this study were retrospectively collected. Secondly, the ANN algorithm itself limited the interpretability of the results. Prospective investigation using considerably larger datasets and more detailed subgroup analysis is required to further validate the robustness and reproducibility of our conclusions. Despite these limitations, we believe the findings are robust and scalable to larger patient populations.
In conclusion, RAS (KRAS and NRAS) and BRAF mutated tumors show discriminative CT radiomics features that, when combined with semantic features, can aid the prediction of tumors harboring RAS and BRAF mutations to ultimately improve patient stratification and individualized treatments. Furthermore, we aim to successfully translate this understanding into clinical practice by allowing oncologists and surgeons to gain critical information regarding the molecular subtype of CRC, that can be adopted to directly inform better decision making in the clinic. These results have promising implications, radiomics and semantic features will require further validation in larger patient cohorts.
Acknowledgements
This work was supported by grants from the National Science and Technology Major Project of the Ministry of Science and Technology of China (No. 2017ZX09304025); Technological Special Project of Liaoning Province of China (2019020176-JH1/103); Science and Technology Plan Project of Liaoning Province (NO. 2013225585); the General Projects of Liaoning Province Colleges and Universities (LFWK201706). The authors thank Yi Liu, Xiaoyi Hao, Zhicheng Chen for the research guidance. The authors also appreciate the experimental assistance given by Xi Chen, Yujia Song, Fang Wang, Liqing Jiang, Yujing Yang, Shiying Tang, Lingzi He, Hui Qu and Yunhui Wang.
Disclosure of conflict of interest
None.
Abbreviations
- CRLM
colorectal cancer with liver metastasis
- ROI
region of interest
- CRC
colorectal cancer
Supporting Information
References
- 1.Sun W, Ren S, Li R, Zhang Q, Song H. LncRNA, a novel target biomolecule, is involved in the progression of colorectal cancer. Am J Cancer Res. 2019;9:2515–2530. [PMC free article] [PubMed] [Google Scholar]
- 2.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2020. CA Cancer J Clin. 2020;70:7–30. doi: 10.3322/caac.21590. [DOI] [PubMed] [Google Scholar]
- 3.Jemal A, Ward EM, Johnson CJ, Cronin KA, Ma J, Ryerson B, Mariotto A, Lake AJ, Wilson R, Sherman RL, Anderson RN, Henley SJ, Kohler BA, Penberthy L, Feuer EJ, Weir HK. Annual report to the nation on the status of cancer, 1975-2014, featuring survival. J Natl Cancer Inst. 2017;109:djx030. doi: 10.1093/jnci/djx030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chen LY, Zhi Z, Wang L, Zhao YY, Deng M, Liu YH, Qin Y, Tian MM, Liu Y, Shen T, Sun LN, Li JM. NSD2 circular RNA promotes metastasis of colorectal cancer by targeting miR-199b-5p-mediated DDR1 and JAG1 signalling. J Pathol. 2019;248:103–115. doi: 10.1002/path.5238. [DOI] [PubMed] [Google Scholar]
- 5.Joung JG, Oh BY, Hong HK, Al-Khalidi H, Al-Alem F, Lee HO, Bae JS, Kim J, Cha HU, Alotaibi M, Cho YB, Hassanain M, Park WY, Lee WY. Tumor heterogeneity predicts metastatic potential in colorectal cancer. Clin Cancer Res. 2017;23:7209–7216. doi: 10.1158/1078-0432.CCR-17-0306. [DOI] [PubMed] [Google Scholar]
- 6.Camidge DR, Doebele RC, Kerr KM. Comparing and contrasting predictive biomarkers for immunotherapy and targeted therapy of NSCLC. Nat Rev Clin Oncol. 2019;16:341–355. doi: 10.1038/s41571-019-0173-9. [DOI] [PubMed] [Google Scholar]
- 7.Pan L, Cai X. PD-(L)1 inhibitors vs targeted therapy vs their combination as second-line treatment in patients with advanced hepatocellular carcinoma previously treated with sorafenib. Ann Oncol. 2019;30(Suppl 4):iv105. [Google Scholar]
- 8.Li Q, Guan X, Chen S, Yi Z, Lan B, Xing P, Fan Y, Wang J, Luo Y, Yuan P, Cai R, Zhang P, Li Q, Zhong D, Zhang Y, Zou J, Zhu X, Ma F, Xu B. Safety, efficacy, and biomarker analysis of pyrotinib in combination with capecitabine in HER2-positive metastatic breast cancer patients: a phase I clinical trial. Clin Cancer Res. 2019;25:5212–5220. doi: 10.1158/1078-0432.CCR-18-4173. [DOI] [PubMed] [Google Scholar]
- 9.Loupakis F, Cremolini C, Salvatore L, Masi G, Sensi E, Schirripa M, Michelucci A, Pfanner E, Brunetti I, Lupi C, Antoniotti C, Bergamo F, Lonardi S, Zagonel V, Simi P, Fontanini G, Falcone A. FOLFOXIRI plus bevacizumab as first-line treatment in BRAF mutant metastatic colorectal cancer. Eur J Cancer. 2014;50:57–63. doi: 10.1016/j.ejca.2013.08.024. [DOI] [PubMed] [Google Scholar]
- 10.Venook AP, Ou FS, Lenz HJ, Kabbarah O, Qu X, Niedzwiecki D, Zemla T, Goldberg RM, Hochster HS, O’Neil BH, Sanoff HK, Mayer RJ, Bertagnolli MM, Blanke CD, Innocenti F. Primary (1°) tumor location as an independent prognostic marker from molecular features for overall survival (OS) in patients (pts) with metastatic colorectal cancer (mCRC): analysis of CALGB/SWOG 80405 (Alliance) J. Clin. Oncol. 2017;35:3503–3503. [Google Scholar]
- 11.Modest DP, Stintzing S, Weikersthal LFv, Decker T, Kiani A, Vehling-Kaiser U, Al-Batran SE, Heintges T, Kahl C, Seipelt G, Kullmann F, Stauch M, Scheithauer W, Held S, Giessen-Jung C, Jung A, Kirchner T, Heinemann V. Primary tumor location and efficacy of second-line therapy after initial treatment with FOLFIRI in combination with cetuximab or bevacizumab in patients with metastatic colorectal cancer- FIRE-3 (AIOKRK0306) J. Clin. Oncol. 2017;35:3525–3525. [Google Scholar]
- 12.Bruera G, Cannita K, Di Giacomo D, Lamy A, Troncone G, Dal Mas A, Coletti G, Frebourg T, Sabourin JC, Tosi M, Ficorella C, Ricevuto E. Prognostic value of KRAS genotype in metastatic colorectal cancer (MCRC) patients treated with intensive triplet chemotherapy plus bevacizumab (FIr-B/FOx) according to extension of metastatic disease. BMC Med. 2012;10:135. doi: 10.1186/1741-7015-10-135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cady B, Jenkins RL, Steele GD Jr, Lewis WD, Stone MD, McDermott WV, Jessup JM, Bothe A, Lalor P, Lovett EJ, Lavin P, Linehan DC. Surgical margin in hepatic resection for colorectal metastasis: a critical and improvable determinant of outcome. Ann Surg. 1998;227:566–571. doi: 10.1097/00000658-199804000-00019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ekberg H, Tranberg KG, Andersson R, Lundstedt C, Hägerstrand I, Ranstam J, Bengmark S. Determinants of survival in liver resection for colorectal secondaries. Br J Surg. 1986;73:727–731. doi: 10.1002/bjs.1800730917. [DOI] [PubMed] [Google Scholar]
- 15.Brudvik KW, Mise Y, Chung MH, Chun YS, Kopetz SE, Passot G, Conrad C, Maru DM, Aloia TA, Vauthey JN. RAS mutation predicts positive resection margins and narrower resection margins in patients undergoing resection of colorectal liver metastases. Ann Surg Oncol. 2016;23:2635–2643. doi: 10.1245/s10434-016-5187-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Xu D, Wang HW, Yan XL, Li J, Wang K, Xing BC. Sub-millimeter surgical margin is acceptable in patients with good tumor biology after liver resection for colorectal liver metastases. Eur J Surg Oncol. 2019;45:1551–1558. doi: 10.1016/j.ejso.2019.03.010. [DOI] [PubMed] [Google Scholar]
- 17.Margonis GA, Buettner S, Andreatos N, Sasaki K, Ijzermans JNM, van Vugt JLA, Pawlik TM, Choti MA, Cameron JL, He J, Wolfgang CL, Weiss MJ. Anatomical resections improve disease-free survival in patients with KRAS-mutated colorectal liver metastases. Ann Surg. 2017;266:641–649. doi: 10.1097/SLA.0000000000002367. [DOI] [PubMed] [Google Scholar]
- 18.Okuno M, Goumard C, Kopetz S, Vega EA, Joechle K, Mizuno T, Omichi K, Tzeng CD, Chun YS, Vauthey JN, Conrad C. RAS mutation is associated with unsalvageable recurrence following hepatectomy for colorectal cancer liver metastases. Ann Surg Oncol. 2018;25:2457–2466. doi: 10.1245/s10434-018-6517-3. [DOI] [PubMed] [Google Scholar]
- 19.Margonis GA, Buettner S, Andreatos N, Kim Y, Wagner D, Sasaki K, Beer A, Schwarz C, Løes IM, Smolle M, Kamphues C, He J, Pawlik TM, Kaczirek K, Poultsides G, Lønning PE, Cameron JL, Burkhart RA, Gerger A, Aucejo FN, Kreis ME, Wolfgang CL, Weiss MJ. Association of BRAF mutations with survival and recurrence in surgically treated patients with metastatic colorectal liver cancer. JAMA Surg. 2018;153:e180996. doi: 10.1001/jamasurg.2018.0996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Douillard JY, Oliner KS, Siena S, Tabernero J, Burkes R, Barugel M, Humblet Y, Bodoky G, Cunningham D, Jassem J, Rivera F, Kocákova I, Ruff P, Błasińska-Morawiec M, Šmakal M, Canon JL, Rother M, Williams R, Rong A, Wiezorek J, Sidhu R, Patterson SD. Panitumumab-FOLFOX4 treatment and RAS mutations in colorectal cancer. N Engl J Med. 2013;369:1023–1034. doi: 10.1056/NEJMoa1305275. [DOI] [PubMed] [Google Scholar]
- 21.Lambin P, Rios-Velazquez E, Leijenaar R, Carvalho S, van Stiphout RG, Granton P, Zegers CM, Gillies R, Boellard R, Dekker A, Aerts HJ. Radiomics: extracting more information from medical images using advanced feature analysis. Eur J Cancer. 2012;48:441–446. doi: 10.1016/j.ejca.2011.11.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Liu H, Zhang C, Wang L, Luo R, Li J, Zheng H, Yin Q, Zhang Z, Duan S, Li X, Wang D. MRI radiomics analysis for predicting preoperative synchronous distant metastasis in patients with rectal cancer. Eur Radiol. 2019;29:4418–4426. doi: 10.1007/s00330-018-5802-7. [DOI] [PubMed] [Google Scholar]
- 23.Forghani R, Chatterjee A, Reinhold C, Perez-Lara A, Romero-Sanchez G, Ueno Y, Bayat M, Alexander JWM, Kadi L, Chankowsky J, Seuntjens J, Forghani B. Head and neck squamous cell carcinoma: prediction of cervical lymph node metastasis by dual-energy CT texture analysis with machine learning. Eur Radiol. 2019;29:6172–6181. doi: 10.1007/s00330-019-06159-y. [DOI] [PubMed] [Google Scholar]
- 24.Hyun SH, Ahn MS, Koh YW, Lee SJ. A machine-learning approach using PET-based radiomics to predict the histological subtypes of lung cancer. Clin Nucl Med. 2019;44:956–960. doi: 10.1097/RLU.0000000000002810. [DOI] [PubMed] [Google Scholar]
- 25.Zhang W, Fang M, Dong D, Wang X, Ke X, Zhang L, Hu C, Guo L, Guan X, Zhou J, Shan X, Tian J. Development and validation of a CT-based radiomic nomogram for preoperative prediction of early recurrence in advanced gastric cancer. Radiother Oncol. 2019;145:13–20. doi: 10.1016/j.radonc.2019.11.023. [DOI] [PubMed] [Google Scholar]
- 26.Wang XH, Long LH, Cui Y, Jia AY, Zhu XG, Wang HZ, Wang Z, Zhan CM, Wang ZH, Wang WH. MRI-based radiomics model for preoperative prediction of 5-year survival in patients with hepatocellular carcinoma. Br J Cancer. 2020;122:978–985. doi: 10.1038/s41416-019-0706-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Antunovic L, De Sanctis R, Cozzi L, Kirienko M, Sagona A, Torrisi R, Tinterri C, Santoro A, Chiti A, Zelic R, Sollini M. PET/CT radiomics in breast cancer: promising tool for prediction of pathological response to neoadjuvant chemotherapy. Eur J Nucl Med Mol Imaging. 2019;46:1468–1477. doi: 10.1007/s00259-019-04313-8. [DOI] [PubMed] [Google Scholar]
- 28.Bhatia A, Birger M, Veeraraghavan H, Um H, Tixier F, McKenney AS, Cugliari M, Caviasco A, Bialczak A, Malani R, Flynn J, Zhang Z, Yang TJ, Santomasso BD, Shoushtari AN, Young RJ. MRI radiomic features are associated with survival in melanoma brain metastases treated with immune checkpoint inhibitors. Neuro Oncol. 2019;21:1578–1586. doi: 10.1093/neuonc/noz141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Park JE, Kim HS, Park SY, Nam SJ, Chun SM, Jo Y, Kim JH. Prediction of core signaling pathway by using diffusion- and perfusion-based MRI radiomics and next-generation sequencing in isocitrate dehydrogenase wild-type glioblastoma. Radiology. 2020;294:388–397. doi: 10.1148/radiol.2019190913. [DOI] [PubMed] [Google Scholar]
- 30.Grossmann P, Stringfield O, El-Hachem N, Bui MM, Rios Velazquez E, Parmar C, Leijenaar RT, Haibe-Kains B, Lambin P, Gillies RJ, Aerts HJ. Defining the biological basis of radiomic phenotypes in lung cancer. Elife. 2017;6:e23421. doi: 10.7554/eLife.23421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Li Y, Eresen A, Shangguan J, Yang J, Lu Y, Chen D, Wang J, Velichko Y, Yaghmai V, Zhang Z. Establishment of a new non-invasive imaging prediction model for liver metastasis in colon cancer. Am J Cancer Res. 2019;9:2482–2492. [PMC free article] [PubMed] [Google Scholar]
- 32.Li Y, Eresen A, Lu Y, Yang J, Shangguan J, Velichko Y, Yaghmai V, Zhang Z. Radiomics signature for the preoperative assessment of stage in advanced colon cancer. Am J Cancer Res. 2019;9:1429–1438. [PMC free article] [PubMed] [Google Scholar]
- 33.Gillies RJ, Kinahan PE, Hricak H. Radiomics: images are more than pictures, they are data. Radiology. 2016;278:563–577. doi: 10.1148/radiol.2015151169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kim SJ, Pak K, Kim K. Diagnostic performance of F-18 FDG PET/CT for prediction of KRAS mutation in colorectal cancer patients: a systematic review and meta-analysis. Abdom Radiol (NY) 2019;44:1703–1711. doi: 10.1007/s00261-018-01891-3. [DOI] [PubMed] [Google Scholar]
- 35.Lovinfosse P, Koopmansch B, Lambert F, Jodogne S, Kustermans G, Hatt M, Visvikis D, Seidel L, Polus M, Albert A, Delvenne P, Hustinx R. (18)F-FDG PET/CT imaging in rectal cancer: relationship with the RAS mutational status. Br J Radiol. 2016;89:20160212. doi: 10.1259/bjr.20160212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.van Helden EJ, Angus L, Menke-van der Houven van Oordt CW, Heideman DAM, Boon E, van Es SC, Radema SA, van Herpen CML, de Groot DJA, de Vries EGE, Jansen MPHM, Sleijfer S, Verheul HMW. RAS and BRAF mutations in cell-free DNA are predictive for outcome of cetuximab monotherapy in patients with tissue-tested RAS wild-type advanced colorectal cancer. Mol Oncol. 2019;13:2361–2374. doi: 10.1002/1878-0261.12550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Modest DP, Ricard I, Heinemann V, Hegewisch-Becker S, Schmiegel W, Porschen R, Stintzing S, Graeven U, Arnold D, von Weikersthal LF, Giessen-Jung C, Stahler A, Schmoll HJ, Jung A, Kirchner T, Tannapfel A, Reinacher-Schick A. Outcome according to KRAS-, NRAS- and BRAF-mutation as well as KRAS mutation variants: pooled analysis of five randomized trials in metastatic colorectal cancer by the AIO colorectal cancer study group. Ann Oncol. 2016;27:1746–1753. doi: 10.1093/annonc/mdw261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Xu Y, Hosny A, Zeleznik R, Parmar C, Coroller T, Franco I, Mak RH, Aerts H. Deep learning predicts lung cancer treatment response from serial medical imaging. Clin Cancer Res. 2019;25:3266–3275. doi: 10.1158/1078-0432.CCR-18-2495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Rios Velazquez E, Parmar C, Liu Y, Coroller TP, Cruz G, Stringfield O, Ye Z, Makrigiorgos M, Fennessy F, Mak RH, Gillies R, Quackenbush J, Aerts H. Somatic mutations drive distinct imaging phenotypes in lung cancer. Cancer Res. 2017;77:3922–3930. doi: 10.1158/0008-5472.CAN-17-0122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Xu Y, Lu L, E LN, Lian W, Yang H, Schwartz LH, Yang ZH, Zhao B. Application of radiomics in predicting the malignancy of pulmonary nodules in different sizes. AJR Am J Roentgenol. 2019;213:1213–1220. doi: 10.2214/AJR.19.21490. [DOI] [PubMed] [Google Scholar]
- 41.Bashir U, Kawa B, Siddique M, Mak SM, Nair A, McLean E, Bille A, Goh V, Cook G. Non-invasive classification of non-small cell lung cancer: a comparison between random forest models utilising radiomic and semantic features. Br J Radiol. 2019;92:20190159. doi: 10.1259/bjr.20190159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Trebeschi S, Drago SG, Birkbak NJ, Kurilova I, Calin AM, Delli Pizzi A, Lalezari F, Lambregts DMJ, Rohaan MW, Parmar C, Rozeman EA, Hartemink KJ, Swanton C, Haanen JBAG, Blank CU, Smit EF, Beets-Tan RGH, Aerts HJWL. Predicting response to cancer immunotherapy using non-invasive radiomic biomarkers. Ann Oncol. 2019;30:998–1004. doi: 10.1093/annonc/mdz108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sandrasegaran K, Lin Y, Asare-Sawiri M, Taiyini T, Tann M. CT texture analysis of pancreatic cancer. Eur Radiol. 2019;29:1067–1073. doi: 10.1007/s00330-018-5662-1. [DOI] [PubMed] [Google Scholar]
- 44.Kirienko M, Cozzi L, Rossi A, Voulaz E, Antunovic L, Fogliata A, Chiti A, Sollini M. Ability of FDG PET and CT radiomics features to differentiate between primary and metastatic lung lesions. Eur J Nucl Med Mol Imaging. 2018;45:1649–1660. doi: 10.1007/s00259-018-3987-2. [DOI] [PubMed] [Google Scholar]
- 45.Aerts HJ, Velazquez ER, Leijenaar RT, Parmar C, Grossmann P, Carvalho S, Bussink J, Monshouwer R, Haibe-Kains B, Rietveld D, Hoebers F, Rietbergen MM, Leemans CR, Dekker A, Quackenbush J, Gillies RJ, Lambin P. Decoding tumour phenotype by noninvasive imaging using a quantitative radiomics approach. Nat Commun. 2014;5:4006. doi: 10.1038/ncomms5006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Vallieres M, Zwanenburg A, Badic B, Cheze Le Rest C, Visvikis D, Hatt M. Responsible radiomics research for faster clinical translation. J Nucl Med. 2018;59:189–193. doi: 10.2967/jnumed.117.200501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Zwanenburg A, Leger S, Vallières M, Löck S. Image biomarker standardisation initiative. Radiotherapy and Oncology. 2016 [Google Scholar]
- 48.Sasaki T, Kinoshita M, Fujita K, Fukai J, Hayashi N, Uematsu Y, Okita Y, Nonaka M, Moriuchi S, Uda T, Tsuyuguchi N, Arita H, Mori K, Ishibashi K, Takano K, Nishida N, Shofuda T, Yoshioka E, Kanematsu D, Kodama Y, Mano M, Nakao N, Kanemura Y. Radiomics and MGMT promoter methylation for prognostication of newly diagnosed glioblastoma. Sci Rep. 2019;9:14435. doi: 10.1038/s41598-019-50849-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ma X, Wei J, Gu D, Zhu Y, Feng B, Liang M, Wang S, Zhao X, Tian J. Preoperative radiomics nomogram for microvascular invasion prediction in hepatocellular carcinoma using contrast-enhanced CT. Eur Radiol. 2019;29:3595–3605. doi: 10.1007/s00330-018-5985-y. [DOI] [PubMed] [Google Scholar]
- 50.Zhao W, Yang J, Ni B, Bi D, Sun Y, Xu M, Zhu X, Li C, Jin L, Gao P, Wang P, Hua Y, Li M. Toward automatic prediction of EGFR mutation status in pulmonary adenocarcinoma with 3D deep learning. Cancer Med. 2019;8:3532–3543. doi: 10.1002/cam4.2233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.van Griethuysen JJM, Fedorov A, Parmar C, Hosny A, Aucoin N, Narayan V, Beets-Tan RGH, Fillion-Robin JC, Pieper S, Aerts HJWL. Computational radiomics system to decode the radiographic phenotype. Cancer Res. 2017;77:e104–e107. doi: 10.1158/0008-5472.CAN-17-0339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Cselényi Z, Olsson H, Farde L, Gulyás B. Wavelet-aided parametric mapping of cerebral dopamine D2 receptors using the high affinity PET radioligand [11C] FLB 457. Neuroimage. 2002;17:47–60. doi: 10.1006/nimg.2002.1152. [DOI] [PubMed] [Google Scholar]
- 53.de Jong EEC, Sanders KJC, Deist TM, van Elmpt W, Jochems A, van Timmeren JE, Leijenaar RTH, Degens JHRJ, Schols AMWJ, Dingemans AC, Lambin P. Can radiomics help to predict skeletal muscle response to chemotherapy in stage IV non-small cell lung cancer? Eur J Cancer. 2019;120:107–113. doi: 10.1016/j.ejca.2019.07.023. [DOI] [PubMed] [Google Scholar]
- 54.Ji GW, Zhang YD, Zhang H, Zhu FP, Wang K, Xia YX, Zhang YD, Jiang WJ, Li XC, Wang XH. Biliary tract cancer at CT: a radiomics-based model to predict lymph node metastasis and survival outcomes. Radiology. 2019;290:90–98. doi: 10.1148/radiol.2018181408. [DOI] [PubMed] [Google Scholar]
- 55.Jiang Y, Wang H, Wu J, Chen C, Yuan Q, Huang W, Li T, Xi S, Hu Y, Zhou Z, Xu Y, Li G, Li R. Noninvasive imaging evaluation of tumor immune microenvironment to predict outcomes in gastric cancer. Ann Oncol. 2020 doi: 10.1016/j.annonc.2020.03.295. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 56.Ravanelli M, Agazzi GM, Tononcelli E, Roca E, Cabassa P, Baiocchi G, Berruti A, Maroldi R, Farina D. Texture features of colorectal liver metastases on pretreatment contrast-enhanced CT may predict response and prognosis in patients treated with bevacizumab-containing chemotherapy: a pilot study including comparison with standard chemotherapy. Radiol Med. 2019;124:877–886. doi: 10.1007/s11547-019-01046-4. [DOI] [PubMed] [Google Scholar]
- 57.Tang W, Ren L, Liu T, Ye Q, Wei Y, He G, Lin Q, Wang X, Wang M, Liang F, Cui Y, Xu J. Bevacizumab plus mFOLFOX6 versus mFOLFOX6 alone as first-line treatment for RAS mutant unresectable colorectal liver-limited metastases: the BECOME randomized controlled trial. J. Clin. Oncol. 2020;38:3175–3184. doi: 10.1200/JCO.20.00174. [DOI] [PubMed] [Google Scholar]
- 58.Goéré D, Glehen O, Quenet F, Guilloit JM, Bereder JM, Lorimier G, Thibaudeau E, Ghouti L, Pinto A, Tuech JJ, Kianmanesh R, Carretier M, Marchal F, Arvieux C, Brigand C, Meeus P, Rat P, Durand-Fontanier S, Mariani P, Lakkis Z, Loi V, Pirro N, Sabbagh C, Texier M, Elias D. Second-look surgery plus hyperthermic intraperitoneal chemotherapy versus surveillance in patients at high risk of developing colorectal peritoneal metastases (PROPHYLOCHIP-PRODIGE 15): a randomised, phase 3 study. Lancet Oncol. 2020;21:1147–1154. doi: 10.1016/S1470-2045(20)30322-3. [DOI] [PubMed] [Google Scholar]
- 59.Nozue M, Oshiro Y, Kurata M, Seino K, Koike N, Kawamoto T, Taniguchi H, Todoroki T, Fukao K. Treatment and prognosis in colorectal cancer patients with bone metastasis. Oncol Rep. 2002;9:109–112. [PubMed] [Google Scholar]
- 60.Ikoma N, You YN, Bednarski BK, Rodriguez-Bigas MA, Eng C, Das P, Kopetz S, Messick C, Skibber JM, Chang GJ. Impact of recurrence and salvage surgery on survival after multidisciplinary treatment of rectal cancer. J. Clin. Oncol. 2017;35:2631–2638. doi: 10.1200/JCO.2016.72.1464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Margalit O, Shacham-Shmueli E, Lawrence YR, Yang YX, Reiss KA, Golan T, Mamtani R, Halpern N, Aderka D, Giantonio B, Boursi B. Lung metastasis predicts better prognosis in metastatic colorectal cancer with mutated KRAS. Clin Colorectal Cancer. 2019;18:e300–e307. doi: 10.1016/j.clcc.2019.06.001. [DOI] [PubMed] [Google Scholar]
- 62.Gagnière J, Dupré A, Gholami SS, Pezet D, Boerner T, Gönen M, Kingham TP, Allen PJ, Balachandran VP, De Matteo RP, Drebin JA, Yaeger R, Kemeny NE, Jarnagin WR, D’Angelica MI. Is hepatectomy justified for BRAF mutant colorectal liver metastases? A multi-institutional analysis of 1497 patients. Ann Surg. 2020;271:147–154. doi: 10.1097/SLA.0000000000002968. [DOI] [PubMed] [Google Scholar]
- 63.Pocock SJ, Stone GW. The primary outcome is positive - is that good enough? N Engl J Med. 2016;375:971–979. doi: 10.1056/NEJMra1601511. [DOI] [PubMed] [Google Scholar]
- 64.Lambin P, Leijenaar RTH, Deist TM, Peerlings J, de Jong EEC, van Timmeren J, Sanduleanu S, Larue RTHM, Even AJG, Jochems A, van Wijk Y, Woodruff H, van Soest J, Lustberg T, Roelofs E, van Elmpt W, Dekker A, Mottaghy FM, Wildberger JE, Walsh S. Radiomics: the bridge between medical imaging and personalized medicine. Nat Rev Clin Oncol. 2017;14:749–762. doi: 10.1038/nrclinonc.2017.141. [DOI] [PubMed] [Google Scholar]
- 65.Parmar C, Rios Velazquez E, Leijenaar R, Jermoumi M, Carvalho S, Mak RH, Mitra S, Shankar BU, Kikinis R, Haibe-Kains B, Lambin P, Aerts HJ. Robust radiomics feature quantification using semiautomatic volumetric segmentation. PLoS One. 2014;9:e102107. doi: 10.1371/journal.pone.0102107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Fave X, Cook M, Frederick A, Zhang L, Yang J, Fried D, Stingo F, Court L. Preliminary investigation into sources of uncertainty in quantitative imaging features. Comput Med Imaging Graph. 2015;44:54–61. doi: 10.1016/j.compmedimag.2015.04.006. [DOI] [PubMed] [Google Scholar]
- 67.Zhao B, Tan Y, Tsai WY, Qi J, Xie C, Lu L, Schwartz LH. Reproducibility of radiomics for deciphering tumor phenotype with imaging. Sci Rep. 2016;6:23428. doi: 10.1038/srep23428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Ng F, Kozarski R, Ganeshan B, Goh V. Assessment of tumor heterogeneity by CT texture analysis: can the largest cross-sectional area be used as an alternative to whole tumor analysis? Eur J Radiol. 2013;82:342–348. doi: 10.1016/j.ejrad.2012.10.023. [DOI] [PubMed] [Google Scholar]
- 69.Benson AB, Venook AP, Al-Hawary MM, Cederquist L, Chen YJ, Ciombor KK, Cohen S, Cooper HS, Deming D, Engstrom PF, Garrido-Laguna I, Grem JL, Grothey A, Hochster HS, Hoffe S, Hunt S, Kamel A, Kirilcuk N, Krishnamurthi S, Messersmith WA, Meyerhardt J, Miller ED, Mulcahy MF, Murphy JD, Nurkin S, Saltz L, Sharma S, Shibata D, Skibber JM, Sofocleous CT, Stoffel EM, Stotsky-Himelfarb E, Willett CG, Wuthrick E, Gregory KM, Freedman-Cass DA. NCCN guidelines insights: colon cancer, version 2.2018. J Natl Compr Canc Netw. 2018;16:359–369. doi: 10.6004/jnccn.2018.0021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Etienne-Grimaldi MC, Formento JL, Francoual M, François E, Formento P, Renée N, Laurent-Puig P, Chazal M, Benchimol D, Delpero JR, Letoublon C, Pezet D, Seitz JF, Milano G. K-Ras mutations and treatment outcome in colorectal cancer patients receiving exclusive fluoropyrimidine therapy. Clin Cancer Res. 2008;14:4830–4835. doi: 10.1158/1078-0432.CCR-07-4906. [DOI] [PubMed] [Google Scholar]
- 71.Artale S, Sartore-Bianchi A, Veronese SM, Gambi V, Sarnataro CS, Gambacorta M, Lauricella C, Siena S. Mutations of KRAS and BRAF in primary and matched metastatic sites of colorectal cancer. J. Clin. Oncol. 2008;26:4217–4219. doi: 10.1200/JCO.2008.18.7286. [DOI] [PubMed] [Google Scholar]
- 72.Knijn N, Mekenkamp LJ, Klomp M, Vink-Börger ME, Tol J, Teerenstra S, Meijer JW, Tebar M, Riemersma S, van Krieken JH, Punt CJ, Nagtegaal ID. KRAS mutation analysis: a comparison between primary tumours and matched liver metastases in 305 colorectal cancer patients. Br J Cancer. 2011;104:1020–1026. doi: 10.1038/bjc.2011.26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Moorcraft SY, Jones T, Walker BA, Ladas G, Kalaitzaki E, Yuan L, Begum R, Eltahir Z, Wotherspoon A, Montero-Fernandez A, Teixeira Mendes LS, Gonzalez de Castro D, Wilson SH, Proszek P, To YM, Hawkes E, Roy A, Cunningham D, Rao S, Watkins D, Starling N, Bowcock AM, Chau I. Molecular profiling of colorectal pulmonary metastases and primary tumours: implications for targeted treatment. Oncotarget. 2017;8:64999–65008. doi: 10.18632/oncotarget.17048. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.