Abstract
Vasculogenic Mimicry (VM) is the main source of blood supply in the early stage of tumor growth. Carcinoma-associated fibroblasts (CAFs) are one of the most important host cells in the tumor microenvironment. Some studies have found that CAFs can promote tumor angiogenesis, but there are few reports on the relationship between CAFs and VM. Tissue samples were collected from 60 cases of hepatocellular carcinoma (HCC) and 10 persons with normal liver function. The relationship between VM expression and clinicopathologic features was analyzed. Furthermore, the relationship between VM expression and vimentin or α-SMA expression was analyzed. Primary culture of hepatocellular CAFs and the collection of conditioned media were carried out. The effects of hepatocellular CAF conditioned medium on the formation of VM and the levels of VM-related proteins and genes in MHCC-97H cells were studied. The positive rate of VM was 35.0% in HCC tissues. There was no VM expression in normal liver tissues. VM expression was related to tumor diameter, Edmondson grade, clinical stage, and liver cirrhosis. The expression of vimentin and α-SMA in VM-positive patients was higher than in VM-negative patients. Different concentrations of hepatocellular CAF conditioned medium could promote the formation of VM and increase the expression of VM-related genes and proteins (MMP2 and EphA2) in MHCC-97H cells. The results show that there was a significant correlation between VM formation and the expression of vimentin or α-SMA in HCC tissues. The conditioned medium of hepatocellular CAFs may promote VM formation and the expression of VM-related genes and proteins (MMP2 and EphA2) in hepatoma cell line MHCC-97H.
Keywords: Hepatocellular carcinoma, carcinoma-associated fibroblasts, vasculogenic mimicry
Introduction
Hepatocellular carcinoma (HCC) is the second leading cause of death due to malignant tumors worldwide [1], and has become a serious public health problem. Risk factors of HCC include chronic hepatitis B and C virus infection, alcohol consumption, smoking, aflatoxin contamination, obesity, and diabetes [2]. Presently, traditional surgical resection is recognized as the most effective treatment option of HCC. In addition, non-operative treatments such as transcatheter arterial chemoembolization, local radiofrequency ablation, radiotherapy, and emerging molecular-targeted therapy have also achieved certain results [3], but do not significantly improve the survival time of HCC patients. Therefore, exploring the molecular mechanism of invasion and metastasis is of great significance for the molecular diagnosis, prevention, and treatment of HCC.
Vasculogenic Mimicry (VM) is a simulation model of the vascular wall structure in which malignant tumor cells imitate endothelial cells and interact with the extracellular matrix (ECM) through self-deformation, in order to reshape the tumor microcirculation. The microvasculature wall in VM is lined by tumor cells without an endothelial cell barrier. After the tumor cells release proteolytic enzymes to dissolve the ECM, they are directly exposed to the bloodstream, which significantly promotes the metastasis of tumor cells [4]. VM may be the main source of blood supply in the early stage of tumor growth. Therefore, anti-VM therapy has become a focus in liver cancer therapy research. Current studies have shown that EphA2, MMPs, VE-cadherin [5], HIF-1α [6], VEGF [7], and PI3K [8] are all involved in VM formation. In addition, epithelial-mesenchymal transformation (EMT) and tumor stem cells (CSCs) are also related to VM formation. EMT-related nuclear transcription factors can inhibit E-cadherin promoter, reduce its transcriptional activity, and promote VM formation [9,10]. Tumor stem cells enhance the plasticity of tumor cells, reshape ECM to form VM, and connect VM ducts to host blood vessels [11]. So far, the research on VM has mainly focused on the morphological structure of VM, the formation mechanism of VM, and the signal pathways involved. Among the various regulatory mechanisms of VM, a highly specific therapeutic target for anti-VM formation has not been found.
In 1993, Anderson et al. put forward the concept of “tumor microenvironment”, which refers to the internal environment in which tumors occur and develop [12]. The tumor microenvironment consists of tumor cells, a variety of stromal cells, hypoxia, and ECM. Stromal cells mainly include CAFs, vascular-associated cells (such as endothelial cells and pericytes), immune cells (such as tumor-associated macrophages), and inflammatory cells [13]. Tumor-associated macrophages can promote the secretion of VEGF-C by tumor cells and the formation of tumor microlymphatic vessels, and also induce lymph node metastases and tumor invasion [14]. Gassmann et al. found that hepatic sinusoidal endothelial cells express chemokine ligand (CXCL12), tumor cells express chemokine receptor (CXCR4), and CXCL12 binds to CXCR4, which mediates intrahepatic metastasis of tumor cells [15]. In hypoxic conditions, the tumor promotes VM formation by inducing HIF-1α factor to regulate the changes of tumor VM-related genes, such as VEGF, VEGFR2, etc. [16]. It can be seen that the tumor microenvironment is closely related to the occurrence, development, invasion, and metastasis of different tumors. CAFs are one of the most important host cells in the tumor microenvironment [17]. Some studies have found that CAFs can activate signal pathways, promote tumor immunosuppression, promote tumor cells to undergo epithelial-mesenchymal transformation, promote tumor invasion and metastasis, and increase the malignant phenotype of tumor cells through direct cell-cell contact, cytokine secretion, and modification of the extracellular matrix components [18-21]. In addition, there is evidence that CAFs can promote the formation of endothelium-dependent blood vessels in tumors [22,23]. However, at present, there are few reports on the effect of CAFs on the formation of VM in HCC. Some scholars have found that the genome of CAFs is more stable than tumor cells, and CAFs are not easily resistant to drugs [24,25]. If CAFs also have a definite effect on VM formation, then tumor-individualized therapies targeting CAFs will bring new opportunities for the treatment of malignant tumors.
The purpose of this study was to explore the effect of CAFs on the formation of VM in HCC, which could contribute to making CAFs a therapeutic target for liver cancer, and provide new ideas and theoretical bases for the treatment of HCC.
Material and methods
Patients and tissue samples
In this study, we included 60 patients with HCC who underwent surgery in the Affiliated Hospital of the Southwest Medical University from November 2015 to December 2016. All patients were being diagnosed for the first time and did not receive hepatic artery embolization, radiotherapy, or chemotherapy before operation. Ten hepatolithiasis patients who underwent peripheral liver tissue resection during this same period were randomly selected to form the control group. Pathology of the resected samples showed a normal liver structure. Tissue samples in both the case and control group were collected. This study was approved by the Ethics Committee of Affiliated Hospital of Southwest Medical University and all participants provided their informed consent to participate in this study.
Immunohistochemical staining of vimentin, α-SMA, and the results analysis
All the specimens were fixed with 10% neutral formaldehyde solution. The samples were dehydrated and embedded in paraffin, and then cut into 4 μm serial sections. Immunohistochemical staining was performed by EnVision two-step method. The sections were boiled in a pressure cooker with citric acid buffer (pH 6.0) for 5 minutes and incubated with a primary antibody at room temperature for 1 hour. The primary antibody included vimentin and α-SMA. The sections were finally displayed in diaminobenzidine (DAB) chromogenic solution. The semi-quantitative method was used to evaluate vimentin and α-SMA expression. Under high magnification, 10 non-overlapping tumor cell visual fields were randomly selected and evaluated according to the staining intensity and the percentage of positive cells (the number of stained cells/the number of tumor cells). The staining of vimentin and α-SMA was located in the cytoplasm. The staining intensity score was 0 for non-staining, 1 for light brown, 2 for tan, and 3 for yellowish brown. The percentage of positive cells score was 0 for non-staining, 1 for <10%, 2 for 10%-30%, and 3 for >30%. A semi-quantitative evaluation was done according to the product of the above two indexes. A score of 0-2 was marked as low expression, and a score ≥3 was marked as high expression.
CD31/Periodic acid-Schiff (PAS) double staining and VM analysis
The immunohistochemical staining method of CD31 was like that for vimentin and α-SMA. After that, the sections were reduced in 0.5% periodate solution for 10 minutes, washed with water for 2 minutes, and then placed in Schiff solution for 15 minutes in a dark environment. Gastric mucinous adenocarcinoma was used as the positive control for PAS and the mucus was stained red. Phosphate-buffered saline (PBS) solution was used instead of a primary antibody as the negative control. The appearance of brown granules in the cell membrane or cytoplasm was recorded as CD31-positive. The lumen of endothelial blood vessels or hepatic sinusoids was surrounded by CD31-positive cells.
VM analysis: Vascular endothelial cells were absent in the lumen which was surrounded by tumor cells. Red blood cells could be seen occasionally in the lumen. The red blood cells and tumor cells were separated by continuous or discontinuous PAS-positive membranes, and there was no obvious necrosis or inflammatory cell infiltration around them [26]. Those with VM were positive and those without VM were negative.
Primary culture of hepatocellular CAFs and the collection of conditioned medium
The tissues used in the primary culture came from the resected liver cancer specimens which were pathologically diagnosed as poorly differentiated HCC. The cells were extracted using the enzyme digestion method and cultured in DMEM/F12 medium containing 20% fetal bovine serum (FBS), 1% double antibody, and 1% L-glutamine. According to the difference in the growth rate and adhesion ability between fibroblasts and tumor cells, a combination of the differential enzyme digestion method and the repeated adhesion method were used to purify cells. The purified CAFs could be obtained after 3 passages. Cell climbing slices were made to identify CAFs, and the immunohistochemical staining steps of vimentin, α-SMA and the analysis of results were the same as before. When the growth of the purified cells reached 70%, the old medium was gently sucked out with a straw, and the cells were washed 3 times with PBS buffer. Then, a 3 ml serum-free medium was added and sucked out after 24 hours of culture. The supernatant was collected after 2000 rpm centrifugation for 5 minutes and filtered using a 0.22 μm microporous membrane.
Cell lines and coculture system
The human normal hepatocyte cell line L02, human hepatoma cell line SMMC-7721 and MHCC-97H were purchased from Beijing Zhongshan Jinqiao Biotechnology Co., Ltd. L02 cells were cultured in RPMI-1640 medium containing 10% FBS. SMMC-7721 cells and MHCC-97H cells were cultured in DMEM high glucose medium containing 10% FBS at 37°C in humidified air with 5% CO2.
Three-dimensional culture
To each 24-well plate, 0.3 ml matrix collagen solution was added, and then the well plate was put into a 37°C incubator for 30 minutes. L02 cells in the logarithmic phase were digested, and the cell density was adjusted to 2×105/ml. The cells were planted in the 24-well plate. Different volumes of conditioned medium were added, and the final concentration was 25%, 50%, and 75% based on which the low, medium, and high concentration groups were respectively constituted. Each concentration was set with three repeat holes. A blank control group was set. The three-dimensional culture model of SMMC-7721 and MHCC-97H were established using the same method. The arrangement and integrity of tubular structures were observed under an inverted microscope. Under a low magnification microscope, the upper, lower, left, right, and center visual fields were randomly selected to record and count the number of tubular structures. The culture was terminated after 5 days.
Real-time PCR steps
MHCC-97H cells were planted in a non-three-dimensional culture. Total RNA was extracted. The RNA concentration was determined and reverse transcription was performed. A pair of primers was designed according to the target genes MMP2 and EphA2, and β-actin was used as the internal reference gene. The real-time PCR system used was SYBR Premix Ex Taq II (Tli RNaseH Plus) (2×) 10 μl, PCR Forward Primer 0.8 μl, PCR Reverse Primer 0.8 μl, DNA template 2 μl, and sterilized water 6.4 μl.
Western blotting steps
The cells of each group were collected. The total protein was extracted, and the protein concentration was measured using the bicinchoninic acid assay method. The primary antibodies; MMP2, EphA2, and β-actin were added and incubated overnight after electrophoresis, membrane transfer, and sealing. The secondary antibody was added to incubate for 1 hour the next day. After rinsing, the PVDF film was immersed in the photoluminescence solution and photographed using the gel imaging system.
Statistical analyses
SPSS 17.0 software was used for statistical analysis of the data. The X 2 test was used to analyze the relationship between VM expression and clinicopathological features, and between VM expression and the expression of vimentin and α-SMA. The continuous variables were expressed as mean ± standard deviation (x ± s). One-way ANOVA was used to compare the mean among groups. The Student-Newman-Keuls (SNK) method was used for pairwise comparison. Levene’s test was used to test the homogeneity of variance. When P<0.05, the difference was considered statistically significant.
Results
Patient characteristics
A total of 60 patients, 48 males and 12 females were included in the study. The clinicopathological data of the patients were intact. The median age of the patients was 46.5 (26-73) years. The tumor diameter was ≥5 cm in 33 cases and <5 cm in 27 cases. Thirty-two cases were HBsAg-positive. Patients with liver cirrhosis, Edmondson grade I-II, Edmondson grade III-IV, TNM stage I-II, and TNM stage III-IV were 25, 27, 33, 24, and 36, respectively.
VM expression in HCC
Among the 60 HCC cases, 21 cases were VM-positive. VM was a lumen-like structure surrounded by tumor cells. There were no CD31-positive endothelial cells on the wall. A PAS-positive ECM could be seen in the periphery of the lumen, and red blood cells could be seen in the lumen occasionally (Figure 1). Thirty-nine HCC cases were VM-negative. The positive expression rate of VM in the cases was 35.0%. All the control tissues were VM-negative. The difference in VM expression between both groups was statistically significant (X 2=5.862, P=0.021; Table 1).
Figure 1.
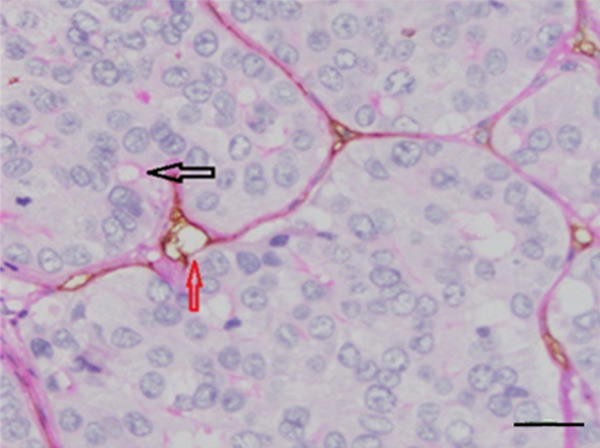
Immunohistochemical double staining of CD31 and PAS in HCC. The black arrow refers to VM. The red arrow refers to the vascular endothelium. Bar=50 μm.
Table 1.
The relationship between VM expression and clinicopathologic features in HCC patients
| Clinicopathological features | N | VM | X 2 | P | ||
|---|---|---|---|---|---|---|
|
| ||||||
| positive | negative | |||||
| Organization category | HCC tissue | 60 | 21 (35.0) | 39 (65.0) | ||
| Control tissue | 10 | 0 (0.0) | 10 (100.0) | 3.472 | 0.021 | |
| Gender | Men | 48 | 16 (33.3) | 32 (66.7) | ||
| Women | 12 | 5 (41.7) | 7 (58.3) | 0.041 | 0.839 | |
| Age (years) | ≥60 | 7 | 1 (14.3) | 6 (85.7) | ||
| <60 | 53 | 20 (37.7) | 33 (62.3) | 0.642 | 0.423 | |
| Tumor size (cm) | ≥5 | 33 | 16 (48.5) | 17 (51.5) | ||
| <5 | 27 | 5 (18.5) | 22 (81.5) | 5.862 | 0.015 | |
| HBsAg (ng/ml) | + | 32 | 12 (37.5) | 20 (62.5) | ||
| - | 28 | 9 (32.1) | 19 (67.9) | 0.188 | 0.664 | |
| Liver cirrhosis | yes | 25 | 13 (52.0) | 12 (48.0) | ||
| no | 35 | 8 (22.9) | 27 (77.1) | 5.444 | 0.020 | |
| Edmondson classification | I+II | 28 | 5 (17.9) | 23 (82.1) | ||
| III+IV | 32 | 16 (50.0) | 16 (50.0) | 6.782 | 0.009 | |
| TNM Staging | I+II | 24 | 4 (16.7) | 20 (83.3) | ||
| III+IV | 36 | 17 (47.2) | 19 (52.8) | 5.910 | 0.015 | |
The relationship between VM expression and clinicopathological features in HCC (Table 1)
There was no significant correlation between VM expression and sex, age, and HBsAg status in HCC (P>0.05). However, VM expression was related to tumor size, Edmondson grade, clinical stage, and liver cirrhosis (X 2=5.862, 6.782, 5.910, 5.444, respectively; P<0.05). The positive rates of VM in samples with tumor diameter ≥5 cm and <5 cm were 48.5% and 18.5%, respectively, and the difference was statistically significant (X 2=5.862, P=0.015). The positive rate of VM in patients with liver cirrhosis was 52.0%, and was significantly higher than in patients without liver cirrhosis (22.9%, X 2=5.444, P=0.020). The positive rate of VM in the Edmondson grade I-II group was 17.9%, and was 50% in the grade III-IV group. There was a significant difference between the two groups (X 2=6.782, P=0.009). The positive rate of VM in the TNM stage I-II group was 16.7%, and was 47.2% in the stage III-IV group. The difference was statistically significant (X 2=5.910, P=0.015).
The relationship between the expression of vimentin, α-SMA, and VM
Among the HCC cases, 39 cases had high vimentin expression and 21 cases had low vimentin expression. The high vimentin expression rate was 65.0%. The high vimentin expression rate in the VM-positive group was 85.7%, and was 53.8% in the VM-negative group. The difference was statistically significant (X 2=6.093, P=0.014; Table 2). Among the HCC patients, 37 cases (61.7%) had high α-SMA expression, and 23 cases had low α-SMA expression. The high α-SMA expression rate in the VM-positive group was 81.0%, and was 51.3% in the VM-negative group. The difference was statistically significant (X 2=5.038, P=0.024; Table 3).
Table 2.
The relationship between vimentin expression and VM (cases, %)
| VM | n | Vimentin | X 2 | P | |
|---|---|---|---|---|---|
|
| |||||
| High expression | low expression | ||||
| Positive | 21 | 18 (85.7) | 3 (14.3) | ||
| negative | 39 | 21 (53.8) | 18 (46.2) | 6.093 | 0.014 |
Table 3.
The relationship between α-SMA expression and VM (cases, %)
| VM | n | α-SMA | X 2 | P | |
|---|---|---|---|---|---|
|
| |||||
| High expression | low expression | ||||
| Positive | 21 | 17 (81.0) | 4 (19.0) | ||
| negative | 39 | 20 (51.3) | 19 (48.7) | 5.038 | 0.024 |
Primary culture and identification of hepatocellular CAFs
After 3 days of culture, a large number of cells adhered to the wall and all kinds of cells were mixed (Figure 2A). The CAFs were fusiform or star-shaped, with multiple protuberances and round or oval nuclei. After purification, there were almost no epithelioid cells in the fourth generation, and the CAFs were arranged in a mat pattern (Figure 2B). The positive expression of vimentin and α-SMA in CAFs resulted in a stained cytoplasm and showed brown granules (Figure 3).
Figure 2.
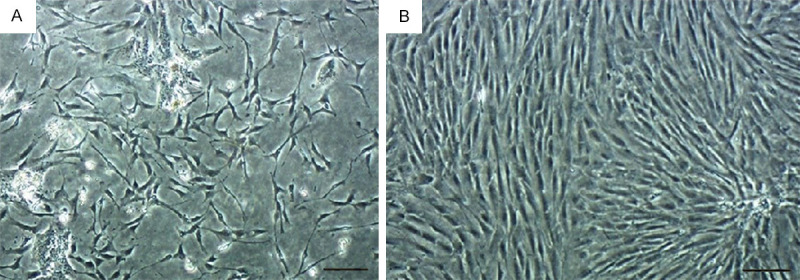
The morphological changes of the primary cell. A. The morphology of CAFs on day 3. B. The highly purified CAFs. Bar=200 μm.
Figure 3.
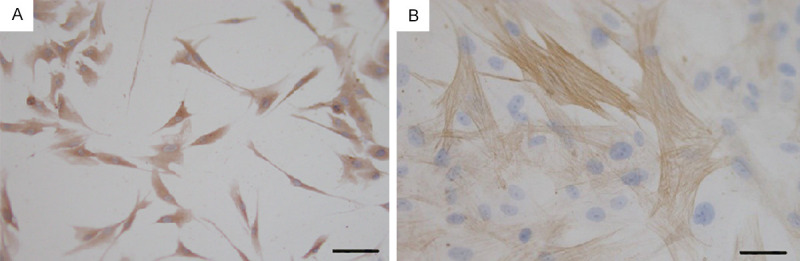
Immunohistochemical staining of CAFs. (A) Positive expression of Vimentin. (B) Positive expression of α-SMA. Bar=50 μm (A), 20 μm (B).
VM formation of three cell lines in three-dimensional culture
For 5 consecutive days, the formation of VM in three kinds of cell lines was observed using an inverted phase contrast microscope. Normal hepatocyte cell line L02 and low metastatic hepatoma cell line SMMC-7721 did not form VM tubular structures, and some cells gathered into cell colonies. Only highly metastatic hepatoma cell line MHCC-97H formed VM tubular structures in the three-dimensional culture (Figure 4). It was also observed that MHCC-97H cells began to protrude slender protuberances between the 6th to 8th hour, and connected to each other into a grid-like or tubular structure. The peak was attained at the 24th hour, and the tubular structures began to loosen after 48 hours.
Figure 4.
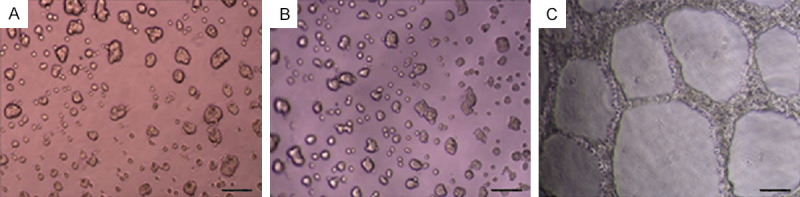
Observing the ability of vasculogenic mimicry in different cell lines in three-dimensional culture. A. L02; B. SMMC-7721; C. MHCC-97H. Bar=100 μm.
The effect of hepatocellular CAF conditioned medium on VM of MHCC-97H cells in three-dimensional culture
After 24 hours of culture, the tubular structures of MHCC-97H cells cultured in different concentrations of CAF conditioned medium were photographed and counted (Table 4). The results showed that the three concentrations of the CAF conditioned medium could promote MHCC-97H cells to form VM, and the tubular structures were smaller than those of the control group. The number of VM formed in each experimental group was significantly different from that in the control group (F=10.889, P<0.01), but there was no significant difference in the three experimental groups (P>0.05, Figures 5, 6).
Table 4.
The effect of different concentrations of the CAF conditioned medium on the formation of VM in MHCC-97H (x̅ ± s)
| Group | Number of VM (24 h) |
|---|---|
| Control | 14.67±1.528 |
| Low concentration | 24.33±2.517 |
| Medium concentration | 25.33±4.041 |
| High concentration | 27.00±3.000 |
| F | 10.889 |
| P | 0.003 |
Figure 5.
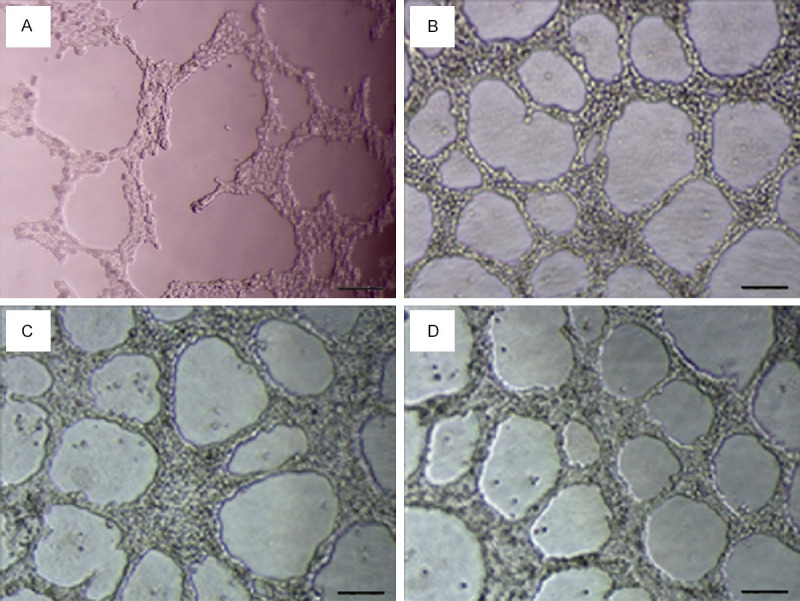
The effect of different concentrations of CAF conditioned medium on the formation of VM in MHCC-97H. A. The control group. B. Low concentration group. C. Medium concentration group. D. High concentration group. Bar=100 μm.
Figure 6.
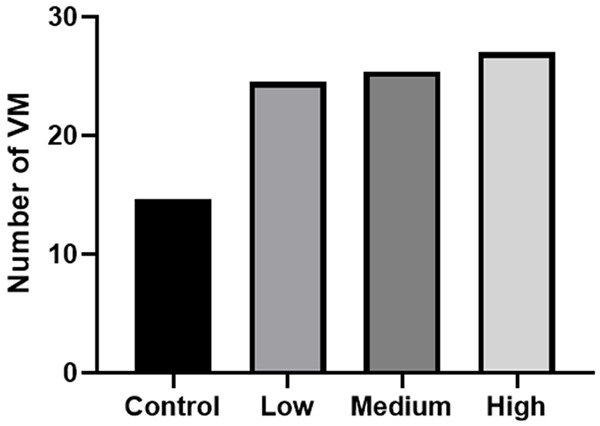
The effect of different concentrations of the CAF conditioned medium on the formation of VM in MHCC-97H.
Detection of the mRNA level expression of VM-related genes MMP2 and EphA2 by real-time PCR
The mRNA levels of VM-related genes, MMP2 and EphA2 in MHCC-97H cells were detected by real-time PCR. The results showed that the mRNA level expression of MMP2 in the three experimental groups of CAF conditioned medium was significantly higher than in the control group (Figure 7A), and there was a statistical significance (F=22.524, P<0.01; Table 5). The mRNA level expression of MMP2 in the medium concentration group was higher than in the high and low concentration groups, but there was no significant difference among the three groups (P>0.05). Compared with the control group, the mRNA level expression of EphA2 in the experimental groups was significantly higher (Figure 7B; Table 5; F=18.784, P<0.01). The expression in the low concentration group was higher than in the medium and high concentration groups, but there was no significant difference among the three groups (P>0.05). The results showed that the CAF conditioned medium could increase the mRNA level expression of MMP2 and EphA2 in MHCC-97H cells, but there was no significant difference in the different concentrations.
Figure 7.
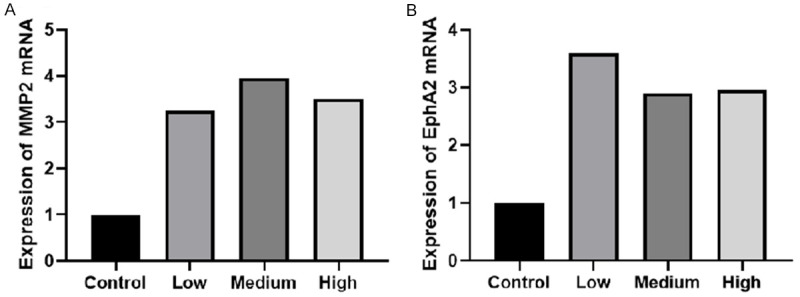
PCR detected the mRNA level expression of MMP2 (A) and EphA2 (B) in MHCC-97H.
Table 5.
PCR detected the mRNA level expression of MMP2 and EphA2 in MHCC-97H (x ± s)
| Group | The mRNA level expression of MMP2 | The mRNA level expression of EphA2 |
|---|---|---|
| Control | 1±0.0000 | 1±0.0000 |
| Low concentration | 3.250±0.672 | 3.619±0.838 |
| Medium concentration | 3.950±0.432 | 2.922±0.281 |
| High concentration | 3.484±0.530 | 2.961±0.188 |
| F | 22.524 | 18.784 |
| P | 0.000 | 0.001 |
Detection of the protein level expression of MMP2 and EphA2 by Western blot
The expression of VM-related proteins MMP2 and EphA2 in MHCC-97H cells were detected by Western Blotting. The results showed that, the expression of MMP2 and EphA2 increased in the experimental groups when compared with the control group (Figure 8). The protein increase of MMP2 in the low concentration group was more obvious than in the medium and high concentration groups. The protein increase of EphA2 in the medium concentration group was more obvious than in the low and high concentration groups. There was no significant difference among the three experimental groups (P>0.05; Figure 9). The results showed that the CAF conditioned medium could increase the protein expression of MMP2 and EphA2 in MHCC-97H cells, but there was no significant difference in different concentrations.
Figure 8.
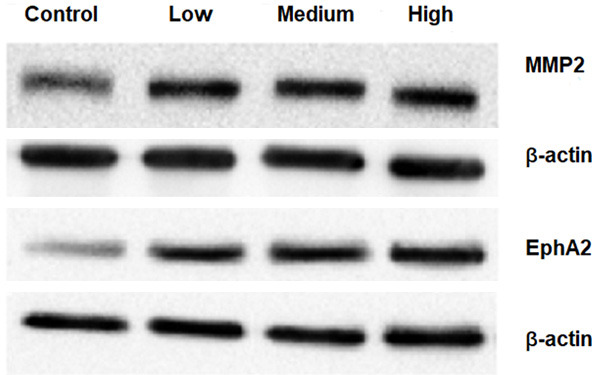
Western Blotting detected the protein level expression of MMP2 and EphA2 in MHCC-97H.
Figure 9.
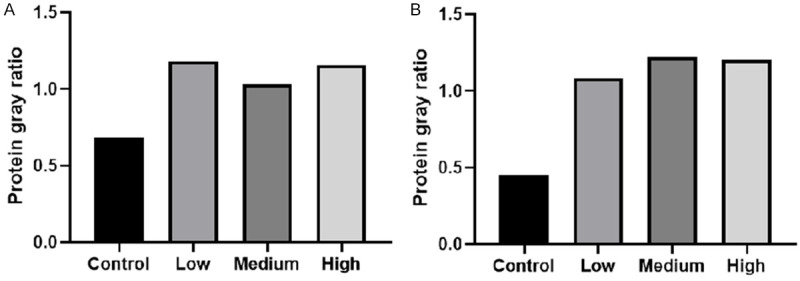
Western Blotting detected the protein level expression of MMP2 (A) and EphA2 (B) in MHCC-97H.
Discussion
Targeted therapy for CAFs can not only inhibit fibrosis, but also inhibit the progression of liver cancer [27]; therefore, CAFs are expected to become a new target for liver cancer. CAFs play an important role in the tumor microenvironment and have become a research hotspot. The premise of research is to obtain purified CAFs. Therefore, the primary culture, purification, and identification of CAFs are necessary. CAFs are activated fibroblasts in the tumor stroma, which have the dual characteristics of smooth muscle cells and fibroblasts in morphology and biological behavior. Vimentin is a marker of mesenchymal cells and mesoderm-derived cells, suggesting that CAFs may be derived from fibroblasts. The CAFs in our study expressed their own specific protein, α-SMA and the expression results were consistent with those reported in previous literature [28,29]. This confirmed that the cells we established were CAFs with high purity. In this study, primary hepatocellular CAFs were successfully cultured and purified using the enzyme digestion method. The experiment was repeatable, and the cells with high vitality and high purity were obtained, which provided a good cell model for the follow-up study.
CAFs are activated fibroblasts which secrete collagen, the main matrix component of many kinds of malignant tumors [30]. More and more literatures have reported that CAFs play a vital role in the development of tumors and may be a potential therapeutic target for tumors. Sukowati et al. [17] co-cultured hepatocellular CAFs and non-tumorous fibroblasts with hepatoma cell lines respectively, and found that CAFs upregulated the gene expression of TGF-β1 and FAP in hepatoma cell lines, while non-tumorous fibroblasts did not induce the expression of the two genes. Oral squamous cell CAFs can promote lymphangiogenesis through the c-Met/PI3K/AKT signaling pathway in vitro [31]. When CAFs were co-cultured with breast cancer cells, CAFs produced higher levels of chemokines such as CCL11 and CXCL14 which promoted the growth, drug resistance, and metastasis of breast cancer cells [32]. Wu et al. [33] also proposed that CAFs can directly provide tumor cells with essential nutrients and energy-rich metabolites, including lactic acid, ketone bodies, fatty acids, glutamine, etc. which can adapt to the metabolic needs of tumor cells in a dynamic way. More importantly, Jine Yang et al. found that co-implantation of CAFs and tumor cells significantly promoted the formation of VM in mouse xenografts. However, when the TGF-β or SDF1 signal is abrogated, the formation of VM promoted by the conditioned medium of cancer-associated fibroblast is attenuated. Recombinant TGF-β1 and SDF1 can induce VM formation. Their findings suggest that the miR-101-TGF-β/SDF1-VE-cadherin/MMP2/LAMC2 network may regulate the formation of VM and is a potential target for tumor therapy [18].
In our study of HCC tissues, we found that the expression of CAF markers, α-SMA and vimentin in the VM-positive group was higher than in the VM-negative group. Through the three-dimensional culture of hepatoma cell lines, we found that only the highly invasive hepatoma cell line MHCC-97H could form VM, while the normal hepatocyte L02 and the low invasive hepatoma cell line SMMC-7721 could not form VM. Therefore, we chose the highly invasive hepatoma cell line MHCC-97H as the main cell line for the follow-up study. The results showed that the hepatocellular CAF conditioned medium could increase the number of VM and up-regulate the mRNA and protein expression of MMP2 and EphA2 in MHCC-97H cells. The effect of the CAF conditioned medium on the formation of VM was not concentration-dependent. MMP2 is an extracellular proteolytic enzyme, which can degrade the tumor-mediated extracellular matrix and is closely related to VM formation and tumor metastasis. A lot of evidence shows that MMP2 activation can promote VM formation [34]. EphA2 is a member of the receptor tyrosine kinase family and is one of the important factors in VM formation. Wang et al. [35] have shown that, VM formation can be prevented by inhibiting the expression of EphA2 in pancreatic cancer. In hepatocellular carcinoma cell lines, Ji-Gang Zhang et al. found that ROCK affects the changes of VM-related factors such as EphA2 and MMP2. RhoC/ROCK2 may have an important effect on VM of hepatocellular carcinoma through ERK/MMPs signal pathway and may become a potential target for liver cancer therapy [36,37]. Research on the molecular mechanism of VM formation is relatively mature, and many researchers have drawn a complete network diagram of the detailed regulation mechanism, though it is not completely unified. Among many signal pathways, most scholars suggest that the key pathway for VM formation may be the VEGF-EphA2-MMPs-VM pathway. The results of this study showed that the transcriptional and protein levels of EphA2 and MMP2 in the experimental groups were higher than in the control group, and the number of VM in the experimental groups was also higher than in the control group.
VM is only a temporary blood supply mode in the process of tumor development, which will be gradually replaced by endothelium-dependent blood vessels. When the tumor is treated by inhibiting the formation of endothelium-dependent blood vessels, the tumor microenvironment will show reactive changes such as aggravation of tumor tissue hypoxia, increase in the expression of angiogenic factors, increase in the coverage of vascular pericytes, promotion of the formation of tumor stem cells, and increase in tumor cell autophagy. In order to get more nutrition to meet the unlimited growth, tumor cells will continue to form VM and increase the ability of tumor cells to invade and metastasize. Therefore, in addition to the traditional anti-angiogenic therapy, the inhibition of VM should be taken into account. The genome of CAFs is relatively stable compared with tumor cells, and CAFs are not easily resistant to drugs. More importantly, it can up-regulate the protein expression of EphA2 and MMP2 in the hepatoma cell line, which is an important target to block the signal pathway in the process of VM formation. However, the current studies tend to focus on the promotion effect of CAFs on endothelium-dependent angiogenesis. There are few studies on the effect of CAFs on VM. This study shows that CAFs can also promote VM formation, which will be a major breakthrough and complement in the mechanism of tumor invasion and metastasis. The mechanism of CAFs promoting VM formation is not clear, and further research is needed.
Recent studies have shown that there are different subsets of CAFs, each of which has its own specific phenotype and function. The emergence of these subsets hinders the application of CAFs in tumor diagnosis and targeted therapy. In various tumors, different CAF subsets secrete different cytokine profiles [38]. Su et al. [39] pointed out that although many literatures have suggested that CAFs play an important role in tumor prognosis, there are two results that have opposite effects on prognosis in different studies. This indicates that different subsets of CAFs may play an opposite role in the occurrence and development of tumors. Therefore, it is necessary to further explore the markers and functions of CAF subsets to accurately target the part of CAF subsets that promote tumor progression.
In this study, only three representative cell lines were selected. In order to obtain more accurate experimental results, we can increase the types of hepatoma cell lines. In addition, we can detect the species and proportion of various substances in the hepatocellular CAF conditioned medium by gene chip technology, and analyze the relationship between these substances and the formation of VM.
Acknowledgements
This work was supported by grants from the Key Project of Sichuan Education Department (13ZA0233), the guiding Scientific Research Plan Project of Luzhou Science and Technology Bureau ([2013] No. 134), and the Joint project of Luzhou City and Southwest Medical University (2015LZCYD-S01 (13/15)).
Disclosure of conflict of interest
None.
References
- 1.Wallace MC, Preen D, Jeffrey GP, Adams LA. The evolving epidemiology of hepatocellular carcinoma: a global perspective. Expert Rev Gastroenterol Hepatol. 2015;9:765–779. doi: 10.1586/17474124.2015.1028363. [DOI] [PubMed] [Google Scholar]
- 2.Baecker A, Liu X, La Vecchia C, Zhang ZF. Worldwide incidence of hepatocellular carcinoma cases attributable to major risk factors. Eur J Cancer Prev. 2018;27:205–212. doi: 10.1097/CEJ.0000000000000428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cabibbo G, Petta S, Maida M, Cammà C. Sorafenib for hepatocellular carcinoma: from randomized controlled trials to clinical practice. Dig Dis. 2015;33:668–674. doi: 10.1159/000438477. [DOI] [PubMed] [Google Scholar]
- 4.El Hallani S, Boisselier B, Peglion F, Rousseau A, Colin C, Idbaih A, Marie Y, Mokhtari K, Thomas JL, Eichmann A, Delattre JY, Maniotis AJ, Sanson M. A new alternative mechanism in glioblastoma vascularization: tubular vasculogenic mimicry. Brain. 2010;133:973–982. doi: 10.1093/brain/awq044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Guo JQ, Zheng QH, Chen H, Chen L, Xu JB, Chen MY, Lu D, Wang ZH, Tong HF, Lin S. Ginsenoside Rg3 inhibition of vasculogenic mimicry in pancreatic cancer through downregulation of VE-cadherin/EphA2/MMP9/MMP2 expression. Int J Oncol. 2014;45:1065–1072. doi: 10.3892/ijo.2014.2500. [DOI] [PubMed] [Google Scholar]
- 6.Tang NN, Zhu H, Zhang HJ, Zhang WF, Jin HL, Wang L, Wang P, He GJ, Hao B, Shi RH. HIF-1α induces VE-cadherin expression and modulates vasculogenic mimicry in esophageal carcinoma cells. World J Gastroenterol. 2014;20:17894–17904. doi: 10.3748/wjg.v20.i47.17894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Liang X, Xu F, Li X, Ma C, Zhang Y, Xu W. VEGF signal system: the application of antiangiogenesis. Curr Med Chem. 2014;21:894–910. doi: 10.2174/09298673113206660264. [DOI] [PubMed] [Google Scholar]
- 8.Delgado-Bellido D, Serrano-Saenz S, Fernández-Cortés M, Oliver FJ. Vasculogenic mimicry signaling revisited: focus on non-vascular VE-cadherin. Mol Cancer. 2017;16:65. doi: 10.1186/s12943-017-0631-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sun D, Sun B, Liu T, Zhao X, Che N, Gu Q, Dong X, Yao Z, Li R, Li J, Chi J, Sun R. Slug promoted vasculogenic mimicry in hepatocellular carcinoma. J Cell Mol Med. 2013;17:1038–1047. doi: 10.1111/jcmm.12087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yang Z, Sun B, Li Y, Zhao X, Zhao X, Gu Q, An J, Dong X, Liu F, Wang Y. ZEB2 promotes vasculogenic mimicry by TGF-β1 induced epithelial-to-mesenchymal transition in hepatocellular carcinoma. Exp Mol Pathol. 2015;98:352–359. doi: 10.1016/j.yexmp.2015.03.030. [DOI] [PubMed] [Google Scholar]
- 11.Liu Z, Sun B, Qi L, Li H, Gao J, Leng X. Zinc finger E-box binding homeobox 1 promotes vasculogenic mimicry in colorectal cancer through induction of epithelial-to-mesenchymal transition. Cancer Sci. 2012;103:813–820. doi: 10.1111/j.1349-7006.2011.02199.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ioannides CG, Whiteside TL. T cell recognition of human tumors: implications for molecular immunotherapy of cancer. Clin Immunol Immunopathol. 1993;66:91–106. doi: 10.1006/clin.1993.1012. [DOI] [PubMed] [Google Scholar]
- 13.Marie-Egyptienne DT, Lohse I, Hill RP. Cancer stem cells, the epithelial to mesenchymal transition (EMT) and radioresistance: potential role of hypoxia. Cancer Lett. 2013;341:63–72. doi: 10.1016/j.canlet.2012.11.019. [DOI] [PubMed] [Google Scholar]
- 14.Kurahara H, Takao S, Maemura K, Mataki Y, Kuwahata T, Maeda K, Sakoda M, Iino S, Ishigami S, Ueno S, Shinchi H, Natsugoe S. M2-polarized tumor-associated macrophage infiltration of regional lymph nodes is associated with nodal lymphangiogenesis and occult nodal involvement in pN0 pancreatic cancer. Pancreas. 2013;42:155–159. doi: 10.1097/MPA.0b013e318254f2d1. [DOI] [PubMed] [Google Scholar]
- 15.Gassmann P, Haier J, Schlüter K, Domikowsky B, Wendel C, Wiesner U, Kubitza R, Engers R, Schneider SW, Homey B, Müller A. CXCR4 regulates the early extravasation of metastatic tumor cells in vivo. Neoplasia. 2009;11:651–661. doi: 10.1593/neo.09272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Li S, Meng W, Guan Z, Guo Y, Han X. The hypoxia-related signaling pathways of vasculogenic mimicry in tumor treatment. Biomed Pharmacother. 2016;80:127–135. doi: 10.1016/j.biopha.2016.03.010. [DOI] [PubMed] [Google Scholar]
- 17.Sukowati CH, Anfuso B, Crocé LS, Tiribelli C. The role of multipotent cancer associated fibroblasts in hepatocarcinogenesis. BMC Cancer. 2015;15:188. doi: 10.1186/s12885-015-1196-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yang J, Lu Y, Lin YY, Zheng ZY, Fang JH, He S, Zhuang SM. Vascular mimicry formation is promoted by paracrine TGF-β and SDF1 of cancer-associated fibroblasts and inhibited by miR-101 in hepatocellular carcinoma. Cancer Lett. 2016;383:18–27. doi: 10.1016/j.canlet.2016.09.012. [DOI] [PubMed] [Google Scholar]
- 19.Deng Y, Cheng J, Fu B, Liu W, Chen G, Zhang Q, Yang Y. Hepatic carcinoma-associated fibroblasts enhance immune suppression by facilitating the generation of myeloid-derived suppressor cells. Oncogene. 2017;36:1090–1101. doi: 10.1038/onc.2016.273. [DOI] [PubMed] [Google Scholar]
- 20.Yamamura Y, Asai N, Enomoto A, Kato T, Mii S, Kondo Y, Ushida K, Niimi K, Tsunoda N, Nagino M, Ichihara S, Furukawa K, Maeda K, Murohara T, Takahashi M. Akt-Girdin signaling in cancer-associated fibroblasts contributes to tumor progression. Cancer Res. 2015;75:813–823. doi: 10.1158/0008-5472.CAN-14-1317. [DOI] [PubMed] [Google Scholar]
- 21.Yu Y, Xiao CH, Tan LD, Wang QS, Li XQ, Feng YM. Cancer-associated fibroblasts induce epithelial-mesenchymal transition of breast cancer cells through paracrine TGF-β signalling. Br J Cancer. 2014;110:724–732. doi: 10.1038/bjc.2013.768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nagasaki T, Hara M, Nakanishi H, Takahashi H, Sato M, Takeyama H. Interleukin-6 released by colon cancer-associated fibroblasts is critical for tumour angiogenesis: anti-interleukin-6 receptor antibody suppressed angiogenesis and inhibited tumour-stroma interaction. Br J Cancer. 2014;110:469–478. doi: 10.1038/bjc.2013.748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Xu LN, Xu BN, Cai J, Yang JB, Lin N. Tumor-associated fibroblast-conditioned medium promotes tumor cell proliferation and angiogenesis. Genet Mol Res. 2013;12:5863–5871. doi: 10.4238/2013.November.22.14. [DOI] [PubMed] [Google Scholar]
- 24.Luo H, Tu G, Liu Z, Liu M. Cancer-associated fibroblasts: a multifaceted driver of breast cancer progression. Cancer Lett. 2015;361:155–163. doi: 10.1016/j.canlet.2015.02.018. [DOI] [PubMed] [Google Scholar]
- 25.Takai K, Le A, Weaver VM, Werb Z. Targeting the cancer-associated fibroblasts as a treatment in triple-negative breast cancer. Oncotarget. 2016;7:82889–82901. doi: 10.18632/oncotarget.12658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Han G, Li Y, Cao Y, Yue Z, Zhang Y, Wang L, Liu J. Overexpression of leptin receptor in human glioblastoma: correlation with vasculogenic mimicry and poor prognosis. Oncotarget. 2017;8:58163–58171. doi: 10.18632/oncotarget.17344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Liu J, Chen S, Wang W, Ning BF, Chen F, Shen W, Ding J, Chen W, Xie WF, Zhang X. Cancer-associated fibroblasts promote hepatocellular carcinoma metastasis through chemokine-activated hedgehog and TGF-β pathways. Cancer Lett. 2016;379:49–59. doi: 10.1016/j.canlet.2016.05.022. [DOI] [PubMed] [Google Scholar]
- 28.Yan Y, Wang R, Guan W, Qiao M, Wang L. Roles of microRNAs in cancer associated fibroblasts of gastric cancer. Pathol Res Pract. 2017;213:730–736. doi: 10.1016/j.prp.2017.02.020. [DOI] [PubMed] [Google Scholar]
- 29.Ji X, Ji J, Shan F, Zhang Y, Chen Y, Lu X. Cancer-associated fibroblasts from NSCLC promote the radioresistance in lung cancer cell lines. Int J Clin Exp Med. 2015;8:7002–7008. [PMC free article] [PubMed] [Google Scholar]
- 30.Kalluri R. The biology and function of fibroblasts in cancer. Nat Rev Cancer. 2016;16:582–598. doi: 10.1038/nrc.2016.73. [DOI] [PubMed] [Google Scholar]
- 31.Gao P, Li C, Chang Z, Wang X, Xuan M. Carcinoma associated fibroblasts derived from oral squamous cell carcinoma promote lymphangiogenesis via c-Met/PI3K/AKT in vitro. Oncol Lett. 2018;15:331–337. doi: 10.3892/ol.2017.7301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Liu Y, Zhang J, Sun X, Su Q, You C. Down-regulation of miR-29b in carcinoma associated fibroblasts promotes cell growth and metastasis of breast cancer. Oncotarget. 2017;8:39559–39570. doi: 10.18632/oncotarget.17136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wu D, Zhuo L, Wang X. Metabolic reprogramming of carcinoma-associated fibroblasts and its impact on metabolic heterogeneity of tumors. Semin Cell Dev Biol. 2017;64:125–131. doi: 10.1016/j.semcdb.2016.11.003. [DOI] [PubMed] [Google Scholar]
- 34.Li Y, Sun B, Zhao X, Wang X, Zhang D, Gu Q, Liu T. MMP-2 and MMP-13 affect vasculogenic mimicry formation in large cell lung cancer. J Cell Mol Med. 2017;21:3741–3751. doi: 10.1111/jcmm.13283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wang H, Lin H, Pan J, Mo C, Zhang F, Huang B, Wang Z, Chen X, Zhuang J, Wang D, Qiu S. Vasculogenic mimicry in prostate cancer: the roles of EphA2 and PI3K. J Cancer. 2016;7:1114–1124. doi: 10.7150/jca.14120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zhang JG, Zhang DD, Liu Y, Hu JN, Zhang X, Li L, Mu W, Zhu GH, Li Q, Liu GL. RhoC/ROCK2 promotes vasculogenic mimicry formation primarily through ERK/MMPs in hepatocellular carcinoma. Biochim Biophys Acta Mol Basis Dis. 2019;1865:1113–1125. doi: 10.1016/j.bbadis.2018.12.007. [DOI] [PubMed] [Google Scholar]
- 37.Zhang JG, Li XY, Wang YZ, Zhang QD, Gu SY, Wu X, Zhu GH, Li Q, Liu GL. ROCK is involved in vasculogenic mimicry formation in hepatocellular carcinoma cell line. PLoS One. 2014;9:e107661. doi: 10.1371/journal.pone.0107661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Öhlund D, Handly-Santana A, Biffi G, Elyada E, Almeida AS, Ponz-Sarvise M, Corbo V, Oni TE, Hearn SA, Lee EJ, Chio II, Hwang CI, Tiriac H, Baker LA, Engle DD, Feig C, Kultti A, Egeblad M, Fearon DT, Crawford JM, Clevers H, Park Y, Tuveson DA. Distinct populations of inflammatory fibroblasts and myofibroblasts in pancreatic cancer. J Exp Med. 2017;214:579–596. doi: 10.1084/jem.20162024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Su S, Chen J, Yao H, Liu J, Yu S, Lao L, Wang M, Luo M, Xing Y, Chen F, Huang D, Zhao J, Yang L, Liao D, Su F, Li M, Liu Q, Song E. CD10(+)GPR77(+) cancer-associated fibroblasts promote cancer formation and chemoresistance by sustaining cancer stemness. Cell. 2018;172:841–856. e816. doi: 10.1016/j.cell.2018.01.009. [DOI] [PubMed] [Google Scholar]


