Abstract
Exosomal PD-L1 (exoPD-L1) is reported to be associated with immunosuppression in various cancers. However, its clinical value in extranodal NK/T cell lymphoma (ENKTL) has not been defined yet. We retrospectively evaluated the prognostic value of pretreatment circulating soluble PD-L1 (sPD-L1) and exosomal PD-L1 (exoPD-L1) in ENKTL patients treated with VIPD-containing chemotherapy. A total of 107 ENKTL patients, including 101 early stage and 6 advanced stage patients were enrolled in our study. ExoPD-L1 and sPD-L1 in the blood were measured by single molecule array (Simoa) and enzyme-linked immunosorbent assay (ELISA), respectively. Compared with the healthy individuals (n=16), the patients with ENKTL (n=107) exhibited significantly elevated exoPD-L1 and sPD-L1 levels in the blood. High pretreatment plasma exoPD-L1 concentration was associated with higher SUVmax level and recurrence rate. Similarly, high sPD-L1 group was also associated with some adverse clinical parameters, including advanced stage, elevated LDH levels, B symptoms, high IPI score and PINK score. The 5-year progression-free survival (PFS) rate and overall survival (OS) rates were 65.2% and 85.7% for the whole cohort, respectively. Patients with a low pretreatment exoPD-L1 level (simoa signal < 1.2) had 5-year OS and PFS rates of 88.1% and 86.1%, respectively, compared with 56.0%. (P=0.012) and 35.7% (P=0.007) in patients with high exoPD-L1 level (simoa signal > 1.2). The 5-year OS and PFS rates for patients with low sPD-L1 group (< 219 pg/mL) was significantly higher than high sPD-L1 group (≥ 219 pg/mL) (OS, 91.3% vs. 55.5%, P < 0.001; PFS, 68.9% vs. 34.6%, P=0.003). However, no correlation was found between circulating exoPD-L1 and sPD-L1 levels. This is the first study to measure plasma exoPD-L1 level on the Quanterix Simoa platform. Our results proved that circulating exoPD-L1 and sPD-L1 levels were significantly elevated in ENKTL and might be potential biomarkers for predicting the survival outcomes of ENKTL patients.
Keywords: NK/T cell lymphoma, exosomal PD-L1, VIPD
Introduction
Extranodal NK/T cell lymphoma (ENKTL) is a distinct type of non-Hodgkin lymphoma with a high degree of malignancy and frequent Epstein-Barr virus (EBV) infection [1]. The 5-year overall survival (OS) rate was lower than 50% in ENKTL patients treated with conventional anthracycline-based chemotherapy [2,3]. In the last decade, the application of L-asparaginase (L-asp) has provided insights into this malignant tumor type and achieved a 5-year OS rate of 64% to 74% in patients with early-stage disease [4-6]. Although the prognosis of ENKTL has improved during the last decade, some patients still experience relapse within 3 years [7,8].
Exosomes are nanovesicles with a diameter of 30-150 nm actively secreted by viable cells. Emerging evidence has demonstrated that lymphoma-derived exosomes play important roles in lymphomagenesis, lymphoma spread and drug resistance [9-11]. The programmed death protein 1 (PD-1)/programmed death ligand 1 (PD-L1) pathway is one of the most critical mechanisms of tumor escape in lymphoma [12]. PD-L1 expressed in tumor cells can inhibit T cell proliferation and induce T cell exhaustion, contributing to immune evasion and cancer development. Recently, a group at the University of Pennsylvania found that PD-L1 present in the exosomal membrane could also mediate immune evasion and was a potential predictor of anti-PD-1 treatment outcomes [13]. Subsequent studies confirmed the role of exosomal PD-L1 (exoPD-L1) in immune escape in various cancers, including head and neck cancer, breast cancer and prostate cancer [14-16]. However, the exact significance and prognostic implications of plasma exoPD-L1 have not been determined in ENKTL.
In this study, we explored the diagnostic and prognostic roles of exoPD-L1 in ENKTL patients. As the predictive value of biological markers can be affected by primary treatment, we conducted this study in patients receiving VIPD-containing chemotherapy.
Materials and methods
Eligibility criteria
This was a retrospective study of newly diagnosed patients with nasal-type NK/T-cell lymphoma between 2010 and 2017 in Fudan university, Shanghai Cancer Center. The inclusion criteria of the current study were as follows: 1) definite diagnosis of ENKTL according to the World Health Organization (WHO) classification of lymphoid neoplasms; 2) the primary tumor site localized in the upper aerodigestive tract; 3) no prior treatment with detailed clinical information and follow-up data available; and 4) an Eastern Cooperative Oncology Group performance status (ECOG PS) score between 0-2. Patients with concomitant malignant tumors or severe organ dysfunction were excluded. This study was performed in accordance with the Declaration of Helsinki and approved by the Ethics Committees of the Fudan University Shanghai Cancer Center, and all the participants provided written informed consent.
Evaluation and treatment
The patients in our cohort were staged based on the Ann Arbor staging system. VIPD (etoposide, ifosfamide, cisplatin and dexamethasone) or L/P-VIPD (L-asparaginase/pegaspargase, etoposide, ifosfamide, cisplatin and dexamethasone) combined with involved-field radiotherapy (IFRT) was delivered as the first-line treatment in early-stage patients. All advanced-stage patients received VIPD chemotherapy. Patient responses were evaluated according to the revised response criteria for malignant lymphoma [17]. All patients in our cohort underwent positron emission tomography-computed tomography (PET/CT) or magnetic resonance imaging (MRI) and/or computed tomography (CT) before treatment, after every two cycles of chemotherapy and after treatment.
Exosome and peripheral blood mononuclear cell (PBMC) isolation from the blood
Plasma samples of 500 μl was thawed on ice and exosomes was then isolated by using exoRNeasy Plasma Midi Kit (Qiagen, #77044). PBMCs were isolated from whole blood with density gradient medium (LymphoprepTM, STEMCELL Technologies, #07851).
Exosome characterization
Exosomes purified from plasma were visualized using transmission electron microscopy (TEM) as described by Thery et al [18]. The size and concentration of exosomes were determined with the Flow NanoAnalyzer (NanoFCM Inc., Xiamen, China) [19]. In addition, the expression of exosome markers such as TSG101 and ALIX was assessed by western blotting. Western blot was performed as described previously [20].
Detection of exoPD-L1 in the plasma based on a single-molecule array (Simoa)
The Simoa HD-1 Analyzer is a digital immunoassay technology used for the detection of various proteins that has significantly higher sensitivity than the ELISA technique [21,22]. CD63 is one of the most important tetraspanins enriched on the exosomal surface and it is considered a specific marker for exosomes [23]. Here, we used two markers, CD63 and PD-L1, to detect PD-L1 expressed on exosomes in the plasma by Simoa.
Preparation of the Simoa Simoa homebrew kits for the detection of PD-L1 positive exosomes were prepared according to the manufacturer’s guidelines. In brief, a capture anti-PD-L1 antibody was diluted to a concentration of 0.2 mg/mL with Bead Conjugation Buffer (Quanterix), and 1-ethyl-3-(3-dimethylaminopropyl) carbodiimide hydrochloride (EDC) (Thermo Fisher Scientific, Waltham, MA, USA) was used to activate paramagnetic carboxylated microparticles (Quanterix). Then, 3 μL of biotin solution (5.2 µg/µl) was added to a detection antibody solution (100 μL, 1.0 mg/mL) to start the biotinylation reaction. The recovered anti-CD63 antibody was adjusted to a concentration of 0.2 mg/mL, and the beads were stored at 4°C. Finally, an assay for detecting PD-L1-positive exosomes via PD-L1-CD63 was developed.
Simoa setup Detection of PD-L1 positive exosomes was performed on a novel automated Simoa HD-1 Analyzer (Quanterix). Microscopic magnetic beads coated with an anti-PD-L1 capture antibody were diluted to 500,000/test, and an anti-CD63 detection antibody was adjusted to a working concentration of 0.3 μg/mL. Streptavidin-β-galactosidase (SBG) was diluted to a working concentration of 150 pM with SBG Diluent (Quanterix). The detection process was a three-step protocol. First, 25 μL of microscopic bead (coated with the anti-PD-L1 capture antibody) solution was incubated in a 100-μL sample for 45 min, followed by three wash steps. Then, 100 μL of anti-CD63 detection antibody was incubated with the microscopic beads for 5 min and 15 s. Finally, 100 μL of SBG was added and incubated for 5 min and 15 s. An RGP substrate solution (20 μL) was mixed with the microscopic beads and loaded into the Simoa disk array. The array was sealed with oil after loading, and the microscopic beads were imaged. Automatic analysis was performed with HD-1 Analyzer software (Quanterix), and the Simoa signal was present in AEB.
Detection of sPD-L1 and exoPD-L1 by ELISA
sPD-L1 in the blood was detected using a commercial ELISA kit (R&D Systems, # DB7H10). Serum samples were available for 96 ENKTL patients and were measured in accordance with the manufacturer’s instructions. For the detection of exoPD-L1, a total of 100 μl of isolated exosomes was added to each well and then tested following the instructions. The minimum detectable level of sPD-L1 was 1.51 pg/ml. Standards and all the samples were tested in duplicate.
Statistical analysis
Overall survival (OS) was defined as the time from the date of diagnosis to the day of last follow-up or death. Progression-free survival (PFS) was defined as the time from the day of diagnosis to the time of first progression, last follow-up or death. Survival analysis was performed using the Kaplan-Meier method, and the log-rank test was used to compare survival curves. Significant factors (P value less than 0.05) in univariate analyses were further examined by multivariate analysis with Cox regression. The prognostic cutoff points of plasma exoPD-L1, sPD-L1 and PET/CT SUVmax were determined by X-tile, and the most discriminant threshold for PFS was selected. The difference of categorical variables between two groups was compared by Pearson Chi-square analysis/Fisher exact test. The significance of group differences was tested using unpaired two-tailed Student’s t test. Spearman correlation analysis was employed to identify correlations between variables. Statistical analysis was performed using GraphPad Prism (version 5, GraphPad Software).
Results
Patient characteristics
Table 1 summarizes patient clinical characteristics. A total of 107 patients with newly diagnosed nasal-type ENKTL including 101 early-stage and 6 advanced-stage patients were enrolled in our study. Most patients in our cohort were middle-aged and young male adults, with a median age of 44 years (range, 17-76 years) and a male to female ratio of approximately 3.7:1. A majority of our patients had normal LDH levels (n=91), low International Prognostic Index (IPI) scores (≤ 1, n=97), low prognostic index of natural killer cell lymphoma (PINK) scores (≤ 1, n=96) and early-stage disease (n=101). B symptoms were present in around half of the patients (n=55) in our cohort. The primary tumor sites included the nasal cavity (n=102) and nasopharynx (n=5). Pretreatment PET/CT was performed in 51 patients, with a median SUVmax value of 9.7 (range 3.2-25.1). Ki-67 was highly expressed in most patients, with a wide range of distribution from 5% to 99% and a median value of 60%. For early stage patients, there is no obvious difference between the baseline clinical characteristics for VIPD group and L/P-VIPD group.
Table 1.
Patient characteristics
| Characteristics | Patient number (n=107) | Early stage nasal type ENKTL (n=101) | ||
|---|---|---|---|---|
|
| ||||
| VIPD Group (n=34) | L/P-VIPD group (n=67) | P value | ||
| Gender | 0.146 | |||
| Male | 84 (78.5) | 29 | 48 | |
| Female | 23 (21.5) | 5 | 19 | |
| Age (years) | 1.000 | |||
| > 60 | 11 (10.3) | 3 | 6 | |
| ≤ 60 | 96 (89.7) | 31 | 61 | |
| Median (years) | 57.0 | 43.5 | 43.0 | |
| ECOG PS | 1.000 | |||
| 0-1 | 106 (99.1) | 34 | 66 | |
| 2-4 | 1 (0.9) | 0 | 1 | |
| B symptoms | 0.404 | |||
| Yes | 55 (51.4) | 15 | 36 | |
| No | 52 (48.6) | 19 | 31 | |
| IPI score | 0.685 | |||
| ≤ 1 | 97 (90.7) | 31 | 63 | |
| > 1 | 10 (9.3) | 3 | 4 | |
| LDH level | 0.066 | |||
| Elevated | 16 (15.0) | 8 | 6 | |
| Normal | 91 (85.0) | 26 | 61 | |
| Primary sites | 0.549 | |||
| Nasal cavity | 102 (95.3) | 34 | 65 | |
| nasopharynx | 5 (4.7) | 0 | 2 | |
| PINK | 0.115 | |||
| 0-1 | 96 (89.7) | 29 | 64 | |
| ≥ 2 | 11 (10.3) | 5 | 3 | |
| Ki-67 ≥ 60% | 56 (52.3) | 14 | 39 | 0.140 |
| EBER + | 99 (92.5) | 31 | 65 | 0.332 |
ECOG PS, Eastern Cooperative Oncology Group performance status; IPI, International Prognostic Index; LDH, lactate dehydrogenase; PINK, Prognostic Index of Natural Killer Lymphoma; EBER, EBV encoded RNA.
Evaluation of efficacy and survival outcomes
Of the 101 patients with early-stage ENKTL, VIPD was used to treat 34 patients, and L/P-VIPD was used to treat 67 patients. All the advanced-stage patients (n=6) received VIPD chemotherapy. The complete response (CR) rate and objective response rate (ORR) for the whole cohort after treatment were 76.2% and 95.2%, respectively, whereas three patients developed progressive disease (PD). Thirty patients (30.8%) relapsed (n=30, 28.0%) or developed PD (n=3, 2.8%) during the follow-up period.
The median follow-up time was 65.0 months (range, 2-119 months) in our study, with 5-year PFS and 5-year OS rates of 65.2% and 85.7%, respectively (Figure 1A, 1B). Compared with VIPD, L/P-VIPD significantly improved the 5-year PFS rate in early-stage patients (73.6% vs. 53.7%, P=0.031). However, no difference in the OS rate was found between the two groups (5-year OS 88.7% vs. 82.5%, P=0.604) (Figure 1C, 1D).
Figure 1.
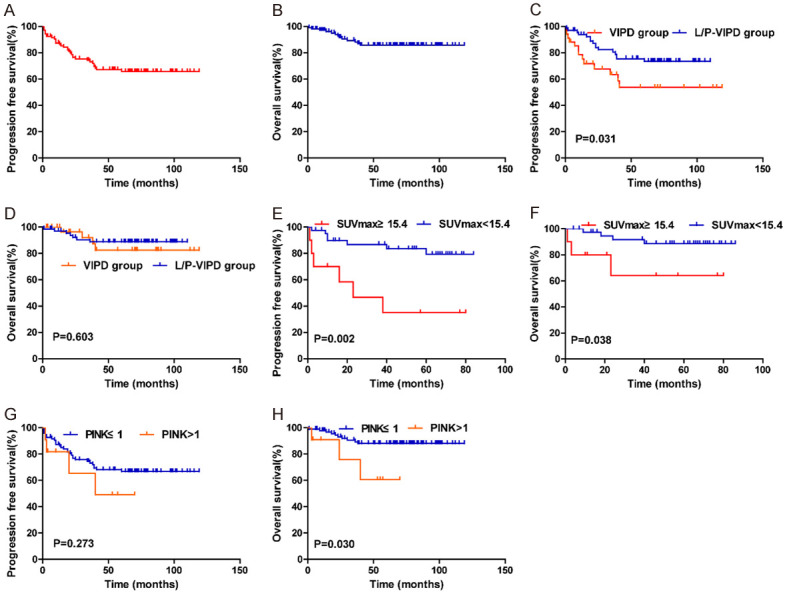
(A, B) Progression-free survival (PFS) and overall survival (OS) for all patients (A, B). (C and D) Comparison of progression-free survival (PFS) (C) and overall survival (OS) (D) between VIPD group and L/P-VIPD group for early stage ENKTL patients. (E and F) Impact of pretreatment SUVmax on the PFS (E) and OS (F) for ENKTL patients. (G and H) Significant impact of PINK on progression-free survival (PFS) (E) and overall survival (OS).
Patients with an SUVmax ≥ 15.4 (n=10) on pretreatment PET/CT had worse 5-year PFS (35.0% vs. 79.3%, P=0.002) and OS (64.0% vs. 88.7%, P=0.038) than those with an SUVmax < 15.4 (n=41) (Figure 1E, 1F). In addition, the 5-year PFS (49.1% vs. 66.7%, P=0.275) and OS rates (60.6% vs. 88.1%, P=0.030) in the high-risk group (PINK ≥ 2) were inferior to those in the low-risk group (PINK ≤ 1) (Figure 1G, 1H).
Detection of PD-L1 on exosomes
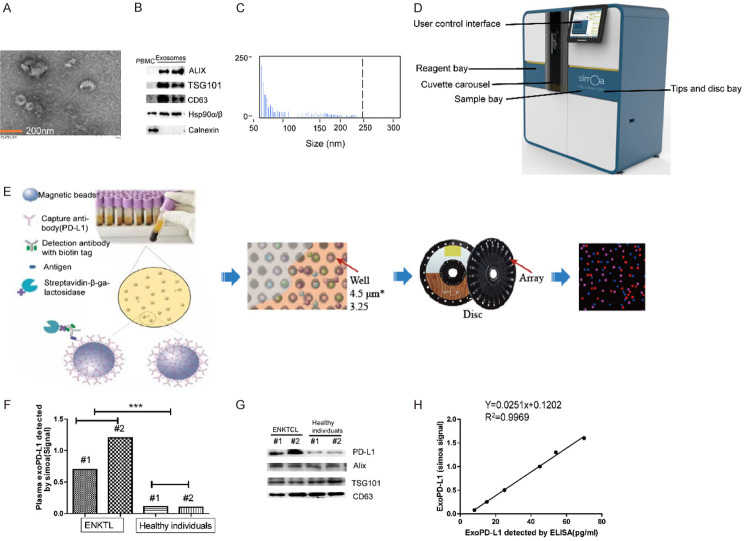
The Simoa HD-1 Analyzer was an ultrasensitive digital technology which could accurately detect exoPD-L1 level with as little as 100 μL plasma. The comparison of this method with exosome kit extraction was performed to further confirm the validity of the PD-L1 level on exosomes. Exosomes from ENKTL patient were isolated by exoRNeasy Plasma Midi Kit. As shown in Figure 2A, the TEM image revealed that exosomes were 30-100nm intact vesicles. Western blotting demonstrated that some specific biomarkers for exosomes, such as TSG101, ALIX, Hsp90α/β and CD63, were enriched in the isolated exosomes (Figure 2B). Flow NanoAnalyzer showed that the isolated exosomes were approximately 50-250 nm in diameter, with a median value of around 70 nm (Figure 2C). Furthermore, western blot analysis established that plasma exoPD-L1 level was significantly higher in ENKTL patients than in healthy subjects, which was in accordance with the signal on the Simoa (Figure 2D-G). A relatively good linear relationship between the simoa signal and the exoPD-L1 concentration quantified by ELISA was obtained (Figure 2H). The above experiments show that plasma exoPD-L1 levels could be reproducibly measured by Simoa.
Figure 2.
(A-C) Characterization of isolated exosomes by transmission electron microscopy (TEM) (A), western blots (B) and size distribution (C). (D, E) Exosomes were captured on microscopic beads coated with anti-PD-L1 mAb and were then stained with labeled biotinylated anti-CD63 mAb, which could bind streptavidin-β-galactosidase (SBG) and catalyze substrate and produce signal after loading on the simoa disc array. (F, G) The plasma exoPD-L1 level of ENKTL patients and healthy individuals analyzed by western blots and simoa. (H) Linear relationship between the simoa signal and the plasma exoPD-L1 concentration detected by ELISA.
Pretreatment exoPD-L1 levels and their correlations with clinical features
A total of 99 ENKTL patients and 16 healthy subjects with available plasma were further tested by simoa. In our study, we found that pretreatment plasma exoPD-L1 levels were significantly higher in our patients than in healthy subjects (P < 0.001) (Figure 3A). Receiver operating characteristic curve (ROC) showed that plasma exoPD-L1 provides an excellent diagnosis accuracy (AUC=0.9968) to differentiate the ENKTL patient (n=99) and healthy control groups (n=16) (Figure 3B). The optimal prognostic cutoff point for the pretreatment plasma exoPD-L1 level measured by simoa was 1.2 (around 43.0 pg/ml) (X-tile, Figure S1) and our cohort was classified into two groups based on this value. To investigate the correlation between plasma exoPD-L1 and prognostic factors, pretreatment exoPD-L1 levels were compared based on the clinical parameters of our cohort (Table 2). Compared with those in the low plasma exoPD-L1 group (simoa signal < 1.2), the patients in the high plasma exoPD-L1 group were more likely to have a higher SUVmax level and a higher recurrence rate (Table 2). However, no correlations were observed between the plasma exoPD-L1 level and age, sex, disease stage, the B symptom status, the LDH level, the IPI score or the PINK score. The pretreatment plasma exoPD-L1 levels of patients with a higher SUVmax were significantly higher than those of patients with a lower SUVmax level (P=0.003) (Figure 3C).
Figure 3.
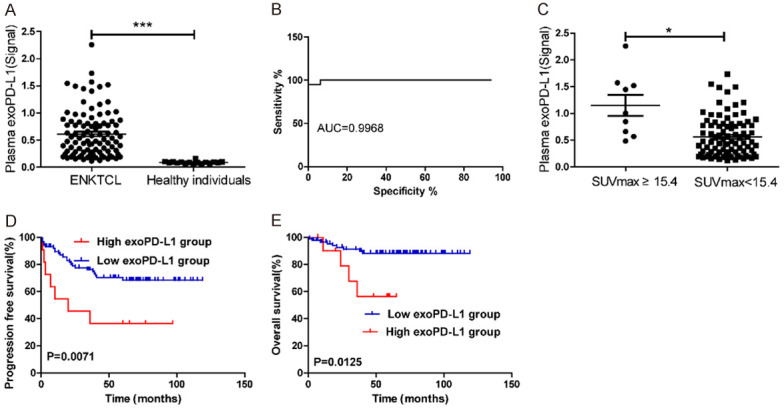
(A) Plasma exoPD-L1 level measured by Simoa in 16 healthy subjects and 99 patients with ENKTL at diagnosis. (B) ROC analysis to evaluate the diagnostic power to detect ENKTL cases (n=99) from the healthy controls (n=16). (C) The exoPD-L1 level are significantly higher in patients with high SUVmax level (≥ 15.4) than those with lower SUVmax level at diagnosis. (D, E) Kaplan-Meier estimates of PFS (D) and OS (E) considering the cutoff point for exoPD-L1 at 1.2 (simoa signal).
Table 2.
Patient characteristics according to the expression of exoPD-L1 and sPD-L1
| Characteristics | ENKTL, nasal type | ENKTL, nasal type | ||||||
|---|---|---|---|---|---|---|---|---|
|
|
|
|||||||
| Total | High exoPD-L1 group | Low exoPD-L1 group | P-Value | Total | High sPD-L1 group | Low sPD-L1 group | P-value | |
| Patient number | 99 | 11 | 88 | 96 | 21 | 75 | ||
| Gender | 0.696 | 0.183 | ||||||
| Male | 78 | 8 (10.3) | 70 (89.7) | 73 | 18 (24.7) | 55 (75.3) | ||
| Female | 21 | 3 (14.3) | 18 (85.7) | 23 | 3 (13.0) | 20 (87.0) | ||
| Age (years) | 1.000 | 0.163 | ||||||
| > 60 | 11 | 1 (9.1) | 10 (90.9) | 7 | 3 (42.9) | 4 (57.1) | ||
| ≤ 60 | 88 | 10 (11.4) | 78 (88.6) | 89 | 18 (20.2) | 71 (79.8) | ||
| Stage | 0.516 | 0.006 | ||||||
| I-II | 93 | 10 (10.8) | 83 (89.2) | 90 | 17 (18.9) | 73 (81.1) | ||
| III-IV | 6 | 1 (16.7) | 5 (83.7) | 6 | 4 (66.7) | 2 (33.3) | ||
| B symptoms | 0.749 | 0.006 | ||||||
| Yes | 54 | 7 (13.0) | 47 (87.0) | 50 | 16 (32.0) | 34 (68.0) | ||
| No | 45 | 4 (8.9) | 41 (91.1) | 46 | 5 (10.9) | 41 (89.1) | ||
| LDH level | 1.000 | < 0.001 | ||||||
| Elevated | 16 | 1 (6.3) | 15 (93.7) | 14 | 10 (71.4) | 4 (28.6) | ||
| Normal | 93 | 10 (10.8) | 83 (89.2) | 82 | 11 (13.4) | 71 (86.6) | ||
| IPI | 0.306 | < 0.001 | ||||||
| 0-1 | 89 | 9 (10.1) | 80 (89.9) | 87 | 14 (16.1) | 73 (83.9) | ||
| ≥ 2 | 10 | 2 (20) | 8 (80) | 9 | 7 (77.8) | 2 (22.2) | ||
| PINK | 1.000 | 0.005 | ||||||
| 0-1 | 88 | 10 (11.4) | 78 (88.6) | 85 | 15 (17.6) | 70 (82.4) | ||
| ≥ 2 | 11 | 1 (9.1) | 10 (90.9) | 11 | 6 (54.5) | 5 (45.5) | ||
| SUVmax | 48 | 5 | 43 | 0.005 | 46 | 9 (19.6) | 37 (80.4%) | 0.024 |
| ≥ 15.4 | 10 | 4 (40.0%) | 6 (60.0%) | 9 | 5 (55.6%) | 4 (44.4%) | ||
| < 15.4 | 38 | 1 (2.6%) | 37 (97.4%) | 37 | 4 (10.8%) | 33 (89.2%) | ||
| Disease progression | 31.3% | 63.6% | 27.3% | 0.033 | 33.3% | 47.6% | 29.3% | 0.126 |
LDH, lactate dehydrogenase; IPI, International Prognostic Index; PINK, Prognostic Index of Natural Killer Lymphoma.
Prognostic value of plasma exoPD-L1 in ENKTL
The prognostic significance of plasma exoPD-L1 was evaluated in our cohort. Patients with a high pretreatment plasma exoPD-L1 level had worse 5-year PFS (35.7% vs. 86.1%, P=0.007) and OS (56.0% vs. 88.1%, P=0.012) than those with a low pretreatment exoPD-L1 level (Figure 3D, 3E).
Correlations between clinical features and pretreatment sPD-L1 levels
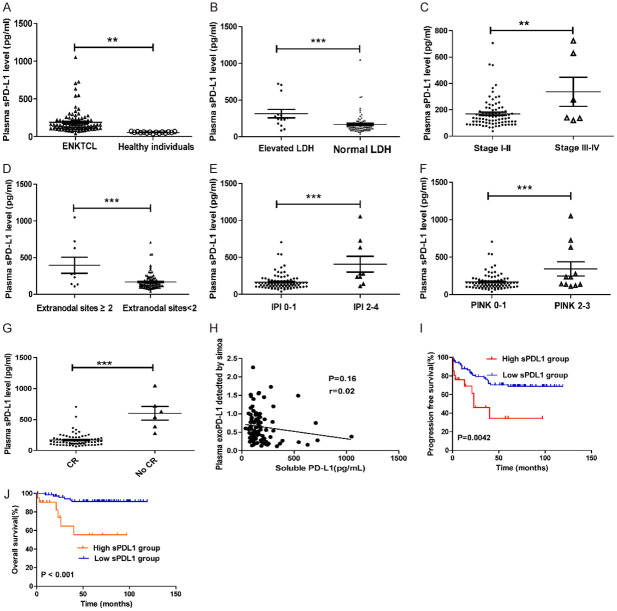
sPD-L1 concentrations in the serum were measured in ENKTL patients and healthy subjects. The sPD-L1 level was significantly higher in the ENKTL patients than in the healthy individuals (Figure 4A, P=0.16). The optimal cutoff point for the pretreatment sPD-L1 level was 219.0 pg/ml (X-tile, Figure S1).
Figure 4.
(A) Circulating sPD-L1 protein measurement in 16 healthy subjects and 96 patients with ENKTL collected at diagnosis. (B-G) The sPD-L1 level are significantly higher in patients with elevated LDH level (B), advanced stage disease (C), extranodal sites ≥ 2 (D), IPI ≥ 2 (E), PINK > 1 (F) and CR status (G) in ENKTL. *, P < 0.05; **, P < 0.001, ***, P < 0.0001. (H) No correlation between the exoPD-L1 in patients’ plasma and the soluble PD-L1 level; Spearman’s correlation at P=0.16, r=0.02. (I, J) Kaplan-Meier estimates of PFS (I) and OS (J) considering the cutoff point for sPD-L1 at 219.0 pg/ml.
The high sPD-L1 group in our cohort was significantly associated with some clinical parameters, including an advanced stage (P=0.0061), an elevated LDH level (P < 0.0001), B symptoms (P=0.0058), a high IPI score (P < 0.0001) and a high PINK score (P < 0.0001) (Table 2). The sPD-L1 level was found to be obviously higher in patients with advanced-stage disease, an elevated LDH level, extranodal sites ≥ 2, an IPI score of 2-4 or a PINK score of 2-4 than in those with early-stage disease (P=0.0020), a normal LDH level (P=0.0008), extranodal sites < 2 (P < 0.0001), an IPI score of 0-1 (< 0.0001) or a PINK score of 0-1 (P=0.0003) (Figure 4B-G).
The correlation between plasma exoPD-L1 and sPD-L1 levels was also explored in our cohort. Interestingly, our results demonstrated that the baseline exoPD-L1 level was not associated with the sPD-L1 level in the blood (P=0.12, r=0.45) (Figure 4H).
Prognostic value of sPD-L1 in the blood in ENKTL
In our study, the low sPD-L1 group (< 219 pg/mL) showed a favorable clinical course, with higher 5-year OS (91.3% vs. 55.5%, P=0.000) and 5-year PFS rates (68.9% vs. 34.6%, P=0.003) than the high sPD-L1 group (≥ 219 pg/mL) (Figure 4I, 4J).
For stage I patients (n=47), subgroup analysis demonstrated that a high sPD-L1 level was an adverse factor affecting the survival outcome. The 5-year PFS and OS rates were 71.6% and 93.9%, respectively, for patients with a low level of sPD-L1 (< 219 pg/mL, n=8), while the rates were 31.3% (P=.0022) and 65.6% (P=0.0082), respectively, for patients with a high pretreatment sPD-L1 level (≥ 219 pg/mL, n=39).
Prognostic factors for PFS and OS
In our study, univariate analysis demonstrated that the baseline SUVmax (P=0.002), plasma exoPD-L1 (P=0.007), sPD-L1 (P=0.003) and an L-asp/peg-containing regimen (P=0.031) were significant factors affecting PFS. Multivariate analysis revealed that the baseline SUVmax (P=0.006) was the only significant independent factor (Table 3).
Table 3.
Univariate and multivariate analysis of prognostic factors for survivals (by Cox regression)
| Clinical factor | Progression-free survival | Overall survival | ||||||
|---|---|---|---|---|---|---|---|---|
|
|
|
|||||||
| Univariate | Multivariate | Univariate | Multivariate | |||||
|
|
|
|
|
|||||
| P | HR (95% CI) | P | HR (95% CI) | P | HR (95% CI) | P | HR (95% CI) | |
| Age ≥ 60 y | 0.450 | 1.736 (0.415-7.254) | 0.433 | 0.548 (0.121-2.471) | ||||
| Gender | 0.270 | 0.585 (0.226-1.516) | 0.497 | 0.593 (0.131-2.679) | ||||
| B symptom | 0.537 | 0.806 (0.406-1.599) | 0.062 | 0.293 (0.081-1.065) | ||||
| LDH | 0.076 | 0.467 (0.201-1.083) | 0.045 | 0.299 (0.092-0.974) | 0.021 | 0.038 (0.002-0.603) | ||
| Ki67 ≥ 60% | 0.693 | 1.159 (0.557-2.411) | 0.136 | 2.546 (0.745-8.701) | ||||
| SUVmax ≥ 15.4 | 0.002 | 0.205 (0.068-0.615) | 0.006 | 0.195 (0.061-0.625) | 0.038 | 0.231 (0.046-1.046) | ||
| IPI score ≤ 1 | 0.146 | 2.187 (0.762-6.281) | 0.010 | 5.570 (1.511-20.531) | ||||
| PINK score ≤ 2 | 0.284 | 1.776 (0.621-5.088) | 0.043 | 3.798 (1.041-13.856) | ||||
| Hemoglobin < 110 g/L | 0.812 | 0.886 (0.326-2.408) | 0.778 | 1.244 (0.272-5.697) | ||||
| Leukopenia < 4×109/L | 0.715 | 0.796 (0.235-2.701) | 0.477 | 0.576 (0.126-2.638) | ||||
| Platelet < 150×109/L | 0.208 | 3.630 (0.488-27.000) | 0.632 | 1.648 (0.213-12.773) | ||||
| ExoPD-L1 (high vs. low) | 0.007 | 0.334 (0.144-0.777) | 0.048 | 0.368 (0.288-3 .856) | ||||
| Serum PD-L1 (high vs. low) | 0.003 | 0.337 (0.157-0.722) | 0.000 | 0.150 (0.048-0.472) | ||||
| L-asp/peg containing regimen | 0.031 | 0.446 (0.224-0.887) | 0.284 | 0.550 (0.184-1.642) | ||||
| CR after treatment | 0.271 | 1.750 (0.646-4.746) | 0.486 | 1.780 (0.351-9.021) | ||||
LDH, lactate dehydrogenase; IPI, International Prognostic Index; PINK, Prognostic Index of Natural Killer Lymphoma; ExoPD-L1, Exosomal programmed death ligand 1; L-asp/peg, L-asparaginase/pegaspargase; CR, Complete remission.
Some significant factors affecting OS were also identified by univariate analysis, including LDH (P=0.045), plasma exoPD-L1 (P=0.007), sPD-L1 (P=0.003), the IPI score (P=0.010) and the PINK score (P=0.043). However, multivariate analysis showed that only the LDH level (P=0.021) remained a significant independent factor for OS in our study (Table 3).
Discussion
ENKTL is a distinct subtype of lymphoma characterized by frequent EBV infection and a propensity for midline facial tissue involvement, with a higher rate of incidence in Asia than in Western countries [1]. VIPD is one of the standard treatments recommended by the National Comprehensive Cancer Network (NCCN) guidelines for the treatment of early-stage ENKTL. In the present study, we reported the clinical efficacy of VIPD-containing regimens in ENKTL with the largest cohort to date. This is the first study to measure plasma exoPD-L1 level on the Quanterix Simoa platform. In addition, we demonstrated for the first time that plasma exoPD-L1 was an independent prognostic factor in ENKTL patients. We also found that exoPD-L1 was not correlated with sPD-L1 in the blood of ENKTL patients. The above results indicate that exoPD-L1 may contribute to immune evasion and cancer development.
VIPD is an effective and well-tolerated treatment regimen for early-stage ENKTL patients [8,24]. A phase II study consisting of 30 stage I/II ENKTL patients demonstrated that 100% of patients achieved a response to VIPD chemotherapy, with 3-year PFS and OS rates of 85.19% and 86.28%, respectively [24]. The response rate of VIPD in our cohort was similar to the rate found in the above study, whereas survival outcomes were even better in our study of early-stage ENKTL patients, which reported a 5-year PFS of 53.7% and 5-year OS of 82.5%. The relatively small sample size and the majority of the enrolled patients in the VIPD group being low risk may partly explain these results.
As lymphoma cells lack L-asparagine synthetase and are unable to synthesize the essential amino acid asparagine, L-asparaginase and pegaspargase can exert anticancer effects by hydrolyzing serum asparagine in ENKTL patients. Some L-asp/Peg based regimens such as GELOXD, SMILE and DDGP have been developed in the last decade and have been proven to be effective in ENKTL patients [1,7,25]. In this study, L/P-VIPD resulted in excellent survival outcomes, with 5-year PFS and 5-year OS rates of 73.6% and 88.7%, respectively, consistent with the results of a previous study [8]. Compared with VIPD, the combination of L-asp/Peg could improve PFS in early-stage patients, indicating that L-asp/Peg treatment may play a key role in the prevention of recurrence.
In recent years, a growing body of evidence showed that exosomes play a key role in cancer development. Several studies have reported that PD-L1 was enriched on the surface of tumor-derived exosomes in various cancers [13,15,16]. However, the concrete mechanism of exoPD-L1 formation has not been fully understood. A recent study proved that exoPD-L1 originated from the surface of prostate cancer cells rather than from the endoplasmic reticulum or Golgi complex, indicating that PD-L1 internalization through the plasma membrane may be the source of exoPD-L1 [16]. In our study, we found that PD-L1-positive exosome level was significantly more abundant in the plasma of ENKTL patients than in that of healthy individuals. Consistent with previous reports [26,27], our data showed that the detection of exoPD-L1 could also differentiate ENKTL patients from a healthy population with high sensitivity and specificity, indicating that the plasma exoPD-L1 level is a potential marker for cancer screening and diagnosis of patients with suspected malignancy.
Some studies have confirmed that PD-L1 expressed on the exosome surface could bind to programmed cell death-1 (PD-1) and suppress the cytotoxicity of CD8 + T cells to promote tumor progression in various cancers, such as melanoma, breast cancer and prostate cancer [14,15,27]. In addition, exoPD-L1 released by tumor cells could be transferred to other cells with low or no PD-L1 expression [15]. Our study proved hat abundant exoPD-L1 was present in the plasma of ENKTL patients and that lymphoma cells might actively release these exosomes to create an immunosuppressive microenvironment to support cancer development. High level of exoPD-L1 in the blood has been demonstrated to be associated with adverse clinicopathologic features, such as advanced disease stage and tumor burden, in various cancers [14]. Our data demonstrated that high level of plasma exoPD-L1 was associated with an elevated baseline SUVmax. PD-L1 expression is associated with glucose metabolism in various cancers, such as lung cancer and cervical cancer [28,29]. The SUVmax in PET/CT was significantly higher in the high exoPD-L1 group and high sPD-L1 group, possibly because lymphoma cells may release PD-L1 to promote glycometabolism.
ExoPD-L1 can also serve as a biomarker for response evaluation and prognosis assessment in some cancers, such as melanoma and head and neck cancer [14,27]. Our research also showed that a high level of plasma exoPD-L1 could predict patient response and prognosis. High expression of plasma exoPD-L1 in ENKTL patients was associated with a poor response and relatively poor prognosis. These results indicate that exoPD-L1 may be a promising biomarker for tumor progression.
Although circulating sPD-L1 level was proved to be significantly higher in lymphoma patients than healthy individuals [30-32], the regulation and source of sPD-L1 remain ambiguous. The stimulation of some inflammatory cytokines such as IFN-γ might promote the secretion of sPD-L1 in cancer cells [33,34]. The correlation between the pretreatment sPD-L1 level and the clinical features are controversial [30,32]. Our study demonstrated that a high baseline sPD-L1 level was correlated with some adverse clinical characteristics, including an advanced stage, an elevated LDH level, B symptoms, a high IPI score and a high PINK score.
The predictive value of pretreatment sPD-L1 has been assessed in previous studies [30,32]. Patients with a high concentration (> 3.23 ng/L, n=34) of sPD-L1 were found to have significantly worse PFS (3-year PFS 85.0 vs. 25.6%, P < 0.001) and OS (3-year OS 51.7 vs. 91.3%, P < 0.001) than those with a low concentration (≤ 3.23 ng/L, n=63) [30]. Consistent with this study, our research found that a high pretreatment level of sPD-L1 was an important prognostic factor for PFS and OS, indicating that the baseline sPD-L1 level is a potential prognostic biomarker in ENKTL patients.
The correlation between exoPD-L1 and sPD-L1 levels in the blood was investigated in a previous study [14]. In this study, no correlation between the exoPD-L1 and sPD-L1 levels in the plasma was observed in the head and neck cancer patients. Although high exoPD-L1 and sPD-L1 levels were associated with a relatively poor prognosis in our study, there was no relationship between these two variables in our patients. The following reasons may explain the above results: 1) PD-L1 on exomes was more stable than sPD-L1 in the plasma; 2) the cell sources of exoPD-L1 and sPD-L1 might be different, and 3) the production and secretion of sPD-L1 by cells was independent of the process of exosome biogenesis.
Blockade of the PD1/PD-L1 pathway is a promising therapeutic approach in non-Hodgkin lymphomas, such as ENKTL and peripheral T cell lymphoma [32]. Clinical trials reported that PD-1 inhibitors were effective in patients with relapsed/refractory NK/T cell lymphoma and achieved an overall response rate of 57%-100% [35,36]. Two studies have demonstrated that high level of exoPD-L1 in the blood was the possible cause for the failure of anti-PD-1 therapies in cancer patients and pretreatment circulating exoPD-L1 level was associated with the tumor response and clinical outcomes of anti-PD-1 therapy [13,37]. The role of sPD-L1 in predicting treatment response remains unclear in ENKTL. In our study, Patients with higher level of sPD-L1 obtained higher complete remission rate and the prognosis of high-level exoPD-L1 and sPD-L1 group was poorer with VIPD-containing regimen, indicating that these patients could receive other treatment regimen to further improve the survival outcome.
Several other risk factors were also identified in our study, including the LDH level, baseline SUVmax, IPI and PINK score. 18F-FDG PET/CT is a standard imaging technology used to stage disease, evaluate responses and monitor for relapse in lymphoma patients [38]. The baseline SUVmax in PET/CT is an important prognostic factor in lymphoma [39,40]. Our results support previous observations that the baseline SUVmax correlates with prognosis in ENKTL [40]. The PINK score represents a new model used to predict outcomes in ENKTL patients treated with non-anthracycline-based therapies, and this model was confirmed in subsequent studies [7,41]. Our research assessed the impact of the PINK on prognosis and found that a high PINK score was associated with a worse outcome.
Conclusions
In summary, our results revealed that VIPD-containing chemotherapy was an effective regimen for patients with nasal-type ENKTL and that abundant PD-L1 was enriched on exosomes derived from patient plasma. In addition, high levels of circulating exoPD-L1 and sPD-L1 were important predictors of adverse survival outcomes, and they could serve as valuable diagnostic and prognostic biomarkers for ENKTL patients.
Acknowledgements
This work was supported by the National Natural Science Foundation of China (No. 81470353, 81870155, 81700195), Innovation Group Project of Shanghai Municipal Health Comission (2019CXJQ03), Shanghai Science and technology development fund (19MC1911000), and Shanghai Municipal Key Clinical Specialty (shslczdzk01301).
Disclosure of conflict of interest
None.
Supporting Information
References
- 1.Yamaguchi M, Suzuki R, Oguchi M. Advances in the treatment of extranodal NK/T-cell lymphoma, nasal type. Blood. 2018;131:2528–2540. doi: 10.1182/blood-2017-12-791418. [DOI] [PubMed] [Google Scholar]
- 2.Wang L, Xia ZJ, Huang HQ, Lu Y, Zhang YJ. Cyclophosphamide, doxorubicin, vincristine, and prednisone (CHOP) in the treatment of stage IE/IIE extranodal natural killer/T cell lymphoma, nasal type: 13-year follow-up in 135 patients. Int J Hematol. 2012;96:617–623. doi: 10.1007/s12185-012-1174-y. [DOI] [PubMed] [Google Scholar]
- 3.Kim BS, Kim TY, Kim CW, Kim JY, Heo DS, Bang YJ, Kim NK. Therapeutic outcome of extranodal NK/T-cell lymphoma initially treated with chemotherapy--result of chemotherapy in NK/T-cell lymphoma. Acta Oncol. 2003;42:779–783. doi: 10.1080/02841860310010682. [DOI] [PubMed] [Google Scholar]
- 4.Zhang L, Jiang M, Xie L, Zhang H, Jiang Y, Yang QP, Liu WP, Zhang WY, Zhuo HY, Li P, Chen NY, Zhao S, Wang F, Zou LQ. Five-year analysis from phase 2 trial of “sandwich” chemoradiotherapy in newly diagnosed, stage IE to IIE, nasal type, extranodal natural killer/T-cell lymphoma. Cancer Med. 2016;5:33–40. doi: 10.1002/cam4.569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tsai HJ, Lin SF, Chen CC, Chen TY, Su WC, Hwang WL, Lin JC, Chiou TJ, Kao WY, Chiu CF, Chang YF, Chang JS, Chang MC, Su IJ. Long-term results of a phase II trial with frontline concurrent chemoradiotherapy followed by consolidation chemotherapy for localized nasal natural killer/T-cell lymphoma. Eur J Haematol. 2015;94:130–137. doi: 10.1111/ejh.12405. [DOI] [PubMed] [Google Scholar]
- 6.Kim SJ, Yang DH, Kim JS, Kwak JY, Eom HS, Hong DS, Won JH, Lee JH, Yoon DH, Cho J, Nam TK, Lee SW, Ahn YC, Suh C, Kim WS. Concurrent chemoradiotherapy followed by L-asparaginase-containing chemotherapy, VIDL, for localized nasal extranodal NK/T cell lymphoma: CISL08-01 phase II study. Ann Hematol. 2014;93:1895–1901. doi: 10.1007/s00277-014-2137-6. [DOI] [PubMed] [Google Scholar]
- 7.Li JW, Li YJ, Zhong MZ, Liu XL, Li J, Li KL, Liu XY, Zhou F, OuYang Z, Sun ZY, Huang LJ, He JQ, Zhou H, Yi PY. Efficacy and tolerance of GELOXD/P-GEMOXD in newly diagnosed nasal-type extranodal NK/T-cell lymphoma: a multicenter retrospective study. Eur J Haematol. 2018;100:247–256. doi: 10.1111/ejh.13004. [DOI] [PubMed] [Google Scholar]
- 8.Dong LH, Zhang LJ, Wang WJ, Lei W, Sun X, Du JW, Gao X, Li GP, Li YF. Sequential DICE combined with l-asparaginase chemotherapy followed by involved field radiation in newly diagnosed, stage IE to IIE, nasal and extranodal NK/T-cell lymphoma. Leuk Lymphoma. 2016;57:1600–1606. doi: 10.3109/10428194.2015.1108415. [DOI] [PubMed] [Google Scholar]
- 9.Rutherford SC, Fachel AA, Li S, Sawh S, Muley A, Ishii J, Saxena A, Dominguez PM, Caldas Lopes E, Agirre X, Chambwe N, Correa F, Jiang Y, Richards KL, Betel D, Shaknovich R. Extracellular vesicles in DLBCL provide abundant clues to aberrant transcriptional programming and genomic alterations. Blood. 2018;132:e13–e23. doi: 10.1182/blood-2017-12-821843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Feng Y, Zhong M, Zeng S, Wang L, Liu P, Xiao X, Liu Y. Exosome-derived miRNAs as predictive biomarkers for diffuse large B-cell lymphoma chemotherapy resistance. Epigenomics. 2018;11:35–51. doi: 10.2217/epi-2018-0123. [DOI] [PubMed] [Google Scholar]
- 11.Li J, Tian T, Zhou X. The role of exosomal shuttle RNA (esRNA) in lymphoma. Crit Rev Oncol Hematol. 2019;137:27–34. doi: 10.1016/j.critrevonc.2019.01.013. [DOI] [PubMed] [Google Scholar]
- 12.Kataoka K, Miyoshi H, Sakata S, Dobashi A, Couronne L, Kogure Y, Sato Y, Nishida K, Gion Y, Shiraishi Y, Tanaka H, Chiba K, Watatani Y, Kakiuchi N, Shiozawa Y, Yoshizato T, Yoshida K, Makishima H, Sanada M, Onozawa M, Teshima T, Yoshiki Y, Ishida T, Suzuki K, Shimada K, Tomita A, Kato M, Ota Y, Izutsu K, Demachi-Okamura A, Akatsuka Y, Miyano S, Yoshino T, Gaulard P, Hermine O, Takeuchi K, Ohshima K, Ogawa S. Frequent structural variations involving programmed death ligands in Epstein-Barr virus-associated lymphomas. Leukemia. 2019;33:1687–1699. doi: 10.1038/s41375-019-0380-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chen G, Huang AC, Zhang W, Zhang G, Wu M, Xu W, Yu ZL, Yang JG, Wang BK, Sun HH, Xia HF, Man QW, Zhong WQ, Antelo LF, Wu B, Xiong XP, Liu XM, Guan L, Li T, Liu SJ, Yang RF, Lu YT, Dong LY, McGettigan S, Somasundaram R, Radhakrishnan R, Mills G, Lu YL, Kim J, Chen YHH, Dong HD, Zhao YF, Karakousis GC, Mitchell TC, Schuchter LM, Herlyn M, Wherry EJ, Xu XW, Guo W. Exosomal PD-L1 contributes to immunosuppression and is associated with anti-PD-1 response. Nature. 2018;560:382–386. doi: 10.1038/s41586-018-0392-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Theodoraki MN, Yerneni SS, Hoffmann TK, Gooding WE, Whiteside TL. Clinical significance of PD-L1(+) exosomes in plasma of head and neck cancer patients. Clin Cancer Res. 2018;24:896–905. doi: 10.1158/1078-0432.CCR-17-2664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yang Y, Li CW, Chan LC, Wei Y, Hsu JM, Xia W, Cha JH, Hou J, Hsu JL, Sun L, Hung MC. Exosomal PD-L1 harbors active defense function to suppress T cell killing of breast cancer cells and promote tumor growth. Cell Res. 2018;28:862–864. doi: 10.1038/s41422-018-0060-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Poggio M, Hu T, Pai CC, Chu B, Belair CD, Chang A, Montabana E, Lang UE, Fu Q, Fong L, Blelloch R. Suppression of exosomal PD-L1 induces systemic anti-tumor immunity and memory. Cell. 2019;177:414–427. e413. doi: 10.1016/j.cell.2019.02.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cheson BD, Pfistner B, Juweid ME, Gascoyne RD, Specht L, Horning SJ, Coiffier B, Fisher RI, Hagenbeek A, Zucca E, Rosen ST, Stroobants S, Lister TA, Hoppe RT, Dreyling M, Tobinai K, Vose JM, Connors JM, Federico M, Diehl V International Harmonization Project on Lymphoma. Revised response criteria for malignant lymphoma. J. Clin. Oncol. 2007;25:579–586. doi: 10.1200/JCO.2006.09.2403. [DOI] [PubMed] [Google Scholar]
- 18.Thery C, Amigorena S, Raposo G, Clayton A. Isolation and characterization of exosomes from cell culture supernatants and biological fluids. Curr Protoc Cell Biol. 2006 doi: 10.1002/0471143030.cb0322s30. Chapter 3: Unit 3.22. [DOI] [PubMed] [Google Scholar]
- 19.Tian Y, Ma L, Gong M, Su G, Zhu S, Zhang W, Wang S, Li Z, Chen C, Li L, Wu L, Yan X. Protein profiling and sizing of extracellular vesicles from colorectal cancer patients via flow cytometry. ACS Nano. 2018;12:671–680. doi: 10.1021/acsnano.7b07782. [DOI] [PubMed] [Google Scholar]
- 20.Jia YJ, Liu ZB, Wang WG, Sun CB, Wei P, Yang YL, You MJ, Yu BH, Li XQ, Zhou XY. HDAC6 regulates microRNA-27b that suppresses proliferation, promotes apoptosis and target MET in diffuse large B-cell lymphoma. Leukemia. 2018;32:703–711. doi: 10.1038/leu.2017.299. [DOI] [PubMed] [Google Scholar]
- 21.Wilson DH, Rissin DM, Kan CW, Fournier DR, Piech T, Campbell TG, Meyer RE, Fishburn MW, Cabrera C, Patel PP, Frew E, Chen Y, Chang L, Ferrell EP, von Einem V, McGuigan W, Reinhardt M, Sayer H, Vielsack C, Duffy DC. The simoa HD-1 analyzer: a novel fully automated digital immunoassay analyzer with single-molecule sensitivity and multiplexing. J Lab Autom. 2016;21:533–547. doi: 10.1177/2211068215589580. [DOI] [PubMed] [Google Scholar]
- 22.Zou J, Guo Y, Wei L, Yu F, Yu B, Xu A. Long noncoding RNA POU3F3 and alpha-synuclein in plasma L1CAM exosomes combined with beta-glucocerebrosidase activity: potential predictors of Parkinson’s disease. Neurotherapeutics. 2020;17:1104–1119. doi: 10.1007/s13311-020-00842-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Andreu Z, Yanez-Mo M. Tetraspanins in extracellular vesicle formation and function. Front Immunol. 2014;5:442. doi: 10.3389/fimmu.2014.00442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kim SJ, Kim K, Kim BS, Kim CY, Suh C, Huh J, Lee SW, Kim JS, Cho J, Lee GW, Kang KM, Eom HS, Pyo HR, Ahn YC, Ko YH, Kim WS. Phase II trial of concurrent radiation and weekly cisplatin followed by VIPD chemotherapy in newly diagnosed, stage IE to IIE, nasal, extranodal NK/T-Cell lymphoma: consortium for improving survival of lymphoma study. J. Clin. Oncol. 2009;27:6027–6032. doi: 10.1200/JCO.2009.23.8592. [DOI] [PubMed] [Google Scholar]
- 25.Li X, Cui Y, Sun Z, Zhang L, Li L, Wang X, Wu J, Fu X, Ma W, Zhang X, Chang Y, Nan F, Li W, Su L, Wang J, Xue H, Zhang M. DDGP versus SMILE in newly diagnosed advanced natural killer/T-cell lymphoma: a randomized controlled, multicenter, open-label study in China. Clin Cancer Res. 2016;22:5223–5228. doi: 10.1158/1078-0432.CCR-16-0153. [DOI] [PubMed] [Google Scholar]
- 26.Pang Y, Shi J, Yang X, Wang C, Sun Z, Xiao R. Personalized detection of circling exosomal PD-L1 based on Fe3O4@TiO2 isolation and SERS immunoassay. Biosens Bioelectron. 2020;148:111800. doi: 10.1016/j.bios.2019.111800. [DOI] [PubMed] [Google Scholar]
- 27.Chen G, Huang AC, Zhang W, Zhang G, Wu M, Xu W, Yu Z, Yang J, Wang B, Sun H, Xia H, Man Q, Zhong W, Antelo LF, Wu B, Xiong X, Liu X, Guan L, Li T, Liu S, Yang R, Lu Y, Dong L, McGettigan S, Somasundaram R, Radhakrishnan R, Mills G, Lu Y, Kim J, Chen YH, Dong H, Zhao Y, Karakousis GC, Mitchell TC, Schuchter LM, Herlyn M, Wherry EJ, Xu X, Guo W. Exosomal PD-L1 contributes to immunosuppression and is associated with anti-PD-1 response. Nature. 2018;560:382–386. doi: 10.1038/s41586-018-0392-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kaira K, Shimizu K, Kitahara S, Yajima T, Atsumi J, Kosaka T, Ohtaki Y, Higuchi T, Oyama T, Asao T, Mogi A. 2-Deoxy-2-[fluorine-18] fluoro-d-glucose uptake on positron emission tomography is associated with programmed death ligand-1 expression in patients with pulmonary adenocarcinoma. Eur J Cancer. 2018;101:181–190. doi: 10.1016/j.ejca.2018.06.022. [DOI] [PubMed] [Google Scholar]
- 29.Wang S, Li J, Xie J, Liu F, Duan Y, Wu Y, Huang S, He X, Wang Z, Wu X. Programmed death ligand 1 promotes lymph node metastasis and glucose metabolism in cervical cancer by activating integrin beta4/SNAI1/SIRT3 signaling pathway. Oncogene. 2018;37:4164–4180. doi: 10.1038/s41388-018-0252-x. [DOI] [PubMed] [Google Scholar]
- 30.Wang H, Wang L, Liu WJ, Xia ZJ, Huang HQ, Jiang WQ, Li ZM, Lu Y. High post-treatment serum levels of soluble programmed cell death ligand 1 predict early relapse and poor prognosis in extranodal NK/T cell lymphoma patients. Oncotarget. 2016;7:33035–33045. doi: 10.18632/oncotarget.8847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rossille D, Gressier M, Damotte D, Maucort-Boulch D, Pangault C, Semana G, Le Gouill S, Haioun C, Tarte K, Lamy T, Milpied N, Fest T Groupe Ouest-Est des Leucémies et Autres Maladies du Sang; Groupe Ouest-Est des Leucémies et Autres Maladies du Sang. High level of soluble programmed cell death ligand 1 in blood impacts overall survival in aggressive diffuse large B-Cell lymphoma: results from a French multicenter clinical trial. Leukemia. 2014;28:2367–2375. doi: 10.1038/leu.2014.137. [DOI] [PubMed] [Google Scholar]
- 32.Nagato T, Ohkuri T, Ohara K, Hirata Y, Kishibe K, Komabayashi Y, Ueda S, Takahara M, Kumai T, Ishibashi K, Kosaka A, Aoki N, Oikawa K, Uno Y, Akiyama N, Sado M, Takei H, Celis E, Harabuchi Y, Kobayashi H. Programmed death-ligand 1 and its soluble form are highly expressed in nasal natural killer/T-cell lymphoma: a potential rationale for immunotherapy. Cancer Immunol Immunother. 2017;66:877–890. doi: 10.1007/s00262-017-1987-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Imai Y, Chiba T, Kondo T, Kanzaki H, Kanayama K, Ao J, Kojima R, Kusakabe Y, Nakamura M, Saito T, Nakagawa R, Suzuki E, Nakamoto S, Muroyama R, Tawada A, Matsumura T, Nakagawa T, Kato J, Kotani A, Matsubara H, Kato N. Interferon-gamma induced PD-L1 expression and soluble PD-L1 production in gastric cancer. Oncol Lett. 2020;20:2161–2168. doi: 10.3892/ol.2020.11757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Chang B, Huang T, Wei H, Shen L, Zhu D, He W, Chen Q, Zhang H, Li Y, Huang R, Li W, Wu P. The correlation and prognostic value of serum levels of soluble programmed death protein 1 (sPD-1) and soluble programmed death-ligand 1 (sPD-L1) in patients with hepatocellular carcinoma. Cancer Immunol Immunother. 2019;68:353–363. doi: 10.1007/s00262-018-2271-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kwong YL, Chan TSY, Tan D, Kim SJ, Poon LM, Mow B, Khong PL, Loong F, Au-Yeung R, Iqbal J, Phipps C, Tse E. PD1 blockade with pembrolizumab is highly effective in relapsed or refractory NK/T-cell lymphoma failing L-asparaginase. Blood. 2017;129:2437–2442. doi: 10.1182/blood-2016-12-756841. [DOI] [PubMed] [Google Scholar]
- 36.Li X, Cheng Y, Zhang M, Yan J, Li L, Fu X, Zhang X, Chang Y, Sun Z, Yu H, Zhang L, Wang X, Wu J, Li Z, Nan F, Tian L, Li W, Young KH. Activity of pembrolizumab in relapsed/refractory NK/T-cell lymphoma. J Hematol Oncol. 2018;11:15. doi: 10.1186/s13045-018-0559-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Cordonnier M, Nardin C, Chanteloup G, Derangere V, Algros MP, Arnould L, Garrido C, Aubin F, Gobbo J. Tracking the evolution of circulating exosomal-PD-L1 to monitor melanoma patients. J Extracell Vesicles. 2020;9:1710899. doi: 10.1080/20013078.2019.1710899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Trotman J, Barrington SF, Belada D, Meignan M, MacEwan R, Owen C, Ptáčník V, Rosta A, Fingerle-Rowson GR, Zhu J, Nielsen T, Sahin D, Hiddemann W, Marcus RE, Davies A PET investigators from the GALLIUM study. Prognostic value of end-of-induction PET response after first-line immunochemotherapy for follicular lymphoma (GALLIUM): secondary analysis of a randomised, phase 3 trial. Lancet Oncol. 2018;19:1530–1542. doi: 10.1016/S1470-2045(18)30618-1. [DOI] [PubMed] [Google Scholar]
- 39.Cheson BD. PET/CT in lymphoma: current overview and future directions. Semin Nucl Med. 2018;48:76–81. doi: 10.1053/j.semnuclmed.2017.09.007. [DOI] [PubMed] [Google Scholar]
- 40.Pak K, Kim BS, Kim K, Kim IJ, Jun S, Jeong YJ, Shim HK, Kim SD, Cho KS. Prognostic significance of standardized uptake value on F18-FDG PET/CT in patients with extranodal nasal type NK/T cell lymphoma: a multicenter, retrospective analysis. Am J Otolaryngol. 2018;39:1–5. doi: 10.1016/j.amjoto.2017.10.009. [DOI] [PubMed] [Google Scholar]
- 41.Chen SY, Yang Y, Qi SN, Wang Y, Hu C, He X, Zhang LL, Wu G, Qu BL, Qian LT, Hou XR, Zhang FQ, Qiao XY, Wang H, Li GF, Zhang YJ, Zhu Y, Cao JZ, Lan SM, Wu JX, Wu T, Zhu SY, Shi M, Xu LM, Yuan ZY, Yahalom J, Tsang R, Song YQ, Zhu J, Su H, Li YX. Validation of nomogram-revised risk index and comparison with other models for extranodal nasal-type NK/T-cell lymphoma in the modern chemotherapy era: indication for prognostication and clinical decision-making. Leukemia. 2020 doi: 10.1038/s41375-020-0791-3. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.