Abstract
Reprogramming of metabolism is one of the hallmarks of cancer, among which glucose metabolism dysfunction is the most prominent feature. The glucose metabolism of tumor cells is significantly different from that of normal cells. Glucose metabolism reprogramming of hepatocellular carcinoma (HCC) has become an important research hotspot in the field of HCC, a variety of tumor metabolic interventions have been applied clinically. Moreover, various Non-coding RNAs (ncRNAs) including microRNAs (miRNAs), long non-coding (lncRNAs) as well as circular RNAs (circRNAs), have recently been proved to play potential roles in glucose metabolism. This review summarizes the effects of ncRNAs on HCC that participate in glucose metabolism and discuss the related mechanisms to find potential and effective targeted treatments for HCC.
Keywords: Hepatocellular carcinoma, glucose metabolism, glycolysis, non-coding RNA, microRNAs, long non-coding RNAs, circular RNAs
Introduction
Liver cancer is the sixth highest-incidence cancer and it is also the 4th most deadly cancer with easy metastasis and poor prognosis. There were approximately 850,000 new cases and 780,000 deaths of liver cancer occurred in 2018. HCC accounts for 75% to 85% of primary liver cancer and more than half of the world’s HCC cases occur in China [1].
For early-stage HCC, a comprehensive treatment plan is mainly based on surgery, combined with transcatheter arterial chemoembolization (TACE) and radiofrequency ablation [2]. However, it is regrettable that most patients have reached an advanced stage or distant metastasis at the first diagnosis, that losing the opportunity for surgery. As for advanced HCC, there is no standard treatment, and the 5-year survival rate of HCC is 3% to 5% [3].
Despite the unremitting efforts of researchers, the key molecular mechanism of HCC development remains inconclusive, limiting the progression of therapeutic regimens. Recently, multiple lines of evidence have shown that metabolic reprogramming is closely related to the occurrence and development of HCC, among which glucose metabolism reprogramming is one of the most prominent features [4]. The mechanisms of glucose metabolic reprogramming often involve gene mutations, especially C-MYC and P53 [5,6]. While changes in the expression or activity of glucose metabolism genes and related glucose metabolism enzymes also have global effects [7].
Non-coding RNAs (ncRNAs) are RNA transcribed from DNA and unable to encode proteins, which account for the majority of RNAs (Figure 1). With the improvement and maturity of ncRNAs identification technology, the crucial role of ncRNAs in tumorigenesis has been increasingly recognized. They participate in almost all biological functions, including proliferation, apoptosis, migration, invasion, EMT, cancer stem cells and drug resistance [8,9].
Figure 1.
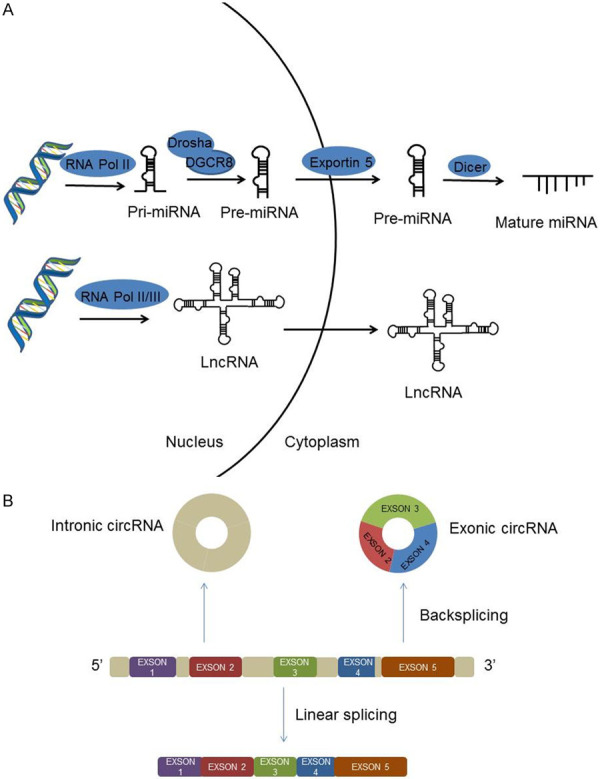
A. The biogenesis of ncRNAs. miRNAs are transcribed into pri-miRNA under the help of RNA Polymerase II, pri-miRNA is processed into a pre-miRNA by Drosha/DGCR8 in the nucleus, and then transported to the cytoplasm via Exportin-5, pre-miRNA is further cleaved by Dicer to form mature miRNA. lncRNAs are transcribed by RNA Polymerase II or III. B. CircRNAs formed by exon circularization.
Multiple lines of evidence have manifested that ncRNAs, mainly miRNAs, lncRNAs and circular RNAs (circRNAs) may play pivotal roles in reprogramming glucose metabolism, including glycolytic pathway, pentose phosphate pathway (PPP) and gluconeogenesis via altering the expression or activity of the glucose metabolism genes and related glucose metabolism enzymes (Figure 2) and regulating the activation of various signaling pathways (Figure 3).
Figure 2.
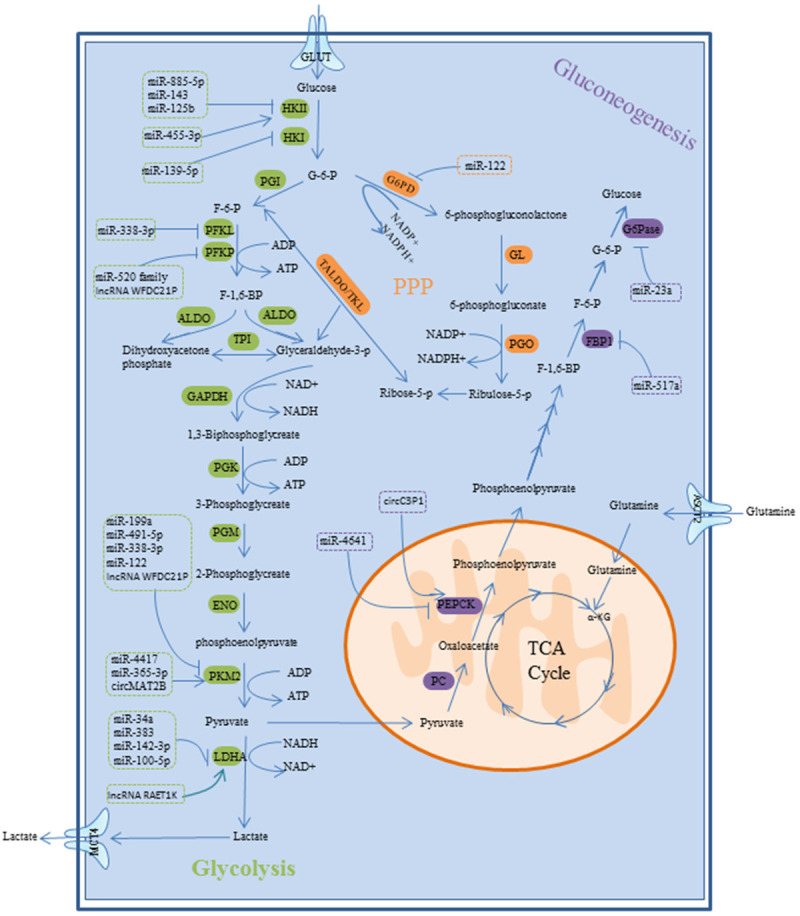
Glycolysis pathway, PPP and gluconeogenesis pathway of HCC, and the role of ncRNAs in glucose metabolism via regulating rate-controlling enzyme and GLUT.
Figure 3.
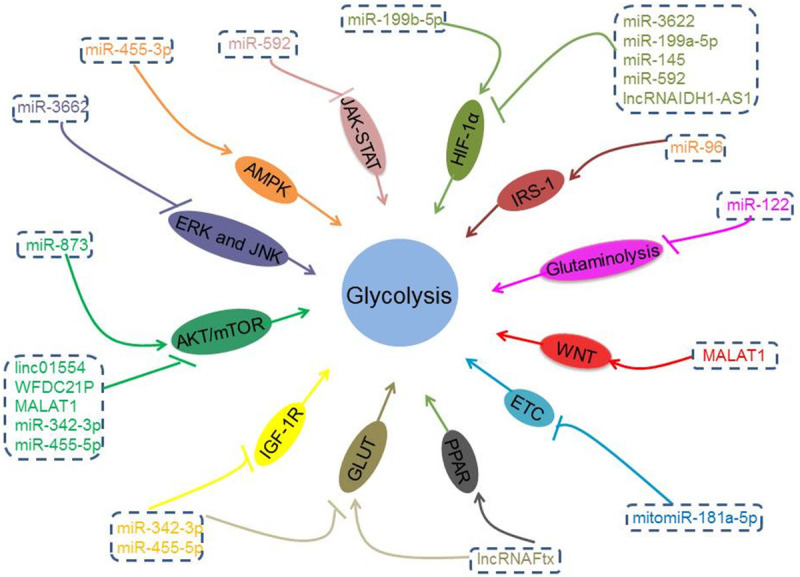
NcRNAs may play an important role in regulating glucose metabolism of HCC through different signal pathways and mechanisms.
MiRNAs
MiRNAs are a class of ncRNAs that about 22 nt in length. MiRNAs completely or incompletely combine with the 3’ untranslated regions (3’-UTR) of the target genes, leading to direct degradation or blocked translation of the target genes, thereby decreasing the expression of these specific mRNAs by either mRNA cleavage or translational repression [10-12]. In recent years, more and more studies have found that miRNAs are involved in the regulation of glucose metabolism, including glycolytic pathway, pentose phosphate pathway (PPP) and gluconeogenesis. Here we discuss the role of miRNAs on glucose metabolism of HCC. The related studies’ contents are summarized in Table 1.
Table 1.
MiRNAs involved in glucose metabolism in HCC
| Glucose metabolism | microRNA | Effect | Target | Mechanism | Reference |
|---|---|---|---|---|---|
| glycolytic pathway | miR-885-5p | Decrease | HKII | - | [23] |
| miR-143 | Decrease | HKII | - | [24] | |
| miR-125b | Decrease | - | Inhibit HKII | [25] | |
| miR-455-3p | Increase | AMPKβ2 | stabilizes HKII protein active AMPK pathway | [26] | |
| miR-139-5p | Decrease | ETS1 | Inhibit HKI and PFKFB3 expression | [27] | |
| miR-520 family | Decrease | PFKP | - | [32] | |
| miR-338-3p | Decrease | PFKL | - | [33] | |
| miR-139-5p | Decrease | PKM2 | - | [27] | |
| miR-199a | Decrease | PKM2 | - | [36] | |
| miR-491-5p | Decrease | PKM2 | - | [37] | |
| miR-338-3p | Decrease | PKM2 | - | [38] | |
| miR-122 | Decrease | PKM2 | - | [39] | |
| miR-4417 | Increase | - | Promote PKM2 phosphorylation | [40] | |
| miR-365a-3p | Increase | - | Inhibit PKM2 degradation | [41] | |
| miR-374b | Decrease | hnRNPA1 | Inhibit PKM2 expression | [42] | |
| miR-142-3p | Decrease | LDHA | - | [44] | |
| miR-383 | Decrease | LDHA | - | [45] | |
| miR-34a | Decrease | LDHA | - | [46] | |
| miR-100-5p | Decrease | LDHA | - | [47] | |
| miR-592 | Decrease | WSB1 | Disrupting HIF-1α stabilization | [51] | |
| miR-199a-5p | Decrease | HIF-1α | - | [52] | |
| miR-3662 | Decrease | HIF-1α | Inactivate ERK and JNK | [53] | |
| miR-145 | Decrease | - | Inhibit HIF-1α and PDK1 expression | [54] | |
| miR-873 | Increase | NDFIP1 | Active AKT/mTOR | [56] | |
| miR-199b-5p | Decrease | - | Up-regulates HIF-1α transcription through overexpression of NPAS2 | [57] | |
| mitomiR-181a-5p | Decrease | - | Impair electron transport chain | [59] | |
| miR-342-3p | Decrease | IGF-1R | Downregulate GLUT1 Inactivate PI3K/AKT | [61] | |
| miR-455-5p | Decrease | IGF-1R | Downregulate GLUT1 Inactivate PI3K/AK | [62] | |
| miR-129-5p | Decrease | PDK4 | - | [55] | |
| pentose phosphate pathway | miR-122 | Decrease | G6PD | - | [65] |
| gluconeogenesis | miR-4641 | Decrease | PCK1 | - | [69] |
| miR-517a | Decrease | FBP1 | - | [72] | |
| miR-23a | Decrease | G6PC PGC-1α | - | [74] | |
| miR-96 | Increase | IRS-1 | - | [76] | |
| miR-122 | Increase | - | Promoted glutaminolysis | [78] |
“-”: unknown. Abbreviations: HCC: Hepatocellular carcinoma; HK: Hexokinase; PFK: 6-phosphate fructokinase; PK: Pyruvate kinase; HIFs: Hypoxia-inducible factors; AMPKβ2: AMP-activated protein kinase subunit beta 2; PFKFB3: 6-phosphofructo-2-kinase/fructose-2,6-biphosphatase 3; PKM2: Pyruvate kinase M2; LDHA: Lactate dehydrogenase A; IGF-1R: Insulin-like growth factor-1 receptor; GLUT: Glucose transporters; PDK4: Pyruvate dehydrogenase kinase 4; PEPCK: Phosphoenolpyruvate carboxylase; FBP1: Fructose 1,6-bisphosphatase; G6PC: Glucose 6-phosphatase; PGC-1α: Peroxisome proliferator-activated receptor gamma, coactivator 1 alpha; IRS-1: Insulin receptor substrate 1.
MiRNAs involved in aerobic glycolysis
Aerobic glycolysis also called the “Warburg effect”, which preference for tumor cells acquire energy through glycolysis rather than oxidative phosphorylation even in the sufficient aerobic and mitochondrial function [13]. Glycolysis also provides raw materials for other anabolic [13]. Furthermore, aerobic glycolysis can produce a large amount of lactic acid and creating an acidic microenvironment, which is conducive to tumor cell invasion and metastasis [15]. Therefore, inhibition of aerobic glycolysis may be a promising anti-tumor therapy [16-19].
The regulation of glycolysis is mainly through manipulating the activity of the rate-controlling enzymes (key enzymes), including hexokinase (HK), 6-phosphate fructokinase (PFK) and pyruvate kinase (PK). Hypoxia-inducible factors (HIFs), particularly HIF-1α is a crucial mediator of hypoxia response, promoting glycolysis process to adapt cancer cells to hypoxic environment [20]. On the one hand, emerging studies demonstrated that alters miRNAs expression profiles may orchestrate the glycolytic pathway of HCC cells by regulating the expression of glycolysis-related enzymes, including HK, PFK, PK, HIFs. On the other hand, different glucose levels also alter the level of miRNAs. For example, the expression of miR-483-3p is low under low glucose, while under high glucose conditions, the expression of miR-483-3p increases and inhibits apoptosis [21].
MiRNAs and HK
HK is the first rate-limiting enzymes of aerobic glycolysis, which catalysis phosphorylation of glucose to glucose 6-phosphate [22]. The overexpression of HK has been highlighted in HCC cells. On the contrary, suppression of HK expression may induce HCC cells apoptosis. Therefore, it is considered to be a vital molecule in the glycolysis pathway and has been proposed as a therapeutic target for cancer.
In mammals, there are four hexokinase isoforms, HKI, HKII, HKIII, and HKIV. On the one hand, miRNAs weaken glycolysis of HCC by directly targeting HKII expressions. For instance, miR-885-5p, miR-143 and miR-125b have proved plays a decisive role in limiting glycolysis in HCC cells by targeting HKII, thereby inhibiting the HCC growth [23-25]. On the other hand, miRNAs elevate glycolysis of HCC by stabilizing HKII expression. For example, miR-455-3p induces cell proliferation, metastasis and glycolysis by increasing HKII expression, which is achieved by stabilizing HKII protein through proteasome. Mir-455-3-p directly targets the 3’UTR of AMP-activated protein kinase subunit beta 2 (AMPKβ2), an important role in the AMPK pathway. Suppress AMPKβ2 expression has been shown to induced p-mTOR, Snail and HKII expressions, leading to enhanced cell glycolysis [26].
Furthermore, HKI has also been found to be involved in the regulation of glycolysis. MiR-139-5p negative regulate HKI and 6-phosphofructo-2-kinase/fructose-2,6-biphosphatase 3 (PFKFB3) expression by directly targeting the ETS1, which is a transcription factor and bound to the promoters of the HKI and PFKFB3 genes [27].
MiRNAs and PFK
PFK, the second rate-limiting enzyme in glycolysis that catalyzes fructose-6-phosphate to fructose-1,6-bisphosphate [28]. Mammals have three PFK isoforms: liver (PFKL), muscle (PFKM) and platelet (PFKP). Expression of PFKP was significantly up-regulated in a variety of cancers, including brain cancer, pancreatic cancer, breast cancer and HCC [29-31].
Recently, several miRNAs have been shown to be involved in regulating glycolysis of HCC by altering the expression of PFK. For instance, miR-520 family, including miR-520a-3p, miR-520b, and miR-520e are indicated to inhibited glycolysis by target the 3’UTR of PFKP in HCC. On the contrary, inhibit the expression of miR-520a/b/e was notably potentiated the rate of glycolysis [32]. MiR-338-3p dampens glycolysis by directly interacted with PFKL. MiR-338-3p act as a tumor suppressor was down-regulated in HCC, while, the expression of miR-338-3p was increased after HCC cells treatment with 125I irradiation, which was considered as a potential strategy for HCC. The up-regulated miR-338-3p elevates the suppression of PFKL expression, thereby inhibiting glycolysis of HCC [33].
Moreover, miRNAs can regulate PFKFB to inhibit glycolysis. For instance, miR-139-5p reduced the expression of HKI and PFKFB3, thereby inhibiting aerobic glycolysis [27]. PFKFB is an allosteric activator of PFK1 and an effective stimulator of glycolysis. PFKFB3 is related to many aspects of tumorigenesis and development. And recent studies also found that PFKFB3 regulates immune response and the sensitivity of sorafenib in HCC [17,34].
MiRNAs and PK
PK is the last rate-limiting enzyme in glycolysis, which transfers phosphate group from phosphoenolpyruvate to adenosine diphosphate (ADP) to produce pyruvate ATP [35]. So it is not surprising that miRNAs can regulate glycolysis and the progression of HCC through manipulating PKM2 expression. For example, miR-199a, miR-491-5p, miR-338-3p, and miR-122 act as a tumor suppressor that induces apoptosis, growth arrest and suppresses glycolysis of HCC cells by direct binding to 3’UTR of PKM2, which is one of isozymes of pyruvate kinase PK, and is universally expressed in embryonic development, tissue repair, and tumors [36-39].
Several miRNAs act as cancer-promoting factors by promoting PKM2 activation. For example, miR-4417 could promote the phosphorylation of PKM2 and facilitates the proliferation and glycolysis of HCC cells [40]. miR-365a-3p dampens PKM2 degradation and provokes Akt/mTOR signaling pathway activation via targeting linc01554, and thereby accelerates glycolytic of HCC cells [41].
MiRNAs not only manipulates the proliferation, metastasis and invasion of HCC through regulating PKM2, but also regulates the drug sensitivity of HCC. MiR-374b reverses the sensitivity of sorafenib-resistant HCC cells to sorafenib by counteracting PKM2-mediated glycolysis pathway via binding to the 3’-UTR of hnRNPA1, which is a RNA-binding protein [42]. It provides new ideas for the treatment of sorafenib resistance.
MiRNAs and lactate dehydrogenase
Lactate dehydrogenase A (LDHA) catalyzes the last key step of glycolysis, catalyzing lactate dehydrogenation to pyruvate. Down-regulation of LDHA expression notably inhibits the proliferation, invasion and migration of HCC cells [43]. Several miRNAs act as tumor suppressors, and decrease the expression of LDHA by binding to the 3’-UTR of LDHA mRNA, thereby inhibiting aerobic glycolysis. For instance, miR-142-3p, miR-383, miR-34a and miR-100-5p inhibit aerobic glycolysis and cell proliferation of HCC by targeting the 3’-UTR of LDHA [44-47].
MiRNAs and HIF-1α
Emerging data have indicated that hypoxia of cancer cells is the initial factor for malignant transformation and even metastasis of tumors, and also one of the key factors that lead to the resistance of tumor cells to radiochemotherapy [48]. Among them, hypoxia-inducible factor-1 alpha (HIF-1α) is an important transcriptional regulator under hypoxia, which has the function of promoting tumor angiogenesis and glucose metabolism, affecting tumor cell proliferation [49]. Under the induction of HIF-1α, glycolytic-related enzyme expression increased, which further increase glycolytic activity, thus improve the imbalance between energy supply and energy consumption caused by hypoxia in tumors [50].
Several miRNAs have been demonstrated to deceased the efficacy of glycolysis by suppressing HIF-1α expression. For instance, miR-592 disrupts HIF-1α protein stabilization and inhibited glycolysis in HCC cells via targeting WSB1 mRNA [51]. Moreover, WSB1 negatively regulates JAK-STAT signaling pathway. MiR-199a-5p inhibits glycolysis by directly targets HIF-1α [52]. Interestingly, HIF-1α overexpression can in turn inhibit the abundance of miR-199a-5p under hypoxic environment. miR-3662 directly targets HIF-1α, and negatively regulates the activation of ERK and JNK signaling pathways in HCC, thereby dampened glycolysis [53]. miR-145 attenuates the expression of HIF-1α and PDK1, opposing glycolysis and suppress cell survival of HCC cells [54].
Other mechanisms
MiR-129-5p targets the mitochondrial matrix protein pyruvate dehydrogenase kinase 4 (PDK4), which diminished phosphorylation of the E1α subunit of pyruvate dehydrogenase (PDH) complex and hinders glycolysis [55].
MiR-873 promotes the Warburg effect through activating AKT/mTOR signaling pathway via targeting NDFIP1, which triggers metabolic shift and NDFIP1 was shown to suppress the PTEN/AKT signaling pathway activation [56].
MiRNAs also involved in glycolysis of HCC by regulating circadian gene expression. For example, miR-199b-5p prevents glycolysis via inhibiting NPAS2 expression, which is a circadian gene and notable boosts glycolysis through elevating the transcription of HIF-1α and downregulated the expression of PGC-1α [57]. Numerous studies have indicated that the consequences of circadian rhythm disturbances are related to many diseases, including obesity, type 2 diabetes and cancer [58]. Additionally, NPAS2 promoted glycolysis by heterodimeric with BMAL1, another core circadian rhythm factor, which regulates the expression of a variety of target genes including glycolysis in HCC cells [57].
MitomiR-181a-5p damages mitochondrial function and accelerates glycolysis in HCC by regulating the electron transport chain (ETC) [59]. MitomiRs refers to miRNAs located in the mitochondria. It is well known that mitochondria are the site of oxidative phosphorylation and adenosine triphosphate (ATP) production, and the explanation of the mechanism of oxidative phosphorylation is mainly based on the electron transport chain [60]. So it is not surprising that dampened ECT can inhibit oxidative phosphorylation and promotes glycolysis in HCC.
miR-342-3p and miR-455-5p attenuate glycolysis of HCC cells by target insulin-like growth factor-1 receptor (IGF-1R), which has been verified to activates the intracellular AKT signaling pathway, then up-regulates the expression of GLUT1 on the plasma membrane and enhances glycolysis in HCC cells [61,62].
MiRNAs involved in PPP
The PPP is also known as hexose phosphate bypass, which is a glucose catabolic pathway commonly found in animals, plants and microorganisms. In addition to providing energy, the PPP provides a variety of raw materials for anabolic metabolism, for example, NADPH and ribose-5-phosphate. Therefore, the PPP is an important multifunctional metabolic pathway [63,64].
Multiple lines of evidence indicated that miRNAs participate in PPP by altering G6PD expression. G6PD is a rate-limiting enzyme of the PPP, and its expression is significantly up-regulated in HCC. MiR-122 plays a tumor suppressive role in HCC and dampens PPP process by targeting G6PD [65].
MiRNAs involved in gluconeogenesis
Gluconeogenesis is the process by which an organism converts a variety of non-carbohydrate carbon substrates into free glucose. In mammals, the liver is the main organ of gluconeogenesis that ensures the blood sugar levels are normal. It has been reported that gluconeogenesis pathway is reduced in HCC. Additionally, Metformin was originally considered as an oral hypoglycemic agent. Recently, metformin has attracted people’s attention due to its anti-tumor therapeutic effect in inhibiting liver gluconeogenesis [22].
Gluconeogenesis and glycolysis are coordinated, glycolysis is extremely active in HCC cells, and thereby the activity of gluconeogenesis is inhibited accordingly. Gluconeogenesis appears to be the reverse reaction of glycolysis, because the seven steps of gluconeogenesis are all reverse reactions of glycolysis and are catalyzed by the same enzymes. But there are three steps in glycolysis, which are irreversible reactions, and these three steps must be bypassed during gluconeogenesis [66].
These three steps are bypassed by the following three rate-controlling enzymes: phosphoenolpyruvate carboxykinase (PEPCK), Fructose 1,6-bisphosphatase (FBP1), and glucose 6-phosphatase (G6Pase) [67]. They can not only affect the overall speed of the entire metabolic pathway but also change the direction of metabolism.
Emerging evident verified that miRNAs play critical roles in gluconeogenesis of HCC by influencing the expression of the rate-controlling enzyme, including PEPCK, FBP1 and G6PC.
MiRNAs and PEPCK
PEPCK is the first rate-controlling enzyme of gluconeogenesis, which catalyzes the irreversible reaction of phosphoenolpyruvate (PEP) and HCO3- to oxaloacetate (OAA) and inorganic phosphoric acid. It is the first and most important reaction in the process of gluconeogenesis [68]. Accumulating evidence has indicated that PCK1 overexpression could block the glycolysis process and initiate the gluconeogenesis process by potentiating PEPCK expression.
MiR-4641 attenuates the expression of PEPCK by targeting PCK1, thereby inhibiting gluconeogenesis and promoting the growth and migration of HCC cells [69]. PCK1 is the coding gene of PEPCK and is widely involved in metabolic and biological processes such as glucose metabolism, lipid metabolism, diabetes, and tumor cell proliferation and apoptosis [70].
MiRNAs and FBP1
FBP1 is the second rate-controlling enzyme of the gluconeogenesis process. It hydrolyzes fructose 1,6-diphosphate (FDP) to phosphoric acid and fructose 6-diphosphate (F6P) [67]. The expression of FBP1 was suppressed in various cancers. In addition, HCC patients with low FBP1 expression have a higher malignant classification, including tumor enlargement, poor differentiation, impaired gluconeogenesis and enhanced glycolysis [71]. Therefore, FBP1 is expected to become a reliable prognostic marker for HCC patients.
MiR-517a inhibits gluconeogenesis, promotes glycolysis of HCC via directly targeting FBP1 [72]. MiR-517a was dominantly overexpressed and FBP1 expression was significantly lower in HCC cells and tissues. Ectopic expression of FBP1 upregulates gluconeogenesis and weaken miR-517a induced cell proliferation.
MiRNAs and G6Pase
G6Pase is the last rate-controlling enzyme of the gluconeogenesis process, which catalyzes glucose 6 phosphates to glucose [67]. G6Pase expression is significantly decreased in HCC cell lines and clinical tissue, G6Pase expression also correlated with tumor grade in HCCs. Moreover, G6Pase deficiency leads to glycogen storage disease type-Ia (GSD-Ia), while HCC is a long-term complication of GSD-Ia. Restore G6Pase expression normalizes glucose homeostasis and prevents the development of HCC in the initial stage [73].
Recently, several miRNAs have been proved to reduce gluconeogenesis of HCC by suppressing the expression of G6Pase. Wang et al. showed that miR-23a inhibits gluconeogenesis and promotes HCC progression by targeting G6PC, which is encoding the key gluconeogenic enzymes G6Pase, thereby suppressing the expression of G6Pase [74]. Moreover, miR-23a inhibits gluconeogenesis via targeting peroxisome proliferator-activated receptor gamma, coactivator 1 alpha (PGC-1α), which has been demonstrated to accelerate hepatic gluconeogenesis in previous studies [75].
Other mechanisms
Jeong et al. found that miR-96 is up-regulated in mitochondrial dysfunction HCC cells and exhibit insulin resistance. Furthermore, mitochondrial dysfunction induced miR-96 overexpression, increase the level of gluconeogenesis in HCC cells via targeting the 3’UTR of Insulin receptor substrate 1 (IRS-1), which resulted in inhibition of gluconeogenesis in HCC cells [76].
It has been affirmed that mammalian cells utilize glutamine (Gln) as an alternative energy source of glucose and as an anaplerotic source for biomass generation. Glutamine-derived oxaloacetate is converted to PEP by PEPCK2, and then participates in the gluconeogenesis and other biosynthesis pathways [77]. Some miRNA can regulate gluconeogenesis by altering glutamine metabolism. For example, the level of miR-122 is reduced in HCC, and its expression is negative correlates with malignant classification. Silence miR-122 expression promoted glutaminolysis but suppressed gluconeogenesis in the mouse model. In contrast, ectopic expression of miR-122 promotes gluconeogenesis [78].
LncRNAs
LncRNAs refers to transcripts longer than 200 nucleotide units and is not involved in protein-coding [79]. LncRNAs regulations are diverse, which have been shown to regulate almost every step of gene expression. LncRNAs may serve as signals, decoys, guides or scaffolds. They also act as “sponge” or competing endogenous RNAs (ceRNAs) through the combination of their complementary miRNA response elements (MREs) and the primary miRNAs, playing a positive or negative role in the processing and expression of mature mRNAs, thereby indirectly participating in a variety of physiological process [80].
Growing researches have reported that lncRNAs play an important role in various tumors, including HCC. Although lncRNAs have been extensively studied in regulating gene expression, the role of lncRNAs in glucose metabolism is not yet clear. The related research contents are listed in Table 2.
Table 2.
LncRNAs and circRNAs involved in glucose metabolism in HCC
| Glucose metabolism | LncRNA/circRNA | Effect | Target | Mechanism | Signaling pathway | Reference |
|---|---|---|---|---|---|---|
| glycolysis | linc01554 | Decrease | - | Promote PKM2 ubiquitination | Inactive Akt/mTOR | [42] |
| lncRNA Ftx | Increase | - | Promote GLUT | active PPARγ | [85] | |
| lncRNA WFDC21P | Decrease | - | Suppress PFKP and PKM2 transcription | - | [86] | |
| linc-RoR | Decrease | miR-145 | Inhibit HIF-1α and PDK1 expression | - | [54] | |
| lncRNAIDH1-AS1 | Decrease | - | Inhibit HIF-1α expression | - | [89] | |
| lncRNA RAET1K | Increase | miR-100-5p | Increase LDHA expression | - | [47] | |
| gluconeogenesis | MALAT1 | Decrease | - | Enhance TCF7L2 translation | Active Wnt and mTOR pathway | [90] |
| glycolysis | circMAT2B | Increase | - | Promote PKM2 expression | - | [39] |
| gluconeogenesis | circC3P1 | Increase | miR-4641 | Promote PCK1 expression | - | [69] |
“-”: unknown. Abbreviations: HCC: Hepatocellular carcinoma; PKM2: Pyruvate kinase M2; HIFs: Hypoxia-inducible factors; GLUT: Glucose transporters; PPARγ: Peroxisome proliferator-activated receptor γ; PFKP: the platelet isoform of phosphofructokinase; PDK1: Pyruvate dehydrogenase kinase; LDHA: Lactate dehydrogenase A; MALAT1: Metastasis-associated lung adenocarcinoma transcript 1; TCF7L2: Transcription factor 7 like 2.
LncRNAs involved in glycolytic pathway
An increasing number of studies have verified that lncRNAs could influence the glycolysis pathway in a variety of tumors by regulating the expression and activation of glycolytic enzymes. We summarize the influence of lncRNAs on glycolytic pathway and related mechanisms, including PI3K/Akt/mTOR pathway, PPARγ, PFK/PKM2, and HIF-1α, so as to find potential and effective targeted therapies for HCC.
LncRNAs and PI3K/Akt/mTOR pathway
The PI3K/AKT/mTOR pathway is an intracellular signaling pathway that is often activated in various types of cancer cells, and its regulatory role in the process of glycolysis has been emphasized in HCC [81].
Linc01554 diminished the rate of glycolytic by accelerating PKM2 degradation and suppresses Akt/mTOR signaling pathway activation. Linc01554 is highly expressed in liver, while it is frequently down-regulated in HCC in the level of protein mRNA and DNA contrast with adjacent normal tissues. Moreover, silence linc01554 was significantly associated with aggressive clinicopathological features [41]. Furthermore, overexpression of linc01554 in combination with Akt inhibitor MK2206 exhibits a synergistic effect compared with used each alone, which shed new light on introducing Akt inhibitor to HCC treatment.
LncRNAs and PPARγ
Peroxisome proliferator-activated receptor γ (PPARγ) belongs to the family of PPARs, which plays a crucial regulatory role in cell differentiation, proliferation, metabolism and tumorigenesis [82]. Currently, PPARγ is the most extensively researched subtype. It has been demonstrated that ectopic expression of PPARγ inhibits WNT/β-catenin pathway and then downregulates PDK1, thus suppressed glycolysis [83].
LncRNA Ftx facilitates glucose consumption through promotes GLUT, including GUL1 and GUL4, and inhibits tumor necrosis factor (TNF) α and leptin expression via targeting PPARγ in HCC cells [84]. Furthermore, lncRNA Ftx potentiates glycolysis of HCC via directly targeting PPARγ, which elevating the activity and expression of glycolytic enzymes (LDH and PFKL) and decreases the activity of Krebs-cycle-associated molecules (TNFα, leptin and PDK1).
LncRNAs and PFK/PKM2
LncRNA WFDC21P diminished glycolysis via decreasing the expression and activity of PFKP and PKM2 [85]. Moreover, lncRNA WFDC21P is positively regulated by Nur77, which is a member of the orphan nuclear receptor NR4A family. Nur77 is down-regulated in HCC and shows the ability to inhibit glycolysis and promotes gluconeogenesis by stabilizing PEPCK1 [86]. Additionally, Linc01554 diminished the rate of glycolytic by accelerating PKM2 degradation [41]. LncRNA Ftx contributes to glycolysis of HCC via enhancing the activity and expression of PFKL [84].
LncRNA and HIF-1α
Recently, Takahashi et al. uncover linc-RoR knockdown significantly decreased HIF-1α expression as well as PDK1 expression, especially under hypoxia stress. Linc-RoR is a hypoxia-responsive lncRNAs, and thereby cancer cells release a large amount of linc-RoR under hypoxia context, facilitating cell survival in recipient cells by promoting glycolysis. In detail, Linc-RoR severs as a miRNA “sponge” to limit miR-145, which attenuates the expression of HIF-1α and PDK1, opposing glycolysis and suppress cell survival of HCC cells [54].
LncRNAIDH1-AS1 potentiates the activity of isocitrate dehydrogenase 1 and augments the production of α-ketoglutarate under normoxia, attenuating the expression of HIF-1α and inhibits glycolysis. Furthermore, MYC-dependent inhibition of lncRNAIDH1-AS1, induction of the Warburg effect by HIF1α [87].
Additionally, HIF1α boosting lncRNA RAET1K expression and facilitates glycolysis by binding to the promoter region of lncRNA RAET1K, and lncRNA RAET1K sponge miR-100-5p, which directly binging to LDHA and significantly inhibits glycolysis [47].
LncRNAs involved in gluconeogenesis
LncRNA metastasis-associated lung adenocarcinoma transcript 1 (MALAT1) acts as an oncogene in HCC. MALAT1 potentiated glycolytic and attenuated gluconeogenesis by enhancing the expression of Transcription factor 7 like 2 (TCF7L2) [88]. TCF7L2 is one of the earliest genes to be found and deeply studied and TCF7L2 transcription factor strongly activates the Wnt signaling pathway. And gluconeogenesis has been shown to be negatively regulated by TCF7L2 [89].
Moreover, MALAT1 activates the mTORC1 pathway by increasing phosphorylation of eIF4E binding protein (4EBP1) and enhancing the expression and function of the splicing oncoprotein SRSF1. 4EBP1 is an important downstream effector of mTORC1 pathway, and SRSF1 has been reported to activate mTOR and protein translation. Both the Wnt and mTOR signaling pathways have been suggested to play a negative regulator role in gluconeogenesis program of HCC [90].
CircRNAs
CircRNAs, a group of endogenous ncRNAs with covalently closed continuous circular structure formed by exon circularization [91]. However, circRNAs were considered to be caused by splicing errors and without function in the previous. Due to the rapid development of high-throughput sequencing, increasingly circRNAs have been discovered and proved to be involved in a variety of biological processes.
The potential functions of circRNAs include: a) served as “miRNA sponge”, inhibit the function of the target miRNAs, one circRNAs may “sponge” multiple mRNAs; b) regulate the splicing of pre-mRNA, thereby affecting protein production; c) interact with proteins; d) translated into protein or polypeptide; e) Regulate the expression of parental genes [92].
Emerging evidence indicates that changes in circRNA expression profiles play pivotal roles in the initiation and development of various cancers, including breast cancer [93], colon cancer [94], gastric cancer [95] and HCC [96]. Even though various studies have highlight miRNAs and lncRNAs partly account for glucose reprogram of HCC, it was very limited research about circRNAs was involved in metabolic regulation in HCC. Recently, Li et al. found that circRNA MAT2B promotes glycolysis and endows HCC cells with clinical aggressiveness under hypoxic [38]. Mechanistically, circMAT2B promotes glycolysis and HCC progression via increasing the abundance of the miR-338-3p, which subsequently blocking PKM2. Moreover, circRNA circC3P1 has been proved to promote the gluconeogenesis process and suppress HCC growth and metastasis through miR-4641/PCK1 pathway [69]. CircC3P1 enhancing the expression of PCK1 by sponging miR-4641 in HCC. PCK1 is the coding gene of PEPCK, which is a rate-controlling enzyme of gluconeogenesis.
Conclusion and future directions
Glucose is the main nutritional component of the animal body, and a unique source of fuel for some organizations to generate and sustain biological function. The reprogramming of glucose metabolism is one of the hallmarks of HCC. This reprogramming is caused by various factors and is closely related to the initiation, development and poor prognosis of HCC. The glucose metabolic differences between HCC and normal cells may become potential new targets. Some related drugs are already undergoing clinical trials and are expected to be used in clinical later (Table 3).
Table 3.
Glucose metabolism targets and drugs which are in preclinical and clinical development for anti-tumor therapy
| Target | Drug | Status | References |
|---|---|---|---|
| GLUTs | Phloretin | Preclinical | [99,100] |
| Fasentin | Preclinical | [101] | |
| STF-31 | Preclinical | [102] | |
| WZB117 | Preclinical | [103] | |
| Ritonavir | Phase III | [104] | |
| Silybin | Phase I | [105] | |
| HKII | 2-Deoxy-D-glucose | Phase II | [106,107] |
| Lonidamine | Phase II | [108] | |
| Genistein-27 | Preclinical | [109] | |
| Benserazide | Preclinical | [110] | |
| Resveratrol | Phase I | [111] | |
| Astragalin | Preclinical | [25] | |
| Chrysin | Preclinical | [112] | |
| PDK | Dichloroacetate | Phase I | [113-115] |
| LDHA | Oxamate | Preclinical | [116] |
| FX11 | Preclinical | [117] | |
| Quinoline-3-sulfonamide | Preclinical | [118] | |
| GNE-140 | Preclinical | [119] | |
| PSTMB | Preclinical | [120] | |
| PKM2 | Shikonin | Preclinical | [121] |
| Benserazide | Preclinical | [122] | |
| PFKFB | 3PO | Preclinical | [123] |
| PFK158 | Preclinical | [124] | |
| GAPDH | Bromopyruvate | Preclinical | [125] |
| IDH | Enasidenib | Approved | [126] |
| Ivosedinib | Approved | [127] | |
| DS-1001b | Preclinical | [128] | |
| Olutasidenib | Preclinical | [129] | |
| GSK864 | Preclinical | [130] | |
| BAY1436032 | Preclinical | [131] | |
| HMS-101 | Preclinical | [132] | |
| I-8 | Preclinical | [133] |
Abbreviations: GLUT: Glucose transporters; HK: Hexokinase; PDK: Pyruvate dehydrogenase kinase; LDHA: Lactate dehydrogenase A; PKM2: Pyruvate kinase M2; PFKFB3: 6-phosphofructo-2-kinase/fructose-2,6-biphosphatase 3; GAPGH: glyceraldehyde-3-phosphate dehydrogenase; IDH: Isocitrate dehydrogenase.
Extensive studies have demonstrated the functions of ncRNAs in glucose reprogramming. It provides us a novel perspective of tumorigenesis and potential therapeutic targets. In addition, the aberrant expression of glucose metabolism related ncRNAs may be serving as biomarkers for the diagnosis or prognosis of HCC. Moreover, some ncRNA-based therapeutics for other diseases has been used in clinical treatment. Although these ncRNAs may only serve as a fine-tuning mechanism, the synergistic effect of multiple ncRNAs may lead to specific major metabolic changes in glucose metabolism in HCC. For example, Tang et al. synthesizes an artificial lncRNA (AlncRNA) that could target multiple sorafenib-resistance-related miRNAs simultaneously, including miR-21, miR-153, miR-216a, miR-217, and miR-494, restore the sensitivity of drug-resistant HCC cells to sorafenib again [97]. It brings a bright research prospect that ncRNAs combined with the glucose-metabolism-related-enzyme inhibitors would be a better choice than utilized inhibitors alone in the battle against HCC.
However, there are still some difficulties remain to be overcome. First, researches on glucose-metabolism-related ncRNAs are still very limited, especially in lncRNAs and circRNAs, which urgently needed to be explored. Second, how to efficiently deliver ncRNA molecules to the target is the biggest problem facing in their clinical application. There are several major problems with ncRNA compounds delivery: 1) Naked single-stranded RNA molecules are easily degraded by nucleases in the physiological environment; 2) RNA molecules are immunogenic and activate the immune system; 3) ncRNAs are biological macromolecules, and they are negatively charged, making it difficult to cross the cell membrane into cells; 4) The toxic effects of ncRNAs are unknown and may overlap the toxicity of existing chemotherapy drugs. 5) Liver is the site where the drug is acting and the site where the drug is metabolized. The amount of medicine, adverse reactions, and treatment of adverse reactions in patients with HCC need attention [98]. Therefore, it is necessary to design a suitable ncRNA delivery method or delivery vector to deliver ncRNAs to the target site to fully realize its huge disease treatment potential.
After decades of searching for ncRNA-based therapeutics, some ncRNA-based therapeutics has been approved for disease treatment. The development of ncRNA-based therapeutics in the future will focus on three aspects: 1) explore more ncRNA molecules that are critical for different glucose metabolism steps. 2) develop chemical modification technology for nucleic acid therapeutics to further improve the efficiency of ncRNA-based therapeutics; 3) develop diverse delivery systems for different types of ncRNA-based therapeutics based on the size and mechanism of action of ncRNAs; 4) combining ncRNA-based therapeutics with a variety of other drugs, such as combining ncRNAs with gene-editing tools, including CRISPR/Cas9-gRNA, antibodies, small molecules, or chemotherapeutics to maximize the effect of HCC treatment; 5) design individualized ncRNA-based therapeutics according to the etiology classification of patients by using gene sequencing technology.
Taken together, ncRNA-based therapies in orchestrates glucose metabolism of HCC have promising prospects. However, the evidence for the practical clinical application of ncRNAs is still very limited and desirable for further investigation.
Acknowledgements
The work was supported by grants from the National Natural Science Foundation of China (No.81772995 and 81472266). The Excellent Youth Foundation of Jiangsu Province, China (BK20140032); Jiangsu Province’s Key Provincial Talents Program (No.ZDRCA2016090).
Consent for publication was obtained from all participants.
Disclosure of conflict of interest
None.
Abbreviations
- HCC
Hepatocellular carcinoma
- ncRNA
Noncoding RNA
- miRNA
MicroRNA
- lncRNA
Long noncoding RNA
- circRNA
Circular RNA
- TACE
Transcatheter arterial chemoembolization
- GLUT
Glucose transporters
- PPP
Pentose phosphate pathway
- ATP
Adenosine triphosphate
- HBP
Hexosamine biosynthetic pathway
- HK
Hexokinase
- PFK
6-phosphate fructokinase
- PK
Pyruvate kinase
- HIFs
Hypoxia-inducible factors
- VDAC
Voltage-dependent anion channel 1
- 3’-UTR
3’-untranslated region
- AMPKβ2
AMP-activated protein kinase subunit beta 2
- PFKFB3
6-phosphofructo-2-kinase/fructose-2,6-biphosphatase 3
- ADP
Adenosine diphosphate
- PKM2
Pyruvate kinase M2
- LDHA
Lactate dehydrogenase A
- SOCS
Suppressor of cytokine signaling
- ETC
Electron transport chain
- IGF-1R
Insulin-like growth factor-1 receptor
- PDK4
Pyruvate dehydrogenase kinase 4
- PDH
Pyruvate dehydrogenase
- PEPCK
Phosphoenolpyruvate carboxylase
- FBP1
Fructose 1,6-bisphosphatase
- G6PC
Glucose 6-phosphatase
- GSD-Ia
Glycogen storage disease type-Ia
- PGC-1α
Peroxisome proliferator-activated receptor gamma, coactivator 1 alpha
- FDP
Fructose 1,6-diphosphate
- PEP
Phosphoenolpyruvate
- OAA
Oxaloacetate
- TCA
Tricarboxylic acid
- IRS-1
Insulin receptor substrate 1
- Gln
Glutamine
- PPARγ
Peroxisome proliferator-activated receptor γ
- TNF
Tumor necrosis factor
- MALAT1
Metastasis-associated lung adenocarcinoma transcript 1
- TCF7L2
Transcription factor 7 like 2
- 4EBP1
eIF4E binding protein
- gRNA
Guide RNA
References
- 1.Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68:394–424. doi: 10.3322/caac.21492. [DOI] [PubMed] [Google Scholar]
- 2.Maluccio M, Covey A. Recent progress in understanding, diagnosing, and treating hepatocellular carcinoma. CA Cancer J Clin. 2012;62:394–399. doi: 10.3322/caac.21161. [DOI] [PubMed] [Google Scholar]
- 3.Heimbach JK, Kulik LM, Finn RS, Sirlin CB, Abecassis MM, Roberts LR, Zhu AX, Murad MH, Marrero JA. AASLD guidelines for the treatment of hepatocellular carcinoma. Hepatology. 2018;67:358–380. doi: 10.1002/hep.29086. [DOI] [PubMed] [Google Scholar]
- 4.Faubert B, Solmonson A, DeBerardinis RJ. Metabolic reprogramming and cancer progression. Science. 2020;368:w5473. doi: 10.1126/science.aaw5473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.David CJ, Chen M, Assanah M, Canoll P, Manley JL. HnRNP proteins controlled by c-Myc deregulate pyruvate kinase mRNA splicing in cancer. Nature. 2010;463:364–368. doi: 10.1038/nature08697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Huang Q, Li J, Xing J, Li W, Li H, Ke X, Zhang J, Ren T, Shang Y, Yang H, Jiang J, Chen Z. CD147 promotes reprogramming of glucose metabolism and cell proliferation in HCC cells by inhibiting the p53-dependent signaling pathway. J Hepatol. 2014;61:859–866. doi: 10.1016/j.jhep.2014.04.035. [DOI] [PubMed] [Google Scholar]
- 7.Baig MH, Adil M, Khan R, Dhadi S, Ahmad K, Rabbani G, Bashir T, Imran MA, Husain FM, Lee EJ, Kamal MA, Choi I. Enzyme targeting strategies for prevention and treatment of cancer: implications for cancer therapy. Semin Cancer Biol. 2019;56:1–11. doi: 10.1016/j.semcancer.2017.12.003. [DOI] [PubMed] [Google Scholar]
- 8.Beermann J, Piccoli M, Viereck J, Thum T. Non-coding RNAs in development and disease: background, mechanisms, and therapeutic approaches. Physiol Rev. 2016;96:1297–1325. doi: 10.1152/physrev.00041.2015. [DOI] [PubMed] [Google Scholar]
- 9.Fan C, Tang Y, Wang J, Xiong F, Guo C, Wang Y, Zhang S, Gong Z, Wei F, Yang L, He Y, Zhou M, Li X, Li G, Xiong W, Zeng Z. Role of long non-coding RNAs in glucose metabolism in cancer. Mol Cancer. 2017;16:130. doi: 10.1186/s12943-017-0699-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pasquinelli AE. MicroRNAs and their targets: recognition, regulation and an emerging reciprocal relationship. Nat Rev Genet. 2012;13:271–282. doi: 10.1038/nrg3162. [DOI] [PubMed] [Google Scholar]
- 11.Sayed D, Abdellatif M. MicroRNAs in development and disease. Physiol Rev. 2011;91:827–887. doi: 10.1152/physrev.00006.2010. [DOI] [PubMed] [Google Scholar]
- 12.Lu J, Clark AG. Impact of microRNA regulation on variation in human gene expression. Genome Res. 2012;22:1243–1254. doi: 10.1101/gr.132514.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Warburg OH. The classic: the chemical constitution of respiration ferment. Clin Orthop Relat Res. 2010;468:2833–2839. doi: 10.1007/s11999-010-1534-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Li J, Liang N, Long X, Zhao J, Yang J, Du X, Yang T, Yuan P, Huang X, Zhang J, He X, Xing J. SDHC-related deficiency of SDH complex activity promotes growth and metastasis of hepatocellular carcinoma via ROS/NFκB signaling. Cancer Lett. 2019;461:44–55. doi: 10.1016/j.canlet.2019.07.001. [DOI] [PubMed] [Google Scholar]
- 15.Peppicelli S, Bianchini F, Calorini L. Extracellular acidity, a “reappreciated” trait of tumor environment driving malignancy: perspectives in diagnosis and therapy. Cancer Metast Rev. 2014;33:823–832. doi: 10.1007/s10555-014-9506-4. [DOI] [PubMed] [Google Scholar]
- 16.Liu J, Pan C, Guo L, Wu M, Guo J, Peng S, Wu Q, Zuo Q. A new mechanism of trastuzumab resistance in gastric cancer: MACC1 promotes the Warburg effect via activation of the PI3K/AKT signaling pathway. J Hematol Oncol. 2016;9:76. doi: 10.1186/s13045-016-0302-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chen D, Ning W, Jiang Z, Peng Z, Zhu L, Zhuang S, Kuang D, Zheng L, Wu Y. Glycolytic activation of peritumoral monocytes fosters immune privilege via the PFKFB3-PD-L1 axis in human hepatocellular carcinoma. J Hepatol. 2019;71:333–343. doi: 10.1016/j.jhep.2019.04.007. [DOI] [PubMed] [Google Scholar]
- 18.Mori Y, Yamawaki K, Ishiguro T, Yoshihara K, Ueda H, Sato A, Ohata H, Yoshida Y, Minamino T, Okamoto K, Enomoto T. ALDH-dependent glycolytic activation mediates stemness and paclitaxel resistance in patient-derived spheroid models of uterine endometrial cancer. Stem Cell Rep. 2019;13:730–746. doi: 10.1016/j.stemcr.2019.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hong W, Cai P, Xu C, Cao D, Yu W, Zhao Z, Huang M, Jin J. Inhibition of glucose-6-phosphate dehydrogenase reverses cisplatin resistance in lung cancer cells via the redox system. Front Pharmacol. 2018;9:43. doi: 10.3389/fphar.2018.00043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wang H, Zhao L, Zhu L, Wang Y, Pan D, Yao J, You Q, Guo Q. Wogonin reverses hypoxia resistance of human colon cancer HCT116 cells via downregulation of HIF-1α and glycolysis, by inhibiting PI3K/Akt signaling pathway. Mol Carcinogen. 2014;53:E107–E118. doi: 10.1002/mc.22052. [DOI] [PubMed] [Google Scholar]
- 21.Li X, Cheng T, He Y, Zhou S, Wang Y, Zhang K, Yu P. High glucose regulates ERp29 in hepatocellular carcinoma by LncRNA MEG3-miRNA 483-3p pathway. Life Sci. 2019;232:116602. doi: 10.1016/j.lfs.2019.116602. [DOI] [PubMed] [Google Scholar]
- 22.DeWaal D, Nogueira V, Terry AR, Patra KC, Jeon S, Guzman G, Au J, Long CP, Antoniewicz MR, Hay N. Hexokinase-2 depletion inhibits glycolysis and induces oxidative phosphorylation in hepatocellular carcinoma and sensitizes to metformin. Nat Commun. 2018;9:446. doi: 10.1038/s41467-017-02733-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Xu F, Yan JJ, Gan Y, Chang Y, Wang HL, He XX, Zhao Q. miR-885-5p negatively regulates Warburg effect by silencing hexokinase 2 in liver cancer. Mol Ther Nucleic Acids. 2019;18:308–319. doi: 10.1016/j.omtn.2019.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fang R, Xiao T, Fang Z, Sun Y, Li F, Gao Y, Feng Y, Li L, Wang Y, Liu X, Chen H, Liu X, Ji H. MicroRNA-143 (miR-143) regulates cancer glycolysis via targeting hexokinase 2 gene. J Biol Chem. 2012;287:23227–23235. doi: 10.1074/jbc.M112.373084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Li W, Hao J, Zhang L, Cheng Z, Deng X, Shu G. Astragalin reduces hexokinase 2 through increasing mir-125b to inhibit the proliferation of hepatocellular carcinoma cellsin vitro and in vivo. J Agr Food Chem. 2017;65:5961–5972. doi: 10.1021/acs.jafc.7b02120. [DOI] [PubMed] [Google Scholar]
- 26.Lin Y, Wu M, Huang Y, Yeh C, Cheng M, Chi H, Tsai C, Chung I, Chen C, Lin K. Taurine up-regulated gene 1 functions as a master regulator to coordinate glycolysis and metastasis in hepatocellular carcinoma. Hepatology. 2018;67:188–203. doi: 10.1002/hep.29462. [DOI] [PubMed] [Google Scholar]
- 27.Hua S, Lei L, Deng L, Weng X, Liu C, Qi X, Wang S, Zhang D, Zou X, Cao C, Liu L, Wu D. miR-139-5p inhibits aerobic glycolysis, cell proliferation, migration, and invasion in hepatocellular carcinoma via a reciprocal regulatory interaction with ETS1. Oncogene. 2018;37:1624–1636. doi: 10.1038/s41388-017-0057-3. [DOI] [PubMed] [Google Scholar]
- 28.Lee J, Liu R, Li J, Zhang C, Wang Y, Cai Q, Qian X, Xia Y, Zheng Y, Piao Y, Chen Q, de Groot JF, Jiang T, Lu Z. Stabilization of phosphofructokinase 1 platelet isoform by AKT promotes tumorigenesis. Nat Commun. 2017;8:949. doi: 10.1038/s41467-017-00906-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yang Y, Ishak Gabra MB, Hanse EA, Lowman XH, Tran TQ, Li H, Milman N, Liu J, Reid MA, Locasale JW, Gil Z, Kong M. MiR-135 suppresses glycolysis and promotes pancreatic cancer cell adaptation to metabolic stress by targeting phosphofructokinase-1. Nat Commun. 2019;10:809. doi: 10.1038/s41467-019-08759-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lee J, Liu R, Li J, Wang Y, Tan L, Li X, Qian X, Zhang C, Xia Y, Xu D, Guo W, Ding Z, Du L, Zheng Y, Chen Q, Lorenzi PL, Mills GB, Jiang T, Lu Z. EGFR-phosphorylated platelet isoform of phosphofructokinase 1 promotes PI3K activation. Mol Cell. 2018;70:197–210. doi: 10.1016/j.molcel.2018.03.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Peng M, Yang D, Hou Y, Liu S, Zhao M, Qin Y, Chen R, Teng Y, Liu M. Intracellular citrate accumulation by oxidized ATM-mediated metabolism reprogramming via PFKP and CS enhances hypoxic breast cancer cell invasion and metastasis. Cell Death Dis. 2019;10:228. doi: 10.1038/s41419-019-1475-7. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 32.Park Y, Kim S, Han HD, Sohn BH, Kim JH, Liang J, Lu Y, Rodriguez-Aguayo C, Lopez-Berestein G, Mills GB, Sood AK, Lee J. Tat-activating regulatory DNA-binding protein regulates glycolysis in hepatocellular carcinoma by regulating the platelet isoform of phosphofructokinase through microRNA 520. Hepatology. 2013;58:182–191. doi: 10.1002/hep.26310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zheng J, Luo J, Zeng H, Guo L, Shao G. 125I suppressed the Warburg effect viaregulating miR-338/PFKL axis in hepatocellular carcinoma. Biomed Pharmacother. 2019;119:109402. doi: 10.1016/j.biopha.2019.109402. [DOI] [PubMed] [Google Scholar]
- 34.Li S, Dai W, Mo W, Li J, Feng J, Wu L, Liu T, Yu Q, Xu S, Wang W, Lu X, Zhang Q, Chen K, Xia Y, Lu J, Zhou Y, Fan X, Xu L, Guo C. By inhibiting PFKFB3, aspirin overcomes sorafenib resistance in hepatocellular carcinoma: inhibiting PFKFB3, ASA overcomes sorafenib resistance. Int J Cancer. 2017;141:2571–2584. doi: 10.1002/ijc.31022. [DOI] [PubMed] [Google Scholar]
- 35.Dayton TL, Jacks T, Vander HM. PKM2, cancer metabolism, and the road ahead. Embo Rep. 2016;17:1721–1730. doi: 10.15252/embr.201643300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zhang LF, Lou JT, Lu MH, Gao C, Zhao S, Li B, Liang S, Li Y, Li D, Liu MF. Suppression of miR-199a maturation by HuR is crucial for hypoxia-induced glycolytic switch in hepatocellular carcinoma. EMBO J. 2015;34:2671–2685. doi: 10.15252/embj.201591803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Xu Q, Dou C, Liu X, Yang L, Ni C, Wang J, Guo Y, Yang W, Tong X, Huang D. Oviductus ranae protein hydrolysate (ORPH) inhibits the growth, metastasis and glycolysis of HCC by targeting miR-491-5p/PKM2 axis. Biomed Pharmacother. 2018;107:1692–1704. doi: 10.1016/j.biopha.2018.07.071. [DOI] [PubMed] [Google Scholar]
- 38.Li Q, Pan X, Zhu D, Deng Z, Jiang R, Wang X. Circular RNA MAT2B promotes glycolysis and malignancy of hepatocellular carcinoma through the miR-338-3p/PKM2 axis under hypoxic stress. Hepatology. 2019;70:1298–1316. doi: 10.1002/hep.30671. [DOI] [PubMed] [Google Scholar]
- 39.Xu Q, Zhang M, Tu J, Pang L, Cai W, Liu X. MicroRNA-122 affects cell aggressiveness and apoptosis by targeting PKM2 in human hepatocellular carcinoma. Oncol Rep. 2015;34:2054–2064. doi: 10.3892/or.2015.4175. [DOI] [PubMed] [Google Scholar]
- 40.Song L, Zhang W, Chang Z, Pan Y, Zong H, Fan Q, Wang L. miR-4417 targets tripartite motif-containing 35 (TRIM35) and regulates pyruvate kinase muscle 2 (PKM2) phosphorylation to promote proliferation and suppress apoptosis in hepatocellular carcinoma cells. Med Sci Monitor. 2017;23:1741–1750. doi: 10.12659/MSM.900296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zheng Y, Li L, Jia Y, Zhang B, Li J, Zhu Y, Li M, He J, Zeng T, Ban X, Yuan Y, Li Y, Guan X. LINC01554-mediated glucose metabolism reprogramming suppresses tumorigenicity in hepatocellular carcinoma via downregulating PKM2 expression and inhibiting Akt/mTOR signaling pathway. Theranostics. 2019;9:796–810. doi: 10.7150/thno.28992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zhang M, Zhang H, Hong H, Zhang Z. MiR-374b re-sensitizes hepatocellular carcinoma cells to sorafenib therapy by antagonizing PKM2-mediated glycolysis pathway. Am J Cancer Res. 2019;9:765. [PMC free article] [PubMed] [Google Scholar]
- 43.Sheng SL, Liu JJ, Dai YH, Sun XG, Xiong XP, Huang G. Knockdown of lactate dehydrogenase A suppresses tumor growth and metastasis of human hepatocellular carcinoma. FEBS J. 2012;279:3898–3910. doi: 10.1111/j.1742-4658.2012.08748.x. [DOI] [PubMed] [Google Scholar]
- 44.Hua S, Liu C, Liu L, Wu D. miR-142-3p inhibits aerobic glycolysis and cell proliferation in hepatocellular carcinoma via targeting LDHA. Biochem Bioph Res Co. 2018;496:947–954. doi: 10.1016/j.bbrc.2018.01.112. [DOI] [PubMed] [Google Scholar]
- 45.Fang Z, He L, Jia H, Huang Q, Chen D, Zhang Z. The miR-383-LDHA axis regulates cell proliferation, invasion and glycolysis in hepatocellular cancer. Iran J Basic Med Sci. 2017;20:187–192. doi: 10.22038/ijbms.2017.8246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Zhang H, Wang Y, Han Y. MicroRNA-34a inhibits liver cancer cell growth by reprogramming glucose metabolism. Mol Med Rep. 2018;17:4483–4489. doi: 10.3892/mmr.2018.8399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Zhou Y, Huang Y, Hu K, Zhang Z, Yang J, Wang Z. HIF1A activates the transcription of lncRNA RAET1K to modulate hypoxia-induced glycolysis in hepatocellular carcinoma cells via miR-100-5p. Cell Death Dis. 2020;11:176. doi: 10.1038/s41419-020-2366-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Liang Y, Zheng T, Song R, Wang J, Yin D, Wang L, Liu H, Tian L, Fang X, Meng X, Jiang H, Liu J, Liu L. Hypoxia-mediated sorafenib resistance can be overcome by EF24 through Von Hippel-Lindau tumor suppressor-dependent HIF-1α inhibition in hepatocellular carcinoma. Hepatology. 2013;57:1847–1857. doi: 10.1002/hep.26224. [DOI] [PubMed] [Google Scholar]
- 49.Semenza GL. Targeting HIF-1 for cancer therapy. Nat Rev Cancer. 2003;3:721–732. doi: 10.1038/nrc1187. [DOI] [PubMed] [Google Scholar]
- 50.Lu H, Forbes RA, Verma A. Hypoxia-inducible factor 1 activation by aerobic glycolysis implicates the Warburg effect in carcinogenesis. J Biol Chem. 2002;277:23111–23115. doi: 10.1074/jbc.M202487200. [DOI] [PubMed] [Google Scholar]
- 51.Jia Y, Zhao J, Li B, Gao K, Song Y, Liu M, Yang X, Xue Y, Wen A, Shi L. miR-592/WSB1/HIF-1α axis inhibits glycolytic metabolism to decrease hepatocellular carcinoma growth. Oncotarget. 2016;7:35257. doi: 10.18632/oncotarget.9135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Li B, He L, Zuo D, He W, Wang Y, Zhang Y, Liu W, Yuan Y. Mutual regulation of mir-199a-5p and hif-1α modulates the warburg effect in hepatocellular carcinoma. J Cancer. 2017;8:940–949. doi: 10.7150/jca.17496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Chen Z, Zuo X, Zhang Y, Han G, Zhang L, Wu J, Wang X. MiR-3662 suppresses hepatocellular carcinoma growth through inhibition of HIF-1α-mediated Warburg effect. Cell Death Dis. 2018;9:549. doi: 10.1038/s41419-018-0616-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Takahashi K, Yan IK, Haga H, Patel T. Modulation of hypoxia-signaling pathways by extracellular linc-RoR. J Cell Sci. 2014;127:1585–1594. doi: 10.1242/jcs.141069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Han H, Li W, Shen H, Zhang J, Zhu Y, Li Y. microRNA-129-5p, a c-Myc negative target, affects hepatocellular carcinoma progression by blocking the Warburg effect. J Mol Cell Biol. 2016;8:400–410. doi: 10.1093/jmcb/mjw010. [DOI] [PubMed] [Google Scholar]
- 56.Zhang Y, Zhang C, Zhao Q, Wei W, Dong Z, Shao L, Li J, Wu W, Zhang H, Huang H, Liu F, Jin S. The miR-873/NDFIP1 axis promotes hepatocellular carcinoma growth and metastasis through the AKT/mTOR-mediated Warburg effect. Am J Cancer Res. 2019;9:927. [PMC free article] [PubMed] [Google Scholar]
- 57.Yuan P, Yang T, Mu J, Zhao J, Yang Y, Yan Z, Hou Y, Chen C, Xing J, Zhang H, Li J. Circadian clock gene NPAS2 promotes reprogramming of glucose metabolism in hepatocellular carcinoma cells. Cancer Lett. 2020;469:498–509. doi: 10.1016/j.canlet.2019.11.024. [DOI] [PubMed] [Google Scholar]
- 58.Masri S, Kinouchi K, Sassone-Corsi P. Circadian clocks, epigenetics, and cancer. Curr Opin Oncol. 2015;27:50–56. doi: 10.1097/CCO.0000000000000153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Zhuang X, Chen Y, Wu Z, Xu Q, Chen M, Shao M, Cao X, Zhou Y, Xie M, Shi Y, Zeng Y, Bu H. Mitochondrial miR-181a-5p promotes glucose metabolism reprogramming in liver cancer by regulating the electron transport chain. Carcinogenesis. 2019;41:972–983. doi: 10.1093/carcin/bgz174. [DOI] [PubMed] [Google Scholar]
- 60.Fan S, Tian T, Chen W, Lv X, Lei X, Zhang H, Sun S, Cai L, Pan G, He L, Ou Z, Lin X, Wang X, Perez MF, Tu Z, Ferrone S, Tannous BA, Li J. Mitochondrial miRNA determines chemoresistance by reprogramming metabolism and regulating mitochondrial transcription. Cancer Res. 2019;79:1069–1084. doi: 10.1158/0008-5472.CAN-18-2505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Liu W, Kang L, Han J, Wang Y, Shen C, Yan Z, Tai Y, Zhao C. miR-342-3p suppresses hepatocellular carcinoma proliferation through inhibition of IGF-1R-mediated Warburg effect. Onco Targets Ther. 2018;11:1643–1653. doi: 10.2147/OTT.S161586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Hu Y, Yang Z, Bao D, Ni J, Lou J. miR-455-5p suppresses hepatocellular carcinoma cell growth and invasion via IGF-1R/AKT/GLUT1 pathway by targeting IGF-1R. Pathol Res Pract. 2019;215:152674. doi: 10.1016/j.prp.2019.152674. [DOI] [PubMed] [Google Scholar]
- 63.Stincone A, Prigione A, Cramer T, Wamelink MM, Campbell K, Cheung E, Olin-Sandoval V, Grüning NM, Krüger A, Tauqeer Alam M, Keller MA, Breitenbach M, Brindle KM, Rabinowitz JD, Ralser M. The return of metabolism: biochemistry and physiology of the pentose phosphate pathway. Biol Rev. 2015;90:927–963. doi: 10.1111/brv.12140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Kowalik MA, Guzzo G, Morandi A, Perra A, Menegon S, Masgras I, Trevisan E, Angioni MM, Fornari F, Quagliata L, Ledda-Columbano GM, Gramantieri L, Terracciano L, Giordano S, Chiarugi P, Rasola A, Columbano A. Metabolic reprogramming identifies the most aggressive lesions at early phases of hepatic carcinogenesis. Oncotarget. 2016;7:32375. doi: 10.18632/oncotarget.8632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Barajas JM, Reyes R, Guerrero MJ, Jacob ST, Motiwala T, Ghoshal K. The role of miR-122 in the dysregulation of glucose-6-phosphate dehydrogenase (G6PD) expression in hepatocellular cancer. Sci Rep. 2018;8:9105. doi: 10.1038/s41598-018-27358-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Pilkis SJ, Granner DK. Molecular physiology of the regulation of hepatic gluconeogenesis and glycolysis. Annu Rev Physiol. 1992;54:885–909. doi: 10.1146/annurev.ph.54.030192.004321. [DOI] [PubMed] [Google Scholar]
- 67.Wang Z, Dong C. Gluconeogenesis in cancer: function and regulation of PEPCK, FBPase, and G6Pase. Trends Cancer. 2019;5:30–45. doi: 10.1016/j.trecan.2018.11.003. [DOI] [PubMed] [Google Scholar]
- 68.Vincent EE, Sergushichev A, Griss T, Gingras MC, Samborska B, Ntimbane T, Coelho PP, Blagih J, Raissi TC, Choinière L, Bridon G, Loginicheva E, Flynn BR, Thomas EC, Tavaré JM, Avizonis D, Pause A, Elder DJ, Artyomov MN, Jones RG. Mitochondrial phosphoenolpyruvate carboxykinase regulates metabolic adaptation and enables glucose-independent tumor growth. Mol Cell. 2015;60:195–207. doi: 10.1016/j.molcel.2015.08.013. [DOI] [PubMed] [Google Scholar]
- 69.Zhong L, Wang Y, Cheng Y, Wang W, Lu B, Zhu L, Ma Y. Circular RNA circC3P1 suppresses hepatocellular carcinoma growth and metastasis through miR-4641/PCK1 pathway. Biochem Biophys Res Commun. 2018;499:1044–1049. doi: 10.1016/j.bbrc.2018.03.221. [DOI] [PubMed] [Google Scholar]
- 70.Shi H, Fang R, Li Y, Li L, Zhang W, Wang H, Chen F, Zhang S, Zhang X, Ye L. The oncoprotein HBXIP suppresses gluconeogenesis through modulating PCK1 to enhance the growth of hepatoma cells. Cancer Lett. 2016;382:147–156. doi: 10.1016/j.canlet.2016.08.025. [DOI] [PubMed] [Google Scholar]
- 71.Hirata H, Sugimachi K, Komatsu H, Ueda M, Masuda T, Uchi R, Sakimura S, Nambara S, Saito T, Shinden Y, Iguchi T, Eguchi H, Ito S, Terashima K, Sakamoto K, Hirakawa M, Honda H, Mimori K. Decreased expression of fructose-1,6-bisphosphatase associates with glucose metabolism and tumor progression in hepatocellular carcinoma. Cancer Res. 2016;76:3265–3276. doi: 10.1158/0008-5472.CAN-15-2601. [DOI] [PubMed] [Google Scholar]
- 72.Zhang D, Li Z, Li T, Luo D, Feng X, Liu Y, Huang J. miR-517a promotes Warburg effect in HCC by directly targeting FBP1. Onco Targets Ther. 2018;11:8025–8032. doi: 10.2147/OTT.S172084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Lee YM, Jun HS, Pan C, Lin SR, Wilson LH, Mansfield BC, Chou JY. Prevention of hepatocellular adenoma and correction of metabolic abnormalities in murine glycogen storage disease type Ia by gene therapy. Hepatology. 2012;56:1719–1729. doi: 10.1002/hep.25717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Wang B, Hsu S, Frankel W, Ghoshal K, Jacob ST. Stat3-mediated activation of miR-23a suppresses gluconeogenesis in hepatocellular carcinoma by downregulating G6PC and PGC-1α. Hepatology. 2012;56:186–197. doi: 10.1002/hep.25632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Sharabi K, Lin H, Tavares CDJ, Dominy JE, Camporez JP, Perry RJ, Schilling R, Rines AK, Lee J, Hickey M, Bennion M, Palmer M, Nag PP, Bittker JA, Perez J, Jedrychowski MP, Ozcan U, Gygi SP, Kamenecka TM, Shulman GI, Schreiber SL, Griffin PR, Puigserver P. Selective chemical inhibition of PGC-1α gluconeogenic activity ameliorates type 2 diabetes. Cell. 2017;169:148–160. doi: 10.1016/j.cell.2017.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Jeong H, Park S, Yang W, Lee W. The induction of miR-96 by mitochondrial dysfunction causes impaired glycogen synthesis through translational repression of IRS-1 in SK-Hep1 cells. Biochem Biophys Res Commun. 2013;434:503–508. doi: 10.1016/j.bbrc.2013.03.104. [DOI] [PubMed] [Google Scholar]
- 77.Altman BJ, Stine ZE, Dang CV. From Krebs to clinic: glutamine metabolism to cancer therapy. Nat Rev Cancer. 2016;16:619–634. doi: 10.1038/nrc.2016.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Sengupta D, Cassel T, Teng KY, Aljuhani M, Chowdhary VK, Hu P, Zhang X, Fan TW, Ghoshal K. Regulation of hepatic glutamine metabolism by miR-122. Mol Metab. 2020;34:174–186. doi: 10.1016/j.molmet.2020.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Matsui M, Corey DR. Non-coding RNAs as drug targets. Nat Rev Drug Discov. 2017;16:167–179. doi: 10.1038/nrd.2016.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Engreitz JM, Ollikainen N, Guttman M. Long non-coding RNAs: spatial amplifiers that control nuclear structure and gene expression. Nat Rev Mol Cell Bio. 2016;17:756–770. doi: 10.1038/nrm.2016.126. [DOI] [PubMed] [Google Scholar]
- 81.Hu H, Juvekar A, Lyssiotis CA, Lien EC, Albeck JG, Oh D, Varma G, Hung YP, Ullas S, Lauring J, Seth P, Lundquist MR, Tolan DR, Grant AK, Needleman DJ, Asara JM, Cantley LC, Wulf GM. Phosphoinositide 3-kinase regulates glycolysis through mobilization of aldolase from the actin cytoskeleton. Cell. 2016;164:433–446. doi: 10.1016/j.cell.2015.12.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Patitucci C, Couchy G, Bagattin A, Cañeque T, de Reyniès A, Scoazec J, Rodriguez R, Pontoglio M, Zucman-Rossi J, Pende M, Panasyuk G. Hepatocyte nuclear factor 1α suppresses steatosis-associated liver cancer by inhibiting PPARγ transcription. J Clin Invest. 2017;127:1873–1888. doi: 10.1172/JCI90327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Zuo Q, He J, Zhang S, Wang H, Jin G, Jin H, Cheng Z, Tao X, Yu C, Li B, Yang C, Wang S, Lv Y, Zhao F, Yao M, Cong W, Wang C, Qin W. PGC1α suppresses metastasis of HCC by inhibiting Warburg effect via PPARγ-dependent WNT/β-catenin/PDK1 axis. Hepatology. 2020 doi: 10.1002/hep.31280. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 84.Li X, Zhao Q, Qi J, Wang W, Zhang D, Li Z, Qin C. lncRNA Ftx promotes aerobic glycolysis and tumor progression through the PPARγ pathway in hepatocellular carcinoma. Int J Oncol. 2018;53:551–566. doi: 10.3892/ijo.2018.4418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Guan Y, Huang Q, Ai Y, Chen Q, Zhao W, Wang X, Wu Q, Chen H. Nur77-activated lncRNA WFDC21P attenuates hepatocarcinogenesis via modulating glycolysis. Oncogene. 2020;39:2408–2423. doi: 10.1038/s41388-020-1158-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Kolluri SK, Zhu X, Zhou X, Lin B, Chen Y, Sun K, Tian X, Town J, Cao X, Lin F, Zhai D, Kitada S, Luciano F, O’Donnell E, Cao Y, He F, Lin J, Reed JC, Satterthwait AC, Zhang X. A short Nur77-derived peptide converts Bcl-2 from a protector to a killer. Cancer Cell. 2008;14:285–298. doi: 10.1016/j.ccr.2008.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Xiang S, Gu H, Jin L, Thorne RF, Zhang XD, Wu M. LncRNA IDH1-AS1 links the functions of c-Myc and HIF1α via IDH1 to regulate the Warburg effect. Proc Natl Acad Sci U S A. 2018;115:E1465–E1474. doi: 10.1073/pnas.1711257115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Malakar P, Stein I, Saragovi A, Winkler R, Stern-Ginossar N, Berger M, Pikarsky E, Karni R. Long noncoding RNA MALAT1 regulates cancer glucose metabolism by enhancing mTOR-mediated translation of TCF7L2. Cancer Res. 2019;79:2480–2493. doi: 10.1158/0008-5472.CAN-18-1432. [DOI] [PubMed] [Google Scholar]
- 89.Shao W, Wang D, Chiang Y, Ip W, Zhu L, Xu F, Columbus J, Belsham DD, Irwin DM, Zhang H, Wen X, Wang Q, Jin T. The Wnt signaling pathway effector TCF7L2 controls gut and brain proglucagon gene expression and glucose homeostasis. Diabetes. 2013;62:789–800. doi: 10.2337/db12-0365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Grant SF, Thorleifsson G, Reynisdottir I, Benediktsson R, Manolescu A, Sainz J, Helgason A, Stefansson H, Emilsson V, Helgadottir A, Styrkarsdottir U, Magnusson KP, Walters GB, Palsdottir E, Jonsdottir T, Gudmundsdottir T, Gylfason A, Saemundsdottir J, Wilensky RL, Reilly MP, Rader DJ, Bagger Y, Christiansen C, Gudnason V, Sigurdsson G, Thorsteinsdottir U, Gulcher JR, Kong A, Stefansson K. Variant of transcription factor 7-like 2 (TCF7L2) gene confers risk of type 2 diabetes. Nat Genet. 2006;38:320–323. doi: 10.1038/ng1732. [DOI] [PubMed] [Google Scholar]
- 91.Vo JN, Cieslik M, Zhang Y, Shukla S, Xiao L, Zhang Y, Wu YM, Dhanasekaran SM, Engelke CG, Cao X, Robinson DR, Nesvizhskii AI, Chinnaiyan AM. The landscape of circular RNA in cancer. Cell. 2019;176:869–881. doi: 10.1016/j.cell.2018.12.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Memczak S, Jens M, Elefsinioti A, Torti F, Krueger J, Rybak A, Maier L, Mackowiak SD, Gregersen LH, Munschauer M, Loewer A, Ziebold U, Landthaler M, Kocks C, le Noble F, Rajewsky N. Circular RNAs are a large class of animal RNAs with regulatory potency. Nature. 2013;495:333–338. doi: 10.1038/nature11928. [DOI] [PubMed] [Google Scholar]
- 93.Liang G, Ling Y, Mehrpour M, Saw PE, Liu Z, Tan W, Tian Z, Zhong W, Lin W, Luo Q, Lin Q, Li Q, Zhou Y, Hamai A, Codogno P, Li J, Song E, Gong C. Autophagy-associated circRNA circCDYL augments autophagy and promotes breast cancer progression. Mol Cancer. 2020;19:65. doi: 10.1186/s12943-020-01152-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Han K, Wang F, Cao C, Ling H, Chen J, Chen R, Feng Z, Luo J, Jin X, Duan J, Li S, Ma N, Yun J, Guan X, Pan Z, Lan P, Xu R, Xie D. CircLONP2 enhances colorectal carcinoma invasion and metastasis through modulating the maturation and exosomal dissemination of microRNA-17. Mol Cancer. 2020;19:60. doi: 10.1186/s12943-020-01184-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Jie M, Wu Y, Gao M, Li X, Liu C, Ouyang Q, Tang Q, Shan C, Lv Y, Zhang K, Dai Q, Chen Y, Zeng S, Li C, Wang L, He F, Hu C, Yang S. CircMRPS35 suppresses gastric cancer progression via recruiting KAT7 to govern histone modification. Mol Cancer. 2020;19:56. doi: 10.1186/s12943-020-01160-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Yu J, Xu QG, Wang ZG, Yang Y, Zhang L, Ma JZ, Sun SH, Yang F, Zhou WP. Circular RNA cSMARCA5 inhibits growth and metastasis in hepatocellular carcinoma. J Hepatol. 2018;68:1214–1227. doi: 10.1016/j.jhep.2018.01.012. [DOI] [PubMed] [Google Scholar]
- 97.Tang S, Tan G, Jiang X, Han P, Zhai B, Dong X, Qiao H, Jiang H, Sun X. An artificial lncRNA targeting multiple miRNAs overcomes sorafenib resistance in hepatocellular carcinoma cells. Oncotarget. 2016;7:73257–73269. doi: 10.18632/oncotarget.12304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Bonetta L. RNA-based therapeutics: ready for delivery? Cell. 2009;136:581–584. doi: 10.1016/j.cell.2009.02.010. [DOI] [PubMed] [Google Scholar]
- 99.Wu K, Ho C, Chen Z, Chen L, Whang-Peng J, Lin T, Ho Y. The apple polyphenol phloretin inhibits breast cancer cell migration and proliferation via inhibition of signals by type 2 glucose transporter. J Food Drug Anal. 2018;26:221–231. doi: 10.1016/j.jfda.2017.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Wu C, Ho Y, Tsai C, Wang Y, Tseng H, Wei P, Lee C, Liu R, Lin S. In vitro and in vivo study of phloretin-induced apoptosis in human liver cancer cells involving inhibition of type II glucose transporter. Int J Cancer. 2009;124:2210–2219. doi: 10.1002/ijc.24189. [DOI] [PubMed] [Google Scholar]
- 101.Wood TE, Dalili S, Simpson CD, Hurren R, Mao X, Saiz FS, Gronda M, Eberhard Y, Minden MD, Bilan PJ, Klip A, Batey RA, Schimmer AD. A novel inhibitor of glucose uptake sensitizes cells to FAS-induced cell death. Mol Cancer Ther. 2008;7:3546–3555. doi: 10.1158/1535-7163.MCT-08-0569. [DOI] [PubMed] [Google Scholar]
- 102.Chan DA, Sutphin PD, Nguyen P, Turcotte S, Lai EW, Banh A, Reynolds GE, Chi JT, Wu J, Solow-Cordero DE, Bonnet M, Flanagan JU, Bouley DM, Graves EE, Denny WA, Hay MP, Giaccia AJ. Targeting GLUT1 and the Warburg effect in renal cell carcinoma by chemical synthetic lethality. Sci Transl Med. 2011;3:94ra70. doi: 10.1126/scitranslmed.3002394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Ojelabi OA, Lloyd KP, Simon AH, De Zutter JK, Carruthers A. WZB117 (2-Fluoro-6-(m-hydroxybenzoyloxy) Phenylm-Hydroxybenzoate) Inhibits GLUT1-mediated sugar transport by binding reversibly at the exofacial sugar binding site. J Biol Chem. 2016;291:26762–26772. doi: 10.1074/jbc.M116.759175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Poordad F, Castro RE, Asatryan A, Aguilar H, Cacoub P, Dieterich D, Marinho RT, Carvalho A, Siddique A, Hu YB, Charafeddine M, Bondin M, Khan N, Cohen DE, Felizarta F. Long-term safety and efficacy results in hepatitis C virus genotype 1-infected patients receiving ombitasvir/paritaprevir/ritonavir + dasabuvir ± ribavirin in the TOPAZ-I and TOPAZ-II trials. J Viral Hepatitis. 2020;27:497–504. doi: 10.1111/jvh.13261. [DOI] [PubMed] [Google Scholar]
- 105.Siegel AB, Narayan R, Rodriguez R, Goyal A, Jacobson JS, Kelly K, Ladas E, Lunghofer PJ, Hansen RJ, Gustafson DL, Flaig TW, Tsai WY, Wu DP, Lee V, Greenlee H. A phase I dose-finding study of silybin phosphatidylcholine (Milk Thistle) in patients with advanced hepatocellular carcinoma. Integr Cancer Ther. 2014;13:46–53. doi: 10.1177/1534735413490798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Raez LE, Papadopoulos K, Ricart AD, Chiorean EG, DiPaola RS, Stein MN, Rocha Lima CM, Schlesselman JJ, Tolba K, Langmuir VK, Kroll S, Jung DT, Kurtoglu M, Rosenblatt J, Lampidis TJ. A phase I dose-escalation trial of 2-deoxy-d-glucose alone or combined with docetaxel in patients with advanced solid tumors. Cancer Chemoth Pharm. 2013;71:523–530. doi: 10.1007/s00280-012-2045-1. [DOI] [PubMed] [Google Scholar]
- 107.Mohanti BK, Rath GK, Anantha N, Kannan V, Das BS, Chandramouli BA, Banerjee AK, Das S, Jena A, Ravichandran R, Sahi UP, Kumar R, Kapoor N, Kalia VK, Dwarakanath BS, Jain V. Improving cancer radiotherapy with 2-deoxy-d-glucose: phase I/II clinical trials on human cerebral gliomas. Int J Radiat Oncol Biol Phys. 1996;35:103–111. doi: 10.1016/s0360-3016(96)85017-6. [DOI] [PubMed] [Google Scholar]
- 108.Berruti A, Bitossi R, Gorzegno G, Bottini A, Alquati P, De Matteis A, Nuzzo F, Giardina G, Danese S, De Lena M, Lorusso V, Farris A, Sarobba MG, DeFabiani E, Bonazzi G, Castiglione F, Bumma C, Moro G, Bruzzi P, Dogliotti L. Time to progression in metastatic breast cancer patients treated with epirubicin is not improved by the addition of either cisplatin or lonidamine: final results of a phase III study with a factorial design. J. Clin. Oncol. 2002;20:4150–4159. doi: 10.1200/JCO.2002.08.012. [DOI] [PubMed] [Google Scholar]
- 109.Tao L, Wei L, Liu Y, Ding Y, Liu X, Zhang X, Wang X, Yao Y, Lu J, Wang Q, Hu R. Gen-27, a newly synthesized flavonoid, inhibits glycolysis and induces cell apoptosis via suppression of hexokinase II in human breast cancer cells. Biochem Pharmacol. 2017;125:12–25. doi: 10.1016/j.bcp.2016.11.001. [DOI] [PubMed] [Google Scholar]
- 110.Li W, Zheng M, Wu S, Gao S, Yang M, Li Z, Min Q, Sun W, Chen L, Xiang G, Li H. Benserazide, a dopadecarboxylase inhibitor, suppresses tumor growth by targeting hexokinase 2. J Exp Clin Canc Res. 2017;36:58. doi: 10.1186/s13046-017-0530-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Dai W, Wang F, Lu J, Xia Y, He L, Chen K, Li J, Li S, Liu T, Zheng Y, Wang J, Lu W, Zhou Y, Yin Q, Abudumijiti H, Chen R, Zhang R, Zhou L, Zhou Z, Zhu R, Yang J, Wang C, Zhang H, Zhou Y, Xu L, Guo C. By reducing hexokinase 2, resveratrol induces apoptosis in HCC cells addicted to aerobic glycolysis and inhibits tumor growth in mice. Oncotarget. 2015;6:13703–13717. doi: 10.18632/oncotarget.3800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Xu D, Jin J, Yu H, Zhao Z, Ma D, Zhang C, Jiang H. Chrysin inhibited tumor glycolysis and induced apoptosis in hepatocellular carcinoma by targeting hexokinase-2. J Exp Clin Canc Res. 2017;36:44. doi: 10.1186/s13046-017-0514-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Xuan Y, Hur H, Ham I, Yun J, Lee J, Shim W, Kim YB, Lee G, Han S, Cho YK. Dichloroacetate attenuates hypoxia-induced resistance to 5-fluorouracil in gastric cancer through the regulation of glucose metabolism. Exp Cell Res. 2014;321:219–230. doi: 10.1016/j.yexcr.2013.12.009. [DOI] [PubMed] [Google Scholar]
- 114.Dunbar EM, Coats BS, Shroads AL, Langaee T, Lew A, Forder JR, Shuster JJ, Wagner DA, Stacpoole PW. Phase 1 trial of dichloroacetate (DCA) in adults with recurrent malignant brain tumors. Invest New Drugs. 2014;32:452–464. doi: 10.1007/s10637-013-0047-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Chu QS, Sangha R, Spratlin J, Vos LJ, Mackey JR, McEwan AJ, Venner P, Michelakis ED. A phase I open-labeled, single-arm, dose-escalation, study of dichloroacetate (DCA) in patients with advanced solid tumors. Invest New Drugs. 2015;33:603–610. doi: 10.1007/s10637-015-0221-y. [DOI] [PubMed] [Google Scholar]
- 116.Zhou M, Zhao Y, Ding Y, Liu H, Liu Z, Fodstad O, Riker AI, Kamarajugadda S, Lu J, Owen LB, Ledoux SP, Tan M. Warburg effect in chemosensitivity: targeting lactate dehydrogenase-A re-sensitizes taxol-resistant cancer cells to taxol. Mol Cancer. 2010;9:33. doi: 10.1186/1476-4598-9-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Le A, Cooper CR, Gouw AM, Dinavahi R, Maitra A, Deck LM, Royer RE, Vander Jagt DL, Semenza GL, Dang CV. Inhibition of lactate dehydrogenase A induces oxidative stress and inhibits tumor progression. Proc Natl Acad Sci U S A. 2010;107:2037–2042. doi: 10.1073/pnas.0914433107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Billiard J, Dennison JB, Briand J, Annan RS, Chai D, Colón M, Dodson CS, Gilbert SA, Greshock J, Jing J, Lu H, McSurdy-Freed JE, Orband-Miller LA, Mills GB, Quinn CJ, Schneck JL, Scott GF, Shaw AN, Waitt GM, Wooster RF, Duffy KJ. Quinoline 3-sulfonamides inhibit lactate dehydrogenase A and reverse aerobic glycolysis in cancer cells. Cancer Metab. 2013;1:19. doi: 10.1186/2049-3002-1-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Purkey HE, Robarge K, Chen J, Chen Z, Corson LB, Ding CZ, DiPasquale AG, Dragovich PS, Eigenbrot C, Evangelista M, Fauber BP, Gao Z, Ge H, Hitz A, Ho Q, Labadie SS, Lai KW, Liu W, Liu Y, Li C, Ma S, Malek S, O Brien T, Pang J, Peterson D, Salphati L, Sideris S, Ultsch M, Wei B, Yen I, Yue Q, Zhang H, Zhou A. Cell active hydroxylactam inhibitors of human lactate dehydrogenase with oral bioavailability in mice. Acs Med Chem Lett. 2016;7:896–901. doi: 10.1021/acsmedchemlett.6b00190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Kim E, Chung T, Han CW, Park SY, Park KH, Jang SB, Ha K. A novel lactate dehydrogenase inhibitor, 1-(phenylseleno)-4-(trifluoromethyl) benzene, suppresses tumor growth through apoptotic cell death. Sci Rep. 2019;9:3969. doi: 10.1038/s41598-019-40617-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Chen J, Xie J, Jiang Z, Wang B, Wang Y, Hu X. Shikonin and its analogs inhibit cancer cell glycolysis by targeting tumor pyruvate kinase-M2. Oncogene. 2011;30:4297–4306. doi: 10.1038/onc.2011.137. [DOI] [PubMed] [Google Scholar]
- 122.Zhou Y, Huang Z, Su J, Li J, Zhao S, Wu L, Zhang J, He Y, Zhang G, Tao J, Zhou J, Chen X, Peng C. Benserazide is a novel inhibitor targeting PKM2 for melanoma treatment. Int J Cancer. 2020;147:139–151. doi: 10.1002/ijc.32756. [DOI] [PubMed] [Google Scholar]
- 123.Clem B, Telang S, Clem A, Yalcin A, Meier J, Simmons A, Rasku MA, Arumugam S, Dean WL, Eaton J, Lane A, Trent JO, Chesney J. Small-molecule inhibition of 6-phosphofructo-2-kinase activity suppresses glycolytic flux and tumor growth. Mol Cancer Ther. 2008;7:110–120. doi: 10.1158/1535-7163.MCT-07-0482. [DOI] [PubMed] [Google Scholar]
- 124.Mondal S, Roy D, Sarkar Bhattacharya S, Jin L, Jung D, Zhang S, Kalogera E, Staub J, Wang Y, Xuyang W, Khurana A, Chien J, Telang S, Chesney J, Tapolsky G, Petras D, Shridhar V. Therapeutic targeting of PFKFB3 with a novel glycolytic inhibitor PFK158 promotes lipophagy and chemosensitivity in gynecologic cancers. Int J Cancer. 2019;144:178–189. doi: 10.1002/ijc.31868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Sun X, Sun G, Huang Y, Hao Y, Tang X, Zhang N, Zhao L, Zhong R, Peng Y. 3-Bromopyruvate regulates the status of glycolysis and BCNU sensitivity in human hepatocellular carcinoma cells. Biochem Pharmacol. 2020;177:113988. doi: 10.1016/j.bcp.2020.113988. [DOI] [PubMed] [Google Scholar]
- 126.Kim ES. Enasidenib: first global approval. Drugs. 2017;77:1705–1711. doi: 10.1007/s40265-017-0813-2. [DOI] [PubMed] [Google Scholar]
- 127.Dhillon S. Ivosidenib: first global approval. Drugs. 2018;78:1509–1516. doi: 10.1007/s40265-018-0978-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Nakagawa M, Nakatani F, Matsunaga H, Seki T, Endo M, Ogawara Y, Machida Y, Katsumoto T, Yamagata K, Hattori A, Fujita S, Aikawa Y, Ishikawa T, Soga T, Kawai A, Chuman H, Yokoyama N, Fukushima S, Yahiro K, Kimura A, Shimada E, Hirose T, Fujiwara T, Setsu N, Matsumoto Y, Iwamoto Y, Nakashima Y, Kitabayashi I. Selective inhibition of mutant IDH1 by DS-1001b ameliorates aberrant histone modifications and impairs tumor activity in chondrosarcoma. Oncogene. 2019;38:6835–6849. doi: 10.1038/s41388-019-0929-9. [DOI] [PubMed] [Google Scholar]
- 129.Caravella JA, Lin J, Diebold RB, Campbell AM, Ericsson A, Gustafson G, Wang Z, Castro J, Clarke A, Gotur D, Josephine HR, Katz M, Kershaw M, Yao L, Toms AV, Barr KJ, Dinsmore CJ, Walker D, Ashwell S, Lu W. Structure-based design and identification of FT-2102 (Olutasidenib), a potent mutant-selective IDH1 inhibitor. J Med Chem. 2020;63:1612–1623. doi: 10.1021/acs.jmedchem.9b01423. [DOI] [PubMed] [Google Scholar]
- 130.Mao MJ, Leonardi DE. Vascular-endothelial response to IDH1 mutant fibrosarcoma secretome and metabolite: implications on cancer microenvironment. Am J Cancer Res. 2019;9:122–133. [PMC free article] [PubMed] [Google Scholar]
- 131.Chaturvedi A, Gupta C, Gabdoulline R, Borchert NM, Goparaju R, Kaulfuss S, Görlich K, Schottmann R, Othman B, Welzenbach J, Panknin O, Wagner M, Geffers R, Ganser A, Thol F, Jeffers M, Haegebarth A, Heuser M. Synergistic activity of IDH1 inhibitor BAY1436032 with azacitidine in IDH1 mutant acute myeloid leukemia. Haematologica. 2020 doi: 10.3324/haematol.2019.236992. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Chaturvedi A, Goparaju R, Gupta C, Weder J, Klünemann T, Araujo Cruz MM, Kloos A, Goerlich K, Schottmann R, Othman B, Struys EA, Bähre H, Grote-Koska D, Brand K, Ganser A, Preller M, Heuser M. In vivo efficacy of mutant IDH1 inhibitor HMS-101 and structural resolution of distinct binding site. Leukemia. 2020;34:416–426. doi: 10.1038/s41375-019-0582-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Jia P, Wu Y, Du H, Yang L, Zhang Z, Ma T, Li S, Yuan S, Lu L, Zha X. I-8, a novel inhibitor of mutant IDH1, inhibits cancer progression in vitro and in vivo. Eur J Pharm Sci. 2019;140:105072. doi: 10.1016/j.ejps.2019.105072. [DOI] [PubMed] [Google Scholar]


