Abstract
Bisphenol A (BPA), an endocrine and metabolic disruptor, is widely used to manufacture polycarbonate plastics and epoxy resins. Accumulating evidence suggests that paternal BPA exposure adversely affects male germlines and results in atypical reproductive phenotypes that might persist for generations to come. Our study investigated this exposure on testicular architecture and sperm quality in mouse offspring, and characterised underlying molecular mechanism(s). A total of 18 immature male Swiss albino mice (3.5 weeks old) were randomly divided into three groups and treated as follows: Group I, no treatment (sham control); Group II, sterile corn oil only (vehicle control); Group III, BPA (400 μg/kg) in sterile corn oil. At 9.5 weeks old, F0 males were mated with unexposed females. F0 offspring (F1 generation) were monitored for postnatal development for 10 weeks. At 11.5 weeks old, the animals were sacrificed to examine testicular architecture, sperm parameters, including DNA integrity, and oxidative stress biomarkers. Results showed that BPA significantly induced changes in the body and testis weights of the F0 and F1 generation BPA lineages compared to F0 and F1 generation control lineages. A decrease in sperm count and motility with further, increased sperm abnormalities, no or few sperm DNA alterations and elevated levels of MDA, PC and NO were recorded. Similar effects were found in BPA exposed F0 males, but were more pronounced in the F0 offspring. In addition, BPA caused alterations in the testicular architecture. These pathological changes extended transgenerationally to F1 generation males’ mice, but the pathological changes were more pronounced in the F1 generation. Our findings demonstrate that the biological and health BPA impacts do not end in paternal adults, but are passed on to offspring generations. Hence, linking observed testis and sperm abnormalities in the F1 generation to BPA exposure of their parental line was evident in this work. The findings also illustrate that oxidative stress appears to be a molecular component of the testis and sperm pathologies.
Keywords: BPA, Endocrine disruptors, Multigenerational, Transgenerational, Testis architecture, Sperm quality, Oxidative stress
1. Introduction
Growing evidence suggests that exposure to a variety of environmental insults, such as stress, nutritional abnormalities, and environmental toxicants adversely affect human health (Nilsson et al., 2019, Rahman et al., 2020, Waterland, 2009). The multigenerational (F0 and F1) and transgenerational (F2 and F3) transmission of environmentally induced phenotypic variations from parents to offspring is increasingly substantiated (Skinner et al., 2010). Such phenotypic abnormalities can involve multigenerational inheritance, where subsequent generations are affected by exposure that occurs only during the first generation (Nilsson et al., 2019, Nilsson and Skinner, 2015, Skinner et al., 2013). Transgenerational inheritance of abnormalities/diseases is described in rats (Manikkam et al., 2012), plants (Henderson and Jacobsen, 2007), nematode worms (Greer et al., 2011), flies (Ruden and Lu, 2008), mice (Guerrero-Bosagna et al., 2012) and humans (Pembrey, 2010). An animal study have established that exposure to environmental toxicants during postnatal life, intrauterine life and early-life; contribute to phenotypic variations and disease susceptibility in later life (Skinner et al., 2013). Phenotypic abnormalities are implicated in abnormalities in immune and reproductive systems (Anway et al., 2006), behavioural and brain abnormalities (Crews et al., 2007), prostate diseases, as well as alterations in kidney (Anway et al., 2006), ovary (Manikkam et al., 2012, Nilsson et al., 2012), testis (Al-Griw et al., 2015, Salian et al., 2009), and liver (Al-Griw et al., 2017). These specific transgenerational alterations may be important biomarkers for paternal/ancestral environmental exposures and abnormalities (Manikkam et al., 2012, Skinner et al., 2013).
The ubiquitous presence of environmental toxicants poses a significant risk to humans and animals (Rattan and Flaws, 2019). The exposure to bisphenol A (BPA) via the contaminated food or water, causes several negative health effects (Björnsdotter et al., 2017, Thoene et al., 2020). BPA, a famous endocrine disruptor, plays important roles in many pathological conditions such as nervous and reproductive system dysfunction, metabolic disorders, obesity, breast cancer, and oxidative stress (Beronius et al., 2010, Birnbaum et al., 2012, Vandenberg et al., 2013). The BPA exposure during development process may negatively alter hormone levels and produce abnormalities within embryonic cells and tissues. Adulthood exposure to BPA can also predispose developing reproductive tissue to diseases/abnormalities that are subsequently being passed down to subsequent generations (Skinner et al., 2013).
Multigenerational and transgenerational actions of the environmental toxicants are extensively studied (Manikkam et al., 2012, Skinner et al., 2013). However, a paucity of data exists for the heritability of BPA effects and its ability to induce testicular architecture abnormalities and reduced sperm quality. Mechanisms of action that underlie the transmission of BPA caused abnormalities to future generations is still unclear, and it is possibly that BPA could act be either genetic or epigenetic alterations or both. The aim of the current study was to explore multigenerational effects of BPA on testicular architecture, sperm quality, and to elucidate the underlying molecular mechanism of testis and sperm pathologies.
2. Materials and methods
2.1. Animals and housing conditions
Ethical approval for animal work was obtained from the Research Ethics Committee at the Biotechnology Research Center (Reference BEC-BTRC 10-2019). A total of 18 male Swiss Albino mice, aged 3.5 weeks and weighing 14.23 ± 1.92 g, were used in this study. Animals were bred and housed under standard conditions of a 12-hour light/dark cycle and temperature (26 ± 2 °C) in the animal facility at the Faculty of Sciences, University of Tripoli. All animals had access to food and water ad libitum.
2.2. Experimental design
The animals were randomly divided into three groups and treated as follows: Group I, no treatment (sham control); Group II, sterile corn oil only (vehicle control); Group III, BPA (BPA dose: 400 μg/ kg, Sigma-Aldrich, Germany) in sterile corn oil. The animals were dosed by intraperitoneal (i.p.) injection twice per week for 6 weeks. BPA dose was selected based on prior studies on BPA adverse effects (Khodayar et al., 2020, Sadowski et al., 2014, Tyl et al., 2008). Treatment lineages were designated ‘control’ and BPA lineage ‘populations’.
At 9.5 weeks old, F0 males were allowed to mate with control (unexposed) females, confirmed for their fertility. Presence of vaginal plugs indicated mating. The day the vaginal plug was noticed was considered the first day of gestation (GD-1). Weights of female mice were measured twice a week during the mating phase. Once impregnated, females were separated and caged individually. Moreover, to confirm pregnancy, F0 dams were monitored and body weights measured daily. Dams were left to deliver naturally, and the day of delivery was considered to be postnatal day 0 (PND-0). After a 14-day mating period, unmated males, designated as infertile, were housed singly. On postnatal day 1 (PND1) weights, sizes, sex ratios and stillbirths of F1 pups were recorded. Per cent of live dead F1pups were calculated on PND-1. F0 offspring (F1 generation) were monitored for postnatal development for 10 weeks.
The male mice treated with BPA were referred to as the F0 generation and their progeny as the F1 generation. F1 offspring were not directly exposed to BPA. Inbreeding artefacts were avoided by preventing cousin or sibling breeding. At 11.5 weeks old, 6 males from each experimental group of F0 and F1 generations were sacrificed for biochemical and histological examination.
2.3. Clinical assessment
Abnormal clinical and behavioural signs were monitored for effects related to F0 male exposure. Survival rate over the course of the study was recorded. Deaths that occurred overnight were documented the following next morning. An independent observer established the cause of death to exclude non-exposure related mortality.
2.4. Body and testis weights
Percent change in the body weights at the beginning and end of the experiment was documented. The testes were dissected, and % relative weight was determined.
2.5. Sample collection
F0 and F1 mice were sacrificed by cervical dislocation, and the target organ was rapidly resected. For sperm analysis, the caudal end of the epididymis was collected. One testis was washed thoroughly with ‘washing buffer’ and homogenised. The homogenate was used for the measurement of oxidative stress indices and other parameters. A portion of tissues was immediately placed in 10% formalin solution and stored until analysed for histological changes. Spermatozoa from control, F0, F1 and BPA groups were obtained by mincing epididymis in normal saline.
2.6. Sperm DNA fragmentation test
Sperm DNA fragmentation was examined as previously described (Kim et al., 2013). Briefly, each raw semen sample (10 μL) was smeared on a slide and air-dried. Slides were fixed by immersion in 1:1 methanol: acetone for 5 min. Following hydrolysis in 0.1% HCL for 2 min, the slides were stained with toluidine blue (Sigma-Aldrich, Germany) for 10 min. Slides were then rinsed in phosphate-buffered saline (PBS) for 1 min. Finally, slides were rinsed in water, air-dried and visualised under a light microscope. In each slide, 200 spermatozoa were randomly counted from different areas at ×1.000 magnification. Immature sperm stained dark blue after eosin counterstaining. Abnormal chromatin condensation in sperm was determined as the ratio of the number of dark blue stained sperm to the total number of sperm analysed.
2.7. Measurement of sperm count and sperm head morphology
The motility and numbers of epididymal sperm were assessed as previously described (Al-Griw et al., 2015, Lane et al., 1982). Briefly, epididymis was placed in a Petri-plate containing 1 ml of normal saline and cut into small pieces. Sperm released into solution were collected by removing cell debris by centrifugation at 1000 rpm for 3 min. Supernatants were used for sperm counting and its quality.
One ml of supernatant was diluted 1:1 and loaded into a counting chamber (Neubauer's haemocytometer, Germany). Sperm present in five squares of the chamber were counted using a 40× objective lens, and the total count was multiplied by 106 to arrive at sperm count per millilitre (sperm/ml). Sperm head morphology was examined by mixing 0.5 ml of the above supernatant and 0.5 ml 2% eosin Y solution and incubated for 60 min. Two to three drops of the mixture were used to prepare a smear. The smear was air-dried and fixed by immersing the slide in absolute methanol for 3 min. Morphological alterations were evaluated under oil immersion light microscope by counting 200 sperm per animal. Data are shown as % abnormal sperm.
2.8. Lipid peroxidation assay
Malondialdehyde (MDA), an index of lipid oxidative stress, was quantified as thiobarbituric acid reactive substrate as previously described (Al-Griw et al., 2016, Alghazeer et al., 2018, Zhang et al., 2004). Briefly, testicular tissues were disrupted with a tissue homogeniser (IKA, RW 20.n, Germany) in 10% (w/v) PBS. Homogenate was centrifuged, and 500 µL of clear supernatant was added to 2 ml of reagent containing thiobarbituric acid 0.37%, 0.24 N HCL and 15% TCA, heated to 100 °C for 15 min, and left to cool. The mixture was centrifuged at 3000 rpm for 10 min, and the absorbance of the supernatant was read at 532 nm. A standard curve was prepared using concentrations of 1, 1, 3, 3-tetramethoxypropane, and the concentration of TBA-MDA adducts in tissues was inferred from the standard curve. MDA level was expressed as nmol/ml.
2.9. Total protein isolation and measurement
Total testicular proteins were measured as previously described (Bradford, 1976, Goa, 1953). In brief, testes from control and BPA treated mice were homogenised in sodium phosphate buffer (pH 7.4) and the homogenate centrifuged at 6000g, for 15 min. The supernatant was separated, and the protein content was estimated using bovine serum albumin as a standard.
2.10. Protein peroxidation assay
Carbonyl concentration in testes was quantified with a protein carbonyl assay as previously described (Wang et al., 2013).
2.11. Nitric oxide (NO) measurement
Testicular NO levels were measured as described previously (Xu et al., 2011) with some modifications. Testis were homogenised in 0.9% saline, and a clear supernatant of the homogenate was obtained after centrifugation at 10,000 rpm for 5 min at 4 °C. One millilitre of the supernatant was mixed with an equal volume of Greiss reagent (0.1% naphthylethylenediamine, 1% sulphanilamide in 5% phosphoric acid). The mixture was left for 30 min at room temperature, and absorbance was measured at 546 nm. NO content was inferred from standard curve prepared using a sodium nitrate solution.
2.12. Histopathological studies and microscopy
For histological analysis, testicular tissues were processed as detailed elsewhere (Al-Griw et al., 2015, Ginsberg et al., 1981). Formalin-fixed testis were dehydrated in a series of graded ethanol solutions and embedded in paraffin. Thin paraffin sections (5 µm) were cut, deparaffinised, hydrated, and stained with H&E. Slides were examined under a light microscopy (Leica, Germany).
Histological analysis was carried out according to the scoring method detailed elsewhere (Almeida et al., 2006, Glander et al., 2000). On the scoring scale from 0 to 10, where 10 indicates the full spermatogenesis; 9, disorganised tubular epithelium; 8, few late spermatids; 7, no late spermatids; 6, few early spermatids; 5, no spermatids; 4, few spermatocytes; 3, spermatogonia only; 2, no germ cells, Sertoli cells only; and 1, no seminiferous epithelial cells. Eight scores were averaged for tissue, and this average was considered as one replicate. Likewise, eight scores were averaged by testis section, and this average was considered as one replicate. Structural changes in testicular tissue from each animal were evaluated blind by a histopathologist. Tissue architecture of testes was examined and documented by light microscope using low- and high-power objectives (Leica, Germany).
2.13. Statistics
Results were analysed using SPSS (version 20.0 Statistical Package for Social Science SPSS Inc., Chicago, IL, USA). Normal distribution was assessed using skewness and kurtosis detection tests. Homogeneity of variances was examined by Livene’s Test. Statistical differences between studied groups were determined by Student’s t-test. Data were expressed as means ± SEM. A p-value of 0.05 was considered statistically significant.
3. Results
3.1. Paternal exposure to BPA-induced abnormalities in F0 male mice reproductive system across generations
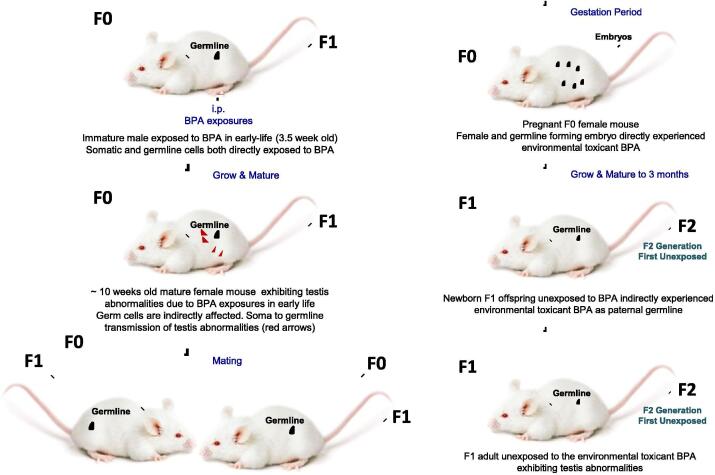
BPA exposure promoted adult onset of testis abnormalities in F0 male mice (Fig. 1). Testis abnormalities increased testicular oxidative injury and damage to sperm parameters in F0 generation were observed. However, testis and sperm abnormalities were more pronounced in the F1 generation. Transgenerational inheritance suggests that toxic effects of the environmental toxicant BPA were manifest in exposed F0 and F1 generations.
Fig. 1.
Schematic diagram of the treatment procedure. Paternal (F0 generation) exposure to BPA induces testis abnormalities in the F1 generation, implying intergenerational transfer of testis abnormalities. Offspring of F0 (F1 generation) were not exposed directly to BPA. F0 male somatic cells (e.g. testis) and germ cells (e.g. sperms) were directly exposed to BPA. Parental BPA exposure caused abnormalities in the germlines (F1 generation).
3.2. F0 paternal BPA exposure did not affect animal survival
Survival/mortality rates of controls and BPA lineage animals were compared. During the course of the study, no mortality among mice any groups and no signs of acute toxicity were observed. No significant differences were found between BPA and control lineages.
3.3. The effect of F0 paternal exposure to BPA on body and testis weights
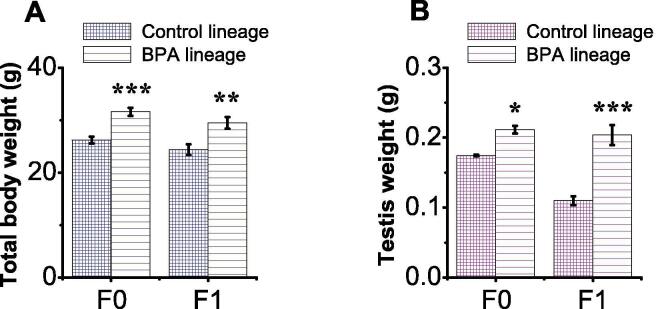
Total body weight (TBW) of each animal was monitored from birth until the end of the study. Testis weight was recorded only at the time of necropsy (Fig. 2). Mean of TBW was significantly increased in the BPA lineage populations compared to sham control (Fig. 2), but sham and vehicle control groups showed no significant difference. Specifically, the F0 generation of BPA treated mice showed a significant (p = 0.0002) difference in the mean TBW compared to respective control (Fig. 2A). Similar effects were observed in F1 mice group (p = 0.009; Fig. 2A). Also, a marked difference was found in the testis weight of the F0 generation BPA lineages compared to respective control (p = 0.0268; Fig. 2B). Similar effects were observed in the F1 generation of BPA-treated mice (p < 0.001; Fig. 2B).
Fig. 2.
BPA exposure increased body and testis weights of F0 and F1 generation BPA lineages. F0 male mice were exposed to the same conditions as sham, vehicle or BPA. (A) Quantification of the TBW. (B) Quantification of the testis weight. Data are presented as mean ± SEM for at least 6 independent biological replicates. (*) indicates p < 0.05, (**) indicates p < 0.001, and (***) indicates p < 0.001.
3.4. F0 paternal BPA exposure did not affect F1 sperm DNA integrity
The percent of abnormal sperm DNA in the BPA and control lineages was measured. Differences between F1 and F0 generation BPA lineage mice compared to respective control lineages were not statistically significant (p > 0.05; data not shown).
3.5. BPA exposure impaired sperm quality of F0 and F1 generation BPA lineages
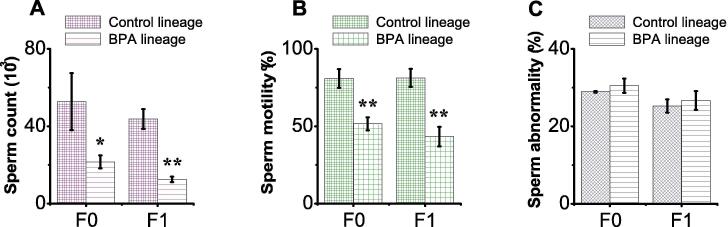
BPA exposure resulted in deleterious effects on the counts, motility, but not in head morphology, of cauda epididymal sperms in F0 generation BPA lineage mice compared to respective control (Fig. 3A). The quantitative assessment showed that both sperm counts and motility in the caudal epididymis in the F0 generation BPA lineage mice was significantly decreased (p = 0.0355, p = 0.0034, respectively; Fig. 3 1A & B). Similar effects were observed in male F1 generation BPA lineage mice (p = 0.0053, p = 0.0073; Fig. 3A & B). BPA exposure had no impact on sperm morphology in either F0 or F1 generation BPA lineages (p = 0.5554, p = 0.6331, respectively; Fig. 3C).
Fig. 3.
BPA exposure reduced quality of sperm parameters of F0 and F1 generation BPA lineage mice. F0 male mice were exposed to the same conditions of sham, vehicle or BPA. Quantifications of (A) sperm counts, (B) sperm motility, and (C) sperm morphology in F0 and F1 generations. Data are expressed as mean ± SEM (n = 6). (*) indicates < 0.05, (**) indicates p < 0.01, and (***) indicates p < 0.001. Data are presented as mean ± SEM for at least 6 independent biological replicates. (*) indicates p < 0.05, (**) indicates p < 0.001, and (***) indicates p < 0.001.
3.6. BPA exposure increased oxidative stress indices in F0 and F1 generations
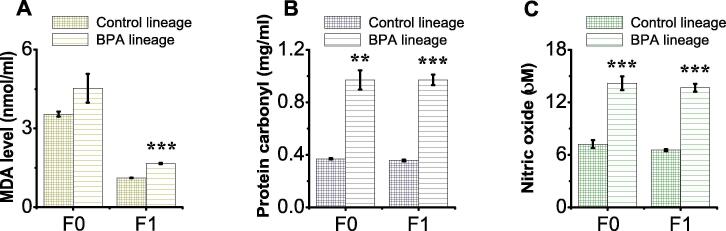
We assessed the possible role of oxidative stress in BPA-induced testis and sperm abnormalities across generations. Lipid peroxidation, measured as MDA level, was notably increased in the testicular tissues of F1 generation BPA lineage mice, but not F0 generation, compared to respective control lineages (p = 0.2591, p < 0.001, respectively; Fig. 4A). Also, a significant increase in protein peroxidation (carbonylation), measured as protein carbonyl (PC) level, was seen in the testicular tissues of both generations of BPA lineages (p = 0.0012, p < 0.001, for F0 and F1, respectively; Fig. 4B). Notably, increases in the MDA and PC levels were more pronounced in the F1 generation BPA lineage (Fig. 4A & B). Results reflect a robust intergeneration toxicological effect of BPA.
Fig. 4.
BPA exposure increased levels oxidative stress indices in F0 and F1 generations. F0 male mice were exposed to the same conditions of sham, vehicle or BPA. Quantification of (A) MDA levels (nmol/ml), (B) protein carbonyl content (mg/ml), and (C) nitric oxide (µM) in F0 and F1 generations. Data are presented as mean ± SEM for at least 6 independent biological replicates. (*) indicates p < 0.05, (**) indicates p < 0.001, and (***) indicates p < 0.001.
Subsequently, we examined inflammatory responses in F0 and F1 generations by measuring NO levels in the testicular tissues. The NO levels were greatly increased (overproduced) in F0 and F1 BPA lineages compared to respective controls (p < 0.0004, p < 0.001, respectively; Fig. 4C). These findings indicate that a pronounced toxicological effect on the F1 generation due to direct paternal BPA exposure.
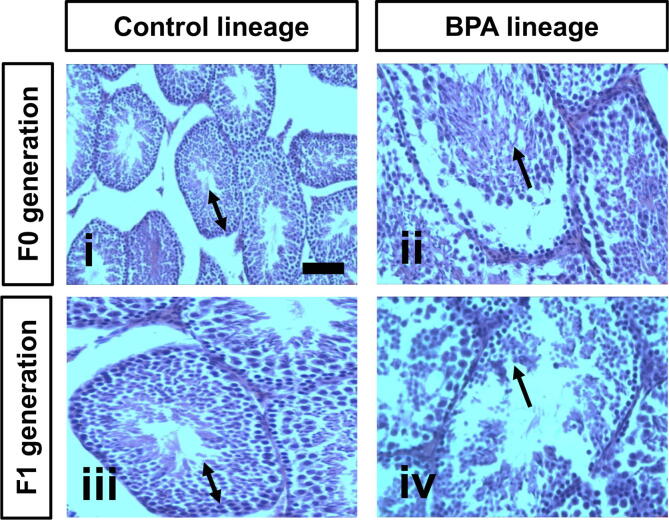
3.7. BPA exposure caused testis pathology in F0 and F1 generations
Histopathological findings demonstrated that BPA exposure induced pathological changes in the tissues of testes of the F0 and F1 BPA lineages compared to respective controls. Specifically, the testis tissues of the control mice of the F0 and F1 generations exhibit normal seminiferous tubules and normal spermatogenesis (panels i and iii; Fig. 5). Testis tissues of the BPA mice of the F1 generation display disorganisation and distortion of the seminiferous tubules and arrest of spermatogenesis at different levels (panel ii, Fig. 5). Similar BPA effects were seen in male F0 BPA lineage mice, but the effects were more pronounced in the F1 generation (panel iv, Fig. 5).
Fig. 5.
Representative photomicrographs of H&E stained sections showing testis pathology in F0 and F1 generation BPA lineages compared to F0 and F1 control lineages. Testis tissues of control group F0 and F1 mice (panels i and iii, respectively) exhibit normal seminiferous tubules and spermatogenesis (→). Testis tissues of BPA treated mice from F0 and F1 generation mice (panels ii and iv, respectively) showing spermatogenesis arrest and disturbances at different levels (↔). Scale bar = 100 µm, X 200.
4. Discussion
The principal findings of this work demonstrate that BPA exposure significantly promoted testis pathology as revealed by biochemical test and histological changes. Specifically, BPA triggered significant alterations in the body and testis mass of the F0 and F1 generations of BPA lineages compared to F0 and F1 generations control lineages. A reduction in sperm count and motility with further increased sperm abnormalities, no/little sperm DNA alterations, and elevated levels of MDA, PC, and NO were recorded. Similar effects were found in BPA exposed F0 males, but were more pronounced in the F0 offspring (F1 generation). In addition, BPA caused alterations in the testicular architecture of the F0 compared to control lineages. These pathological changes extended transgenerationally to F1 generation males’ mice, but the pathological alterations were more pronounced in the F1 generation. The findings also illustrate that oxidative stress appears to be a molecular component of the testis and sperm pathologies.A growing body of evidence suggests that exposures to environmental toxicants, abnormal nutrition, or stress lead to increased risks of developing abnormalities/diseases later in life (Al-Griw et al., 2016, Anway et al., 2005, Kent, 2012, Manikkam et al., 2012, Pan et al., 2019, Salian et al., 2009). Such abnormalities/diseases can be passed to subsequent generations, based on substantial evidence (Hou et al., 2012). Parental and/or ancestral environmental toxicant exposures adversely affect male germlines and results in atypical reproductive phenotypes that might persist for generations to come (Al-Griw et al., 2016, Manikkam et al., 2012, Nilsson et al., 2018). Previously, we reported that exposure to the environmental toxicant trichloroethylene induced testis and sperm abnormalities (Al-Griw et al., 2016, Al-Griw et al., 2015).Exposure to environmental toxicants results in atypical reproductive phenotypes that might persist for generations to come. Exposure of F0 generation gestating rodents to a well-known environmental toxicant BPA led to reduced fertility in F3 generation males (Salian et al., 2009), prostate, kidney diseases, tumours, immune system abnormalities, polycystic ovarian disease, and uterine haemorrhage during pregnancy (Guerrero-Bosagna et al., 2012, Nilsson et al., 2012) as well as abnormalities in methylation of imprinted genes in sperm from F3 generation male mice. The possible effect of paternal germ cell exposure on the health of offspring remains a major public health issue worldwide, and a topic of interest to the broader scientific community. Recently, BPA exposures of rats at puberty (F0) produce higher degrees of damage to germ cells of F1 offspring than to old rats (Almeida et al., 2018, Dobrzyńska et al., 2018). However, our understanding of the effects of BPA exposure on the first waves of spermatogenesis before puberty is still limited. The present work was envisaged to evaluate the impacts of paternal BPA exposure on testis architecture and sperm quality of male mouse offspring (F1 generation). Our findings clearly demonstrate that BPA exposure promotes the transmission of testis and sperm phenotypes/abnormalities between generations. Exposure to environmental factors can impair spermatogenesis (Al-Griw et al., 2015a). Altered sperm count, morphology, and motility are useful markers to assess fertilisation potential (Zribi et al., 2011). In this study, we found that BPA exposure reduced sperm counts and motility with little to no impact on the sperm morphology in the F0 offspring (F1 generation) compared to their respective control lineage. These abnormalities were also seen in F0 generation, but the effect was more pronounced in F1 generation. Inheritance of detrimental phenotypes independently of direct exposure to environmental toxicants is attributed to multigenerational and transgenerational impacts (Anway et al., 2006). Two mechanisms are proposed: (1) alterations in the parental germ cell genetic material and (2) alteration of epigenetic information, including histone modification and DNA methylation (Nadeau, 2009, Skinner, 2007). Altered DNA structure due to DNA single- or double-strand breaks may contribute switching reproductive status between fertility and infertility (Zribi et al., 2011). Recently, BPA exposure was found to induce genetic damage in sperm (Wu et al., 2011), and reduced sperm chromatin integrity (Pan et al., 2019). Exposure of male zebrafish to BPA (100 µg/kg) during mitosis in spermatogenesis caused a slight increase in sperm DNA fragmentation, and upregulated DNA repair activity in embryos (Pan et al., 2019). In contrast, poor sperm integrity was seen in mice exposed to 2000 μg/kg BPA (Lombó et al., 2019). Damaging of sperm DNA integrity may result from environmental factors, and genetic abnormalities might be repaired or inherited in the next generation (Henkel and Franken, 2011). Our findings demonstrate that BPA exposure promoted sperm DNA fragmentation in the F0 generation compared to respective control lineages, but this difference was not statistically significant. These abnormalities were also seen in the F0 generation, but the effect was more pronounced in F1 generation. Establishment and maintenance of epigenetic patterns in germ cells are affected by the impacts of environmental exposures on genome transcription and translation (Guerrero-Bosagna et al., 2012). These changes may be passed on to subsequent generations, even in subjects unexposed to environmental toxicants (Guerrero-Bosagna et al., 2012). The primary exposure of soma to environmental toxicants either directly or indirectly can lead to development heritable phenotypic changes (Jablonka, 2012, Sharma, 2013). Factors such as RNAs, neurohormones and neuropeptides were shown earlier to contribute to epigenetic inheritance through communication between soma and germline (Jablonka, 2012). The flow of information from somatic cells to germ cells may be carried out by exosomes that carry RNA shed by somatic cells (Cossetti et al., 2014). Hereditary information transfer from soma to germline can be relayed despite Weismann barrier principles that indicate that genetic alterations cannot be passed from somatic cells to the germline (Cossetti et al., 2014). The mechanism(s) underlying reproductive system abnormalities/diseases caused by BPA exposures are completely different in F0 generation and their offspring (F1 generation) (Cossetti et al., 2014, Guerrero-Bosagna et al., 2012). Our findings showed that testes of the F0 generation carried defected sperm produced by enhanced apoptosis. This enhanced apoptosis was carried over to F1 generation, and in outcrossed offspring, following non-Mendelian genetic inheritance. Male F0 generation mice were subjected to direct BPA exposure. In addition, F0 generation germ cells that were directly exposed to BPA are responsible for the creation of F0 offspring (F1 generation). Therefore, the F0 generation that is directly exposed to BPA exhibits transgenerational inheritance of vulnerability to abnormalities/diseases. Another major finding in this work is how BPA exposure contributes to testis and sperm pathologies. Exposure to BPA induces oxidative stress damage later in male rodent offspring (Song et al., 2014). Several sperm parameters, including counts, morphology and motility, are significantly susceptible to oxidative stress which can reduce sperm fertility (Dobrzyńska et al., 2018, Saleh and Agarwal, 2002). The findings of this work demonstrate that early-life BPA exposure promoted testicular oxidative damage in later life in the F0 generation. The findings also demonstrate that oxidative stress is passed on to F1 generation (F0 offspring). Specifically, BPA induced a significant increase in MDA and PC in F0 generation BPA lineages. There was also a significant increase in the NO level. These effects were more pronounced in F1 mice. Taken together, these findings suggest that oxidation-reduction processes in BPA exposed F0 males are affected and produce testis and sperm abnormalities in F1 males (F0 offspring).
5. Conclusion
Our findings illustrate that paternal BPA exposure promotes testis and sperm abnormalities later in life, and that the impacts of BPA exposure do not end in paternal adults, but are passed on to F0 offspring (F1 generation). Hence, the linkage of observed testis and sperm abnormalities in the F1 generation to BPA exposure of their parental line was evident in this study. Although this study is not intended for risk assessment; still, our findings have implications for subjects exposed to environmental toxicants, particularly people with deficient fertility and increased adult onset of abnormalities/diseases, with an increased risk of transmitting to future generations.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial or not-for profit sectors.
CRediT author statement
Mohamed A.Al-Griwa: Conceptualization, Data curation, Formal analysis, Funding acquisition, Investigation, Methodology, Project administration, Resources, Software, Supervision, Validation, Visualization, Writing - original draft, Writing - review & editing. Rabia O. Alghazeer: Data curation, Formal analysis, Funding acquisition, Investigation, Methodology, Resources, Software, Visualization, Writing - original draft, Writing - review & editing. Naser M.Salama: Data curation, Formal analysis, Investigation, Methodology, Resources, Software, Validation, Visualization, Writing - original draft, Writing - review & editing. Bashir A.Lwaleed: Formal analysis, Resources, Software, Writing - original draft, Writing - review & editing. Areej A.Eskandrani: Formal analysis, Software, Writing - original draft, Writing - review & editing. Wafa S.Alansari: Formal analysis, Resources, Writing - original draft, Writing - review & editing. Afnan M.Alnajeebi: Formal analysis, Resources, Software, Writing - original draft, Writing - review & editing. Nouf A.Babteen: Formal analysis, Resources, Software, Writing - original draft, Writing - review & editing. Abdul HakimElnfatia: Data curation, Formal analysis, Investigation, Methodology, Resources, Software, Validation, Visualization, Writing - original draft, Writing - review & editing.
Declaration of Competing Interest
The authors declare no conflict of interest.
Acknowledgments
The authors would like to express their deepest appreciation to the University of Tripoli for supporting this work.
Footnotes
Peer review under responsibility of King Saud University.
References
- Al-Griw M.A., Treesh S.A., Alghazeer R.O., Regeai S.O. Environmentally toxicant exposures induced intragenerational transmission of liver abnormalities in mice. Open Vet. J. 2017;7(3):244. doi: 10.4314/ovj.v7i3.8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Al-Griw M.A., Alghazeer R.O., Al-Azreg S.A., Bennour E.M. Cellular and molecular etiology of hepatocyte injury in a murine model of environmentally induced liver abnormality. Open Vet. J. 2016;6(3):150. doi: 10.4314/ovj.v6i3.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Al-Griw M.A., Salama N., Treesh S., Elnfati A. Transgenerational Genetic Effect of Trichloroethane (TCE) on phenotypic variation of acrosomal proteolytic enzyme and male infertility risk. Int. J. Genet. Genom. 2015;3:43–49. [Google Scholar]
- Alghazeer R., Elgahmasi S., Elnfati A., Elhensheri M., Al-Griw M., Awayn N., El- Nami M. Antioxidant Activity and Hepatoprotective Potential of Flavonoids from Arbutus pavarii against CCl4 Induced Hepatic Damage. BJI. 2018;21(1):1–12. doi: 10.9734/BJI/2018/39528. [DOI] [Google Scholar]
- Almeida F.F., Leal M.C., França L.R. Testis morphometry, duration of spermatogenesis, and spermatogenic efficiency in the wild boar. Biol. Reprod. 2006;75:792–799. doi: 10.1095/biolreprod.106.053835. [DOI] [PubMed] [Google Scholar]
- Almeida S., Raposo A., Almeida-González M., Carrascosa C. Bisphenol A: Food Exposure and Impact on Human Health: Bisphenol A and human health effect…. Compr. Rev. Food Sci. Food Saf. 2018;17(6):1503–1517. doi: 10.1111/1541-4337.12388. [DOI] [PubMed] [Google Scholar]
- Anway M.D., Cupp A.S., Uzumcu M., Skinner M.K. Epigenetic transgenerational actions of endocrine disruptors and male fertility. Science. 2005;308:1466–1469. doi: 10.1126/science.1108190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anway M.D., Leathers C., Skinner M.K. Endocrine Disruptor Vinclozolin Induced Epigenetic Transgenerational Adult-Onset Disease. Endocrinology. 2006;147(12):5515–5523. doi: 10.1210/en.2006-0640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beronius A., Rudén C., Håkansson H., Hanberg A. Risk to all or none? Reprod. Toxicol. 2010;29(2):132–146. doi: 10.1016/j.reprotox.2009.11.007. [DOI] [PubMed] [Google Scholar]
- Birnbaum L.S., Bucher J.R., Collman G.W., Zeldin D.C., Johnson A.F., Schug T.T., Heindel J.J. Consortium-Based Science: The NIEHS’s Multipronged, Collaborative Approach to Assessing the Health Effects of Bisphenol A. Environ. Health Perspect. 2012;120(12):1640–1644. doi: 10.1289/ehp.1205330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Björnsdotter M.K., de Boer J., Ballesteros-Gómez A. Bisphenol A and replacements in thermal paper: A review. Chemosphere. 2017;182:691–706. doi: 10.1016/j.chemosphere.2017.05.070. [DOI] [PubMed] [Google Scholar]
- Bradford M.M. A rapid and sensitive method for the quantification of microgram quantities of protein utilizing the principle of protein-dye binding. Analy. Biochem. 1976;72:248–254. doi: 10.1006/abio.1976.9999. [DOI] [PubMed] [Google Scholar]
- Cossetti C., Lugini L., Astrologo L., Saggio I., Fais S., Spadafora C., Busson P. Soma-to-Germline Transmission of RNA in Mice Xenografted with Human Tumour Cells: Possible Transport by Exosomes. PLoS ONE. 2014;9(7):e101629. doi: 10.1371/journal.pone.0101629.s002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crews D., Gore A.C., Hsu T.S., Dangleben N.L., Spinetta M., Schallert T., Anway M.D., Skinner M.K. Transgenerational epigenetic imprints on mate preference. Proc. Natl. Acad. Sci. 2007;104(14):5942–5946. doi: 10.1073/pnas.0610410104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dobrzyńska M.M., Gajowik A., Jankowska-Steifer E.A., Radzikowska J., Tyrkiel E.J. Reproductive and developmental F1 toxicity following exposure of pubescent F0 male mice to bisphenol A alone and in a combination with X-rays irradiation. Toxicology. 2018;410:142–151. doi: 10.1016/j.tox.2018.10.007. [DOI] [PubMed] [Google Scholar]
- Ginsberg L.C., Johnson S.C., Salama N., Ficsor G. Acrosomal proteolytic assay for detection of mutagens in mammals. Mutat. Res. Lett. 1981;91(4-5):413–418. doi: 10.1016/0165-7992(81)90024-5. [DOI] [PubMed] [Google Scholar]
- Glander H.J., Horn L.C., Dorschner W., Paasch U., Kratzsch J. Probability to retrieve testicular spermatozoa in azoospermic patients. Asian J. Androl. 2000;2:199–205. [PubMed] [Google Scholar]
- Goa J. A Micro Biuret Method for Protein Determination Determination of Total Protein in Cerebrospinal Fluid. Scand. J. Clin. Lab. Invest. 1953;5(3):218–222. doi: 10.3109/00365515309094189. [DOI] [PubMed] [Google Scholar]
- Greer E.L., Maures T.J., Ucar D., Hauswirth A.G., Mancini E., Lim J.P., Benayoun B.A., Shi Y., Brunet A. Transgenerational epigenetic inheritance of longevity in Caenorhabditis elegans. Nature. 2011;479(7373):365–371. doi: 10.1038/nature10572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guerrero-Bosagna C., Covert T.R., Haque M.M., Settles M., Nilsson E.E., Anway M.D., Skinner M.K. Epigenetic transgenerational inheritance of vinclozolin induced mouse adult onset disease and associated sperm epigenome biomarkers. Reprod. Toxicol. 2012;34(4):694–707. doi: 10.1016/j.reprotox.2012.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henderson I.R., Jacobsen S.E. Epigenetic inheritance in plants. Nature. 2007;447(7143):418–424. doi: 10.1038/nature05917. [DOI] [PubMed] [Google Scholar]
- Henkel R.R., Franken D.R. Sperm DNA Fragmentation: Origin and Impact on Human Reproduction. J. Reprod. Stem Cell Biotechnol. 2011;2(2):88–108. doi: 10.1177/205891581100200204. [DOI] [Google Scholar]
- Hou L., Zhang X., Wang D., Baccarelli A. Environmental chemical exposures and human epigenetics. Int. J. Epidemiol. 2012;41(1):79–105. doi: 10.1093/ije/dyr154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jablonka E. Epigenetic inheritance and plasticity: the responsive germline. Prog. Biophys. Mol. Biol. 2012 doi: 10.1016/jpbiomolbio201208014. [DOI] [PubMed] [Google Scholar]
- Kent A.L. Developmental origins of health and adult disease: What should neonatologists/paediatricians be considering about the long-term health of their patients? J. Paediatr. Child Health. 2012;48(9):730–734. doi: 10.1111/j.1440-1754.2012.02541.x. [DOI] [PubMed] [Google Scholar]
- Khodayar M.J., Kalantari H., Mahdavinia M., Khorsandi L., Alboghobeish S., Samimi A., Alizadeh S., Zeidooni L. Protective effect of naringin against BPA-induced cardiotoxicity through prevention of oxidative stress in male Wistar rats. Drug Chem. Toxicol. 2020;43(1):85–95. doi: 10.1080/01480545.2018.1504958. [DOI] [PubMed] [Google Scholar]
- Kim H.-S., Kang M.J., Kim S.A., Oh S.K., Kim H., Ku S.-Y., Kim S.H., Moon S.Y., Choi Y.M. The utility of sperm DNA damage assay using toluidine blue and aniline blue staining in routine semen analysis. Clin. Exp. Reprod. Med. 2013;40(1):23. doi: 10.5653/cerm.2013.40.1.23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lane R., Riddle B., Borzelleca J. Effects of 1,2-dichloroethane and 1,1,1-trichloroethane in drinking water on reproduction and development in mice. Toxicol. Appl. Pharmacol. 1982;63:409–421. doi: 10.1016/0041-008x(82)90270-8. [DOI] [PubMed] [Google Scholar]
- Lombó M., Fernández-Díez C., González-Rojo S., Herráez M.P. Genetic and epigenetic alterations induced by bisphenol A exposure during different periods of spermatogenesis: from spermatozoa to the progeny. Sci. Rep. 2019;9(1) doi: 10.1038/s41598-019-54368-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manikkam M., Guerrero-Bosagna C., Tracey R., Haque M.M., Skinner M.K., Shioda T. Transgenerational Actions of Environmental Compounds on Reproductive Disease and Identification of Epigenetic Biomarkers of Ancestral Exposures. PLoS ONE. 2012;7(2):e31901. doi: 10.1371/journal.pone.0031901.s009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nadeau J.H. Transgenerational genetic effects on phenotypic variation and disease risk. Hum. Mol. Genet. 2009;18(R2):R202–R210. doi: 10.1093/hmg/ddp366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nilsson E., Larsen G., Manikkam M., Guerrero-Bosagna C., Savenkova M.I., Skinner M.K., Shioda T. Environmentally Induced Epigenetic Transgenerational Inheritance of Ovarian Disease. PLoS ONE. 2012;7(5):e36129. doi: 10.1371/journal.pone.0036129.s005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nilsson E., Maamar M.B., Skinner M.K. Definition of epigenetic transgenerational inheritance and biological impacts. Transgenerational Epigenetics. Academic Press. 2019;1:13–24. [Google Scholar]
- Nilsson E.E., Sadler-Riggleman I., Skinner M.K., Mansuy I. Environmentally induced epigenetic transgenerational inheritance of disease. Environ. Epigenet. 2018;4(2) doi: 10.1093/eep/dvy016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nilsson E.E., Skinner M.K. Environmentally induced epigenetic transgenerational inheritance of disease susceptibility. Trans. Res. 2015;165(1):12–17. doi: 10.1016/j.trsl.2014.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan D., Feng D., Ding H., Zheng X., Ma Z., Yang B., Xie M. Effects of bisphenol A exposure on DNA integrity and protamination of mouse spermatozoa. Andrologia. 2019 doi: 10.1111/andr.12694. [DOI] [PubMed] [Google Scholar]
- Pembrey M.E. Male-line transgenerational responses in humans. Human Fertility. 2010;13(4):268–271. doi: 10.3109/14647273.2010.524721. [DOI] [PubMed] [Google Scholar]
- Rahman M.S., Pang W.-K., Ryu D.-Y., Park Y.-J., Pang M.-G. Multigenerational and transgenerational impact of paternal bisphenol A exposure on male fertility in a mouse model. Hum. Reprod. 2020;35(8):1740–1752. doi: 10.1093/humrep/deaa139. [DOI] [PubMed] [Google Scholar]
- Rattan S., Flaws J.A. The epigenetic impacts of endocrine disruptors on female reproduction across generations†. Biol. Reprod. 2019;101(3):635–644. doi: 10.1093/biolre/ioz081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruden D.M., Lu X. Hsp90 affecting chromatin remodeling might explain transgenerational epigenetic inheritance in Drosophila. Curr. Genomics. 2008;9:500–508. doi: 10.2174/138920208786241207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sadowski R.N., Wise L.M., Park P.Y., Schantz S.L., Juraska J.M. Early exposure to bisphenol A alters neuron and glia number in the rat prefrontal cortex of adult males, but not females. Neuroscience. 2014;279:122–131. doi: 10.1016/j.neuroscience:2014.08.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saleh R.A., Agarwal A. Oxidative stress and male infertility: from research bench to clinical practice. J. Androl. 2002;23:737–752. [PubMed] [Google Scholar]
- Salian S., Doshi T., Vanage G. Impairment in protein expression profile of testicular steroid receptor coregulators in male rat offspring perinatally exposed to Bisphenol A. Life Sci. 2009;85(1-2):11–18. doi: 10.1016/j.lfs.2009.04.005. [DOI] [PubMed] [Google Scholar]
- Sharma A. Transgenerational epigenetic inheritance: Focus on soma to germline information transfer. Prog. Biophys. Mol. Biol. 2013;113(3):439–446. doi: 10.1016/j.pbiomolbio.2012.12.003. [DOI] [PubMed] [Google Scholar]
- Skinner M.K. Endocrine Disruptors and Epigenetic Transgenerational Disease Etiology. Pediatr. Res. 2007;61(5 Part 2):48R–50R. doi: 10.1203/pdr.0b013e3180457671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skinner M.K., Manikkam M., Guerrero-Bosagna C. Epigenetic transgenerational actions of environmental factors in disease etiology. Trends Endocrinol. Metab. 2010;21(4):214–222. doi: 10.1016/j.tem.2009.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skinner M.K., Manikkam M., Tracey R., Guerrero-Bosagna C., Haque M., Nilsson E.E. Ancestral dichlorodiphenyltrichloroethane (DDT) exposure promotes epigenetic transgenerational inheritance of obesity. BMC Med. 2013;11(1) doi: 10.1186/1741-7015-11-228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song S., Zhang L., Zhang H., Wei W., Jia L. Perinatal BPA Exposure Induces Hyperglycemia, Oxidative Stress and Decreased Adiponectin Production in Later Life of Male Rat Offspring. IJERPH. 2014;11(4):3728–3742. doi: 10.3390/ijerph110403728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thoene M., Dzika E., Gonkowski S., Wojtkiewicz J. Bisphenol S in food causes hormonal and obesogenic effects comparable to or worse than Bisphenol A: A literature review. Nutr. 2020;12:532. doi: 10.3390/nu12020532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tyl R.W., Myers C.B., Marr M.C., Sloan C.S., Castillo N.P., Veselica M.M., Seely J.C., Dimond S.S., Van Miller J.P., Shiotsuka R.N., Beyer D., Hentges S.G., Waechter J.M., Jr Two-Generation Reproductive Toxicity Study of Dietary Bisphenol A in CD-1 (Swiss) Mice. Toxicol. Sci. 2008;104(2):362–384. doi: 10.1093/toxsci/kfn084. [DOI] [PubMed] [Google Scholar]
- Vandenberg L.N., Ehrlich S., Belcher S.M., Ben-Jonathan N., Dolinoy D.C., Hugo E.R., Hunt P.A., Newbold R.R., Rubin B.S., Saili K.S., Soto A.M., Wang H.-S., vom Saal F.S. Low dose effects of bisphenol A: An integrated review of in vitro, laboratory animal, and epidemiology studies. Endocrine Disruptors. 2013;1(1):e26490. doi: 10.4161/endo.26490. [DOI] [Google Scholar]
- Wang G., Wang J., Ma H., Ansari G.A.S., Khan M.F. N-Acetylcysteine protects against trichloroethene-mediated autoimmunity by attenuating oxidative stress. Toxicol. Appl. Pharmacol. 2013;273(1):189–195. doi: 10.1016/j.taap.2013.08.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waterland R.A. Is Epigenetics an Important Link between Early Life Events and Adult Disease? Horm. Res. Paediatr. 2009;71(1):13–16. doi: 10.1159/000178030. [DOI] [PubMed] [Google Scholar]
- Wu D.H., Leung Y.-K., Thomas M.A., Maxwell R., Ho S.-M. Bisphenol A (BPA) confers direct genotoxicity to sperm with increased sperm DNA fragmentation. Fertil. Steril. 2011;96(3):S5–S6. doi: 10.1016/j.fertnstert.2011.07.030. [DOI] [Google Scholar]
- Xu Y., Zhao H., Zhang M., Li C.-J., Lin X.-Z., Sheng J., Shi W. Variations of Antioxidant Properties and NO Scavenging Abilities during Fermentation of Tea. IJMS. 2011;12(7):4574–4590. doi: 10.3390/ijms12074574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y.-T., Zheng Q.-S., Pan J., Zheng R.-L. Oxidative Damage of Biomolecules in Mouse Liver Induced by Morphine and Protected by Antioxidants. Pharmacol. Toxicol. 2004;95(2):53–58. doi: 10.1111/j.1742-7843.2004.950202.x. [DOI] [PubMed] [Google Scholar]
- Zribi N., Chakroun N., Elleuch H., Abdallah F., Ben Hamida A., Gargouri J., Fakhfakh F., Keskes L. Sperm DNA fragmentation and oxidation are independent of malondialdheyde. Reprod. Biol. Endocrinol. 2011;9(1):47. doi: 10.1186/1477-7827-9-47. [DOI] [PMC free article] [PubMed] [Google Scholar]