Abstract
Ethnobotanical and phytochemical studies are useful to discover new drugs. Phytochemical screening is an important step in the detection of the bioactive components existing in medicinal plants that are used in traditional medicine. Very few phytochemical studies investigating medicinal plants used in traditional medicine exist in Saudi Arabia. Eighty-five medicinal plants used in traditional medicine in Jeddah, Saudi Arabia are investigated here for the first time. This research aims to screen of 85 medicinal plants used in traditional medicine in Jeddah for the presence of secondary metabolites, and to answer the following question: Is the ethnomedicinal importance of medicinal plants used in Jeddah conform to their secondary metabolite content. Ethnobotanical fieldwork took place in Jeddah from August 2018 to September 2019. Eighty-five different plant species belonging to 37 families were identified. Screening of 85 medicinal plants was performed for the presence of alkaloids, glycosides, flavonoids, tannins, saponins and resins using standard methods. The most commonly distributed phytochemical compounds among medicinal plants used were glycosides (82%; 70 species), tannins (68%; 58 species), alkaloids (56%; 48 species), saponins (52%, 44 species) and flavonoids (35%; 30 species). On the other hand, the least commonly distributed compounds were resins (31%; 26 species). All the six groups of secondary metabolites were found in seeds of Cuminum cyminum L., Pimpinella anisum L. and Trigonella foenum-graecum L. It can be said that the ethnomedicinal importance of these 85 medicinal plants used in Jeddah conform to their secondary metabolite content. More research should be carried out on the quantitative analysis of phytochemicals in these 85 medicinal plants used in traditional medicine in Jeddah. Furthermore, there is a need to focus phytochemical screening on ethnobotanical studies to complete research into traditional medicine which leads to the discovery of new drugs.
Keywords: Ethnomedicine, Pharmacology, Secondary metabolites, Traditional knowledge
1. Introduction
Medicinal plants are an important source of medicines which are effective in the therapy of various diseases, especially in traditional medicine (Bako et al., 2005, Borokini and Omotayo, 2012). Different parts of the plant are used in traditional medicine, including barks, flowers, fruits, leaves, resins, rhizomes, roots, seeds, and stems (Alqethami et al., 2017). The medicinal value of these plant lies on certain chemical effective compounds in different parts of the plant which produce a specific physiological effect in the human body (Kumar and Satapathy, 2011). Plant compounds are bioactive compounds found naturally in plants. These plant substances are often divided into primary and secondary metabolites (Balandrin et al., 1985).
Primary metabolites are essential to plants and are involved in the primary metabolic processes of plant cell building and retention (Briskin, 2000). They are widely distributed in nature and occur in one form or another in all organisms. Among higher plants they are often located within seeds and vegetative storage organs. They are widely used as foods like carbohydrates or food additives, as well as raw industrial resources. For example, fatty acids and plant oils used to produce soaps and cleansers (Balandrin et al.,1985).
On the other hand, secondary metabolites are essential, due to their medicinal value (Borokini and Omotayo, 2012), which can be defined as biological active compounds, found in plant, derived from primary metabolites and have pharmacological effects in the human body (Balandrin et al., 1985, Bernhoft et al., 2010). Secondary metabolites are not specifically necessary for the plant's basic photosynthetic or respiratory metabolism such primary metabolites (Theis and Lerdau, 2003, Bernhoft et al., 2010), but many of them seem to have ecological function and important roles in plants such as protection, chemical defenses from microorganism, insect or even higher predators, also as pollinator attractants (Balandrin et al., 1985, Bernhoft et al., 2010). Based on chemical structure, secondary metabolites could be classified into a range of different groups, which largely distinct in terms of the essence of their ecological functions (Kennedy and Wightman, 2011). The most important phytochemical groups in this regard are alkaloids, glycosides, flavonoids, tannins, saponins and resins which have medicinal properties.
Plants which are found to be medicinally effective and frequently traditionally administered may include substances that are possible drug candidates. Therefore, they should be intensively reviewed. In different countries, scientific research of medicinal plants has been conducted due to their effective role to health care (Heinrich et al., 1998, Cunningham, 1993, Gazzaneo et al., 2005, Cai et al., 2006, Aqil et al., 2006). The active compounds differ from plant to plant due to their biodiversity and they produce a definite physiological action on the human body (Edeoga et al., 2006, Borokini and Omotayo, 2012). Moreover, the distribution of these chemical compounds in the different plant parts differs (Bako et al., 2005, Borokini and Omotayo, 2012). Thus, the medicinal content in medicinal plants is affected primarily by cultivation period, season of collection (Nalawade and Tsay, 2004). Therefore, phytochemical screening must be carried out regularly, even on plants whose secondary metabolites are known.
This work focus on one of the Saudi Arabian cities with highest population growth, Jeddah. Aiming to screen of 85 medicinal plants used in traditional medicine in Jeddah for the presence of secondary metabolites, and to answer the following question: Is the ethnomedicinal importance of medicinal plants used in Jeddah conform to their secondary metabolite content. The choice of 85 medicinal plants for this study was based on a recent study carried out by Alqethami et al. (2020) where it was reported that there are 85 different medicinal plants traditionally used in Jeddah.
2. Methods
2.1. Study area
Jeddah is the second-largest city in the Kingdom of Saudi Arabia (KSA). It is situated on the western coast of KSA at latitude 29.21 north and longitude 39.7 east, in the center of the east coast of the Red Sea, 12 m above sea level. Jeddah's urban boundary is about 1765 km2. The climate in Jeddah is a desert climate with high humidity, the average annual temperature is approximately 28 °C, and in summer temperatures can rise to 40 °C. It has Red Sea's biggest seaport (UN-Habitat, 2018).
2.2. Plant collection and identification
Samples of 85 different plants (Table 1) were collected based on ethnobotanical fieldwork which took place over a year in Jeddah, from August 2018 to September 2019. The ethical guidelines of the American Anthropological Association (2012) and the Code of Ethics of the International Society of Ethnobiology (2006) were followed. Approval from the Ethics Committee of the Unit of Biomedical Ethics Research Committee, King Abdulaziz University, was obtained (Reference No 671-19). Plant specimens were mounted and were identified in the King Abdulaziz University herbarium (KAUH) using specimens of herbarium, Flora of KSA (Chaudhary, 1999, 2000, 2001) and the Flora of KSA (Migahid, 1978). Voucher specimens were deposited in KAUH. Identification was validated by authors. Nomenclature and families follow Catalogue of Life (2015). The list was cross-checked with the online checklist of the Flora of KSA (Thomas, 2011), as well as with (Chaudhary, 1999, 2000, 2001).
Table 1.
A comprehensive inventory of phytochemical screening of the 85 medicinal plants used in traditional medicine in Jeddah including scientific name, family, voucher specimen number, common name(s), part(s) tested. (+) = Present/(−) = Absent.
| Scientific name (family, voucher specimen) | Common name | Part tested | Alkaloid | Glycosides | Flavonoid | Tannins | Saponin | Resin |
|---|---|---|---|---|---|---|---|---|
| Allium cepa L. (Amaryllidaceae, AQJ_2) | Onion | Bulb | − | + | + | − | − | − |
| Allium sativum L. (Amaryllidaceae, AQJ_1) | Garlic | Bulb | − | + | − | − | + | − |
| Aloe vera (L.) Burm.f. (Asphodelaceae, AQJ_3) | Aloe | Leaves | − | + | − | + | − | − |
| Anastatica hierochuntica L. (Brassicaceae, AQJ_4) | Flower of Mariyam | All plant | + | + | − | + | + | − |
| Anethum graveolens L. (Apiaceae, AQJ_5) | Dill-weed | Leaves | − | + | − | + | + | − |
| Seed | − | + | + | − | − | − | ||
| Angelica sinensis (Oliv.) Diels (Apiaceae, AQJ_6) | Dong Quai | Stem | + | + | − | − | + | − |
| Apium graveolens L. (Apiaceae, AQJ_7) | Wild Celery | Leaves | − | + | − | + | − | − |
| Aucklandia costus Falc. (Asteraceae, AQJ_8) | Costus | Root | + | + | − | + | + | − |
| Azadirachta indica A.Juss. (Meliaceae, AQJ_9) | Neem | Leaves | − | + | − | + | − | − |
| Beta vulgaris L. (Amaranthaceae, AQJ_10) | Beet | Root | + | + | + | + | − | − |
| Boswellia sacra Flückiger-Dupiron (Burseraceae, AQJ_11) | Frankincense | Resin | + | − | − | − | + | + |
| Camellia sinensis (L.) Kuntze (Theaceae, AQJ_12) | Tea | Leaves | − | + | + | + | − | − |
| Carum carvi L. (Apiaceae, AQJ_13) | Caraway | Seed | + | + | + | + | − | + |
| Chenopodium quinoa Willd. (Amaranthaceae, AQJ_14) | Quinoa | Seed | + | − | + | − | + | + |
| Cinnamomum cassia (L.) Presl (Lauraceae, AQJ_15) | Cinnamon | Bark | + | − | + | + | − | − |
| Cinnamomum tamala (Buch-Ham.) Th. G. G. Nees (Lauraceae, AQJ_16) | Indian bay leaf | Leaves | + | + | − | + | + | − |
| Citrus aurantium L. (Rutaceae, AQJ_18) | Orange | Fruit | + | + | + | + | − | − |
| Citrus limon (L.) Burm. fil. (Rutaceae, AQJ_17) | Lemon | Fruit | − | + | + | + | − | − |
| Coffea arabica L. (Rubiaceae, AQJ_19) | Arabian Coffee Plant | Seed's peel | − | + | − | + | − | − |
| Commiphora gileadensis (L.) C. Christ. (Burseraceae, AQJ_21) | Balsam of Gilead | Stem | + | − | − | + | + | − |
| Commiphora myrrha (Nees) Engl. (Burseraceae, AQJ_20) | Myrrha | Resin | − | − | − | + | + | + |
| Coriandrum sativum L. (Apiaceae, AQJ_22) | Coriander | Leaves | − | + | − | + | + | − |
| Crocus sativus L. (Iridaceae, AQJ_23) | Saffron crocus | Stigma in flower | + | + | − | − | − | − |
| Cucumis sativus L. (Cucurbitaceae, AQJ_24) | Garden cucumber | Fruit | + | + | − | − | − | − |
| Cuminum cyminum L. (Apiaceae, AQJ_25) | Cumin | Seed | + | + | + | + | + | + |
| Curcuma longa L. (Zingiberaceae, AQJ_26) | Turmeric | Rhizome | + | + | − | + | + | − |
| Daucus carota L. (Apiaceae, AQJ_27) | Carrot | Root | + | + | + | − | − | − |
| Elettaria cardamomum (L.) Maton (Zingiberaceae, AQJ_28) | Cardamom | Fruit | − | + | − | + | − | − |
| Eruca vesicaria (L.) Cav. (Brassicaceae, AQJ_29) | Salad rocket | Leaves | − | + | − | + | + | − |
| Ficus carica L. (Moraceae, AQJ_30) | Common fig | Fruit | + | + | − | + | − | − |
| Foeniculum vulgare Mill. (Apiaceae, AQJ_31) | Fennel | Seed | + | + | + | − | + | − |
| Glycyrrhiza glabra L. (Fabaceae, AQJ_32) | Licorice | Root | − | + | − | + | + | + |
| Helianthus annuus L. (Asteraceae, AQJ_33) | Common sunflower | Seed | − | + | + | − | − | + |
| Hibiscus sabdariffa L. (Malvaceae, AQJ_34) | Roselle | Flowers | + | − | − | + | − | + |
| Hordeum vulgare L. (Poaceae, AQJ_35) | Barley | Seed | + | − | − | − | − | − |
| Hyphaene thebaica (L.) Mart. (Arecaceae, AQJ_36) | Doom palm | Seed | − | + | − | + | − | − |
| Lavandula atriplicifolia Benth. (Lamiaceae, AQJ_37) | Lavender | Flowers | − | + | − | − | + | − |
| Lawsonia inermis L. (Lythraceae, AQJ_38) | Henna | Leaves | − | + | − | + | − | + |
| Lepidium sativum L. (Brassicaceae, AQJ_39) | Garden cress | Seed | + | + | + | + | − | + |
| Linum usitatissimum L. (Linaceae, AQJ_40) | Flax | Seed | + | − | − | − | + | + |
| Luffa aegyptiaca Mill. (Cucurbitaceae, AQJ_41) | Sponge gourd | Fruit | + | + | + | − | + | − |
| Lupinus albus L. (Fabaceae, AQJ_42) | Lupin | Seed | + | + | + | − | + | + |
| Malus pumila Mill. (Rosaceae, AQJ_43) | Apple | Fruit | − | + | + | + | − | − |
| Matricaria aurea (L.) Sch. Bip. (Asteraceae, AQJ_44) | Golden Chamomile | Flowers | + | + | − | + | + | − |
| Mentha spicata L. (Lamiaceae, AQJ_45) | Mint | Leaves | + | + | + | + | − | − |
| Momordica charantia L. (Cucurbitaceae, AQJ_46) | Bitter melon | Fruit | + | + | − | − | + | − |
| Moringa oleifera Lam. (Moringaceae, AQJ_47) | Moringa | Leaves | − | + | − | + | − | − |
| Morus nigra L. (Moraceae, AQJ_48) | Black Mulberry | Leaves | − | + | − | + | − | + |
| Myristica fragrans Houtt. (Myristicaceae, AQJ_49) | Nutmeg | Seed & Fruit | + | + | − | − | − | + |
| Myrtus communis L. (Myrtaceae, AQJ_50) | Myrtle | Leaves | − | + | − | + | + | − |
| Nigella sativa L. (Ranunculaceae, AQJ_51) | Nigella | Seed | + | − | + | + | + | − |
| Ocimum americanum L. (Lamiaceae, AQJ_52) | Hoary basil | Leaves | + | + | − | + | − | − |
| Ocimum basilicum L. (Lamiaceae, AQJ_53) | Basil | Leaves | − | + | − | − | + | − |
| Olea europaea L. (Oleaceae, AQJ_54) | Olive | Leaves | − | − | − | + | + | + |
| Fruit | − | − | + | − | + | − | ||
| Origanum syriacum L. (Lamiaceae, AQJ_55) | Syrian oregano | Leaves | + | + | − | − | − | − |
| Panax ginseng C.A.Mey. (Araliaceae, AQJ_56) | Ginseng | Root | + | + | + | − | + | + |
| Petroselinum crispum (Mill.) Fuss (Apiaceae, AQJ_57) | Parsley | Leaves | + | + | − | + | − | − |
| Phoenix dactylifera L. (Arecaceae, AQJ_58) | Date palm | Fruit | − | + | − | + | − | − |
| Pimpinella anisum L. (Apiaceae, AQJ_59) | Anise | Seed | + | + | + | + | + | + |
| Piper nigrum L. (Piperaceae, AQJ_60) | Black Pepper | Seed | + | + | − | + | − | − |
| Pistacia lentiscus L. (Anacardiaceae, AQJ_61) | Mastic | Resin | + | + | − | + | + | + |
| Plectranthus aegyptiacus (Forssk.) C.Chr. (Lamiaceae, AQJ_62) | Silver spur flower | Leaves | − | + | − | − | − | − |
| Prunus mahaleb L. (Rosaceae, AQJ_63) | Mahaleb cherry | Seed | + | + | − | − | + | + |
| Psidium guajava L. (Myrtaceae, AQJ_64) | Guava | Leaves | − | − | − | + | − | + |
| Punica granatum L. (Lythraceae, AQJ_65) | Pomegranate | Peel | + | + | − | + | + | − |
| Rosa damascena P. Mill. (Rosaceae, AQJ_66) | Damask rose | Flowers | − | + | + | + | − | − |
| Salvadora persica L. (Salvadoraceae, AQJ_67) | Arak | Stem | − | + | − | + | + | − |
| Salvia officinalis L. (Lamiaceae, AQJ_68) | Garden sage | Leaves | + | + | − | + | − | − |
| Salvia rosmarinus Schleid. (Lamiaceae, AQJ_69) | Rosemary | Leaves | + | + | − | + | + | + |
| Senegalia senegal (L.) Britton (Fabaceae, AQJ_70) | Gum Arabic tree | Resin | − | + | − | − | + | + |
| Senna alexandrina Mill. (Fabaceae, AQJ_71) | Senna | Leaves | − | + | + | + | + | − |
| Senna italica Mill. (Fabaceae, AQJ_72) | Italian Senna | Fruit | − | + | − | + | + | − |
| Seriphidium herba-alba (Asso) J. Soják (Asteraceae, AQJ_73) | White Wormwood | Leaves | − | + | − | + | + | + |
| Sesamum indicum L. (Pedaliaceae, AQJ_74) | Sesame | Seed | + | − | + | − | − | − |
| Syzygium aromaticum (L.) Merr. & L.M.Perry (Myrtaceae, AQJ_75) | Cloves | Flower buds | + | − | + | + | − | + |
| Tamarindus indica L. (Fabaceae, AQJ_76) | Tamarind | Fruit | − | + | − | + | + | − |
| Thymus vulgaris L. (Lamiaceae, AQJ_77) | Common thyme | Leaves | − | + | − | − | + | − |
| Trachyspermum ammi (L.) Sprague (Apiaceae, AQJ_78) | Ajwain | Seed | + | + | + | + | − | − |
| Trigonella foenum-graecum L. (Fabaceae, AQJ_79) | Fenugreek | Seed | + | + | + | + | + | + |
| Tripleurospermum auriculatum (Boiss.) Rech. fil. (Asteraceae, AQJ_80) | Mayweed | Leaves | + | + | − | + | + | − |
| Vigna radiata (L.) R. Wilczek (Fabaceae, AQJ_81) | Green Gram | Seed | + | + | − | − | + | − |
| Vitellaria paradoxa C.F.Gaertn. (Sapotaceae, AQJ_82) | Shea | Seed | + | + | + | − | − | + |
| Vitis vinifera L. (Vitaceae, AQJ_83) | Grape | Fruit | − | − | − | + | − | − |
| Zingiber officinale Roscoe (Zingiberaceae, AQJ_84) | Ginger | Root | − | + | + | + | + | − |
| Ziziphus spina-christi (L.) Desf. (Rhamnaceae, AQJ_85) | Christ's thorn jujube | Leaves | + | − | − | + | + | − |
| Total of species | – | – | 48 | 70 | 30 | 58 | 44 | 26 |
2.3. Preparation of plant extracts
Plant materials were prewashed, dried and grinded to get a homogeneous sample (Thangaraj, 2016). For different extraction, plant powdered was divided into two sections: Aqueous extraction and solvent extraction (Fig. 1).
Fig. 1.

Plants extraction.
2.3.1. Aqueous extraction
Two hundred ml of distilled water was mixed with five grams of plant samples. This mixture was heated for 20 min with continuous stirring at 30–40 °C. The aqueous extract was filtered by Whatman No.1 filter paper (Yadav and Agarwala, 2011).
2.3.2. Solvent extraction
Ten grams of plant material was taken in one hundred ml of solvent (solvents used were methanol and ethanol) in a conical flask, plugged with cotton and then kept for 24 h and filtered using Whatman No. 1 filter paper (Thangaraj, 2016).
2.4. Chemicals and reagents
Methanol, ethanol, hydrochloric acid (HCl), glacial acetic acid, ferric chloride (FeCl3), concentrated sulphuric acid (H2SO4), magnesium ribbon, Hager’s reagent, sodium hydroxide solution (NaOH) and distilled water.
2.5. Phytochemical analysis
Qualitative analysis of phytochemical contents was performed according to published methods as following; alkaloids (Pandey and Tripathi, 2014, Yadav et al., 2014, Thangaraj, 2016), glycosides (Parekh and Chanda, 2007, Yadav and Agarwala, 2011, Pandey and Tripathi, 2014), flavonoids (Kumar et al., 2007, Pandey and Tripathi, 2014), tannins (Kumar et al., 2007, Parekh and Chanda, 2007, Yadav et al., 2014), resin (Ukoha et al., 2011, Aldhebiani and Mufarah, 2017) and saponin (Parekh and Chanda, 2007, Ukoha et al., 2011, Yadav et al., 2014). Each plant sample was analyzed 3 times.
2.5.1. Detection of alkaloids
(Hager’s test)
Ethanol extract was dissolved in a few ml dilute Hydrochloric acid and filtered. Few drops of Hager’s reagent (saturated aqueous solution of picric acid) were added to 2 ml of filtrate. A prominent yellow precipitate indicates the test as positive (Pandey and Tripathi, 2014, Yadav et al., 2014, Thangaraj, 2016).
2.5.2. Detection of glycosides
(Kellar – Kiliani test)
One ml of glacial acetic acid, one ml of FeCl3 and one ml of H2SO4 were added to two ml of filtrate (ethanol extract). A green-blue color indicates the presence of glycosides (Parekh and Chanda, 2007, Yadav and Agarwala, 2011).
2.5.3. Detection of flavonoids
(Shinoda test)
A piece of magnesium ribbon and one ml of concentrated HCl were applied to two–three ml of (methanol extract) filtrate. The solution's pink-red or red coloration indicates the presence of flavonoids (Kumar et al., 2007).
(Alkaline Reagent test)
Few drops of NaOH solution were added to a few ml of (methanol extract) filtrate, and then a few ml of diluted HCl was added. Flavonoid existence suggests a yellow solution with NaOH that becomes colorless after adding diluted HCl (Pandey and Tripathi, 2014).
2.5.4. Detection of tannins
(Braymer’s test)
Few drops of FeCl3 (10%) solution were applied to two–three ml of (ethanol extract) filtrate. The existence of tannins suggests a solution of greenish grey or dark blue color (Kumar et al., 2007, Parekh and Chanda, 2007, Yadav et al., 2014).
2.5.5. Detection of saponins
(Foam test)
Five ml of distilled water was mixed to five ml of filtrate (aqueous extract) with vigor shaking. The existence of saponins suggests a stable froth (Parekh and Chanda, 2007, Ukoha et al., 2011, Yadav et al., 2014).
2.5.6. Detection of resins
(Precipitate test)
Twenty ml distilled water was added to fifteen ml of filtrate (ethanol extract). A precipitate indicating the presence of resins (Ukoha et al., 2011, Aldhebiani and Mufarah, 2017).
3. Results
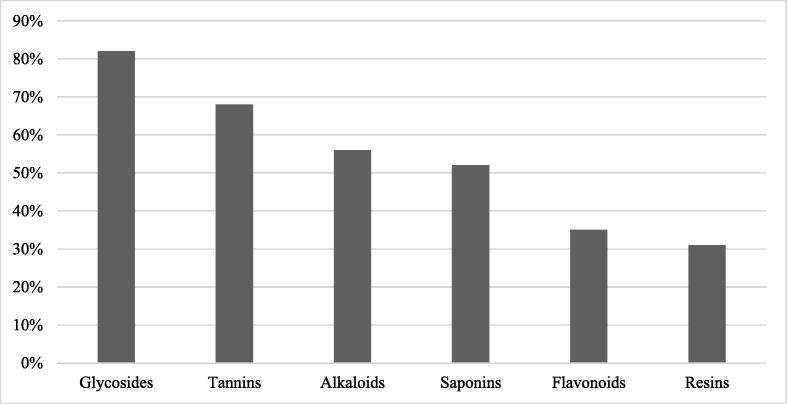
A total of 85 different medicinal plants belong to 37 families used in the traditional treatment in Jeddah were tested for the presence of the phytochemical content including alkaloids, glycosides, flavonoids, tannins, saponins and resins (Table 1). The most commonly distributed compounds among medicinal plants used in the traditional treatment in Jeddah were glycosides (82%; 70 species), tannins (68%; 58 species), alkaloids (56%; 48 species), saponins (52%, 44 species) and flavonoids (35%; 30 species). On the other hand, the least commonly distributed compounds were resins (31%; 26 species; Fig. 2). All species of Apiaceae, Lamiaceae, Zingiberaceae, Fabaceae and Asteraceae in the current study had glycosides. Plants representing Apiaceae in this study had the highest number of phytochemicals groups. All the six groups of chemical compounds were found in seeds of Cuminum cyminum L., Pimpinella anisum L. and Trigonella foenum-graecum L.
Fig. 2.
The percentage of distribution of phytochemical groups among medicinal plants used in the traditional treatment in Jeddah.
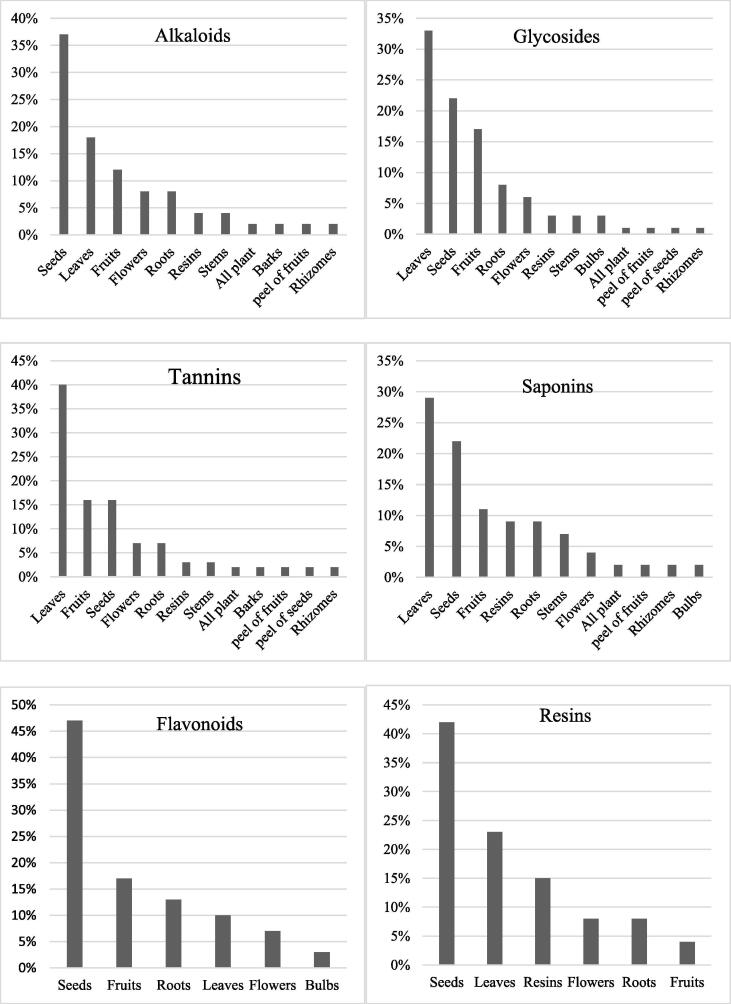
Thirteen various parts of plants were tested including (all plant, bark, bulbs, flowers, fruits, leaves, peel of seeds, peel of fruits, resins, rhizomes, roots, seeds and stems). Most of plant species were tested one part of the plant only, while two species were tested two parts of the plant used in medicine. Out of the 70 species had glycosides, the most parts of these species had glycosides were leaves (33%; 24 species) followed by seeds (22%; 16 species) and fruits (17%; 12 species). Of the 58 species which had tannins, most of the tannins were found in leaves (40%; 23 species) followed by fruits and seeds (16%; 9 species each). Of the 48 species that had alkaloids, the majority of alkaloids were found in seeds (37%; 18 species) followed by leaves (18%; 9 species) and fruits (12%; 6 species). Out of the 44 species had saponins, the most parts of these species had saponins were leaves (29%; 13 species) followed by seeds (22%; 10 species) and fruits (11%; 5 species). Of the 30 species which had flavonoids, the most parts of these species had flavonoids were seeds (47%; 14 species) followed by fruits (17%; 5 species) and roots (13%; 4 species). Out of 26 species had resins, the most parts of these species had resins were seeds (42%; 11 species) followed by leaves (23%; 6 species) and resins (15%; 4 species; Fig. 3).
Fig. 3.
The percentage of distribution of each phytochemical group among different parts of plants.
4. Discussion
Traditional uses in folk medicine are justified scientifically. The reasons behind using these medicinal plants in traditional treatment in Jeddah could be either cultural or chemical reasons, or both. In the light of the findings of the current study, the chemical reasons are highlighted. Secondary metabolites including glycosides, tannins, alkaloids, saponins, flavonoids and resins contribute significantly towards medicinal as well as physiological activities (Yadav and Agarwala, 2011). Glycosides were the most commonly distributed compounds among these medicinal plants. As well as, all species of Apiaceae, Lamiaceae, Zingiberaceae, Fabaceae and Asteraceae in this study had glycosides. Glycosides have wide medicinal efficacy as they exist in almost all medicinal plants (Yadav et al., 2014). According to previous studies, it has been shown that glycosides have sedative and digestives properties (Galvano et al., 2004, Güçlü-Üstündağ and Mazza, 2007), anticancer (Zhou et al., 2013) and expectorant (Kabera et al., 2014). Therefore, these could be reasons for these families which are considered the most commonly used families and have a high number of uses that are reported in traditional treatment in Jeddah (Alqethami et al., 2020).
The second most commonly distributed compounds among medicinal plants used in folk medicine in Jeddah were tannins. Tannins possess extremely stringent effects. They help in wound healing and inflamed mucosal membranes (Yadav et al., 2014). The tannin containing plant extracts are used as astringents, against diarrhea, as diuretics, against stomach and duodenal tumors, and as anti-inflammatory, antiseptic, and hemostatic pharmaceuticals (Khanbabaee and van Ree, 2001). Alkaloids, saponins and flavonoids are also present in medicinal plants used in traditional medicine in Jeddah. Alkaloids have beneficial effects. They are also known as their sedative properties and they have a powerful effect on the nervous system (Renu, 2005). Saponins have been used for medicinal reasons in plants. Most herbal medicines include saponin (Lacaille-Dubois and Wagner, 1996, Kareru et al., 2008). Flavonoids have antioxidant potentials hence could offer protection against heart disease and cancer (Noroozi et al.,1998). Resins were also found but least common in these medicinal plants. Many resins are antimicrobial and aid in healing wounds (Bernhoft et al., 2010). Hence, the presence of these groups seems to support the efficacy of the use of these medicinal plants in ethnomedicinal practice in Jeddah.
According to Alqethami et al. (2020) most popular medicinal species have wide medicinal uses in traditional medicine in Jeddah were Zingiber officinale Roscoe, followed by Mentha spicata L., Pimpinella anisum, Cuminum cyminum and Foeniculum vulgare Mill. These plants were also widely used in herbal medicine. All the six groups of chemical compounds were found in the seeds of Cuminum cyminum and Pimpinella anisum, which agree with findings of previous studies by (Mushtaq et al., 2014, Al-Snafi, 2016, Islam et al., 2016). In addition, four group of chemical compounds were found together in Zingiber officinale, which were glycosides, flavonoid, tannins and saponin; a similar finding was reported by (Zeeshan et al., 2018). In Mentha spicata all of alkaloids, glycosides, flavonoids and tannins were found, this result is in agreement with the finding reported by Khan et al. (2011). On the other hand, all of alkaloid, glycosides, flavonoid and saponin were found in Foeniculum vulgare, which is in agreement with findings by Beyazen et al. (2017). Hence, the presence of all the six groups of secondary metabolites or the most of them in these five plants could explain why these plants are the most popular medicinal species used in Jeddah.
There are striking similarities in the phytochemical components of plants belonging to the same family. For example, in the current study, Apiaceae was represented by 11 species, all of which tested positive for the presence of glycosides, eight of these species contain alkaloid, eight had tannins, seven contain flavonoid, six contain saponin and only three of these species had Resin. Another example is Lamiaceae, which was represented by 9 species, all of which tested positive for the presence of glycosides. In contrast, all of them lacked resin except one species contain resin. Furthermore, the two species that represent the Arecaceae (Hyphaene thebaica (L.) Mart. and Phoenix dactylifera L.) contain glycosides and tannins, while they lacked alkaloid, flavonoid, saponin and resin.
On the other hand, there are relatively wide differences in the secondary metabolites of plants belonging to the same family. For example, the three representatives of the Myrtaceae had relatively wide differences in the secondary metabolites they contain. This observation is the same for the two species representing the Amaranthaceae. The similarity and wide diversity in the distribution of secondary metabolites among plants in the same family, as revealed in this study, are of taxonomic importance. As a result, the use of phytochemical constituents in plants as a taxonomic tool in classification has wide acceptability (Borokini and Omotayo, 2012).
Secondary metabolites were found in different parts of plants. Leaves and seeds were parts of plants that have the most secondary metabolites compared with other plant parts. Leaves were the most parts have glycosides, tannins and saponins and this conforms with the report of (Aththorick and Berutu, 2018). According to Tripathy (2015) leaves are rich in therapeutically active secondary metabolites and essential oils. In contrast, seeds were the most parts have alkaloids, flavonoids and resins. As Shirley (1998) found that, the majority of seeds are rich in flavonoids. This could explain that why leaves and seeds are the most parts were used in traditional medicine in Jeddah (Alqethami et al., 2020). Plant parts that contain a lot of secondary metabolites will be widely used in traditional medicine (Aththorick and Berutu, 2018).
5. Conclusion
The findings of this study show a wide diversity in secondary metabolite spread between the 85 medicinal plants used in traditional medicine in Jeddah. In addition, it can be said the ethnomedicinal importance of these 85 medicinal plants conform to their secondary metabolite content. Therefore, more research should be carried out on the quantitative analysis of phytochemicals in these 85 medicinal plants used in traditional medicine in Jeddah. For example, estimating the phytochemicals which have antioxidant. Furthermore, there is a need to focus on phytochemical screening on ethnobotanical studies to complete research into traditional medicine which leads to the discovery of new drugs.
6. Notes
The present study was conducted as a part of the doctoral dissertation work by Afnan Alqethami and supervised by Amal Aldhebiani which focused on the diversity of medicinal plants traditionally used in Jeddah city in Saudi Arabia.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
The authors would like to thank the members of the herbarium of King Abdulaziz University for their collaboration and support.
Footnotes
Peer review under responsibility of King Saud University.
Contributor Information
Afnan Alqethami, Email: amqethami@uqu.edu.sa.
Amal Y. Aldhebiani, Email: aaldhebiani@kau.edu.sa.
References
- Al-Snafi A.E. The pharmacological activities of Cuminum cyminum-A review. IOSR J. Pharm. 2016;6(6):46–65. [Google Scholar]
- Aldhebiani A.Y., Mufarah N. Phytochemical screening of some wild plants from Wadi Yalmlam, Saudi Arabia. J. Pharm. Biol. Sci. 2017;12(4):25–27. [Google Scholar]
- Alqethami A., Hawkins J.A., Teixidor-Toneu I. Medicinal plants used by women in Mecca: urban, Muslim and gendered knowledge. J. Ethnobiol. Ethnomed. 2017;13(1):62. doi: 10.1186/s13002-017-0193-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alqethami A., Aldhebiani A.Y., Teixidor-Toneu I. Medicinal plants used in Jeddah, Saudi Arabia: A gender perspective. J. Ethnopharmacol. 2020;257:112899. doi: 10.1016/j.jep.2020.112899. [DOI] [PubMed] [Google Scholar]
- American Anthropological Association, 2012. Statement on ethics: principles of responsibility. Access date, August 25, 2018, from: http://www.aaanet.org/profdev/ethics/
- Aqil F., Ahmad I., Mehmood Z. Antioxidant and free radical scavenging properties of twelve traditionally used Indian medicinal plants. Turkish J. Biol. 2006;30(3):177–183. [Google Scholar]
- Aththorick T.A., Berutu L. Ethnobotanical study and phytochemical screening of medicinal plants on Karonese people from North Sumatra, Indonesia. J. Phys.: Conf. Ser. 2018;1116:052008. doi: 10.1088/1742-6596/1116/5/052008. [DOI] [Google Scholar]
- Bako S.P., Bakfur M.J., John I., Bala E.I. Ethnomedicinal and Phytochemical profile of some savanna plant species in Nigeria. Int. J. Botany. 2005;1(2):147–150. [Google Scholar]
- Balandrin M., Klocke J., Wurtele E., Bollinger W. Natural plant chemicals: sources of industrial and medicinal materials. Science. 1985;228(4704):1154–1160. doi: 10.1126/science.3890182. [DOI] [PubMed] [Google Scholar]
- Bernhoft A., Siem H., Bjertness E., Meltzer M., Flaten T., Holmsen E. The Norwegian Academy of Science and Letters; Oslo: 2010. Bioactive compounds in plants–benefits and risks for man and animals. [Google Scholar]
- Beyazen A., Dessalegn E., Mamo W. Phytochemical screening and biological activities of leaf of Foeniculum vulgare (Ensilal) World J. Agric. Sci. 2017;13(1):01–10. [Google Scholar]
- Borokini T.I., Omotayo F.O. Phytochemical and ethnobotanical study of some selected medicinal plants from Nigeria. J. Med. Plants Res. 2012;6(7):1106–1118. [Google Scholar]
- Briskin D.P. Medicinal plants and phytomedicines. Linking plant biochemistry and physiology to human health. Plant Physiol. 2000;124(2):507–514. doi: 10.1104/pp.124.2.507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cai Y.Z., Sun M., Xing J., Luo Q., Corke H. Structure–radical scavenging activity relationships of phenolic compounds from traditional Chinese medicinal plants. Life Sci. 2006;78(25):2872–2888. doi: 10.1016/j.lfs.2005.11.004. [DOI] [PubMed] [Google Scholar]
- Catalogue of Life, 2015. Access date March 27, 2019, from: http://www.catalogueoflife.org.
- Chaudhary, S.A., 1999, 2000, 2001. Flora of the Kingdom of Saudi Arabia, Riyadh: Ministry of Agriculture and Water.
- Cunningham A.B. United Nations Educational, Scientific and Cultural Organization; Paris: 1993. African medicinal plants. [Google Scholar]
- Edeoga H.O., Omosun G., Uche L.C. Chemical composition of Hyptis suaveolens and Ocimum gratissimum hybrids from Nigeria. Afr. J. Biotechnol. 2006;5(10):892–895. [Google Scholar]
- Galvano F., La Fauci L., Lazzarino G., Fogliano V., Ritieni A., Ciappellano S., Battistini N.C., Tavazzi B., Galvano G. Cyanidins: metabolism and biological properties. J. Nutritional Biochem. 2004;15(1):2–11. doi: 10.1016/j.jnutbio.2003.07.004. [DOI] [PubMed] [Google Scholar]
- Gazzaneo L.R.S., De Lucena R.F.P., de Albuquerque U.P. Knowledge and use of medicinal plants by local specialists in an region of Atlantic Forest in the state of Pernambuco (Northeastern Brazil) J. Ethnobiol. Ethnomed. 2005;1(1):9. doi: 10.1186/1746-4269-1-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Güçlü-Üstündağ Ö., Mazza G. Saponins: properties, applications and processing. Crit. Rev. Food Sci. Nutr. 2007;47(3):231–258. doi: 10.1080/10408390600698197. [DOI] [PubMed] [Google Scholar]
- Heinrich M., Ankli A., Frei B., Weimann C., Sticher O. Medicinal plants in Mexico: healers' consensus and cultural importance. Soc. Sci. Med. 1998;47(11):1859–1871. doi: 10.1016/s0277-9536(98)00181-6. [DOI] [PubMed] [Google Scholar]
- International Society of Ethnobiology, 2006. ISE Code of Ethics (with 2008 additions). Access date, August 25, 2018, from: http://www.ethnobiology.net/code-of-ethics/
- Islam Z.M., Khan K., Rakhshanda S., Mahdi R., Chowdhury I.M. Antibacterial and phytochemical screening of Pimpinella anisum through optimized extraction procedure. Asian J. Sci. Technol. 2016;7(11):3912–3918. [Google Scholar]
- Kabera J.N., Semana E., Mussa A.R., He X. Plant secondary metabolites: biosynthesis, classification, function and pharmacological properties. J. Pharm. Pharmacol. 2014;2:377–392. [Google Scholar]
- Kareru P.G., Keriko J.M., Gachanja A.N., Kenji G.M. Direct detection of triterpenoid saponins in medicinal plants. Afr. J. Tradit. Complement. Altern. Med. 2008;5(1):56–60. doi: 10.4314/ajtcam.v5i1.31257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kennedy D.O., Wightman E.L. Herbal extracts and phytochemicals: plant secondary metabolites and the enhancement of human brain function. Adv. Nutrit. 2011;2(1):32–50. doi: 10.3945/an.110.000117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khan F.A., Hussain I., Farooq S., Ahmad M., Arif M., Rehman I.U. Phytochemical screening of some Pakistanian medicinal plants. Middle-East J. Sci. Res. 2011;8(3):575–578. [Google Scholar]
- Khanbabaee K., van Ree T. Tannins: classification and definition. Royal Soc. Chem. 2001;18(6):641–649. doi: 10.1039/b101061l. [DOI] [PubMed] [Google Scholar]
- Kumar G.S., Jayaveera K.N., Kumar C.K., Sanjay U.P., Swamy B.M., Kumar D.V. Antimicrobial effects of Indian medicinal plants against acneinducing bacteria. Trop. J. Pharm. Res. 2007;6(2):717–723. [Google Scholar]
- Kumar S., Satapathy M.K. Medicinal plants in an Urban environment; herbaceous medicinal flora from the campus of Regional Institute of Education, Bhubaneswar, Odisha. Int. J. Pharm. Life Sci. 2011;2(10):1206–1210. [Google Scholar]
- Lacaille-Dubois M.A., Wagner H. A review of the biological and pharmacological activities of saponins. Phytomedicine. 1996;2(4):363–386. doi: 10.1016/S0944-7113(96)80081-X. [DOI] [PubMed] [Google Scholar]
- Migahid A.M. King Saud University Press; Riyadh: 1978. Flora of Saudi Arabia. [Google Scholar]
- Mushtaq A., Ahmad M., Jabeen Q., Saqib A., Wajid M., Akram M.A. Hepatoprotective investigations of Cuminum cyminum dried seeds in nimesulide intoxicated albino rats by phytochemical and biochemical methods. Int. J. Pharm. Pharm. Sci. 2014;6(4) [Google Scholar]
- Nalawade S.M., Tsay H.-S. In vitro propagation of some important Chinese medicinal plants and their sustainable usage. In Vitro Cell. Dev. Biol. - Plant. 2004;40(2):143–154. [Google Scholar]
- Noroozi M., Angerson W.J., Lean M.E. Effects of flavonoids and vitamin C on oxidative DNA damage to human lymphocytes. Am. J. Clin. Nutrit. 1998;67(6):1210–1218. doi: 10.1093/ajcn/67.6.1210. [DOI] [PubMed] [Google Scholar]
- Pandey A., Tripathi S. Concept of standardization, extraction and pre phytochemical screening strategies for herbal drug. J. Pharmacognosy Phytochem. 2014;2(5):115–119. [Google Scholar]
- Parekh J., Chanda S. In vitro antimicrobial activity and phytochemical analysis of some Indian medicinal plants. Turkish J. Biol. 2007;31(1):53–58. [Google Scholar]
- Renu R.S. Useful metabolites from plant tissue cultures. Biotechnology. 2005;4(2):79–93. [Google Scholar]
- Shirley B.W. Flavonoids in seeds and grains: physiological function, agronomic importance and the genetics of biosynthesis. Seed Sci. Res. 1998;8(4):415–422. [Google Scholar]
- Thangaraj P. Springer; Geneva: 2016. Pharmacological assays of plant-based natural products. [Google Scholar]
- Theis N., Lerdau M. The evolution of function in plant secondary metabolites. Int. J. Plant Sci. 2003;164(S3):S93–S102. [Google Scholar]
- Thomas, J., 2011. Flora of Saudi Arabia—checklist, 2011. Access date, 25 September 2019, from: http://www.plantdiversityofsaudiarabia.info/Biodiversity-Saudi-Arabia/Flora/Checklist/Cheklist.htm
- Tripathy S. Importance of plants and animals in medicine. J. Exp. Zool. India. 2015;18(2):531–543. [Google Scholar]
- Ukoha P.O., Cemaluk E.A., Nnamdi O.L., Madus E.P. Tannins and other phytochemical of the Samanaea saman pods and their antimicrobial activities. Afr. J. Pure Appl. Chem. 2011;5(8):237–244. [Google Scholar]
- UN-Habitat, 2018. Jeddah CPI profile 2018, future Saudi Cities Programme, Ministry of Municipal and Rural Affairs.
- Yadav M., Chatterji S., Gupta S.K., Watal G. Preliminary phytochemical screening of six medicinal plants used in traditional medicine. Int. J. Pharm. Pharm. Sci. 2014;6(5):539–542. [Google Scholar]
- Yadav R.N.S., Agarwala M. Phytochemical analysis of some medicinal plants. J. Phytol. 2011;3(12):10–14. [Google Scholar]
- Zeeshan U., Barkat M.Q., Mahmood H.K. Phytochemical and antioxidant screening of Cassia angustifolia, Curcuma zedoaria, Embelia ribes, Piper nigrum, Rosa damascena, Terminalia belerica, Terminalia chebula, Zingiber officinale and their effect on stomach and liver. Matrix Sci. Pharma. 2018;2(2):15–20. [Google Scholar]
- Zhou Q., Liang D., Deng A., Zhang J., Wu C., Nie Z., jiang J., Wang Y.i. Antitussive, expectorant and bronchodilating effects of ethanol extract of Sorghum bicolor (L.) Moench roots. J. Ethnopharmacol. 2013;149(1):297–302. doi: 10.1016/j.jep.2013.06.038. [DOI] [PubMed] [Google Scholar]