Abstract
The aim of the present study was to investigate the comparative effects of pesticides Chlorfenapyr, Dimethoate and Acetamiprid on the health of Cirrhinus mrigala under long term exposure. Eighty C. mrigala were divided in four equal groups; one control and three treated groups. The blood was collected from both control and treated groups at intervals of 10th, 20th and 30th days for hemato-biochemistry and histopathological alterations. The result indicates significant difference (P < 0.05) in RBCs, Hb, PCV and MCHC whereas elevation in WBCs and Platelets counts were recorded. In 10th day sampling, MCV value of Dimethoate and Acetamiprid treatment had no difference in comparison with the control group, however it is significantly increased (P < 0.05) in rest of sampling. The MCH value of exposed fish showed significant increased (P < 0.05) after 20th and 30th days for Chlorfenapyr and after 30th days for Acetamiprid exposure while insignificantly increased for rest of sampling. It was also found that these pesticides significantly decrease (p < 0.05) the T3 and T4 levels while increase in the TSH, cortical, ALP, AST, ALT and LDH levels in the serum of the treated fishes in contrast to control group. Similarly, histopathological analysis of gills and liver showed significant alterations in all the treated groups. Toxicity trends of these pesticides was ranked as Chlorfenapyr > Acetamiprid > Dimethoate. It is concluded that indiscriminate use of such pesticides poses a noxious threat to non-target organisms, harm the ecosystems and jeopardizes human health.
Keywords: C. mrigala, Chlorfenapyr, Acetamiprid, Dimethoate, Histopathology and hematology
1. Introduction
Cirrhinus mrigala is an indigenous major carp widely found in freshwater of Indo-Pak sub-continent is also called Mrigal or Nain. It is bottom feeder and significantly important species in polyculture (Singh et al., 2018, Ujjania and Soni, 2018). Pesticide residues enter into the aquatic environment along with water runoff and where they seriously affect the non-target species like fishes and finally enter to the food chain threatening the biodiversity of the nature and ecological equilibrium (Dar et al., 2016). In addition, the pesticides also possess bioaccumulation property and bioaccumulate in the tissues of aquatic organisms (Maurya and Malik, 2016, Yadav et al., 2018). It is estimated that the total pollution surface water receive about 50% from agricultural sources (Gavrilescu et al., 2015). Chlorfenapyr is a halogenated pyrrole-based insecticide which is presently applied on different crops for the control insects and mites (Ullah et al., 2016). Acetamiprid is a neonicotinoid chemical pesticide (Shengyun et al., 2005) which is extensively applied in agriculture for pest management in numerous countries (Zhang et al., 2011). Dimethoate is an organophosphorus insecticide used against a wide range of insects and mites, which is sanctioned for application in the European Union and in other different countries of the world (Tarbah et al., 2007). As fishes are very sensitive to the presence of pollutants in water, so they can be used as bioindicator in the study of different aquatic ecosystems. In comparison to other aquatic bioindicators fishes occupy the top position of the aquatic trophic, therefor they offer an integrated image of whole aquatic ecosystem. They represents a risk to human health when these fishes are used as a source of food (Abdel-Moneim et al., 2012). Fish have the ability to accumulate toxic substances from environment in different body parts (Aghoghovwia et al., 2016, Izah and Angaye, 2016). Hematological investigation offers a blueprint of health condition and internal environment of the animal which indicate internal alterations before the animal display any external indications of toxic anxiety (Harabawy and Ibrahim, 2014, Kumar et al., 2011, Narra et al., 2015, Osman et al., 2010, Saravanan et al., 2011). Histopathological alterations have been commonly used as bio-indicators in assessing the health of fish exposed to pollutants, both in the laboratory as well as field studies. Therefor histopathological alterations can be used as indicators for monitoring the effects of numerous contaminants on organisms and are a reflection of the overall health status of the whole ecosystem (Drishya et al., 2016, Olaniyi, 2020). To evaluate the health status of aquatic organisms, alterations in biochemical parameters could be used as biomarker (Poopal et al., 2017). The important function thyroid hormone is regulates the growth and development in fishes especially in early stages of life, therefor thyroid disruption due to pollutants hinder the growth in both cultured and wild fish species, reduce fish seed production which lead to decline in wild fisheries (Nugegoda and Kibria, 2017). Cortisol is one of the most active glucocorticoids hormone produced by adrenal cortex and is involved in the metabolism of protein, lipids and carbohydrates (Simonato et al., 2013). During stress condition serum cortisol level increases to convert proteins into glucose for more energy production (Bhanu, 2016). The present study aims to evaluate time-dependent negative effect of Chlorfenapyr, Dimethoate and Acetamiprid exposure on different health biomarkers of on a particular freshwater fish (C. mrigala) during chronic toxicity.
2. Materials and methods
2.1. Fish collection
One hundred healthy freshwater fish C. mrigala an Indian major carp having average weight 70 ± 8.0 g was collected from fish Farm service road Karma Panjab, Pakistan which is relatively free from pollutants. Fish were transported to the laboratory of Zoology Department, Govt Postgraduate College Haripur, within well packed polythene bags containing aerated water.
2.2. Acclimatization
The fish were acclimatized in laboratory environment for 14 days before beginning of the experiment and fed with commercial fish food. The aquariums were connected with constant system of aeration with a 12 ± 1:12 ± 1 light–dark cycle. Dissolved oxygen concentration was retained from 93% to 98%, temperature from 20 to 22 °C and pH from 7.0 to 7.6. In order to reduce the metabolic waste contamination, the water was renewed after every 24 h.
2.3. Chemicals
Three different formula grade pesticides namely Chlorfenapyr, Acetamiprid and Dimethoate were used in the present study purchased from local Agro-chemical market Haripur KP, Pakistan.
2.4. Experimental design
The experimental fish was exposed to three different formula grade pesticides namely Chlorfenapyr, Acetamiprid and Dimethoate in water at concentration of 3 ppb for each chemical. The acclimatized fish was randomly divided into four groups with 20 fish in each group. Group-I serve as primary control group and maintained under normal conditions of control water for the whole periods of the experiment. The Groups-II, III and IV was exposed to Chlorfenapyr, Dimethoate and Acetamiprid respectively. Every effort was made to provide optimal condition for fish in order to avoid mortality during experimental time period. The experiment was conducted for a period of 30-day with 10 days sampling frequency.
2.5. Hematological analysis
Five fish were randomly selected from both control and treated groups upon completion of the sublethal exposure period of 10th, 20th and 30th days to pesticides, and anesthetized with clove oil. Blood was collected from the caudal vein by the using heparin coated syringe and shifted to EDTA tubes for estimation of total erythrocyte count, total leucocyte count, hemoglobin percentage, hematocrit, MCV, MCH, MCHC and platelets count through hematology analyzer.
2.6. Biochemical analysis
Similarly, blood was collected from the caudal vein by using syringe and shifted to clotted tubes. Centrifugation of collected blood was done at 3000 rpm for 10 min in order to obtained the serum, for analysis of T3, T4, TSH, Cortisol ALP, AST, ALT and LDH through biochemical analyzer.
2.7. Histological analysis
For histological investigation gills and liver samples were collected from both control and pesticides treated groups after each sampling period (10th, 20th & 30th days). The collected tissues were rinsed with saline solution (0.9% NaCl) to remove, blood, mucus and debris stick to the tissues and fixed in 10% formaldehyde. After this, these tissues were dehydrated through a graded alcohol series as dehydrating agent. The dehydrated tissues were cleared in xylene and then embedded in paraffin wax. Tissue sections were cut at 5 μm using a rotary microtome and stained with hematoxylin and eosin. The prepared slides were analyzed under an Olympus optical microscope and photographed at 40× and 100× magnification.
2.8. Statistical analysis
By using SPSS software (version 24) statistical significance between groups was determined. The data was expressed as mean ± SD. Significant differences between groups were identified using t-test. The significance level was set at P < 0.05.
3. Results
3.1. Physico-chemical properties of water
The physico-chemical properties of water were examined during experiment at regular interval. The recorded values of Mean ± SD values of temperature, dissolve oxygen, pH, conductivity and total hardness were range from 22 ± 2.76 °C, 6 ± 1.92 mg/L, 7.3 ± 0.9, 180 ± 25.13 μM cm−1 and 130 ± 15.45 mg/l respectively.
3.2. Behavioural response of C. mrigala
The behavioural response of C. mrigala on exposure to Chlorfenapyr, Dimethoate and Acetamiprid started after 1-week of treatment. The change in the behavior pattern of C. mrigala exposed to these pesticides as well as control group are summarized in Table 1. No mortality was recorded in Dimethoate treated group, while in Chlorfenapyr and Acetamiprid treated group mortality was recorded after 22 and 26 days respectively.
Table 1.
Impact of Chlorfenapyr, Acetamiprid and Dimethoate on the behavioural pattern of C. mrigala for 30 days.
| Parameters | Duration | Control | Chlorfenapyr | Dimethoate | Acetamiprid |
|---|---|---|---|---|---|
| Colour of skin | 10 days | – | * | – | – |
| 20 days | – | ** | – | * | |
| 30 days | – | *** | * | ** | |
| Loss of balance | 10 days | – | * | – | – |
| 20 days | – | ** | – | * | |
| 30 days | – | *** | * | ** | |
| Hyperactivity | 10 days | – | ** | – | * |
| 20 days | – | * | * | ** | |
| 30 days | – | – | ** | * | |
| Rate of swimming | 10 days | – | ** | – | – |
| 20 days | – | * | * | ** | |
| 30 days | – | * | ** | * | |
| Rate of opercular activity | 10 days | – | ** | * | * |
| 20 days | – | ** | * | ** | |
| 30 days | – | * | ** | * | |
| Surfacing activity | 10 days | – | ** | * | * |
| 20 days | – | * | * | * | |
| 30 days | – | ** | * | ** | |
| Convulsions | 10 days | – | – | – | – |
| 20 days | – | * | – | * | |
| 30 days | – | ** | * | * |
(*) sign indicate abnormal behavioural parameters while (–) sign indicate normal behaviour of C. mrigala.
3.3. Hematological analysis
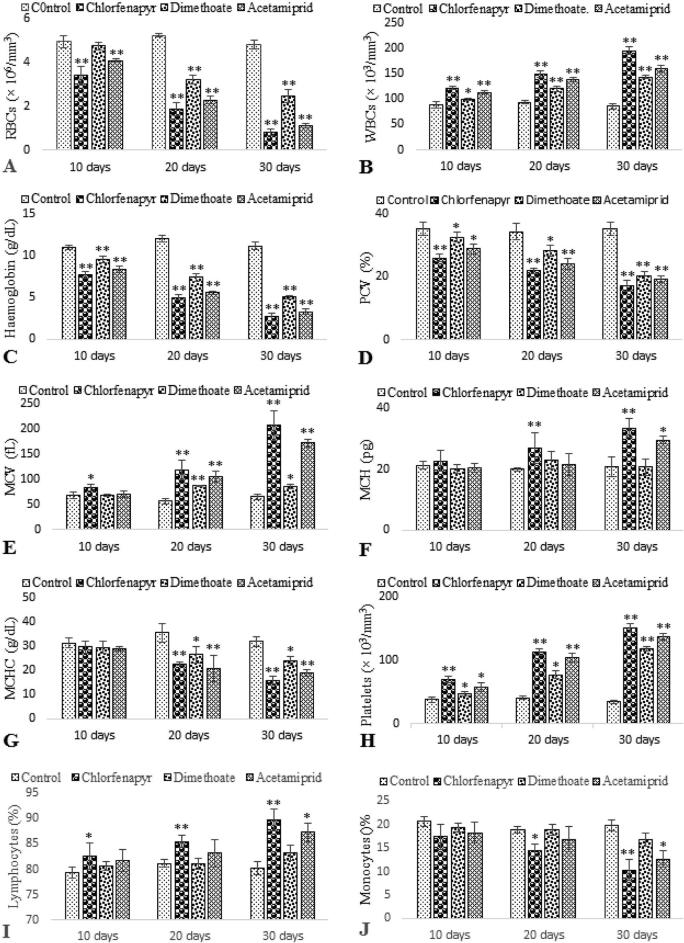
In present study haematological variations in the C. mrigala were evaluated on exposure to sub-lethal concentration of Chlorfenapyr, Dimethoate and Acetamiprid at long term (10, 20 and 30 days) periods for each chemical. Effect of these chemicals on blood parameters including RBCs, WBCs, Hb, Hct, MCV, MCH, MCHC and platelets of C. mrigala are presented in Fig. 1. A time dependent significant decrease in RBCs, Hb and PCV values was observed in blood of C. mrigala after 10th, 20th and 30th days pesticides exposure (Fig. 1. A, C & D). RBCs was insignificantly decrease after 10th exposure to dimethoate. Non-significant decrease in the value of MCHC after 10th days while significant increased after 20th and 30th days were observed for each pesticide (Fig. 1. G). In 10th day sampling, MCV value of Dimethoate and Acetamiprid treatment had no difference in comparison with the control group. However, it is significantly increased in rest of sampling on treatment with Chlorfenapyr, Dimethoate and Acetamiprid compared to control group (Fig. 1. E). A significant increase in MCH level was recorded after 20th and 30th days to Chlorfenapyr and 30th days to Acetamiprid while non-significant increase for rest of sampling frequencies (Fig. 1. F). The values of WBCs and platelets of C. mrigala exposed to pesticides (Chlorfenapyr, Dimethoate and Acetamiprid) exhibited significant increase as compared to control for 10th, 20th and 30th days of exposure (Fig. 1. B & H). Maximum increase or decrease in the values of various haematological parameters was recorded at Chlorfenapyr followed by Acetamiprid and Dimethoate treatment respectively.
Fig 1.
RBC, WBC, Hb, Hct, MCV, MCH, MCHC and platelets of C. mrigala after Chlorfenapyr, Dimethoate and Acetamiprid exposure. Values are expressed as mean ± SE from triplicate groups. Bars with * indicate significant differences while Bars with ** indicate highly significant differences between control and treated groups.
3.4. Biochemical analysis
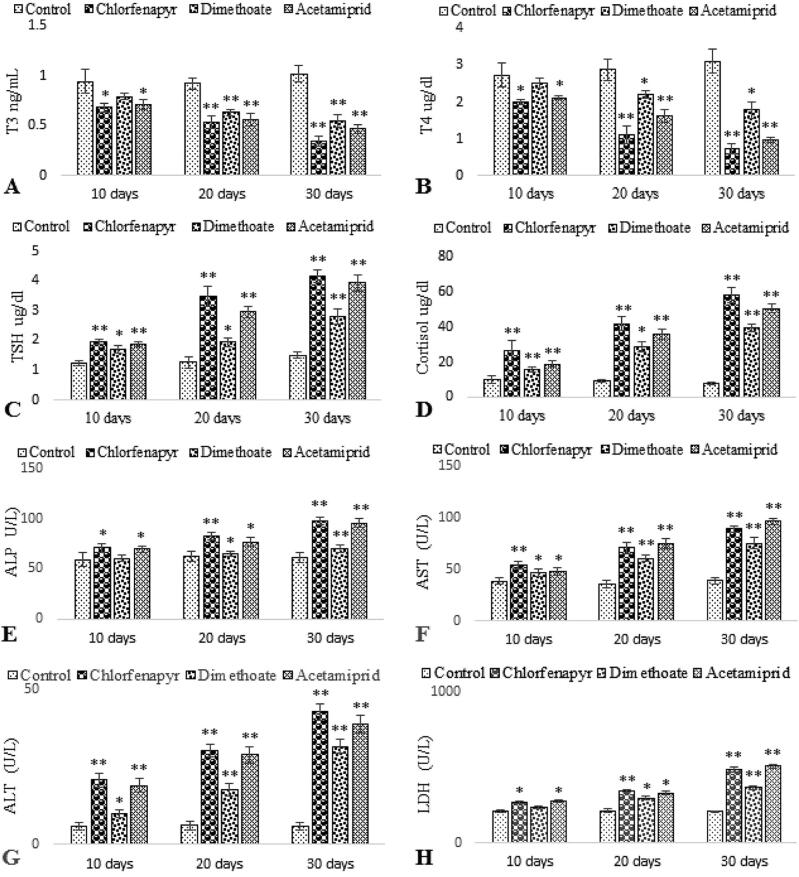
It was evident from the present study that on exposure to Chlorfenapyr, Dimethoate and Acetamiprid during chronic toxicity C. mrigala shows time dependent significant reduction in triiodothyronine (T3) and tetraiodothyronine (T4) activity except for Dimethoate in 10th day sample where they show non-significant decrease (Fig. 2. A & B). In contrast significant increase in the levels of (thyroid stimulating hormone) TSH and cortisol was recorded in all the treated groups as compared to control group (Fig. 2 C & D). The activities of hepatic function enzymes alkaline phosphate (ALP), aspartate aminotransferase (AST) and Alanine aminotransferase (ALT) were significantly elevated on exposure to Chlorfenapyr, Dimethoate and Acetamiprid 10th, 20th and 30th days in contrast to control group (Fig. 2. E, F & G). Similarly, serum lactate dehydrogenase (LDH) was also significantly higher than the control group except for Dimethoate in 10th day sampling where it shows non-significant increase (Fig. 2. H).
Fig 2.
Serum T3, T4, TSH, ALP, AST, ALT and LDH cortisol levels of C. mrigala after Chlorfenapyr, Dimethoate and Acetamiprid exposure. Values are expressed as mean ± SE from triplicate groups. Bars with * indicate significant differences while Bars with ** indicate highly significant differences between control and treated groups.
3.5. Liver histopathology
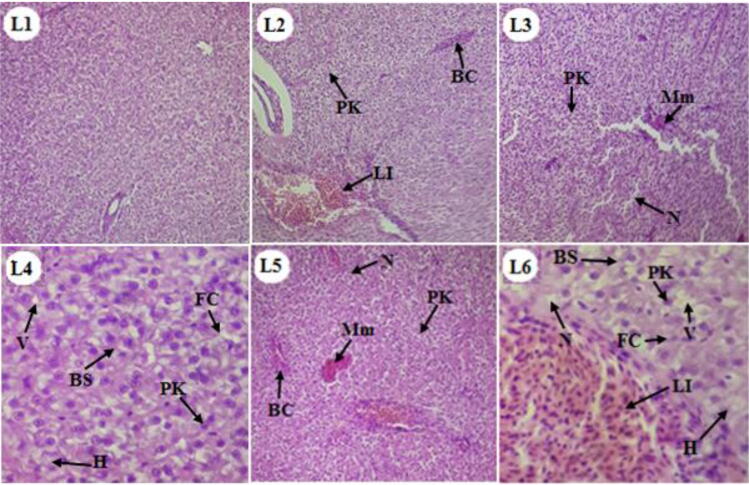
Liver sections of control group fish revealed polygonal hepatocytes containing spherical, large centrally placed nucleus with homogeneous cytoplasm. Sinusoids are seen as communication channels between the hepatocytes containing blood cells and giving chord like structure to the hepatic parenchyma (Fig. 3. L1). After 10 days, the treated fish liver exhibited blood congestion, lymphocytes infiltration, pyknotic nuclei with mild necrosis (Fig. 3. L2). After 20 days, the pesticide inflicted aggregation of melanomacrophage, foamy cells, blood sinusoid dilation, vacuolation and hypertrophy along with blood congestion, lymphocytes infiltration, pyknotic nuclei and necrosis (Fig. 3. L3 & L4). Prolonged treatment with pesticides for 30 days caused severe histopathological alterations in liver (Fig. 3. L5 & L6).
Fig 3.
Light micrographs of H & E stained sections of liver of Cirrhinus mrigala: (L1) Control group liver showing normal structure, (L2 to L6) treated groups liver showing various histopathological alterations including blood congestion (BC), lymphocytes infiltration (LI), pyknotic nuclei (PK), aggregation of melanomacrophage (Mm), necrosis (N), foamy cells (FC), blood sinusoid dilation (BS), vaculation (V) and hypertrophy (H).
3.6. Gills histopathology
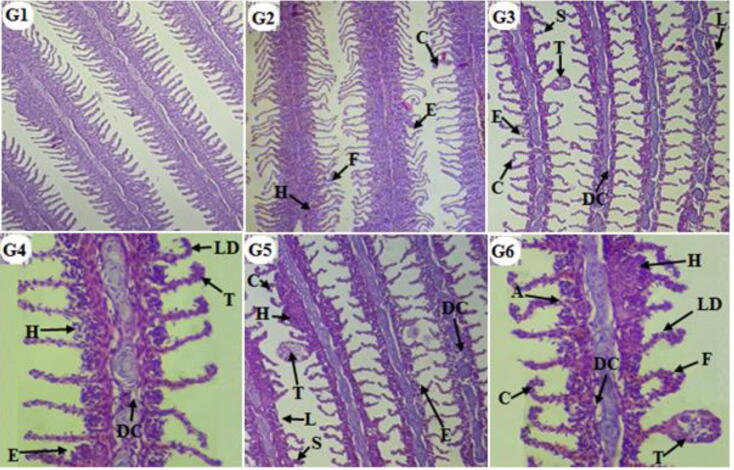
A histological sections of control group gills do not show any morphological anomalies throughout the experimental period (Fig. 4. G1). Compared to control group pesticides treated groups revealed significant histopathological changes in the gill architecture. After 10 days, the treated fish gills revealed fusion of secondary gill lamellae, epithelial lifting of secondary gill lamellae, hyperplasia of gill epithelium and curling of secondary gill lamellae (Fig. 4. G2). The severity of the histopathological alterations was increased in all treated groups after 10 days (Fig. 2. G3 & G4) and 30 days (Fig. 4. G5 & G6) including telangiectasia, shortening of secondary gill lamellae, disruption of cartilaginous core, lamellar disorganization and lamellar atrophy along with fusion of secondary gill lamellae, epithelial uplifting of secondary gill lamellae, curling of secondary gill lamellae and hyperplasia of gill epithelium.
Fig 4.
Light micrographs of H & E stained sections of Gills of Cirrhinus mrigala: (G1) Control group gills showing normal structure, (G2 to G6) treated groups gills showing various histopathological alterations including fusion of secondary gill lamellae (F), epithelial uplifting of secondary gill lamellae (E) hyperplasia of gill epithelium (H), curling of secondary gill lamellae (C), telangiectasia (T), shortening of secondary gill lamellae (S), disruption of cartilaginous core (DC), lamellar disorganization (LD) and lamellar atrophy (A).
4. Discussion
The current investigation was conducted to understand the poisonous level induced by Chlorfenapyr, Acetamiprid and Dimethoate pesticide on hemato-biochemistry and histopathological aspects of C. mrigala. The C. mrigala in the control group were observed to be normal and no mortality was recorded in it while under Chlorfenapyr, Acetamiprid and Dimethoate intoxication it showed several abnormal behaviours i.e. Rapid swimming, surface activity, increase in opercular activity, convulsions, hyperactivity and loss of balance with time. The results were in line with (Ghelichpour et al., 2020) reporting similar results in Cyprinus carpio exposed to lufenuron. Similar behavioral changes were also testified earlier in the Oreochromis mossambicus treated with chlorpyrifos (Ghayyur et al., 2019).
Hematology is among one of the most key method in evaluating the hazard effects of pollutants on aquatic organisms (Hedayati et al., 2019), because blood variables respond to low doses of pollutants (Osman et al., 2018). In our finding, a time dependent significant decline in RBCs count, Hb and PCV was observed among all the treated groups compared to control group, which are in accordance with the study of (Woryi et al., 2020) who also testified significant reduction in RBC count, Hb level and PCV in Clarias gariepinus treated with Paraquat during chronic toxicity. RBCs along with Hb play a key part in providing oxygen to various parts of the body, while packed cell volume (PCV) is the percentage of RBCs in the blood which depend upon the number as well as size of RBCs. Therefore, any change in the size and number of RBCs or Hb change the PCV percentage. Similar to present study, (Ghayyur et al., 2019) found that Chlorpyrifos treated fish had low RBC count, Hb level and PCV. Similarly, (Ko et al., 2019) also reported significant reduction in RBCs count, Hb and PCV of Platichthys stellatus after four weeks exposure to hexavalent chromium. The raise in WBCs number can be linked with more antibodies formation, which aids in recovery and survival of the fish exposed to different pesticides (Malik and Maurya, 2014). In the present investigation significant elevation in total WBC & platelets count was noted in all the treated groups after 10th, 20th and 30th days but maximum elevation was observed in Chlorfenapyr treated group. A similar trend in WBCs & platelets count was reported when O. mossambicus were treated with different concentrations of Chlorpyrifos (Ghayyur et al., 2019). Our results are supported by the similar investigations of (Hasan et al., 2015) in Ctenopharyngodon idella exposed to Endosulfan during acute toxicity. The hematological alterations in mean cell hemoglobin (MCH), mean cell volume (MCV), and mean cell hemoglobin concentration MCHC values also change because the calculation of these parameters depend on RBCs, Hb and PCV values. The selected pesticides tested in this study had significantly increased MCH and MCV values while reduced MCHC value. Similar observations are reported in O. mossambicus exposure to chlorpyrifos (Ghayyur et al., 2019) and C. carpio exposed to silver nanoparticles (Vali et al., 2020). The increase in MCV and MCH values on treatment with pesticides designates that decrease in RBCs count may be resulted from destruction of RBCs or their lessened synthesis in bone marrow.
The findings of present investigation displayed significant declining trend of both T3 & T4 plasma levels with significant elevation plasma TSH level of pesticides exposed fish in contrast to control group. This result was in agreement with that reported by (Bhanu, 2016) in C. carpio treated with cypermethrin. The result of the effect lufenuron in common carp plasma T3 level was in line with the present findings (Ghelichpour et al., 2020). The decrease in plasma T3 level occur due to the reduction in T4 synthesis or its secretion (Li et al., 2008). In fishes thyroid hormones regulate osmoregulation, growth, development, skin pigmentation, reproduction, metamorphosis, behaviour and all aspects of protein and lipid metabolism (Lacasana et al., 2010, Yu et al., 2015). But a number of pesticides are found to decrease the activity levels of T3 and T4 in freshwater fishes (Ghelichpour et al., 2017, Khatun and Mahanta, 2014, Nugegoda and Kibria, 2017, Zhang et al., 2013). Cortisol is an adrenocortical steroid hormone mainly involve in ions regulation and energy metabolism, now a day it is frequently used as marker in ecotoxicological studies because it indicates the degree of stress faced by an organism (Ramesh et al., 2009). In our study, a significant increase in cortisol level was found in the serum of pesticides exposed fishes. This finding agrees with a recent study of (Woo and Chung, 2020) who reported a significant increase in cortisol level of C. carpio treated with various concentrations of trichlorfon. Similar observations were also reported by many researchers in various fish species like O. mossambicus treated with Chlorpyrifos (Ghayyur et al., 2019) Chanos chanos treated with endosulfan (Kumar et al., 2016), Scatophagus argus treated with dazinon (Ghasemzadeh et al., 2015) and Labeo rohita treated with deltamethrin (Suvetha et al., 2015). As liver of fish is one of the prime targeted organ for various types of water pollutants due to its involvement in removal and detoxification of harmful substances showing alterations in biochemistry (Salamat and Zarie, 2012). In this investigation, pesticides treated fish exhibited significant elevation in serum ALT, AST, ALP, and LDH level. Increase in the level of these enzymes is due to oxidative stress caused by pesticides treatment which result in the cell membrane destruction of hepatocytes and outflow of these enzymes to the blood (Rahman et al., 2019). The significant increase in serum ALT, AST, ALP, and LDH level was testified in C. carpio treated with profenofos (Rahman et al., 2020). Similar findings were also reported in serum of Oreochromis niloticus exposed to endosulfan, C. carpio exposed to lufenuron, Oreochromis niloticus exposed to deltamethrin (Dawood et al., 2020a, Ghelichpour et al., 2020, Hussein et al., 2019).
Our results demonstrate that Chlorfenapyr, Dimethoate and Acetamiprid severely affects the gills and liver of C. mrigala and the degree of histopathological alterations are time dependent i.e. the severity of alterations increased with time of exposure. Liver is an important organ of the body controlling many functions like homeostasis, detoxification, enzyme production and metabolism etc therefore serve as a reliable biomarker organ for eco-toxicological studies (Sharma et al., 2019). The histopathological alterations detected in liver during the present investigation are blood congestion, lymphocytes infiltration, pyknotic nuclei, aggregation of melanomacrophage, necrosis, foamy cells, blood sinusoid dilation, vacuolation and hypertrophy. These alterations are in agreement with those observed in O. niloticus treated with deltamethrin (Dawood et al., 2020b) and in Venus verrucosa exposed to lambda-cyhalothrin (Fouzai et al., 2020). Our findings are also coherent with (Sharma and Jindal, 2020), reported similar alteration in Catla catla treated with 0.21 μg/L and 0.41 μg/L of cypermethrin for 45 days. Similar liver lesions in fish liver were also reported on exposure to different pesticide (Gunal et al., 2020, Hasan et al., 2015, Khan et al., 2019, Qureshi et al., 2016). Due to large surface area of gills and their direct communication with the outside aquatic environment, fish gills are considered a chief target organ of pollutants and fluctuations in water quality (Macirella et al., 2019). In current investigations significant histopathological alterations were reported in the gill architecture of all the treated groups, the alterations in the gills are very much similar to alterations caused by cypermethrin in C. catla at the concentration of 0.12 μg/L and 0.41 μg/L for a period of 15th, 30th and 45th day treatment (Sharma and Jindal, 2020). Recent research using deltamethrin (Dawood et al., 2020b) also validated similar alterations in gills architecture of O. niloticus. All these morphological changes in the gills especially gill epithelium uplifting and hyperplasia of the epithelium cells dilate the space among the exterior medium and the blood flow which act as an obstacle to prevent the uptake of unwanted substances (Raibeemol and Chitra, 2016). Similar alterations were also reported in gills of Carassius auratus treated with chlorpyrifos (Macirella et al., 2020), C. carpio treated with endosulfan (Khan et al., 2019) and L. rohita treated with sub-lethal concentration of cypermethrin.
5. Conclusion
This investigation clearly demonstrated the adverse impacts of Chlorfenapyr, Dimethoate and Acetamiprid pesticides on selected hormonal, haematological and histopathological biomarker of a freshwater fish C. mrigala. It is concluded that parameter studied in present investigations can be used effectively as potential biomarkers of pesticides toxicity to the fish as well as other aquatic organisms in the field of environmental biomonitoring.
Acknowledgments
The work presented here was conducted by the author Mr. Shehzad Ghayyur for the fulfillment of his PhD (Zoology) Dissertation. This study was supported by the Higher Education Commission of Pakistan (10108).
Footnotes
Peer review under responsibility of King Saud University.
Contributor Information
Muhammad Fiaz Khan, Email: fiazkhanhu333@gmail.com.
Samina Qamer, Email: saminabee@gmail.com.
References
- Abdel-Moneim A.M., Al-Kahtani M.A., Elmenshawy O.M. Histopathological biomarkers in gills and liver of Oreochromis niloticus from polluted wetland environments, Saudi Arabia. Chemosphere. 2012;88(8):1028–1035. doi: 10.1016/j.chemosphere.2012.04.001. [DOI] [PubMed] [Google Scholar]
- Aghoghovwia O.A., Ohimain E.I., Izah S.C. Bioaccumulation of Heavy metals in different tissues of some commercially important fish species from Warri River, Niger Delta, Nigeria. Biotechnol. Res. 2016;2(1):25–32. [Google Scholar]
- Bhanu A.P. Disrupting action of cypermethrin on thyroid and cortisol hormones in the serum of Cyprinus carpio. J. Entomol. Zool. Studi. 2016;4:340–341. [Google Scholar]
- Dar S.A., Yousuf A.R., Balkhi M. An introduction about genotoxicology methods as tools for monitoring aquatic ecosystem: present status and future perspectives. Fish Aquac. J. 2016;7(1):1–11. [Google Scholar]
- Dawood M.A., AbdEl-Kader M.F., Moustafa E.M., Gewaily M.S., Abdo S.E. Growth performance and hemato-immunological responses of Nile tilapia (Oreochromis niloticus) exposed to deltamethrin and fed immunobiotics. Environ. Sci. Pollut. Res. 2020:1–10. doi: 10.1007/s11356-020-07775-8. [DOI] [PubMed] [Google Scholar]
- Dawood M.A., Abdo S.E., Gewaily M.S., Moustafa E.M., SaadAllah M.S., AbdEl-Kader M.F., Hamouda A.H., Omar A.A., Alwakeel R.A. The influence of dietary β-glucan on immune, transcriptomic, inflammatory and histopathology disorders caused by deltamethrin toxicity in Nile tilapia (Oreochromis niloticus) Fish Shellfish Immunol. 2020;98:301–311. doi: 10.1016/j.fsi.2020.01.035. [DOI] [PubMed] [Google Scholar]
- Drishya M., Ambikadevi A., Aswin B. Histopathological changes in the gills of fresh water fish, Catla catla exposed to electroplating effluent. Int. J. Fisheries Aquatic. 2016;4:13–16. [Google Scholar]
- Fouzai C., Trabelsi W., Bejaoui S., Telahigue K., Rabeh I., Nechi S., Chelbi E., Soudani N. Cellular toxicity mechanisms of lambda-cyhalothrin in Venus verrucosa as revealed by fatty acid composition, redox status and histopathological changes. Ecol. Ind. 2020;108 [Google Scholar]
- Gavrilescu M., Demnerová K., Aamand J., Agathos S., Fava F. Emerging pollutants in the environment: present and future challenges in biomonitoring, ecological risks and bioremediation. New Biotechnol. 2015;32(1):147–156. doi: 10.1016/j.nbt.2014.01.001. [DOI] [PubMed] [Google Scholar]
- Ghasemzadeh J., Sinaei M., Bolouki M. Biochemical and histological changes in fish, spotted scat (Scatophagus argus) exposed to diazinon. Bull. Environ. Contamin. Toxicol. 2015;94(2):164–170. doi: 10.1007/s00128-014-1454-8. [DOI] [PubMed] [Google Scholar]
- Ghayyur S., Tabassum S., Ahmad M.S., Akhtar N., Khan M.F. Effect of Chlorpyrifos on Hematological and Seral Biochemical Components of Fish Oreochromis mossambicus. Pakistan J. Zool. 2019;51(3):1047–1052. [Google Scholar]
- Ghelichpour M., Mirghaed A.T., Hoseini S.M., Jimenez A.P. Plasma antioxidant and hepatic enzymes activity, thyroid hormones alterations and health status of liver tissue in common carp (Cyprinus carpio) exposed to lufenuron. Aquaculture. 2020;516 [Google Scholar]
- Ghelichpour M., Taheri Mirghaed A., Mirzargar S.S., Joshaghani H., Ebrahimzadeh Mousavi H. Plasma proteins, hepatic enzymes, thyroid hormones and liver histopathology of Cyprinus carpio (Linnaeus, 1758) exposed to an oxadiazin pesticide, indoxacarb. Aquac. Res. 2017;48(11):5666–5676. [Google Scholar]
- Gunal A.C., Erkmen B., Pacal E., Arslan P., Yildirim Z., Erkoc F. Sub-lethal Effects of Imidacloprid on Nile Tilapia (Oreochromis niloticus) Water Air Soil Pollut. 2020;231(1):4. [Google Scholar]
- Harabawy A.S., Ibrahim A.T.A. Sublethal toxicity of carbofuran pesticide on the African catfish Clarias gariepinus (Burchell, 1822): Hematological, biochemical and cytogenetic response. Ecotoxicol. Environ. Saf. 2014;103:61–67. doi: 10.1016/j.ecoenv.2013.09.022. [DOI] [PubMed] [Google Scholar]
- Hasan Z., Ghayyur S., Hassan Z.U., Rafique S. Histomorphometric and Hematological Profile of Grass Carp (Ctenopharyngodon idella) during Acute Endosulfan Toxicity. Pakistan Veterinary J. 2015;35(1):23–27. [Google Scholar]
- Hedayati S.A., Farsani H.G., Naserabad S.S., Hoseinifar S.H., Van Doan H. Protective effect of dietary vitamin E on immunological and biochemical induction through silver nanoparticles (AgNPs) inclusion in diet and silver salt (AgNO3) exposure on Zebrafish (Danio rerio) Comp. Biochem. Physiol. C: Toxicol. Pharmacol. 2019;222:100–107. doi: 10.1016/j.cbpc.2019.04.004. [DOI] [PubMed] [Google Scholar]
- Hussein M.M., Elsadaawy H.A., El-Murr A., Ahmed M.M., Bedawy A.M., Tukur H.A., Swelum A.-A.-A., Saadeldin I.M. Endosulfan toxicity in Nile tilapia (Oreochromis niloticus) and the use of lycopene as an ameliorative agent. Comp. Biochem. Physiol. C: Toxicol. Pharmacol. 2019;224 doi: 10.1016/j.cbpc.2019.108573. [DOI] [PubMed] [Google Scholar]
- Izah S.C., Angaye T.C. Heavy metal concentration in fishes from surface water in Nigeria: Potential sources of pollutants and mitigation measures. Sky J. Biochem. Res. 2016;5(4):31–47. [Google Scholar]
- Khan M.F., Tabassum S., Sadique H., Sajid M., Ghayyur S., Dil K., Badshah N.K., Ullah I. Hematological, Biochemical and Histopathological Alterations in Common Carp during Acute Toxicity of Endosulfan. Int. J. Agric. Biol. 2019;22(4):703–709. [Google Scholar]
- Khatun N., Mahanta R. A study on the effect of chlorpyrifos (20% EC) on thyroid hormones in freshwater fish, Heteropneustes fossilis (Bloch.) by using EIA technique. Sci. Probe. 2014;2(2):8–16. [Google Scholar]
- Ko H.D., Park H.J., Kang J.C. Change of growth performance, hematological parameters, and plasma component by hexavalent chromium exposure in starry flounder, Platichthys stellatus. Fisheries Aquatic Sci. 2019;22(1):9. [Google Scholar]
- Kumar N., Ambasankar K., Krishnani K., Gupta S., Bhushan S., Minhas P. Acute toxicity, biochemical and histopathological responses of endosulfan in Chanos chanos. Ecotoxicol. Environ. Saf. 2016;131:79–88. doi: 10.1016/j.ecoenv.2016.05.013. [DOI] [PubMed] [Google Scholar]
- Kumar N., Prabhu P.A.J., Pal A., Remya S., Aklakur M., Rana R., Gupta S., Raman R., Jadhao S. Anti-oxidative and immuno-hematological status of Tilapia (Oreochromis mossambicus) during acute toxicity test of endosulfan. Pestic. Biochem. Physiol. 2011;99(1):45–52. [Google Scholar]
- Lacasana M., Lopez-Flores I., Rodríguez-Barranco M., Aguilar-Garduno C., Blanco-Munoz J., Pérez-Mendez O., Gamboa R., Bassol S., Cebrian M.E. Association between organophosphate pesticides exposure and thyroid hormones in floriculture workers. Toxicol. Appl. Pharmacol. 2010;243(1):19–26. doi: 10.1016/j.taap.2009.11.008. [DOI] [PubMed] [Google Scholar]
- Li D., Xie P., Zhang X. Changes in plasma thyroid hormones and cortisol levels in crucian carp (Carassius auratus) exposed to the extracted microcystins. Chemosphere. 2008;74(1):13–18. doi: 10.1016/j.chemosphere.2008.09.065. [DOI] [PubMed] [Google Scholar]
- Macirella R., Madeo G., Sesti S., Tripepi M., Bernabò I., Godbert N., La Russa D., Brunelli E. Exposure and post-exposure effects of chlorpyrifos on Carassius auratus gills: An ultrastructural and morphofunctional investigation. Chemosphere. 2020;251 doi: 10.1016/j.chemosphere.2020.126434. [DOI] [PubMed] [Google Scholar]
- Macirella R., Sesti S., Bernabò I., Tripepi M., Godbert N., Brunelli E. Lead toxicity in seawater teleosts: A morphofunctional and ultrastructural study on the gills of the Ornate wrasse (Thalassoma pavo L.) Aquat. Toxicol. 2019;211:193–201. doi: 10.1016/j.aquatox.2019.04.009. [DOI] [PubMed] [Google Scholar]
- Malik D., Maurya P.K. Heavy metal concentration in water, sediment, and tissues of fish species (Heteropneustis fossilis and Puntius ticto) from Kali River, India. Toxicol. Environ. Chem. 2014;96(8):1195–1206. [Google Scholar]
- Maurya P.K., Malik D. Distribution of heavy metals in water, sediments and fish tissue (Heteropneustis fossilis) in Kali River of western UP India. Int. J. Fish. Aquat. Stud. 2016;4(2):208–215. [Google Scholar]
- Narra M.R., Rajender K., Reddy R.R., Rao J.V., Begum G. The role of vitamin C as antioxidant in protection of biochemical and haematological stress induced by chlorpyrifos in freshwater fish Clarias batrachus. Chemosphere. 2015;132:172–178. doi: 10.1016/j.chemosphere.2015.03.006. [DOI] [PubMed] [Google Scholar]
- Nugegoda D., Kibria G. Effects of environmental chemicals on fish thyroid function: implications for fisheries and aquaculture in Australia. Gen. Comp. Endocrinol. 2017;244:40–53. doi: 10.1016/j.ygcen.2016.02.021. [DOI] [PubMed] [Google Scholar]
- Olaniyi K. Histopathology Studies of Selected Organs of Hemichromis Fasciatus [1] Inhabiting Igun Gold Mining and Opa Reservoirs, South Western Nigeria: A Comparative Study. Int. J. Adv. Life Sci. Technol. 2020;4(1):1–10. [Google Scholar]
- Osman A.G., AbouelFadl K.Y., Abd El Baset M., Mahmoud U.M., Kloas W., Moustafa M.A. Blood Biomarkers in Nile tilapia Oreochromis niloticus niloticus and African Catfish Clarias gariepinus to Evaluate Water Quality of the River Nile. J. FisheriesSciences. com. 2018;12(1):1–15. [Google Scholar]
- Osman A.G., Koutb M., Sayed A.-E.-D.-H. Use of hematological parameters to assess the efficiency of quince (Cydonia oblonga Miller) leaf extract in alleviation of the effect of ultraviolet–A radiation on African catfish Clarias gariepinus (Burchell, 1822) J. Photochem. Photobiol., B. 2010;99(1):1–8. doi: 10.1016/j.jphotobiol.2010.01.002. [DOI] [PubMed] [Google Scholar]
- Poopal R., Ramesh M., Maruthappan V., Rajendran R.B. Potential effects of low molecular weight phthalate esters (C 16 H 22 O 4 and C 12 H 14 O 4) on the freshwater fish Cyprinus carpio. Toxicol. Res. 2017;6(4):505–520. doi: 10.1039/c7tx00084g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qureshi I.Z., Bibi A., Shahid S., Ghazanfar M. Exposure to sub-acute doses of fipronil and buprofezin in combination or alone induces biochemical, hematological, histopathological and genotoxic damage in common carp (Cyprinus carpio L.) Aquat. Toxicol. 2016;179:103–114. doi: 10.1016/j.aquatox.2016.08.012. [DOI] [PubMed] [Google Scholar]
- Rahman A.N.A., ElHady M., Hassanin M.E., Mohamed A.-A.-R. Alleviative effects of dietary Indian lotus leaves on heavy metals-induced hepato-renal toxicity, oxidative stress, and histopathological alterations in Nile tilapia, Oreochromis niloticus (L.) Aquaculture. 2019;509:198–208. [Google Scholar]
- Rahman A.N.A., Mohamed A.-A.-R., Mohammed H.H., Elseddawy N.M., Salem G.A., El-Ghareeb W.R. The ameliorative role of geranium (Pelargonium graveolens) essential oil against hepato-renal toxicity, immunosuppression, and oxidative stress of profenofos in common carp, Cyprinus carpio (L.) Aquaculture. 2020;517 [Google Scholar]
- Raibeemol K., Chitra K. Histopathological alteration in gill of the freshwater fish Pseudetroplus maculatus (Bloch, 1795) under chlorpyrifos toxicity. Int. J. Adv. Res. Biol. Sci. 2016;3(12):141–146. [Google Scholar]
- Ramesh M., Saravanan M., Kavitha C. Hormonal responses of the fish, Cyprinus carpio, to environmental lead exposure. Afr. J. Biotechnol. 2009;8(17):4154–4158. [Google Scholar]
- Salamat N., Zarie M. Using of fish pathological alterations to assess aquatic pollution: a review. World J. Fish Mar. Sci. 2012;4(3):223–231. [Google Scholar]
- Saravanan M., Karthika S., Malarvizhi A., Ramesh M. Ecotoxicological impacts of clofibric acid and diclofenac in common carp (Cyprinus carpio) fingerlings: hematological, biochemical, ionoregulatory and enzymological responses. J. Hazard. Mater. 2011;195:188–194. doi: 10.1016/j.jhazmat.2011.08.029. [DOI] [PubMed] [Google Scholar]
- Sharma P., Chadha P., Saini H.S. Tetrabromobisphenol A induced oxidative stress and genotoxicity in fish Channa punctatus. Drug Chem. Toxicol. 2019;42(6):559–564. doi: 10.1080/01480545.2018.1441864. [DOI] [PubMed] [Google Scholar]
- Sharma R., Jindal R. Assessment of cypermethrin induced hepatic toxicity in Catla catla: A multiple biomarker approach. Environ. Res. 2020;184 doi: 10.1016/j.envres.2020.109359. [DOI] [PubMed] [Google Scholar]
- Shengyun S., Xiaoming L., Renfeng W., Yong W. Efficacy of Several Insecticides on the Control of Myzus persicae and Lipaphis erysimi. Pesticide Sci. Administr. 2005;7 [Google Scholar]
- Simonato J.D., Fernandes M.N., Martinez C.B. Physiological effects of gasoline on the freshwater fish Prochilodus lineatus (Characiformes: Prochilodontidae) Neotrop. Ichthyol. 2013;11(3):683–691. [Google Scholar]
- Singh G., Bhatnagar A., Alok K., Ajay S. Enzymatic Profiling and Feeding Preferences of Catla: Catla Catla, Rohu: Labeo rohita and Mrigala: Cirrhinus mrigala in Rural Polyculture Ponds. J. Aquac Res. Develop. 2018;9(553):2. [Google Scholar]
- Suvetha L., Saravanan M., Hur J.-H., Ramesh M., Krishnapriya K. Acute and sublethal intoxication of deltamethrin in an Indian major carp, Labeo rohita: Hormonal and enzymological responses. J. Basic Appl. Zool. 2015;72:58–65. [Google Scholar]
- Tarbah F., Shaheen A., Benomran F., Hassan A., Daldrup T. Distribution of dimethoate in the body after a fatal organphosphateintoxication. Forensic Sci. Int. 2007;170(2–3):129–132. doi: 10.1016/j.forsciint.2007.04.232. [DOI] [PubMed] [Google Scholar]
- Ujjania, N., Soni, N., 2018. Use of scale for the growth study of Indian major carp (Cirrhinus mrigala Ham., 1822) in tropical freshwater.
- Ullah S., Shah R.M., Shad S.A. Genetics, realized heritability and possible mechanism of chlorfenapyr resistance in Oxycarenus hyalinipennis (Lygaeidae: Hemiptera) Pestic. Biochem. Physiol. 2016;133:91–96. doi: 10.1016/j.pestbp.2016.02.007. [DOI] [PubMed] [Google Scholar]
- Vali S., Mohammadi G., Tavabe K.R., Moghadas F., Naserabad S.S. The effects of silver nanoparticles (Ag-NPs) sublethal concentrations on common carp (Cyprinus carpio): Bioaccumulation, hematology, serum biochemistry and immunology, antioxidant enzymes, and skin mucosal responses. Ecotoxicol. Environ. Saf. 2020;194 doi: 10.1016/j.ecoenv.2020.110353. [DOI] [PubMed] [Google Scholar]
- Woo S.J., Chung J.K. Effects of trichlorfon on oxidative stress, neurotoxicity, and cortisol levels in common carp, Cyprinus carpio L., at different temperatures. Comp. Biochem. Physiol. C: Toxicol. Pharmacol. 2020;229:1–9. doi: 10.1016/j.cbpc.2019.108698. [DOI] [PubMed] [Google Scholar]
- Woryi J., Ugbomeh A., Gabriel U., Daka E. Chronic effects of Paraquat on haematology of African Catfish (Clarias gariepinus) Int. J. Innov. Sci. Eng. Technol. 2020;7(3):73–83. [Google Scholar]
- Yadav K.K., Gupta N., Kumar V., Khan S.A., Kumar A. A review of emerging adsorbents and current demand for defluoridation of water: bright future in water sustainability. Environ. Int. 2018;111:80–108. doi: 10.1016/j.envint.2017.11.014. [DOI] [PubMed] [Google Scholar]
- Yu L., Han Z., Liu C. A review on the effects of PBDEs on thyroid and reproduction systems in fish. Gen. Comp. Endocrinol. 2015;219:64–73. doi: 10.1016/j.ygcen.2014.12.010. [DOI] [PubMed] [Google Scholar]
- Zhang J.-J., Yi W., Xiang H.-Y., Li M.-X., Li W.-H., Wang X.-Z., Zhang J.-H. Oxidative stress: role in acetamiprid-induced impairment of the male mice reproductive system. Agric. Sci. China. 2011;10(5):786–796. [Google Scholar]
- Zhang X., Tian H., Wang W., Ru S. Exposure to monocrotophos pesticide causes disruption of the hypothalamic–pituitary–thyroid axis in adult male goldfish (Carassius auratus) Gen. Comp. Endocrinol. 2013;193:158–166. doi: 10.1016/j.ygcen.2013.08.003. [DOI] [PubMed] [Google Scholar]