Abstract
Obesity is one of the most serious health problems in the world, increasing the risk of other chronic diseases. Alterations in fatty acid synthesis related genes are crucially involved in obesity progression. Diosgenin (DG) was one of the phytosterols compounds with vital activity against lipid disorders. Therefore, this study was intended to evaluate the protective effect of DG on lipogenesis in the high-fat diet (HFD)-induced obesity in mice, via investigating the expression of two of the fatty acid synthesis–involved genes; sterol regulatory element-binding protein (SREBP-1c) and fatty acid synthase (FASN) genes. Thirty adult male mice were divided into 3 groups. Control group, fed with normal diet; HFD group, mice fed with a high-fat diet and HFD + DG group, mice fed with a high-fat diet and supplemented in parallel with DG for 6 consecutive weeks. The effect of DG on Body weights, liver enzymes, lipid profile, were evaluated. Histopathological fatty changes as well as SREBP-1c and FASN gene expression were also investigated. DG significantly alleviated body weight gain, adjusted liver enzymes, and improved lipid profile. Additionally, DG ameliorated the histopathological changes by reducing the lipid vacuoles and hence the hepatosteatosis. Accordingly, DG significantly downregulated the two-fold increase in the SREBP-1c and FASN gene expression observed in the HFD group. In conclusion, DG possesses a beneficial impact against diet-induced obesity in mice, which makes it a good candidate for NAFLD and obesity prevention.
Keywords: Diosgenin, FASN, SREBP-1c, Non-alcoholic fatty liver disease, Obesity
1. Introduction
Obesity is the growing fundamental problem of human diseases in developing and underdeveloped countries. Universally, more than 650 million people are affected by obesity. Since it is related to complications of hypertension, cardiovascular, type 2 diabetes mellitus, and liver diseases (Al-Nbaheen, 2020). Moreover, it is associated with multiple dysfunctions of metabolism and is characterized as chronic low-grade inflammation and oxidative stress (Iyer et al., 2010).
Obesity is defined as the accumulation of excess weight deposited in the form of fats in different parts of the human body (Leal-Ugarte et al., 2019). The liver is the most favorable part of fat accumulation in the body (Byrne and Targher, 2014). High dietary levels of fat consumed led to excessive lipid accumulation in the liver and consequently non-alcoholic fatty liver disease (NAFLD). Unfortunately, the pathology of the liver in obesity is not limited to initial NAFLD stages, but also can lead to cirrhosis and may develop into hepatocellular carcinoma (Marchesini et al., 2008).
Fatty acid synthase (FASN), is considered a central enzyme in fatty acid synthesis. It is distributed consistently in different human tissues, with the highest level in liver and adipose tissue (Postic and Girard, 2008). The gene encoding the FASN enzyme was known as a candidate gene for the determination of body fat. Its expression is highly regulated by the transcription factor sterol regulatory element-binding protein (SREBP-1c), which makes it a vital factor for TG synthesis (Dorn et al., 2010). FASN is involved in obesity development and plays an important role in the regulation of body weight (Kovacs et al., 2004). Therefore, targeting the fatty acid synthesis-involved genes may be a new promising approach for fatty liver accumulation and obesity prevention. So, this study was planned to target the expression of two of the lipogenic –involved genes; SREBP-1c and FASN utilizing a specific natural agent to prevent obesity and its related fat accumulation in the liver.
Global research efforts are seeking to find therapeutic options responsible for the reducing prevalence of obesity in the population. Nevertheless, synthetic agents that are currently available for obesity treatment can cause abnormal echocardiograms and acute kidney injury (Xiao et al., 2019). Therefore, the use of phytosterols may be considered as an excellent alternative strategy for developing a new promising, safe, and effective agent for obesity and fat accumulation treatment (Mayer et al., 2009). Phytosterols, one of the natural phytochemical classes, are similar in structure to mammalian cholesterol (Rideout et al., 2010). It is the collective name for the plant-derived sterols and stanols compounds. Stanols are the predominant form occurring in nature (Marangoni and Poli, 2010). They are abundant in vegetable oils, nuts, cereals, and legumes. Diosgenin (DG), a steroidal saponin widely found in nature, has many biological activities in recent years (Sangeetha et al., 2013). DG has been shown to exert a great effect on obesity. It is known to possess anti-inflammatory and antioxidant properties and can be useful in various diseases, including obesity, insulin resistance, metabolic syndrome, and NAFLD (Izar et al., 2011). Besides, it plays a vital role as a key regulator in lipid metabolism (Gupta et al., 2011). Therefore, the present study aims to elucidate the genetic alterations in diet-inducing obesity and investigate the protective effect of DG on HFD-induced fat accumulation and obesity in mice via elucidating its impact on the expression of fatty acid-involved genes; FASN and its transcription factor, SREBP-1c.
2. Materials and methods
2.1. Chemicals
Diosgenin, 98% was purchased from Sigma Chemical Co. (St. Louis, MO). It was dissolved in 0.5% DMSO in saline before use. Serum alanine transaminase (ALT) analysis kit and serum aspartate aminotransferase (AST) analysis kit were purchased from Abcam plc, UK. Paraffin, hematoxylin and eosin were purchased from Merck KGaA, Darmstadt, Germany. All other chemicals used in this study were of high-quality analytical grade.
2.2. Animals and experimental design
All experimental procedures of the current study were carried out according to the guidelines' principles of CIOMS (Council for International Organizations for Medical Science) and ICLAS (International Council for Laboratory Animal Science) for Biomedical Research Involving Animals.
Thirty adult male C57BL/6J mice, 6–8 weeks old, with weight 24–26 g were purchased from the research unit of pharmacology and chemistry, Misr University Science and Technology, Cairo, Egypt. Animals were housed in the breeding unit of the Medical Research Center (Faculty of Medicine, Ain Shams University, Cairo, Egypt). Room temperature, relative humidity, and light/dark conditions were set to be maintained between 21 ± 2 °C; 30–70%, and 12-h light/12-h dark respectively for all mice throughout the experiment. Animals were randomized into three groups: (I) Control group, mice fed with normal diet (approximately, 12% dietary fat) and ingested orally by gavage for 6 consecutive weeks with the vehicle solution, 0.5% DMSO in saline; (2) HFD group, mice fed with high-fat diet (60% fat) for 6 consecutive weeks; (3) HFD + DG group, mice fed with high-fat diet (60% fat) and supplemented in parallel with DG in 0.5% DMSO in saline (80 mg/kg b.w./day) (Liu et al., 2015), orally by gavage for 6 consecutive weeks. At the end of the experimental period, animals were fasted overnight (only water was allowed), euthanized by sodium thiopental (30 mg/kg in saline) (Brookes et al., 2000). Blood was allowed to clot, centrifuged and the serum obtained was stored at −20 °C until use. At necropsy, livers were collected immediately, weighed, and divided into two parts. The first part (~100 mg) was immediately cut on ice and then preserved at −20 °C for the molecular studies. Although for histological investigations the second component was soaked in a fixative solution.
2.3. Body and liver weights determination:
The weights of all mice from different groups were observed at the beginning of the experiment, taken regularly once a week during the study, and at the end of the experiment. The fasted animals were weighed individually using an electronic weighing balance. Likewise, after necroscopy, the weight of the liver from all the groups was reported.
2.4. Biochemical analysis
2.4.1. Liver function tests:
Serum alanine transaminase (ALT) and aspartate aminotransferase (AST) activities were assayed by the colorimetric methods of (Reitman and Frankel, 1957), and (Bessey et al., 1946) respectively.
2.4.2. Lipid profile
Total cholesterol (TC) and high-density lipoprotein cholesterol (HDL-c) were determined in serum according to the method described by (Allain et al., 1974) and (Finley et al., 1978), respectively. Serum triglycerides (TGs) were evaluated using the method described by Fossati and Prencipe (Fossati and Prencipe, 1982).
2.5. Histological investigation:
Fixed liver tissue samples were enclosed in paraffin cubes, cut into serial 4 μm-thick sections. Then, the parts of tissue collected were stained with stains of hematoxylin and eosin (H&E), examined, and photographed under a light microscope (Mepham, 1991).
2.6. Real-time quantitative reverse transcriptase-polymerase chain reaction (qRT-PCR)
Total RNA was isolated from liver tissue samples using the BIOLINE TRIsure™ kit (Cat.no. BIO-38032) according to the manufacturer’s instructions. For cDNA synthesis, one microgram of the total RNA was used using the BIOLINE SensiFast™ cDNA synthesis kit (Cat. no. BIO-65053) following the manufacturer’s instructions. The relative expression levels of mRNA encoding the sterol element-binding protein (SEBP-1c), fatty acid synthase (FASN) and the housekeeping gene; β-actin was assessed using the SensiFAST™ SYBR® No-ROX kit (2X) (Cat. no. BIO-98005), according to the manufacturer’s protocol. The results were computerized using the Stratagene (Mx 3000PTM) machine. The expression level of the target SEBP-1c and FASN genes were normalized to the β-actin and presented as fold change relative to the control group. The primers used for qRT-PCR in this study are illustrated in Table 1.
Table 1.
Primer sequences for the studied genes.
| Gene | Accession number | Forward | Reverse |
|---|---|---|---|
| FASN | NM_007988 | 5′-TTGCTGGCACTACAGAATGC-3′ | 5′-AACAGCCTCAGAGCGACAAT-3′ |
| SREBP-1c | NM_001358314.1 | 5′-GGGGCCTGACAGGTGAAATC-3′ | 5′- TGTCAGCAGCAGTGAGTCTG-3′ |
| β-actin | NM_031144 | 5′-AGCCATGTACGTAGCCATCC-3′ | 5′-CTCTCAGCTGTGGTGGTGAA-3′ |
2.7. Statistical analysis
The software program, Statistical Package for Social Science (SPSS), version 23.0 for Windows (SPSS® Chicago, USA), analyzed all data. Data were expressed as mean ± Standard Deviation (SD) for quantitative descriptive parametric results. Statistical analysis of differences in means of variables between groups was performed using the Student t-test; the probability of P < 0.05 was considered statistically significant.
3. Results
3.1. Effect of diosgenin on body and liver weight
Although all groups of mice were matched by weight with no substantial difference among their mean initial bodyweights, results showed a significant increase (P < 0.05) in the bodyweight of the HFD group, compared to the control group (Fig. 1-i). In contrast, the group of animals receiving DG along with HFD showed a significant reduction in their body weight which almost rebounding to the normal control weight. In addition, the liver weight in the HFD-induced obesity animals was significantly increased as compared to the control group. However, treatment with DG has brought back the liver weight to near the control group in addition to inhibition of obesity-induced by HFD in mice (Fig. 1-ii).
Fig. 1.
Effect of Diosgenin on (i) body and (ii) liver weight. Data are represented by mean ± SD (n = 10). A significant difference is indicated as * when p-value < 0.05 corresponding to the control group. The significant difference corresponding to the HFD group is indicated as # when P-value < 0.05.
3.2. Effect of diosgenin on liver enzymes activities
As shown in Fig. 2; the ALT and AST activities showed a significant increase in p < 0.001, approximately two-fold elevation levels in mice fed with HFD, compared to the control group. However, these enzymes’ activities were significantly decreased (p < 0.001) in the HFD + DG group, compared to the HFD group. These results indicate the DG impact in rebounding the liver enzymes to almost their normal levels and its protective effect against liver injury while consuming HFD.
Fig. 2.
Effect of Diosgenin on Liver enzymes activities. Data are represented by mean ± SD (n = 10). A significant difference relative to the control group is indicated as *: p < 0.001 compared to the control group. A significant difference relative to the HFD group is indicated as #: P < 0.001. ALT: alanine transaminase; AST: aspartate transaminase.
3.3. Lipid profile
Results showed that the group of mice supplemented with DG along with HFD (HFD + DG group) exhibited significant reduction (p < 0.001) in TC and TG levels, compared to the group of mice fed with HFD only (HFD group). Accordingly, a significant increase (p < 0.001) in the HDL-c level was detected in the HFD + DG group, compared to the HFD group. These findings confirmed the effect of DG in alleviating the dyslipidemia state induced by HFD consumption for 6 consecutive weeks (Fig. 3).
Fig. 3.
Effect of Diosgenin on serum triglycerides (TG), total cholesterol (TC), and high-density lipoprotein-cholesterol (HDL-c) in mice. Data are represented by mean ± SD (n = 10). A significant difference relative to the control group is indicated as *: p < 0.001 compared to the control group. A significant difference relative to the HFD group is indicated as #: P < 0.001.
3.4. Molecular studies
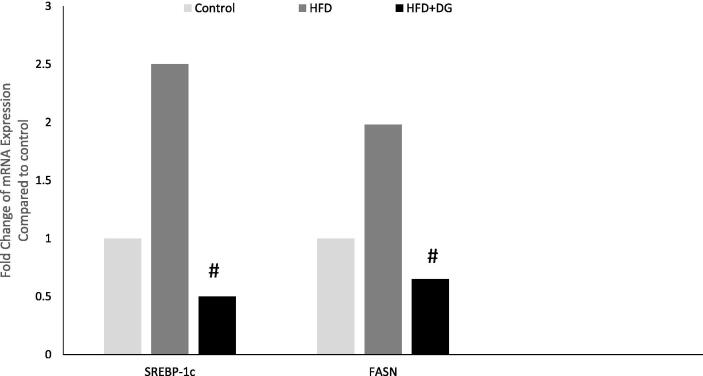
The expression level of SREBP-1c and FASN mRNA was analyzed in the three studied groups. The ß-actin gene was used as an internal standard for normalization of the target gene expression levels. As shown in Fig. 4, the expression of SREBP-1c and FASN genes were significantly increased by 2.5 and 2-folds respectively, in the livers of mice ingested with HFD (HFD group), compared to the control group. However, daily supplementation of diosgenin with HFD ingestion was shown to significantly downregulated the mRNA levels of the SREBP-1C and FASN gene expression to a level below that expressed in mice with a normal diet with low-fat level (12%). These results confirm the protective effect of diosgenin in regulating fatty acid synthesis-involved genes and hence the lipogenesis via regulating the SREBP-1c and FASN gene expression at the mRNA levels.
Fig. 4.
Effects of diosgenin on the mRNA expression of SREBP-1c and FASN genes. Data are presented as the mean ± SD (n = 6 mice from each group). * P < 0.001, compared to the control group; # P < 0.001, compared to the HFD group.
3.5. Histopathological examination of liver
Histological analysis of the liver tissue samples from the studied groups was performed to determine the active role of DG on hepatic steatosis. Control group mice showed normal-sized hepatocytes, an average portal tract (PT) with normal portal veins (PV), bile ducts and average central vein (CV) (Fig. 5; i&ii). In contrast, the HFD group's liver revealed many histological changes, including dilation in the portal and central veins, widespread of lipid vacuoles, degeneration in many hepatocytes with marked microvascular steatosis in peri-portal and peri-venular zones (Fig. 5; iii&iv). However, the HFD + DG group showed decreased lipid accumulation, lesser damage, and near-normal hepatocytes with fewer and small cytoplasmic lipid vacuoles in the peri-venular area with an average portal and central veins compared to the HFD group (Fig. 5; v&vi). These findings indicate the impact of DG on the improvement of HFD-induced histopathological alterations.
Fig. 5.
Histopathological observation of hepatic tissues. Control group: (i) liver showing average portal tract (black arrow), average CV (red arrow), and average hepatocytes (blue arrow) (H&E X 200)., (ii) another view showing average CV surrounded by average hepatocytes arranged in single-cell cords (red arrow) with average intervening blood sinusoids (black arrow) (H&E X 400). HFD group: (iii) liver showing average portal tract (red arrow), dilated CV and scattered vacuolated hepatocytes (black arrows) (H&E X 200); (iv) high power view showing dilated PV, average bile duct (red arrows), and hepatocytes showing micro-vesicular steatosis (black arrows) (H&E X 400). HFD + DG group: (v) liver showing portal tract with mildly dilated PV and average bile duct, (black arrow), average CV, and average surrounding hepatocytes (red arrow) (H&E X 200), (vi) liver showing markedly dilated PV with average CV (black arrow) and average surrounding hepatocytes (red arrow) (H&E X 200). CV: central vein; PV: portal vein.
4. Discussion
High levels of dietary fat consumption can trigger obesity. It leads to excessive fat accumulation and promotes lipogenesis via enhancing fatty acid synthesis (Kumashiro et al., 2011). Thus, the development of novel drugs targeting fatty acid synthesis is a promising strategy for treating obesity. Diosgenin, one of the phytosterol agents was attracted great attention due to its activity against various diseases; including hyperlipidemia, in addition to the safety of its oral administration in doses of up to ~500 mg/kg (Lima et al., 2013). The present study focused on the protective effects of diosgenin on HFD-induced obesity. This protection was achieved by regulating the fatty acid synthesis-involved gene expression and can be managed by bodyweight evaluation, biochemical parameters assessment, and histopathological features investigations.
As shown in the results, by the end of the 6th week of HFD consumption; ~1.5 folds increase was detected in the weight of this group. This weight gained reflected the exceeding anabolic rate of fatty acid synthesis over the catabolic one. Moreover, it indicated fat accumulation as a consequence of HFD consumption. However, DG co-administration along with HFD reduce this gained weight and almost normalized it. This indicates the DG impact against fat accumulation and obesity via its improving effect in regulating bodyweight during HFD consumption.
Concerning the relationship between obesity and liver injury, the present results revealed two-fold elevations in the ALT and AST enzymes’ activities in the group of mice consuming HFD. These findings confirmed the closely associated link between obesity and the risk of elevated liver enzymes since an increase in AST and ALT levels are among the sensitive biochemical markers of liver injury and the main glue of obesity (Marchesini et al., 2008). Accordingly, histopathological alterations in hepatocyte’s structure clarified these elevations by the hepatic steatosis observed in the HFD group. However, DG supplementation in parallel with HFD (HFD + DG group) was shown to reduce this dramatic increase by returning the enzymes’ activities to almost their normal levels and improve the histological structure of hepatocytes.
An abnormal lipid profile is the first hallmark indicating obesity. The current study showed that animals consumed HFD for 6 consecutive weeks (HFD group) trigger dyslipidemia, as illustrated by the two-fold increase in the serum TC and TG, besides the dramatic decrease in the HDL-c levels. However, DG supplementation along with HFD (HFD + DG group) was significantly reduced the TC and TG, restored the HDL-c to almost their normal levels. DG can reduce the serum total cholesterol by its ability to bind the bile acids in the digestive tract, causing a decline in their levels and that in turn increases their production from cholesterol. At the same time, DG interferes with the enterohepatic circulation. Therefore, the absorbed cholesterol via the gastrointestinal tract was inhibited and secreted into the feces (Katzung, 2012). Accordingly, DG showed a 63% reduction in serum triglycerides, as shown in the HFD + DG group. Gong et al. (2010), previously reported the dual effect of DG on lowering the total blood cholesterol and triglycerides levels by 35%. Similarly, Uemura et al. (2011) demonstrated that DG extracted from fenugreek decreased the hepatic and plasma triglyceride. Furthermore, the capability of DG to restore the HDL-c levels was closely related to the inhibition of cholesterol and fat absorption, since, HDL transports cholesterol from the body tissues to the liver and hence, prevents its accumulation (Gong et al., 2010, Mann and Truswell, 2017, Uemura et al., 2011). Histopathological fatty changes in the liver of studied groups revealed the abundance of cytoplasmic fatty vacuoles in the majority of hepatocytes, as a result of fat accumulation. Moreover, the marked liver steatosis observed in the group of mice fed with HFD (HFD group) indicates that the rate of fatty acid anabolism exceeds that of catabolism (Musso et al., 2009). In other words, the histological alterations in the liver of the HFD group reflect the uncontrolled expression of fatty acid synthesis-involved genes. On the other hand, DG supplementation along with HFD (HFD + DG group) markedly improve these histopathological fatty changes alterations, by reducing the number of fatty vacuoles and improving liver steatosis.
Regarding the fatty acid synthesis progression, expression of two of the fatty acid-involved genes revealed the upregulation of SREBP-1c and FASN by 2.5 and 2-folds respectively, as a consequence of HFD consumption for 6 consecutive weeks (HFD group). SREBP-1c, a well-recognized nuclear transcription factor involved in the biosynthesis of cholesterol, fatty acid, and triglyceride in mammals; plays an important role in regulating the transcription of its target genes, among which the FASN gene (Horton et al., 2002). Abnormal expression of this SREBP-1c gene was greatly associated with the pathogenesis of fat accumulation and NAFLD since its overexpression in the liver was shown to produce a TG-enriched fatty liver (Li et al., 2016). It is noteworthy to denote that Diosgenin supplementation along with high dietary fats significantly suppressed the expression of SREBP-1c and FASN genes. In line with, Moriwaki et al. (2014), previously reported that Yamogenin, a diastereomer of diosgenin in fenugreek, inhibited lipid accumulation through the suppression of SREBP-1c in hepatocytes (Moriwaki et al., 2014). The present findings demonstrated that the protective effect of diosgenin against fat accumulation and NAFLD may occur via suppressing two of the central genes involved in the fatty acid synthesis; SREBP-1c and FASN.
5. Conclusion
In conclusion, the current study demonstrated the protective effect of diosgenin on obesity and its related fat accumulation in mice. This was proved by the diosgenin’s ability to evoke gradual weight loss, ameliorate the liver enzymes’ levels, and regulate the blood lipid profile. Furthermore, diosgenin can improve the histopathological fatty changes as a result of inhibiting the hepatic fatty acid and triglyceride accumulation via suppressing the SREBP-1c and FASN genes. Consequently, DG agents can be developed as a new promising and efficient candidate for obesity and NAFLD prevention.
Acknowledgments
Acknowledgement
The authors greatly thank and acknowledge Taif University, for its support.
Funding
This research has been supported by Taif University Research Supporting Project number (TURSP-2020/104) Taif University, Taif, Saudi Arabia.
Footnotes
Peer review under responsibility of King Saud University.
References
- Al-Nbaheen M.S. Impact of weight loss predictors in severe-morbid obesity patients in the Saudi population. Saudi J. Biol. Sci. 2020;27:2509–2513. doi: 10.1016/j.sjbs.2020.03.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allain C.C., Poon L.S., Chan C.S., Richmond W., Fu P.C. Enzymatic determination of total serum cholesterol. Clin. Chem. 1974;20:470–475. [PubMed] [Google Scholar]
- Bessey O.A., Lowry O.H., Brock M.J. A method for the rapid determination of alkaline phosphates with five cubic millimeters of serum. J. Biol. Chem. 1946;164:321–329. [PubMed] [Google Scholar]
- Brookes Z.L., Brown N.J., Reilly C.S. Intravenous anaesthesia and the rat microcirculation: the dorsal microcirculatory chamber. Br. J. Anaesth. 2000;85:901–903. doi: 10.1093/bja/85.6.901. [DOI] [PubMed] [Google Scholar]
- Byrne C.D., Targher G. Ectopic fat, insulin resistance, and nonalcoholic fatty liver disease: implications for cardiovascular disease. Arterioscler Thromb. Vasc. Biol. 2014;34:1155–1161. doi: 10.1161/ATVBAHA.114.303034. [DOI] [PubMed] [Google Scholar]
- Dorn C., Riener M.O., Kirovski G., Saugspier M., Steib K., Weiss T.S., Gäbele E., Kristiansen G., Hartmann A., Hellerbrand C. Expression of fatty acid synthase in nonalcoholic fatty liver disease. Int. J. Clin. Exp. Pathol. 2010;3:505–514. [PMC free article] [PubMed] [Google Scholar]
- Finley P.R., Schifman R.B., Williams R.J., Lichti D.A. Cholesterol in high-density lipoprotein: use of Mg2+/dextran sulfate in its enzymic measurement. Clin. Chem. 1978;24:931–933. [PubMed] [Google Scholar]
- Fossati P., Prencipe L. Serum triglycerides determined colorimetrically with an enzyme that produces hydrogen peroxide. Clin. Chem. 1982;28:2077–2080. [PubMed] [Google Scholar]
- Gong G., Qin Y., Huang W., Zhou S., Wu X., Yang X., Zhao Y., Li D. Protective effects of diosgenin in the hyperlipidemic rat model and in human vascular endothelial cells against hydrogen peroxide-induced apoptosis. Chem. Biol. Interact. 2010;184:366–375. doi: 10.1016/j.cbi.2010.02.005. [DOI] [PubMed] [Google Scholar]
- Gupta A.K., Savopoulos C.G., Ahuja J., Hatzitolios A.I. Role of phytosterols in lipid-lowering: current perspectives. QJM. 2011;104:301–308. doi: 10.1093/qjmed/hcr007. [DOI] [PubMed] [Google Scholar]
- Horton J.D., Goldstein J.L., Brown M.S. SREBPs: activators of the complete program of cholesterol and fatty acid synthesis in the liver. J. Clin. Investig. 2002;109:1125–1131. doi: 10.1172/JCI15593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iyer A., Fairlie D.P., Prins J.B., Hammock B.D., Brown L. Inflammatory lipid mediators in adipocyte function and obesity. Nat. Rev. Endocrinol. 2010;6:71–82. doi: 10.1038/nrendo.2009.264. [DOI] [PubMed] [Google Scholar]
- Izar M.C., Tegani D.M., Kasmas S.H., Fonseca F.A. Phytosterols and phytosterolemia: gene-diet interactions. Genes Nutr. 2011;6:17–26. doi: 10.1007/s12263-010-0182-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katzung B.G. Mc Graw Hill; 2012. Basic and Clinical Pharmacology. [Google Scholar]
- Kovacs P., Harper I., Hanson R.L., Infante A.M., Bogardus C., Tataranni P.A., Baier L.J. A novel missense substitution (Val1483Ile) in the fatty acid synthase gene (FAS) is associated with percentage of body fat and substrate oxidation rates in nondiabetic Pima Indians. Diabetes. 2004;53:1915–1919. doi: 10.2337/diabetes.53.7.1915. [DOI] [PubMed] [Google Scholar]
- Kumashiro N., Erion D.M., Zhang D., Kahn M., Beddow S.A., Chu X., Still C.D., Gerhard G.S., Han X., Dziura J., Petersen K.F., Samuel V.T., Shulman G.I. Cellular mechanism of insulin resistance in nonalcoholic fatty liver disease. Proc. Natl. Acad. Sci. USA. 2011;108:16381–16385. doi: 10.1073/pnas.1113359108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leal-Ugarte E., Peralta-Leal V., Meza-Espinoza J.P., Durán-González J., Macías-Gómez N., Bocanegra-Alonso A., Lara-Ramos J.R. Association of the MTHFR 677C>T polymorphism with obesity and biochemical variables in a young population of Mexico. J. Med. Biochem. 2019;38:461–467. doi: 10.2478/jomb-2018-0046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li J., Ding L., Song B., Xiao X., Qi M., Yang Q., Yang Q., Tang X., Wang Z., Yang L. Emodin improves lipid and glucose metabolism in high fat diet-induced obese mice through regulating SREBP pathway. Eur. J. Pharmacol. 2016;770:99–109. doi: 10.1016/j.ejphar.2015.11.045. [DOI] [PubMed] [Google Scholar]
- Lima C.M., Lima A.K., Melo M.G., Serafini M.R., Oliveira D.L., de Almeida E.B., Barreto R.S., Nogueira P.C., Moraes V.R., Oliveira E.R., de Albuquerque R.L., Jr., Quintans L.J., Júnior, Araújo A.A. Bioassay-guided evaluation of Dioscorea villosa - an acute and subchronic toxicity, antinociceptive and anti-inflammatory approach. BMC Complem. Altern. Med. 2013;13:195. doi: 10.1186/1472-6882-13-195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu M., Xu L., Yin L., Qi Y., Xu Y., Han X., Zhao Y., Sun H., Yao J., Lin Y., Liu K., Peng J. Potent effects of dioscin against obesity in mice. Sci. Rep. 2015;5:7973. doi: 10.1038/srep07973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mann J., Truswell A.S. Oxford University Press; 2017. Essentials of human nutrition. [Google Scholar]
- Marangoni F., Poli A. Phytosterols and cardiovascular health. Pharmacol. Res. 2010;61:193–199. doi: 10.1016/j.phrs.2010.01.001. [DOI] [PubMed] [Google Scholar]
- Marchesini G., Moscatiello S., Di Domizio S., Forlani G. Obesity-associated liver disease. J. Clin. Endocrinol. Metab. 2008;93:S74–S80. doi: 10.1210/jc.2008-1399. [DOI] [PubMed] [Google Scholar]
- Mayer M.A., Hocht C., Puyó A., Taira C.A. Recent advances in obesity pharmacotherapy. Curr. Clin. Pharmacol. 2009;4:53–61. doi: 10.2174/157488409787236128. [DOI] [PubMed] [Google Scholar]
- Mepham, B.L., 1991, Theory and practice of histological techniques, 3rd ed. J.D. Bancroft, A. Stevens (Eds). Churchill Livingstone, Edinburgh, 1990. No. of pages: 740. Price: £55. J. Pathol. 164, 281–281. ISBN: 0 443 03559 8.
- Moriwaki S., Murakami H., Takahashi N., Uemura T., Taketani K., Hoshino S., Tsuge N., Narukami T., Goto T., Kawada T. Yamogenin in fenugreek inhibits lipid accumulation through the suppression of gene expression in fatty acid synthesis in hepatocytes. Biosci. Biotechnol. Biochem. 2014;78:1231–1236. doi: 10.1080/09168451.2014.915736. [DOI] [PubMed] [Google Scholar]
- Musso G., Gambino R., Cassader M. Recent insights into hepatic lipid metabolism in non-alcoholic fatty liver disease (NAFLD) Prog. Lipid Res. 2009;48:1–26. doi: 10.1016/j.plipres.2008.08.001. [DOI] [PubMed] [Google Scholar]
- Postic C., Girard J. Contribution of de novo fatty acid synthesis to hepatic steatosis and insulin resistance: lessons from genetically engineered mice. J. Clin. Investig. 2008;118:829–838. doi: 10.1172/JCI34275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reitman S., Frankel S. A colorimetric method for the determination of serum glutamic oxalacetic and glutamic pyruvic transaminases. Am. J. Clin. Pathol. 1957;28:56–63. doi: 10.1093/ajcp/28.1.56. [DOI] [PubMed] [Google Scholar]
- Rideout T.C., Harding S.V., Jones P.J. Consumption of plant sterols reduces plasma and hepatic triglycerides and modulates the expression of lipid regulatory genes and de novo lipogenesis in C57BL/6J mice. Mol. Nutr. Food Res. 2010;54:S7–S13. doi: 10.1002/mnfr.201000027. [DOI] [PubMed] [Google Scholar]
- Sangeetha M.K., ShriShri Mal N., Atmaja K., Sali V.K., Vasanthi H.R. PPAR's and Diosgenin a chemico biological insight in NIDDM. Chem. Biol. Interact. 2013;206:403–410. doi: 10.1016/j.cbi.2013.08.014. [DOI] [PubMed] [Google Scholar]
- Uemura T., Goto T., Kang M.S., Mizoguchi N., Hirai S., Lee J.Y., Nakano Y., Shono J., Hoshino S., Taketani K. Diosgenin, the main aglycon of fenugreek, inhibits LXRα activity in HepG2 cells and decreases plasma and hepatic triglycerides in obese diabetic mice. J. Nutr. 2011;141:17–23. doi: 10.3945/jn.110.125591. [DOI] [PubMed] [Google Scholar]
- Xiao S., Zhang Z., Chen M., Zou J., Jiang S., Qian D., Duan J. Xiexin Tang ameliorates dyslipidemia in high-fat diet-induced obese rats via elevating gut microbiota-derived short chain fatty acids production and adjusting energy metabolism. J. Ethnopharmacol. 2019;241:112032. doi: 10.1016/j.jep.2019.112032. [DOI] [PubMed] [Google Scholar]