Abstract
Toxoplasma gondii is a protozoan parasite distributed globally. It causes toxoplasmosis, which is prevalent in animals, birds, and soil. T. gondii infection leads to severe pathological impacts in immunodeficient patients and congenital cases. This review indicated that high prevalence groups had close contact with cats, dogs, consumed uncooked raw fruits, meat, or vegetables and the socio-economic level noted to be one of the crucial factors that influence toxoplasmosis. Toxoplasmosis infection is high in low-income countries and low in developed European countries. Immunosuppressed groups and pregnant women were the highly vulnerable groups. The epidemiology of the parasite enumerated various routes of infections; but consumption of T. gondii contaminated food was the major route of disease transmission. However, the role of meat and meat-producing animals on disease transmission remained unclear. Unfiltered water acts as the primary reservoir of toxoplasmosis transmission. The diagnostic methods for determining T. gondii infection are not the gold standard, and different approaches have been prescribed to analyze the infected populations based on the organs affected. Although toxoplasmosis was reported before 70 years, no appropriate solution noted to be recommended to treat this disease. Based on the present analyses, it concluded that the eradication of toxoplasmosis would be challenging from the world until people's socio-economic level is improved. The main aim of the present study was to analyze and update the disease transmission, epidemiology, and possible clinical interventions of toxoplasmosis.
Keywords: Toxoplasma gondii, Socio-economic disease, Toxoplasmosis, Immunosuppressed individuals
1. Introduction
Toxoplasmosis is a parasitic infection caused by the obligate intracellular parasite Toxoplasma gondii; it causes various clinical symptoms in birds, animals, sea mammals, and humans. T. gondii has been isolated from various geographical regions except Antarctica (Dubey, 2010). There are three infectious forms of T. gondii, including sporozoites that found in oocysts, tachyzoites and bradyzoites living in tissue cysts (Montoya and Remington, 2008, Ozgonul and Besirli, 2017). In humans, toxoplasmosis is reported to be transmitted via mother-to-child transmission, undercooked meat infested with latent cysts, contaminated water with sporulated oocysts and ingested contaminated food (Tenter et al., 2000). However, acquisition via various laboratories working on Toxoplasma sp. or by accidental ingestion or via blood products is rare. It was noted that T. gondii infected more than 60 million, and a majority of the infected cases are asymptomatic. At the acute stage, this infection is mainly asymptomatic in immunocompetent patients; however, ocular disease or cervical lymphadenopathy could occur. In Latin America, the virulent T. gondii strains caused toxoplasmosis was noted to be more prevalent among immunocompetent individuals and it can also cause disseminated disease, severe pneumonia, and even death (Demar et al., 2007). Acute toxoplasmosis infection in the immunocompetent individual leads to asymptomatic infection in skeletal and encysts in cardiac muscles, retina, parenchyma, and brain tissues. The latent infection has been reported among such patients, had a rapid conversion of bradyzoites into tachyzoites leading to severe mortality if not treated. Latent infection was reported among immunocompetent patients' retina, leading to a severe loss of visual acuity. Symptoms such as headache, fever, and muscle pain were reported, lasting for a few weeks. Though the severity of this disease is reported to be controlled by mediation, prevention is recommended to avoid any parasitic exposure (Alavi and Alavi, 2010).
T. gondii was mainly isolated from pork, lamb, undercooked meat, and cat feces. This potentially causes congenital disabilities or fetal mortality. Despite the asymptomatic nature of this disease, a major burden due to the intracellular parasite necessitates the recommendation of various effective steps for the management, diagnosis, and prevention of the disease. Serological assays and Polymerase Chain Reaction (PCR) are recommended for the diagnosis of congenital infection. There are many serological methods have been developed for toxoplasmosis diagnosis, and these methods confirmed good outcomes.
Moreover, the novel, reliable, sensitive, and specific approaches to determine T. gondii infection using serodiagnosis, which could highly be useful to differentiate chronic and acute disease, are available (Ybañez et al., 2020). This review analysed the current trends in human toxoplasmosis and gives an overview of the application of various recombinant proteins and their applications to detect the parasite infection in serodiagnosis and early diagnostic approaches enabling the treatment of the infection.
2. History of toxoplasmosis disease
Nicolle and Manceaux initially reported T. gondii in 1908 (Nicolle and Manceaux, 1908) from the North African rodent, Ctenodactylus gondii (gundi), and rabbits in Brazil by Splendore (Ferguson, 2004) and was widely recognized as a common infection in various warm-blood animals, including rodents and mammals. The clinical implications of toxoplasmosis were first reported in early 1920s among children with encephalitis, retinochoroiditis and hydrocephalus. In the 1980s, it was observed as one of the major opportunistic infections among patients with immune suppression due to HIV infection (Lim and Othman, 2014). Similarly, Remington et al. (2006) reported the clinical significance of the infection in various immune-suppressed patients, including patients undergoing organ transplant or cancer treatment. In general, toxoplasmosis is unnoticed in individuals from the immunocompetent group; however, lymphadenopathy, non-specific flu-like symptoms, and some complications associated with primary infection were also reported. Among pregnant women, the primary infection noted to be highly prevalent; the intracellular parasite crosses the placenta frequently, affects the fetus's health, and reportedly causes seizures, mental retardation, and hydrocephaly retinochoroiditis, and cause death to the fetus (Weiss and Kim, 2007). The risk and severity of symptoms and congenital infection mainly depended on the age of gestation. Early diagnosis of toxoplasmosis and health care management of congenital and maternal toxoplasmosis were highly useful to prevent sequelae. It was reported that most affected neonates were mainly asymptomatic at birth, and visual impairment was also reported after a few years (Peyron et al., 2011).
Toxoplasmic encephalitis resulting from reactivation of a latent infection of toxoplasmosis was also reported, and if not appropriately treated, the disease reportedly causes serious complications. Toxoplasmic encephalitis, is prevalent and highly common in various countries, especially among HIV patients and recent findings revealed that suitable antiretroviral therapy could be useful to reduce the incidence of toxoplasmic encephalitis. (Mohraz et al., 2011). Toxoplasmic retinochoroiditis is one of the eye diseases and leads to a heavy health burden throughout the world. Among immunocompromised patients, ocular toxoplasmosis has been reported in posterior uveitis due to acquired (Balasundaram et al., 2010, Palanisamy et al., 2006) and congenital infections. It may cause either chronic or recurrent after primary infection of T. gondii (Remington et al., 2006). Although this disease was reported as early as in the 1920s, the parasite’s life cycle was outlined in the late 1960s (Dubey and Frenkel, 1972, Frenkel et al., 1970, Hutchison et al., 1970). Cat has been reported to play a central role in transmitting the parasite as this animal is the host for the parasitic cycle and rapidly spread oocysts through its feces. T. gondii infected both females and males without any difference in all age groups. This parasitic infection is highly prevalent in humid and warm climates (Bojar and Szymańska, 2010). However, this parasitic infection's prevalence mainly depended on the hygiene and quality of water resources accessed (Jones and Dubey, 2010). Many findings revealed that domesticated cats did not lead to T. gondii infection among humans (Flegr et al., 1998); however, few studies indicated a high risk of parasitic infection through such cats (Jones et al., 2009). About 14%−77% of pregnant women suffered due to the seroprevalence of T. gondii in most developing and developed countries (Montoya and Liesenfeld, 2004). Toxoplasmosis is one of the serious problems among livestock, and the economic impact of this disease is a known sequence. The outcome and risk of toxoplasmosis infection are mainly based on the genotypes of T. gondii, and three clonal lineages were reported among the strains (Howe et al., 1997). The type strains I, II were isolated from patients with HIV infection; however, only the type strain III was reported from animals.
3. The life cycle of T. gondii
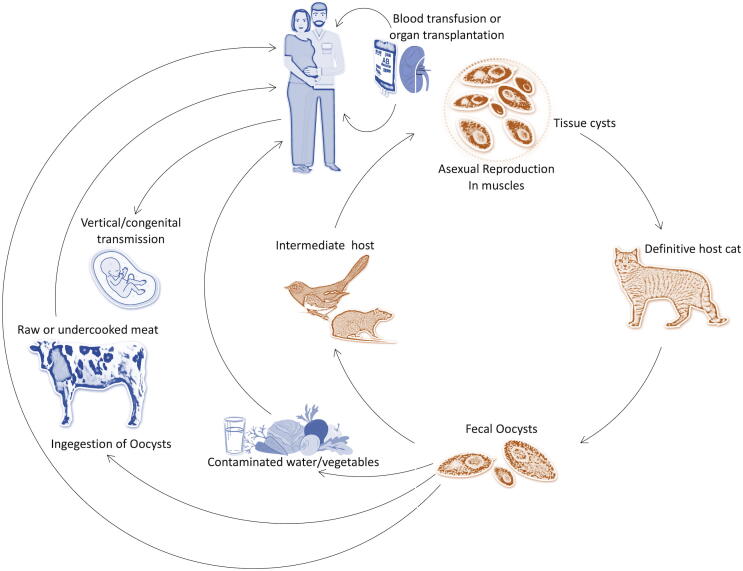
The lifecycle of T. gondii is unique among the parasite and includes asexual as well as sexual reproduction (see Fig. 1). Further, three different infective stages representing invasive tachyzoite (rapidly dividing), bradyzoite in tissue cysts (slowly dividing), and the sporozoite (environmental stage) are common. Sporozoite form of life is protected inside the oocysts and is an environmental stage. T. gondii at various stages are approximately 2 m wide and 5 m long, crescent-shaped cells, a rounded posterior end, and a pointed apical end. The motility and structural integrity of the cells are governed by the cytoskeleton. They contain apicoplast, a multiple-membrane-bound plastid-like organelle, endoplasmic reticulum, ribosomes, Golgi complex, and a mitochondrion. This parasite is also associated with endosymbiosis of various algae (Roos et al., 1999). The parasite's cytoskeletal structure helps to invade the cells and various secretary organelles such as micronemes, dense granules, and rhoptries (Dubey, 1998, Henriquez, 2008, Weiss and Kim, 2007).
Fig. 1.
Modes of primary transmission of T. gondii.
Tachyzoites are a highly active form of parasitic life, and they can invade effectively in almost all vertebrate cell types. Bradyzoites stage was developed from tachyzoites and form cysts among various tissues. These cysts are elongated in muscular cells or less spheroid in brain cells. The size of younger cysts and older cysts varied widely. The size is only 10 m in the case of most immature cysts; however, 100 m size was reported in the earlier stages. The older cysts consist of thousands of densely packed bradyzoites. Many invaginations and granular materials have been reported on the cyst wall (Ferguson, 2004). Bradyzoites mode of life is well adapted to survive in a long time, and the cysts could survive in the intracellular region. Bradyzoites resist the pepsin enzyme of the host organism and survive 1–2 h in the presence of pepsin-HCl. Sporozoites are found in 12- to 13-m sized oocysts. The wall of the oocyst is a multilayer structure protecting the organism from chemical and mechanical changes. This multilayer complex allows the parasite to survive more than a year (Mai et al., 2009). The mode of disease transmission also varied among the groups. Toxoplasmosis is transmitted between intermediate hosts via the asexual cycle (carnivorism), sexual cycle (definitive hosts), and also by intermediate hosts (Figure). The asexual and sexual cycles and disease transmission patterns in a survival environment vary based on the physical characters and structures of both definitive and intermediate host populations (Afonso et al., 2006). Schwartzman et al. (1948) reported congenital toxoplasmosis in humans, and the trend of clinical toxoplasmosis has been pictured in recent years (Weiss and Dubey, 2009). For the past two decades, field studies and the advancement of novel genotyping brought out the details of the evolution of T. gondii (Mercier et al., 2011).
4. Mode of toxoplasmosis transmission
Transmission of the disease reported to occur mainly through water, from contaminated food, ingestion of the tissue cysts, congenital transmission, or ingestion of oocysts (Hill and Dubey, 2002). However, this disease's transmission varied widely among populations, mainly based on food habits and culture. The infection can get through unpasteurized milk and contamination of blood products by tachyzoites (Tenter et al., 2000). Tissue cysts are located in the brain and muscle of most of the intermediate hosts. Cats reported to acquire toxoplasmosis through contaminated prey such as birds or rodents and act as hosts to continue sexual reproduction, enteroepithelial cycle, and oocyst production (Dubey et al., 1970). Carnivorous animals from wild habitats such as skunks, raccoon, fox, bears also acquire toxoplasmosis through the ingestion of cysts from tissues. Human beings acquire toxoplasmosis by consuming half-cooked contaminated meat, such as lamb or pork (Dubey et al., 2005, Hill et al., 2010). The ingested tissue cyst is digested by the action of various proteolytic enzyme activities in the stomach of the host organism and release the parasite in the form of bradyzoites. The parasite is observed to be highly resistant to protease and survive in the host organism's small intestine. A report estimated more than 10 million cyst removal through fecal materials in cats within two weeks of ingestion. The released oocyst sporulates within five days of release in the environment. In the soil sample, the sporulated organism remains alive for about one year (Dubey, 1998).
Generally, eating contaminated vegetables, fruits, or contaminated food with oocysts were the major risk factors of disease transmission. These risk factors and modes of actions were assessed previously by Dubey (2010) and Pereira et al. (2010). In human beings, oocyst induced infections are highly complex and more severe than cyst acquired infections. However, water-borne infections noted to be sporadic (Balasundaram et al., 2010, Palanisamy et al., 2006), and in the USA, outbreak of toxoplasmosis was reported due to marine animals, and this disease outbreak was linked with contamination of oocyst from the municipal water reservoir in Canada (Dubey, 2004). Among cats, oocyst shedding happens for a short duration, and the fastidious nature of cats, the passing of non-infective oocysts, direct contact with cats is no risk to any kind of infection from cats (Elmore et al., 2010). In seawater, oocysts are stable for six months, and this revealed that the coastal environment is an essential source of infection. Also, the oocysts transfer from one host to another (Lindsay and Dubey, 2009). Oocysts could tolerate a wide environment and survive in fishes such as sardines and anchovies (filter feeders), and it remained in the alimentary canal of the organism and be viable (Massie et al., 2010).
The congenital transmission is also reported, and this stage of transmission happens when the time of toxoplasmosis is at the acute phase. Tachyzoites form of parasite from the mother crosses the placenta and causes infection in the fetus (Jones et al., 2003, Montoya and Remington, 2008). The effect of maternal toxoplasmosis is based on the stage of pregnancy. During the earlier stages of pregnancy (first trimester), maternal toxoplasmosis is very low; however, at the later stages of pregnancy, the transmission rate increased by about 80% (Jones et al., 2003, Ortiz-Alegría et al., 2010). Maternal toxoplasmosis in earlier stages caused various problems, including mental retardation, hydrocephaly, and spontaneous abortion. However, during the final trimester transmission rate was very high, and various subclinical interventions such as recurrent chorioretinitis, subclinical interventions potentially blindness or vision problems reported in children (Montoya and Liesenfeld, 2004). Based on previous studies conducted by Tenter et al. (2000) and Dubey et al. (2011), congenital toxoplasmosis among most groups of populations the incidence was about 1 in 1000 to 1 in 10,000 birth. In a study, Hide et al. (2007) used PCR to detect vertical transmission through umbilical cord tissue, and the transmission rate was about 19.8%. In a mouse model, the transmission rate was about 75%; however, it was reported to be declining to 65% in sheep. These findings suggested that the vertical transmission rate in humans was not clearly understood as most of the studies were based on serological methods (Hide et al., 2009). Oocysts are available in large quantities in various environments; hence, analysis of oocyst in the environment is critical to analyze the health risks. The recent findings indicated that marine, aquatic and terrestrial environments are contaminated with oocysts (Du et al., 2012, Gao et al., 2016, VanWormer et al., 2016). In T. gondii oocysts, the survival ability was mainly influenced by various environmental and geological characteristics such as soil texture, temperature, and chemistry (Lélu et al., 2012, Lindsay and Dubey, 2009). The research on the persistence of oocysts in the soil sample, survival rate, and pathogenic properties, including tissue cysts' infectivity, is limited (Lélu et al., 2012).
5. Epidemiology of toxoplasmosis
Toxoplasmosis infection was reported in warm-blooded animals and humans, and the distribution was reported throughout the world. It affected almost all species, from mammals to various bird species (Dubey et al., 2005). In the case of humans, toxoplasmosis was reported in nearly all parts of the world, and about one-third of human populations were vulnerable to latent infection. The incidence of toxoplasmosis in developing and underdeveloped nations is reported to be very high; however, it is noted that the infections were low in developed countries. High prevalence of toxoplasmosis was reported in Africa, parts of south-east Asia, Middle East, parts of Central/Eastern Europe, and Latin America. In the US, the toxoplasmosis rate was between 15 and 22%, and the proportion was lesser than the previous years. The same trend was determined in Europe (Pappas et al., 2009). The prevalence could be influenced by various factors such as cultural, climatic, ethical practices, and cats' presence. Oocysts' survival rate was high in the environment and has been described by Dabritz and Conrad (2010). In European countries, due to cold climatic conditions, the prevalence is very low and higher in warm climatic conditions. Based on climatic conditions, the prevalence of toxoplasmosis in cats was reported to be about 40%; however, the percentage decreased to 16.1% in southwestern regions and high prevalence (59.2%) in Hawaii (Elmore et al., 2010). Food preparing habits such as thoroughly cooked foods (meat) or half-cooked were the critical factors influencing transmission (Tenter, 2009, Tenter et al., 2000). Eating raw mussels, clams, and oysters have been predicted as a significant risk factor of toxoplasmosis in various countries, including the USA (Jones et al., 2009). It was estimated that about 25–30% of human populations infected with T. gondii (Montoya and Liesenfeld, 2004). Many climatic factors that influence the survival rate of oocysts and ruminants play a significant role in transmitting disease. Also, seroprevalence was observed to be higher with aged people (Wyman et al., 2017); however, the infection rate was largely associated with older age adults and varied based on individuals' socio-economic conditions. Water carries oocysts, and the consumption of unfiltered water or water in recreation facilities contributed to disease transmission (Bahia-Oliveira et al., 2003, Jones and Dubey, 2010). The socio-economic level has been an influencing factor on toxoplasmosis infection. In Brazil, seroprevalence was 23% in the upper socio-economic groups, 62% in middle socio-economic groups, and 84% among the lower socio-economic group. Consuming unfiltered water could increase the risk of Toxoplasma infection. These analyses were highly relevant in poor and developing countries. However, in the United States, toxoplasmosis infection was mainly associated with poverty (Hotez, 2008). Also, in the United States, toxoplasmosis infection varied among various populations (Jones et al., 2007). Improvement of socio-economic conditions, quality drinking water, hygienic level, the consumption of frozen meat, changes in farming systems, and feeding of cats with autoclaved diet have decreased the toxoplasmosis rate in various countries for the past two decades. T. gondii infection was documented as 14.1% in US-born citizens aged from 12 to 49 years in 1988 to 1994, and decreased by 9% between 1999 and 2004 (Jones et al., 2007). In France, about 80% of pregnant women was affected by toxoplasmosis in 1960s and decreased by 44% in 2003 (Villena et al., 2010). This declining trend was reported in almost all countries in Europe. In the Netherlands, the disease's seroprevalence decreased by 50% from 1995 to 2007 among women of active reproductive age group (Hofhuis et al., 2011).
6. Transmission and diagnosis
The impacts of toxoplasmosis differ depending on the parasite's characteristic features such as inoculums size, the virulence of the infecting strain, host factors such as immune status, and genetic background of the individuals (Weiss and Dubey, 2009). Three types of genetic variance were reported from the T. gondii (type 1, 2, and 3) and the three type strains varied in the epidemiological pattern of occurrence and virulence (Dardé, 2008). In animals, T. gondii caused clinical disease with various range of clinical symptom in definitive or intermediate hosts or caused subclinical infections. The prevalence of toxoplasmosis among wild and domestic animals has been reported in various studies (Smith, 2009). Seropositive ruminants were at high risk of transmitting the disease to the individuals, either through farming or direct contact. Horses, goats, sheep, poultry, cattle, and pigs were identified to be important sources for infection (Suzán et al., 2015). It was reported that ingestion of half-boiled or half-cooked meat contaminated with T. gondii tissue cysts, mainly from chicken, goats, lambs, and pigs or consumption of water and food with oocysts from the fecal material of cats as the general route of infection. Possible sources of parasite infection for pigs included cannibalism, eating infected rodents, and food contaminated with cat feces. Pigs slaughtered in contaminated conditions generally increased the transmission of disease (Kagira et al., 2003).
Chicken infected by this parasite acts as a reservoir of parasitic infection. Free-range chickens mainly infected by feeding on open grounds are highly contaminated with oocysts. Hence, the presence of T. gondii in bird is an efficient indicator of oocyst load in a particular environment (Dubey et al., 2005, Mose et al., 2016). Toxoplasmosis in animals leads to economic losses due to failure in reproduction, including barrenness, fetal resorption, and abortion. However, studies shown that the occurrence of T. gondii in ruminants and other meat-producing animals substantially decreased for the past 20 years (Ajioka and Morrissette, 2009). The host organism and its affected organ by T. gondii summarized in Table 1 and clearly showed that various organisms were infected by different forms of T. gondii.
Table 1.
Summary of host organisms involved in toxoplasmosis.
| Sl. No | Host organism | Organ Affected | Stage of T. gondii | Reference |
|---|---|---|---|---|
| 1 | Cats | Brain | Tachyzoites | Paul (1999) |
| 2 | Dogs | Brain | Tachyzoites | Roghmann et al. (1999) |
| 3 | Marsupials | Tissues | Tissue cyst | Dubey (1996) |
| 4 | Sheep | Tissues | Tissue Cyst | Munday and Corbould (1979) |
| 5 | Goat | Tissues | Tissue cyst | Wallace (1969) |
| 6 | Domestic cat | Parasitic mode | Oocyst | Dubey (1996) |
| 7 | Pigs | Parasitic mode | Oocyst | Arias et al. (1994) |
| 8 | Cockroaches | Gut | Oocyst | Chinchilla et al. (1994) |
| 9 | Wild felines | Internal tissues | Oocyst | Valcavi et al. (1995) |
| 10 | Pregnant women | Fetus | Primary stage | Zemene et al. (2012) |
| 11 | Goat | Placenta | NA | Dubey (1982) |
| 12 | Human | Eye | Tissue cyst | Balasundaram et al., 2010, Balasundaram et al., 2010, Palanisamy et al., 2006) |
| 13 | Human | Brain | Encephalitis | Toxoplasmosis of Animals and Man (1988) |
| 14 | Human | Blood cells | Tachyzoites | Sabin (1953) |
Although various methods have been suggested to screen the infection, there is no standard method for determining the Toxoplasma species. The specificity and sensitivity of the methods mainly based on the cutoff values. In recent years, a modified agglutination test has been recommended to identify toxoplasmosis (Dubey, 2010) and enzyme-linked immunosorbent assays used for the diagnosis of toxoplasmosis among various domestic animals. These tests were initially developed to analyze serum; however, these methods have been modified to determine Toxoplasma from meat samples (Dubey et al., 2011, Halos et al., 2010). Analysis of Toxoplasma antibodies from meat samples was a very less sensitive method.
Moreover, the survey of disease by the serological methods alone may not be adequate to determine infection by viable parasites as Toxoplasma strains were screened and isolated from animals with seronegative cases (Dubey et al., 2011). In some cases, the direct determination of parasites showed negative results in most seropositive animals (Opsteegh et al., 2011). Bioassays are the most sensitive methods useful for the determination of cysts in animal tissues. In the case of parasite determination from the mouse, tissues were dissected out and digested with trypsin or pepsin or with acid before the inoculation of parasites into mice. Then it was subjected to symptom analysis and seroconversion as described by Dubey (Dubey, 1998). In the case of cat bioassays, the method is highly sensitive than elsewhere; however, it is highly expensive than bioassays with mouse models. This procedure consisted of inoculating tissue samples with the parasite and analyzing the fecal sample for excretion of oocytes for one to two weeks post-inoculation. These methods are time-consuming and laborious, however; these were noted to be easy to screen a large number of samples within a short period. Hence, PCR-based methods were recommended to detect the DNA of the parasite among test samples.
However, PCR based methods could be less sensitive owing to the heterogenous distribution of the cyst abundance, and small sample size. The magnetic capture of sequence-specific DNA and amplification was developed to enhance the sensitivity of the determination of parasite DNA in the tissues. This method allowed the analysis of about 100 g tissue samples, which enhanced the possibility of detecting cysts (Opsteegh et al., 2010). The genotypes of T. gondii were differentiated based on restriction fragment length polymorphism (RFLP) analysis using SAG2 gene analyses. Further, various high-resolution methods have been reported for type strain analysis of T. gondii using peptide mapping and microsatellite analysis (Su et al., 2010). Novel prevention methods of emerging infectious diseases, predictive tools, and mathematical modeling have been evolved during the past two decades. These tools allow for improved prediction of disease dynamics, enhanced characterization, and to analyze disease behavior (Aguirre et al., 2016, Guo et al., 2016, Vinetz et al., 2005).
Improved disease diagnostic method has been used in recent years to perform seroepidemiological analyses in a wide range of animals and humans, which proved a strong evidence of a diverse distribution of the parasite among the infected human population. The rate of seroprevalence of the disease varied widely among various countries, geographical locations, and ethnic populations living in a particular area. Hence, antibodies of T. gondii have been found from 0 to 100% of the human population (Pappas et al., 2009). This neglected zoonotic disease distributed in patches covers about 20 countries in North America and the Middle East region. This disease was prevalent in pregnant women who were immunocompromised with HIV infection (Hotez et al., 2012). In New Zealand and Australia, this disease's prevalence was up to 50%, and prevalence rates were high in India and China (Zarkovic et al., 2007). Toxoplasmosis also complicated the health condition of recipient after organ transplantation.
Transplantation or infected organ with T. gondii reactivates the disease (Morris et al., 2010). Increased awareness among the populations and improved practices in animal husbandry management and awareness of the risk of eating half boiled meat and undercooked meat showed a sharp decrease in the prevalence of this parasitic infection (Tenter, 2009). One health approach has been proposed to deal with this parasitic infection and reported to be effective in handling rabies virus control in Sri Lanka; echinococcosis in North America (Häsler et al., 2014, Massolo and Liccioli, 2016).
Sociobiologists, veterinarians, public health officials, and epidemiologists need to redress reliable inference methods, including disease inference techniques and model selection, to apply a rapid approach to manage this disease (Azeez and Prabhakar, 2016). Long-term survival of the parasite in oocyst form or infectivity of tissue cyst or the duration of survival has not yet been understood remarkably. These knowledge gaps undoubtedly require collaborations from scientists from various disciplines, including health science, engineering, social, ecological, biological, environmental, and earth sciences (Aguirre and Wilcox, 2008).
7. Management of toxoplasmosis
The inhibitors of dihydrofolate reductase, dihydropteroate synthetase were recommended to treat toxoplasmosis. Pyrimethamine is one of the inhibitors of dihydrofolate reductase prescribed as an effective agent to treat this disease. Drugs such as trimethoprim and sulfamethoxazole were also used to treat this infection (Gallant, 2015). Almost all drugs prescribed were effective against the tachyzoite stage and not cysts containing bradyzoites (Khan and Araujo, 1996). Among immunocompetent patients, cysts remained dormant in retina/uvea, and frequent reactivation of bradyzoites was reported. No effective treatment has been reported to eradicate oocysts (Tedford and McConkey, 2017).
In some cases, toxoplasmosis affected eyes, pyrimethamine with either clindamycin or sulfadiazine were useful in managing the disease. In the case of a weakened immune system, pyrimethamine with sulfadiazine was useful. The natural choice is sulfadiazine, combined with pyrimethamine. Drugs such as pyrimethamine and trimethoprim-sulfamethoxazole were also recommended to treat this disease (Konstantinovic et al., 2019). Vaccination was also recommended to resist parasitic infection of the host cell (Lim and Othman, 2014) among immunocompromised hosts. The recombinant anti-Toxoplasma antibodies prevent parasite invasion at an early stage or tachyzoite replication (Fu et al., 2011). Although effective therapy has been reported, the drug safety, length of therapy, and drug potency were the main concerns in treating Toxoplasma infection. Spiramycin was useful in pregnant women until week 16 of pregnancy, and sulfadiazine, pyrimethamine, and folinic acid were recommended at various combinations (Hotop et al., 2012). In order to curtail mother to child transmission, spiramycin has been widely used. Sulfadiazine and pyrimethamine were used for the treatment of neonatal infections. However, the effectiveness of the drugs was unclear. To treat ocular toxoplasmosis, pyrimethamine, and sulfadiazine, combined with corticosteroids, were reported to be highly effective (Serranti et al., 2011).
8. Conclusions
The review on toxoplasmosis analyzed the disease's epidemiology, it's transmission, and seroprevalence among diverse populations. Infection with Toxoplasma spread mainly through livestock, free-roaming wildlife, and birds. Ingested uncooked or semi-cooked foods with T. gondii could transmit the disease. The socio-economic level is ascertained to be one of the determining factors concerning disease epidemiology. This review would be beneficial to academicians, policymakers, health-care staff, and veterinary personnel. Toxoplasmosis is one of the neglected infections that should be brought to the attention of the common men. Therefore, enhanced attention should be paid to educate people on T. gondii caused toxoplasmosis.
Acknowledgment
The Authors would like to thank Deanship of Scientific Research at Majmaah University, Al Majmaah, 11952, Saudi Arabia for supporting this work.
Footnotes
Peer review under responsibility of King Saud University.
References
- Afonso E., Thulliez P., Gilot-Fromont E. Transmission of Toxoplasma gondii in an urban population of domestic cats (Felis catus) Int. J. Parasitol. 2006;36:1373–1382. doi: 10.1016/j.ijpara.2006.07.010. [DOI] [PubMed] [Google Scholar]
- Aguirre A., Wilcox B.A. EcoHealth: Envisioning and Creating a Truly Global Transdiscipline. Ecohealth. 2008;5:238–239. doi: 10.1007/s10393-008-0197-6. [DOI] [PubMed] [Google Scholar]
- Aguirre A.A., Beasley V.R., Augspurger T., Benson W.H., Whaley J., Basu N. One health-Transdisciplinary opportunities for SETAC leadership in integrating and improving the health of people, animals, and the environment. Environ. Toxicol. Chem. 2016;35:2383–2391. doi: 10.1002/etc.3557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ajioka J.W., Morrissette N.S. A century of Toxoplasma research. Int. J. Parasitol. 2009;39:859–860. doi: 10.1016/j.ijpara.2009.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alavi S.M., Alavi L. Treatment of toxoplasmic lymphadenitis with co-trimoxazole: double-blind, randomized clinical trial. Int. J. Infect. Dis. 2010;14(Suppl 3):e67–e69. doi: 10.1016/j.ijid.2009.11.015. [DOI] [PubMed] [Google Scholar]
- Azeez R., Prabhakar A. A Review on Disease Inference Techniques. Int. J. Sci. Res. 2016;5:514–518. doi: 10.21275/v5i1.nov152786. [DOI] [Google Scholar]
- Bahia-Oliveira L.M.G., Jones J.L., Azevedo-Silva J., Alves C.C.F., Oréfice F., Addiss D.G. Highly Endemic, Waterborne Toxoplasmosis in North Rio de Janeiro State. Brazil. Emerg. Infect. Dis. 2003;9:55–62. doi: 10.3201/eid0901.020160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balasundaram, M.B., Andavar, R., Palaniswamy, M., Venkatapathy, N., 2010. Outbreak of acquired ocular toxoplasmosis involving 248 patients. Arch. Ophthalmol. (Chicago, Ill. 1960) 128, 28–32. https://doi.org/10.1001/archophthalmol.2009.354. [DOI] [PubMed]
- Chinchilla M., Guerrero O.M., Castro A., Sabah J. Cockroaches as transport hosts of the protozoan Toxoplasma gondii. Revista de Biologia Trop. 1994;42(1–2):329–331. [PubMed] [Google Scholar]
- Bojar I., Szymańska J. Environmental exposure of pregnant women to infection with Toxoplasma gondii–state of the art. Ann. Agric. Environ. Med. 2010;17:209–214. [PubMed] [Google Scholar]
- Dabritz H.A., Conrad P.A. Cats and Toxoplasma : Implications for Public Health. Zoonoses Public Health. 2010;57:34–52. doi: 10.1111/j.1863-2378.2009.01273.x. [DOI] [PubMed] [Google Scholar]
- Dardé M.L. Toxoplasma gondii, “new” genotypes and virulence. Parasite. 2008;15:366–371. doi: 10.1051/parasite/2008153366. [DOI] [PubMed] [Google Scholar]
- Demar M., Ajzenberg D., Maubon D., Djossou F., Panchoe D., Punwasi W., Valery N., Peneau C., Daigre J.-L., Aznar C., Cottrelle B., Terzan L., Darde M.-L., Carme B. Fatal Outbreak of Human Toxoplasmosis along the Maroni River: Epidemiological, Clinical, and Parasitological Aspects. Clin. Infect. Dis. 2007;45:e88–e95. doi: 10.1086/521246. [DOI] [PubMed] [Google Scholar]
- Du F., Feng H.L., Nie H., Tu P., Zhang Q.L., Hu M., Zhou Y.Q., Zhao J.L. Survey on the contamination of Toxoplasma gondii oocysts in the soil of public parks of Wuhan. China. Vet. Parasitol. 2012;184:141–146. doi: 10.1016/j.vetpar.2011.08.025. [DOI] [PubMed] [Google Scholar]
- Dubey J.P. Repeat transplacental transfer of Toxoplasma gondii in dairy goats. J. Am. Veterinary Med. Assoc. 1982;180:1220–1221. [PubMed] [Google Scholar]
- Dubey J.P. Strategies to reduce transmission of Toxoplasma gondii to animals and humans. Vet Parasitol. 1996;64:65–70. doi: 10.1016/0304-4017(96)00961-2. [DOI] [PubMed] [Google Scholar]
- Dubey J.P. 2nd ed. CRC Press; Boca Raton, FL: 2010. Toxoplasmosis of animals and humans. [Google Scholar]
- Dubey J.P. Toxoplasmosis – a waterborne zoonosis. Vet. Parasitol. 2004;126:57–72. doi: 10.1016/j.vetpar.2004.09.005. [DOI] [PubMed] [Google Scholar]
- Dubey J.P. Refinement of pepsin digestion method for isolation of Toxoplasma gondii from infected tissues. Vet. Parasitol. 1998;74:75–77. doi: 10.1016/S0304-4017(97)00135-0. [DOI] [PubMed] [Google Scholar]
- Dubey J.P., Frenkel J.K. Cyst-Induced Toxoplasmosis in Cats*. J. Protozool. 1972;19:155–177. doi: 10.1111/j.1550-7408.1972.tb03431.x. [DOI] [PubMed] [Google Scholar]
- Dubey J.P., Hill D.E., Jones J.L., Hightower A.W., Kirkland E., Roberts J.M., Marcet P.L., Lehmann T., Vianna M.C.B., Miska K., Sreekumar C., Kwok O.C.H., Shen S.K., Gamble H.R. Prevalence of viable toxoplasma gondii in beef, chicken, and pork from retail meat stores in the united states: risk assessment to consumers. J. Parasitol. 2005;91:1082–1093. doi: 10.1645/GE-683.1. [DOI] [PubMed] [Google Scholar]
- Dubey J.P., Miller N.L., Frenkel J.K. Toxoplasma gondii life cycle in cats. J. Am. Vet. Med. Assoc. 1970;157:1767–1770. [PubMed] [Google Scholar]
- Dubey J.P., Rajendran C., Ferreira L.R., Martins J., Kwok O.C.H., Hill D.E., Villena I., Zhou H., Su C., Jones J.L. High prevalence and genotypes of Toxoplasma gondii isolated from goats, from a retail meat store, destined for human consumption in the USA. Int. J. Parasitol. 2011;41:827–833. doi: 10.1016/j.ijpara.2011.03.006. [DOI] [PubMed] [Google Scholar]
- Elmore S.A., Jones J.L., Conrad P.A., Patton S., Lindsay D.S., Dubey J.P. Toxoplasma gondii: epidemiology, feline clinical aspects, and prevention. Trends Parasitol. 2010;26:190–196. doi: 10.1016/j.pt.2010.01.009. [DOI] [PubMed] [Google Scholar]
- Ferguson D.J. Use of molecular and ultrastructural markers to evaluate stage conversion of Toxoplasma gondii in both the intermediate and definitive host. Int. J. Parasitol. 2004;34:347–360. doi: 10.1016/j.ijpara.2003.11.024. [DOI] [PubMed] [Google Scholar]
- Flegr J., Hrdá S., Tachezy J. The role of psychological factors in questionnaire-based studies on routes of human toxoplasmosis transmission. Cent. Eur. J. Public Health. 1998;6:45–50. [PubMed] [Google Scholar]
- Frenkel J.K., Dubey J.P., Miller N.L. Toxoplasma gondii in Cats: Fecal Stages Identified as Coccidian Oocysts. Science (80-. 1970;). 167:893–896. doi: 10.1126/science.167.3919.893. [DOI] [PubMed] [Google Scholar]
- Fu Y.-F., Feng M., Ohnishi K., Kimura T., Itoh J., Cheng X.-J., Tachibana H. Generation of a Neutralizing Human Monoclonal Antibody Fab Fragment to Surface Antigen 1 of Toxoplasma gondii Tachyzoites. Infect. Immun. 2011;79:512–517. doi: 10.1128/IAI.00969-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallant J. Get Rich Quick With Old Generic Drugs! The Pyrimethamine Pricing Scandal. Open Forum. Infect. Dis. 2015;2:ofv177. doi: 10.1093/ofid/ofv177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao X., Wang Hongbin, Wang Huan, Qin H., Xiao J. Land use and soil contamination with Toxoplasma gondii oocysts in urban areas. Sci. Total Environ. 2016;568:1086–1091. doi: 10.1016/j.scitotenv.2016.06.165. [DOI] [PubMed] [Google Scholar]
- Guo M., Mishra A., Buchanan R.L., Dubey J.P., Hill D.E., Gamble H.R., Jones J.L., Pradhan A.K. A Systematic Meta-Analysis of Toxoplasma gondii Prevalence in Food Animals in the United States. Foodborne Pathog. Dis. 2016;13:109–118. doi: 10.1089/fpd.2015.2070. [DOI] [PubMed] [Google Scholar]
- Halos L., Thébault A., Aubert D., Thomas M., Perret C., Geers R., Alliot A., Escotte-Binet S., Ajzenberg D., Dardé M.-L., Durand B., Boireau P., Villena I. An innovative survey underlining the significant level of contamination by Toxoplasma gondii of ovine meat consumed in France. Int. J. Parasitol. 2010;40:193–200. doi: 10.1016/j.ijpara.2009.06.009. [DOI] [PubMed] [Google Scholar]
- Häsler B., Hiby E., Gilbert W., Obeyesekere N., Bennani H., Rushton J. A One Health Framework for the Evaluation of Rabies Control Programmes: A Case Study from Colombo City. Sri Lanka. PLoS Negl. Trop. Dis. 2014;8 doi: 10.1371/journal.pntd.0003270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henriquez, F.L., 2008. Toxoplasma. Molecular and Cellular Biology, Parasitology. Horizon Bioscience, Norfolk, UK. https://doi.org/10.1017/S0031182008004861.
- Hide G., Gerwash O., Morley E.K., Williams R.H., Hughes J.M., Thomasson D., Elmahaishi M.S., Elmahaishi K.H., Terry R.S., Smith J.E. Does vertical transmission contribute to the prevalence of toxoplasmosis? Parassitologia. 2007;49:223–226. [PubMed] [Google Scholar]
- Hide G., Morley E.K., Hughes J.M., Gerwash O., Elmahaishi M.S., Elmahaishi K.H., Thomasson D., Wright E.A., Williams R.H., Murphy R.G., Smith J.E. Evidence for high levels of vertical transmission in Toxoplasma gondii. Parasitology. 2009;136:1877–1885. doi: 10.1017/S0031182009990941. [DOI] [PubMed] [Google Scholar]
- Hill D., Dubey J.P. Toxoplasma gondii: transmission, diagnosis and prevention. Clin. Microbiol. Infect. 2002;8:634–640. doi: 10.1046/j.1469-0691.2002.00485.x. [DOI] [PubMed] [Google Scholar]
- Hill D.E., Haley C., Wagner B., Gamble H.R., Dubey J.P. Seroprevalence of and Risk Factors for Toxoplasma gondii in the US Swine Herd Using Sera Collected During the National Animal Health Monitoring Survey (Swine 2006) Zoonoses Public Health. 2010;57:53–59. doi: 10.1111/j.1863-2378.2009.01275.x. [DOI] [PubMed] [Google Scholar]
- Hofhuis A., Van Pelt W., Van Duynhoven Y.T.H.P., Nijhuis C.D.M., Mollema L., Van Der Klis F.R.M., Havelaar A.H., Kortbeek L.M. Decreased prevalence and age-specific risk factors for Toxoplasma gondii IgG antibodies in The Netherlands between 1995/1996 and 2006/2007. Epidemiol. Infect. 2011;139:530–538. doi: 10.1017/S0950268810001044. [DOI] [PubMed] [Google Scholar]
- Hotez P.J. Neglected Infections of Poverty in the United States of America. PLoS Negl. Trop. Dis. 2008;2 doi: 10.1371/journal.pntd.0000256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hotez P.J., Savioli L., Fenwick A. Neglected Tropical Diseases of the Middle East and North Africa: Review of Their Prevalence, Distribution, and Opportunities for Control. PLoS Negl. Trop. Dis. 2012;6 doi: 10.1371/journal.pntd.0001475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hotop A., Hlobil H., Gross U. Efficacy of Rapid Treatment Initiation Following Primary Toxoplasma gondii Infection During Pregnancy. Clin. Infect. Dis. 2012;54:1545–1552. doi: 10.1093/cid/cis234. [DOI] [PubMed] [Google Scholar]
- Howe D.K., Honoré S., Derouin F., Sibley L.D. Determination of genotypes of Toxoplasma gondii strains isolated from patients with toxoplasmosis. J. Clin. Microbiol. 1997;35:1411–1414. doi: 10.1128/JCM.35.6.1411-1414.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hutchison W.M., Dunachie J.F., Siim J.C., Work K. Coccidian-like Nature of Toxoplasma gondii. BMJ. 1970;1:142–144. doi: 10.1136/bmj.1.5689.142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones J., Lopez A., Wilson M. Congenital toxoplasmosis. Am. Fam. Physician. 2003;67:2131–2138. [PubMed] [Google Scholar]
- Jones J.L., Dargelas V., Roberts J., Press C., Remington J.S., Montoya J.G. Risk Factors for Toxoplasma gondii Infection in the United States. Clin. Infect. Dis. 2009;49:878–884. doi: 10.1086/605433. [DOI] [PubMed] [Google Scholar]
- Jones J.L., Dubey J.P. Waterborne toxoplasmosis – Recent developments. Exp. Parasitol. 2010;124:10–25. doi: 10.1016/j.exppara.2009.03.013. [DOI] [PubMed] [Google Scholar]
- Jones J.L., Kruszon-Moran D., Sanders-Lewis K., Wilson M. Toxoplasma gondii infection in the United States, 1999 2004, decline from the prior decade. Am. J. Trop. Med. Hyg. 2007;77:405–410. https://doi.org/17827351. [PubMed] [Google Scholar]
- Kagira J.M., Kanyari P.W.N., Munyua W.K., Waruiru R.M. The control of parasitic nematodes in commercial piggeries in Kenya as reflected by a questionnaire survey on management practices. Trop. Anim. Health Prod. 2003;35:79–84. doi: 10.1023/a:1022031806486. [DOI] [PubMed] [Google Scholar]
- Khan A.A., Araujo F.G. Recent developments in the search for therapeutic interventions against Toxoplasma gondii infection. Recent Res. Dev. Antimicrob. agents Chemother. 1996:65–77. [Google Scholar]
- Konstantinovic N., Guegan H., Stäjner T., Belaz S., Robert-Gangneux F. Treatment of toxoplasmosis: Current options and future perspectives. Food Waterborne Parasitol. 2019;15 doi: 10.1016/j.fawpar.2019.e00036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lélu M., Villena I., Dardé M.-L., Aubert D., Geers R., Dupuis E., Marnef F., Poulle M.-L., Gotteland C., Dumètre A., Gilot-Fromont E. Quantitative Estimation of the Viability of Toxoplasma gondii Oocysts in Soil. Appl. Environ. Microbiol. 2012;78:5127–5132. doi: 10.1128/AEM.00246-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim S.S.-Y., Othman R.Y. Recent Advances in Toxoplasma gondii Immunotherapeutics. Korean J. Parasitol. 2014;52:581–593. doi: 10.3347/kjp.2014.52.6.581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindsay D.S., Dubey J.P. Long-Term Survival of Toxoplasma gondii Sporulated Oocysts in Seawater. J. Parasitol. 2009;95:1019–1020. doi: 10.1645/GE-1919.1. [DOI] [PubMed] [Google Scholar]
- Mai K., Sharman P.A., Walker R.A., Katrib M., Souza D. De, McConville M.J., Wallach M.G., Belli S.I., Ferguson D.J., Smith N.C. Oocyst wall formation and composition in coccidian parasites. Mem. Inst. Oswaldo Cruz. 2009;104:281–289. doi: 10.1590/S0074-02762009000200022. [DOI] [PubMed] [Google Scholar]
- Massie G.N., Ware M.W., Villegas E.N., Black M.W. Uptake and transmission of Toxoplasma gondii oocysts by migratory, filter-feeding fish. Vet. Parasitol. 2010;169:296–303. doi: 10.1016/j.vetpar.2010.01.002. [DOI] [PubMed] [Google Scholar]
- Massolo A., Liccioli S. Applying a One Health, multi-scale approach to understanding and preventing zoonotic parasite transmission in urban ecosystems: Echinococcus multilocularis and alveolar echinococcosis in North America. In: Cork Susan, David Hall K.L., editors. One Health Case Studies: Addressing Complex Problems in a Changing World. 5M Publishing Ltd.; Sheffield, UK: 2016. pp. 40–53. [Google Scholar]
- Mercier A., Ajzenberg D., Devillard S., Demar M.P., de Thoisy B., Bonnabau H., Collinet F., Boukhari R., Blanchet D., Simon S., Carme B., Dardé M.-L. Human impact on genetic diversity of Toxoplasma gondii: example of the anthropized environment from French Guiana. Infect. Genet. Evol. 2011;11:1378–1387. doi: 10.1016/j.meegid.2011.05.003. [DOI] [PubMed] [Google Scholar]
- Mohraz M., Mehrkhani F., Jam S., SeyedAlinaghi S., Sabzvari D., Fattahi F., Jabbari H., Hajiabdolbaghi M. Seroprevalence of toxoplasmosis in HIV(+)/AIDS patients in Iran. Acta Med. Iran. 2011;49:213–218. https://doi.org/21713730. [PubMed] [Google Scholar]
- Montoya J.G., Liesenfeld O. Toxoplasmosis. Lancet (London, England) 2004;363:1965–1976. doi: 10.1016/S0140-6736(04)16412-X. [DOI] [PubMed] [Google Scholar]
- Montoya J.G., Remington J.S. Clinical Practice: Management of Toxoplasma gondii Infection during Pregnancy. Clin. Infect. Dis. 2008;47:554–566. doi: 10.1086/590149. [DOI] [PubMed] [Google Scholar]
- Morris M.I., Fischer S.A., Ison M.G. Infections Transmitted by Transplantation. Infect. Dis. Clin. North Am. 2010;24:497–514. doi: 10.1016/j.idc.2010.02.002. [DOI] [PubMed] [Google Scholar]
- Mose J.M., Kagira J.M., Karanja S.M., Ngotho M., Kamau D.M., Njuguna A.N., Maina N.W. Detection of Natural Toxoplasma gondii Infection in Chicken in Thika Region of Kenya Using Nested Polymerase Chain Reaction. Biomed Res. Int. 2016;2016:1–5. doi: 10.1155/2016/7589278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munday B., Corbould A. Serological responses of sheep and cattle exposed to natural Toxoplasma infection. Austral. J. Exp. Biol. Med. Sci. 1979;57:141–145. doi: 10.1038/icb.1979.14. [DOI] [PubMed] [Google Scholar]
- Nicolle C., Manceaux L. Sur une infection à corps de Leishman (ou organismes voisins) du gondi. C R Hebd Séances Acad Sci. 1908;147:763–766. [Google Scholar]
- Opsteegh, M., Langelaar, M., Sprong, H., den Hartog, L., De Craeye, S., Bokken, G., Ajzenberg, D., Kijlstra, A., der Giessen, J. van, 2010. Direct detection and genotyping of Toxoplasma gondii in meat samples using magnetic capture and PCR. Int. J. Food Microbiol. 139, 193–201. https://doi.org/10.1016/j.ijfoodmicro.2010.02.027. [DOI] [PubMed]
- Opsteegh M., Teunis P., Züchner L., Koets A., Langelaar M., van der Giessen J. Low predictive value of seroprevalence of Toxoplasma gondii in cattle for detection of parasite DNA. Int. J. Parasitol. 2011;41:343–354. doi: 10.1016/j.ijpara.2010.10.006. [DOI] [PubMed] [Google Scholar]
- Ortiz-Alegría L.B., Caballero-Ortega H., Cañedo-Solares I., Rico-Torres C.P., Sahagún-Ruiz A., Medina-Escutia M.E., Correa D. Congenital toxoplasmosis: candidate host immune genes relevant for vertical transmission and pathogenesis. Genes Immun. 2010;11:363–373. doi: 10.1038/gene.2010.21. [DOI] [PubMed] [Google Scholar]
- Ozgonul C., Besirli C.G. Recent Developments in the Diagnosis and Treatment of Ocular Toxoplasmosis. Ophthalmic Res. 2017;57:1–12. doi: 10.1159/000449169. [DOI] [PubMed] [Google Scholar]
- Palanisamy M., Madhavan B., Balasundaram M., Andavar R., Venkatapathy N. Outbreak of ocular toxoplasmosis in Coimbatore. India. Indian J. Ophthalmol. 2006;54:129. doi: 10.4103/0301-4738.25839. [DOI] [PubMed] [Google Scholar]
- Pappas G., Roussos N., Falagas M.E. Toxoplasmosis snapshots: Global status of Toxoplasma gondii seroprevalence and implications for pregnancy and congenital toxoplasmosis. Int. J. Parasitol. 2009;39:1385–1394. doi: 10.1016/j.ijpara.2009.04.003. [DOI] [PubMed] [Google Scholar]
- Paul M. Immunoglobulin G avidity in diagnosis of toxoplasmic lymphadenopathy and ocular toxoplasmosis. Clin. Diagn. Lab. Immunol. 1999;6:514–518. doi: 10.1128/cdli.6.4.514-518.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pereira, K.S., Franco, R.M.B., Leal, D.A.G., 2010. Transmission of Toxoplasmosis (Toxoplasma gondii) by Foods. pp. 1–19. https://doi.org/10.1016/S1043-4526(10)60001-0. [DOI] [PubMed]
- Peyron F., Garweg J.G., Wallon M., Descloux E., Rolland M., Barth J. Long-term Impact of Treated Congenital Toxoplasmosis on Quality of Life and Visual Performance. Pediatr. Infect. Dis. J. 2011;30:597–600. doi: 10.1097/INF.0b013e31820bb5f3. [DOI] [PubMed] [Google Scholar]
- Remington, J., McLeod, R., Thulliez, P., Desmonts, G., 2006. Toxoplasmosis. In: Remington, J.S., Klein, J.O., Wilson, C.B., B.C. (Ed.),. Elsevier-Saunders Company, Philadelphia, U.S.A., pp. 947 – 1091.
- Roghmann M.C., Faulkner C.T., Lefkowitz A., Patton S., Zimmerman J., Morris J.G., Jr. Decreased seroprevalence for Toxoplasma gondii in Seventh Day Adventists in Maryland. Am. J. Trop. Med. Hyg. 1999;60:790–792. doi: 10.4269/ajtmh.1999.60.790. [DOI] [PubMed] [Google Scholar]
- Roos D.S., Crawford M.J., Donald R.G., Kissinger J.C., Klimczak L.J., Striepen B. Origin, targeting, and function of the apicomplexan plastid. Curr. Opin. Microbiol. 1999;2:426–432. doi: 10.1016/S1369-5274(99)80075-7. [DOI] [PubMed] [Google Scholar]
- Sabin A.B. Toxoplasmosis: Current Status and Unsolved Problems. Introductory Remarks. Am. J. Trop. Med. Hygiene. 1953;2:360–364. doi: 10.4269/ajtmh.1953.2.360. [DOI] [PubMed] [Google Scholar]
- Schwartzman J., Maffia A., Crusius M.E., Brunhoffer A. Congenital toxoplasmosis. J. Pediatr. 1948;33:66–73. doi: 10.1016/S0022-3476(48)80154-X. [DOI] [PubMed] [Google Scholar]
- Serranti D., Buonsenso D., Valentini P. Congenital toxoplasmosis treatment. Eur. Rev. Med. Pharmacol. Sci. 2011;15:193–198. [PubMed] [Google Scholar]
- Smith, J.E., 2009. Chapter 6 Tracking Transmission of the Zoonosis Toxoplasma gondii. pp. 139–159. https://doi.org/10.1016/S0065-308X(08)00606-4. [DOI] [PubMed]
- Su C., Shwab E.K., Zhou P., Zhu X.Q., Dubey J.P. Moving towards an integrated approach to molecular detection and identification of Toxoplasma gondii. Parasitology. 2010;137:1–11. doi: 10.1017/S0031182009991065. [DOI] [PubMed] [Google Scholar]
- Suzán G., García-Peña G.E., Castro-Arellano I., Rico O., Rubio A.V., Tolsá M.J., Roche B., Hosseini P.R., Rizzoli A., Murray K.A., Zambrana-Torrelio C., Vittecoq M., Bailly X., Aguirre A.A., Daszak P., Prieur-Richard A.-H., Mills J.N., Guégan J.-F. Metacommunity and phylogenetic structure determine wildlife and zoonotic infectious disease patterns in time and space. Ecol. Evol. 2015;5:865–873. doi: 10.1002/ece3.1404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tedford, E., McConkey, G., 2017. Neurophysiological Changes Induced by Chronic Toxoplasma gondii Infection. Pathog. (Basel, Switzerland) 6. https://doi.org/10.3390/pathogens6020019. [DOI] [PMC free article] [PubMed]
- Tenter A.M. Toxoplasma gondii in animals used for human consumption. Mem. Inst. Oswaldo Cruz. 2009;104:364–369. doi: 10.1590/s0074-02762009000200033. [DOI] [PubMed] [Google Scholar]
- Tenter A.M., Heckeroth A.R., Weiss L.M. Toxoplasma gondii: from animals to humans. Int. J. Parasitol. 2000;30:1217–1258. doi: 10.1016/s0020-7519(00)00124-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toxoplasmosis of Animals and Man. By J. P. Dubey and C. P. Beattie., 1988. CRC Press, Boca Raton, 1988. doi:10.1017/S0031182000078914.
- Valcavi P.P., Natali A., Soliani L., Montali S., Dettori G., Cheezi C. Prevalence of anti-Toxoplasma gondii antibodies in the population of the area of Parma (Italy) Eur. J. Epidemiol. 1995;11:333–337. doi: 10.1007/BF01719439. [DOI] [PubMed] [Google Scholar]
- VanWormer E., Carpenter T.E., Singh P., Shapiro K., Wallender W.W., Conrad P.A., Largier J.L., Maneta M.P., Mazet J.A.K. Coastal development and precipitation drive pathogen flow from land to sea: evidence from a Toxoplasma gondii and felid host system. Sci. Rep. 2016;6:29252. doi: 10.1038/srep29252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Villena, I., Ancelle, T., Delmas, C., Garcia, P., Brezin, A.P., Thulliez, P., Wallon, M., King, L., Goulet, V., Toxosurv network and National Reference Centre for Toxoplasmosis, 2010. Congenital toxoplasmosis in France in 2007: first results from a national surveillance system. Euro Surveill. 15. https://doi.org/10.2807/ese.15.25.19600-en. [DOI] [PubMed]
- Vinetz J.M., Wilcox B.A., Aguirre A., Gollin L.X., Katz A.R., Fujioka R.S., Maly K., Horwitz P., Chang H. Beyond Disciplinary Boundaries: Leptospirosis as a Model of Incorporating Transdisciplinary Approaches to Understand Infectious Disease Emergence. Ecohealth. 2005;2:291–306. doi: 10.1007/s10393-005-8638-y. [DOI] [Google Scholar]
- Wallace G.D. Sabin-Feldman dye test for toxoplasmosis. The use of sodium citrate in accessory factor, and a method for collecting and storing blood on paper discs. Am. J. Trop. Med. Hygiene. 1969;18:395–398. http://www.ncbi.nlm.nih.gov/pubmed/5768773 Retrieved from. [PubMed] [Google Scholar]
- Weiss L.M., Dubey J.P. Toxoplasmosis: A history of clinical observations. Int. J. Parasitol. 2009;39:895–901. doi: 10.1016/j.ijpara.2009.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss, L.M, Kim, K., 2007. Toxoplasma gondii: the model apicomplexan. Perspectives and methods. Academic Press Inc, London, United Kingdom.
- Wyman C.P., Gale S.D., Hedges-Muncy A., Erickson L.D., Wilson E., Hedges D.W. Association between Toxoplasma gondii seropositivity and memory function in nondemented older adults. Neurobiol. Aging. 2017;53:76–82. doi: 10.1016/j.neurobiolaging.2017.01.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ybañez R.H.D., Ybañez A.P., Nishikawa Y. Review on the Current Trends of Toxoplasmosis Serodiagnosis in Humans. Front. Cell. Infect. Microbiol. 2020;10:204. doi: 10.3389/fcimb.2020.00204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zarkovic A., McMurray C., MacMurray C., Deva N., Ghosh S., Whitley D., Guest S. Seropositivity rates for Bartonella henselae, Toxocara canis and Toxoplasma gondii in New Zealand blood donors. Clin. Experiment. Ophthalmol. 2007;35:131–134. doi: 10.1111/j.1442-9071.2006.01406.x. [DOI] [PubMed] [Google Scholar]
- Zemene E., Yewhalaw D., Abera S., Belay T., Samuel A., Zeynudin A. Seroprevalence of Toxoplasma gondii and associated risk factors among pregnant women in Jimma town. Southwestern Ethiopia. BMC Infect. Diseases. 2012;12:337. doi: 10.1186/1471-2334-12-337. [DOI] [PMC free article] [PubMed] [Google Scholar]