Abstract
Background
This study examined usefulness and efficiency of Lurasidone in appraisal with the placebo as for the treatment of Bipolar Disorders.
Methods
Seven treatment centers in Pakistan were selected for the purpose of starting a six week-long control trial (randomized and double-blind placebo). 76 subjects, already diagnosed with Bipolar I or II based on DSM 5 diagnosis, were selected after randomization. Patients were allocated in one of the two groups. Primary efficacy of the drug was measured using Young Mania Rating Scale. Positive response of the drug was defined as 50% reduction in symptoms from the baseline/13 point less than the baseline score on Young Mania Rating Scale. Efficacy and safety of the drug was assessed using variety of markers such as administering extra-pyramidal symptoms rating scale, adverse side effects reported, electrocardiograms, body weight, vital signs changes, and laboratory investigations.
Results
Patients treated with Lurasidone showed enhanced improvement in their overall health and symptoms manifestation in comparison to patients who were given placebo. Lurasidone treated patients showed a better response to the drug (66%), in comparison with the placebo treated patients (42%).
Limitations
Study was conducted on small scale due to complexity.
Conclusion
Patients treated with Lurasidone showed reduction in bipolar symptoms and tolerate the drug well.
Keywords: Bipolar affective disorders, Pakistan, Mania, Lurasidone
1. Introduction
Geller, and Luby (1997), reports that a number of mood stabilizers and combination therapies are used to treat Bipolar disorders, however in spite of the notable morbidity analogous with Bipolar Disorder, minimal progress still needs to be enhanced. Only limited research is available to assess the safety and efficiency of drugs most commonly used to Bipolar Disorder. Many studies have highlighted the efficacy of the drug lithium for treatment of bipolar disorder, however, the use of antipsychotics is still mainstay in the treatment of Mania and that too leads to depressive symptoms later on, which unfortunately is more difficult to treat (Osby, Brandt, Correia, Ekbom, and Sparen, 2001) (Latuda, 2013, Harvey et al., 2011).
In attempted to get a better insight about the therapeutic agents of mania in Pakistan, we tried to study the Lurasidone a newly launched and recent most antipsychotics which is considered to be effective in Bipolar Depression in the west as well. Literature shows the partial efficacy of drugs most commonly used for bipolar disorder (carbamazepine, lithium, carbonate, and valproate) in adult population (Barnes, 1989) (European Medicines Agency, 2014). However these drugs exhibited greater rates for relapse and slow onset of action (Suppes, Kroger, Pikalov, &Loebel, 2016) (Nuechterlein et al., 2008). There is a notion related to the limited use of antipsychotics, prevalently used in the acute and chronic long-term treatment of adults and children with mania, mainly due to the consequent occurrence of neuroleptic side effects in general and, in particular, tradive dyskinesia. (Tohen, Chengappa, Baker, Zarate, Sachs, Kupfer, Ghaemi, Risser, Evans, & Calabrese, 2004) (Citrome, 2011). Late investigations have risen, reporting the viability of the atypical neuroleptics and Lurasidone especially, in the treatment of BPD in grown-ups. Lurasidone has been seen as valuable in the treatment of grown-ups BPD as a mono-therapeutic operator in Europe and United States (Tohen et al. 2004) (Keefe et al., 2006).
Prior to the testing and evaluation studies were conducted on randomized clinical trials, controlled clinical trials were viewed as the best quality level test for the assessment of any treatment for a psychological issue (Citrome, 2012a). Several preliminary steps are required in order to characterize the advantages just as the dangers of the proposed new treatment in our populace (Miller, & Bauer, 2014) (Citrome et al., 2014a). Pakistan is considered to be a country, which is in Asia, however they are different in body morphology and enzyme systems than other comparable Asian population. We attempted to compare the Lurasidone with Placebo in a small sample in seven different centers of Pakistan.
2. Methodology
The investigation was led in 7 treatment centers, six week long, double blind control trial of Lurasidone in the treatment of Bipolar I and Bipolar II focusing to assess the effectiveness and security, impact size, and time course of treatment reaction of the drug (Citrome, 2012b). Institutional Review Board at Gujranwala Medical and Dental College reviewed the protocol. Informed consent was taken from all the patient and their guardians respectively.
2.1. Subjects
Out of the 102 participants, screened for the investigation from 7 treatment centers, 8 (7.84%) were rejected. Out of the final 94 eligible participants, only 76 subjects fulfilled the inclusion criteria.
All the selected participants had been diagnosed with of Bipolar I and Bipolar II as per guidelines of DSM5, ranging in age between 19 and 59 years, both male and female, were selected from OPDs of the 7 selected treatment centers, through the referrals from practicing psychiatrists. Based on careful clinical evaluation, the Bipolar Disorder diagnosis was established by the psychiatrist, later confirmed and substantiated by semi-structured interview (Tandon, 2014).
Notwithstanding fulfilling full indicative BPD criteria, an absolute rating score of 15 at Young Mania Rating Scale (YMRS) was required for qualified and consenting patients to be part of the trial (Citrome et al., 2014b). Exclusion criteria included all subjects with predisposition to drug reactions, suicidality, unstable medical or neurological condition, pregnancy, allergies, lactation and active drug abuse and treated with a depot neuroleptic (Loebel, 2014).
2.2. Screening and selection
Preceding consideration in the investigation, patients experienced a standard clinical appraisal comprising of a mental assessment by a psychiatrist that included acquiring a full history by means of a meeting with the relative or parental figure.
Furthermore, a structured interview, a general physical assessment, and lab appraisals were done (prolactin levels, thyroid function tests, LFTs, glucose, total cholesterol, creatinine kinase, hepatitis screening and a total platelet count) (Sunovion, 2015).
Young Mania Rating Scale was administered as well as demographic details were obtained. In addition, baseline electrocardiograms were done where it was indicated clinically along with the measurement of supine and standing blood pressure, weight, height, and heart rate. Seriousness of manic symptomatology was watched utilizing the YMRS. These appraisals were performed at baseline and week by week all through the investigation.
Security was checked by leading a general physical test pursued by ECG, blood pressure and pulse at the baseline, then on a week by week premise. A vigilant metabolic battery (LFTs; hematology; and urinalysis was conducted at baseline weeks 2, 4, and 6 and towards the end of the investigation; prolactin levels were inspected at standard, week 2. Extrapyramidal symptoms were surveyed at benchmark and at every week by week visit utilizing the Extra-Pyramidal Rating Scale (Loebel et al., 2014).
2.3. Procedure
2-to 7-day screening period was set for the screening procedure of the participant. During the screening period all the participants experienced screening, tests, a mental history and interview and a physical assessment. Subjects were advised to end all medications for the given multi week time span.
Lurasidone was started at 40 mg/day and the dosage augmented by 40 mg/day if needed on day 6 based on assessments such as adverse reactions and drug response. Lurasidone was recommended to be taken once in a day in most of the cases immediately after the evening meals.
On account of antagonistic events, if necessary procyclidine or any benzodiazepines required were endorsed. Treatment response was characterized as a 50% reduction in YMRS score acquired at the start of the trial (visit 2) to the end of the investigation (visit 6). All patients were living with family or they were in-patient psychiatric unit so compliance was not an issue.
2.4. Study design
Patients were randomized in a 1:1 proportion to Lurasidone and placebo treatment. Computer created codes were utilized to make randomized squares of clinical trial material kits before the investigation began. Each square contained 2 Lurasidone and 2 Placebo treatment kits. Each kit contained all the clinical trial material utilized by a subject all through the span of the investigation. Individual at the site appointed the patient the following accessible kit. Lurasidone was initially prescribed at 40 mg per day which was subject to increase to 80 mg per day on the 6th day of the treatment.
2.5. Statistical analysis
Analysis was done utilizing the last observation carried forward method (LOCF). LOCF assessed the mean changes from and between the baseline and end of the investigation. Furthermore, analysis based on visits was also conducted, baseline, with at least one assessment done after the baseline measurements were noted for the purpose of the analysis.
Statistics and figures for all enlisted patients were investigated for treatment and reported antagonistic effects. Changes in the laboratory analysis, vital analysis and extra pyramidal symptoms were analyzed. Continuous data assessment was done on the mean and standard deviations. Within group changes were assessed using the Student’s t test.
3. Results
Out of the 76 patients selected for the trial, meeting the criteria for BPD, 44% had psychotic symptoms as well. Table 1 presents the demographic and clinical characteristics of the participants of the trial. Among the participants, 54 were noted as males and the remaining 22 as females. Mean age of onset was noted to 32, number of patients having prior unsuccessful medication trials was noted to be 19.
Table 1.
Patient demographics (n 76).
| Age at time of study (years) | 19–59 |
| Punjab | 59 (77.6%) |
| Baluchistan | 8 (10.5%) |
| KPK | 9 (11.8%) |
| Mania Characteristics | |
| Onset age | 32+_8 |
| First depressive episode before mania | 22 (29%) |
| Mean illness period in years | 4.3 |
| Manic/hypomanic episodes | 6.97 |
| BPD running in family | 9.88 (13%) |
| Depression history in family | 42.56(52%) |
| History of substance abuse in family | 36.48 (48%) |
| Psychotic | 33.44 (44%) |
| First episode | 16.72 (22%) |
| Patients were missing data. | 2(2.6%) |
Lurasidone was started at a mean dosage of 80 mg/day by the end of the trial. 72 participants took Lurasidone once per day (qhs). The remaining four participants that were recommended twice day by day dosing (with the higher portion given at night) were generally young (1 participant of 23 years old, 2 participants were 27 years old, and one participant was 29 years old). Accompanying prescriptions utilized during the investigation included mood stabilizers (35%), benzodiazepines (13%), and benztropine (9%).
3.1. Analysis of efficacy
A critical improvement was seen in the side effects of bipolar, as reflected in the YMRS absolute score (LOCF) decline of 19 (62% improvement) from initial ratings to the end of the trial (p 0.001) in the group of patients receiving Lurasidone. Evidential enhancement was seen towards the end of the trial, which in this manner improved all through the month and a half of the investigation (Fig. 1). Participants demonstrated an improvement by the end of the trial with respect to the YMRS scores (Ishibashi et al., 2010). Noteworthy improvement was found in the accompanying YMRS items (p 0.001), and insight (p > 0.005). Compared to placebo the scores either remained stationary or overall deterioration of the symptoms especially in YMRS scores was seen.
Fig. 1.
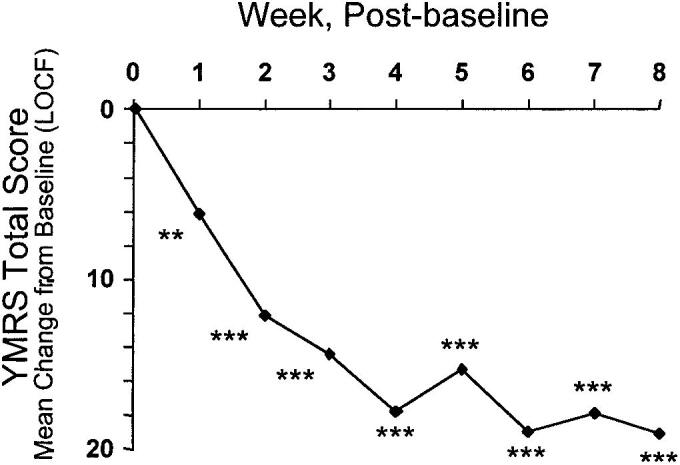
Mean alteration from starting point (last observation carried forward; LOCF) in mean Young Mania Rating Scale (YMRS) total score in 76 patients with bipolar disorder receiving Lurasidone.
3.2. Safety analysis
Lurasidone treatment was noted being well tolerated. After 6 weeks of therapy, only two patients discontinued the research owing to enhanced depressive symptoms with subsequent suicidal thoughts, resulting in hospital admission. There were no important changes in the EPS ratings from initial assessment to the last assessment carried (Leucht et al., 2013). There were no significant baseline-to-endpoint changes in the EPS rating scale. However, as opposed to placebo, 9 participants (11.8 percent) showed treatment-emergent Akathisia (Horiguchi et al., 2011).
As shown in Table 2, abdominal pain (n 7), somnolence (n 4) and depression (n 6) were the most commonly reported negative occurrences. None of the patients recorded or measured weight gain on either the placebo or the Lurasidone group during the six-week trial.
Table 2.
Treatment-emernt adverse events as reported by the patients (20%).
| Event | Number | Percentage |
|---|---|---|
| Reduced Appetite | 3 | 3.9 |
| Somnolence | 4 | 5.2 |
| Abdominal discomfort | 7 | 9.2 |
| Depression | 6 | 7.8 |
| Diarrhea | 5 | 6.5 |
| Fever | 3 | 3.9 |
| EPS | 9 | 11.8 |
Whereas in hematocrit, hemoglobin, and mean cell volume, a slight but statistically significant decline from baseline to end-point was found, but all end-point hematological evaluation showed the values within normal ranges in comparison to the placebo. A metaphorically significant mean alter in prolactin concentrations from initial assessment to final assessment (0.4 6 0.5 mmol / L, p 0.002) and above-range final assessment values in 21 out of 76 patients (27.6%) with ordinary baseline concentrations relative to placebo was noted. Among the six instances of enhanced prolactin emerging therapy, there was a single condition where the amount of prolactin (2.18 mmol / L) surpassed the reference range's upper limit twice (Cates et al., 2013). There were 13 males and 8 women in the 21 instances of treatment-emergent hyperprolactinemia. Diagnostically, during the period of trial, no subject reported any signs or symptoms associated with prolactin elevation. It is however recommended that prolactin levels be monitored long-term.
Supine pulse rates indicated a slight but highly significant initial assessment to final assessment elevation (baseline, 84.5 6 13.5 beats per minute [bpm]; change, 9.8 6 14.5 bpm; p 5 0.004) and pulse rates (baseline, 97.8 6 18.9 bpm; change, 10.3 6 13.0 bpm; p 0.001). Similarly, results from electrocardiography showed statistically significant elevated heart rate (baseline, 82.7 6 16.8 bpm; change, 11.3 6 14.9 bpm; p 5 0.002) as compared to the placebo. All other parameters in ECG were normal and showed no changes (Blier and Ward, 2003).
4. Discussion
This research was intended to assess Lurasidone's security and tolerability (20–80 mg / day) in patients with bipolar disorder who in a randomized, double-blind trial finished 6 weeks of therapy with Lurasidone or placebo (Ohno, 2011). Mood stabilizer collateral treatment was allowed. A main focus was on analyzing mood normalization, with therapeutic effectiveness as a secondary matter of fact (Preskorn et al., 2013).
The present research findings indicate that Lurasidone carries a comparatively small prospective danger for weight gain and negative metabolic impacts in the daily dose range of 40–80 mg (Chiu et al., 2014). Alterations in BMI, weight, glycemic indices, prolactin, and lipids were low, but not clinically noteworthy in addition to assisting individuals boost mood in comparison with participants receiving placebo (Ogasa et al., 2013).
This data confirm that trials in schizophrenia and bipolar disorder conducted in west and other countries of the world, where it was launched two years ago, are comparable and in line to those conducted in Pakistan (Nakamura et al., 2009). It also indicates that Lurasidone seems to have a reduced risk of serious weight gain or loss, lipid and glucose-related impacts (Ketter, Sarma, Silva, Kroger, & Cucchiaro, 2016) (Nasrallah et al., 2013).
Cessation related to negative event precipitation throughout the 6 weeks of Lurasidone trial was comparatively insignificant (6.6%) (Meltzer et al., 2011). Rate of development of adverse effects was significantly lower when compared to other atypical antipsychotic drugs and to placebo (Loebel, Cucchiaro, & Sarma, 2014). In the present research, a lower percentage of patients revealed extrapyramidal symptom-related adverse events than in earlier reported West and Schizophrenia research (Loebel et al., 2013).
In this bipolar cluster, the negative effects of Lurasidone have been resolutely analogous to those recorded in prior research of Lurasidone in schizophrenics (Correll, Cucchiaro, Silva, Hsu, Pikalov, & Loebel, 2016) (McEvoy et al., 2013). The percentage of participants reporting manic remission has been 35–38 percent, with established remission based on strict YRMS standards indicating the lack of bipolar symptom severity (Citrome et al., 2012). Similarly, double-blind, placebo-controlled relapse avoidance studies in people with bipolar disorder also record relapse scores in the range of 18–31 percent over 1.5–2 years of therapy with atypical antipsychotics which were significantly greater than that of the existing findings (Tohen et al. 2004) (Loebel et al., 2013).
5. Study limitations
The constraints of the research included restricted and small sample sizes and the layout of the research, absence of comparisons with other antipsychotics used in Bipolar Disorder (Harvey et al., 2013). Furthermore, another limitation was the short period of time and allocation of restricted placebo and Lurasidone availability. In this population of bipolar disorder, the treatment with Lurasidone in a six week trial have been discovered to be secure and very well condoned., compatible with prior studies in participants in other areas of the globe with a similar diagnosis of Bipolar Disorder.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Footnotes
Study sponsored by Helix Pharmaceuticals Pvt Ltd, Karachi Pakistan. Who provided the Lurasidone and the exact replica placebo.
Peer review under responsibility of King Saud University.
References
- Latuda® (lurasidone HCl) tablets [package insert] Marlborough, MA: Sunovion Pharmaceuticals Inc, 2013. [Accessed March 25, 2015]. Available from: http://www.latuda.com/LatudaPrescribingInformation.pdf.
- European Medicines Agency. Annex I: Summary of Product Characteristics. London, UK: European Medicines Agency, 2014. [Accessed March 25, 2015]. Available from: http://www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Product_Information/human/002713/WC500164683.pdf.
- Citrome L. Lurasidone for schizophrenia: a review of the efficacy and safety profile for this newly approved second-generation antipsychotic. Int. J. Clin. Pract. 2011;65(2):189–210. doi: 10.1111/j.1742-1241.2010.02587.x. [PubMed] [DOI] [PubMed] [Google Scholar]
- Citrome L. Lurasidone in schizophrenia: new information about dosage and place in therapy. Adv. Ther. 2012;29(10):815–825. doi: 10.1007/s12325-012-0052-6. [PubMed] [DOI] [PubMed] [Google Scholar]
- Citrome L. Lurasidone for the acute treatment of adults with schizophrenia: what is the number needed to treat, number needed to harm, and likelihood to be helped or harmed? Clin. Schizophr. Relat. Psychoses. 2012;6(2):76–85. doi: 10.3371/CSRP.6.2.5. [PubMed] [DOI] [PubMed] [Google Scholar]
- Citrome L., Ketter T.A., Cucchiaro J., Loebel A. Clinical assessment of lurasidone benefit and risk in the treatment of bipolar I depression using number needed to treat, number needed to harm, and likelihood to be helped or harmed. J. Affect Disord. 2014;155:20–27. doi: 10.1016/j.jad.2013.10.040. [PubMed] [DOI] [PubMed] [Google Scholar]
- Ishibashi T., Horisawa T., Tokuda K. Pharmacological profile of lurasidone, a novel antipsychotic agent with potent 5-hydroxytryptamine 7 (5-HT7) and 5-HT1A receptor activity. J. Pharmacol. Exp. Ther. 2010;334(1):171–181. doi: 10.1124/jpet.110.167346. [PubMed] [DOI] [PubMed] [Google Scholar]
- Horiguchi M., Huang M., Meltzer H.Y. The role of 5-hydroxytryptamine 7 receptors in the phencyclidine-induced novel object recognition deficit in rats. J. Pharmacol. Exp. Ther. 2011;338(2):605–614. doi: 10.1124/jpet.111.180638. [PubMed] [DOI] [PubMed] [Google Scholar]
- Cates L.N., Roberts A.J., Huitron-Resendiz S., Hedlund P.B. Effects of lurasidone in behavioral models of depression. Role of the 5-HT7 receptor subtype. Neuropharmacology. 2013;70:211–217. doi: 10.1016/j.neuropharm.2013.01.023. [PubMed] [DOI] [PubMed] [Google Scholar]
- Blier P., Ward N.M. Is there a role for 5-HT1A agonists in the treatment of depression? Biol. Psychiatry. 2003;53(3):193–203. doi: 10.1016/s0006-3223(02)01643-8. [PubMed] [DOI] [PubMed] [Google Scholar]
- Ohno Y. Therapeutic role of 5-HT1A receptors in the treatment of schizophrenia and Parkinson’s disease. CNS Neurosci. Ther. 2011;17(1):58–65. doi: 10.1111/j.1755-5949.2010.00211.x. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Preskorn S., Ereshefsky L., Chiu Y.Y., Poola N., Loebel A. Effect of food on the pharmacokinetics of lurasidone: results of two randomized, open-label, crossover studies. Hum. Psychopharmacol. 2013;28(5):495–505. doi: 10.1002/hup.2338. [PubMed] [DOI] [PubMed] [Google Scholar]
- Chiu Y.Y., Ereshefsky L., Preskorn S.H., Poola N., Loebel A. Lurasidone drug-drug interaction studies: a comprehensive review. Drug Metabol. Drug Interact. 2014;29(3):191–202. doi: 10.1515/dmdi-2014-0005. [PubMed] [DOI] [PubMed] [Google Scholar]
- Ogasa M., Kimura T., Nakamura M., Guarino J. Lurasidone in the treatment of schizophrenia: a 6-week, placebo-controlled study. Psychopharmacology (Berl) 2013;225(3):519–530. doi: 10.1007/s00213-012-2838-2. [PMC free article] [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamura M., Ogasa M., Guarino J. Lurasidone in the treatment of acute schizophrenia: a double-blind, placebo-controlled trial. J. Clin. Psychiatry. 2009;70(6):829–836. doi: 10.4088/JCP.08m04905. [PubMed] [DOI] [PubMed] [Google Scholar]
- Nasrallah H.A., Silva R., Phillips D. Lurasidone for the treatment of acutely psychotic patients with schizophrenia: a 6-week, randomized, placebo-controlled study. J. Psychiatr. Res. 2013;47(5):670–677. doi: 10.1016/j.jpsychires.2013.01.020. [PubMed] [DOI] [PubMed] [Google Scholar]
- Meltzer H.Y., Cucchiaro J., Silva R. Lurasidone in the treatment of schizophrenia: a randomized, double-blind, placebo-and olanzapine-controlled study. Am. J. Psychiatry. 2011;168(9):957–967. doi: 10.1176/appi.ajp.2011.10060907. [PubMed] [DOI] [PubMed] [Google Scholar]
- Loebel A., Cucchiaro J., Sarma K. Efficacy and safety of lurasidone 80 mg/day and 160 mg/day in the treatment of schizophrenia: a randomized, double-blind, placebo-and active-controlled trial. Schizophr. Res. 2013;145(1–3):101–109. doi: 10.1016/j.schres.2013.01.009. [PubMed] [DOI] [PubMed] [Google Scholar]
- McEvoy J.P., Citrome L., Hernandez D. Effectiveness of lurasidone in patients with schizophrenia or schizoaffective disorder switched from other antipsychotics: a randomized, 6-week, open-label study. J. Clin. Psychiatry. 2013;74(2):170–179. doi: 10.4088/JCP.12m07992. [PubMed] [DOI] [PubMed] [Google Scholar]
- Citrome L., Cucchiaro J., Sarma K. Long-term safety and tolerability of lurasidone in schizophrenia: a 12-month, double-blind, active-controlled study. Int. Clin. Psychopharmacol. 2012;27(3):165–176. doi: 10.1097/YIC.0b013e32835281ef. [PubMed] [DOI] [PubMed] [Google Scholar]
- Loebel A., Cucchiaro J., Xu J., Sarma K., Pikalov A., Kane J.M. Effectiveness of lurasidone vs quetiapine XR for relapse prevention in schizophrenia: a 12-month, double-blind, noninferiority study. Schizophr. Res. 2013;147(1):95–102. doi: 10.1016/j.schres.2013.03.013. [PubMed] [DOI] [PubMed] [Google Scholar]
- Harvey P.D., Siu C.O., Hsu J., Cucchiaro J., Maruff P., Loebel A. Effect of lurasidone on neurocognitive performance in patients with schizophrenia: a short-term placebo-and active-controlled study followed by a 6-month double-blind extension. Eur. Neuropsychopharmacol. 2013;23(11):1373–1382. doi: 10.1016/j.euroneuro.2013.08.003. [PubMed] [DOI] [PubMed] [Google Scholar]
- Harvey P.D., Ogasa M., Cucchiaro J., Loebel A., Keefe R.S. Performance and interview-based assessments of cognitive change in a randomized, double-blind comparison of lurasidone vs ziprasidone. Schizophr. Res. 2011;127(1–3):188–194. doi: 10.1016/j.schres.2011.01.004. [PubMed] [DOI] [PubMed] [Google Scholar]
- Nuechterlein K.H., Green M.F., Kern R.S. The MATRICS Consensus Cognitive Battery, part 1: test selection, reliability, and validity. Am. J. Psychiatry. 2008;165(2):203–213. doi: 10.1176/appi.ajp.2007.07010042. [PubMed] [DOI] [PubMed] [Google Scholar]
- Keefe R.S., Poe M., Walker T.M., Kang J.W., Harvey P.D. The Schizophrenia Cognition Rating Scale: an interview-based assessment and its relationship to cognition, real-world functioning, and functional capacity. Am. J. Psychiatry. 2006;163(3):426–432. doi: 10.1176/appi.ajp.163.3.426. [PubMed] [DOI] [PubMed] [Google Scholar]
- Citrome L., Weiden P.J., McEvoy J.P. Effectiveness of lurasidone in schizophrenia or schizoaffective patients switched from other antipsychotics: a 6-month, open-label, extension study. CNS Spectr. 2014;19(4):330–339. doi: 10.1017/S109285291300093X. [PMC free article] [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tandon, R., Loebel, A., Phillips, D., et al., 2014. EPA-1722 – A double-blind, placebo-controlled, randomized withdrawal study of lurasidone for the maintenance of efficacy in patients with schizophrenia [abstract]. Eur. Psychiatry 29(Supplement 1), S372. [DOI] [PMC free article] [PubMed]
- Loebel A. Dose escalation in early nonresponders to 80 mg/day of lurasidone: The Optimize study; Presented at the annual meeting of the American College of Neuropsychopharmacology. Phoenix. 2014 [Google Scholar]
- Sunovion ProFile™ [webpage on the Internet] Marlborough, MA: Sunovion Pharmaceuticals Inc; 2015. [Accessed March 25, 2015]. (Metabolic parameters: metabolic parameters evaluated in multiple bipolar depression studies for 6 weeks and 12 weeks). Available from: https://www.sunovionprofile.com/sp/latuda-bp/metabolic-parameters.html.
- Loebel A.D., Siu C.O., Cucchiaro J.B., Pikalov A.A., Harvey P.D. Daytime sleepiness associated with lurasidone and quetiapine XR: results from a randomized double-blind, placebo-controlled trial in patients with schizophrenia. CNS Spectr. 2014;19(2):197–205. doi: 10.1017/S1092852913000904. [PMC free article] [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leucht S., Cipriani A., Spineli L. Comparative efficacy and tolerability of 15 antipsychotic drugs in schizophrenia: a multiple-treatments meta-analysis. Lancet. 2013;382(9896):951–962. doi: 10.1016/S0140-6736(13)60733-3. [PubMed] [DOI] [PubMed] [Google Scholar]
Further Reading
- Stahl S.M., Cucchiaro J., Simonelli D., Hsu J., Pikalov A., Loebel A. Effectiveness of lurasidone for patients with schizophrenia following 6 weeks of acute treatment with lurasidone, olanzapine, or placebo: a 6-month, open-label, extension study. J. Clin. Psychiatry. 2013;74(5):507–515. doi: 10.4088/JCP.12m08084. [PubMed] [DOI] [PubMed] [Google Scholar]


