Abstract
Diabetes is a major cause of morbidity and mortality worldwide. It can affect many organs and, over time, leads to serious complications. Diabetic retinopathy (DR), a specific ocular complication of diabetes, remains the leading cause of vision loss and vision impairment in adults. This work is the first in Eastern Morocco aimed at identifying the different stages of DR and to determine their frequencies and associated risk factors. It is a case-control study conducted from December 2018 to July 2019 at the ophthalmology department of Al-Irfane Clinic (Oujda). Data were obtained from a specific questionnaire involving 244 diabetic patients (122 cases with retinopathy vs 122 controls without retinopathy). All results were analyzed by the EPI-Info software. This study shows a predominance of proliferative diabetic retinopathy (PDR) with 57.4% of cases (uncomplicated proliferative diabetic retinopathy (UPDR): 23.8%; complicated proliferative diabetic retinopathy (CPDR): 33.6%). The non-proliferative diabetic retinopathy (NPDR) represents 42.6% (minimal NPDR: 8.2%; moderate NPDR: 26.2%; severe NPDR: 8.2%). The determinants of DR were insulin therapy, high blood pressure, poor glycemic control and duration of diabetes. Regarding the chronological evolution, retinopathy precedes nephropathy. Diabetic nephropathy (DN) was present in 10.6% of cases especially in patients with PDR. In summary, the frequency of PDR was higher than that of NPDR. DR appears before DN with a high frequency of DN in patients with PDR. Good glycemic control and blood pressure control, as well as early diagnosis are the major preventive measures against DR.
Keywords: Diabetic retinopathy, Diabetic nephropathy, Eastern Morocco, Risk factors
1. Introduction
In 2019, approximately 463 million people worldwide were living with diabetes; this number will rise to 578 million by 2030 and 700 million by 2045 (Saeedi et al., 2019). In Morocco, the national prevalence of diabetes among adults aged 20 years and over ranged from 6.6% in 2000 (Tazi et al., 2003) to 12.4% in 2016 (WHO, 2016). Other research and regional studies conducted in different areas of the country have identified high rates. Rguibi and Belahsen (2004) found a prevalence of 11.9% in 2001–2002 among a sample of 249 no pregnant women aged 15 years and older living in urban areas of Laayoune city in southern Morocco. A survey realized by our team showed that the frequency of type 2 diabetes in eastern Morocco was 10.2% (Ramdani et al., 2012). A study carried out in two towns in central Morocco (Khemisset and El Jadida) which aims the estimation of the prevalence of chronic kidney disease, hypertension, diabetes and obesity among the adult population of Morocco reported that diabetes was at 13.4% of the population surveyed (Gharbi et al., 2016). With the increasing diabetes rates worldwide, the prevalence of diabetes-related complications is growing, especially that of diabetic retinopathy (DR) (the global prevalence of DR during the period 2015–2019 was 27.0%) (Sabanayagam et al., 2019, Thomas et al., 2019). DR is the main microvascular retinal complication of diabetes, and is currently the leading cause of visual impairment and blindness in working population (Cheung et al., 2010, Yau et al., 2012). In the initial stages of disease, DR is often asymptomatic, but if untreated, it can impair vision and progress to blindness (Frank, 2004, Cheung et al., 2010). The DR has two major types: the Non Proliferative Diabetic Retinopathy (NPDR) and Proliferative Diabetic Retinopathy (PDR) (Memon et al., 2017). Non-proliferative retinopathy is the earliest stage of DR which advances from minimal to moderate and severe non proliferative diabetic retinopathy (Kaur and Mittal, 2018). Minimal non-proliferative diabetic retinopathy is identified by the presence of at least one micro aneurysms. Moderate non-proliferative diabetic retinopathy is characterized by the presence of few hemorrhages, hard exudates and cotton wool spots, while these lesions are present in greater quantity in severe NPDR. Hemorrhages are of various forms including ‘‘dot’’, ‘‘blot’’ and ‘‘flame’’ hemorrhages (Kaur and Mittal, 2018). PDR is the advanced stage of DR that ultimately causes neovascularization, a natural formation of new blood vessels in the form of functional microvascular networks that develop on the inside surface of the retina (Qummar et al., 2019). The pathological mechanisms that lead to the occurrence of DR are complicated and multifactorial (Wang and Lo, 2018, Whitehead et al., 2018). Several genes are associated with the development of DR such as growth factor gene polymorphisms, oxidative stress genes, cytokines… (Petrovič, 2013). In addition, various risk factors are involved in the progression of DR such as: uncontrolled hyperglycemia (glycosylated hemoglobin HbA1c > 7.0%), duration of diabetes, hypertension, hyperlipidemia, and treatment with insulin (Elwali et al., 2017, Zhang et al., 2017, Song et al., 2018).
The analysis of such factors will facilitate the identification of people at risk of DR by knowledge of its biomarkers, and will help to explore its pathogenesis and to provide recommendations for its prevention. Furthermore, early detection and treatment of DR is very important as it is a progressive disease and its severity is dependent on the number and the characteristics of lesions present in the fundus image. The early detection of the DR giving opportunity for medical practitioners to treat and cure this medical problem at an early stage with higher accuracy. The more time the disease remains unrecognized, the consequences could be more serious. The DR treatment plays a major role in the prevention of complications of PDR, stabilization and improvement of visual acuity.
Diabetic nephropathy (DN) is a complication of diabetes characterized by structural and functional pathological changes in the kidneys of diabetic patients (type 1 and type 2). These changes are characterized by the presence of proteinuria, hypertension and progressive reductions in kidney function (Umanath and Lewis, 2018). Nearly, 30% to 40% of diabetic patients will progress to nephropathy (Umanath and Lewis, 2018). Among patients with type 1 diabetes (T1D), the prevalence of DN is around 40% (Andersen et al., 1983). Hence, ten years after the diagnosis of type 2 diabetes (T2D), approximately 25% of patients will develop DN (Adler et al., 2003). Several studies have shown a close association between DR and DN in the majority of diabetic patients (the presence of DR may expose patients at risk for DN) (Villar et al., 1999; El-asrar et al., 2001; Rossing et al., 2002, Chandy et al., 2008). Previous work has indicated that DR is significantly associated with deterioration of kidney function and patients with DN have known a higher incidence of DR than patients without DN (Wong et al., 2004, Edwards et al., 2005, Pedro et al., 2010, Park et al., 2015, Jeng et al., 2016).
In Eastern Morocco, the only study that describes the epidemiological aspects of diabetic complications was published by our team in 2018 (Hammoudi et al., 2018). This study which included 2401 diabetic patients showed that DR was the most common complication with a frequency of 16.8% followed by DN (12.4%). These results have encouraged us to deepen our work on this pathology to look for the description of its clinical features, the estimation of the frequency of its stages and the identification of its associated risk factors for the first time in Eastern Morocco.
2. Material and methods
2.1. Study population
Our case–control study was conducted at the ophthalmology department of a private structure, Al-Irfane clinic in Oujda (capital of Eastern Morocco) from December 2018 to July 2019. Cases were diabetic patients with DR and controls were diabetic patients without DR. The sample size for this study is calculated using the following formula: n = Z2 * p (1 – p) / i2 where n = sample size; Z = confidence level according to the reduced normal centered law (for a confidence level of 95%, Z = 1,96); p = estimated proportion of the population with complication (p = 16,8%); i = tolerated margin of error (5%).
The calculated sample size was estimated at 215 patients and the study sample included 244 patients. All recruited patients were diabetic and came from different parts of Eastern Morocco, for an eye exam. Inclusion criteria were: patients with T1D and T2D presenting for a medical examination for whatever reason, all diabetics aged 18 and over regardless of sex with DR (cases), without DR (controls) and with regular medical follow-up (at least one consultation per year). Exclusion criteria were: all patients with other types of diabetes (gestational diabetes, drug-induced diabetes…), with retinopathy due to other causes than diabetes, or with irregular medical follow-up.
2.2. Data collection procedure
All 244 diabetic patients participated in this study had a full eye exam including the measurement of visual acuity performed by an optician and an examination of the fundus using a slit lamp (to detect the presence or the absence of DR) performed by an ophthalmologist. The examination using a slit lamp included analysis of the macular region, the papilla, and the retina. Complementary examinations (optical coherence tomography and fluorescein angiography) were performed in patients with manifest RD lesions. The fluorescein angiography examination confirms the diagnosis of area of the retina affected, the area to be treated and the choice of therapeutic management. The examination of optical coherence tomography helps to determine the character of the macular edema. A questionnaire was used for data collection from individual interviews with patients. The questionnaire consisted of several items: socio-demographic data (age, education level, profession and residence), data related to diabetes (type of diabetes, diabetes duration, treatment), anthropometric and bioclinical data: 1- Weight in (kg), height in (m) and body mass index (BMI = weight / (height) 2) in kg / m2. The WHO BMI classification has been adopted (WHO, 1998). 2- The waist size and hip circumference were measured for each patient. Abdominal obesity is defined by a value for waist circumference ⁄ hip circumference (waist-hip ratio (WHR)) greater than 0.85 for women and 1 for men (OMS, 2003). 3-Hypertension was defined according to the criteria of the WHO classification of 1999 (Chalmers et al., 1999), by a value of systolic blood pressure ≥140 mmHg and / or a diastolic blood pressure ≥90 mmHg. 4- Glycated hemoglobin (HbA1c) (HbA1c ≤ 7.0% was considered as a good glycemic control (ADA, 2019)). Our questionnaire also included data associated with the lifestyle of patients, especially sleeping. Regarding the information associated with complications, we have detailed the data related to DN. Thus, the presence and or the absence of DN has been determined based on the patient's statement. All DR data have been reported by the ophthalmologist and the stages were divided as follows: 1- non-proliferative diabetic retinopathy (NPDR): minimal non-proliferative diabetic retinopathy (minimal NPDR), moderate non-proliferative diabetic retinopathy (moderate NPDR), severe non-proliferative diabetic retinopathy (severe NPDR); and 2-proliferative diabetic retinopathy (PDR): uncomplicated proliferative diabetic retinopathy (UPDR) and complicated proliferative diabetic retinopathy (CPDR).
2.3. Ethical considerations
The study's protocol was approved by the Ethics Committee for Biomedical Research of Oujda (CERBO) of the Faculty of Medicine and Pharmacy, Mohammed First Oujda University, under the order number 03/2018. The recruited patients received a letter of information about the research project, written in Arabic or French depending on the participant's choice and explained in Arabic or Berber for illiterate patients. The informed consent form was attached to the letter of information. In addition, the collected data in questionnaires were coded and stored in a protected Excel file.
2.4. Statistical analysis
All statistical analysis were performed using EPI-INFO software version 3.5.4; July 30, 2012. Quantitative variables were expressed as means ± standard deviation, and qualitative variables as percentages. Student t-test was used for the comparison of means, and chi-2 test for the comparison of qualitative variables. We performed a step-down multivariate logistic regression for variables that were statistically significant in the univariate logistic analysis to identify risk factors for DR. A p-value < 0.05 was considered significant.
3. Results
During the period of our study (from December 2018 to July 2019), we collected a total of 244 diabetic patients. This population is characterized by a slight female predominance (56.1% of women against 43.9% of men) with a sex ratio (W/M) of 1.3. The average age was not significantly different (P > 0.05) between cases (63.5 ± 11.7 years) and controls (65.0 ± 12.2 years). The results describing the comparison of the 122 diabetic cases (presenting with DR) with the data of the 122 diabetic controls characterized by the absence of DR are presented in Table 1. The characteristics of controls and cases were similar for sex and age (Table 1). The frequency of T1D was significantly higher in cases compared to controls (12.3% versus 3.3%; P < 0.01). The same finding was observed regarding insulin therapy (38.5% versus 7.4%; P < 0.001), hypertension (86.9% versus 64.8%; P < 0.0001), and glycemic control (HbA1c > 7.0%) (77.9% against 54.9%; P < 0.0001) (Table 1). We have also observed an increase in the rate of DR with an increasing of diabetes duration (Fig. 1). Indeed, the percentage of DR increased from 22.1% for a diabetes duration of less than 10 years to 53.3% for a diabetes duration more than 15 years (Fig. 1). The frequency of patients with sleep duration less than 6 h (38.5%) was higher in cases compared to controls (26.2%; P < 0.05) (Table 1).
Table 1.
Comparison of characteristics of cases and controls.
| Variables | Cases (n = 122) |
Controls (n = 122) |
P-value | ||
|---|---|---|---|---|---|
| N | % | N | % | ||
| Gender | |||||
| Women | 67 | 54.9 | 70 | 57.4 | |
| Men | 55 | 45.1 | 52 | 42.6 | NS |
| Age (Years) | |||||
| <40 | 5 | 4.1 | 5 | 4.1 | |
| 40–50 | 9 | 7.4 | 8 | 6.6 | |
| 50–60 | 32 | 26.2 | 29 | 23.8 | |
| 60–70 | 41 | 33.6 | 40 | 32.8 | |
| >70 | 35 | 28.7 | 40 | 32.7 | NS |
| Types of diabetes | |||||
| T1D | 15 | 12.3 | 4 | 3.3 | |
| T2D | 107 | 87.7 | 118 | 96.7 | <0.01 |
| Use of OADs | |||||
| Yes | 37 | 30.3 | 87 | 71.3 | |
| No | 85 | 69.7 | 35 | 28.7 | <0.001 |
| Insulin therapy | |||||
| Yes | 47 | 38.5 | 9 | 7.4 | |
| No | 75 | 61.5 | 113 | 92.6 | <0.001 |
| Combined treatment (OADs + Insulin) | |||||
| Yes | 38 | 31.1 | 18 | 14.8 | |
| No | 84 | 68.9 | 104 | 85.2 | <0.01 |
| Overweight and/ or Obesity | |||||
| Yes | 89 | 73 | 99 | 81.1 | |
| No | 33 | 27 | 23 | 18.9 | NS |
| Abdominal obesity | |||||
| Yes | 113 | 92.6 | 112 | 91.8 | |
| No | 9 | 7.4 | 10 | 8.2 | NS |
| Hypertension | |||||
| Yes | 106 | 86.9 | 79 | 64.8 | |
| No | 16 | 13.1 | 43 | 35.2 | <0.0001 |
| HbA1c (%) | |||||
| Poor glycemic control (HbA1c > 7%) | 95 | 77.9 | 67 | 54.9 | |
| Good glycemic control (HbA1c ≤ 7%) | 27 | 22.1 | 55 | 45.1 | <0.0001 |
| Number of hours of sleep | |||||
| <6H | 47 | 38.5 | 32 | 26.2 | |
| ≥6H | 75 | 61.5 | 90 | 73.8 | <0.05 |
| Diabetic Nephropathy | |||||
| Yes | 13 | 10.6 | 0 | 0 | |
| No | 109 | 89.4 | 122 | 100 | <0.001 |
N: number of patients; OADs: oral antidiabetic drugs; HbA1c: glycated haemoglobin; NS: not significant. P-value: Comparison of cases to controls.
Fig. 1.
Distribution of cases and controls according to diabetes duration. *P < 0.0001 cases compared to controls for diabetes duration >15 years.
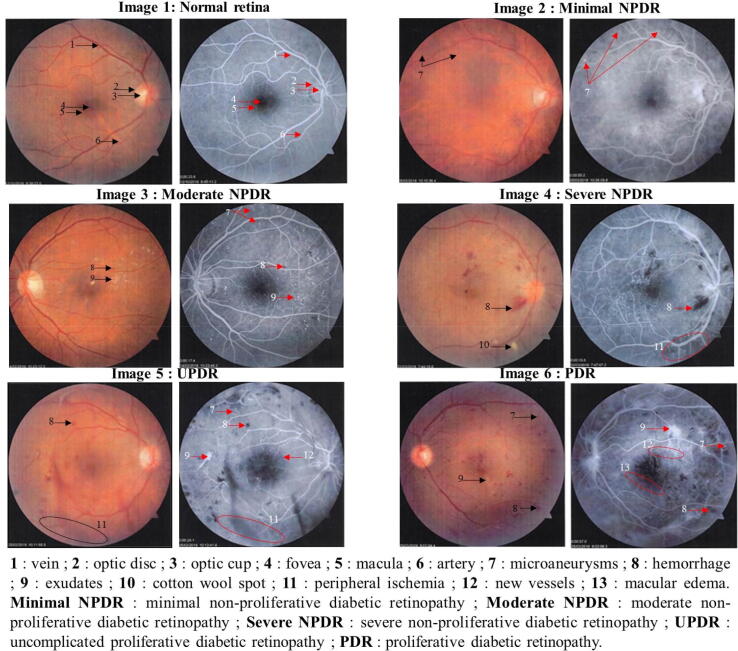
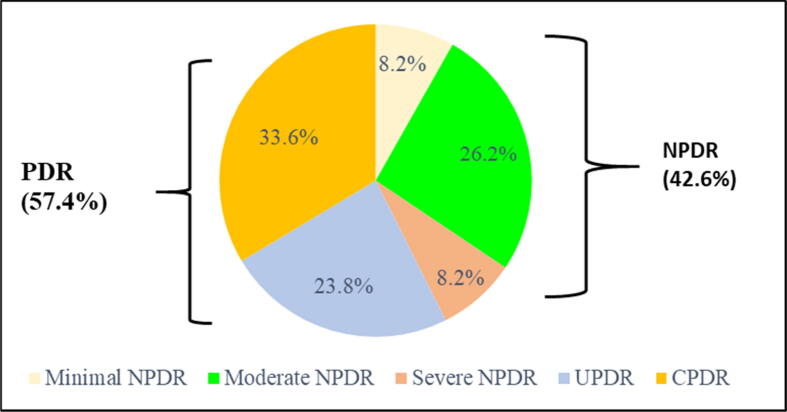
The clinical features of DR are shown in Fig. 2. DR is divided into two major forms: non-proliferative diabetic retinopathy (NPDR) (Fig. 2: images 2, 3 and 4) and proliferative diabetic retinopathy (PDR) (Fig. 2: images 5 and 6). NPDR is manifested in the retina by the presence of microaneurysms (Fig. 2: image 2), dot and blot hemorrhages and exudates (Fig. 2: images 3 and 4). PDR is the more advanced stage of DR, characterized by abnormal retinal neovascularization (formation of new blood vessels) and vitreous/preretinal hemorrhages (Fig. 2: images 5 and 6). The frequencies of non-proliferative diabetic retinopathy (NPDR) and proliferative diabetic retinopathy (PDR) were 42.6% and 57.4% respectively (Fig. 3). Complicated proliferative diabetic retinopathy (CPDR) is the most common DR class (33.6%), followed by uncomplicated proliferative diabetic retinopathy (UPDR) (23.8%), moderate non-proliferative diabetic retinopathy (moderate NPDR) (26.2%), minimal non-proliferative diabetic retinopathy (minimal NPDR) (8.2%) and severe non-proliferative diabetic retinopathy (severe NPDR) (8.2%) (Fig. 3). Table 2 shows the associations of the independent variables with DR. In univariate analysis, the strong association of DR was insulin therapy (OR = 7.87; 95% CI = [3.64–17.00]; P < 0.0001), followed by hypertension, HbA1c (>7.0% / ≤7.0%), combined treatment, diabetes duration, hours of sleep (≥6 h), types of diabetes and use of OADs. After multivariate logistic regression analysis, the risk factors for DR were insulin therapy (OR = 3.82; 95% CI = [1.53–9.54]; P < 0.001), hypertension (OR = 2.67; 95% CI = [1.26–5.62]; P < 0.01), HbA1c > 7% (OR = 2.50; 95% CI = [1.30–4.84]; P < 0,01) and diabetes duration (OR = 1.13; 95% CI = [1.07–1.18]; P < 0.0001) (Table 2).
Fig. 2.
Fluorescein angiography showing clinical features of non-proliferative (minimal, moderate and severe stages) and proliferative diabetic retinopathy (red images before fluorescein injection; black and white images after fluorescein injection).
Fig. 3.
Frequency of DR stages. NPDR: non-proliferative diabetic retinopathy; Minimal NPDR: minimal non-proliferative diabetic retinopathy; Moderate NPDR: moderate non-proliferative diabetic retinopathy; Severe NPDR: severe non-proliferative diabetic retinopathy; PDR: proliferative diabetic retinopathy; UPDR: uncomplicated proliferative diabetic retinopathy; CPDR: complicated proliferative diabetic retinopathy.
Table 2.
Univariate and multivariate logistic regression analyses showing associations of DR with independent variables.
| Dependent variable | Diabetic retinopathy | |||
|---|---|---|---|---|
| Independent variables | Crude OR [95% CICI] | P-value | Adjusted OR [95% CI] | P-value |
| Insulin therapy (Yes/No) | 7.87 [3.64–17.00] | <0.0001 | 3.82 [1.53–9.54] | <0.001 |
| Hypertension (Yes/No) | 3.60 [1.90–6.86] | <0.0001 | 2.67 [1.26–5.62] | <0.01 |
| HbA1c (%) (>7%/≤7%) | 2.88 [1.65–5.04] | <0.0001 | 2.50 [1.30–4.84] | <0.01 |
| Combined treatment (OADs + Insulin) (Yes/No) | 2.61 [1.40–4.90] | <0.01 | – | |
| Diabetes duration (Years) | 1.17 [1.11–1.22] | <0.0001 | 1.13 [1.07–1.18] | <0.0001 |
| Number of hours of sleep (≥6h/<6h) | 0.56 [0.32–0.97] | <0.05 | – | |
| Types of diabetes (T2D/T1D) | 0.24 [0.07–0.75] | <0.05 | – | |
| Use of OADs (Yes/No) | 0.17 [0.10–0.30] | <0.05 | – | |
CI: confidence interval; OADs: oral antidiabetic drugs; HbA1c: glycated haemoglobin; T1D: type 1 diabetes; T2D: type 2 diabetes; OR: Odds ratio.
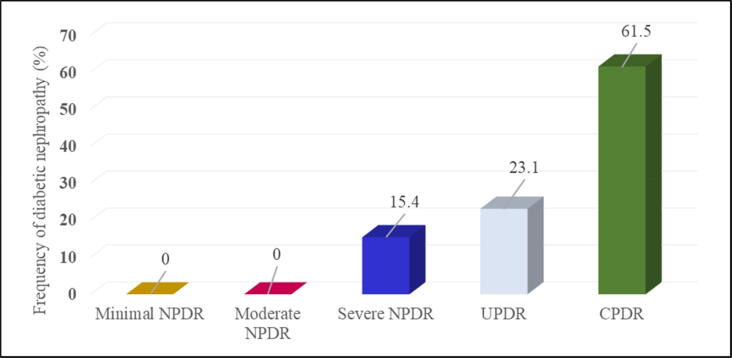
Different types of self-reported complications (diabetic nephropathy, diabetic neuropathy, diabetic foot, cardiovascular complications) have been identified in both groups (cases and controls). However, diabetic nephropathy (DN) was the complication that was present only in cases with a frequency of (10.6%) and absent in controls (0%) (Table 1). The highest percentages of DN were in subjects with CPDR with a value of 61.5%, followed by patients with UPDR (23.1%) and diabetics with severe NPDR (15.4%). However, no cases of DN have been recorded in patients with minimal and moderate NPDR (Fig. 4). In order to verify the chronological order of the appearance of DN and DR, we determined the duration of DN based on the duration of DR; for the 13 cases with both complications (DR and DN), DR precedes the onset of DN by about 5–6 years on average.
Fig. 4.
Frequency of diabetic nephropathy according to DR stages. It should be noted that the DN was based only on patient's statement. Minimal NPDR: minimal non-proliferative diabetic retinopathy; Moderate NPDR: moderate non-proliferative diabetic retinopathy; Severe NPDR: severe non-proliferative diabetic retinopathy; UPDR: uncomplicated proliferative diabetic retinopathy; CPDR: complicated proliferative diabetic retinopathy.
4. Discussion
DR is one of the main microvascular complications of diabetes, which poses a threat to progressive vision loss (Magliah et al., 2018). Our study is a case-control epidemiological survey of 244 diabetics (122 diabetics with DR “cases” and 122 diabetics without DR “controls”). In a descriptive analysis, although the frequency of DR was high among women (54.9% versus 45.1% among men) and among age groups (60–70 and >70 years), gender and age variables were not statistically associated with DR (Table 1). Similarly, in several studies, no association was found between DR and sex, age or age group (Jost et al., 2010, Ahmed et al., 2011, Yang et al., 2013, Abougalambou and Abougalambou, 2015, Abdellaoui et al., 2016, Boulbaroud et al., 2018). However, some research teams have found an independent association in men (Zhang et al., 2010, Semeraro et al., 2011, Hussain et al., 2013, Cherchi et al., 2020), and others in women with DR (Memon et al., 2014).
The overall stage frequencies of DR in our study were 42.6% for NPDR, which was classified as minimal in 8.2%, moderate in 26.2% and severe in 8.2% of patients. PDR was found in 57.4% of cases, 23.8% with UPDR and 33.6% with CPDR. These results were similar to those found by Sayad and colleagues in 2008 in Marrakech (Morocco) (NPDR = 47.0%; PDR = 53.0%) (Sayad et al., 2010) but different from other studies [(NPDR: 63.4%; PDR: 36.6%) (Jingi et al., 2014); (NPDR: 18.8%; PDR: 2.7%) (Shani et al., 2018); (NPDR (minimal NPDR: 28.4%; moderate NPDR: 27.1%; severe NPDR: 4.6%); PDR: 0.6%) (Hatz et al., 2019); (NPDR: 83.0%; PDR: 17.0%) (Yang et al., 2019). This difference in the frequency of DR stages can be largely explained by the methodology of each study (sample size, inclusion and exclusion criteria…), the different classification of DR stages and the specific characteristics of the population studied (elderly population).
In this work, T1D was more common in patients with DR (12.3%) compared to controls (3.3%) (Table 1). This finding is similar to those of other studies (Wong et al., 2006, Yau et al., 2012, Konstantinidis et al., 2017). The high frequency of DR in T1D patients can be explained by other factors such as diabetes duration, insulin use, hypertension and HbA1c.
Several studies showed that diabetes duration is an important risk factor for DR (Yau et al., 2012, Jingi et al., 2014, Rajaona et al., 2016, Liu et al., 2017, Magliahet al., 2018, Bandello et al., 2019). Nearly all patients with T1D and more than three-quarters of patients with T2D will have some form of DR after 20 years of disease (Bandello et al., 2019). The WESDR study showed that in young diabetics, the prevalence of retinopathy progressively increases by 8.0% at 3 years, 25.0% at 5 years, 60.0% at 10 years and 80.0% at 15 years of diabetes duration (Klein et al., 1984). T1D with more than 20 years of diabetes are 8.7 times more likely to develop DR than T2D patients with less than 10 years of disease (Yau et al., 2012). Our data are consistent with these reports. In fact, the frequency of DR increases with the diabetes duration, rising from 22.1% for less than 10 years, to 24.6% between 10 and 15 years and 53.3% for ˃ at 15 years (Fig. 1). Thus, statistical analysis showed that the risk of developing DR increases by 0.13 times each year (OR = 1.13; 95% CI = [1.07–1.18]; P < 0.0001) in the diabetics of our sample.
The rate of people with diabetes on insulin therapy and/or combined therapy (Insulin + OADs) was higher compared to those treated with OADs in cases than controls; these results are similar to those of other researchers (Donnio-Cordoba et al., 2001, West et al., 2002). Our findings are identical to those in the literature regarding the association between insulin therapy and progression of DR (Wong et al., 2006, Silpa-Archa and Sukhawarn, 2012, Zhao et al., 2014, Magliahet al., 2018). Indeed, patients on insulin have a higher risk of DR compared to those treated with OADs (OR = 3.82; 95% CI = [1.53–9.54]; P < 0.001) in our study. The mechanisms by which insulin increases the risk of DR need more research, especially because insulin use is considered a risk factor for certain diseases such as colorectal cancer (Wang et al., 2013, Yin et al., 2014). In this work, the high frequency of DR in insulin-dependent patients can be explained firstly by the poor management of insulin use by the diabetic. This means that the patient does not know how to adjust insulin doses according to his diet and physical activity, which always leads to hypoglycaemia, hyperglycaemia and therefore micro and/or macrovascular complications. Secondly, patients who take insulin have a long duration of diabetes, mainly because they are affected by diabetes at a very young age. Thus, This diabetes duration may have an impact on the development of diabetes related complications as known in the scientific literature (Maghbooli et al., 2014, Zoungas et al., 2014, Nanayakkara et al., 2018).
In this research, Crude multivariate regression analysis indicated that patients taking OADs were less exposed (OR = 0.17; 95% CI = [0.10–0.30]; P < 0.05) to the development of DR compared to those treated with insulin or a combination of insulin and hypoglycemic agents. This may suggest that it is easier to reduce blood glucose to near-normal levels and to achieve good glycemic control with OADs than with insulin therapy (UKPDS, 1995, Adham et al., 2010, Huri et al., 2015).
The statistical analysis of our database has also demonstrated that the progression of DR was closely related to high blood pressure (OR = 2.67; 95% CI = [1.26–5.62]; P < 0.01). Poor blood pressure control exacerbates the risk of DR (Ting et al., 2016). Therefore, it was shown that the prevalence of DR increases from 30.8% in diabetics with blood pressure ≤140 / 90 mmHg to 39.6% in diabetics with hypertension (BP > 140 / 90 mmHg) (Yau et al., 2012). The relative risk of having a DR is 1.5 times higher with a systolic pressure between 125 and 139 mmHg and 2.8 times greater with a systolic pressure >140 mmHg (Bandello et al., 2019).
The pathway by which hypertension increases DR is still unknown. Both diabetes and hypertension are risk factors for endothelial dysfunction (Mohamed et al., 2012). Hypertension can lead to impaired retinal vascular self-regulation, especially in the presence of high blood glucose (Rassam et al., 1995), and may promote oxidative stress and inflammation related to the damage caused by diabetes (Mohamed et al., 2012). The renin-angiotensin-aldosterone system (with angiotensin II) that controls blood pressure is implicated in the development of the microvascular alterations of DR. In addition to being a potent vasoconstrictor of arterioles, angiotensin II stimulates VEGF secretion which is known as a primary initiator of PDR, and as a potential mediator of NPDR (Bandello et al., 2019).
The American Diabetes Association has recommended that HbA1c levels should be maintained at or below 7.0% in all patients with diabetes (T1D and T2D) in order to prevent and to reduce the long-term complications of diabetes, including DR (ADA, 2015). In the same context, the results of this study demonstrated that HbA1c > 7.0% was an independent risk factor for DR (OR = 2.50; 95% CI = [1.30–4.84]; P < 0,01). Indeed, it has been reported that the prevalence of DR increased from 18.0 to 51.2% when HbA1c increased by ≤7.0% to a value ≥9.0% (Yau et al., 2012). The WESDR study showed that in T1D, for every 1% increase in HbA1c, the risk of progression to DR increased by 1.21 times (Klein et al., 1984).
The results of this study showed that there was no significant association between DR and some parameters known as risk factors for this disease, especially overweight and abdominal obesity. These results are consistent with some studies (Manaviat et al., 2008, Lima et al., 2016, Magliahet al., 2018) but differ from others (Chaturvedi et al., 2001, Bastawrous et al., 2017) that found a positive correlation between increased BMI and WHR with increased risk of DR. The lack of correlation with anthropometric parameters in our study is probably related to the characteristics of our sample: elderly population (mean age: 64.2 ± 12.0 years) and 77.0% of patients have a high BMI. Some other variables such as tobacco, alcohol consumption and nutrition (dietary compliance) did not show any significant association with the development of DR.
Sleep disorders have been linked to impaired glucose metabolism and an increased risk of diabetes (Grandner et al., 2016). Various epidemiological studies have indicated that sleep duration is a risk factor for insulin resistance and T2D (Donga and Romijn, 2014, Shan et al., 2015, Rudnicka et al., 2017). Statistical treatment of our variables revealed an association between sleep duration and DR. Patients who have a sleep duration ≥6 h are at lower risk of developing DR (OR = 0.56; 95% CI = [0.32–0.97]; P < 0.05), which is consistent with other research findings (Tan et al., 2018, Chew et al., 2020).
The retinal and renal complications of diabetes result from damage to the small vessels in these organs. These diabetic microvascular complications can have devastating effects, including blindness and end-stage renal failure. Some authors have noted associations between the two complications (DR and DN) and one complication may be a risk factor for the other (Lee et al., 2014). In our sample, DN was only present in cases with a frequency of 10.6%. This frequency was comparable to that observed (12.4%) in our previous study at the reference center of diabetology and chronic diseases in Eastern Morocco (Hammoudi et al., 2018). In addition, the most important percentages of nephropathy were in patients with PDR (0.0% for minimal and moderate NPDR, 15.4% for severe NPDR, 23.1% for UPDR and finally 61.5% for CPDR). These findings are consistent with the results of Al-Rubeaan et al. (2015).
Kofoed-enevoldsen et al., 1987, Klein et al., 2005 reported that similar molecular pathways appear to govern the development of diabetic renal and retinal microvascular lesions. Yang et al. noted that urinary haptoglobin, which is specific to ocular lesions, is a clinical biomarker for predicting diabetes-related kidney damage (Yang et al., 2017). Different studies have shown that DR as well as DN are multifactorial diseases involving multiple pathways (polyol pathway, advanced glycation products, oxidative stress…) (Sheetz and King, 2002, Caldwell et al., 2005, Fearn and Sheerin, 2015, Keir et al., 2017). Hence, the recent study by Martinez and Peplow (2019) discussed the importance of specific molecules such as micro-RNAs that may be a good biomarker for screening for DR (Martinez and Peplow, 2019). Our study has identified the chronological relationship between DR and DN; the development of retinopathy precedes the appearance of DN by a few years (an average of 5–6 years), which is in accordance with the results of other studies (Kofoed-enevoldsen et al., 1987, Ha et al., 2019). In the present series, the occurrence of DR before DN could be linked to the early diagnosis of DR, which is easier and faster: the signs of retinal damage can be recognized and detected at the time of diagnosis by a simple examination of the eye fundus. On the contrary, the diagnosis of DN, requiers clinical and biological tests (albuminuria, creatinine, albumin/creatinine ratio, and GFR) (Persson and Rossing, 2018, Thomas and Karalliedde, 2019) which do not provide insight into the stage of renal damage. Therefore, only a histological analysis of renal biopsy can provide a definitive diagnosis of DN. If renal biopsies could be performed in all patients, many patients would likely be diagnosed with early stages of DN (Dhaun et al., 2014, Persson and Rossing, 2018). Several new biomarkers (fetuin A, endostatin, cystatin C, haptoglobin, CKD273, VEGF…) have been studied to improve the prognostic and the diagnostic accuracy of DN, but none have yet been implemented in routine clinical care (Inoue et al., 2013, Papadopoulou-Marketou et al., 2017, Colhoun and Marcovecchio, 2018, Persson and Rossing, 2018).
5. Study limitations
This study had certain limitations that are worthy of comment:
First, the presence or absence of DN was based on patient's statement and therefore the stage of DN could not be determined. Second, some important parameters such as blood lipids, creatinine, albuminuria and GFR were not available.
6. Conclusion
This study is the first of its kind to focus on the pathology of DR in Eastern Morocco. It is a case-control epidemiological study carried out in the ophthalmology department of the Al-Irfane clinic (Oujda) on a total of 244 diabetics (122 cases and 122 controls). Among diabetics with retinopathy, we detected a predominance of PDR with 57.4% of cases distributed as follows: 23.8% UPDR and 33.6% CPDR. NPDR was present in 42.6% of cases (8.2% minimal NPDR; 26.2% moderate NPDR; 8.2% severe NPDR). The main risk factors for the appearance and progression of DR were insulin therapy, hypertension, poor glycemic control and diabetes duration. Our results also showed that DR precedes DN and that the latter was present in 10.6% of diabetic cases with DR (especially in patients with PDR). Finally, this work may constitute the starting point of future studies targeting the exploration of new indicators and biomarkers responsible for the occurrence of these two pathologies in order to improve the diagnosis and management of DR and DN.
Funding
None.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
We thank the team of Dr. El Habri CHELQI especially Nawal FENNOUCHI, Mounia BERTAOUI, Aicha MRAH, Safae OUINESS and Kaoutar AZZA, for their help and support to ensure the success of this project. We thank Pr. BOUJAMAA El Koy (Professor of english literature at the multidisciplinary faculty of Nador (FPN) for the english revision.
Footnotes
Peer review under responsibility of King Saud University.
References
- Abdellaoui M., Marrakchi M., Benatiya I.A., Tahri H. Dépistage de la rétinopathie diabétique par un rétinographe non mydriatique dans la région de Fès. Journal Français d'Ophtalmologie. 2016;39(1):48–54. doi: 10.1016/j.jfo.2015.08.009. [DOI] [PubMed] [Google Scholar]
- Abougalambou S.S.I., Abougalambou A.S. Risk factors associated with diabetic retinopathy among type 2 diabetes patients at teaching hospital in Malaysia. Diabetes Metabolic Syndrome: Clin. Res. Rev. 2015;9(2):98–103. doi: 10.1016/j.dsx.2014.04.019. [DOI] [PubMed] [Google Scholar]
- ADA (American Diabetes Association), 2015. 6. Glycemic targets. Diabetes care. 38(Suppl 1), S33–S40. 10.2337/dc15-S009. [DOI] [PubMed]
- ADA (American Diabetes Association), 2019. 6. Glycemic targets: standards of medical care in diabetes-2019. Diabetes Care. 42(Suppl 1), S61–S70. 10.2337/dc19-S006. [DOI] [PubMed]
- Adham M., Froelicher E.S., Batieha A., Ajlouni K. Glycaemic control and its associated factors in type 2 diabetic patients in Amman, Jordan. East. Mediterr. Health. J. 2010;16(7):732–739. https://apps.who.int/iris/handle/10665/117965 [PubMed] [Google Scholar]
- Adler Amanda I., Stevens Richard J., Manley Sue E., Bilous Rudy W., Cull Carole A., Holman Rury R., UKPDS Group Development and progression of nephropathy in type 2 diabetes: The United Kingdom Prospective Diabetes Study (UKPDS 64) Kidney Int. 2003;63(1):225–232. doi: 10.1046/j.1523-1755.2003.00712.x. [DOI] [PubMed] [Google Scholar]
- Ahmed Kazi R., Karim Md N., Bukht Mohammad S., Bhowmik Bishwajit, Acharyya Amitava, Ali Liaquat, Hussain Akhtar. Risk factors of diabetic retinopathy in Bangladeshi type 2 diabetic patients. Diabetes Metabolic Syndrome: Clin. Res. Rev. 2011;5(4):196–200. doi: 10.1016/j.dsx.2012.02.020. [DOI] [PubMed] [Google Scholar]
- Al-Rubeaan Khalid, Abu El-Asrar Ahmed M., Youssef Amira M., Subhani Shazia N., Ahmad Najlaa A., Al-Sharqawi Ahmad H., Alguwaihes Abdullah, Alotaibi Metib S., Al-Ghamdi Ali, Ibrahim Heba M. Diabetic retinopathy and its risk factors in a society with a type 2 diabetes epidemic: a Saudi National Diabetes Registry-based study. Acta Ophthalmol. 2015;93(2):e140–e147. doi: 10.1111/aos.12532. [DOI] [PubMed] [Google Scholar]
- Andersen A.R., Christiansen J.S., Andersen J.K., Kreiner S., Deckert T. Diabetic nephropathy in Type 1 (insulin-dependent) diabetes: an epidemiological study. Diabetologia. 1983;25(6):496–501. doi: 10.1007/BF00284458. [DOI] [PubMed] [Google Scholar]
- Bandello, F., Zarbin, M. A., Lattanzio, R., Zucchiatti, I., 2019. Clinical strategies in the management of diabetic retinopathy. A step-by-step guide for ophthalmologists. 2nd ed. Springer, Nature Switzerland AG. 10.1007/978-3-319-96157-6. [DOI]
- Bastawrous A., Mathenge W., Wing K., Bastawrous M., Rono H., Weiss H.A., Macleod D., Foster A., Peto T., Blows P., Burton M., Kuper H. The incidence of diabetes mellitus and diabetic retinopathy in a population-based cohort study of people age 50 years and over in Nakuru, Kenya. BMC. Endocr. Disord. 2017;17:19. doi: 10.1186/s12902-017-0170-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boulbaroud Z., Bensbaa S., El Aziz S., Chadli A. Dépistage de la rétinopathie diabétique par rétinographie réalisée par le médecin endocrinologue au sein du CHU de Casablanca. Ann. Endocrinol. 2018;79(4):464. doi: 10.1016/j.ando.2018.06.883. [DOI] [Google Scholar]
- Caldwell R.B., Bartoli M., Behzadian M.A., El-Remessy A.E., Al-Shabrawey M., Platt D.H., Liou G.I., Caldwell R.W. Vascular endothelial growth factor and diabetic retinopathy: role of oxidative stress. Curr. Drug. Targets. 2005;6:511–524. doi: 10.2174/1389450054021981. [DOI] [PubMed] [Google Scholar]
- Chalmers, J. O. H. N., MacMahon, S., Mancia, G., Whitworth, J., Beilin, L., Hansson, L., Neal, B., Rodgers, A., Mhurchu, C. Ni., Clark, T., 1999. 1999 World Health Organization-International Society of hypertension guidelines for the management of hypertension. Guidelines sub-committee of the World Health Organization. Clin. Exp. Hypertens (New York, NY, 1993). 21(5-6), 1009–60. doi: 10.3109/10641969909061028. [DOI] [PubMed]
- Chandy A., Pawar B., John M., Isaac R. Association between diabetic nephropathy and other diabetic microvascular and macrovascular complications. Saudi. J. Kidney. Dis. Transpl. 2008;19(6):924–1228. http://www.sjkdt.org/text.asp?2008/19/6/924/43466 [PubMed] [Google Scholar]
- Chaturvedi N., Sjoelie A.-K., Porta M., Aldington S.J., Fuller J.H., Songini M., Kohner E.M. Markers of insulin resistance are strong risk factors for retinopathy incidence in type 1 diabetes: the EURODIAB prospective complications study. Diabetes Care. 2001;24(2):284–289. doi: 10.2337/diacare.24.2.284. [DOI] [PubMed] [Google Scholar]
- Cherchi S., Gigante A., Spanu M.A., Contini P., Meloni G., Fois M.A., Pistis D., Pilosu R.M., Lai A., Ruiu S., Campesi I., Tonolo G. Sex-gender differences in diabetic retinopathy. Int. J. Diabetol. 2020;1(1):1–10. doi: 10.3390/diabetology1010001. [DOI] [Google Scholar]
- Cheung Ning, Mitchell Paul, Wong Tien Yin. Diabetic retinopathy. The Lancet. 2010;376(9735):124–136. doi: 10.1016/S0140-6736(09)62124-3. [DOI] [PubMed] [Google Scholar]
- Chew Merwyn, Tan Nicholas Y.Q., Lamoureux Ecosse, Cheng Ching-Yu, Wong Tien Yin, Sabanayagam Charumathi. The associations of objectively measured sleep duration and sleep disturbances with diabetic retinopathy. Diabetes Res. Clin. Pract. 2020;159:107967. doi: 10.1016/j.diabres.2019.107967. [DOI] [PubMed] [Google Scholar]
- Colhoun H.M., Marcovecchio M.L. Biomarkers of diabetic kidney disease. Diabetologia. 2018;61(5):996–1011. doi: 10.1007/s00125-018-4567-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dhaun Neeraj, Bellamy Christopher O., Cattran Daniel C., Kluth David C. Utility of renal biopsy in the clinical management of renal disease. Kidney Int. 2014;85(5):1039–1048. doi: 10.1038/ki.2013.512. [DOI] [PubMed] [Google Scholar]
- Donga E., Romijn J.A. Sleep characteristics and insulin sensitivity in humans. Handb. Clin. Neurol. 2014;124:107–114. doi: 10.1016/B978-0-444-59602-4.00007-1. [DOI] [PubMed] [Google Scholar]
- Donnio-Cordoba A., Richer R., Spinelli F., Merle H. La rétinopathie diabétique en Martinique: résultats d'une enquête transversale sur 771 patients. J. Fr. ophtalmol. 2001;24(6):603. doi: JFO-06-2001-24-6-0181-5512-101019-ART6. [PubMed] [Google Scholar]
- Edwards Matthew S., Wilson David B., Craven Timothy E., Stafford Jeanette, Fried Linda F., Wong Tien Y., Klein Ronald, Burke Gregory L., Hansen Kimberley J. Associations between retinal microvascular abnormalities and declining renal function in the elderly population: the cardiovascular health study. Am. J. Kidney Dis. 2005;46(2):214–224. doi: 10.1053/j.ajkd.2005.05.005. [DOI] [PubMed] [Google Scholar]
- El-asrar A.M.A., Al-Rubeaan K.A., Al-Amro S.A., Moharram O.A., Kangave D. Retinopathy as a predictor of other diabetic complications. Int. Ophthalmol. 2001;24(1):1–11. doi: 10.1023/A:1014409829614. [DOI] [PubMed] [Google Scholar]
- Elwali E.S., Almobarak A.O., Hassan M.A., Mahmooud A.A., Awadalla H., Ahmed M.H. Frequency of diabetic retinopathy and associated risk factors in Khartoum, Sudan: population based study. Int. J. Ophthalmol. 2017;10(6):948–954. doi: 10.18240/ijo.2017.06.18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fearn A., Sheerin N.S. Complement activation in progressive renal disease. World. J. Nephrol. 2015;4:31–40. doi: 10.5527/wjn.v4.i1.31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frank Robert N. Diabetic retinopathy. N. Engl. J. Med. 2004;350(1):48–58. doi: 10.1056/NEJMra021678. [DOI] [PubMed] [Google Scholar]
- Gharbi M.B., Elseviers M., Zamd M., Alaoui A.B., Benahadi N., Trabelssi E.H., Bayahia R., Ramdan B., De Broe M.E. Chronic kidney disease, hypertension, diabetes, and obesity in the adult population of Morocco: how to avoid “over”- and “under”-diagnosis of CKD. Kidney Int. 2016;89(6):1363–1371. doi: 10.1016/j.kint.2016.02.019. [DOI] [PubMed] [Google Scholar]
- Grandner M.A., Seixas A., Shetty S., Shenoy S. Sleep duration and diabetes risk: population trends and potential mechanisms. Curr. Diab. Rep. 2016;16(11):106. doi: 10.1007/s11892-016-0805-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ha M., Choi S.Y., Kim M., Na J.K., Park Y.H. Diabetic nephropathy in type 2 diabetic retinopathy requiring panretinal photocoagulation. Korean. J. Ophthalmol. 2019;33(1):46–53. doi: 10.3341/kjo.2018.0034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammoudi Jamila, Dahmani Hassana, Bouanani Nourel Houda, Nouayti Hamid, Mekhfi Hassane, Legssyer Abdelkhaleq, Bnouham Mohamed, Ziyyat Abderrahim. Risk factors and diabetes related complications frequency in the population of the northeastern Morocco. OJEpi. 2018;08(03):164–185. doi: 10.4236/ojepi.2018.83014. [DOI] [Google Scholar]
- Hatz Katja, Minder Anna Elisabeth, Lehmann Roger, Drescher Tilman, Gerendas Bianca, Schmidt-Erfurth Ursula, Kaider Alexandra, Pruente Christian, Zulewski Henryk. The prevalence of retinopathy in patients with type 1 diabetes treated with education-based intensified insulin therapy and its association with parameters of glucose control. Diabetes Res. Clin. Pract. 2019;148:234–239. doi: 10.1016/j.diabres.2019.01.016. [DOI] [PubMed] [Google Scholar]
- Huri H.Z., Ling D.Y.H., Ahmad W.A.W. Association between glycemic control and antidiabetic drugs in type 2 diabetes mellitus patients with cardiovascular complications. Drug. Des. Devel. Ther. 2015;9:4735. doi: 10.2147/dddt.s87294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hussain S., Qamar M.R., Iqbal M.A., Ahmad A., Ullah E. Risk factors of retinopathy in type 2 diabetes mellitus at a tertiary care hospital, Bahawalpur Pakistan. Pak. J. Med. Sci. 2013;29(2):536–539. doi: 10.12669/pjms.292.3066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inoue Kentaro, Wada Jun, Eguchi Jun, Nakatsuka Atsuko, Teshigawara Sanae, Murakami Kazutoshi, Ogawa Daisuke, Terami Takahiro, Katayama Akihiro, Tone Atsuhito, Iseda Izumi, Hida Kazuyuki, Yamada Masao, Ogawa Tomohisa, Makino Hirofumi, Yagihashi Soroku. Urinary Fetuin-A is a novel marker for diabetic nephropathy in type 2 diabetes identified by lectin microarray. PLoS ONE. 2013;8(10):e77118. doi: 10.1371/journal.pone.0077118.t008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeng Chi-Juei, Hsieh Yi-Ting, Yang Chung-May, Yang Chang-Hao, Lin Cheng-Li, Wang I-Jong, Tzekov Radouil. Diabetic retinopathy in patients with diabetic nephropathy: development and progression. PLoS ONE. 2016;11(8):e0161897. doi: 10.1371/journal.pone.0161897.t004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jingi A.M., Noubiap J.J.N., Ellong A., Bigna J.J.R., Mvogo C.E. Epidemiology and treatment outcomes of diabetic retinopathy in a diabetic population from Cameroon. BMC. Ophthalmol. 2014;14(1):1–5. doi: 10.1186/1471-2415-14-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jost B.S., Hilgemberg É., Rodrigues E.B., Daniotti A.F., Bonamigo E.L. Prevalência de retinopatia diabética na população portadora de diabetes mellitus tipo 2 do município de Luzerna–SC. Arq. Bras. Oftalmol. 2010;73(3):259–265. doi: 10.1590/S0004-27492010000300010. [DOI] [PubMed] [Google Scholar]
- Kaur Jaskirat, Mittal Deepti. Estimation of severity level of non-proliferative diabetic retinopathy for clinical aid. Biocybernet. Biomed. Eng. 2018;38(3):708–732. doi: 10.1016/j.bbe.2018.05.006. [DOI] [Google Scholar]
- Keir Lindsay S., Firth Rachel, Aponik Lyndsey, Feitelberg Daniel, Sakimoto Susumu, Aguilar Edith, Welsh Gavin I., Richards Anna, Usui Yoshihiko, Satchell Simon C., Kuzmuk Valeryia, Coward Richard J., Goult Jonathan, Bull Katherine R., Sharma Ruchi, Bharti Kapil, Westenskow Peter D., Michael Iacovos P., Saleem Moin A., Friedlander Martin. VEGF regulates local inhibitory complement proteins in the eye and kidney. J. Clin. Investig. 2017;127(1):199–214. doi: 10.1172/JCI86418DS1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein R., Klein B.E., Moss S.E., Davis M.D., DeMets D.L. The Wisconsin epidemiologic study of diabetic retinopathy. II: prevalence and risk of diabetic retinopathy when age at diagnosis is less than 30 years. Arch. Ophthalmol. 1984;102:520–526. doi: 10.1001/archopht.1984.01040030398010. [DOI] [PubMed] [Google Scholar]
- Klein R., Zinman B., Gardiner R., Suissa S., Donnelly S.M., Sinaiko A.R., Kramer M.S., Goodyer P., Moss S.E., Strand T., Mauer M. The relationship of diabetic retinopathy to preclinical diabetic glomerulopathy lesions in type 1 diabetic patients: the renin-angiotensin system study. Diabetes. 2005;54(2):527–533. doi: 10.2337/diabetes.54.2.527. [DOI] [PubMed] [Google Scholar]
- Kofoed-enevoldsen A., Jensen T., Borch-johnsen K., Deckert T. Incidence of retinopathy in type 1 (insulin-dépendent) diabetes: association with clinical nephropathy. Diabet. Complicat. 1987;1:96–99. doi: 10.1016/S0891-6632(87)80064-8. [DOI] [PubMed] [Google Scholar]
- Konstantinidis L., Carron T., de Ancos E., Chinet L., Hagon-Traub I., Zuercher E., Peytremann-Bridevaux I. Awareness and practices regarding eye diseases among patients with diabetes: a cross sectional analysis of the CoDiab-VD cohort. BMC. Endocr. Disord. 2017;17(1):56. doi: 10.1186/s12902-017-0206-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee W.J., Sobrin L., Lee M.J., Kang M.H., Seong M., Cho H. The relationship between diabetic retinopathy and diabetic nephropathy in a population-based study in Korea (KNHANES V-2, 3) Invest. Ophthalmol. Vis. Sci. 2014;55(10):6547–6553. doi: 10.1167/iovs.14-15001. [DOI] [PubMed] [Google Scholar]
- Lima V.C., Cavalieri G.C., Lima M.C., Nazario N.O., Lima G.C. Risk factors for diabetic retinopathy: a case–control study. Int. J. Retin. Vitr. 2016;2(1):21. doi: 10.1186/s40942-016-0047-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Yan, Yang Jiarui, Tao Liyuan, Lv Huibin, Jiang Xiaodan, Zhang Mingzhou, Li Xuemin. Risk factors of diabetic retinopathy and sight-threatening diabetic retinopathy: a cross-sectional study of 13 473 patients with type 2 diabetes mellitus in mainland China. BMJ Open. 2017;7(9):e016280. doi: 10.1136/bmjopen-2017-016280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maghbooli Z., Pasalar P., Keshtkar A., Farzadfar F., Larijani B. Predictive factors of diabetic complications: a possible link between family history of diabetes and diabetic retinopathy. J. Diabetes. Metab. Disord. 2014;13(1):55. doi: 10.1186/2251-6581-13-55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magliah S.F., Bardisi W., Al Attah M., Khorsheed M.M. The prevalence and risk factors of diabetic retinopathy in selected primary care centers during the 3-year screening intervals. J. Family. Med. Prim. Care. 2018;7(5):975–981. doi: 10.4103/jfmpc.jfmpc_85_18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manaviat M.R., Rashidi M., Afkhami-Ardekani M. Four years incidence of diabetic retinopathy and effective factors on its progression in type II diabetes. Eur. J. Ophthalmol. 2008;18(4):572–577. doi: 10.1177/112067210801800412. [DOI] [PubMed] [Google Scholar]
- Martinez B., Peplow P.V. MicroRNAs as biomarkers of diabetic retinopathy and disease progression. Neural. Regen. Res. 2019;14(11):1858–1869. doi: 10.4103/1673-5374.259602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Memon S., Ahsan S., Riaz Q., Basit A., Sheikh S.A., Fawwad A., Shera A.S. Frequency, severity and risk indicators of retinopathy in patients with diabetes screened by fundus photographs: a study from primary health care. Pak. J. Med. Sci. 2014;30(2):366–372. doi: 10.12669/pjms.302.4086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Memon W.R., Lal B., Sahto A.A. Diabetic retinopathy. Prof. Med. J. 2017;24(2):234–238. doi: 10.17957/TPMJ/17.3616. [DOI] [Google Scholar]
- Mohamed I.N., Soliman S.A., Alhusban A., Matragoon S., Pillai B.A., Elmarkaby A.A., El-Remessy A.B. Diabetes exacerbates retinal oxidative stress, inflammation, and microvascular degeneration in spontaneously hypertensive rats. Mol. Vis. 2012;18:1457–1466. http://www.molvis.org/molvis/v18/a152 [PMC free article] [PubMed] [Google Scholar]
- Nanayakkara Natalie, Ranasinha Sanjeeva, Gadowski Adelle, Heritier Stephane, Flack Jeff R., Wischer Natalie, Wong Jencia, Zoungas Sophia. Age, age at diagnosis and diabetes duration are all associated with vascular complications in type 2 diabetes. J. Diabetes Complicat. 2018;32(3):279–290. doi: 10.1016/j.jdiacomp.2017.11.009. [DOI] [PubMed] [Google Scholar]
- OMS, 2003. Obésité: prévention et prise en charge de l‘épidémie mondiale. OMS, série de rapports techniques 894. Rapport d’une consultation de l’OMS. Organisation mondiale de la santé, Genève.
- Papadopoulou-Marketou Nektaria, Kanaka-Gantenbein Christina, Marketos Nikolaos, Chrousos George P., Papassotiriou Ioannis. Biomarkers of diabetic nephropathy: a 2017 update. Crit. Rev. Clin. Lab. Sci. 2017;54(5):326–342. doi: 10.1080/10408363.2017.1377682. [DOI] [PubMed] [Google Scholar]
- Park Young-Hoon, Shin Jeong Ah, Han Jae-Hyung, Park Yong-Moon, Yim Hyeon Woo, Mischak Harald. The association between chronic kidney disease and diabetic retinopathy: the Korea national health and nutrition examination survey 2008-2010. PLoS ONE. 2015;10(4):e0125338. doi: 10.1371/journal.pone.0125338.s001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pedro Romero-Aroca, Ramon Sagarra-Alamo, Marc Baget-Bernaldiz, Juan Fernández-Ballart, Isabel Méndez-Marin. Prevalence and relationship between diabetic retinopathy and nephropathy, and its risk factors in the north-east of Spain, a population-based study. Ophthalmic Epidemiol. 2010;17(4):251–265. doi: 10.3109/09286586.2010.498661. [DOI] [PubMed] [Google Scholar]
- Persson Frederik, Rossing Peter. Diagnosis of diabetic kidney disease: state of the art and future perspective. Kidney Int. Suppl. 2018;8(1):2–7. doi: 10.1016/j.kisu.2017.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petrovič Daniel. Candidate genes for proliferative diabetic retinopathy. Biomed Res. Int. 2013;2013:1–9. doi: 10.1155/2013/540416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qummar Sehrish, Khan Fiaz Gul, Shah Sajid, Khan Ahmad, Shamshirband Shahaboddin, Rehman Zia Ur, Ahmed Khan Iftikhar, Jadoon Waqas. A deep learning ensemble approach for diabetic retinopathy detection. IEEE Access. 2019;7:150530–150539. doi: 10.1109/ACCESS.2019.2947484. [DOI] [Google Scholar]
- Rajaona R.A., Volamarina R.F., Andriamahenina A.M., Raobela L., Bernardin P., Andriantsoa V. Aspect épidémiologique de la rétinopathie diabétique, étude bicentrique à Antananarivo (Madagascar), à propos de 158 cas. J. Fr. Ophtalmol. 2016;39(5):e137–e138. doi: 10.1016/J.JFO.2015.11.012. [DOI] [PubMed] [Google Scholar]
- Ramdani Noureddine, Vanderpas Jean, Boutayeb Abdeslam, Meziane Abdelouafi, Hassani Benyounès, Zoheir Jaouhar, Legssyer Abdelkhaleq, Aziz Mohammed, Mekhfi Hassane, Bnouham Mohammed, Ziyyat Abderrahim. Diabetes and obesity in the eastern Morocco. Mediterr. J. Nutr. Metab. 2012;5(2):149–155. doi: 10.1007/s12349-011-0087-2. [DOI] [Google Scholar]
- Rassam S.M., Patel V., Kohner E.M. The effect of experimental hypertension on retinal vascular autoregulation in humans: a mechanism for the progression of diabetic retinopathy. Exp. Physiol. 1995;80(1):53–68. doi: 10.1113/expphysiol.1995.sp003834. [DOI] [PubMed] [Google Scholar]
- Rguibi M., Belahsen R. Overweight and obesity among urban Sahraoui women of South Morocco. Ethn. Dis. 2004;14:542–547. https://pubmed.ncbi.nlm.nih.gov/15724774/ [PubMed] [Google Scholar]
- Rossing P., Hougaard P., Parving H.-H. Risk factors for development of incipient and overt diabetic nephropathy in type 1 diabetic patients: a 10-year prospective observational study. Diabetes Care. 2002;25(5):859–864. doi: 10.2337/diacare.25.5.859. [DOI] [PubMed] [Google Scholar]
- Rudnicka Alicja R., Nightingale Claire M., Donin Angela S., Sattar Naveed, Cook Derek G., Whincup Peter H., Owen Christopher G. Sleep duration and risk of type 2 diabetes. Pediatrics. 2017;140(3):e20170338. doi: 10.1542/peds.2017-0338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sabanayagam Charumathi, Banu Riswana, Chee Miao Li, Lee Ryan, Wang Ya Xing, Tan Gavin, Jonas Jost B, Lamoureux Ecosse L, Cheng Ching-Yu, Klein Barbara E K, Mitchell Paul, Klein Ronald, Cheung C M Gemmy, Wong Tien Y. Incidence and progression of diabetic retinopathy: a systematic review. Lancet Diab. Endocrinol. 2019;7(2):140–149. doi: 10.1016/S2213-8587(18)30128-1. [DOI] [PubMed] [Google Scholar]
- Saeedi Pouya, Petersohn Inga, Salpea Paraskevi, Malanda Belma, Karuranga Suvi, Unwin Nigel, Colagiuri Stephen, Guariguata Leonor, Motala Ayesha A., Ogurtsova Katherine, Shaw Jonathan E., Bright Dominic, Williams Rhys. Global and regional diabetes prevalence estimates for 2019 and projections for 2030 and 2045: Results from the International Diabetes Federation Diabetes Atlas, 9th edition. Diabetes Res. Clin. Pract. 2019;157:107843. doi: 10.1016/j.diabres.2019.107843. [DOI] [PubMed] [Google Scholar]
- Sayad N.-O., Errajraji A., Benfdil N., Baha A., Moutaouakil A., Essaadouni L. Aspects épidémiologiques et angiofluographiques de la rétinopathie diabétique à Marrakech (Maroc) Médecine des Maladies Métaboliques. 2010;4(6):700–703. doi: 10.1016/S1957-2557(10)70168-1. [DOI] [Google Scholar]
- Semeraro Francesco, Parrinello Giovanni, Cancarini Anna, Pasquini Luisa, Zarra Emanuela, Cimino Antonio, Cancarini Giovanni, Valentini Umberto, Costagliola Ciro. Predicting the risk of diabetic retinopathy in type 2 diabetic patients. J. Diabetes Complicat. 2011;25(5):292–297. doi: 10.1016/j.jdiacomp.2010.12.002. [DOI] [PubMed] [Google Scholar]
- Shan Zhilei, Ma Hongfei, Xie Manling, Yan Peipei, Guo Yanjun, Bao Wei, Rong Ying, Jackson Chandra L., Hu Frank B., Liu Liegang. Sleep duration and risk of type 2 diabetes: a meta-analysis of prospective studies. Dia Care. 2015;38(3):529–537. doi: 10.2337/dc14-2073. [DOI] [PubMed] [Google Scholar]
- Shani Michal, Eviatar Tali, Komaneshter Doron, Vinker Shlomo. Diabetic retinopathy –incidence and risk factors in a community setting- a longitudinal study. Scand. J. Prim. Health Care. 2018;36(3):237–241. doi: 10.1080/02813432.2018.1487524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheetz M.J., King G.L. Molecular understanding of hyperglycemia’s adverse effects for diabetic complications. JAMA. 2002;288:2579–2588. doi: 10.1001/jama.288.20.2579. [DOI] [PubMed] [Google Scholar]
- Silpa-Archa S., Sukhawarn R. Prevalence and associated factors of diabetic retinopathy in Chandrubeksa Hospital, Directorate of Medical Services, Royal Thai Air Force. J. Med. Assoc. Thai. 2012;95(Suppl 4):S43–S49. https://pubmed.ncbi.nlm.nih.gov/22696851/ [PubMed] [Google Scholar]
- Song Peige, Yu Jinyue, Chan Kit Yee, Theodoratou Evropi, Rudan Igor. Prevalence, risk factors and burden of diabetic retinopathy in China: a systematic review and meta-analysis. J. Glob Health. 2018;8(1) doi: 10.7189/jogh.08.010803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan N.Y.Q., Chew M., Tham Y.C., Nguyen Q.D., Yasuda M., Cheng C.Y., Wong T.Y., Sabanayagam C. Associations between sleep duration, sleep quality and diabetic retinopathy. PLoS ONE. 2018;13(5) doi: 10.1371/journal.pone.0196399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tazi Mohammed A, Abir-Khalil Saädia, Chaouki Noureddine, Cherqaoui Sanaa, Lahmouz Fatima, Sraïri Jamal E, Mahjour Jaouad. Prevalence of the main cardiovascular risk factors in Morocco: results of a National Survey, 2000. J. Hypertens. 2003;21(5):897–903. doi: 10.1097/00004872-200305000-00013. [DOI] [PubMed] [Google Scholar]
- Thomas R.L., Halim S., Gurudas S., Sivaprasad S., Owens D.R. IDF Diabetes Atlas: A review of studies utilising retinal photography on the global prevalence of diabetes related retinopathy between 2015 and 2018. Diabetes Res. Clin. Pract. 2019;157:107840. doi: 10.1016/j.diabres.2019.107840. [DOI] [PubMed] [Google Scholar]
- Thomas Stephen, Karalliedde Janaka. Diabetic nephropathy. Medicine. 2019;47(2):86–91. doi: 10.1016/j.mpmed.2018.11.010. [DOI] [Google Scholar]
- Ting Daniel Shu Wei, Cheung Gemmy Chui Ming, Wong Tien Yin. Diabetic retinopathy: global prevalence, major risk factors, screening practices and public health challenges: a review: global burden of diabetic eye diseases. Clin. Exp. Ophthalmol. 2016;44(4):260–277. doi: 10.1111/ceo.12696. [DOI] [PubMed] [Google Scholar]
- UKPDS (UK Prospective Diabetes Study Group), 1995. Overview of 6 years therapy of type II diabetes: a progressive disease. UK Prospective Diabetes Study 16. Diabetes. 44, 1249–58. https://pubmed.ncbi.nlm.nih.gov/7589820/. [PubMed]
- Umanath Kausik, Lewis Julia B. Update on diabetic nephropathy: core curriculum 2018. Am. J. Kidney Dis. 2018;71(6):884–895. doi: 10.1053/j.ajkd.2017.10.026. [DOI] [PubMed] [Google Scholar]
- Villar G., Garcia Y., Goicolea I., Vazquez J.A. Determinants of development of microalbuminuria in normotensive patients with type 1 and type 2 diabetes. Diabetes Metab. 1999;25:246–254. https://pubmed.ncbi.nlm.nih.gov/10499194/ [PubMed] [Google Scholar]
- Wang Lei, Cai Shuang, Teng Zan, Zhao Xin, Chen Xinyue, Bai Xiaojuan. Insulin therapy contributes to the increased risk of colorectal cancer in diabetes patients: a meta-analysis. Diagn. Pathol. 2013;8(1):180. doi: 10.1186/1746-1596-8-180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang W., Lo A.C.Y. Diabetic retinopathy: pathophysiology and treatments. Int. J. Mol. Sci. 2018;19(6):1816. doi: 10.3390/ijms19061816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- West Sheila K, Munoz Beatriz, Klein Ronald, Broman Aimee T, Sanchez Rosario, Rodriguez Jorge, Snyder Robert. Risk factors for type ii diabetes and diabetic retinopathy in a mexican-american population: proyecto ver. Am. J. Ophthalmol. 2002;134(3):390–398. doi: 10.1016/S0002-9394(02)01595-7. [DOI] [PubMed] [Google Scholar]
- Whitehead Michael, Wickremasinghe Sanjeewa, Osborne Andrew, Van Wijngaarden Peter, Martin Keith R. Diabetic retinopathy: a complex pathophysiology requiring novel therapeutic strategies. Expert Opin. Biol. Ther. 2018;18(12):1257–1270. doi: 10.1080/14712598.2018.1545836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WHO, 2018. Consultation on Obesity, & Division of Non communicable Diseases World Health Organization. Obesity: preventing and managing the global epidemic; report of a WHO Consultation on Obesity, Geneva, 3-5 June 1997. WHO. https://apps.who.int/iris/handle/10665/63854.
- WHO, 2016. World Health Organization. Country Profiles for Diabetes. Available at: http://www.who.int/diabetes/countryprofiles/mar_en.pdf.
- Wong T.Y., Coresh J., Klein R., Muntner P., Couper D.J., Sharrett A.R., Klein B.E.K., Heiss G., Hubbard L.D., Duncan B.B. Retinal microvascular abnormalities and renal dysfunction: the atherosclerosis risk in communities study. J. Am. Soc. Nephrol. 2004;15(9):2469–2476. doi: 10.1097/01.ASN.0000136133.28194.E4. [DOI] [PubMed] [Google Scholar]
- Wong T.Y., Klein R., Islam F.A., Cotch M.F., Folsom A.R., Klein B.E., Sharrett A.R., Shea S. Diabetic retinopathy in a multi-ethnic cohort in the United States. Am. J. Ophthalmol. 2006;141(3):446–455.e1. doi: 10.1016/j.ajo.2005.08.063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Jin-Kui, Wang Ying-Ying, Liu Chang, Shi Ting-Ting, Lu Jing, Cao Xi, Yang Fang-Yuan, Feng Jian-Ping, Chen Chen, Ji Li-Nong, Xu Aimin. Urine proteome specific for eye damage can predict kidney damage in patients with type 2 diabetes: a case-control and a 5.3-year prospective cohort study. Dia Care. 2017;40(2):253–260. doi: 10.2337/dc16-1529. [DOI] [PubMed] [Google Scholar]
- Yang Ju Yean, Kim Na Kyung, Lee Yun Jeong, Noh Jung Hyun, Kim Dae Jung, Ko Kyung Soo, Rhee Byoung Doo, Kim Dong-Jun. Prevalence and factors associated with diabetic retinopathy in a Korean adult population: The 2008–2009 Korea National Health and Nutrition Examination Survey. Diabetes Res. Clin. Pract. 2013;102(3):218–224. doi: 10.1016/j.diabres.2013.10.016. [DOI] [PubMed] [Google Scholar]
- Yang Q.H., Zhang Y., Zhang X.M., Li X.R. Prevalence of diabetic retinopathy, proliferative diabetic retinopathy and non-proliferative diabetic retinopathy in Asian T2DM patients: a systematic review and Meta-analysis. Int. J. Ophthalmol. 2019;12(2):302–311. doi: 10.18240/ijo.2019.02.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yau J.W.Y., Rogers S.L., Kawasaki R., Lamoureux E.L., Kowalski J.W., Bek T., Chen S.-J., Dekker J.M., Fletcher A., Grauslund J., Haffner S., Hamman R.F., Ikram M.K., Kayama T., Klein B.E.K., Klein R., Krishnaiah S., Mayurasakorn K., O'Hare J.P., Orchard T.J., Porta M., Rema M., Roy M.S., Sharma T., Shaw J., Taylor H., Tielsch J.M., Varma R., Wang J.J., Wang N., West S., Xu L., Yasuda M., Zhang X., Mitchell P., Wong T.Y. Global prevalence and major risk factors of diabetic retinopathy. Diabetes Care. 2012;35(3):556–564. doi: 10.2337/dc11-1909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yin S., Bai H., Jing D. Insulin therapy and colorectal cancer risk among type 2 diabetes mellitus patients: a systemic review and meta-analysis. Diagn. Pathol. 2014;9(1):91. doi: 10.1186/1746-1596-9-91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Guihua, Chen Haoyu, Chen Weiqi, Zhang Mingzhi. Prevalence and risk factors for diabetic retinopathy in China: a multi-hospital-based cross-sectional study. Br. J. Ophthalmol. 2017;101(12):1591–1595. doi: 10.1136/bjophthalmol-2017-310316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X., Saaddine J.B., Chou C.F., Cotch M.F., Cheng Y.J., Geiss L.S., Gregg E.W., Albright A.L., Klein B.E.K., Klein R. Prevalence of diabetic retinopathy in the United States, 2005–2008. JAMA. 2010;304(6):649–656. doi: 10.1001/jama.2010.1111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao Chun, Wang Weifang, Xu Ding, Li Hui, Li Min, Wang Fang. Insulin and risk of diabetic retinopathy in patients with type 2 diabetes mellitus: data from a meta-analysis of seven cohort studies. Diagn. Pathol. 2014;9(1):130. doi: 10.1186/1746-1596-9-130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zoungas, S., Woodward, M., Li, Q., Cooper, M. E., Hamet, P., Harrap, S., Heller, S., Marre, M., Patel, A., Poulter, N., Williams, B., Chalmers, J., The ADVANCE Collaborative group, 2014. Impact of age, age at diagnosis and duration of diabetes on the risk of macrovascular and microvascular complications and death in type 2 diabetes. Diabetologia, 57(12), 2465–74. 10.1007/s00125-014-3369-7. [DOI] [PubMed]