Abstract
The beneficial live microbes of humans and animals are termed probiotics, and the chemical compounds that improve the growth of probiotics are known as prebiotics. Paraprobiotics and postbiotics refer to dead or inactivated living cells of probiotics and healthful metabolic products that are produced by the living cells of probiotics, respectively. Although the healthful, functional, nutritional, and immune benefits of probiotics and prebiotics are scientifically well established beyond a reasonable doubt, their potential biological roles against COVID-19 infection still warrant further clinical and laboratory investigation.
Keywords: Probiotics, Prebiotics, Paraprobiotics, Postbiotics, COVID-19, SARS-CoV-2
Abbreviations: FAO, Food and Agriculture Organization; WHO, World Health Organization
1. Introduction
Historically, a probiotic, as a scientific term, indicates some chemical compound produced by one of the protozoans that has an ability to stimulate another, and the term has been applied to the supplements that are added to animal feed to improve the health of the animal through the beneficial effects on their gut flora. The description of probiotics as “organisms and chemical supplements make to create a microbial balance in the intestine” is completely inaccurate in scientific terms. In 1989, Fuller revised the term probiotic and emphasized that probiotics must be live microbial cells that have the biological activity to improve the microbial balance in the host intestine (AFRC, 1989). This definition has gradually developed to involve specific beneficial effects such as improving the host’s immune system (Isolauri et al., 2004). Ilya Ilyich Mechnikov (discoverer of phagocytes) hinted that live microbes have a biological role in improving human health during his study about longevity. According to the theory of Elie Metchnikoff, the manipulation in the microbiomes of animal and human gastrointestinal tract using beneficial microbes cultivated in yogurt can enhance health and delay aging (Mackowiak, 2013).
Over a century earlier, there were several scientific questions concerning probiotics that still require interpretations and answers. At a global level, the aspects of identification, production, biological features, advantages, disadvantages, competition with pathogens, passing through the gastrointestinal tract, colonization of the intestinal area, nutritional value, and induction of immunity system mechanisms have been well investigated by scientists. Today, researchers are seeking to investigate the use of probiotics to fight against the SARS-CoV-2 (COVID-19 infection) endemic and pandemic (Infusino et al., 2020, He et al., 2020). This article is aimed to review the scientific facts related to this topic.
2. What are probiotics, prebiotics, paraprobiotics, and postbiotics?
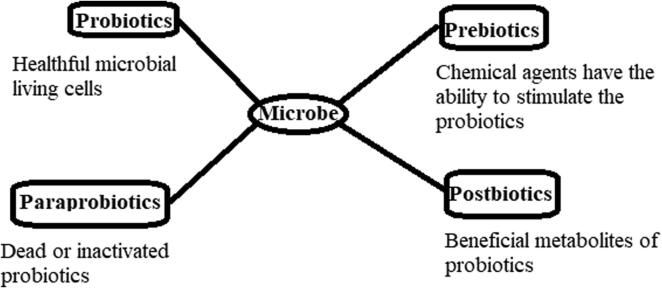
In 2001, the joint FAO and WHO Expert Consultation agreed on a specific definition of the probiotic, i.e., “live microorganisms which when administered in adequate amounts confer a health benefit on the host.” (Hotel and Cordoba, 2001). The beneficial live microorganisms of the host are carried through dairy and nondiary fermented products (Rivera-Espinoza and Gallardo-Navarro, 2010). The selection of probiotics depends on biological (food safety and functional aspects) and technical characteristics. The vital aspects concerning the selection and production of probiotics include the pathogenicity, resistance to antibiotics, viability and stability in the gastrointestinal tract, beneficial modulations of the immune system, antagonistic features, and genetic and physiological stability (Saarela et al., 2000). The concept of prebiotics has been revisited currently, leading to their definition as a most suitable fermented component that provides the opportunity for particular biological modifications, both in the constituents and/or performance in the microbiota of the gastrointestinal tract that has healthy benefits on the host (Roberfroid, 2007). Recently, the terms paraprobiotics and postbiotics have emerged in some literature, where the first term refers to dead or inactivated living cells of probiotics, and the second term refers to healthful metabolic products that are produced by the living cells of probiotics (Barros et al., 2020). Fig. 1 summarizes the major differences among probiotics, prebiotics, paraprobiotics, and postbiotics.
Fig. 1.
The major differences among probiotics, prebiotics, paraprobiotics, and postbiotics.
3. The most important probiotics
Several microbes are used as probiotics, and several products have been claimed to contain some of those microbes. The most common types of probiotics belong to the genera Bifidobacterium, Lactobacillus, and Saccharomyces, and the most common seven types of probiotics include B. animalis subsp. animalis, B. animalis subsp. lactis, B. longum, L. acidophilus, L. reuteri, L. casei, and S. boulardii.
* Bifidobacterium spp. are Gram-positive, nonspore-forming, anaerobic, frequently branched with bifid shape, nonmotile bacteria. They inhabit mammals and have been frequently isolated from the human gastrointestinal tract, breastfed infants’ faces, mouth, vagina, and dental caries (CROCIANI et al., 1996, GAVINI et al., 2006, Hadadji et al., 2005). B. animalis subsp. animalis and B. animalis subsp. lactis possess the ability to grow well on milk culture and resistance to acidity and oxidative stress and exert several biological functions such as immunomodulation, adherence to human epithelial cells, and improvement of intestinal barrier function. In this regard, B. animalis subsp. lactis BB-12 strain and B. animalis subsp. lactis DN-173 010 strain are the two commercial probiotic strains that have been widely investigated (Quigley, 2017). These strains can be distinguished using subspecies-specific biomarker peaks observed in mass spectrometry, and tuf (elongation factor Tu) and atpD (ATP synthase beta subunit) gene sequencing can also be applied for the identification of these strains (Schumann and Maier, 2014). The bacterium B. longum includes three biotypes that were previously classified into the following three independent species: B. infantis, B. suis, and B. longum. This reclassification was based on the analysis of 16S RNA gene sequence, 16S-23S ITS sequence, DNA–DNA hybridization, and HSP60 (heat shock protein 60) gene sequence (Mattarelli et al., 2008). It has been reported that numerous strains of B. longum can colonize the intestine due to their ability to resist bile salts and gastric acid in the gastrointestinal tract (Xiao et al., 2003). Moreover, a recent research reported that some strains of B. longum can metabolize several oligosaccharides to produce acetic acid (primary end product) and lactic and formic acids (Ruiz-Aceituno et al., 2020).
* Lactobacillus spp. are Gram-positive bacteria that do not form endospores and possess the ability to tolerate oxygen (or microaerophilic bacteria). There are more than 200 species belonging to the genus Lactobacillus, and several Lactobacillus species are known to inhabit the human body, constituting a group of the most important microbiota, and are considered as the most common probiotics that are added to several dairy and nondairy products. Some Lactobacillus species can ferment sugar into lactic acid (homofermentation), and other species can also produce lactic acid or alcohol (homofermentation) (Hammes and Vogel, 1995, Sun et al., 2015). L. acidophilus strains metabolize sugar into lactic acid (homofermentation) and can grow in low-pH environments. Based on the oxygen requirement, these strains are classified as microaerophilic bacteria (Bâati et al., 2000). L. reuteri is identified as an indigenous human intestinal microbiome and has also been isolated from other mammals and food. This probiotic is a heterofermentative bacterium with the ability to produce reuterin that exhibits a broad-spectrum antimicrobial property (Britton, 2017, Lee et al., 2017). The L. casei group has been reclassified genotypically and phenotypically to consist of the following three facultative heterofermentative bacterial species: L. casei, L. rhamnosus, and L. paracasei. This group can produce several bioactive metabolic compounds that are beneficial for humans and animals (Hill et al., 2018).
* S. boulardii is an S. cerevisiae strain that has particular genome characteristics such as chromosome IX trisomy and some distinctive genes and also exerts a biological activity against numerous stomach and intestinal disorders and diarrhea associated with antibiotics. This strain has distinctive physiological features such as tolerance to differences in pH, temperature, and gastrointestinal stresses such as enzymes, acids, and bile salts. It also has unique adhesion proteins that function to obstruct pathogenic microbial adhesion to the mucosal membrane in the stomach and the intestine (Edwards-Ingram et al., 2007, Fietto et al., 2004, Khatri et al., 2017, Tiago et al., 2012).
4. Coronavirus disease 2019 (COVID-19)
Coronaviruses (CoV) are a large family of viruses that have distinctive crown-like appendages on their surface. Human coronaviruses (CoV) were identified in the mid-1960 s, and currently the following seven types of CoV have been identified as having the ability to cause several mild, moderate, and severe symptoms: 229E (alpha), NL63 (alpha), OC43 (beta, HKU1 (beta), MERS-CoV (Middle East respiratory syndrome, or MERS), SARS-CoV (severe acute respiratory syndrome, or SARS), and SARS-CoV-2 (COVID-19) (https://www.cdc.gov/coronavirus/types.html, Page last reviewed on February 15, 2020). Based on the last update on July 22, 2020, 03:00 GMT + 3, the number of confirmed cases and confirmed deaths caused by the novel coronavirus (COVID-19) globally had reached 14,73,563 and 611,284, respectively (https://www.who.int/emergencies/diseases/novel-coronavirus-2019). The treatment strategies that could be applied for coronavirus disease 2019 (COVID-19) include artificial ventilation and antiviral agents. In addition to the universal attempts made to develop and produce vaccines, the biological agents needed to treat the viral infection using immunomodulation have received prime attention (Cao, 2020). Immunomodulation refers to alteration of the immune response or the functional immune system by the action of a substance that affects the functioning of the immune system, which is termed as an immunomodulator (Bondy and Pestka, 2000, Hubbell et al., 2009).
5. Immunomodulation by probiotics and prebiotics
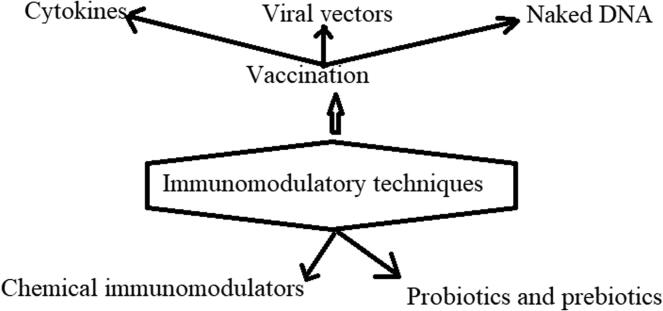
Immunomodulation includes the natural and therapeutic processes that are aimed at modifying the immune response (Gea-Banacloche, 2006). The immunomodulatory techniques used currently are shown in Fig. 2. Immunomodulators can be classified into the following two major groups: immunostimulants and immunosuppressants that respectively function to stimulate and suppress the animal immune system (Nair et al., 2019). It has been reported that in the gastrointestinal tract of humans and animals, peptidoglycan (released from the cell walls of both Gram-positive and Gram-negative bacteria) and lipopolysaccharide (released from the cell wall of only Gram-negative bacteria) of the intestinal bacterial flora could play a functionally significant role in improving, conservation, and biological functions of the immune system (Hamann et al., 1998).
Fig. 2.
The most efficient immunomodulatory tools applied for modifying the human immune response. The information is extracted from (Erickson and Hubbard, 2000, Gea-Banacloche, 2006).
6. Probiotics against the COVID-2019 pandemic
There is extensive research investigating the biological roles of the gut microbiota in influencing lung disorders that include asthma, chronic obstructive pulmonary disease, chronic bronchitis, emphysema, lung cancer, pneumonia, pleural effusion, viral and infection (Han et al., 2007). It is also recognized that viral infections in the respiratory tract cause a disturbance in the gut microbiota (Dhar and Mohanty, 2020). On May 8, 2020, (Baud et al., 2020) listed the most important probiotics that could be related to decreasing the burden of the COVID-19 pandemic, which included L. casei, L. gasseri, B. longum, B. bifidum, L. rhamnosus, L. plantarum, B. breve, Pediococcus pentosaceus, and Leuconostoc mesenteroides. All these probiotics have been added to several products such as DanActive/Actimel fermented drink (Danone), Tribion harmonis (Merck), Shirota, Morinaga, and Medipharm (Baud et al., 2020). A recent study conducted in China confirmed that COVID‐19 infection affects the balance of natural microbiota in the human intestine based on the observation of reduced counts of Bifidobacterium spp. and Lactobacillus spp. in patients infected by COVID‐19 (Xu et al. (2020a)). In another recent study, the authors reported that the proportion of patients with COVID-19 who suffered from antibiotic‐related diarrhea could reach 36% (Mak et al., 2020). These studies indicate the urgent need to support the balance of microbiota in patients with COVID-19 using some probiotics (Mak et al., 2020, Xu et al., 2020a). In another recently published retrospective study from Zhejiang province, China, the authors found that most of the patients with relatively mild symptoms had received probiotics along with the established treatment protocols, which included interferon-alpha inhalation, lopinavir, ritonavir, and arbidol (Xu et al., 2020b). It has also been reported that COVID-19 can cause severe hypoxemia and changes in the balance of gut microorganisms. On the other hand, a significant reduction of probiotics (Bifidobacterium spp., Lactobacillus spp., and Eubacterium spp.) was found to significantly increase the number of pathogens (such as Corynebacterium spp., Actinobacteria spp., and Ruthenibacterium spp.) (Yu et al., 2020). Ceccarelli et al., (2020) reported that COVID-19 can cause disorders in the human stomach and intestines, however, there is no scientific evidence about the role of COVID-19 in host-microbial flora disorders. Some probiotics that belong to the genus of Lactobacillus and bifidobacterium biological activity to control the gastrointestinal dysbiosis caused by the severe acute respiratory syndrome coronavirus 2 (Sundararaman et al., 2020).
7. Reducing the cytokine storm associated with COVID-19 using probiotics
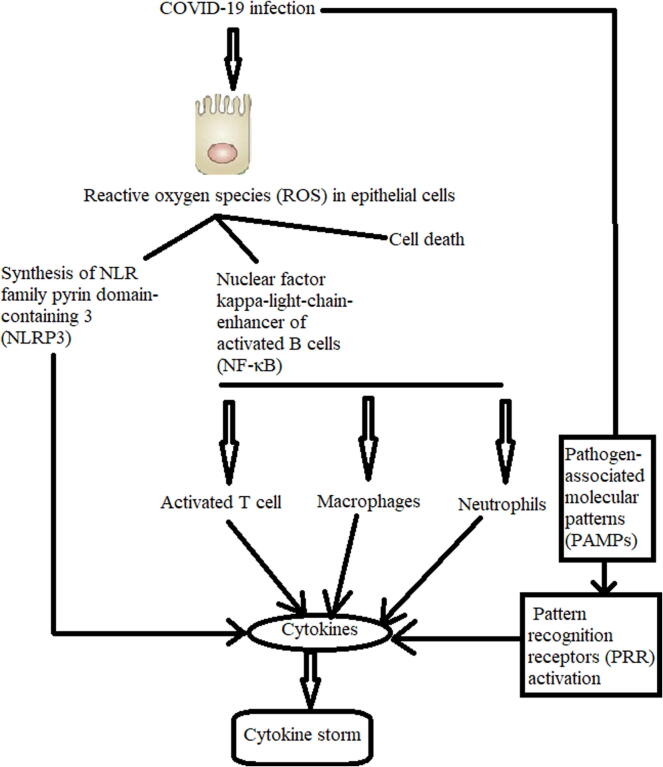
The cytokine storm is an offensive inflammatory response resulting from COVID-19 infection in some patients, which is caused due to the production of a large quantity of proinflammatory cytokines (Huang et al., 2020, Ragab et al., 2020) (Fig. 3). This cytokine storm can damage the lung, gastrointestinal tract, brain, cardiovascular system, liver, kidney, microcirculation, and eyes (Bhaskar et al., 2020). Extensive research suggests that probiotics have the ability to regulate functional and mucosal immune cells and epithelial cells in the human intestinal tract. Probiotics exert functional roles in achieving and preserving the precise equilibrium between the necessary and unnecessary protective mechanisms, including all the immune responses (innate and adaptive) (Yan and Polk, 2011). According to Yan and Polk (2011), regulation of the immune response by probiotics is always performed through several biological interactions as follows:
-
-
direct interaction with epithelial cells in the intestinal tract
-
-
interaction with dendritic cells
-
-
interaction with follicle-associated epithelial cells
-
-
-interaction with macrophages
-
-
interaction with T and B lymphocytes
-
-
interaction with gene expression
-
-
interaction with signaling pathways (Yan and Polk, 2011)
Fig. 3.
Cytokine storm resulting from SARS-CoV-2 infection. The data are extracted from (Bhaskar et al., 2020).
A biological role of probiotics in fighting the cytokine storm related to COIVD-19 can be deduced from the available theoretical evidence concerning their role in regulating the immune response; however, there still exists a need for clinical and laboratory evidence (Akour, 2020, Baud et al., 2020, Mak et al., 2020). Therapeutic and precautionary applications of Weissella cibaria (a Gram-positive bacterial probiotic) and heat-killed W. cibaria in inflammatory diseases have been evaluated by (Yu et al., 2019), wherein the results indicated that W. cibaria possesses biological activity as an anti-inflammatory agent, which is associated with the repression of NF-κB. Another study conducted by (Schmitter et al., 2018) concluded that some strains of L. paracasei and L. plantarum have the potential capacity of reducing the immune inflammatory response. The probiotic L. reuteri and some Lactobacillus strains can form biofilms and produce some biological factors that possess anti-inflammatory characteristics (Ayyanna et al., 2018, Jones and Versalovic, 2009). In another recent study, it was mentioned that one of the prophylactic methods that can reduce the impact of COVID-19 on immunocompromised individuals is to improve human immunity through probiotic supplementations (Dhar and Mohanty, 2020).
In the event of a failure to produce a vaccine, it is believed that the best approach to fight COVID-19 infection is by improving the immune system using probiotics and prebiotics that have the potential to minimize the inflammation caused by COVID-19 infection (Conte and Toraldo, 2020). In August 2020, (Bottari et al., 2020) reported that immune benefits of probiotics in COVID-19 infection could be obtained by developing mucosal immunity through the stimulation of IgA secretion, improvement of the biological functions of phagocytosis and macrophages, and adjustment of regulatory cells. Moreover, it has been reported that there is scientific evidence to confirm the role of probiotics and some nutrition elements (such as vitamins, trace elements, and nutraceuticals) in enhancing the immune functions, which could play a role in the obstruction and management of the viral infection caused by COVID-19 (Jayawardena et al., 2020).
8. Conclusion
The probiotics are live microbes which when administered in acceptable quality and quantity amounts confer a health benefit on the host. Coronavirus disease (COVID-19) is a contagious illness caused by a recently identified coronavirus. This disease caused health, economic, and social problems, and the number of cases still continuously increasing. Although the healthful, functional, nutritional, and immune benefits of probiotics and prebiotics are unquestionable, including their roles in fighting some viral infections such as seasonal virus infections, their potential roles against COVID-19 infection still warrant more clinical and laboratory investigations.
9. Ethical issues
There are no ethical issues
Declaration of Competing Interest
There are no competing interests.
Acknowledgements
The author extends their appreciation to the Deputyship for Research & Innovation, “Ministry of Education” in Saudi Arabia for funding this research work through the project number IFKSURP-1438-091. The author thanks the Deanship of Scientific Research and RSSU at King Saud University for their technical support.
Footnotes
Peer review under responsibility of King Saud University.
References
- Afrc, R.F., 1989. Probiotics in man and animals. J. Appl. Bacteriol.. 66, 365-378 [PubMed]
- Akour, A., 2020. Probiotics and COVID‐19: is there any link? Lett. Appl. Microbiol. 71, 229--234 [DOI] [PMC free article] [PubMed]
- Ayyanna R., Ankaiah D., Arul V. Anti-inflammatory and antioxidant properties of probiotic bacterium Lactobacillus mucosae AN1 and Lactobacillus fermentum SNR1 in Wistar albino rats. Front. Microbiol. 2018;9:3063. doi: 10.3389/fmicb.2018.03063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bâati, L.l., Fabre-Gea, C., Auriol, D., Blanc, P.J., 2000. Study of the cryotolerance of Lactobacillus acidophilus: effect of culture and freezing conditions on the viability and cellular protein levels. Int. J. Food Microbiol. 59, 241-247. [DOI] [PubMed]
- Barros C.P., Guimarães J.T., Esmerino E.A., Duarte M.C.KH., Silva M.C., Silva R., Ferreira B.M., Sant’Ana A.S., Freitas M.Q., Cruz A.G. Paraprobiotics and postbiotics: concepts and potential applications in dairy products. Current Opin. Food Sci. 2020;32:1–8. [Google Scholar]
- Baud, D., Agri, V.D., Gibson, G.R., Reid, G., Giannoni, E., 2020. Using Probiotics to Flatten the Curve of Coronavirus Disease COVID-2019 Pandemic. Frontiers in Public Health 8,186, https://doi: 10.3389/fpubh.2020.00186 [DOI] [PMC free article] [PubMed]
- Bhaskar, S., Sinha, A., Banach, M., Mittoo, S., Weissert, R., Kass, J.S., Rajagopal, S., Pai, A.R., Kutty, S., 2020. Cytokine Storm in COVID-19—Immunopathological Mechanisms, Clinical Considerations, and Therapeutic Approaches: The REPROGRAM Consortium Position Paper. Front. Immunol. 11, 1648. https://doi.org/10.3389/fimmu.2020.01648 [DOI] [PMC free article] [PubMed]
- Bondy G.S., Pestka J.J. Immunomodulation by fungal toxins. J. Toxicol. Environ. Health Part B: Critical Rev. 2000;3:109–143. doi: 10.1080/109374000281113. [DOI] [PubMed] [Google Scholar]
- Bottari B., Castellone V., Neviani E. Probiotics and Covid-19. Int. J. Food Sci. Nutr. 2020;12:1–7. doi: 10.1080/09637486.2020.1807475. [DOI] [PubMed] [Google Scholar]
- Britton R. Lactobacillus reuteri, In: The microbiota in gastrointestinal pathophysiology: implications for human health, prebiotics, probiotics, and dysbiosis. Academic Press; 2017. pp. 89–97. [Google Scholar]
- Cao X. COVID-19: immunopathology and its implications for therapy. Nat. Rev. Immunol. 2020;20(5):269–270. doi: 10.1038/s41577-020-0308-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ceccarelli G., Scagnolari C., Pugliese F., Mastroianni C.M., d'Ettorre G. Probiotics and COVID-19. Lancet Gastroenterol. Hepatol. 2020;5(8):721–722. doi: 10.1016/S2468-1253(20)30196-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conte, L., Toraldo, D.M., 2020. Targeting the gut–lung microbiota axis by means of a high-fibre diet and probiotics may have anti-inflammatory effects in COVID-19 infection. Ther. Adv. Respir. Dis. https://doi.org/10.1177/1753466620937170 [DOI] [PMC free article] [PubMed]
- Crociani F., Biavati B., Alessandrini A., Chiarini C., Scardovi V. Bifidobacterium inopinatum sp. nov. and Bifidobacterium denticolens sp. nov., Two New Species Isolated from Human Dental Caries. Int. J. Syst. Bacteriol. 1996;46(2):564–571. doi: 10.1099/00207713-46-2-564. [DOI] [PubMed] [Google Scholar]
- Dhar D., Mohanty A. Gut microbiota and Covid-19- possible link and implications. Virus Res. 2020;285:198018. doi: 10.1016/j.virusres.2020.198018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edwards-Ingram L., Gitsham P., Burton N., Warhurst G., Clarke I., Hoyle D., Oliver S.G., Stateva L. Genotypic and Physiological Characterization of Saccharomyces boulardii, the Probiotic Strain of Saccharomyces cerevisiae. AEM. 2007;73(8):2458–2467. doi: 10.1128/AEM.02201-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erickson, K.L., Hubbard, N.E., 2000. Probiotic immunomodulation in health and disease. J. Nutr. 130, 403S-409S. [DOI] [PubMed]
- Fietto J.L.R., Araújo R.S., Valadão F.N., Fietto L.G., Brandão R.L., Neves M.J., Gomes F.C.O., Nicoli J.R., Castro I.M. Molecular and physiological comparisons between Saccharomyces cerevisiae and Saccharomyces boulardii. Can. J. Microbiol. 2004;50(8):615–621. doi: 10.1139/w04-050. [DOI] [PubMed] [Google Scholar]
- Gavini, F., Delcenserie, V., Kopeinig, K., Pollinger, S., Beerens, H., Bonaparte, C., Upmann, M., 2006. Bifidobacterium species isolated from animal feces and from beef and pork meat. J. Food Prot. 69, 871-877. [DOI] [PubMed]
- Gea-Banacloche J.C. Immunomodulation. In: Runge M.S., Cam P., editors. Principles of molecular medicine. Springer. Springer Science and Business Media; 2006. pp. 893–904. [Google Scholar]
- Hadadji M., Benama R., Saidi N., Henni D.E., Kihal M. Identification of cultivable Bifidobacterium species isolated from breast-fed infants feces in West-Algeria. Afr. J. Biotechnol. 2005;4:422–430. [Google Scholar]
- Hamann L., EL-Samalouti V., Ulmer A.J., Flad H.-D., Rietschel E.T. Components of gut bacteria as immunomodulators. Int. J. Food Microbiol. 1998;41(2):141–154. doi: 10.1016/s0168-1605(98)00047-6. [DOI] [PubMed] [Google Scholar]
- Hammes W.P., Vogel R.F. The genus lactobacillus. In: Wood B.J.B., Holzapfel W.H., editors. The genus lactobacillusThe genera of lactic acid bacteria. Springer; 1995. pp. 19–54. [Google Scholar]
- Han M.K., McLaughlin V.V., Criner G.J., Martinez F.J. Pulmonary diseases and the heart. Circulation. 2007;116(25):2992–3005. doi: 10.1161/CIRCULATIONAHA.106.685206. [DOI] [PubMed] [Google Scholar]
- He L.H., Ren L.F., Li J.F., Wu Y.N., Li X., Zhang L. Intestinal flora as a potential strategy to fight SARS-CoV-2 infection. Front Microbio. 2020;11(1388) doi: 10.3389/fmicb.2020.01388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill D., Sugrue I., Tobin C., Hill C., Stanton C., Ross R.P. The Lactobacillus casei group: history and health related applications. Front. Microbiol. 2018;9:2107. doi: 10.3389/fmicb.2018.02107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hotel A.C.P., Cordoba A. Health and nutritional properties of probiotics in food including powder milk with live lactic acid bacteria. Prevention. 2001;5:1–10. [Google Scholar]
- Huang C., Wang Y., Li X., Ren L., Zhao J., Hu Y.i., Zhang L.i., Fan G., Xu J., Gu X., Cheng Z., Yu T., Xia J., Wei Y., Wu W., Xie X., Yin W., Li H., Liu M., Xiao Y., Gao H., Guo L.i., Xie J., Wang G., Jiang R., Gao Z., Jin Q.i., Wang J., Cao B. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. The Lancet. 2020;395(10223):497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hubbell J.A., Thomas S.N., Swartz M.A. Materials engineering for immunomodulation. Nature. 2009;462(7272):449–460. doi: 10.1038/nature08604. [DOI] [PubMed] [Google Scholar]
- Infusino, F., Marazzato, M., Mancone, M., Fedele, F., Mastroianni, C.M., Severino, P., Ceccarelli, G., Santinelli, L., Cavarretta, E., Marullo, A.G., Miraldi, F., 2020. Diet Supplementation, Probiotics, and Nutraceuticals in SARS-CoV-2 Infection: A Scoping Review. Nutrients, 12(6), p.1718. [DOI] [PMC free article] [PubMed]
- Isolauri E., Salminen S., Ouwehand A.C. Best Pract Res Clin Gastroenterol. 2004;18:299–313. doi: 10.1016/j.bpg.2003.10.006. [DOI] [PubMed] [Google Scholar]
- Jayawardena R., Sooriyaarachchi P., Chourdakis M., Jeewandara C., Ranasinghe P. Enhancing immunity in viral infections, with special emphasis on COVID-19: A review. Diabet. Metabol. Syndrome Clin. Res. Rev. 2020;14(4):367–382. doi: 10.1016/j.dsx.2020.04.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones S.E., Versalovic J. Probiotic Lactobacillus reuteri biofilms produce antimicrobial and anti-inflammatory factors. BMC Microbiol. 2009;9(1):35. doi: 10.1186/1471-2180-9-35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khatri I., Tomar R., Ganesan K., Prasad G.S., Subramanian S. Complete genome sequence and comparative genomics of the probiotic yeast Saccharomyces boulardii. Sci. Rep. 2017;7(1) doi: 10.1038/s41598-017-00414-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee J.-Y., Han G.G., Choi J., Jin G.-D., Kang S.-K., Chae B.J., Kim E.B., Choi Y.-J. Pan-Genomic Approaches in Lactobacillus reuteri as a Porcine Probiotic: Investigation of Host Adaptation and Antipathogenic Activity. Microb. Ecol. 2017;74(3):709–721. doi: 10.1007/s00248-017-0977-z. [DOI] [PubMed] [Google Scholar]
- Mackowiak P.A. Recycling Metchnikoff: probiotics, the intestinal microbiome and the quest for long life. Front. Public Health. 2013;62:1–3. doi: 10.3389/fpubh.2013.00052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mak J.W.Y., Chan F.K.L., Ng S.C. Probiotics and COVID-19: one size does not fit all. Lancet Gastroenterol. Hepatol. 2020;5(7):644–645. doi: 10.1016/S2468-1253(20)30122-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mattarelli P., Bonaparte C., Pot B., Biavati B. Proposal to reclassify the three biotypes of Bifidobacterium longum as three subspecies: Bifidobacterium longum subsp. longum subsp. nov., Bifidobacterium longum subsp. infantis comb. nov. and Bifidobacterium longum subsp. suis comb. nov. Int. J. Systemat. Evolution. Microbiol. 2008;58(4):767–772. doi: 10.1099/ijs.0.65319-0. [DOI] [PubMed] [Google Scholar]
- Nair A., Chattopadhyay D., Saha B. New Look to Phytomedicine. Elsevier; 2019. Plant-derived immunomodulators; pp. 435–499. [Google Scholar]
- Quigley, E. 2017. Bifidobacterium animalis spp. lactis, In: The Microbiota in Gastrointestinal Pathophysiology, edited by Floch, M., Yehuda R. and Walker W.A Elsevier, 127-130.
- Ragab, D., Salah Eldin, H., Taeimah, M., Khattab, R., Salem, R., 2020. The COVID-19 cytokine storm; what we know so far. Front. Immunol. https://doi.org/10.3389/fimmu.2020.01446. [DOI] [PMC free article] [PubMed]
- Rivera-Espinoza Y., Gallardo-Navarro Y. Non-dairy probiotic products. Food Microbiol. 2010;27(1):1–11. doi: 10.1016/j.fm.2008.06.008. [DOI] [PubMed] [Google Scholar]
- Roberfroid, M., 2007. Prebiotics: the concept revisited. J. Nutr. 137, 830S-837S. [DOI] [PubMed]
- Ruiz-Aceituno L., Esteban-Torres M., James K., Moreno F.J., van Sinderen D. Metabolism of biosynthetic oligosaccharides by human-derived Bifidobacterium breve UCC2003 and Bifidobacterium longum NCIMB 8809. Int. J. Food Microbiol. 2020;316:108476. doi: 10.1016/j.ijfoodmicro.2019.108476. [DOI] [PubMed] [Google Scholar]
- Saarela M., Mogensen G., Fondén R., Mättö J., Mattila-Sandholm T. Probiotic bacteria: safety, functional and technological properties. J. Biotechnol. 2000;84(3):197–215. doi: 10.1016/s0168-1656(00)00375-8. [DOI] [PubMed] [Google Scholar]
- Schmitter T., Fiebich B.L., Fischer J.T., Gajfulin M., Larsson N., Rose T., Goetz M.R. Ex vivo anti-inflammatory effects of probiotics for periodontal health. J. Oral Microbiol. 2018;10(1):1502027. doi: 10.1080/20002297.2018.1502027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schumann P., Maier T. MALDI-TOF mass spectrometry applied to classification and identification of bacteria. Meth. Microbiol. 2014;41:275–306. [Google Scholar]
- Sun Z., Harris H.M.B., McCann A., Guo C., Argimón S., Zhang W., Yang X., Jeffery I.B., Cooney J.C., Kagawa T.F., Liu W., Song Y., Salvetti E., Wrobel A., Rasinkangas P., Parkhill J., Rea M.C., O’Sullivan O., Ritari J., Douillard F.P., Paul Ross R., Yang R., Briner A.E., Felis G.E., de Vos W.M., Barrangou R., Klaenhammer T.R., Caufield P.W., Cui Y., Zhang H., O’Toole P.W. Expanding the biotechnology potential of lactobacilli through comparative genomics of 213 strains and associated genera. Nat. Commun. 2015;6(1) doi: 10.1038/ncomms9322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sundararaman A., Ray M., Ravindra P.V., Halami P.M. Role of probiotics to combat viral infections with emphasis on COVID-19. Appl. Microbiol. Biotechnol. 2020;104(19):8089–8104. doi: 10.1007/s00253-020-10832-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tiago, F.d.C.P., Martins, F.d.S., Souza, E., Pimenta, P.F.P., Araújo, H.R.C., Castro, I.d.M., Brandão, R.L., Nicoli, J.R., 2012. Adhesion to the yeast cell surface as a mechanism for trapping pathogenic bacteria by Saccharomyces probiotics. J. Med. Microbiol. 61, 1194-1207. [DOI] [PubMed]
- Xiao J.Z., Kondo S., Takahashi N., Miyaji K., Oshida K., Hiramatsu A., Iwatsuki K., Kokubo S., Hosono A. Effects of Milk Products Fermented by Bifidobacterium longum on Blood Lipids in Rats and Healthy Adult Male Volunteers. J. Dairy Sci. 2003;86(7):2452–2461. doi: 10.3168/jds.S0022-0302(03)73839-9. [DOI] [PubMed] [Google Scholar]
- Xu K., Cai H., Shen Y., Ni Q., Chen Y., Hu S., Li J., Wang H., Yu L., Huang H. Management of corona virus disease-19 (COVID-19): the Zhejiang experience. J. Zhejiang Univ. (medical science) 2020;49 doi: 10.3785/j.issn.1008-9292.2020.02.02. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu, X.-W., Wu, X.-X., Jiang, X.-G., Xu, K.-J., Ying, L.-J., Ma, C.-L., Li, S.-B., Wang, H.-Y., Zhang, S., Gao, H.-N., 2020b. Clinical findings in a group of patients infected with the 2019 novel coronavirus (SARS-Cov-2) outside of Wuhan, China: retrospective case series. bmj http://dx.doi.org/10.1136/bmj.m606. [DOI] [PMC free article] [PubMed]
- Yan F., Polk D.B. Probiotics and immune health: Current Opin. Gastroenterol. 2011;27(6):496–501. doi: 10.1097/MOG.0b013e32834baa4d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu H.-S., Lee N.-K., Choi A.-J., Choe J.-S., Bae C.H., Paik H.-D. Anti-inflammatory potential of probiotic strain Weissella cibaria JW15 isolated from Kimchi through regulation of NF-κB and MAPKs pathways in LPS-induced RAW 264. 7 Cells. J. Microbiol. Biotechn. 2019;29:1022–1032. doi: 10.4014/jmb.1903.03014. [DOI] [PubMed] [Google Scholar]
- Yu, L., Tong, Y., Shen, G., Fu, A., Lai, Y., Zhou, X., Yuan, Y., Wang, Y., Pan, Y., Yu, Z., 2020. Immunodepletion with Hypoxemia: A Potential High Risk Subtype of Coronavirus Disease 2019. medRxiv. https://doi.org/10.1101/2020.03.03.20030650.