Abstract
Liver fibrosis occurs due to liver injuries and toxins. Silymarin (SMR) extracted by the milk thistle seeds, is widely used such as herbal drug for its hepatoprotective properties. The purpose of this study to assess the properties of an optimized dose of encapsulated crude SMR on antidiabetic activity and liver fibrosis induced by paracetamol in male albino rat. Hepatic fibrosis was assessed by measuring liver enzymes. Results revealed that the consumption of encapsulated SMR, can effectively affluence the target and avoid the degradation of bioactive compound. Body weight of animal also significantly increased in each group during all the period. According to our optimized study, the long-term induction of SMR (300 mg/kg) significantly amplified survival time of rats with paracetamol induced hepatic injuries. The changes of liver fibrosis and the significant increase of hepatic enzyme biomarkers were also observed. In conclusion, the results suggest that SMR acts as a hepatoprotective agent by inhibiting the fibrogenisis and apoptosis in liver, as well as insulin resistance.
Keywords: Hepatic enzymes, Fibrogenisis, Hepatoprotective, Insulin resistance
1. Introduction
Silymarin, a hepatoprotective agent and bioactive compound of Silybum Marianum (family Asteraceae), is a light yellow incompact powder obtained from the milk thistle seed, extracted by using organic solvent. SMR is renowned for its high clinical efficacy and use in herbal treatment of chronic liver disease (Weiss and Meuss, 2001). No health hazards are known in aggregation, along with properly managed compounds (Shaker et al., 2010). The main constituents of SMR are flavonolignans, which include toxifolin, silychristin, SDN, silybinin A, silybinin B, isosilybinin A and isosilybinin B (Li and Hu, 2004) (Campodónico et al., 2001). SMR has poor aqueous solubility; solid dispersions are generally used to improve the suspension and bioavailability in digestion (Blumenthal et al., 2000). Demonstration of modern pharmaceutical technology to increase the bioavailability of SMR compared to suspension containing raw materials (Wu et al., 2006, Woo et al., 2007, Javed et al., 2011, Hwang et al., 2014). SMR acts as an antioxidant, and promotes the growth of new liver cells (Sarwar et al., 2011) while also acting as a stimulant to the regeneration and detoxification of the liver (Bhattacharya, 2011). SMR and its isomers are used to treat hepatitis and liver damage due to excessive intake of alcohol. SMR work against oxidative stress, inflammatory responses and benzoil peroxide induced fibrosis promote in the mice
SMR effect against the Oxidative Stress, Inflammatory Response and Fibrosis of Benzoic Acid Peroxide tempted fibrosis stimulate in Mice (Katiyar, 2005). A standardized extract should contain 99% of SMR. The usual dosage of milk thistle extract is approximately 300–600 mg/day. SMR is very effective against hepatotoxicity caused by carbon tetrachloride (CCL4) and paracetamol due to the high antioxidant assets (Dumbravă et al., 2008). SMR are accomplished through numerous mechanisms, including the inhibition of lipid peroxidation, antioxidant, and protection of glutathione depletion also enhancing Liver Detoxification by Inhibiting First phase detoxification (Batakov, 2001).
Due to the limited availability of data on optimized and standard dosage of SMR, an initial dose-ranging study was performed to identify these measurements. In turn, the optimized dosage was used to enhance the stability of SMR when digested in an encapsulated form, and to prevent the degradation of the bioactive compound. The sole focus of our research is on assessing the potential role of SMR capsules as a protective agent against paracetamol drugs that lead to liver fibrosis and necrosis.
2. Material and methods
Milk thistle seed were collect from Xian, China. Laboratory animal were purchased from NIAB Laboratory Islamabad, Pakistan. Paracetamol purchased from SIGMA pharmaceutical. Complete blood count (CBCs) were tested from laboratory, Pakisan. Histopathological analysis was examined by Ishak-modified histological index scoring system.
2.1. Formulation of SMR encapsulates
SMR extraction was carried out with 80% ethanol, and lyophilized. SMR encapsulation was prepared by the following method (Gorbunova et al., 2018). Briefly, 1 g SMR and 0.1 g sodium alginate was mixed in 100 mL deionized water with constant exciting for 1 h at 500 rpm and 1 h at rest, to allow any of bubbles to the surface. Once homogenized, it was drip into a 100 mL cold solution containing 1.5calcium chloride dissolved in 100 mL deionized water, and kept in 5 C for 2 h. Beads formed in the solution remained at rest for 30 min, then were filter and dried in open air for 10 h. Vacuum drying followed for 4 h, and stored in airtight containers for further experiments.
2.2. Phytochemical screening
The content of some phytochemicals i.e saponins, tannins, alkaloids, steroids, cardiac glycosides and flavonoids in ethanol extracted alginate based beads of SMR was determined by standard procedure.
2.3. Animals
96 Male Albino rats, age 4 weeks (180–200 g), were attained from the Agriculture Research Center (Islamabad, Pakistan). Animals were located under precise without pathogen conditions with 12 h light-dark cycles, with 50% relative humidity and constant temperature (25 ± 2 °C). They received human care in compliance with the National Institutes of Health guide for care and use of laboratory animals (no.8023, revised 1978) at Ayub Research Center (Pakistan). The basal diet ratio: CHO 58%, protein 17.5%, fat 3.4%, moisture 11% cellulose 3.1%, minerals 1.49%, 0.9 and 0.59% calcium and phosphorus 0.59%
2.4. Experimental plan of study
Encapsulated SMR was mixed with rat pellet diet and administered to the animals orally. SMR as average defense was managed to the rats. Paracetamol was obtained from SIGMA pharmaceuticals.. The animals were introduced a 2 week acclimation period. During this, they maintained a normal rat pellet food, with 3 groups having a mean weight 170 ± 2 g. Furthermore, 6 treatments were randomly allocated to the mean weight delivery as follows:
Group 1: Normal rats fed with basal diet and SMR
Group 2: Hepatic impaired rats fed with basal diet and paracetamol 2 mg/kg for 2 week, SMR capsules
Group 3: Diabetic rats fed with basal diet with high sucrose + paracetamol 2 mg/kg for 2 week, SMR capsules
After 5 weeks of administering the drug, collect the blood and serum had separated. The liver and kidney was instantly removed and fixed into 10% formalin for the histopathological examination.
2.5. Paracetamol induction of hepatotoxicity
Hepatotoxicity was induced in rats with the method (Zakaria et al., 2011). Paracetamol used in this study was kept from SIGMA pharmaceuticals and attained as a pure concentration 100%.
2.6. Acute toxicity studies
Acute toxicity studies were approved to assess the approximate of the average median lethal dose of SMR extracts in albino rats (25–30 g). Divide rats into 8 groups, 3 rats per group, SML and SMS extracts (100, 300, 500, 700, 900 mg/kg) were given intraperitoneally. Treated animals were monitored for 24 h to understand toxic mortality and behavioral changes.
2.7. Biochemical analysis
The experiment continued for 35 days, and concluded with an assessment of food ingesting and body weight. The animals stood sacrificed, besides blood sampling occurred through a cardiac puncture into the orbital plexus. Once the blood began to clot, it was done centrifuged at 3000 rpm for 15 min, and the serum was reserved at 20 °C until required. The rats were euthanasia, their liver and kidneys were carefully excised, thoroughly rinsed with saline, and histopathologically examined. The biochemical markers from the blood serum included RBCs, WBCs, Platelate count, Glutamic-oxaloacetic (AST), glutamic-pyruvic (ALT) transaminase and alkaline phosphatase (ALP). They determined through the use of commercial kits.
2.8. Glutathione and liver TBARS estimation
An estimate of 5,5-dithio-2-nitrobenzoic acid (DTNB) in blood and liver glutathione was made according to method describe in kit. Using TBARS as catalogue of lipid peroxidation was determined by an improved with kit method. Protein in the tissue homogenization was also assessed. The level of TBARS was nmol MDA/mg protein.
2.9. Histopathological examination
Autopsy samples were taken from the liver and kidneys of each group of rats and fixed in 10% of the official saline for 24 h. The sample is rinsed in distilled water and then dehydrated by continuous dilution of absolute ethanol. The specimen was removed in xylene and embedded in 560C paraffin for 24 h in a hot air oven. The tissue was collected on a glass slide and stained with deparaffinized and stained by both hematoxylin and for histopathological investigation. The tissue was collected on a glass slide and stained with oxygenated nuclide. The assessment was done through a light microscope, following the Ishak-modified histological guide scoring system (Ishak et al., 1995), reported by Prof. Dr. Naveed Shezaad, Dept., Histophathology.
2.10. Statistical analysis
The results were median is standard deviation (*SD). The group mean was compared using the one-way analysis of variance (ANOVA). The values of histopathological examination were analyzed by nonparametric Mann-Whitney U test. P < 0.05 is considered to be an indication of a statistically significant difference.
3. Results
3.1. Phytochemical constituents in SMR beads
Preliminary phytochemical screening of extracts from SMR showed the presence of alkaloids, reducing sugar, saponins and tannins (Table 1). SMR extract contained more saponin and flavonoids, with respect to other qualitative tests.
Table 1.
Phytochemical constituents of sodium alginate based SMR beads.
| Phytochemical | Observation | SMR |
|---|---|---|
| Flavonoids | Resultant solution turn yellow | +++ |
| Sponins | Persistent froth unbroke upon standing | ++ |
| Tannin | Blue black precipitate | + |
| Reducing sugar | Reddish brown precipitate upon heating | + |
(+) to (+++) = detected in moderate to abundant quantities.
3.2. Acute toxicity of SMR in rats
The treated animals were monitored for mortality, and after a single dose (intraperitneally) extraction of SMR extract (100–900 mg/kg) from the abdominal cavity, No signs of toxicity were observed for even 24 h after administration of extract. Therefore, we choose 5 doses, 100–500 maximum dose tests (900 mg/kg) as the experimental dose.
3.3. Hepatoprotection study
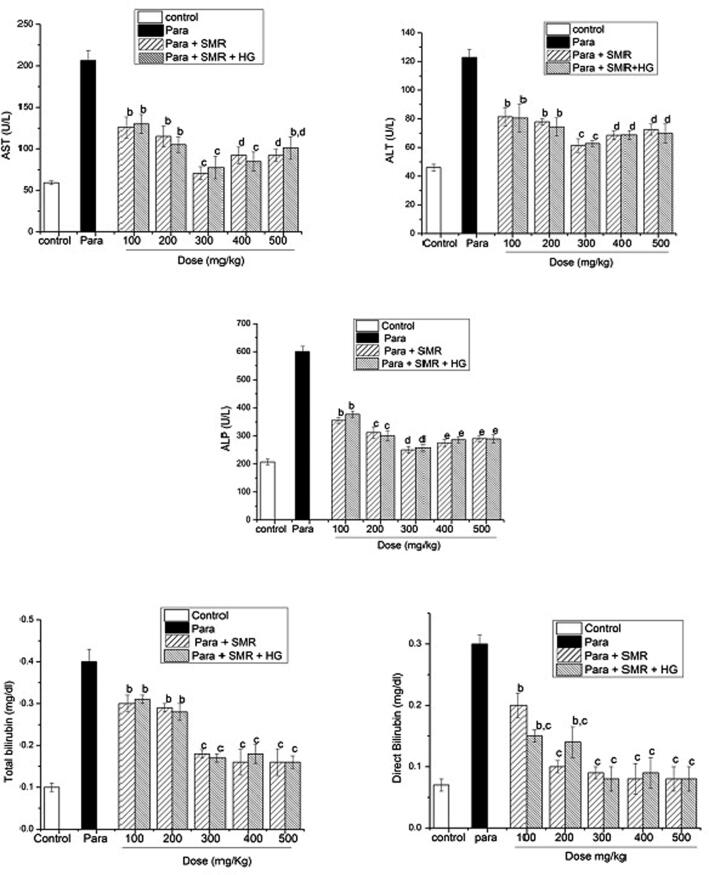
According to our optimized study, the long-term introducing of SMR significantly increased survival time of animals with paracetamol induced liver injuries. It was a challenge to induce the toxicity by paracetamol and elevation of increase in hepatic enzymes about two fold from normal (Fig. 1). As shown in the figure, paracetamol was administered for 10–14 days. The value of ALT indicates the amount of liver damage (Table.2). Serum ALT levels differ significantly (median, 45.98 ± 2.3 U/L placebo; 61.33 ± 4.7 U/L for 300 mg SMR with hepatic; and 62.78 ± 2.03 U/L for 300 mg SMR with hepatic and diabetic rats; P = 0.90) across the treatment group compared to the paracetamol group (122 ± 5.65 U/L for ALT), (206.33 ± 11.98 U/L for AST) and (601 ± 18.25 for ALP U/L), p < 0.05.
Fig. 1.
LFTs in paracetamol induced toxicity and SMR treatment.
Table 2.
Effect of SMR (300 mg/kg) on serum alanine aminotransferase (ALT), aspartate amino transferase (AST), alkaline phosphatase (ALP) activities in paracetamol induced liver fibrosis in rat.
| Groups | AST Median ± SD |
ALP Median ± SD |
ALT Median ± SD |
HCV | GSH Median ± SD |
TBARs Median ± SD |
|---|---|---|---|---|---|---|
| Control | 59.24 ± 1.89a | 206.89 ± 9.761a | 45.98 ± 2.35a | Negative | 0.535 ± 0.08a | 61.358 ± 21.22a |
| Paracetamol | 206.33 ± 11.98b | 601 ± 18.25b | 122 ± 5.65b | Positive | 0.237 ± 0.06b | 11.128 ± 56.55b |
| SMR + Paracetamol | 70.44 ± 7.84bc | 250 ± 11.97bc | 61.33 ± 4.78bc | Negative | 0.307 ± 0.10bc | 56.55 ± 15.78bc |
| SMR + paracetamol + HG | 77.31 ± 13.5 cd | 258 ± 12.76 cd | 62.78 ± 2.03 cd | Negative | 0.389 ± 0.08 cd | 60.061 ± 12.49 cd |
Values expressed as mean ± SD (n = 6). Significant difference vs. a respective control, b respective paracetamol, c represented SMR, d respective diabetic groups, each at p < 0.05.
Significant reduction in tissue TBARS by introduce paracetamol. GSH was also significantly increased in silymarin induced tissues (P < 0.05) (Table 1). In group of silymarin and diabetics significantly increased the tissue TBARS. Glutathione is an important endogenous antioxidant system originates in particularly high concentrations in the liver and is identified to have key functions during the protective process. The reduction form of GSH can be easily oxidized to GSSG when it interacts with free radicals. Overproduction of free radicals can prime to oxidative stress, damage of macromolecules such as lipids, and induce lipid peroxidation in the body.
In present study, galactosamine treatment produced level of TBARS and exhaustion in glutathione (GSH) consumption in altitude. TBARS levels were significantly decreased and GSH concentrations were increased in rats treated with SMR capsules. These results revealed that the hepatoprotective outcome of SMR may be because the presence of high level of antioxidants, i.e. flavonoids, vitamin A, vitamin C and α- and β-carotenes. The level of ALT enzyme indicates the degree of cell membrane damage, whereas the level of AST indicates mitochondrial damage. Significant results seen after 5 weeks, SMR protect the liver cells against paracetamol induced and also reduced the levels of total bilirubin (Fig. 1).
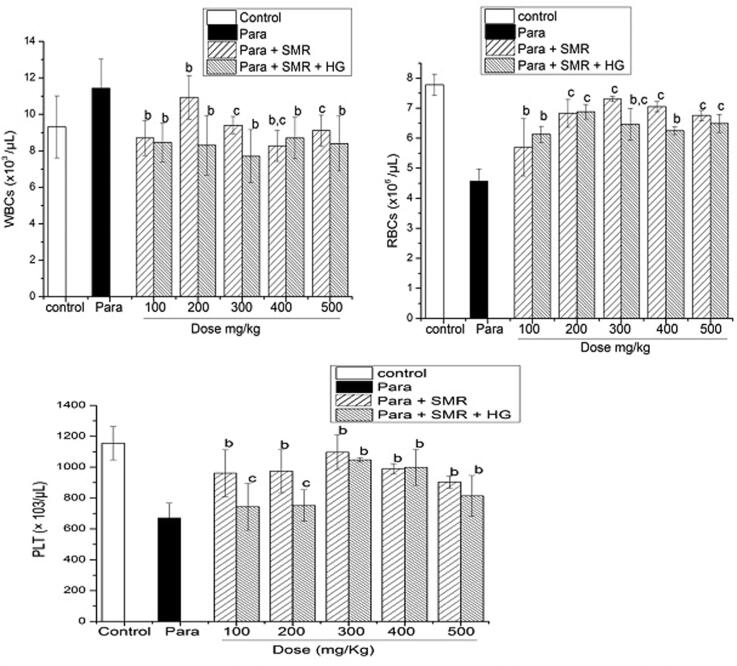
However, markers suggested advanced liver disease (serum bilirubin levels, Platelet counts and CBCs Complete blood cell counts) differed across the treatment groups. TBCs and blood markers in the paracetamol induced rats given the SMR treatment also indicated change (Fig. 2). Values expressed as mean displayed significant differences p < 0.05 (Table 3).
Fig. 2.
Total blood count TBCs in paracetamol induced toxicity and SMR treatment.
Table 3.
Effect of SMR (300 mg/kg) on total blood count (TBCs) in paracitamol induced liver fibrosis in rat.
| Groups | Control | Paracetamol | SMR + Paracetamol | SMR + Paracetamol + HG |
|---|---|---|---|---|
| Sugar | 92 ± 3.4 | 136 ± 11.2 | 90 ± 6.89 | 110 ± 5.76 |
| Cholesterol | 155 ± 6.71 | 206 ± 2.7 | 199 ± 1.33 | 200 ± 3.71 |
| HGB g/dL | 14.4 ± 1.08 | 13.9 ± 2.14 | 14.1 ± 2.35 | 12.7 ± 1.44 |
| HCT % | 38.4 ± 2.98 | 45.0 ± 7.2 | 43.3 ± 5.65 | 39.7 ± 3.89 |
| MCV(fL) | −54.9 ± 7.84 | −65.0 ± 3.4 | 61.33 ± 4.78 | 63.8 ± 7.6 |
| LYM % | 87.7 ± 11.5 | 92.9 ± 12.76 | 80.9 ± 2.03 | 78.5 ± 5.4 |
| MXD % | 0.0 ± 0.001 | 0.0 ± 0.00 | 0.0 ± 0.00 | 0.0 ± 0.001 |
| NEUT (%) | 12.3 ± 0.07 | 7.1 ± 0.02 | 19.1 ± 0.04 | 21.5 ± 1.22 |
| RDW-CV % | 19.1 ± 0.05 | 21.9 ± 0.03 | 21.2 ± 0.01 | 20.0 ± 0.07 |
| P-LCR % | 5.7 ± 0.021 | 5.2 ± 0.014 | 5.4 ± 0.019 | 6.3 ± 0.014 |
| PCT % | 0.70 ± 0.01 | 0.66 ± 0.003 | 0.61 ± 0.005 | 0.81 ± 0.012 |
Values expressed as mean ± SD (n = 6) each at p < 0.05.
3.4. Histopathological analysis
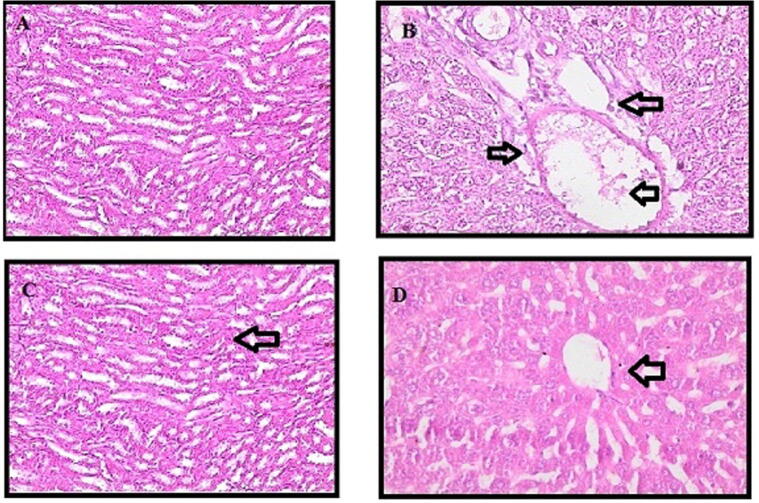
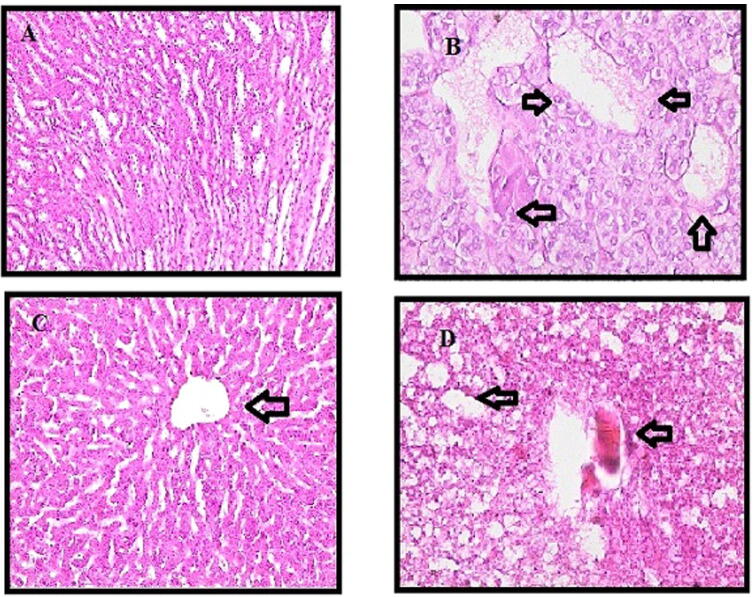
Histologic examination confirmed the results we obtained from serum and tissue analysis. Paracetamo intoxication demonstrates fat accumulation and liver cell fibrosis. In the control group, liver (Fig. 3) and kidney (Fig. 4) segments had normal hepatic cells with conserved cytoplasm and the central vein. The stained slides para +100 mg the SMR group showed fibrous expansion of more portal areas, with occasional portal to portal bridging. SMR group para +300 mg showed some portal areas of fibrous dilation have no fibrosis of the septum. Fibrosis scoring following the Ishak score table is shown below (Table 4).
Fig. 3.
Histopathological capture picture of smooth muscles actin (α- SMA) protein expression in liver parenchyma.
Fig. 4.
Representative capture picture showing effect of SMR on paracetamol induced changes of kidney tissues.
Table 4.
Effect of SMR (300 mg/kg) on fibrosis and necroinflamation scores in paracetamol induced liver fibrosis in rats.
| Groups | Fibrosis Mean ± SD |
Necroinflammation Mean ± SD |
|---|---|---|
| Control | 0.0 | 0.0 |
| Paracetamol | 3.23 ± 0.015 | 7.8 ± 0.18 |
| SMR + Paracetamol | 1.89 ± 0.31 | 4.11 ± 0.28 |
| SMR + paracetamol + diabetic | 2.01 ± 0.35 | 3.63 ± 0.3 |
Values expressed as Mean ± SD (n = 6) significance difference at p < 0.05.
The results also revealed that ballooning degeneration is present under all doses in both the hepatic and diabetic groups, and also indicated necrosis in some areas. However, the SMR treatment produced no inflammation. Therefore, some scattered apoptotic bodies are seen in the para group, but the architecture is preserved and no apoptosis is seen in the 300 mg SMR + para group. Treatment with 300 mg SMR in both hepatic and hepatic-diabetic groups revealed that fatty accumulation, sinusoidal congestion, and dilatation in the central vein were all prevented.
4. Discussion
Increasing stability and bioavailability through the incorporation of extracts into a biopolymer matrix is an effective way to protect health benefits (Nedovic et al., 2011, Munin and Edwards-Lévy, 2011, Nedovic et al., 2011, Fang and Bhandari, 2010). Liver fibrosis is the foremost cause of morbidity and mortality worldwide, its most common association being chronic liver damage. It may develop into cirrhosis within 1–10 years (Hernandez-Gea and Friedman, 2011). Therefore, preventing the progression of fibrosis may be an effective survival strategy. The present study is intended to evaluate the antifibrotic and hepatoprotective effect of SMR with the optimized dose against hepatocytotoxin in paracetamol induced liver fibrosis in rats.
A high dose of paracetamol may causes the toxicity in liver by forming toxic metabolites (Bhondave et al., 2014). This is decontaminated in the body by the development of a conjugate, correlated with membrane damage and rise in AST, ALT and bilirubin levels. Subsequently, non-metabolic toxicity products bind to protein covalently and leading to the toxicity (Hsiang et al., 2015, Kumar et al., 2014). Paracetamol has been described to produced strong inflammatory penetration in the liver parenchyma and surrounding areas. In this study, paracetamol induced (Group II) exhibited high toxicity in liver, with strong penetration of inflammatory cells around the portal vein and also in the hepatic parenchyma cells. The liver of rats was protected from the histopathological changes induced by paracetamol by 1 week of pretreatment with SMR.
In one vitro study (Sonnenbichler et al., 1999), the results showed that the management of silybin before and after chemical injury could reduce nephrotoxicity and sugar levels. Similarly, SMR reduces sugar levels and the overall toxic effect. Paracetamol toxication resulted ballooning and necrosis in the hepatocytes of the liver parenchyma cells. These findings justify that SMR is effective in healing hepatocytes injuries, and serves as a preventative measure against the risk of diabetes. These results recommend that SMR has an obvious defensive effect on paracetamol-induced hepatotoxicity in rats. Since this experimental model is surprisingly similar to human viral hepatitis
5. Conclusion
SMR can exert membrane stability and antioxidant activity. It promotes hepatocyte regeneration and reduces the risk factors of type 2 diabetes. In addition, it inhibits liver fibrosis. According to our optimized study, long-term use of SMR can significantly increased the survival time of rats with paracetamol-induced liver injury. SMR can reduce tumor cell proliferation, as well as insulin resistance. The compound has beneficial effects on the balance of cell survival, fibrosis and apoptosis.
Ethics approval and consent to participate
Animal treated as ethics of National Institutes of Health guide for care and use of laboratory animals (no.8023, revised 1978).
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgement
The authors extend their appreciation to the Researchers supporting project number (RSP-2020/94) King Saud University, Riyadh, Saudi Arabia.
Footnotes
Peer review under responsibility of King Saud University.
Contributor Information
Shanza Mukhtar, Email: 2015208034@njau.edu.cn.
Muhammad Samee Mubarik, Email: sameech2002@yahoo.com.
References
- Batakov E.A. Effect of Silybum marianum oil and legalon on lipid peroxidation and liver antioxidant systems in rats intoxicated with carbon tetrachloride. Eksp. Klin. Farmakol. 2001;64:53–55. [PubMed] [Google Scholar]
- Bhattacharya, S., 2011. Milk thistle (Silybum marianum L. Gaert.) seeds in health. In: Nuts and Seeds in Health and Disease Prevention. Elsevier, pp. 759–766.
- Bhondave P.D., Devarshi P.P., Mahadik K.R., Harsulkar A.M. ‘Ashvagandharishta’ prepared using yeast consortium from Woodfordia fruticosa flowers exhibit hepatoprotective effect on CCl4 induced liver damage in Wistar rats. J. Ethnopharmacol. 2014;151:183–190. doi: 10.1016/j.jep.2013.10.025. [DOI] [PubMed] [Google Scholar]
- Blumenthal, M., Goldberg, A., Brinckmann, J., 2000. Herbal Medicine. Expanded Commission E monographs. Integrative Medicine Communications.
- Campodónico A., Collado E., Ricci R., Pappa H., Segall A., Pizzorno M.T. Dissolution test for silymarin tablets and capsules. Drug Dev. Ind. Pharm. 2001;27:261–265. doi: 10.1081/ddc-100000244. [DOI] [PubMed] [Google Scholar]
- Dumbravă D.G., Hădărugă N.G., Hădărugă D.I., Gruia A., Tatu C., Păunescu V., Lupea A.X. Antioxidant activity of some celandine (Chelidonium majus L.) carotenoidic extracts. J. Agroaliment. Process. Technol. 2008;14:433–441. [Google Scholar]
- Fang Z., Bhandari B. Encapsulation of polyphenols–a review. Trends Food Sci. Technol. 2010;21:510–523. [Google Scholar]
- Gorbunova N., Bannikova A., Evteev A., Evdokimov I., Kasapis S. Alginate-based encapsulation of extracts from beta Vulgaris cv. beet greens: stability and controlled release under simulated gastrointestinal conditions. LWT. 2018;93:442–449. [Google Scholar]
- Hernandez-Gea V., Friedman S.L. Pathogenesis of liver fibrosis. Annu. Rev. Pathol. Mech. Dis. 2011;6:425–456. doi: 10.1146/annurev-pathol-011110-130246. [DOI] [PubMed] [Google Scholar]
- Hsiang C.-Y., Lin L.-J., Kao S.-T., Lo H.-Y., Chou S.-T., Ho T.-Y. Glycyrrhizin, silymarin, and ursodeoxycholic acid regulate a common hepatoprotective pathway in HepG2 cells. Phytomedicine. 2015;22:768–777. doi: 10.1016/j.phymed.2015.05.053. [DOI] [PubMed] [Google Scholar]
- Hwang D.H., Kim Y.-I., Cho K.H., Poudel B.K., Choi J.Y., Kim D.-W., Shin Y.-J., Bae O.-N., Yousaf A.M., Yong C.S. A novel solid dispersion system for natural product-loaded medicine: silymarin-loaded solid dispersion with enhanced oral bioavailability and hepatoprotective activity. J. Microencapsul. 2014;31:619–626. doi: 10.3109/02652048.2014.911375. [DOI] [PubMed] [Google Scholar]
- Ishak K., Baptista A., Bianchi L., Callea F., De Groote J., Gudat F., Denk H., Desmet V., Korb G., MacSween R.N.M. Histological grading and staging of chronic hepatitis. J. Hepatol. 1995;22:696–699. doi: 10.1016/0168-8278(95)80226-6. [DOI] [PubMed] [Google Scholar]
- Javed S., Kohli K., Ali M. Reassessing bioavailability of silymarin. Altern. Med. Rev. 2011;16:239–250. [PubMed] [Google Scholar]
- Katiyar S.K. Silymarin and skin cancer prevention: anti-inflammatory, antioxidant and immunomodulatory effects. Int. J. Oncol. 2005;26:169–176. [PubMed] [Google Scholar]
- Kumar N., Rai A., Reddy N.D., Raj P.V., Jain P., Deshpande P., Mathew G., Kutty N.G., Udupa N., Rao C.M. Silymarin liposomes improves oral bioavailability of silybin besides targeting hepatocytes, and immune cells. Pharmacol. Reports. 2014;66:788–798. doi: 10.1016/j.pharep.2014.04.007. [DOI] [PubMed] [Google Scholar]
- Li F.-Q., Hu J.-H. Improvement of the dissolution rate of silymarin by means of solid dispersions. Chem. Pharm. Bull. 2004;52:972–973. doi: 10.1248/cpb.52.972. [DOI] [PubMed] [Google Scholar]
- Munin A., Edwards-Lévy F. Encapsulation of natural polyphenolic compounds; a review. Pharmaceutics. 2011;3:793–829. doi: 10.3390/pharmaceutics3040793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nedovic V., Kalusevic A., Manojlovic V., Levic S., Bugarski B. An overview of encapsulation technologies for food applications. Procedia Food Sci. 2011;1:1806–1815. [Google Scholar]
- Sarwar M., Attitalla I.H., Abdollahi M. A review on the recent advances in pharmacological studies on medicinal plants: animal studies are done but clinical studies needs completing. Asian J. Anim. Vet. Adv. 2011;6:867–883. [Google Scholar]
- Shaker E., Mahmoud H., Mnaa S. Silymarin, the antioxidant component and Silybum marianum extracts prevent liver damage. Food Chem. Toxicol. 2010;48:803–806. doi: 10.1016/j.fct.2009.12.011. [DOI] [PubMed] [Google Scholar]
- Sonnenbichler J., Scalera F., Sonnenbichler I., Weyhenmeyer R. Stimulatory effects of silibinin and silicristin from the milk thistle Silybum marianum on kidney cells. J. Pharmacol. Exp. Ther. 1999;290:1375–1383. [PubMed] [Google Scholar]
- Weiss R.F., Meuss A.R. Thieme; 2001. Weiss’s herbal medicine. [Google Scholar]
- Woo J.S., Kim T.-S., Park J.-H., Chi S.-C. Formulation and biopharmaceutical evaluation of silymarin using SMEDDS. Arch. Pharm. Res. 2007;30:82–89. doi: 10.1007/BF02977782. [DOI] [PubMed] [Google Scholar]
- Wu W., Wang Y., Que L. Enhanced bioavailability of silymarin by self-microemulsifying drug delivery system. Eur. J. Pharm. Biopharm. 2006;63:288–294. doi: 10.1016/j.ejpb.2005.12.005. [DOI] [PubMed] [Google Scholar]
- Zakaria, Z.A., Rofiee, M.S., Somchit, M.N., Zuraini, A., Sulaiman, M.R., Teh, L.K., Salleh, M.Z., Long, K., 2011. Hepatoprotective activity of dried-and fermented-processed virgin coconut oil. Evidence-Based Complement. Altern. Med. 2011. [DOI] [PMC free article] [PubMed]