Abstract
Memory impairment (MI) is one of the predominant criteria generally used to identify schizophrenia, dementia and amnesia that are associated with neurodegenerative disorders by evaluating patient’s cognitive symptoms. To date, there is no available treatment that can completely mitigate MI. Currently, there is a trend in recent investigations towards symptomatic therapy approaches using a variety of natural compounds. Mangiferin is one of them that have been investigated extensively. Mangiferin is a naturally occurring potent glucoxilxanthone and is mainly isolated from the Mangifera indica (Mango) plant. This review is aimed at providing a comprehensive overview on the efficacy of mangiferin on MI, based on in-vivo animal studies. After screening through articles identified from Scopus and PubMed based on the inclusion and exclusion criteria, a total of 11 articles between 2009 and 2019 were included. The minimum and maximum dose of mangiferin were 10 and 200 mg/kg respectively and administered over the period of 12–154 days. The results of 11 articles showed that mangiferin effectively improved spatial recognition, episodic aversive events, short- and long-term memories primarily occurring via its antioxidant and anti-inflammatory effects. The outcomes of the review revealed that mangiferin improves memory and cognitive impairment in different animal models, indicating that it has potential preventive and therapeutic roles in MI.
Keywords: Cognitive, Mangiferin, Mangifera indica, Memory impairment, Neuroprotective, Natural product
1. Introduction
Memory refers to the mental process or ability to acquire, encode, retain and retrieve information (Stern and Alberini, 2013). It accounts for all information received from past experiences including facts, events and known or learned knowledge and skills (Brewer et al., 2007). Memory disorder or memory impairment (MI) is one of the major criteria commonly used to diagnose schizophrenia, dementia and amnesia that are related with neurodegenerative disorders, including Alzheimer’s disease (AD), Parkinson’s disease (PD) and Huntington’s disease (HD) (Bonam and Muller, 2020, Head et al., 2001). Studies indicated that 1) 8.4% of newly diagnosed PD patients suffered from MI at some point of their lives, 2) 40% of multiple sclerosis patients live with MI and 3) MI are often said to be the consequences of disease progression in patients diagnosed with traumatic brain injury (TBI) (Hildebrandt, 2019).
MI is determined from putative cognitive symptoms associated with behavioral outcomes in humans. These behavior include emotional distress, cognitive problem, disturbances in focusing, being forgetful, lack of ability to live independently or lack of acquisition of occupational skills (Tam et al., 2015). Hippocampus is a complex brain structure, which plays a decisive role, particularly in memory and learning. Pathologically, MI can result from the damage in the hippocampal region or possibly the frontal lobe, which is responsible for cognitive functions (Festini and Reuter-Lorenz, 2015). In the absence of brain trauma or neurodegenerative disorders, MI may occur due to various factors including 1) side effects of certain drugs, 2) stress, 3) depression, 4) aging, 5) exposure to environmental risk factors, like heavy metals as well as air pollution and 6) unhealthy lifestyles, such as high fat intake, high alcohol consumption, sedentary lifestyle and tobacco use (Sanei and Saberi-Demneh, 2019).
To date, there is no definite treatment available to completely mitigate the progression of MI. However, therapies in terms of memory enhancement with the purpose of confronting MI risk factors, are worthy of consideration for maintaining a patient’s cognitive function. A plethora of traditional treatments using natural products, such as resveratrol, berberine, galantamine, and others, have been shown to possess a wide range of therapeutic window for neurological disorders based on in-vivo and in-vitro models (Doreddula et al., 2014). One such promising natural product is mangiferin.
Mangiferin (molecular formula: C19H18O11; structural name: 1,3,6,7-tetrahydroxyxanthone C2-β-D-glucoside) (Fig. 1) is a naturally occurring glucoxilxanthone, predominantly isolated from the Mangifera indica (Mango) plant, which belongs to the Anacardiaceae family (Matkowski et al., 2013, Sekar, 2015). Mangiferin is derived from various plant parts, including the leaves, fruits, flowers, seeds, roots and stem bark (Khare and Shanker, 2016). In China, mangiferin is included in many traditional remedies containing Iris domestica, Folium pyrrosiae, Gentiana scabra and Anemarrhena asphodeloides. Worldwide, mangiferin is extracted from plants, such as Zizyphus cambodiana, Bombax ceida, Trichomanes reniforme, Rhizoma anemarrhenae and Arrabidaea patellifera (Saha et al., 2016).
Fig. 1.
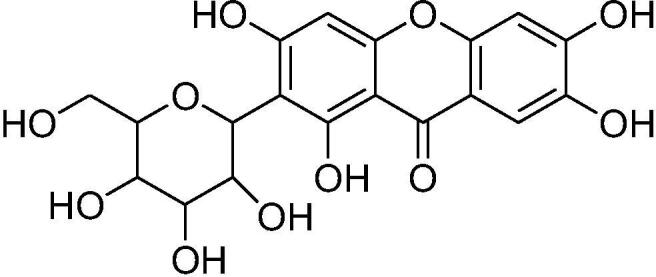
Chemical structure of mangiferin.
Mangiferin is believed to pass through the blood–brain barrier to exert some putative neuroprotective effect. Therefore, it has been extensively investigated against neurological disorders. Further, findings supported its neuroprotective effect, which is mediated via immunomodulation, anti-inflammation, antioxidant and anti-apoptosis (Benard and Chi, 2015). Motivated by these positive effects, recent investigation has been devoted to its administration to ameliorate MI. Overall, mangiferin has been found to confer its protection against MI predominantly via 1) antioxidant system, by free radical scavenging, reduction in reactive oxygen species (ROS) and oxidative stress, 2) reduction of lipid peroxidation, 3) preservation of the mitochondrial function, 4) protection against inflammation, 5) preservation of brain-derived neurotrophic factor (BDNF), 6) cholinergic enhancement and 7) reduction of noradrenaline as well as dopamine (Biradar et al., 2012, Feng et al., 2017, Fu et al., 2015, Jangra et al., 2014, Jung et al., 2009, Li et al., 2013, Satish et al., 2009).
To gain insight into the potential roles of mangiferin in ameliorating MI, an overview of various studies on mangiferin against MI is presented in this review. We attempt to outline the study design of each relevant study on MI in terms of the animal models, memory testing procedures and mangiferin’s dose. Subsequently, a summary of information gathering the effectiveness of mangiferin against MI is presented. The possible protective mechanisms conferred by mangiferin are also highlighted and discussed, in order to reduce the gap of knowledge regarding its application as an alternative medicine for MI.
2. Methods
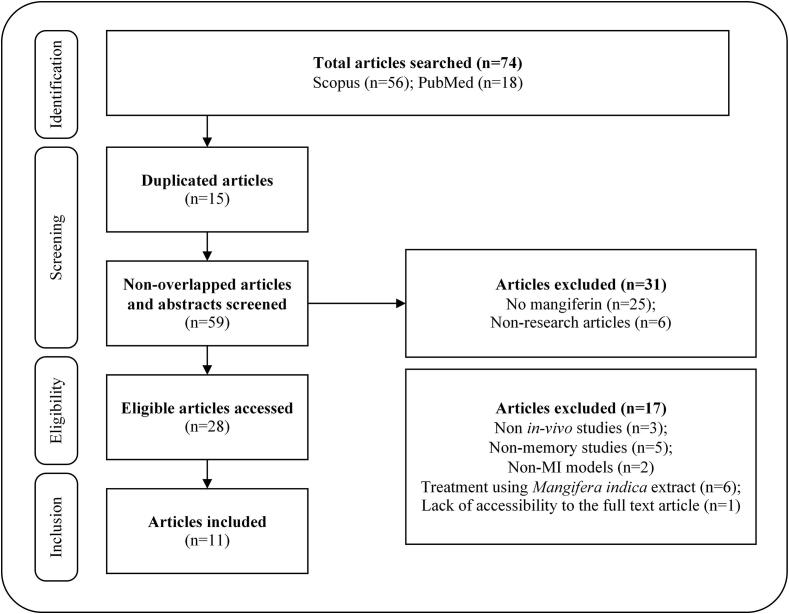
For this review, the following terms were used for the search: “Mangiferin” OR “Mangifera indica” OR “Mango extract” AND “Memory” OR “Cognitive” OR “Dementia”. Studies relevant to mangiferin used in the treatment of MI and published between 2009 and 2019 were initially extracted from two major scientific databases (Scopus and PubMed). A total of 74 articles were identified. After removing the duplicated articles, the remaining articles were further screened based on their titles and abstracts. Subsequently, 28 articles were deemed as eligible. From this number, 17 articles had to be excluded due to their non-specificity; being either an in-vitro study, a non-memory study, non-MI model as well as incorporating treatment with Mangifera indica extract. The study selection was not restricted to any particular mangiferin dose, route of administration and duration of treatment. The characteristics of studied animal type, MI model, mangiferin administration regiment and memory testing procedure were reviewed. Finally, a total of 11 articles fulfilling the criteria were included (Fig. 2).
Fig. 2.
Literature screening process.
3. Description of study design
3.1. Animals
Most studies utilized mice (n = 8) and rats (n = 3) (Table 1). All experimental protocols were approved by respective committees from the relevant institution for animal welfare and were conducted in compliance with the guidelines for use and care of laboratory animals.
Table 1.
A summary of in-vivo studies investigating the efficacy of mangiferin on memory impairment.
| Animal, sex | MI model | Mangiferin dose (mg/kg/day) | Route | Duration (days) | Memory type | Memory parameters | Remarks | References |
|---|---|---|---|---|---|---|---|---|
| SAMP8 mice, male | Aging | 100, 200 | p.o. | 60 | Spatial memory | MWM | Memory and learning abilities ↑ | Du et al. (2019) |
| Swiss albino mice, male | SD | 40 | p.o. | 14 | Recognition memory | MWM, NOR | Memory impairment ↓ | Feng et al. (2017) |
| APP/PS1 mice, male and female | Transgenic with APP/PS1 | 50 | p.o. | 154 | Episodic, spatial memory | MWM, NOD | Episodic and spatial memory impairment ↓ | Infante-Garcia et al. (2017) |
| Kunming mice, male | LPS | 50 | p.o. | 12 | – | MWM | Memory and learning deficits ↓ | Fu et al. (2015) |
| Swiss albino mice, male | AlCl3 | 20, 40* | p.o. | 21 | Recognition memory | MWM, NOR | Cognitive dysfunction ↓ |
Kasbe et al. (2015) |
| Sprague-dawley rat, male | Scopolamine | 50, 100* | p.o. | 14 | – | MWM | Memory-dependent learning abilities ↑ | Sethiya and Mishra (2014) |
| Fo66 mice, female | Transgenic with SCA-2 | 10 | p.o. | – | Recognition and aversive memory | NOR, IA | Recognition memory ↑ No effect on aversive memory |
Maurmann et al. (2014) |
| Wistar rat, male and female | Lead | 50, 100, 200* | p.o. | 84 | Spatial memory | MWM | Spatial learning abilities ↑ | Li et al. (2013) |
| Sprague-dawley rat, male | Streptozotocin | 15, 30, 60 | p.o. | 63 | – | MWM | Cognitive impairment ↓ |
Liu et al. (2013) |
| Swiss albino mice | Scopolamine, aging | 10, 20, 40 | i.p. | 14 | Short-term, long-term memory | EPM, PAT | Memory deficits ↓ | Biradar et al. (2012) |
| ICR mice, male | Scopolamine | 10, 20*, 40 | p.o. | – | Long-term memory | MWM, PAT | Memory impairment ↓ | Jung et al. (2009) |
Abbreviations: AlCl3, Aluminum chloride; EPM, Elevated plus maze; i.p., Intraperitoneal injection; IA, Inhibitory avoidance task; ICR, Institute of Cancer Research; LPS, Lipopolysaccharide; MI, Memory impairment; MWM, Morris water maze; NOD, New object discrimination; NOR, New object recognition; p.o., Oral administration Per os; PAT, Passive avoidance test; SAMP8, Senescence-accelerated mouse prone 8; SCA-2, Spinocerebellar ataxia type 2; SD, Sleep deprivation.
*Most effective dose.
3.2. MI models
Three studies investigated on scopolamine-induced memory deficits (Biradar et al., 2012, Jung et al., 2009, Sethiya and Mishra, 2014) while another two employed aging mice (Biradar et al., 2012, Du et al., 2019). On the other hand, Feng et al. (2017) conducted sleep deprivation (SD)-induced MI while Infante-Garcia et al. (2017) employed amyloid precursor protein/presenilin 1 (APP/PS1) double transgenic mouse models to investigate the efficacy of mangiferin in cognitive deficits due to Alzheimer’s disease. Maurmann et al. (2014) utilized spinocerebellar ataxia type 2 (SCA-2) transgenic mice to evaluate the effect of mangiferin in improving aversive memory. Other MI models studied including cognitive dysfunction as induced by lipopolysaccharide (LPS), aluminum chloride (AlCl3) and lead. In another study, streptozotocin (STZ)-induced cognitive impairment in diabetic rats was investigated by Liu et al. (2013).
3.3. Mangiferin dose
Among the selected relevant studies, eight studies have utilized purchased mangiferin (≥98% purity) for treatment effects, whereas three studies isolated mangiferin from medicinal plants. Most studies administered mangiferin at a minimum dose of 10 mg/kg while the maximum dose was 200 mg/kg. Among the various doses, 10 and 50 mg/kg were the most common doses selected in the treatment of MI in-vivo model. Besides, ten studies have administered mangiferin orally (p.o.) while a single study have utilized the intraperitoneal injection (i.p.) route. Mangiferin treatment was administered for a minimum of 12 days and the maximum of 154 days prior to the behavioral tests.
3.4. Toxicity profile of mangiferin
Very recently, Gelabert-Rebato et al. (2019) studied the effect of mangiferin for 15-days to be used as a supplement in men [140 and 420 mg/day of mango leaves extract (MLE, Zynamite®) containing 100 and 300 mg/day of mangiferin, respectively], to determine enhancement in exercise performance. The dose of mangiferin for this study was selected based on the pharmacokinetic study reported by Hou et al. (2012), which showed that a single dose of mangiferin (900 mg) in humans did not produce any side effects or changes in clinical symptoms and blood biochemical variables (Hou et al., 2012). The findings were supported by Na et al. (2015) who evaluated the effects of mangiferin (300 mg/day) on serum lipid profiles in overweight patients with hyperlipidemia and did not observe any side effects or changes in liver enzymes or kidney variables among the participants following a 12-week intervention. Apart from these studies, an in-vivo acute toxicity study on experimental animals was conducted by Reddeman et al. (2019). Accordingly, the mango leaves extract containing 60% of mangiferin (up to 2000 mg/kg for 90 days in repeated dose) did not show any mortality and toxic changes up to 2000 mg/kg/day in male and female Wistar rats. In another toxicity study, the rats which were orally treated with mangiferin (up to 1000 mg/kg) for 28 days did not show any abnormal clinical signs or hematology alterations (Prado et al., 2015). Based on all these reports, we found that mangiferin is safe at the selected dose levels, did not produce any side effects in animals as well as in humans.
3.5. Memory testing procedure
The neuronal process of learning, memory and attention is determined using animal models of cognitive dysfunction. These cognitive dysfunction animal models are majorly categorized as (i) pharmacological animal models, (ii) toxicological animal models and (iii) genetic animal models (Levin and Buccafusco, 2006a).
The pharmacological animal models are developed based on a neurotransmitter system involved in cognitive function. The cholinergic system (nicotinic and muscarinic receptors) and glutamatergic system (mainly NMDA receptors) are mainly involved in learning and memory processes in the brain. The antagonists of nicotinic receptors like mecamylamine (a noncompetitive antagonist); chlorisondamine and d-tubocurarine (non-specific nicotinic antagonists); dihydro-β-erythroidine hydrobromide [DHβE] (a receptor-subunit-specific α4β2 antagonist); methyllycaconitine [MLA] (a receptor-subunit-specific α7 antagonist) are used to impair cognitive function in rats (Roegge and Levin, 2006). The various antimuscarinic agents like scopolamine, atropine, pirenzepine, trihexyphenidyl. benztropine, biperidine and dicyclomine are used as amnestic agents to impair cognitive function in rats (Terry et al., 2006). Similarly, NMDA-receptor antagonists like ketamine, dizocilpine (MK-801), 2-amino-5-phosphonopentanoate (AP5), phencyclidine (PCP), and 2-methyl-6-(phenylethynyl)-pyridine (MPEP), a selective mGlu5-receptor antagonist have been demonstrated for amnestic effect in rodents (Rezvani, 2006). Moreover, MI in laboratory animals can be induced by benzodiazepines like diazepam, and benzodiazepine-induced MI is reversed by benzodiazepine receptor antagonist, i.e., flumazenil and benzodiazepine inverse agonist, i.e., beta-carbolines (Dhingra and Kumar, 2012). The putative mechanism of action of nootropic agents can be elucidated using these pharmacological MI animal models.
To date, toxicological MI animal models have been developed based on neurotoxicity induced in the brain using a variety of neurotoxicants like lead, mercury, AlCl3, polychlorinated biphenyls (PCBs), and 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP) (Levin and Buccafusco, 2006a). The cell death or apoptosis caused by heavy metals like lead and mercury is mainly due to the induction of oxidative stress in which upregulation of reactive oxygen species (ROS), such as O2., OH, NO., RO., ONOO., and H2O2 and downregulation of antioxidant defense agents like superoxide dismutase (SOD), glutathione (GSH), glutathione S-transferase (GST) and catalase (CAT) have been reported (Jaishankar et al., 2014). PCBs and its metabolites are known to induce oxidative stress by the upsurge in ROS (H2O2 and O2–) thereby causing cell injury and death (Zhu et al., 2009). MPTP is neurotoxic to various tissues like cortical brain tissue, striatum, and especially to dopaminergic neurons in the substantia nigra of the brain. MPTP is oxidized to an active toxic metabolite, MPP+ (1-methyl-4-phenylpyridinium), which generates ROS by mitochondrial dysfunction (Guo et al., 2018). Furthermore, other neurotoxicants, such as LPS and STZ are used to induce cognitive impairment in rodents. LPS activates the innate immune system through toll-like receptor (TLR)-4 thereby stimulating various inflammatory cytokines interleukin-1β (IL-1β), IL-6, tumor necrosis factor-α (TNF-α) via NF-κB (the nuclear factor-kappa B)-mediated signaling (Lee et al., 2020). These pro-inflammatory cytokines increase the production of nitric oxide (NO) and prostanoids (PG) by stimulating nitric oxide synthase-2 (NOS-2) and cyclooxygenase-2 (COX-2) enzymes respectively, thereby causing nitrosative and oxidative stresses (Lee et al., 2020).
STZ, a commonly used diabetogenic agent in rodents is known to cause hypoinsulinemia and hyperglycemia. The hippocampus and cerebral cortex of the brain possess insulin and insulin receptors that also regulates the cognitive function, such as learning and memory. Downregulation of insulin secretion in the brain can lead to MI caused by STZ (Tamaddonfard et al., 2013). Moreover, STZ generates ROS and reactive nitrogen species (RNS) leads to oxidative and nitrosative stresses (Raza and John, 2012). It has also been reported that chronic restraint stress (CRS; 6 h per day for 21 days) decreased BDNF in both the hippocampus and cerebral cortex and can induce MI in rats (Zhang et al., 2017). CRS induces oxidative stress via ROS production, mitochondrial dysfunction and apoptosis, and c-Jun N-terminal kinase (JNK) activation in the hippocampus thereby impairing learning and memory (Zhang et al., 2020). In summary, the oxidative stress and nitrosative stress induced by various neurotoxicants impair the cognitive function in experimental animals. Therefore, the drugs which possess high antioxidant potential are expected to preserve the cognitive function that is damaged by neurotoxicants.
Genetically manipulated mice (transgenic mice) are used to study the cognitive function specifically to mimic Alzheimer’s disease and age-related cognitive dysfunction. Cholinergic receptors knockout (KO) mice (muscarinic receptors KO, nicotinic receptors KO and acetylcholinesterase KO) and APP transgenic mice are commonly utilized to evaluate the therapeutic potential of drugs to treat Alzheimer’s disease (Levin and Buccafusco, 2006b). On the other hand, memory was accessed using different experimental procedures, including 1) Morris water maze (MWM), 2) new object recognition (NOR), 3) new object discrimination (NOD), 4) elevated plus maze (EPM), 5) inhibitory avoidance (IA)/passive avoidance test (PAT). To date, among these studies, MWM and NOR are the most commonly used memory testing procedures.
3.5.1. Morris water maze (MWM)
MWM is used to evaluate the spatial learning and memory function of animals (Morris, 1984). Briefly, the water maze which is a system with a circular water tank as a pool is filled with water at 26 °C. The pool is then divided into four quadrants (i.e., NW, NE, SW and SE). A platform is submerged (1–2 cm below the water surface) at the center of the tank. The platform is kept in the same place throughout the experimental session (Li et al., 2013). Subsequently, a place navigation test is conducted to access the extent of the learning function where the animals first received a training trial for 4–5 days. The animals are placed into the water tank one day prior to the training trials, to allow proper adaptation to the environment.
During the trial, each animal is released into the pool, in the direction of the wall tank. The animal is allowed to explore the escape platform for 90 s with a time interval of 30 s each time. The number of times of crossing the platform is recorded and the time taken for each animal to reach the platform is expressed as “escape latency”. If the animal failed to search the platform, it is guided back or is placed onto the platform (Feng et al., 2017). To access the memory function of the animals when retrieving information, each animal is subjected to the spatial probe test without platform after 24 h of the final training trial. During the probe trial, each animal is placed into the quadrant opposite to the former quadrant with a platform. The time spent in a quadrant is recorded as retention time, indicating the degree of memory of an animal has after the learning session. In addition, the duration of swimming directly in/over the space previously shared by the escape platform was recorded. The data confirms how the animals know precisely where the platform was placed during the training trials (Liu et al., 2013).
3.5.2. New object recognition (NOR)
In order to evaluate the recognition memory of animals, NOR is conducted in an apparatus set-up that is mainly equipped with an easily cleaned wooden box. The memory test is conducted in three phases: habituation, familiarization and test trials (Andreu et al., 2010, Feng et al., 2017). In the habituation phase, individual animal is released into an open filed area for exploration (5–10 min). After an open field exploration, a familiarization session is performed by placing each animal into the same field that is occupied by two objects (objects A1 and A2) positioned adjacently at the two corners of the box. The animal is allowed to begin the object exploration for 5–10 min by touching or sniffing the object with forepaws or nose. Subsequently, a long-term memory test for each animal is done in the same area in the presence of a familiar (A1) and a novel (B, replacement of A2) object. The animal is given 5–10 min to explore and recall the two objects they were familiarized with, within the previous training session. All objects used in the experiment are distinctive in shape but are similar in color, texture and shape. To avoid the effect of natural olfactory cues innate in animals, the explored areas and objects are cleaned with 70% ethanol solution between the trial sessions. The time spent to explore novel object B (TB) and familiar object A1 (TA) is recorded. Recognition index (%) is calculated and is expressed by the ratio of [TB/(TB + TA)] (Andreu et al., 2010).
3.5.3. New object discrimination (NOD)
NOD is employed to access the episodic memory of animals. Similar to NOR, NOD testing procedures consisted of habituation, familiarization and test trial phases (Dere et al., 2005).
Day 1: Habituation of animals to the environment (a transparent box).
Day 2: Habituation of animals to the objects.
Day 3: Memory testing session comprised of two sample trials followed by a test trial.
First sample trial: Each animal is released to the same environment in the presence of three copies of a new object positioned in a triangle configuration for exploration for 5 min.
Second sample trial: Each animal is allowed 5 min to explore four new objects positioned in a quadratic configuration.
Test trial: Each animal received two copies of the “familiar” object from the first sample trial (the first placed at the same position as the “familiar non-displaced” object while the second is placed at a new position as a “familiar displaced” object).
In the testing trials, episodic memory is accessed by analyzing “what” (the time difference in exploring familiar and recent objects), “where” (the time difference in exploring non-displaced and displaced objects) and “when” (the time difference in exploring familiar non-displaced and recent non-displaced objects (Infante-Garcia et al., 2017).
3.5.4. Elevated plus maze (EPM)
EPM which belongs to the exteroceptive animal behavioural model, is used to evaluate learning and memory in rats and mice. The dimensions of two open arms (16 cm × 5 cm) and two closed arms (16 cm × 5 cm × 12 cm) elevated to 25 cm from the floor for mice while the dimension of two open arms (50 cm × 10 cm) and two closed arms (50 cm × 10 cm × 30 cm) elevated from the floor for rats are commonly adopted. Generally, the testing sessions are conducted on the day in which the final test dose is administered. Each animal is released at the end of an open arm, facing away from the central platform. The time spent by the animal for moving from the open arm into one of the closed arms is recorded as the acquisition transfer latency time. The animal is then allowed to explore the maze for 2 min before returning to its home cage. After 24 h of trials, memory retention of each individual animal is examined, and the time taken to complete the learned task is recorded as the “retention transfer latency” (RTL). A significant reduction in transfer latency in the acquisition trial and memory retention trial indicates an improvement of learning and memory, respectively (Biradar et al., 2012).
3.5.5. Inhibitory avoidance (IA)/Passive avoidance test (PAT)
IA/PAT is performed to emotionally motivate fear-associated memory of animals (McGaugh, 2000). Typically, the apparatus consists of an acrylic box, featuring parallel stainless-steel bars (1 mm in diameter, distanced 1 cm apart) and a platform (2 cm in height) erected on the floor. During the training session, each animal is placed on the platform. Subsequently, their step-down latency with four paws on the grid floor is recorded. Instantly after stepping down, an electrical foot shock (0.6 mA/1.0 s) is administered. In the retention test (24 h after training sessions), the animal does not receive any foot shock and the latency stepping down is then measured as retention (Maurmann et al., 2011).
Another fear response “freezing time”, is also measured and a ceiling (300 s) is used in the test. For certain PAT, the testing model is comprised of a light compartment equipped with a light bulb and a dark compartment featured with a grid floor (Kim et al., 2007). Guillotine door is used to separate both compartments. In the training trial, each animal walks from the illuminated to the non-illuminated compartment. An electrical shock is administered once the animal enters the dark compartment after the door is automatically closed. The time taken for the animal to approach and enter the dark compartment is recorded as the latency time where similar retention trials are performed with no electrical shock given (Jung et al., 2009).
4. Mangiferin effectiveness
4.1. Aging
In the study by Du et al. (2019), the anti-dementia properties of mangiferin were investigated using six-months-old senescence-accelerated mouse prone 8 (SAMP8) model, a mice model exhibiting a series of pathological characteristics that are associated with rapid aging, progressive Aβ deposition, tau (τ) protein expression and dementia. Subsequently, low (100 mg/kg) and high doses of mangiferin (200 mg/kg), were administrated p.o. for 60 days. To evaluate the protective potential of mangiferin on aging-induced memory and learning impairment, the mice were subjected to the MWM test for seven consecutive days. The mangiferin-treated group demonstrated a decrease in the swimming distance in the MWM trials as compared with the SAMP8 group, thus confirming a dose-dependent restoration of navigation and learning ability by mangiferin.
4.2. Sleep deprivation (SD)
Feng et al. (2017) conducted a study on SD-induced memory loss in Swiss albino male mice using mangiferin pre-treatment [(40 mg/kg), p.o., 14 days] before SD treatment (five days). The memory performance of the mice was evaluated by performing MWM and NOR after 24 h of sleep deprivation. The results indicated that mangiferin induced a significant reduction in escape latency along with an increase in the number of crossing over the platform position during MWM. Additionally, the decrease in the recognition index was ameliorated by mangiferin administration without influencing the total exploratory time in the NOR test. The findings confirmed that pre-administration of mangiferin can improve MI.
4.3. APP/PS1 mice
In another study, the protective role of mangiferin in AD pathology was investigated in four weeks old APP/PS1 transgenic mice by Infante-Garcia et al. (2017). The treatment group received a long-term treatment of mangiferin (50 mg/kg), p.o. daily through the intake of food pellets for 22 weeks. Following intervention, the NOD testing session was performed before the commencement of the MWM test. MWM training was started for four consecutive days accompanied by four trials/day. Following treatment of mangiferin, spatial and episodic memory of APP/PS1 mice was markedly improved along with the amelioration of other pathological features.
4.4. Lipopolysaccharide (LPS)
An in-vivo study was conducted by Fu et al. (2015) to investigate the potential of mangiferin towards LPS-triggered memory and learning deficits based on the literature that suggests a chronic infusion of lipopolysaccharide (LPS) leads to neuronal damage associated with cognitive deficits (Nolan et al., 2004). In the study, Kunming male mice (one-month-old) were used as the animal model. LPS injection (1 mg/kg), i.p. was administered once daily for five consecutive days. Mangiferin was administered at 50 mg/kg, by oral gavage for a total of 12 days. To access their cognitive function, MWM was performed via a navigation test (day 1–4) and a spatial probe test (day 5). A significant decrease in the escape latency and the time spent in the quadrant among the mangiferin treatment group was observed, confirming the ability of mangiferin to alleviate LPS-induced memory dysfunction and learning disability in mice.
4.5. Aluminum chloride (AlCl3)
To investigate the neuroprotective effect of mangiferin against aluminum chloride (AlCl3)-induced cognitive dysfunction and neurotoxicity, AlCl3 was given by oral gavage to male Swiss albino mice once daily for a total of 42 days (Kasbe et al., 2015). Meanwhile, mangiferin (20 and 40 mg/kg), p.o. was administered to the treatment mice groups in the final 21 days of the experiment. The cognitive abilities of the mice were accessed by MWM and NOR tests. Administration of mangiferin at 40 mg/kg, reduced the retention latency and prevented the reduction in the number of crossing over a platform as well as the recognition index. Nevertheless, mangiferin is not effective for cognitive improvement when administered at 20 mg/kg. Overall, it was suggested that mangiferin ameliorates AlCl3-induced memory and cognitive deficits when administered orally at 40 mg/kg.
4.6. Scopolamine
Mounting evidence confirmed the degeneration of cholinergic neurons in AD patients. Sethiya and Mishra (2014) treated male Sprague-Dawley rats with mangiferin (50 and 100 mg/kg), p.o. whereas in the study by Jung et al. (2009), male ICR swiss albino mice were given mangiferin (10, 20 and 40 mg/kg), p.o. for 14 days. Both findings confirmed the potent effect of mangiferin in reversing the scopolamine-induced cognitive deficits by 1) significant reduction of escape latency, 2) increasing the number of crossing over the platform in MWM and 3) increasing the step-through latency in PAT.
4.7. Spinocerebellar ataxia type-2 (SCA-2)
Spinocerebellar ataxia type-2 (SCA-2) is an autosomal dominant neurological disorder associated with the progressive loss of function in the cerebellum caused by oxidative stress, mitochondrial dysfunction and neurotoxicity, with no specific treatment available. Maurmann et al. (2014) evaluated the protective role of mangiferin against SCA-2 induced memory outcomes where female SCA-2 transgenic mice were administrated with mangiferin (10 mg/kg), p.o. daily for 12 months. The findings from both NOR and IA indicated that mangiferin improve memory on object recognition but did not influence the memory on inhibitory avoidance in SCA-2 mice.
4.8. Lead
Li et al. (2013) investigated the alleviative potential of mangiferin on lead-induced neurotoxicity. Mangiferin (50, 100 and 200 mg/kg), p.o. was administered to Wistar rats for 12 weeks. MWM was employed to evaluate the spatial memory of mangiferin-treated rats where it was found to cause a dose-dependent improvement on the spatial memory of rats. Nevertheless, the higher dose (200 mg/kg) yielded better performance in spatial learning, in which there was a shorter time latency in finding the hidden platform.
4.9. Streptozotocin (STZ)
Liu et al. (2013) investigated diabetes-related cognitive deficits and the protective mechanism of mangiferin on STZ-induced cognitive decline in diabetic rats. STZ was administered i.p. to 10 weeks old male Sprague-Dawley rats and the development of diabetes was confirmed by a higher fasting blood glucose level (>11.1 mmol/L). Mangiferin (15, 30 and 60 mg/kg), was given by oral gavage to diabetic rats for 9 weeks. A dose-dependent reduction in the escape latency is accompanied by the increase in the percentage of the time spent in the quadrant while the number of crossing platform indicates improvement in cognitive performances.
4.10. Scopolamine and aging
Biradar et al. (2012) investigated the neuropharmacological effect of mangiferin in both young (3 months old) and aged (14 months old) Swiss albino mice where the mice in the treatment groups were administrated with mangiferin (10, 20 and 40 mg/kg), i.p. for 14 consecutive days. MI agents, scopolamine and aging, were employed to induce amnesia in young and aged mice groups, respectively. EPM and PAT trials were performed on the 14th day while the retention test was conducted on the 15th day. Following the above three regimens, mangiferin was found to remarkably reverse memory deficits induced by scopolamine and natural aging, as evidenced by reduced transfer latency in EPM and increased step-down latency in PAT.
5. Discussion
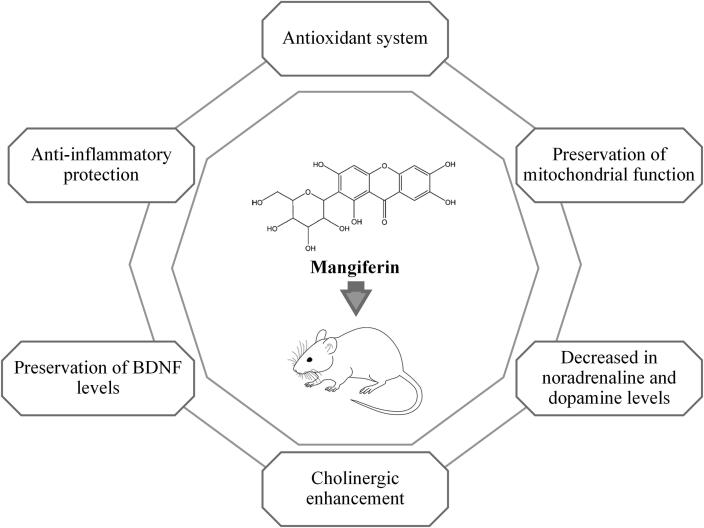
Several evidence-based studies have revealed that MI is contributed by many risk factors including aging, unhealthy lifestyles (i.e., sleep deprivation and high-fat diet) (Feng et al., 2017, Fu et al., 2015), environmental pollution (i.e., lead and aluminum exposure) (Kasbe et al., 2015, Li et al., 2013), neurodegenerative disorders (i.e., AD, PD and HD) (Biradar et al., 2012) and certain non-communicable diseases (i.e., diabetes) (Liu et al., 2013). Indeed, relevant studies have reported that mangiferin efficiently improves spatial recognition, episodic aversive events, short-term and long-term memories. Mangiferin confers some protection against memory loss by acting via the antioxidant system (Li et al., 2013), (Biradar et al., 2012, Feng et al., 2017), preservation of mitochondrial function (Jangra et al., 2014), reduction of lipid peroxidation (Satish et al., 2009), anti-inflammatory protection (Fu et al., 2015, Jung et al., 2009), preservation of BDNF activity (Feng et al., 2017, Fu et al., 2015, Kasbe et al., 2015), cholinergic enhancement (Jung et al., 2009) and reduction of noradrenaline and dopamine (Biradar et al., 2012) (Fig. 3).
Fig. 3.
Possible protective mechanism of mangiferin against MI.
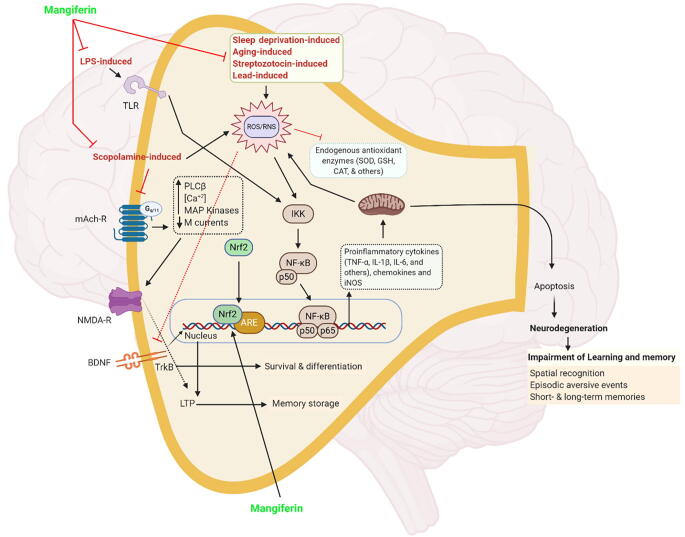
ROS (i.e., hydroxyl and superoxide radicals) play essential roles in counteracting cellular antioxidant defense status in the central nervous system (CNS) (Brand, 2010). Physiologically, ROS induction is depends on the amount of the endogenous antioxidants present. However, numerous studies have reported that chronic sleep deprivation induces higher ROS generation associated with neurodegeneration and memory deficits leading to AD (Zhang et al., 2013). When ROS levels overwhelm the endogenous antioxidant capacity, it creates a state of “oxidative stress” in the brain (Pohl and Paul, 2018), which is one of the convergent mechanisms closely correlated with memory loss in various memory impairment animal models, such as sleep deprivation-induced, ageing-induced, STZ-induced, and lead-induced animal models. Oxidative/nitrosative stress hinders the endogenous antioxidant enzymes function, and sequentially activates the secretion of pro-inflammatory cytokines followed by dysregulation of mitochondrial function (Bhat et al., 2015). Since mitochondria are the major site of production for adenosine triphosphate (ATP), its dysfunction directly impairs energy metabolism by reducing intracellular ATP (Bhangale et al., 2016, Malik et al., 2017). The effects cumulatively lead to a programmed cell death (apoptosis) that causes neurodegeneration in the hippocampus, which facilitates memory impairment.
LPS-induced model directly activates the pro-inflammatory cytokine signaling via the formation of the LPS-CD14 complex, which induces the interaction with TLR-4 on microglia membranes subsequent by the activation of microglia and astrocytes (Zakaria et al., 2017). The direct action induces the release of pro-inflammatory cytokines and complement proteins along with a series of chronic inflammation pathways, which in turn contributes to neuronal death and hippocampal memory impairment. In scopolamine-induced models, scopolamine inhibits central cholinergic activity by blocking the muscarinic acetylchonine receptor and also by enhancing the activity of acetylcholinesterase (AChE) and diminishing choline acetyltransferase (ChAT), as a result impairing long term potentiation, thereby induced memory deficit in mammals. In the hippocampus of mice treated with scopolamine, the mRNA level of ChAT and the concentration of acetylcholine were significantly decreased, whereas, AChE activity was found to be increased. Moreover, mRNA level of BDNF and other memory related signaling molecules such as Ca2+/calmodulin–dependent protein kinase (CaMK), cAMP response element binding protein (CREB), extracellular–regulated protein kinase (ERK) and phosphoinositide 3–kinase (PI3K) in hippocampus of scopolamine-injected mice was significantly decreased when compared with vehicle-treated mice (Hu et al., 2019). BDNF is known to upregulate the hippocampal NMDA receptors by which it facilitates long-term potentiation and memory process. CREB is an important transcription factor in long term memory formation in the hippocampal region (Hu et al., 2019). The low level maintenance of the brain's acetylcholine concentration leads to hippocampal neuronal injury, which results in the impairment of synaptic transmission and loss of learning and memory (Heo et al., 2014).
Overall, mounting evidences have indicated that mangiferin exerts its antioxidant mechanism (Fig. 4) by 1) sequestering free radicals (Kasi et al., 2010), 2) reversing the decreased level of cellular antioxidants (Amazzal et al., 2007), 3) attenuating the elevated level of cellular oxidative stress by neutralizing excess ROS (Feng et al., 2017). The endogenous antioxidative defense system induced by mangiferin occurs via activation of the nuclear factor erythroid 2–related factor 2 (Nrf2) signaling pathway as well as upregulation of the enzymatic antioxidants, such as superoxide dismutase (SOD), catalase (CAT) and reduced glutathione (GSH). Additionally, lipid peroxidation is reduced along with the reduction of malondialdehyde (MDA) (Satish et al., 2009). Finally, mangiferin can restore the mitochondrial membrane potential (ΔΨ) by normalizing Ca2+ influxes and ATP synthesis, thus preventing mitochondrial dysfunction. Based on all of the effects, collectively, the integrity and function of the distinctive cells and neurons are protected against oxidative stress-induced MI (Jangra et al., 2014).
Fig. 4.
Molecular mechanism(s) of mangiferin. Induction of oxidative/nitrosative stress is a key factor in various memory impairment animal models, such as sleep deprivation-induced, ageing-induced, STZ-induced, and lead-induced models. Oxidative/nitrosative stress inhibits the endogenous antioxidant enzymes, and sequentially activates the proinflammatory cytokines secretion followed by dysregulation of mitochondrial function. The effects cumulatively lead to a programmed cell death (apoptosis) that causes neurodegeneration in hippocampus which facilitates memory impairment. In addition, LPS-induced model directly activates the pro-inflammatory cytokine signalling without activating ROS/RNS. Furthermore, scopolamine induces dysregulation of cholinergic activity and contribute to induction of oxidative stress. Mangiferin exerts its neuroprotective by inhibiting the oxidative/nitrosative stress and elevating the antioxidant mechanism. Finally, the endogenous anti-oxidative defense system induced by mangiferin occurs via activation of the nuclear factor erythroid 2–related factor 2 (Nrf2) signalling pathway.Abbreviations, ARE, antioxidant response element, LTP, long-term potentiation., mAch-R, muscarinic acetylcholine receptor, PLC, phospholipase C, TrkB, tropomyosin receptor kinase B, other abbreviations are available in the main text.
Many studies have confirmed that the pathological mechanisms of AD, PD and HD extensively involves the elevation of pro-inflammatory markers (i.e., IL-1β, IL-6, TNF-α), which impacts the cognitive function in patients (Khemka et al., 2014). Moreover, current findings demonstrated that administration of LPS (Fu et al., 2015) and induced chronic sleep deprivation (Li et al., 2013) provokes an increase in various pro-inflammatory cytokines in both plasma and brain regions of mice, thus, strongly supporting the fact that inflammation plays a decisive role in MI and that mangiferin’s anti-inflammatory effects decrease neuro-inflammation-induced MI. In response to the inflammatory conditions, mangiferin hinders the activation of NF-κB and blocks the NF-κB pathway. In lieu with this, the release of pro-inflammatory cytokines is inhibited, ultimately resulting in the attenuation of memory deterioration (Fu et al., 2015). Similarly, Jung et al. (2009) confirmed that mangiferin can reduce the expression of TNF-α in scopolamine-induced mice(Jung et al., 2009). Additionally, mangiferin can upregulate the expression of heme oxygenase 1 (HO-1), thereby diminishing the level of pro-inflammatory factors (Fu et al., 2015).
Sleep-regulated BDNF secretion plays a pivotal role in maintaining the normal memory and cognitive performances (Budni et al., 2015). In fact, many studies have revealed that reduced BDNF level is closely related to memory deficits as induced by chronic sleep deprivation and AD in animal models (Biradar et al., 2012, Feng et al., 2017). Accumulating literatures support the neuroprotective role of mangiferin in up-regulating the hippocampal BDNF level in SD- (Feng et al., 2017), LPS- (Fu et al., 2015) and AlCl3-induced MI models (Kasbe et al., 2015). Furthermore, it is plausible that the attenuation of the brain cholinergic system leads to MI as confirmed by a significant reduction in brain acetylcholine level in scopolamine treated mice (Jung et al., 2009). Mangiferin’s facilitatory effect on cholinergic system in the brain is said to reverse scopolamine-induced long-term memory deficits occurring via inhibition of acetylcholinesterase (AChE) activation (Jung et al., 2009).
Biradar et al. (2012) reported that mangiferin (40 mg/kg, i.p.) decreased dopamine and noradrenaline levels in the brain of normal young mice although the changes are non-significant. Scopolamine-treated and natural aged mice showed a memory deficit in which a significant increase of dopamine and non-significant increase of noradrenaline was observed. Mangiferin (10, 20 and 40 mg/kg, i.p.) non-significantly decreased the elevated dopamine and noradrenaline levels seen. Piracetam a standard nootropic drug is also reported to decrease the levels of biogenic amines (dopamine, noradrenaline and 5-hydroxytryptamine [5-HT]) in the brain which supports a working model of mangiferin in alleviating MI by regulation of dopamine and noradrenaline levels in the mouse brain.
To date, although the involvement of brain biogenic systems in the cognitive function is demonstrated, there are conflicting findings. The biogenic amines including dopamine, noradrenaline and dopamine play a vital role in the brain cognitive process. It has been reported that direct administration of noradrenaline into the hippocampal CA1 region, amygdala and entorhinal cortex can improve learning and memory in rats (Madhyastha et al., 2002) indicating the facilitatory roles of noradrenaline in cognitive function. Similarly, dopamine also showed a facilitatory effect on learning and memory where the administration of dopamine receptor agonists into the prefrontal cortex was found to improve spatial memory in rats. In humans, dopamine D2 receptor antagonists can impair learning and memory (Madhyastha et al., 2002). Moreover, a similar severe memory deficit was observed when 5-HT level was reduced by 60% (fronto-parietal cortex) and 80% (hippocampus). Therefore, reduced serotonergic activity in the brain can lead to memory deficits.
Intraventricular administration of methotrexate can impair learning and memory by depleting biogenic amines in the hippocampal region of the rat brain (Madhyastha et al., 2002). Conversely, benzo[a]pyrene (B[a]P) an environmental toxicant impaired the learning and memory with increased activity of monoamines (noradrenaline, dopamine and serotonin/5-HT) and its metabolites [dihydroxyphenylacetic acid (DOPAC) and 5-hydroxyindoleacetic acid (5-HIAA) in the hippocampus] in rats (Xia et al., 2011). Moreover, an inverted ‘U’ shaped dose–response curve for learning and memory (i.e.) enhancement of memory has been reported at moderate doses but caused a decline in memory when used in higher doses (Xia et al., 2011).
Overall, mangiferin exhibited nootropic activity in all three categories of MI animal models (pharmacological, toxicological and genetic models). Therefore, mangiferin can be a novel therapeutic and prophylactic agent to treat MI. It is concluded that the protection against neuronal damage and death along with the attenuation of MI can be conferred by either a single or combined neuropharmacological mechanism of mangiferin.
6. Conclusion
The review presents a comprehensive account of the neuroprotective efficacy of mangiferin against MI based on in-vivo models. Following mangiferin administration, memory and learning deficits are improved mainly through the antioxidant system, via free radical scavenging, reduction of ROS, amelioration of oxidative stress, reduction of lipid peroxidation, preservation of mitochondrial function, anti-inflammatory protection, preservation of BDNF, cholinergic enhancement as well as reduction of noradrenaline and dopamine. Nevertheless, the exact molecular mechanism behind the ability of mangiferin to improve memory function needs to be fully elucidated. For example, the effect of mangiferin on autophagy and inflammasome signaling which play vital roles in neurodegenerative diseases should be explored. Additionally, the efficacy and safety of mangiferin needs to be further confirmed in clinical trials, either for short or long-term usages before it can be recommended for improvement in MI. Further dedicated work on investigating the neuroprotective potential of mangiferin towards other neurological disorders is required to confirm its utility in the development of novel and potent therapeutic agents for neurodegenerative diseases. Since it is difficult to obtain mangiferin which may be present in variable amounts based on the food source and the season, the development of mangiferin health supplements are more realistic for consistent deliveries.
Declaration of Competing Interest
The authors declared that there is no conflict of interest.
Acknowledgments
Acknowledgment
The authors would like to thank the Ministry of Higher Education (MOHE) Malaysia for the financial support provided via the Fundamental Research Grant Scheme [Ref: FRGS/1/2020/SKK06/UNIKL/02/4]. The author VP would like to express his gratitude to the Science and Engineering Research Board, India (CRG/2018/000813) for the financial support.
The funding sources are not involved in study design, data collection and analysis, decision to publish, or preparation of the manuscript. The figure was created with the support of BioRender.com under a paid subscription. The authors also would like to thank Universiti Kuala Lumpur Royal College of Medicine Perak, Malaysia for providing the necessary facilities and resources to complete the study.
Footnotes
Peer review under responsibility of King Saud University.
Contributor Information
Pei Teng Lum, Email: peiteng1013@gmail.com.
Mahendran Sekar, Email: mahendransekar@unikl.edu.my.
Siew Hua Gan, Email: gan.siewhua@monash.edu.
Vijayapandi Pandy, Email: pandiphd@gmail.com.
Srinivasa Reddy Bonam, Email: bsrpharmacy90@gmail.com.
References
- Amazzal L., Lapôtre A., Quignon F., Bagrel D. Mangiferin protects against 1-methyl-4-phenylpyridinium toxicity mediated by oxidative stress in N2A cells. Neurosci. Lett. 2007;418:159–164. doi: 10.1016/j.neulet.2007.03.025. [DOI] [PubMed] [Google Scholar]
- Andreu G.L.P., Maurmann N., Reolon G.K., de Farias C.B., Schwartsmann G., Delgado R., Roesler R. Mangiferin, a naturally occurring glucoxilxanthone improves long-term object recognition memory in rats. Eur. J. Pharmacol. 2010;635:124–128. doi: 10.1016/j.ejphar.2010.03.011. [DOI] [PubMed] [Google Scholar]
- Benard O., Chi Y. Medicinal properties of mangiferin, structural features, derivative synthesis, pharmacokinetics and biological activities. Mini Rev. Med. Chem. 2015;15:582–594. doi: 10.2174/1389557515666150401111410. [DOI] [PubMed] [Google Scholar]
- Bhangale J.O., Acharya N.S., Acharya S.R. Protective effect of Ficus religiosa (L.) against 3-nitropropionic acid induced Huntington disease. Orient. Pharm. Exp. Med. 2016;16:165–174. [Google Scholar]
- Bhat A.H., Dar K.B., Anees S., Zargar M.A., Masood A., Sofi M.A., Ganie S.A. Oxidative stress, mitochondrial dysfunction and neurodegenerative diseases; a mechanistic insight. Biomed. Pharmacother. 2015;74:101–110. doi: 10.1016/j.biopha.2015.07.025. [DOI] [PubMed] [Google Scholar]
- Biradar S.M., Joshi H., Chheda T.K. Neuropharmacological effect of Mangiferin on brain cholinesterase and brain biogenic amines in the management of Alzheimer's disease. Eur. J. Pharmacol. 2012;683:140–147. doi: 10.1016/j.ejphar.2012.02.042. [DOI] [PubMed] [Google Scholar]
- Bonam S.R., Muller S. Parkinson’s disease is an autoimmune disease: A reappraisal. Autoimmun. Rev. 2020:102684. doi: 10.1016/j.autrev.2020.102684. [DOI] [PubMed] [Google Scholar]
- Brand M.D. The sites and topology of mitochondrial superoxide production. Exp. Gerontol. 2010;45:466–472. doi: 10.1016/j.exger.2010.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brewer J.B., Gabrieli J.D.E., Preston A.R., Vaidya C.J., Rosen A.C. Chapter 5 - Memory. In: Goetz C.G., editor. Textbook of Clinical Neurology. Third Edition. Saunders; Philadelphia, W.B: 2007. pp. 63–78. [Google Scholar]
- Budni J., Bellettini-Santos T., Mina F., Garcez M.L., Zugno A.I. The involvement of BDNF, NGF and GDNF in aging and Alzheimer’s disease. Aging Dis. 2015;6:331. doi: 10.14336/AD.2015.0825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dere E., Huston J.P., Silva M.A.D.S. Episodic-like memory in mice: simultaneous assessment of object, place and temporal order memory. Brain Res. 2005;16:10–19. doi: 10.1016/j.brainresprot.2005.08.001. [DOI] [PubMed] [Google Scholar]
- Dhingra D., Kumar V. Memory-enhancing activity of palmatine in mice using elevated plus maze and Morris water maze. Adv. pharmacol. sci. 2012;2012 doi: 10.1155/2012/357368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doreddula, S.K., Bonam, S.R., Gaddam, D.P., Desu, B.S.R., Ramarao, N., Pandy, V., 2014. Phytochemical analysis, antioxidant, antistress, and nootropic activities of aqueous and methanolic seed extracts of ladies finger (Abelmoschus esculentus L.) in mice. Sci. World J. 2014. [DOI] [PMC free article] [PubMed]
- Du Z., Fanshi F., Lai Y.-H., Chen J.-R., Hao E., Deng J., Hsiao C.-D. Mechanism of anti-dementia effects of mangiferin in a senescence accelerated mouse (SAMP8) model. Biosci. Rep. 2019;39:1–10. doi: 10.1042/BSR20190488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng X., Xue J.H., Xie K.X., Liu S.P., Zhong H.P., Wang C.C., Feng X.Q. Beneficial effect of Mangiferin against sleep deprivation-induced neurodegeneration and memory impairment in mice. Biomed. Res. 2017;28:769–777. [Google Scholar]
- Festini S.B., Reuter-Lorenz P.A. Dysexecutive amnesia. In: Wright J.D., editor. International Encyclopedia of the Social & Behavioral Sciences. Second Edition. Elsevier; Oxford: 2015. pp. 717–723. [Google Scholar]
- Fu Y., Liu H., Song C., Zhang F., Liu Y., Wu J., Wen X., Liang C., Ma K., Li L. Mangiferin regulates cognitive deficits and heme oxygenase-1 induced by lipopolysaccharide in mice. Int. Immunopharmacol. 2015;29:950–956. doi: 10.1016/j.intimp.2015.10.035. [DOI] [PubMed] [Google Scholar]
- Gelabert-Rebato M., Wiebe J.C., Martin-Rincon M., Galvan-Alvarez V., Curtelin D., Perez-Valera M., Juan Habib J., Pérez-López A., Vega T., Morales-Alamo D. Enhancement of exercise performance by 48 hours, and 15-day supplementation with mangiferin and luteolin in men. Nutrients. 2019;11:344. doi: 10.3390/nu11020344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo J.D., Zhao X., Li Y., Li G.R., Liu X.L. Damage to dopaminergic neurons by oxidative stress in Parkinson's disease. Int. J. Mol. Med. 2018;41:1817–1825. doi: 10.3892/ijmm.2018.3406. [DOI] [PubMed] [Google Scholar]
- Head E., Milgram N.W., Cotman C.W. 30 - Neurobiological models of aging in the dog and other vertebrate species. In: Hof P.R., Mobbs C.V., editors. Functional Neurobiology of Aging. Academic Press; San Diego: 2001. pp. 457–468. [Google Scholar]
- Heo Y.-M., Shin M.-S., Lee J.-M., Kim C.-J., Baek S.-B., Kim K.-H., Baek S.-S. Treadmill exercise ameliorates short-term memory disturbance in scopolamine-induced amnesia rats. Int. Neurourol. J. 2014;18:16–22. doi: 10.5213/inj.2014.18.1.16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hildebrandt H. Chapter II - Rehabilitation of moderately to mildly impaired memory functions. In: Hildebrandt H., editor. Cognitive Rehabilitation of Memory. Academic Press; 2019. pp. 71–147. [Google Scholar]
- Hou S., Wang F., Li Y., Li Y., Wang M., Sun D., Sun C. Pharmacokinetic study of mangiferin in human plasma after oral administration. Food Chem. 2012;132:289–294. doi: 10.1016/j.foodchem.2011.10.079. [DOI] [PubMed] [Google Scholar]
- Hu J.R., Chun Y.S., Kim J.K., Cho I.J., Ku S.K. Ginseng berry aqueous extract prevents scopolamine-induced memory impairment in mice. Exp. Ther. Med. 2019;18:4388–4396. doi: 10.3892/etm.2019.8090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Infante-Garcia C., Ramos-Rodriguez J.J., Delgado-Olmos I., Gamero-Carrasco C., Fernandez-Ponce M.T., Casas L., Mantell C., Garcia-Alloza M. Long-term mangiferin extract treatment improves central pathology and cognitive deficits in APP/PS1 mice. Mol. Neurobiol. 2017;54:4696–4704. doi: 10.1007/s12035-016-0015-z. [DOI] [PubMed] [Google Scholar]
- Jaishankar M., Tseten T., Anbalagan N., Mathew B.B., Beeregowda K.N. Toxicity, mechanism and health effects of some heavy metals. Interdiscip. Toxicol. 2014;7:60–72. doi: 10.2478/intox-2014-0009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jangra A., Lukhi M.M., Sulakhiya K., Baruah C.C., Lahkar M. Protective effect of mangiferin against lipopolysaccharide-induced depressive and anxiety-like behaviour in mice. Eur. J. Pharmacol. 2014;740:337–345. doi: 10.1016/j.ejphar.2014.07.031. [DOI] [PubMed] [Google Scholar]
- Jung K., Lee B., Han S.J., Ryu J.H., Kim D.-H. Mangiferin ameliorates scopolamine-induced learning deficits in mice. Biol. Pharm. Bull. 2009;32:242–246. doi: 10.1248/bpb.32.242. [DOI] [PubMed] [Google Scholar]
- Kasbe P., Jangra A., Lahkar M. Mangiferin ameliorates aluminium chloride-induced cognitive dysfunction via alleviation of hippocampal oxido-nitrosative stress, proinflammatory cytokines and acetylcholinesterase level. J. Trace Elem. Med. Biol. 2015;31:107–112. doi: 10.1016/j.jtemb.2015.04.002. [DOI] [PubMed] [Google Scholar]
- Kasi V.E., Nageshwar R.B., Satish R.B.S. Antigenotoxic effect of mangiferin and changes in antioxidant enzyme levels of Swiss albino mice treated with cadmium chloride. Hum. Exp. Toxicol. 2010;29:409–418. doi: 10.1177/0960327110361752. [DOI] [PubMed] [Google Scholar]
- Khare P., Shanker K. Mangiferin: A review of sources and interventions for biological activities. BioFactors. 2016;42:504–514. doi: 10.1002/biof.1308. [DOI] [PubMed] [Google Scholar]
- Khemka V.K., Ganguly A., Bagchi D., Ghosh A., Bir A., Biswas A., Chattopadhyay S., Chakrabarti S. Raised serum proinflammatory cytokines in Alzheimer’s disease with depression. Aging Dis. 2014;5:170. doi: 10.14336/AD.2014.0500170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim D.H., Jeon S.J., Son K.H., Jung J.W., Lee S., Yoon B.H., Lee J.-J., Cho Y.-W., Cheong J.H., Ko K.H. The ameliorating effect of oroxylin A on scopolamine-induced memory impairment in mice. Neurobiol. Learn. Mem. 2007;87:536–546. doi: 10.1016/j.nlm.2006.11.005. [DOI] [PubMed] [Google Scholar]
- Lee B., Yeom M., Shim I., Lee H., Hahm D.-H. Inhibitory effect of carvacrol on lipopolysaccharide-induced memory impairment in rats. Korean J. Physiol. Pharmacol. 2020;24:27–37. doi: 10.4196/kjpp.2020.24.1.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levin E.D., Buccafusco J.J. Introduction. In: Levin E.D., Buccafusco J.J., editors. Animal Models of Cognitive Impairment. CRC Press; Boca Raton (FL): 2006. [Google Scholar]
- Levin E.D., Buccafusco J.J. Section III: Mouse Genetic Models. In: Levin E.D., Buccafusco J.J., editors. Animal Models of Cognitive Impairment. CRC Press; Boca Raton (FL): 2006. [Google Scholar]
- Li H.-W., Deng J.-G., Du Z.-C., Yan M.-S., Long Z.-X., Thi P.-T.-P., Yang K.-D. Protective effects of mangiferin in subchronic developmental lead-exposed rats. Biol. Trace Elem. Res. 2013;152:233–242. doi: 10.1007/s12011-013-9610-2. [DOI] [PubMed] [Google Scholar]
- Liu Y.-W., Zhu X., Yang Q.-Q., Lu Q., Wang J.-Y., Li H.-P., Wei Y.-Q., Yin J.-L., Yin X.-X. Suppression of methylglyoxal hyperactivity by mangiferin can prevent diabetes-associated cognitive decline in rats. Psychopharmacology. 2013;228:585–594. doi: 10.1007/s00213-013-3061-5. [DOI] [PubMed] [Google Scholar]
- Madhyastha S., Somayaji S., Rao M., Nalini K., Bairy K.L. Hippocampal brain amines in methotrexate-induced learning and memory deficit. Can. J. Physiol. Pharmacol. 2002;80:1076–1084. doi: 10.1139/y02-135. [DOI] [PubMed] [Google Scholar]
- Malik J., Karan M., Dogra R. Ameliorating effect of Celastrus paniculatus standardized extract and its fractions on 3-nitropropionic acid induced neuronal damage in rats: Possible antioxidant mechanism. Pharm. Biol. 2017;55:980–990. doi: 10.1080/13880209.2017.1285945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matkowski A., Kus P., Goralska E., Wozniak D. Mangiferin–a bioactive xanthonoid, not only from mango and not just antioxidant. Mini Rev. Med. Chem. 2013;13:439–455. [PubMed] [Google Scholar]
- Maurmann N., de Farias C.B., Schwartsmann G., Roesler R., Delgado-Hernández R., Pardo-Andreu G.L. Mangifera indica L. extract (Vimang) improves the aversive memory in spinocerebellar ataxia type 2 transgenic mice. J. Pharm. Pharmacogn. Res. 2014;2:63–72. [Google Scholar]
- Maurmann N., Reolon G.K., Rech S.B., Fett-Neto A.G., Roesler R. A valepotriate fraction of Valeriana Glechomifolia shows sedative and anxiolytic properties and impairs recognition but not aversive memory in mice. Evid. Based Complementary Altern. Med. 2011;2011:1–7. doi: 10.1093/ecam/nep232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGaugh J.L. Memory–A century of consolidation. Science. 2000;287:248–251. doi: 10.1126/science.287.5451.248. [DOI] [PubMed] [Google Scholar]
- Morris R. Developments of a water-maze procedure for studying spatial learning in the rat. J. Neurosci. Methods. 1984;11:47–60. doi: 10.1016/0165-0270(84)90007-4. [DOI] [PubMed] [Google Scholar]
- Na L., Zhang Q., Jiang S., Du S., Zhang W., Li Y., Sun C., Niu Y. Mangiferin supplementation improves serum lipid profiles in overweight patients with hyperlipidemia: a double-blind randomized controlled trial. Sci. Rep. 2015;5:1–9. doi: 10.1038/srep10344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nolan Y., Martin D., Campbell V.A., Lynch M. Evidence of a protective effect of phosphatidylserine-containing liposomes on lipopolysaccharide-induced impairment of long-term potentiation in the rat hippocampus. J. Neuroimmunol. 2004;151:12–23. doi: 10.1016/j.jneuroim.2004.02.001. [DOI] [PubMed] [Google Scholar]
- Pohl F., Paul K.T.L. The potential use of plant natural products and plant extracts with antioxidant properties for the prevention/treatment of neurodegenerative diseases: in vitro, in vivo and clinical trials. Molecules. 2018;23:3283. doi: 10.3390/molecules23123283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prado Y., Merino N., Acosta J., Herrera J.A., Luque Y., Hernández I., Prado E., Garrido G., Delgado R., Rodeiro I. Acute and 28-day subchronic toxicity studies of mangiferin, a glucosyl xanthone isolated from Mangifera indica L. stem bark. J. Pharm. Pharmacogn. Res. 2015;3:13–23. [Google Scholar]
- Raza H., John A. Streptozotocin-induced cytotoxicity, oxidative stress and mitochondrial dysfunction in human hepatoma HepG2 cells. Int. J. Mol. Sci. 2012;13:5751–5767. doi: 10.3390/ijms13055751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reddeman R.A., Glávits R., Endres J.R., Clewell A.E., Hirka G., Vértesi A., Béres E., Szakonyiné I.P. A toxicological evaluation of mango leaf extract (Mangifera indica) containing 60% mangiferin. J. Toxicol. 2019;2019:1–14. doi: 10.1155/2019/4763015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rezvani A.H. Involvement of the NMDA System in Learning and Memory. In: Levin E.D., Buccafusco J.J., editors. Animal Models of Cognitive Impairment. CRC Press; Boca Raton (FL): 2006. pp. 37–48. [Google Scholar]
- Roegge C.S., Levin E.D. Nicotinic receptor antagonists in rats. In: Levin E.D., Buccafusco J.J., editors. Animal models of cognitive impairment. CRC Press; Boca Raton (FL): 2006. pp. 21–36. [Google Scholar]
- Saha S., Sadhukhan P., Sil P.C. Mangiferin: A xanthonoid with multipotent anti-inflammatory potential. BioFactors. 2016;42:459–474. doi: 10.1002/biof.1292. [DOI] [PubMed] [Google Scholar]
- Sanei M., Saberi-Demneh A. Effect of curcumin on memory impairment: A systematic review. Phytomedicine. 2019;52:98–106. doi: 10.1016/j.phymed.2018.06.016. [DOI] [PubMed] [Google Scholar]
- Satish R.B.S., Sreedevi M.V., Nageshwar Rao B. Cytoprotective and antigenotoxic potential of Mangiferin, a glucosylxanthone against cadmium chloride induced toxicity in HepG2 cells. Food Chem. Toxicol. 2009;47:592–600. doi: 10.1016/j.fct.2008.12.017. [DOI] [PubMed] [Google Scholar]
- Sekar M. Molecules of Interest–Mangiferin–A Review. Annu Res Rev Biol. 2015;5:307–320. [Google Scholar]
- Sethiya N.K., Mishra S. Investigation of mangiferin, as a promising natural polyphenol xanthone on multiple targets of Alzheimer's disease. JBAPN. 2014;4:111–119. [Google Scholar]
- Stern S.A., Alberini C.M. Mechanisms of memory enhancement. WIREs Syst. Biol. Med. 2013;5:37–53. doi: 10.1002/wsbm.1196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tam S.K.E., Pritchett D., Brown L.A., Foster R.G., Bannerman D.M., Peirson S.N. Chapter Fifteen - Sleep and circadian rhythm disruption and recognition memory in schizophrenia. In: Sehgal A., editor. Methods in Enzymology. Academic Press; 2015. pp. 325–349. [DOI] [PubMed] [Google Scholar]
- Tamaddonfard E., Farshid A.A., Asri-Rezaee S., Javadi S., Khosravi V., Rahman B., Mirfakhraee Z. Crocin improved learning and memory impairments in streptozotocin-induced diabetic rats. Iran J Basic Med Sci. 2013;16:91–100. [PMC free article] [PubMed] [Google Scholar]
- Terry, A.V., Roegge, C.S., Levin, E.D., RAM, R.A.M., Task, T.-P.R., Alternation, T.-M., Maze, W., Learning, A., Rezvani, A.H., Paule, M.G., 2006. Muscarinic Receptor Antagonists in Rats, in: Levin, E.D., Buccafusco, J.J. (Eds.), Animal Models of Cognitive Impairment. CRC press, Boca Raton (FL).
- Xia Y., Cheng S., He J., Liu X., Tang Y., Yuan H., He L., Lu T., Tu B., Wang Y. Effects of subchronic exposure to benzo [a] pyrene (B [a] P) on learning and memory, and neurotransmitters in male Sprague-Dawley rat. Neurotoxicology. 2011;32:188–198. doi: 10.1016/j.neuro.2010.12.015. [DOI] [PubMed] [Google Scholar]
- Zakaria R., Wan Yaacob W., Othman Z., Long I., Ahmad A., Al-Rahbi B. Lipopolysaccharide-induced memory impairment in rats: a model of Alzheimer's disease. Physiol. Res. 2017;66 doi: 10.33549/physiolres.933480. [DOI] [PubMed] [Google Scholar]
- Zhang H., Wei M., Sun Q., Yang T., Lu X., Feng X., Song M., Cui L., Fan H. Food Chem. Toxicol.; 2020. Lycopene ameliorates chronic stress-induced hippocampal injury and subsequent learning and memory dysfunction through inhibiting ROS/JNK signaling pathway in rats; p. 111688. [DOI] [PubMed] [Google Scholar]
- Zhang L., Zhang H.-Q., Liang X.-Y., Zhang H.-F., Zhang T., Liu F.-E. Melatonin ameliorates cognitive impairment induced by sleep deprivation in rats: role of oxidative stress. BDNF and CaMKII. Behav. Brain Res. 2013;256:72–81. doi: 10.1016/j.bbr.2013.07.051. [DOI] [PubMed] [Google Scholar]
- Zhang Q., Wang X., Bai X., Xie Y., Zhang T., Bo S., Chen X. Resveratrol reversed chronic restraint stress-induced impaired cognitive function in rats. Mol. Med. Rep. 2017;16:2095–2100. doi: 10.3892/mmr.2017.6851. [DOI] [PubMed] [Google Scholar]
- Zhu Y., Kalen A.L., Li L., Lehmler H.-J., Robertson L.W., Goswami P.C., Spitz D.R., Aykin-Burns N. Polychlorinated-biphenyl-induced oxidative stress and cytotoxicity can be mitigated by antioxidants after exposure. Free Radic. Biol. Med. 2009;47:1762–1771. doi: 10.1016/j.freeradbiomed.2009.09.024. [DOI] [PMC free article] [PubMed] [Google Scholar]