Abstract
The mass organic compound 4-nitrophenol with low molecular is involved in many chemicals processes and most common organic pollutants. 4-Nitrophenol (4-NP) existing in soils and water bodies, thereby causing severe environmental impact and health risk. Even low concentrations are harmful to health and potential mutagenic and carcinogenic. Though the existing methods of biodegradation though effective, their popularity is hindered due to high cost. Hence, in the present study a less expensive method involving the use of Pseudomonas sp. with gum arabic (PAA) was tested. The biodegradation of 4-NP was thoroughly investigated by progressive characterization methods. The promising Pseudomonas sp. YPS 3 was identified with biochemical and molecular identification process. The average particle sizes of stable crystalline PAA was 8–20 nm. The experiments were conducted with optimized parameters viz., pH (7.0), concentration (30 ppm), temperature (37 °C) and time (6 h). The study was tested as adsorbent particle size on 4-NP concurrent adsorption-biodegradation. In addition, these Pseudomonas sp. YPS3 and its PAA are used as an eco-friendly for removal of toxic organic 4-NP pollutant from the ecosystems.
Keywords: 4-Nitrophenol, Biodegradation, Pseudomonas sp., Immobilized cells, Gum arabic
1. Introduction
One of the most precious natural resources in our planet is water and this made of 70% earth’s surface. Now-a-days, water pollution is the widespread crisis. The squalor of the atmosphere with synthetic unrefined compounds has turn into huge quandary with urbanization and substantial industrialization (Munoz et al., 2016, Monsalvo et al., 2012). The normal water bodies are tainted by a variety of toxic chemical causing pollutions. As a results, need for safe, pure and potable water is fetching a vital focus on worldwide (Wang et al., 2016a). 4-Nitrophenol (4-NP) is a phenol compound and is used in different industries. Nitrophenol is one of the most obstinate compounds owing to their elevated stability and solubility in wastewater content. 4-NP is mostly used to manufacturing of drugs, fungicides, insecticides, synthetic dyes, darken leather, and used in petrochemical plants and textile industries. 4-NP can enter into body through skin or inhalation and passes into the blood stream. Once after inside it is biotransformed into toxic species, which causes several adverse effects. Acute inhalation or intake of 4-NP can cause symptoms such as headaches, drowsiness, nausea and cyanosis in lips, ears, and finger nails. And also, contact with eyes causes exasperation to the humans (Dhorabe et al., 2016).
In recent years, the number of microorganisms are resistive to phenol and are capable of degrading phenol related chemical compounds. Moreover, some yeast such as Cupriavidus sp. (Min et al., 2020), Pseudomonas sp. (Das et al., 2020) is also known to be competent phenolic compounds degraders. Among, all the Pseudomonas species encompasses significant bacteria with environmental application in bioremediation (Kimura et al., 2014, Kowalczyk et al., 2015). Thus, from a chemical point of view, resistance to low absorption are essential for these compounds to enter the distal sections of the intestine where they can be fermented by the microbiota, which in effect is selectively changed in the phase. Such additional prebiotic acts aim to improve the mucosa's capacity to absorb luminous micro-organisms and their components, i.e., intestinal barrier function (IBF) (Kumar et al., 2005, Rodriguez et al., 2006, Wang et al., 2016b). However, several species that are immune to phenol and are capable of degrading phenol. Pure bacterial culture such as Acinebacter sp., (Abd-El-Haleem et al., 2003), Nocardioidess (Cho et al., 2000), P. fluorescens (Ojumu et al., 2005), P. pictorum (Annadurai et al., 2000), P. putida (Reardon et al., 2000), P. resinovorans (Yang and Lee, 2007), was degraded phenolic compounds aerobically.
The immobilization of microbial cells with sufficient adsorbent and it increases the efficiency of removal (Wang et al., 2016b). This transform is due to the creation of a bio-layer on the adsorbent bed and, at the same time, to adsorption- biodegradation (Chen et al., 2016, Zhao et al., 2015, Sengupta and Balomajumder, 2014). The different study reports were done with adsorption of phenol from polluted water by using different types of biosorbant. The effectiveness of adsorption is based on the effect of pH, and adsorbent concentration (Singh and Balomajumder, 2016, Van Tran et al., 2020). Due to the substantial adsorption capacity for removing various aquatic pollutants, Acacia nilotica tree gum, has gained broad attention as an important biosorbant (Wen et al., 2013). Cost efficiency, ample supply, and renewability make it an affordable choice for the treatment of water and waste. Phenol degradation using Pseudomonas sp., immobilized with Acacia nilotica gum in the form of powder was investigated.
There are very few studies on bioremediation potential of Pseudomonas sp., from the results obtained; the finest experimental settings were explored in order to assess the feasibility of their combination for the complete removal of the pollutant and detoxification of the effluent relative to the PAA duckweed. The aim of the present study was to evaluate the 4-nitrophenol biodegradation potential using microbes. The degradation of 4-NP was confirmed by different analysis such as characterization of morphology, size and pores. The effect on 4-NP was studied of different parameters including different time, temperature, dose and concentration. Outcomes of this current study depicts innovation in micro reactor based degradation techniques with economic practicality, non-toxic and ultrahigh speed catalyst method.
2. Materials and methods
2.1. Chemicals and adsorbent
Analytical grade of 4-NP (99% pure chemical grad) chemical used as mineral salt medium. The adsorbent Acacia nilotica gum is used as support matrices for the immobilization of microbial cells and was obtained from Yercaud, Eastern Ghats, South India.
2.2. Isolation and identification of organisms
The rhizosphere soil sample was collected from biodiversity garden in latitude 11.7184°N, 78.0771°E, South India. The soil was sieved and stored in sterile cover. The sample was serially diluted with up to 10−7 dilution. Sample was speeded on a plate of nutrient agar and incubated at 37 °C for 24 h. The isolated colonies were sub-cultured on Pseudomonas isolation agar medium. After that, the isolated colonies were stored for further study.
2.3. Identification of Pseudomonas sp.
2.3.1. Morphology, biochemical and molecular characterization
The most promising strain was characterized by standard microscopic observation, biochemical and physiological activities were scrutinized by the following protocol Cappuccino and Sherman.
The genomic DNA was isolated from the bacterium using modified protocol by Kumar et al. (2016) and molecular identification of 4-NP removal bacterial strains was done. In addition, the isolated strain was identified by 16S rDNA sequencing which is followed by Kalaimurugan et al. The bacterial strain was taxonomically classified by sequencing analysis with BLAST (NCBI) and phylogenetic tree was constructed using Neighbor-joining method using MEGA 6 software.
2.4. Preparation of 4-NP aqueous solution
For screening of degrading bacteria, 100 mg/L of 4-NP solutions stock solution) were prepared as a stock according to the method described by Balance (1996). The stock solution was diluted further different working concentrations from 20, 40, 60, 80 and 100 mg/L.
2.5. Preparation and pretreatment of adsorbent
Acacia nilotica gum (ANG) was sundried and grinded using serrated disk grinder to obtain small-sized particles. The complete methodology was followed as per Shazryenna et al. (2015). In order to attain the powdered particles, these particles were sieved and desired average particle sizes ranges from 0.152 to 0.422 mm. Further analysis revealed the chemical composition that ANG is mainly composed of lignin. Briefly, one gram of ANG was dissolved in 100 ml of 1 M HCl, treated for 24 h and then kept in water bath for 30 min at 70 °C. Later, it was neutralized by refrigeration with 50 ml of 1 M NaOH. The filtrates were separated and then dried in oven for 5 h at 60 °C. Throughout the test the pretreated powdered ANG was used as an adsorbent.
2.6. Batch culture study
Batch mode studies were performed with adsorption by inoculating known volume 1 × 106 CFU/ml of Pseudomonas sp., YPS3 in a number of conical flasks comprising a known amount of ANG biosorbant (1 g). 4-NP was performed to examine the effect of pH (4, 5, 6, 7 and 9), contact time (1–6 h), adsorbent of different temperature (25, 30, 35, 40 and 45), different concentration (10, 20, 30, 40 and 50 ppm) and different dose (100, 200, 300, 400 and 500 mg). Solution containing adsorption ANG was taken in 100 ml Erlenmeyer flasks and uptight at 160 rpm using an orbital shaker at encoded time intervals (Kalaimurugan et al., 2020). The supernatant was alienated from the biosorbant with spinning for 15 min at 3000 rpm. Then, the supernatant was separated and determined under UV–Visible spectrophotometer (Shimadzu, India) at 590 nm (Moharami and Jalali, 2013).
2.7. Characterization of PAA
FTIR analysis was performed by using Perkin Elemer Spectrum-1, and categorizes the chemical in the Mid Infrared (MIR) region of 400–4000 cm−1 of the ANG adsorbent from bacteria extracted samples. XRD pattern of the ANG adsorbent were measured by Phillips PW 1830 instrument. 40 kV operating voltage and 30 mA current with 0.1541 nm wavelength kα radiation, in the 2θ territories 10–80°, advance size 0.02/θ. SEM analysis was also done by FEI QUANTA 200 FE-SEM model, operated at a working distance of 8 mm (30 kV). Thin films of the sample were carbon coated and an extremely tiny amount of the specimen were placed on the sample holder. Blotting paper was used to eliminate the spare solution and the film on the FE-SEM was allowed to dry by 5 min placed under a mercury lamp (Durairaj et al., 2019).
2.8. Statistical analysis
Three replicates were produced per treatment and Analysis of variance was performed on data. The collected data was analysed by using PRISM software, version 18.0.
3. Results and discussion
3.1. Isolation of bacterial strain
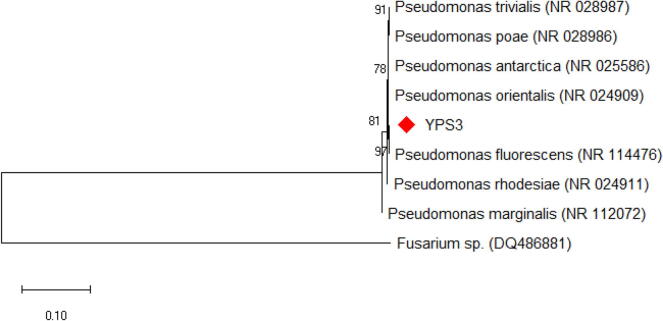
The strain Pseudomonas sp. YPS3 was recognized by standard cultural, morphological and biochemical characteristics features and it was gram-negative, rod-shaped bacterium (Table 1 and Fig. 1). The genomic DNA extracted from the Pseudomonas sp. YPS3 and it was used as a 16S rRNA amplification template for PCR. For the amplification and sequencing of the 16S rRNA gene fragment the universal primers 27F and 1429R have been used. The optimum temperature for the annealing was found at 55 °C. The PCR sample was subjected to sequencing from both forward and reverse directions using the BDT V3.1 process sequencing package on the ABI 3730 XL genetic analyses. The collected sequences were aligned using BLAST alignment tool system of NCBI database. The obtained results confirmed 99–100% similarity and the neighbor-joining method were used to construct phylogenetic tree (Fig. 2) with Two-parameter model Kimura using Super 6.0 software. The aligned sequence was submitted in NCBI database with accession number of MH580200.
Table 1.
Results of biochemical tests Pseudomonas sp. YPS3.
| Biochemical Test | Observations |
|---|---|
| Pseudomonas sp. YPS3 | |
| Indole production test | Negative |
| Methyl red test | Negative |
| Voges proskauer test | Negative |
| Citrate utilization test | Positive |
| Catalase test | Positive |
| Oxidase test | Positive |
Fig. 1.
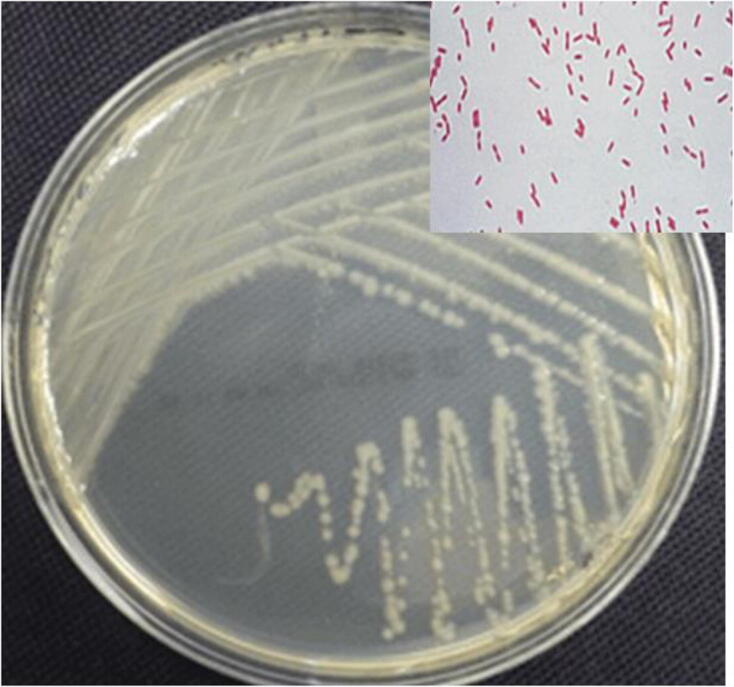
Bacterial Strain in Pseudomonas sp. YPS3 Gram negative rod shaped bacteria.
Fig. 2.
Phylogenetic tree of Pseudomonas sp. YPS3.
3.2. Batch culture study
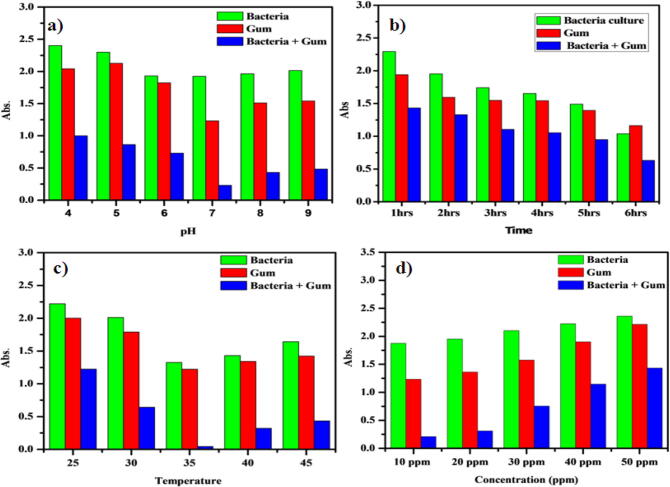
The influence of pH on the 4-NP degradation is shown in Fig. 3a. Initial pH of the 4-nitrophenol solution has essentially no effect on the 4-NP removal by Pseudomonas sp. YPS3 with Acacia gum (PAA). High 4-NP removal efficiency was observed by the use of PAA. More than 98% removal values were reached at pH 7 values. The time influence on the removal of 4-NP by PAA was displayed in Fig. 3b. The equilibrium time value was measured as 6 h with time increase the quantity of 4-NP sorbet increased. The 4-NP solution temperature essentially has no effect on the removal of 4-NP by PAA. More than 98% removal values were reached at 35 °C temperature values. The effect of temperature the 4-NP removal is shown in Fig. 3c. The 4-NP solutions with different concentrations with adsorbents were used to investigate the impact of concentrations on removal of 4-NP at 37 °C. At concentrations of 20, 40, 50, 60, 80 and 100 ppm/L, the adsorbent values were 99. 41, 99. 1699. 2699. 0798. 52 and 98.07% respectively. The percentage uptake of 4-NP decreased by increasing initial concentration (Fig. 3d). It was owing of the saturation of resin adsorption sites as the solution concentration increased. The 4-NP solutions of different adsorbent dose were used to investigate the impact of dose on the removal of 4-NP at 35 °C. At dose of 100, 200, 300, 400 and 500 mg/L, the adsorbent values were 76. 41%, 84. 16%, 91. 26%, 96% and 98. 47% respectively. It has been attributed to the degradation of the resin adsorption sites as the solution dose increased (Fig. 4).
Fig. 3.
a) Effect of pH adsorption of 4-Nitrophenol by PAA b) Effect of Time adsorption of 4-Nitrophenol by PAA c) Effect of Temperature adsorption of 4-Nitrophenol by PAA d) Effect of different concentration adsorption of 4-Nitrophenol by PAA.
Fig. 4.
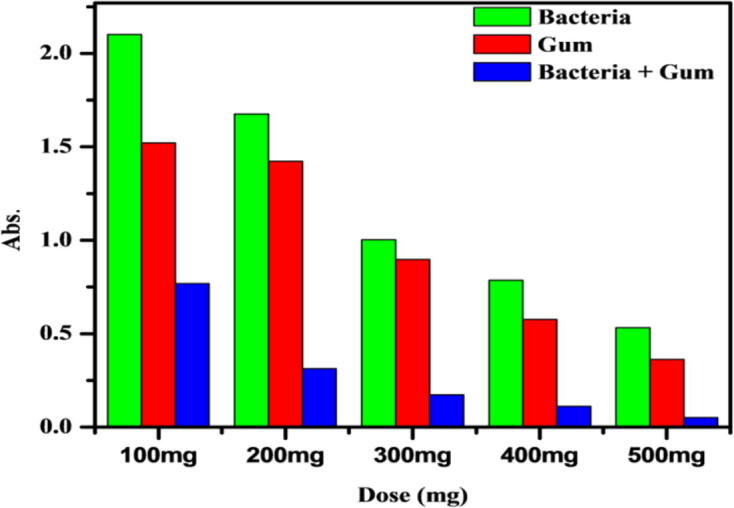
Effect of different dose adsorption of 4-Nitrophenol by PAA.
The optimum temperature for biodegradation of 4-NP was 25–35 °C range. The efficiency of P-NP degradation is known to be optimum at 30 °C temperature (Leilei et al., 2012). Dey et al. reported that 4- NP degraded by Arthrobacter sp., was revealed the extreme at 30 °C. The 96% of 4-NP was degraded at pH 9 by the particular strain by 3 days of incubation at 30 °C. The outcomes were supported by the early report of Yang and Lee, (2007). Further, the degradation of 4-NP was intensified at high pH owing of enhanced bioavailability and declined toxicity of phenol.
Pseudomonas sp. is considered one of the most common species of bacteria degrading the isolated phenolic compounds from polluted sites (Duan et al., 2016, Cherifi et al., 2014, Gu et al., 2014, Liu et al., 2014). The Pseudomonas species exhibited their ability to degrade 2, 4, 6-TCP, 2, 4-DCP, and pentachlorophenol (El-Naas et al., 2009, Prieto et al., 2002). The isolate of P. fluorescens was able to use a wide variety of phenolic compounds with various rates of halo-substitution as the carbon sources. P. fluorescens isolate had an increased ability to use 2, 4, 6-TCP and 2, 6-DCP as a growth-based substratum. The concentration of substrates increased the growth of P. fluorescens further increasing the concentrations it weakens the growth (Kim et al., 2002, Ahmaruzzaman, 2008).
Phenolic compounds are capable of disrupting membrane functions and inducing cell death. As observed in this study and others, Pseudomonas sp., grow better at pH values within the range of 6–9 (Ksibi et al., 2003). The current results showed phenol concentration exceeded 480 mg/L and it was the highest degradation reported rate per cell by Pseudomonas sp. This results were accordance with early investigation by Whitney et al. (2001). The concentration of phenol was found to be play a crucial role in degradation by study reports (Dabhade et al., 2009).
Current study, the rate of biodegradation of 4-NP was significantly affected by pH, time, incubation temperature and 4-NP concentration which is used as a carbon and energy source. The optimized method was observed that the growth of microorganism and gum increases as pH increases. The optimum conditions for the removal of 4-NP were found to be pH 7, temperature 37 °C and 30 ppm of 4-NP concentration.
3.3. Characterization of PAA
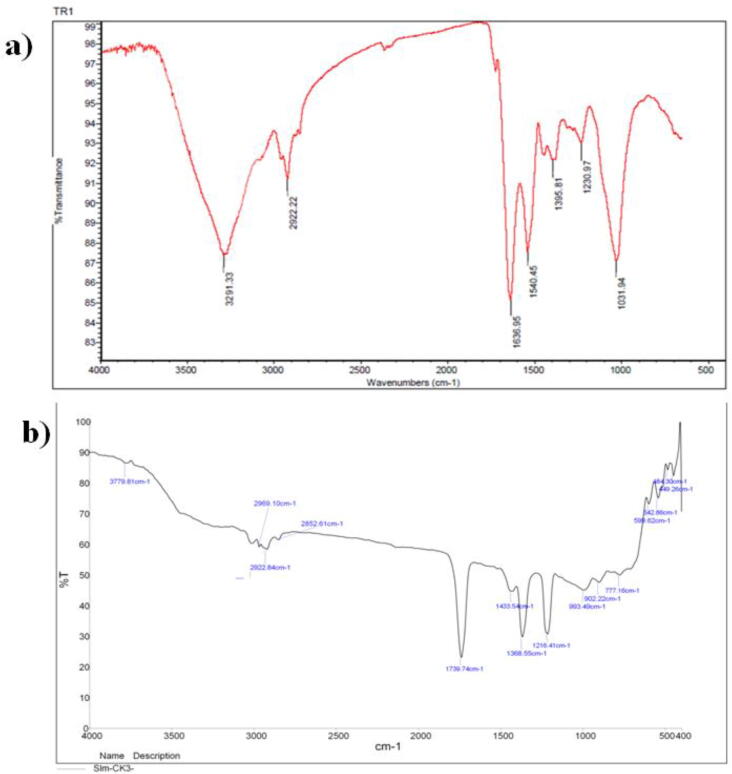
FTIR has been used to evaluate the form of the biosorbant functional groups. FTIR was also used to obtain details about the existence of potential adsorbent interactions with adsorbents. FTIR spectra of 4000–400 cm−1 for the virgin pine bark powder and 4-nitrophenol loaded pine bark powder are shown in (Fig. 5a). The adsorption before FTIR Spectroscopic characteristics functional on are shown in (Table 2). After adsorption, the FTIR spectrum of pine bark powder (Fig. 5b) showed a board adsorption peak of 2237 cm−1, corresponding to the overlapping —OH and —NH levels. The C—H group represents for a peak at 2147 cm−1.
Fig. 5.
FT-IR of PAA a) before the adsorption of PAA b) after the adsorption of PAA.
Table 2.
FT-IR functional group’s analysis of PAA for 4-nitrophenol removal before.
| Vibrational assignment | Observed wave number (cm−1) | Functional groups | Visible intensity |
|---|---|---|---|
| O—H stretching | 3291.33 | Carboxylic acids | Wide peak |
| C—H stretching | 2922.22 | Aldehyde | Small medium peak |
| NO2 asymmetric stretching | 1636.95 | Nitrate | Sharp peak |
| NO2 asymmetric stretching | 1540.45 | Aromatic nitro compound | Small medium peak |
| N N stretching | 1395.81 | Azo compound | Small peak |
| C—O stretching | 1230.97 | Esters | Sharp peak |
| C—O stretch | 1031.94 | Alcohol and phenols | Medium sharp peak |
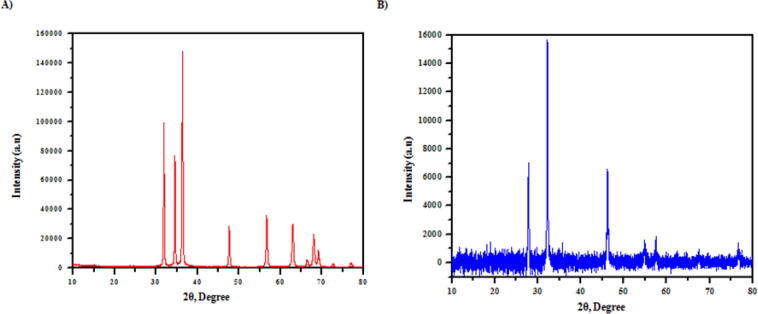
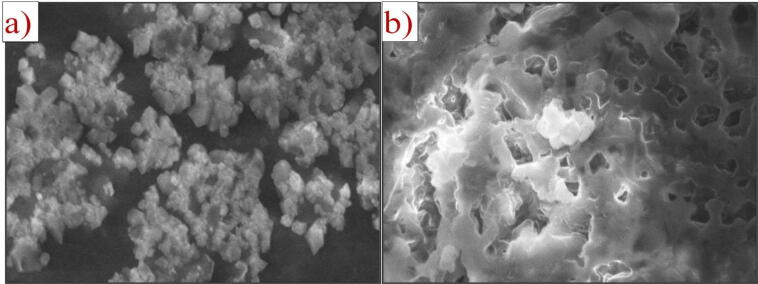
The pure pine bark powder stretching for C O was at 1617 cm−1. The presence of —C—N linkages from the peaks at 1058 cm−1 is confirmed. Several peaks have been significantly moved and extended (Table 3). These results confirm the presence of the pine bark powder group of amino, carboxyl, and hydroxyl as possible active binding sites for 4-nitrophenol adsorption. An X-ray diffraction was carried out on the culture of bacteria and the gum. The PAA exhibited a board peak at 20 = 26.8° (Fig. 6a, b), indicating the amorphous state of the adsorption. FESEM analysis of the bacterial culture with gum consisted of a type of brick, plate-like structure (Fig. 7a), with an agglomerated and irregular surface structure. The rough surfaces of bacterial culture and Acacia gum have an irregular shape, suggesting high porosity and thus allowing the adsorption of 4-nitrophenol on various adsorbent sections (Fig. 7b). Another peak occurs at 1384 cm−1 in the IR spectra, which allocated for tri-coordinated boron vibration (Feng et al., 2011). These types of vibrational modes occur only with doped samples in the range of 1200–1750 cm−1 (Sharma et al., 2006). This result is consistent with the research reported earlier, in which the rutile phase of concentration of boron dopant was observed (Chen et al., 2006). The pore size distribution of microbes indicates the existence of mesoporous of pore diameter in the range of 2–12 nm, while the TiO2 and BT7 sample pore size distributions are large (2–15 nm) in length (Yadav et al., 2020).
Table 3.
FT-IR functional group’s analysis of PAA for 4-nitrophenol removal after.
| Vibrational assignment | Observed wave number (cm−1) | Functional groups | Visible intensity |
|---|---|---|---|
| O—H stretching | 3779.81 | Alcohol | Very Small peak |
| C—H stretch | 2922.22 | Aldehyde | Small peak |
| C O stretching | 1739.07 | Carboxylic acids | Sharp peak |
| N N stretching | 1433.54 | Azo compound | Small medium peak |
| NO2 symmetric stretching | 1368.55 | Aromatic nitro compound | Medium sharp peak |
| C—O stretching | 1216.41 | Esters | Medium sharp peak |
| C—O stretching | 993.49 | Alcohol and phenols | Small peak |
| N—H wagging | 777.16 | Secondary amide | Very small peak |
Fig. 6.
XRD a) before and b) after the adsorption of PAA.
Fig. 7.
a) SEM image of Acacia gum b) 4-nitrophenol adsorption of PAA.
4. Conclusion
In the present study the Pseudomonas sp. YPS3 culture immobilized with Acacia gum (PAA) was used for biodegradation of 4-NP. The optimized biodegradation capabilities PAA was pH 7.0, at 37 °C with 30 ppm concentration. PAA adsorption has demonstrated exceptional catalytic activity when used at room temperature to reduce 4-NP. The outcome of this study clearly showed that 4-NP reduction had been completed in 6 h. This work recommended that degradation of 4-NP by biosynthesized PAA, is useful in remove harmful 4-NP and its derivatives from wastewater and Pseudomonas sp. YPS3 as a promising candidature. Further, the future study was investigated with their enzymes responsible for biodegrading and computational studies for effective removal of toxic 4-NP and its derivatives from the ecosystems.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
D. K also thanks Dr. M.S. Shivakumar, Assistant professor, Department of Biotechnology, Periyar University, Salem for critically going through the manuscript and offering valuable suggestions and comments. This project was supported by Researchers Supporting Project number (RSP-2020/5) King Saud University, Riyadh, Saudi Arabia.
Footnotes
Peer review under responsibility of King Saud University.
References
- Abd-El-Haleem D., Beshay U., Abdelhamid A.O., Moawad H., Zaki S. Effects of mixed nitrogen sources on biodegradation of phenol by immobilized Acinetobacter sp. strain W-17. Africa. J. Biotech. 2003;2(1):8–12. [Google Scholar]
- Ahmaruzzaman M. Adsorption of phenolic compounds on low-cost adsorbents: a review. Adv. Coll. Inter. Sci. 2008;143(1–2):48–67. doi: 10.1016/j.cis.2008.07.002. [DOI] [PubMed] [Google Scholar]
- Annadurai G., Balan S.M., Murugesan T. Design of experiments in the biodegradation of phenol using immobilized Pseudomonas pictorum (NICM-2077) on activated carbon. Bioprod. Eng. 2000;22:101–107. [Google Scholar]
- Balance, R., 1996. In: Chapter 7 - Physical and Chemical Analyses, Water Quality Monitoring - A Practical Guide to the Design and Implementation of Freshwater Quality Studies and Monitoring Programmes Edited by Jamie Bartram and Richard Balance. United Nations Environment Programme and the World Health Organization.
- Chen J.Z., Shi Y.L., Wang L., Yan F.Y., Zhang F.Q. Preparation and properties of hydroxyapatite-containing titania coating by micro-arc oxidation. Mater. Lett. 2006;60(20):2538–2543. [Google Scholar]
- Chen Y., Yu B., Lin J., Naidu R., Chen Z. Simultaneous adsorption and biodegradation (SAB) of diesel oil using immobilized Acinetobacter venetianus on porous material. Chem. Eng. J. 2016;289:463–470. [Google Scholar]
- Cherifi H., Bentahar F., Hanini S. Biosorption of phenol by dried biomass. Desalin. Water Treat. 2014;52(7–9):1699–1704. [Google Scholar]
- Cho Y.-G., Rhee S.-K., Lee S.-T. Influence of phenol on biodegradation of p-nitrophenol by freely suspended and immobilized Nocardioides sp. NSP41. Biodegrada. 2000;11:21–28. doi: 10.1023/a:1026512922238. [DOI] [PubMed] [Google Scholar]
- Dabhade M.A., Saidutta M.B., Murthy D.V.R. Adsorption of phenol on granular activated carbon from nutrient medium: equilibrium and kinetic study. Int. J. Environ. Res. 2009;3(4):557–568. [Google Scholar]
- Das A., Dey A. P-Nitrophenol-Bioremediation using potent Pseudomonas strain from the textile dye industry effluent. J. Environ. Chem. Eng. 2020:103830. [Google Scholar]
- Dhorabe P.T., Lataye D.H., Ingole R.S. Removal of 4-nitrophenol from aqueous solution by adsorption onto activated carbon prepared from Acacia glauca sawdust. Water Sci. Tech. 2016;73(4):955–966. doi: 10.2166/wst.2015.575. [DOI] [PubMed] [Google Scholar]
- Duan L., Wang H., Sun Y., Xie X. Biodegradation of phenol from wastewater by microorganism immobilized in bentonite and carboxymethyl cellulose gel. Chem. Eng. Commun. 2016;203(7):948–956. [Google Scholar]
- Durairaj K., Senthilkumar P., Velmurugan P., Dhamodaran K., Kadirvelu K., Kumaran S. Sol-gel mediated synthesis of silica nanoparticle from Bambusa vulgaris leaves and its environmental applications: kinetics and isotherms studies. J. Sol-Gel Sci. Technol. 2019;90(3):653–664. [Google Scholar]
- El-Naas M.H., Al-Muhtaseb S.A., Makhlouf S. Biodegradation of phenol by Pseudomonas putida immobilized in polyvinyl alcohol (PVA) gel. J. Hazard. Mater. 2009;164(2–3):720–725. doi: 10.1016/j.jhazmat.2008.08.059. [DOI] [PubMed] [Google Scholar]
- Feng Q., Chen F., Wu H. Preparation and characterization of a temperature-sensitive lignin-based hydrogel. Bio Resources. 2011;6(4):4942–4952. [Google Scholar]
- Gu S., Wunder S., Lu Y., Ballauff M., Fenger R., Rademann K., Jaquet B., Zaccone A. Kinetic analysis of the catalytic reduction of 4-nitrophenol by metallic nanoparticles. J. Phys. Chem. C. 2014;118(32):18618–18625. [Google Scholar]
- Kalaimurugan D., Durairaj K., Kumar A.J., Senthilkumar P., Venkatesan S. Novel preparation of fungal conidiophores biomass as adsorbent for removal of phosphorus from aqueous solution. Environ. Sci. Poll. Res. 2020:1–13. doi: 10.1007/s11356-020-08307-0. [DOI] [PubMed] [Google Scholar]
- Kim J.H., Oh K.K., Lee S.T., Kim S.W., Hong S.I. Biodegradation of phenol and chlorophenols with defined mixed culture in shake-flasks and a packed bed reactor. Pro. Biochem. 2002;37(12):1367–1373. [Google Scholar]
- Kimura N., Kitagawa W., Kamagata Y. In: Biological Remediation of Explosive Residues. Environmental Science and Engineering. Singh S., editor. Springer; Cham: 2014. Biodegradation of nitrophenol compounds. [Google Scholar]
- Kowalczyk A., Eyice O., Schäfer H., Price O.R., Finnegan C.J., van Egmond R.A., Shaw L.J., Barrett G., Bending G.D. Characterization of para-nitrophenoldegrading bacterial communities in river water by using functional markers and stable isotope probing. Appl. Environ. Microbiol. 2015;81:6890–6900. doi: 10.1128/AEM.01794-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ksibi M., Zemzemi A., Boukchina R. Photocatalytic degradability of substituted phenols over UV irradiated TiO2. J. Photochem. Photobiol. Chem. 2003;159(1):61–70. [Google Scholar]
- Kumar A., Kumar S., Kumar S. Biodegradation kinetics of phenol and catechol using Pseudomonas putida MTCC 1194. Biochem. Eng. J. 2005;22:151–159. [Google Scholar]
- Kumar S., Stecher G., Tamura K. MEGA7: molecular evolution– ary genetics analysis version 7.0 for bigger datasets. Mol. Biol. Evol. 2016;33:1870–1874. doi: 10.1093/molbev/msw054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leilei Z., Mingxin H., Suiyi Z. Biodegradation of p-nitrophenol by immobilized Rhodococcus sp. strain Y-1. Chem. Biochem. Eng. Quart. 2012;26(2):137–144. [Google Scholar]
- Liu X.Z., Zhang Q., Liang Y.X., Zhang R.F. Biodegradation of 2-chlorophenol by laccase immobilized on large-sized macroporous silica. Adv. Mater. Res. 2014;1010:830–834. [Google Scholar]
- Min J., Xu L., Fang S., Chen W., Hu X. Microbial degradation kinetics and molecular mechanism of 2, 6-dichloro-4-nitrophenol by a Cupriavidus strain. Environ. Pollu. 2020;258 doi: 10.1016/j.envpol.2019.113703. [DOI] [PubMed] [Google Scholar]
- Moharami S., Jalali M. Removal of phosphorus from aqueous solution by Iranian natural adsorbents. Chem. Eng. J. 2013;223:328–339. [Google Scholar]
- Monsalvo V.M., Mohedano A.F., Rodriguez J.J. Adsorption of 4-chlorophenol by inexpensive sewage sludge-based adsorbents. Chem. Eng. Res. Des. 2012;90(11):1807–1814. [Google Scholar]
- Munoz M., Kaspereit M., Etzold B.J.M. Deducing kinetic constants for the hydrodechlorination of 4-chlorophenol using high adsorption capacity catalysts. Chem. Eng. J. 2016;285:228–235. [Google Scholar]
- Ojumu T.V., Bello O.O., Sonibare J.A., Solomon B.O. Evaluation of microbial systems for bioremediation of petroleum refinery effluents in Nigeria. Afri. J. Biotech. 2005;4(1):31–35. [Google Scholar]
- Prieto M., Hidalgo A., Rodriguez-Fernandez C., Serra J., Llama M. Biodegradation of phenol in synthetic and industrial wastewater by Rhodococcus erythropolis UPV-1 immobilized in an air-stirred reactor with clarifier. Appl. Microbiol. Biotech. 2002;58(6):853–860. doi: 10.1007/s00253-002-0963-2. [DOI] [PubMed] [Google Scholar]
- Reardon K.F., Mosteller D.C., Rogers J.D.B. Biodegradation kinetics of benzene, toluene, and phenol as single and mixed substrates for Pseudomonas putida F1. Biotech. Bioeng. 2000;69(4):385–400. doi: 10.1002/1097-0290(20000820)69:4<385::aid-bit5>3.0.co;2-q. [DOI] [PubMed] [Google Scholar]
- Rodriguez G.V., Youssef C.B., Vilanova J.W. Two-step modeling of the biodegradation of phenol by an acclimated activated sludge. Chem. Eng. J. 2006;117(3):245–252. [Google Scholar]
- Sengupta P., Balomajumder C. Potential of corn husk leaves for the co-removal of phenol and cyanide from waste water using simultaneous adsorption and biodegradation. IJRET. 2014;3:700–707. [Google Scholar]
- Sharma R.K., Agrawal M., Marshall F. Heavy metal contamination in vegetables grown in wastewater irrigated areas of Varanasi, India. Bull. Environ. Contam. Toxicol. 2006;77(2):312–318. doi: 10.1007/s00128-006-1065-0. [DOI] [PubMed] [Google Scholar]
- Shazryenna D., Ruzanna R., Jessica M.S., Piakong M.T. Phenol biodegradation by free and immobilized candida tropicalis RETL-Crl on coconut husk and loofah packed in biofilter column. IOP Conf. Ser.: Mater. Sci. Eng. 2015;78 [Google Scholar]
- Singh N., Balomajumder C. Simultaneous biosorption and bioaccumulation of phenol and cyanide using coconut shell activated carbon immobilized Pseudomonas putida (MTCC 1194) J. Environ. Chem. Eng. 2016;4(2):1604–1614. [Google Scholar]
- Van Tran T., Dai Cao V., Nguyen V.H., Hoang B.N., Vo D.V.N., Nguyen T.D., Bach L.G. MIL-53 (Fe) derived magnetic porous carbon as a robust adsorbent for the removal of phenolic compounds under the optimized conditions. J. Environ. Chem. Eng. 2020;8(1) [Google Scholar]
- Wang M., Fang G., Liu P., Zhou D., Ma C., Zhang D., Zhan J. Fe3O4@b-CD nanocomposite as heterogeneous Fenton-like catalyst for enhanced degradation of 4-chlorophenol (4-CP) Appl. Catal. B. Environ. 2016;188:113–122. [Google Scholar]
- Wang Y., Chen H., Liu Y.X., Ren R.P., Lv Y.K. An adsorption-release-biodegradation system for simultaneous biodegradation of phenol and ammonium in phenol-rich wastewater. Bioresou. Technol. 2016;211:711–719. doi: 10.1016/j.biortech.2016.03.149. [DOI] [PubMed] [Google Scholar]
- Wen Q., Yang T., Wang S., Chen Y., Cong L., Qu Y. Dechlorination of 4- chlorophenol to phenol in bioelectrochemical systems. J. Hazard. Mater. 2013;244–245:743–749. doi: 10.1016/j.jhazmat.2012.11.002. [DOI] [PubMed] [Google Scholar]
- Whitney, J.M., Davis, G.C., Snowden, A.C., General Electric Co, 2001. Phenolic compounds, polymers derived there from and method. U.S. Patent. 6 (255), 438.
- Yadav V., Verma P., Sharma H., Tripathy S., Saini V.K. Photodegradation of 4-nitrophenol over B-doped TiO 2 nanostructure: effect of dopant concentration, kinetics, and mechanism. Environ. Sci. Poll. Res. 2020:1–15. doi: 10.1007/s11356-019-06674-x. [DOI] [PubMed] [Google Scholar]
- Yang C.F., Lee C.M. Enrichment, isolation, and characterization of phenol degrading Pseudomonas resinovorans strain P-1 and Brevi bacillus sp. strain P-6. Inter. Biodeter. Biodegra. 2007;59:206–210. [Google Scholar]
- Zhao Q., Han H., Jia S., Zhuang H., Hou B., Fang F. Adsorption and bioregeneration in the treatment of phenol, indole, and mixture with activated carbon. Desalin. Water Treat. 2015;55(7):1876–1884. [Google Scholar]