Abstract
The quantification, localization, production, function, and regulation of irisin/FNDC5 in camel species have not been previously studied. The objective of this study was to detect the irisin content in Arabian camel blood and tissues and study the gene expression of FNDC5 and PGC-1α in camel skeletal muscles and white adipose tissue depots under basal conditions. To monitor if exercise influences blood and tissue irisin protein levels as well as FNDC5 and PGC-1α gene expression levels, we analyzed irisin concentrations in the serum, skeletal muscles (soleus and gastrocnemius), and white adipose tissues (hump, subcutaneous, visceral, epididymal, and perirenal) in both control (n = 6) and exercised group (n = 6) using ELISA and determined the cellular localization of irisin/FNDC5 and the mRNA levels of FNDC5 and PGC-1α in skeletal muscles and adipose tissues via immunohistochemistry and real-time PCR, respectively. The possible regulatory roles of exercise on some hormones and metabolites as well as the detection of links between serum irisin and other circulating hormones (insulin, leptin, and cortisol) and metabolites (glucose, free fatty acids, triglycerides, and ATP) were explored for the first time in camels. Our results indicated that exercise induces tissue-specific regulation of the camel irisin, FNDC5, and PGC-1α levels, which subsequently regulates the circulating irisin level. Significant associations were detected between the levels of irisin/FNDC5/PGC-1α in camels and the metabolic and hormonal responses to exercise. Our study suggested that irisin regulates, or is regulated by, glucose, FFA, insulin, leptin, and cortisol in camels. The novel results of the present study will serve as baseline data for camels.
Keywords: PGC-1α, Glucose, Insulin, Leptin, Exercise
1. Introduction
The dromedary camel (Camelus dromedarius; also called the Arabian camel or one-humped camel) is unique and the most famous member of the family Camelidae (Bornstein, 1990). The dromedary occupies an exclusive place among all domesticated animals due to its tolerance and adaptation to extremely arid conditions using a variety of anatomical and physiological mechanisms (Gebreyohanes and Assen, 2017). Camels develop markedly higher basal blood glucose levels than those of monogastrics and two-fold higher than other ruminants (Elmahdi et al., 1997, Abdel-Fattah et al., 1999). However, the whole-body insulin sensitivity of camels is lower than that of adult ruminants (Kaske et al., 2001, Abdel-Fattah et al., 1999). Moreover, camels do not develop diabetes. Their high basal blood glucose level and low insulin sensitivity might be considered adaptation mechanisms that help camels survive in dry and harsh conditions (Elmahdi et al., 1997, Abdel-Fattah et al., 1999). Therefore, detecting the factors and understanding the mechanisms that are involved in glucose homeostasis in camels are crucial.
Irisin is a recently reported novel myokine and adipokine generated by the cleavage of its precursor protein fibronectin type III domain-containing protein 5 (FNDC5) prior to being released into the circulation (Boström et al., 2012, Roca-Rivada et al., 2013). Recent studies have confirmed that exercise induced the expression of peroxisome proliferator-activated receptor gamma co-activator 1-α (PGC-1α) in skeletal muscle, which regulates the conversion of FNDC5 to irisin (Boström et al., 2012, Brenmoehl et al., 2014, Norheim et al., 2014). Various studies on humans and rodents (Boström et al., 2012, Perakakis et al., 2017, Mahgoub et al., 2018) proposed that irisin is involved in energy homeostasis and is a promising regulator of glucose metabolism that mediates the beneficial effects of exercise on metabolism.
To date, conclusions on the presence of irisin, FNDC5, and PGC-1α have been derived from rodents, humans, and only one study of cattle. The presence of irisin, FNDC5, and PGC-1α, the regulation of irisin/FNDC5 production, and the function of irisin in camel species are far from being elucidated. Therefore, the principal aims of this study were to quantify the irisin protein content in camel blood and tissues and examine the gene expression levels of FNDC5 and PGC-1α in camel skeletal muscles and white adipose depots under basal conditions. We also explored the possible regulatory effects of exercise on the circulating and tissue irisin content, the expression profiles of FNDC5 and PGC-1α, and the cellular localization pattern of irisin/FNDC5 protein in skeletal muscles and white adipose tissues of camels. Finally, we examined the effect of exercise on camel metabolism and clarify the possible involvement of the PGC-1α/FNDC5/irisin pathway in camel metabolism. Using this approach, we attempted to gain a better understanding of the role of irisin in camel physiology.
2. Methods
2.1. Animals
Twelve healthy male dromedary camels (1–2 years old) were used in this study. All camels were kept under hygienic conditions and veterinary supervision at an ambient temperature of 30 ± 2 °C and 35–50% humidity in a shaded corral in Badaway village, Mansoura District, Dakahlia Governorate. Camels were allowed to fed hay and barley twice daily at 6:00 and 17:00 o'clock, while drinking water was available ad libitum.
2.2. Exercise protocol
Six male dromedary camels were subjected to a 5 km warm-up by walking for 30 min, followed by running for 1.5 km for 7 min in an agricultural open area away from traffics. The control group (n = 6) did not undergo any exercise training. On the day before the experiment, the camels of each group were food-deprived for 16 h (from about 18:00 o'clock). The experimental protocol used in the present study was approved by the Ethics of Animal Use in Research Committee of Zagazig University (approval number: ZU-IACUC/2/F/110/2019).
2.3. Sample collection and preparation
Blood samples were withdrawn from the camels’ jugular veins using a 20 mL syringe from camels (n = 6) just prior to and immediately after exercise. For serum preparation, the samples were centrifuged at 3000 rpm for 10 min. The serum samples were stored at −20 °C for hormone and metabolite analyses. Tissue samples from skeletal muscles (soleus and gastrocnemius) and white adipose tissues (hump, subcutaneous, visceral, epididymal, and perirenal) of the exercised and control camels were collected immediately after slaughtering by bleeding from the carotid artery to be used for immunohistochemistry, real-time PCR, and biochemical analyses. The tissues were washed in ice-cold saline and snap-frozen in liquid nitrogen and stored at −40 °C until processing to analyze protein and mRNA. For tissue homogenate, 100 mg were removed from each tissue and placed in a tube containing 500 KIU of aprotinin before careful homogenization in phosphate-buffered saline (PBS, pH7.4) solution. The tissue homogenates were then centrifuged at 4000 rpm for 10 min to get the supernatant that was stored at −40 °C for the biochemical analyses. For immunohistochemical study, the tissue samples were immediately placed in 10% neutral-buffered formalin for 24 h at room temperature and dehydrated with graded ethanol, cleared in xylene, and embedded in paraffin.
2.4. Biochemical measurements
The serum concentrations of hormones and metabolites examined in this study were measured in triplicate using commercially available kits.
2.5. Hormonal analyses
The irisin protein levels were measured in the camel serum and tissue supernatants using a commercially available irisin recombinant (human, rat, mouse, and canine) ELISA kit (EK-067-29, Phoenix, AZ, USA). Data of this kit showed that the cross-reactivity is 100% with irisin (42-112) (human, rat, mouse, and canine). The intra-assay and inter-assay values are <10% and <15%, respectively. The quantitation range is between 0.1 and 1000 ng/ml. The assay was conducted according to the manufacturer’s instructions. Since previous evidence indicated that irisin levels show a discrepancy depending on the commercial ELISA kits used (Polyzos and Mantzoros, 2015, Winn et al., 2017), we also measured the irisin levels with a Cell Biolabs irisin ELISA kit (cat. no.: MET-5089, San Diego, CA, USA), which had a detection sensitivity limit of 6.25 ng/ml irisin.
The serum insulin level was measured using a commercially available ELISA kit (cat. no: RSHAKRIN010TR) according to the manufacturer’s instructions (BioVendor Laboratory Medicine, Inc., Brno, Czech Republic). Furthermore, the serum levels of cortisol and leptin were measured using commercially available ELISA kits (cat. no: CSB-E05112r and CSB-E07433r, respectively) according to the manufacturer’s instructions (Cusabio, Wuhan, China).
2.6. Metabolite analyses
The serum levels of glucose and triglycerides were quantified using GOD-POD based kits (Spinreact SAU, Sant Esteve de Bas, Spain) according to the manufacturer’s instructions. The quantitative measurement of ATP in the serum was determined using an ELISA kit (cat. no.: KT-59182, Kamiya Biomedical Co., Seattle, WA, USA) according to the manufacturer’s protocols. The serum free fatty acid level was determined using an ELISA kit (cat. no: CSB-E08770r; Cusabio, Wuhan, China). Muscle lactate was estimated using a commercially available lactate assay kit (cat. no.: MET-5012, Cell Biolabs, Inc.).
2.7. Immunohistochemical analysis
The immunohistochemical localization of irisin/FNDC5 protein in formalin fixed-paraffin embedded samples from skeletal muscles (soleus and gastrocnemius) and white adipose tissues (hump, subcutaneous, visceral, epididymal, and perirenal) of the exercised and control camels was conducted using an UltraVision LP large volume detection system (cat. no. TL-125-HL; Thermo Fisher Scientific, Fremont, CA, USA) according to the manufacturer’s recommended protocols. Briefly, 4 μm tissue sections were deparaffinized followed by antigen retrieval by heating for 15 min in a microwave in the presence of sodium citrate buffer (0.01 m and pH 6.0). Endogenous peroxidase was blocked by 10 min incubation in H2O2 block. After washing, Ultra V block was applied for 10 min at room temperature to block non-specific binding. The sections were incubated for 1 h at room temperature in rabbit anti-irisin/FNDC5 antibody (against the amino acids 32–143 of the human irisin/FNDC5 protein; cat. no. NBP2-59680, Novus Biologicals, Centennial, CO USA). The antibody was diluted 1:100 with 0.1 M of PBS (pH 7.2) containing 0.25% sodium azide and 2.5% bovine serum albumin (BSA). The sections were then washed (4 times) with PBS and treated with horseradish peroxidase (HRP) polymer for 15 min at room temperature. The sections were then immersed in 0.5% (w/v) 3,3′-diaminobenzidine tetrachloride (Kanto Chemical Co., Inc., Tokyo, Japan) in PBS containing 0.01% H2O2 to visualize the bound antibody. After washing with distilled water, the sections were counterstained with Mayer's hematoxylin, dehydrated, and coverslipped with mounting medium. Negative immunohistochemical controls were included in each staining run. These controls involved the omission of the primary antibody as well as the use of irisin/FNDC5 primary antibody that had been preabsorbed with 10 μg/ml of protein antigen. For histological examination, the sections were stained with hematoxylin and eosin (H&E). The sections were examined using an Olympus BX-43 (DP 72) microscope (Olympus Corp., Tokyo, Japan), evaluated, and photographed.
2.8. Cross-reactivity of the ELISA kit and immunohistochemistry antibodies with camel irisin/FNDC5 protein
FNDC5 protein contains an N-terminal signal peptide (aa 1–28), an FNIII domain (aa 33–124), a transmembrane domain (aa 150–170), and a cytoplasmic tail (aa 171–209) (www.uniprot.org) and by its proteolytic cleavage, irisin containing 112 amino acids (aa 29–140) is generated and released into the circulation (Wrann, 2015). Homology searches using GENETYX-MAC software version 12.25 (GENETYX, Tokyo, Japan) and BLAST (http://blast.ncbi.nlm.nih.gov/Blast.cgi) verified that the immunogen sequence of the anti-human irisin/FNDC5 antibody used for IHC as well as the immunogen sequence of irisin antibody ELISA kit showed 99% and 100% homology, respectively with the analogous region derived from the predicted Camelus dromedarius FNDC5 (GenBank accession no. XP_010974361) and cross-reacted with the camel irisin/FNDC5 protein.
2.9. RNA extraction, cDNA Synthesis, and quantitative real-time polymerase chain reaction
Total RNA was prepared from skeletal muscles (soleus and gastrocnemius) and white adipose tissues (hump, subcutaneous, visceral, epididymal, and perirenal) of the exercised and control camels using easy-RED total RNA extraction kit (cat. no. 17063; iNtRON Biotechnology, Inc., Seoul, South Korea) following the manufacturer’s instructions. The total RNA was quantified using a Genova nano spectrophotometer (Jenway, Staffordshire, UK) and reverse transcribed to cDNA using a HiSenScript RH(−) cDNA synthesis kit (cat. no. 25014; iNtRON Biotechnology, Inc.) according to the manufacturer’s protocols. Real-time quantitative PCR was conducted using a T100 Thermal Cycler (Bio-Rad, Hercules, CA, USA) using TOPreal qPCR 2X PreMIX (SYBR Green with low ROX) (cat. no. RT500M; Enzynomics, Daejeon, South Korea) according to the manufacturer's guidelines. To measure the gene expression levels of FNDC5 and PGC-1α, the oligonucleotide primer sequences were used as follows: FNDC5 primer pairs were derived from the predicted Camelus dromedaries FNDC5 (GenBank accession no. XM_010976059) (sense: 5′-CACTGTCAGGCATCTCAAGGCCA-3′; antisense: 5′-TCATATCTTGCTGCGGAGAAGACC-3′). PGC-1α primer pairs were derived from Homo sapiens PGC-1α (GenBank accession no. NR_148983.1) (sense: 5′-CCTCTTGCAAGACTGTGGTGCC-3′; antisense: 5′-CACCGGTCTTGTCTGCTTCGTC-3′). The house keeping gene; glyceraldehyde-3-phosphate dehydrogenase (GAPDH) primer sets were designed from the predicted Camelus dromedarius GAPDH sequences (GenBank accession no. XM_010990867.1) (sense: 5′-ATGGTGAAGGTCGGAGTGAACGG-3′; antisense: 5′-GCAGAGATGATGACCCTCTTGGC-3′). After the reaction, the threshold cycle (Ct) was obtained for each sample and a ΔCt value was calculated by subtracting the Ct value of the sample’s housekeeping gene from the Ct value of each sample’s gene. The relative target mRNA expression levels were calculated as 2−ΔCt and normalized to endogenous control GAPDH in the camel skeletal muscles and white adipose tissues.
2.10. Statistical analysis
Statistical evaluations were conducted using SPSS version 20.0 for Windows (SPSS, Chicago, IL, USA). The data were compared using Student’s t test or analysis of variance (ANOVA) as appropriate. All of the assays were conducted at least in duplicate and expressed as means ± standard deviation (SD). The relationship between variables was analyzed by Pearson’s bivariate correlation test. P values of <0.05, <0.01, and <0.001 were considered statistically significant for all of the analyses. Different letters or symbols above the bars indicate statistical significance.
3. Results
First we studied the possible presence, localization, and measurement of irisin protein (via immunohistochemical and ELISA analyses) in the camel blood, skeletal muscles, and white adipose tissues. Then we examined the presence, distribution pattern, and mRNA expression levels of the irisin precursor (FNDC5) along with the PGC-1α mRNA by quantitative real-time PCR in the camel skeletal muscles and white adipose depots.
3.1. Expression and cellular localization of irisin/FNDC5 protein in the camel skeletal muscles
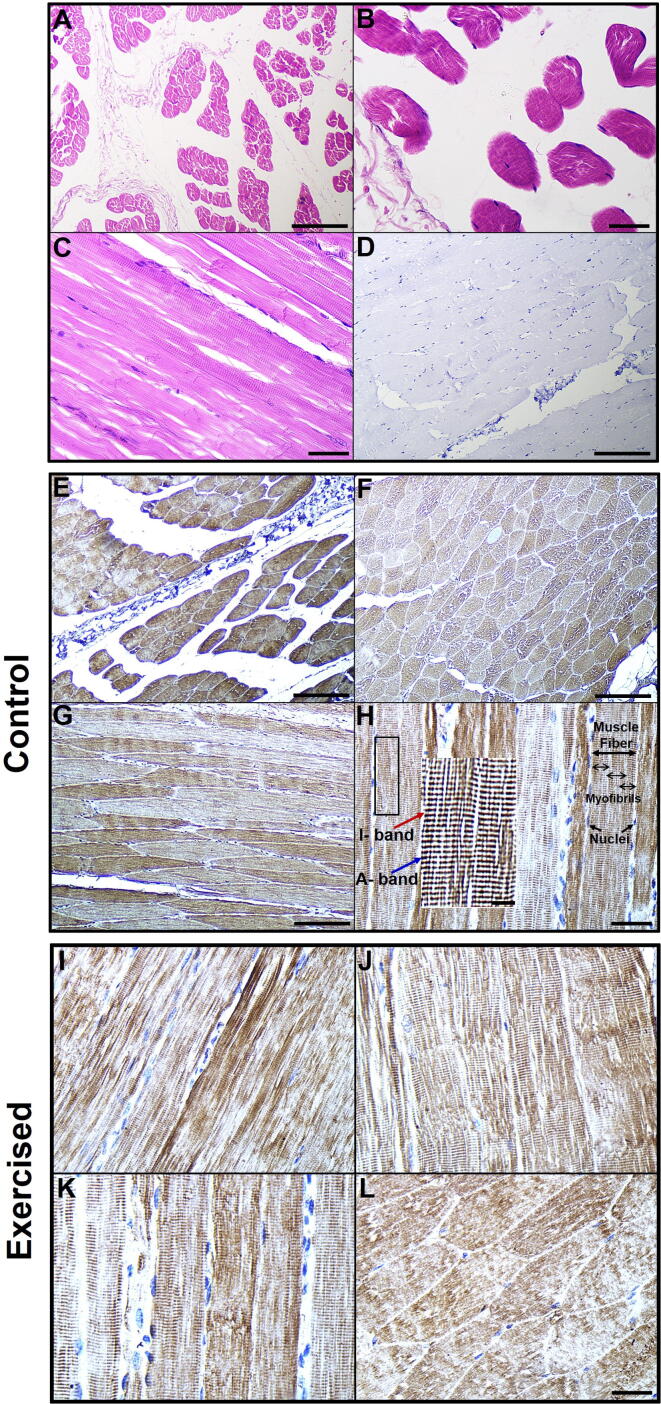
Fig. 1A–C represents various histological sections of camel skeletal muscle fibers under low-power microscope showing longitudinal and cross-arrangements of gastrocnemius muscle bundles. The muscle fibers appear as elongated cylindrical multinucleated cells with acidophilic sarcoplasm and regular transverse striations (Fig. 1C). Each muscle fiber had many myofibrils, and the nuclei were pushed to the side (Fig. 1C). The dark and light bands in one myofibril with those of adjacent myofibrils provide the characteristic cross-striations (Fig. 1C). The muscle fibers in the cross-sections (Fig. 1A and B) were polyhedral in shape and the myofibrils appear as fine dots in the sarcoplasm.
Fig. 1.
Cellular localization of irisin/FNDC5 in the gastrocnemius muscles of the control and exercised camels. (A–C) Light microscopy micrographs at different magnifications of the control camels’ gastrocnemius muscles stained with H&E. (D) Representative negative control showing no irisin/FNDC5 immunoreactivity. (E–L) Immunohistochemical images of irisin/FNDC5 positivity (brown) in various sections of the gastrocnemius muscles of the control (E–H) and exercised (I–L) camels. Inset in H shows irisin/FNDC5 on the myofibrils’ A bands. Scale bar, 40 μm (A, D, and E–G), 20 μm (B, C, H, and I–L), and 10 μm (inset in H).
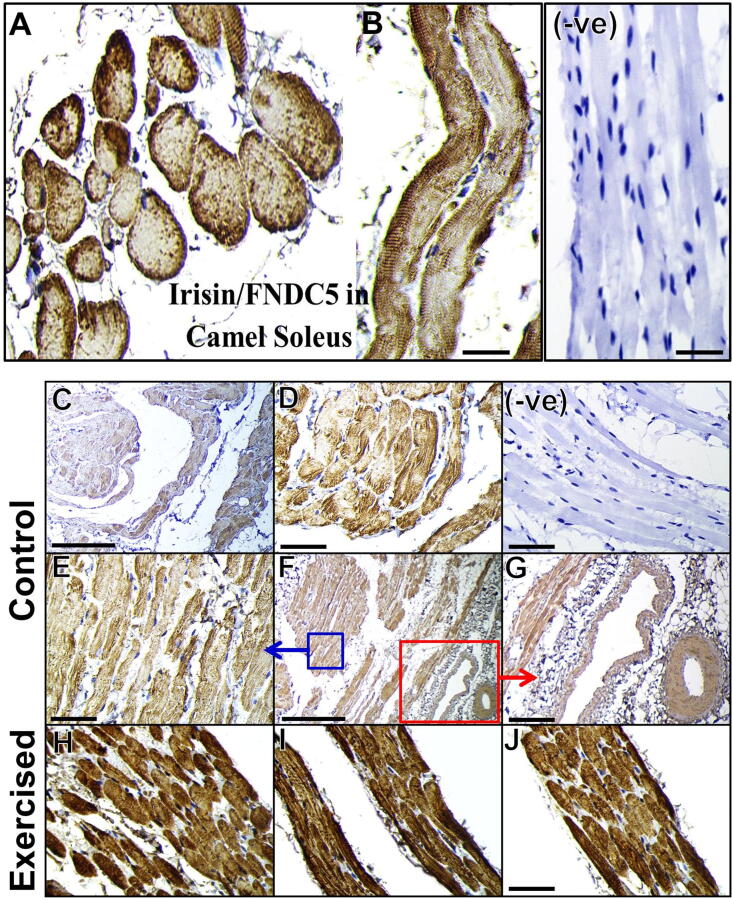
In the control camels, the immunoreactive positivities for irisin/FNDC5 antibody in the cross-sections and longitudinal sections of camel fast glycolytic muscle fiber (gastrocnemius; Fig. 1E–H) and slow oxidative muscle fiber (soleus; Fig. 2A–G) were predominantly abundant in the sarcolemma and sarcoplasm. As shown in the immunostained sections of camel skeletal muscle cells (Figs. 1E–H and 2A–G), the myofibrils were positively visible by the signals of irisin/FNDC5 reactivity. The irisin/FNDC5 protein appeared to be concentrated on the A band of the myofibrils, which resulted in clearly demarcated repetitive A and I bands forming the myofibrils’ striated staining patterns (Figs. 1H, 2B, D, and E). Notably, positive irisin/FNDC5 staining was not observed in all of the muscle fibers (Figs. 1E–H and 2A–G). We also recognized the irisin/FNDC5 protein as indicated by positive staining in the blood vessels (Fig. 2F and G).
Fig. 2.
Cellular localization of irisin/FNDC5 in the soleus muscles of the control and exercised camels. Representative different magnification immunohistochemical images are shown for irisin/FNDC5 protein in the soleus muscle fibers of the control (A and G) and exercised camels (H–J). Note the positive reactivity of irisin/FNDC5 in the endothelial lining of the blood vessels (F and G). Negative control (−ve) showing no reaction for irisin/FNDC5. Scale bar, 50 μm (C and F) and 20 μm (A, B, D, E, G-J, and −ve).
Under the effect of exercise, irisin/FNDC5 immunoreactivities became extremely obvious and showed more dense brown staining in both the gastrocnemius (Fig. 1I–L) and soleus camel muscle fibers (Fig. 2H–J) than that of the control gastrocnemius (Fig. 1E–H) and soleus muscles (Fig. 2A–G). Moreover, irisin/FNDC5 was more intensely localized and distributed in the muscle fiber membranes, sarcoplasm, and myofibrils of exercised gastrocnemius skeletal muscle fibers (Fig. 1I–L) and soleus (Fig. 2H–J) compared with their respective controls (Figs. 1E–H and 2A–G). Additionally, the numbers of immune-reacted muscle fibers with the irisin/FNDC5 antibody in the exercised camel skeletal muscles (Figs. 1I–L and 2H–J) were highly increased compared with the controls (Figs. 1E-H and 2A-G). No immunostaining for irisin/FNDC5 was seen in the negative control slides (Figs. 1D and 2−ve).
Using a graduated scale ruler, we noticed a marked increase in the width (1.4 ± 0.2 vs 0.5 ± 0.1 cm; P < 0.001) and length (5.2 ± 0.2 vs 2.5 ± 0.1 cm; P < 0.001) of the soleus muscles in the exercised camels in comparison with that of the controls.
3.2. Expression and cellular localization of irisin/FNDC5 protein in the camel white adipose tissue depots
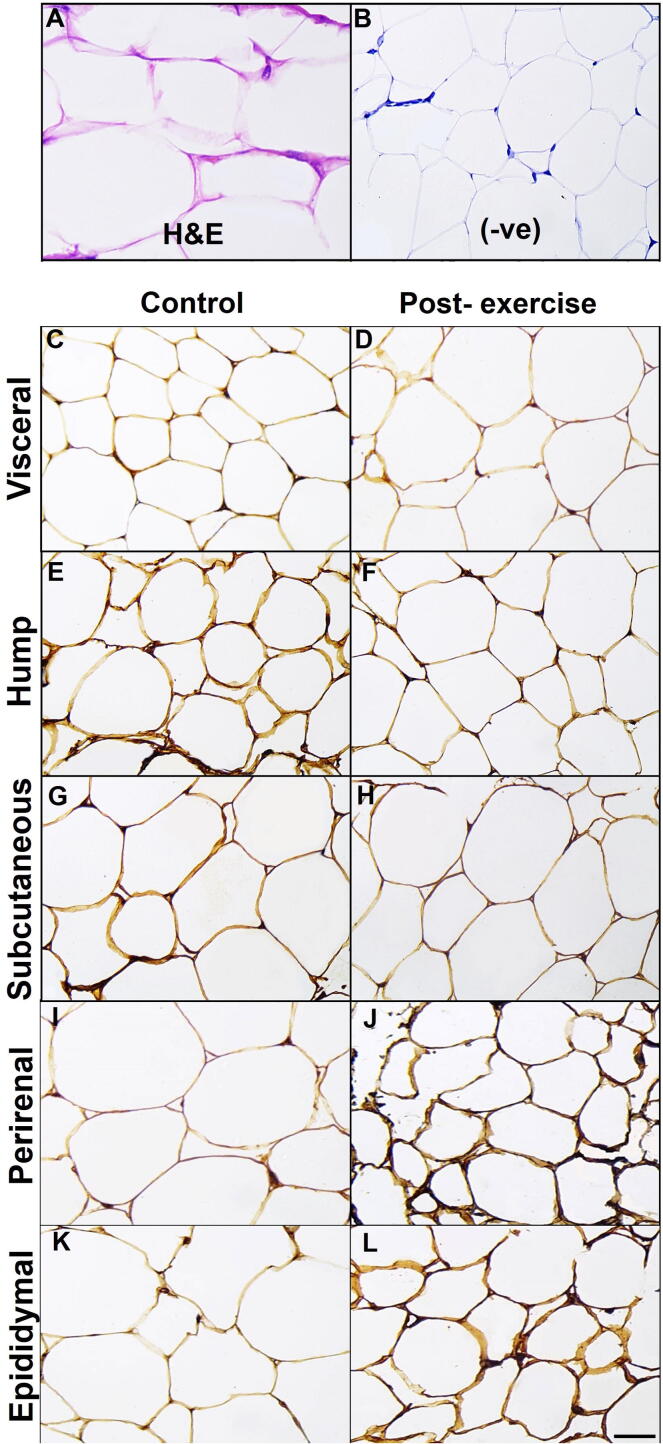
Histologically, adipose tissue contained a large number of fat cells or adipocytes arranged into lobules (Fig. 3A). Each adipocyte contained a large lipid droplet that fills the bulk of the cell. The cytoplasm and nucleus of fat cells were confined to the periphery (Fig. 3A).
Fig. 3.
Cellular localization of irisin/FNDC5 in the white adipose tissues of the control and exercised camels. (A) Histological section of visceral adipose tissue in the control camels. Immunohistochemical images are shown for irisin/FNDC5 protein in various white adipose tissues of the control (C–K) and exercised camels (D–L). (B) Representative negative control section. Scale bar, 20 μm.
Immunohistochemical detection of irisin/FNDC5 protein was studied in five white adipose depots (hump, subcutaneous, visceral, epididymal, and perirenal) of the camels. As shown in Figure (3), irisin/FNDC5 immunopositive signals were confined to the adipocyte cell membrane in all of the examined white adipose depots of the camels.
Interestingly, the adipocytes in the hump and subcutaneous tissues of the exercised camels (Fig. 3F and H, respectively) displayed less positive staining intensity for irisin/FNDC5 than the controls (Fig. 3E and G, respectively). Conversely, the irisin/FNDC5 in the perirenal and epididymal adipocytes of the exercised camels (Fig. 3J and L, respectively) exhibited darker and stronger intensity as well as an increase in the number of immunopositive adipocytes compared with the controls (Fig. 3I and K, respectively). No differences could be seen between the visceral adipocytes of the exercised (Fig. 3D) and control (Fig. 3C) camels for irisin/FNDC5. Negative control preparations revealed no immunostaining for irisin/FNDC5 in the camel adipocytes (Fig. 3B).
3.3. Baseline concentrations of irisin hormone in the camels’ circulating serum, skeletal muscles, and white adipose tissues
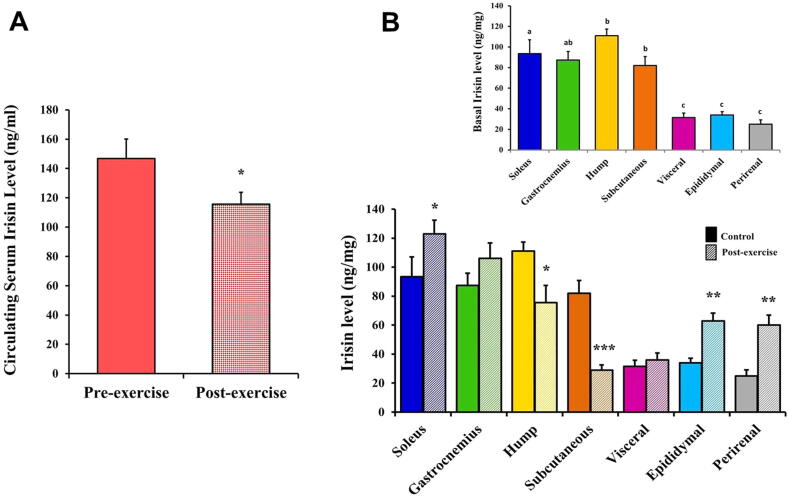
The baseline values of the irisin protein levels were detected using ELISA kits and measured, for the first time, in the camel serum (Fig. 4A) and tissue supernatant of the skeletal muscles (soleus and gastrocnemius) and white adipose tissues (hump, subcutaneous, visceral, epididymal, and perirenal) (inset in Fig. 4B). The camel irisin levels using EK-067-29 Phoenix and MET-5089 Cell Biolabs irisin ELISA kits showed relatively similar results (data not shown).
Fig. 4.
Circulating and tissue irisin levels under the effect of exercise in the camels. (A) Irisin levels (ng/ml) in the serum of the pre- and post-exercised camels. (B) Irisin levels (ng/mg) in the skeletal muscles and white adipose tissues of the control and exercised camels. Inset in B: Basal irisin concentrations in the skeletal muscles and white adipose tissue depots of the camels. Values with different letters are significantly different. Significant difference was identified at *P < 0.05, **P < 0.01, and ***P < 0.001 compared to the control values.
As shown in Fig. 4A, the camels’ serum irisin level was 146.87 ± 13.2 ng/ml. Regarding the tissue irisin level in the examined control camel samples (inset in Fig. 4B), irisin in the soleus muscles (93.5 ± 13.5 ng/mg) did not significantly differ (P ˃ 80.05) from the level in the gastrocnemius muscles (87.33 ± 8.4 ng/mg).
Among the various camel white adipose tissue depots, the hump exhibited significantly (P < 0.01) higher irisin concentrations (111 ± 6.4 ng/mg) than the level in the subcutaneous adipose (82 ± 8.76 ng/mg) (inset in Fig. 4B). No significant difference (P ˃ 0.05) was observed in the irisin concentration between the visceral (31.5 ± 4.28 ng/mg), epididymal (34 ± 3.12 ng/mg), and perirenal (25 ± 4.2 ng/mg) adipose tissues (inset in Fig. 4B). In the white adipose tissues of the control camels, the irisin levels in the hump and subcutaneous adipose tissue were significantly (P < 0.01) higher than in the visceral, epididymal, and perirenal adipose tissues (inset in Fig. 4B). The irisin level in the hump was significantly (P < 0.05) greater than that in the gastrocnemius muscles. Moreover, there was no statistically significant difference (P ˃ 0.05) in the irisin levels between the hump and soleus muscles or between the gastrocnemius muscles and subcutaneous adipose (Inset in Fig. 4B).
Collectively, among the examined samples in the control camel group, the irisin protein levels were in the following order: serum ˃ hump ≥ soleus ≥ gastrocnemius ≥ subcutaneous ˃ epididymal ≥ visceral ≥ perirenal (inset in Fig. 4B).
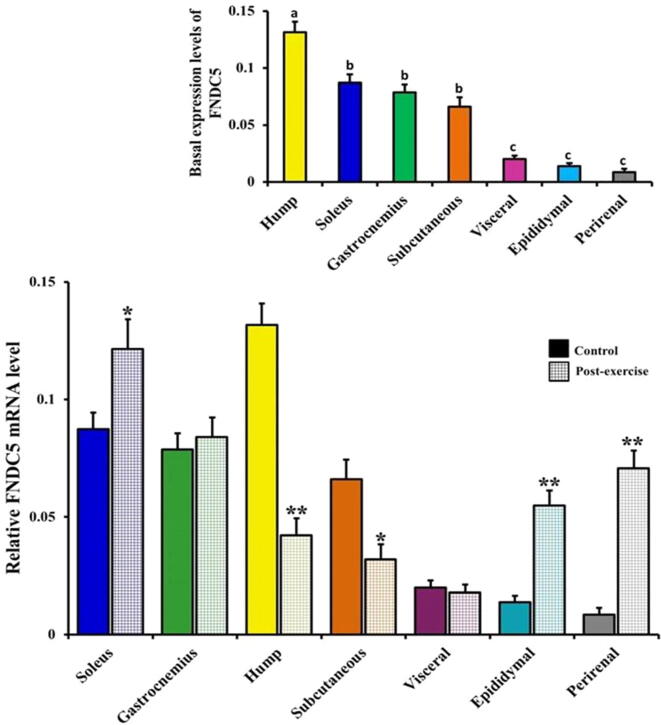
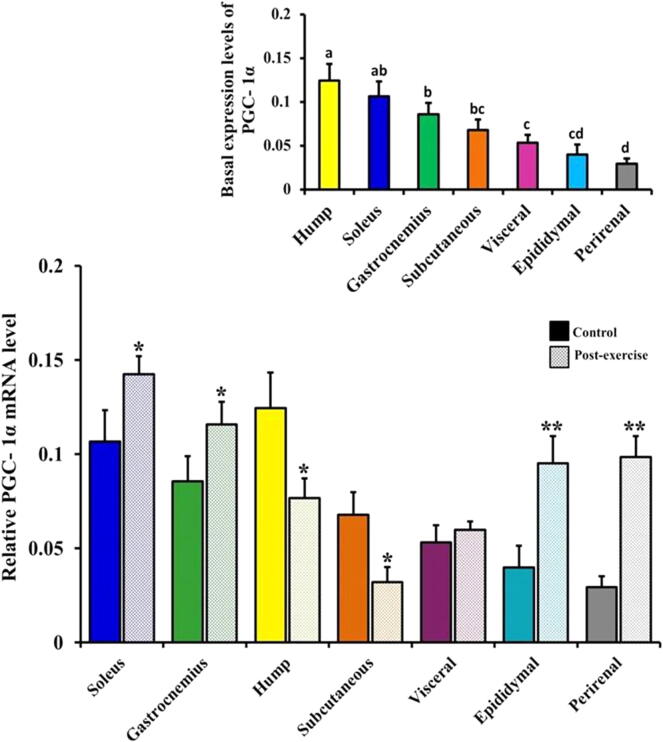
3.4. Baseline expression levels of FNDC5 and PGC-1α genes in the camel skeletal muscles and white adipose tissues
The expression and normal distribution patterns of FNDC5 mRNA (inset in Fig. 5) and PGC-1α mRNA (inset in Fig. 6) were clearly shown in the various skeletal and white adipose tissues of the control camels as assessed via real-time RT-PCR. The differential mRNA expression levels in both FNDC5 (inset in Fig. 5) and PGC-1α (inset in Fig. 6) displayed the highest expression levels in the hump (0.1316 ± 0.0091 and 0.1244 ± 0.019, respectively), subcutaneous adipose (0.0661 ± 0.0082 and 0.0678 ± 0.012, respectively), soleus (0.0874 ± 0.0070 and 0.1066 ± 0.0167, respectively), and gastrocnemius (0.0788 ± 0.0069 and 0.0857 ± 0.0132, respectively) as well as modest expression levels in the depots of the epididymal (0.0137 ± 0.0028 and 0.0398 ± 0.0116, respectively), visceral (0.0201 ± 0.0030 and 0.0532 ± 0.009, respectively), and perirenal adipose tissues (0.0084 ± 0.0033 and 0.0295 ± 0.0057, respectively).
Fig. 5.
Tissue distribution and differential gene expression level of FNDC5 in the skeletal muscles and white adipose tissues of the control and exercised camels. Real-time PCR analysis of FNDC5 expression level in the skeletal muscles (soleus and gastrocnemius) and white adipose tissue depots (hump, subcutaneous, visceral, epididymal, and perirenal) in the control and exercised camels are shown. Inset: Basal expression level of FNDC5 in the camels’ skeletal muscles and white adipose tissue depots. Values with different letters are significantly different. Significant difference was identified at *P < 0.05 and **P < 0.01 compared to the control values.
Fig. 6.
Tissue distribution and differential gene expression level of PGC-1α in the skeletal muscles and white adipose tissues of the control and exercised camels. Real-time PCR analysis of the PGC-1α expression level in the skeletal muscles (soleus and gastrocnemius) and white adipose tissue depots (hump, subcutaneous, visceral, epididymal, and perirenal) in the control and exercised camels are shown. Inset: Basal expression level of PGC-1α in the camels’ skeletal muscles and white adipose tissue depots. Values with different letters are significantly different. Significant difference was identified at *P < 0.05 and **P < 0.01 compared to the control values.
3.5. Effect of exercise on camels
In the second part of our study, we explored the effect of exercise on camels by investigating the effect of acute exercise on the levels of irisin protein, FNDC5 mRNA, and PGC-1α mRNA. We also studied the effect of exercise on some hormones and energetic parameters involved in energy regulation in camels.
3.6. Effect of exercise on the circulating serum irisin level in camels
As presented in Figure (4A), the camels’ serum irisin level was significantly (P < 0.05) lower post-exercise than pre-exercise (115.52 ± 8.31vs 146.87 ± 13.2 ng/ml).
3.7. Effect of exercise on irisin production and gene expression of FNDC5 and PGC-1α in the camels
The irisin protein levels in the examined camel tissues were altered in a different manner (unchanged, reduced, or elevated) by acute exercise. The irisin concentrations significantly decreased post-exercise in the supernatants of the hump and subcutaneous white adipose tissues of camels compared with the control levels (75.5 ± 11.8 vs 111 ± 6.4 ng/mg; P < 0.05 and 29 ± 3.64 vs 82 ± 8.76 ng/mg; P < 0.001, respectively) (Fig. 4B). The irisin levels were significantly elevated in soleus muscles (123 ± 9.44 vs 93.5 ± 13.5 ng/mg; P < 0.05) as well as in the epididymal (63 ± 5.28 vs 34 ± 3.12 ng/mg; P < 0.01) and perirenal (60 ± 6.8 vs 25 ± 4.2 ng/mg; P < 0.01) white adipose tissues of the camels post-exercise compared with the corresponding control levels (Fig. 4B).
However, no significant changes (P ˃ 0.05) were observed post-exercise in the irisin protein content in the gastrocnemius muscles (106 ± 10.8 vs 87.33 ± 8.4 ng/mg) and visceral adipose tissue (36 ± 4.68 vs 31.5 ± 4.28 ng/mg) compared with the control levels (Fig. 4B).
The gene expression levels of FNDC5 and PGC-1α were regulated by exercise in a tissue-specific manner in the camel skeletal muscles and white adipose tissues (Fig. 5, Fig. 6). Under the effect of acute exercise, the FNDC5 (Fig. 5) and PGC-1α (Fig. 6) mRNA levels significantly decreased in the camel hump compared to their respective levels in the controls (0.0422 ± 0.0072 vs 0.1316 ± 0.0091; P < 0.01 and 0.0768 ± 0.0103 vs 0.1244 ± 0.019; P < 0.05, respectively). A significant decrease (P < 0.05) was also detected in the subcutaneous FNDC5 (Fig. 5) and PGC-1α (Fig. 6) mRNA levels in the exercised camels compared to the controls (0.0319 ± 0.0065 vs 0.0661 ± 0.0082 and 0.032 ± 0.0081 vs 0.0678 ± 0.012, respectively).
Conversely, the mRNA levels of FNDC5 and PGC-1α (Fig. 5, Fig. 6) were significantly higher in the soleus muscles, epididymal, and perirenal adipose tissues of the exercised camels than the corresponding controls (0.1214 ± 0.0127 vs 0.0874 ± 0.0070; P < 0.05 and 0.1424 ± 0.0096 vs 0.1066 ± 0.0167; P < 0.05, respectively, in the soleus muscles; 0.0549 ± 0.0063 vs 0.0137 ± 0.0028; P < 0.01 and 0.0952 ± 0.0145 vs 0.0398 ± 0.0116; P < 0.01, respectively, in the epididymal adipose tissues, and 0.0707 ± 0.0075 vs 0.0084 ± 0.0033; P < 0.01 and 0.0985 ± 0.011 vs 0.0295 ± 0.0057; P < 0.01, respectively, in the perirenal adipose tissues). As shown in Fig. 5, Fig. 6, the gastrocnemius muscles of the exercised camels had a non-significant increase in the FNDC5 mRNA levels compared to the levels in the control group (0.0840 ± 0.0083 vs 0.0788 ± 0.0069; P ˃ 0.05) and a significant increase in the PGC-1α mRNA levels compared with the respective control levels (0.1159 ± 0.0119 vs 0.0857 ± 0.0132; P < 0.05).
Changes in the levels of FNDC5 and PGC-1α mRNAs (Fig. 5, Fig. 6) were not significantly obvious in the visceral adipose tissues of the exercised camels compared with the respective controls (0.0178 ± 0.0034 vs 0.0201 ± 0.0030; P ˃ 0.05 and 0.0598 ± 0.0045 vs 0.0532 ± 0.009; P ˃ 0.05, respectively).
3.8. Effect of exercise on the circulating levels of various hormones involved in the energy metabolism of camels
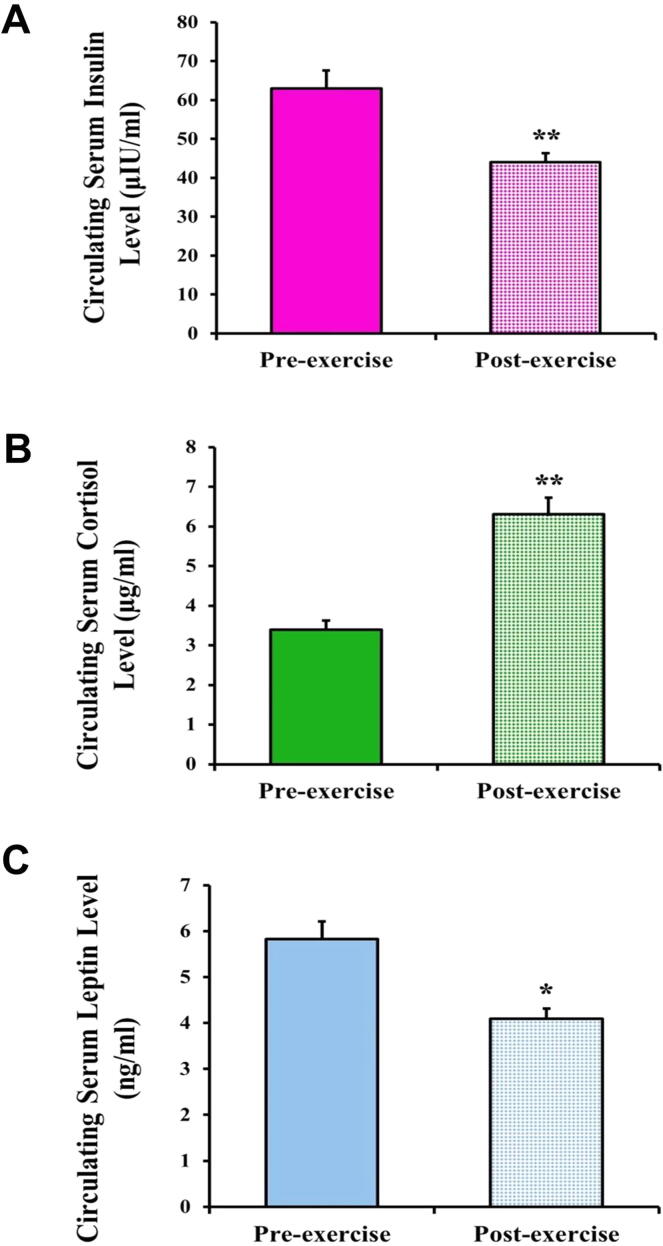
As a result of acute exercise, the camel serum circulating insulin levels were significantly decreased compared with the pre-exercise values (44 ± 2.3 vs 63 ± 4.6; P < 0.01) (Fig. 7A). Furthermore, the camels post-exercise had significantly lower circulating leptin levels than pre-exercise (4.09 ± 0.22 vs 5.83 ± 0.38; P < 0.05) (Fig. 7C). However, the cortisol levels in the post-exercise camels were significantly elevated compared to those measured pre-exercise (6.3 ± 0.43vs 3.4 ± 0.23; P < 0.01) (Fig. 7B).
Fig. 7.
Effect of exercise on the levels of circulating hormones involved in energy metabolism regulation in the camels. Effect of exercise on the camels’ serum levels of (A) insulin, (B) cortisol, and (C) leptin are shown. Data are expressed as means ± SD. *P < 0.05, **P < 0.01.
3.9. Effect of exercise on some energetic parameters in camels
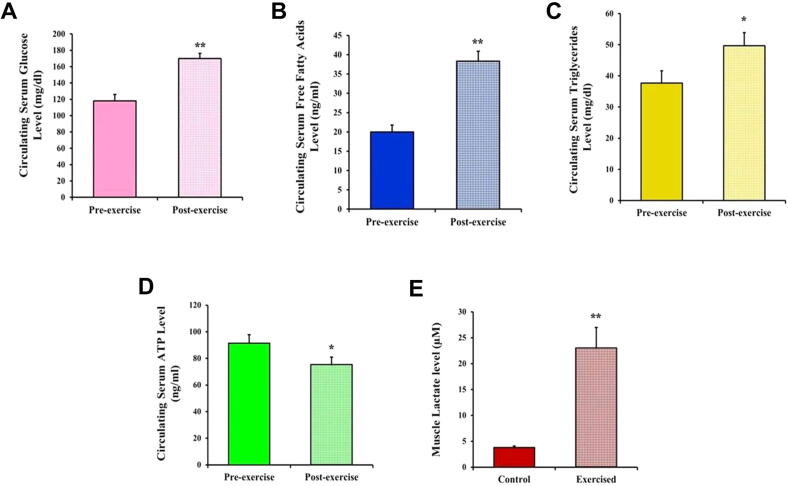
Following exercise, the camels’ blood glucose levels were significantly higher compared to the pre-exercise concentrations (170 ± 6.3 vs 118 ± 7.8; P < 0.01), as presented in Fig. 8A. The camels’ post-exercise serum FFA concentrations were significantly higher than the respective pre-exercise values (38.33 ± 2.6 vs 20 ± 1.8; P < 0.01) (Fig. 8B). Additionally, after exercise, serum triglycerides showed a significant increase compared with the pre-exercise levels (49.67 ± 4.215 vs 37.72 ± 3.891; P < 0.05) (Fig. 8C). The serum ATP levels were significantly decreased post-exercise compared with the pre-exercise values (75.25 ± 5.8 vs 91.5 ± 6.3; P < 0.05) (Fig. 8D). Moreover, muscle lactate was significantly increased over the pre-exercise values (23 ± 4.0 vs 3.8 ± 0.12; P < 0.01) (Fig. 8E).
Fig. 8.
Effect of exercise on the levels of some energetic metabolites in the camels. Effect of exercise on the camels’ (A) glucose, (B) free fatty acids, (C) triglycerides, (D) ATP, and (E) muscle lactate are shown. Data are expressed as means ± SD. *P < 0.05, **P < 0.01.
3.10. Correlations between the examined variables in the camels
A significant positive correlation has been detected between the circulating irisin levels and each of the irisin content in the hump and subcutaneous adipose tissues of the exercised camels (Table 1). Whereas, the circulating irisin levels were inversely correlated with the irisin content in the soleus and gastrocnemius muscles as well as with the epididymal and perirenal adipose tissues of the exercised camels (Table 1).
Table 1.
Pearson correlations (r) of tissue irisin contents in the skeletal muscle and adipose tissues of the exercised camels with the gene expression levels of FNDC5 and PGC-1α and the circulating serum irisin.
| Circulating Irisin | FNDC5 | PGC-1α | |||
|---|---|---|---|---|---|
| Tissue Irisin | Soleus | r | −0.408 | 0.803 | 0.776 |
| P | 0.0425 | 0.0121 | 0.0132 | ||
| Gastrocnemius | r | −0.369 | 0.745 | 0.666 | |
| P | 0.0480 | 0.0174 | 0.0293 | ||
| Hump | r | 0.877 | 0.698 | 0.898 | |
| P | 0.0011 | 0.0201 | 0.00097 | ||
| Subcutaneous | r | 0.792 | 0.555 | 0.788 | |
| P | 0.0124 | 0.0483 | 0.0115 | ||
| Visceral | r | −0.141 | 0.562 | 0.311 | |
| P | 0.1355 | 0.0463 | 0.0092 | ||
| Epididymal | r | −0.495 | 0.911 | 0.688 | |
| P | 0.0462 | 0.001 | 0.0233 | ||
| Perirenal | r | −0.586 | 0.887 | 0.705 | |
| P | 0.0431 | 0.0011 | 0.0201 | ||
Significant difference was identified at P < 0.05, P < 0.01, and P < 0.001. n = 6.
As presented in Table 1, the irisin content in the skeletal muscles and white adipose depots of exercised camels was significantly and positively associated with the corresponding FNDC5 and PGC-1α gene expression levels. Additionally, the FNDC5 mRNA levels also showed significant positive correlation with the PGC-1α mRNA levels in all the examined tissues of exercised camels (Table 2).
Table 2.
Pearson correlations (r) between gene expression levels of FNDC5 and PGC-1α in the skeletal muscle and adipose tissues of the exercised camels.
| PGC-1α | |||
|---|---|---|---|
| Tissue FNDC5 | Soleus | r | 0.436 |
| P | 0.0482 | ||
| Gastrocnemius | r | 0.389 | |
| P | 0.0222 | ||
| Hump | r | 0.887 | |
| P | 0.0010 | ||
| Subcutaneous | r | 0.892 | |
| P | 0.0006 | ||
| Visceral | r | 0.454 | |
| P | 0.0175 | ||
| Epididymal | r | 0.855 | |
| P | 0.0017 | ||
| Perirenal | r | 0.686 | |
| P | 0.0227 | ||
Significant difference was identified at P < 0.05, P < 0.01, and P < 0.001. n = 6.
In camels, the difference between the post- and pre-exercise values (post-pre exercise changes) in the serum irisin exhibited significantly positive correlations with the corresponding serum levels of insulin, leptin, and ATP and significantly negative correlations with the corresponding levels of glucose, FFA, triglycerides, and cortisol (Table 3).
Table 3.
Pearson’s correlations between circulating irisin and other circulating variables in camels under the effect of exercise.
| Circulating Hormones & Metabolites | |||
|---|---|---|---|
| Circulating IRISIN | Insulin | r | 0.513 |
| P | 0.0456 | ||
| Cortisol | r | −0.522 | |
| P | 0.0409 | ||
| Leptin | r | 0.560 | |
| P | 0.041 | ||
| Glucose | r | −0.533 | |
| P | 0.0372 | ||
| Free fatty acids | r | −0.693 | |
| P | 0.0132 | ||
| Triglycerides | r | −0.520 | |
| P | 0.0415 | ||
| ATP | r | 0.558 | |
| P | 0.0469 | ||
Correlation was carried out using the difference between post- and pre- exercise values
Significant difference was identified at P < 0.05.
Table 4 showed that the post-pre exercise changes in camel circulating insulin levels were significantly and negatively correlated with the corresponding changes in serum glucose, FFA, and cortisol levels and significantly positive correlated with the serum leptin levels. The difference between post and pre exercise values of circulating cortisol levels showed significantly positive correlations with the corresponding changes in serum levels of glucose and FFA, whereas it had significantly negative correlations with the camels’ serum leptin levels (Table 4). Moreover, the correlation of the post-pre exercise changes in the camel serum leptin levels was significantly negative with the corresponding serum glucose and FFA levels (Table 4).
Table 4.
Pearson’s correlations (r) between circulating hormones and metabolites levels in camels under the effect of exercise.
| Circulating Hormones |
|||||
|---|---|---|---|---|---|
| Insulin | Cortisol | Leptin | |||
| Circulating Hormones & Metabolites | Cortisol | r | −0.860 | ||
| P | 0.0001 | ||||
| Leptin | r | 0.68 | −0.720 | ||
| P | 0.0153 | 0.0094 | |||
| Glucose | r | −0.811 | 0.847 | −0.849 | |
| P | 0.0007 | 0.0003 | 0.001 | ||
| Free fatty acids | r | −0.79 | 0.853 | −0.624 | |
| P | 0.0036 | 0.0009 | 0.0493 | ||
Correlation was carried out using the difference between post- and pre- exercise values.
Significant difference was identified at P < 0.05 and P < 0.001.
4. Discussion
The main highlights of the present study are: (i) irisin is present in the camel circulation; (ii) irisin protein as well as FNDC5 and PGC-1α genes are present in the skeletal muscles and white adipose tissues of camels; (iii) irisin/FNDC5 protein is cellularly localized on the sarcolemma, myofibrils, and sarcoplasm of camel skeletal muscle fibers as well as on the adipocyte cell membranes of camels’ white adipose tissues; (iv) the baseline concentration of irisin protein content was quantified in the camel blood, skeletal muscles, and various white adipose tissue depots in the following order: serum ˃ hump ≥ soleus ≥ gastrocnemius ≥ subcutaneous ˃ epididymal ≥ visceral ≥ perirenal; (v) the profiles of the FNDC5 and PGC-1α mRNAs consistently matched the profile of the irisin protein levels in all of the examined camel tissues; (vi) exercise significantly decreased the camels’ circulating irisin levels; (vii) exercise induced tissue-specific changes in the irisin protein and gene expression of the camels’ FNDC5 and PGC-1α, which subsequently regulated the circulating irisin content; (viii) under the effect of exercise, among the examined camel tissues, the irisin contents and expression levels of FNDC5 and PGC-1α in the hump and subcutaneous white adipose tissues were downregulated and positively associated with the decreased circulating irisin level; (ix) exercise significantly upregulated the gene expression of FNDC5 and PGC-1α as well as the irisin levels in the soleus muscles and epididymal and perirenal white adipose depots; (x) in the camels, acute exercise significantly increased the serum glucose, FFA, triglycerides, and cortisol levels; (xi) the serum insulin and leptin levels significantly decreased in the camels post-exercise; (xii) in vivo FNDC5 and PGC-1α gene expression and irisin secretion were regulated by endogenous signals linked with exercise in the camels; and (xiii) the overall associations of the metabolic and hormonal responses to exercise with the expression patterns of irisin/FNDC5/PGC-1α related specifically to camels suggest the potential involvement of glucose and FFA as well as insulin, leptin, and cortisol in the regulation of the PGC-1α/FNDC5/irisin pathway in the camels.
4.1. Irisin/FNDC5 is localized in skeletal muscles and white adipose tissues of camels
The immunohistochemical images (Fig. 1, Fig. 2, Fig. 3) gave evidence for the first time of the presence and location of irisin/FNDC5 protein in the skeletal muscle and white adipose depots of camels. As expected for a transmembrane protein, the predominant location of irisin/FNDC5 on the muscle fiber and adipocyte cell membranes suggested that it may play distinct potential physiological roles in camel metabolism.
The immunohistochemical findings (Fig. 1, Fig. 2) were in accordance with others in humans, mice, and cattle. For instance, FNDC5 was detected in the sarcolemma and cytosol of cattle semitendinosus muscle (Komolka et al., 2014). In humans, irisin was detected in skeletal muscles (Piya et al., 2014) and irisin/FNDC5 was identified in visceral and subcutaneous adipose tissue sections (Pérez-Sotelo et al., 2017). In mice, immunohistochemical analysis confirmed the presence of irisin/FNDC5 in skeletal muscles (Dun et al., 2013, Brenmoehl et al., 2014, Amengual et al., 2018) and inguinal and epididymal white adipose tissues (Amengual et al., 2018). Conversely, irisin immunoreactivity was absent from skeletal muscles of young and old rats, in which traces of irisin were shown only in the perimysium of skeletal muscles (Aydin et al., 2014).
4.2. Basal irisin levels were quantified in camel serum, skeletal muscles, and white adipose tissues
The immunohistochemical data (Fig. 1, Fig. 2, Fig. 3) were further confirmed by the biochemical detection of irisin protein content in the camels (Fig. 4). This study provides novel information regarding the presence and measurement of basal irisin levels in camel blood, skeletal muscles, and various white adipose depots at the intracellular level using ELISA (Fig. 4A and inset in B).
Consistent with our findings, the circulating basal irisin levels have been detected in rodents (Aydin et al., 2014, Varela-Rodríguez et al., 2016) and humans (Huh et al., 2012, Kurdiova et al., 2014). In contrast to our results in camels, irisin in cattle was neither detectable in the circulating plasma nor in muscles under resting conditions despite the abundant presence of FNDC5 mRNA in cattle skeletal muscles (Komolka et al., 2014).
Results from rodents and humans have shown that irisin/FNDC5 is not only present in skeletal muscles but is also expressed and secreted by adipose tissues (Huh et al., 2012, Roca-Rivada et al., 2013, Moreno-Navarrete et al., 2013, Kurdiova et al., 2014, Varela-Rodríguez et al., 2016, Amengual et al., 2018). This was also demonstrated by our findings (inset in Fig. 4B) suggesting a role of irisin as an adipo-myokine in camels. Interestingly, we confirmed that the basal level of irisin in the examined camel tissues was highest in the hump, soleus muscles, and relatively high and almost comparable in the gastrocnemius muscles and subcutaneous adipose tissues (inset in Fig. 4B). However, the camels’ visceral, epididymal, and perirenal adipose tissues exhibited significantly lower irisin protein expression levels (Inset in Fig. 4B). These results were in line with the preliminary observations of Kurdiova et al. (2014), who indicated that levels of irisin/FNDC5 protein detected by EIA assay in human subcutaneous adipose tissues might be comparable to those in muscle.
The present findings (inset in Fig. 4B) were consistent with the immunoblotting results, which revealed that rat skeletal muscle secreted more irisin/FNDC5 protein than subcutaneous and visceral adipose tissues and subcutaneous adipose expressed and secreted more irisin/FNDC5 than visceral adipose tissue in rats (Roca-Rivada et al., 2013).
Specifically for camels, our results (inset in Fig. 4B) verified that hump adipose tissues expressed significantly more irisin levels than the examined skeletal muscles and other white adipose depots along with the high irisin content in subcutaneous adipose, supporting the idea that the regulation of irisin production/release in/from adipose tissues might contribute to circulating irisin levels in camels.
4.3. FNDC5 and PGC-1α genes are expressed in skeletal muscles and white adipose of camels
Another new finding in the present study that could add knowledge to camel research was the presence of FNDC5 and PGC-1α genes in the camels (Fig. 5, Fig. 6). Notably, the FNDC5 and PGC-1α levels in the camels’ skeletal muscles and white adipose tissues under basal conditions correlated well and clearly matched the same irisin order, in which tissues with very high irisin content also showed markedly high levels of FNDC5 and PGC-1α gene expression and vice versa (inset in Fig. 5, Fig. 6).
Our results confirmed the previous study of Wrann et al. (2013), who reported that FNDC5 gene expression correlated with PGC-1α expression levels in various mice tissues. In agreement with earlier reports, among the tissues examined in humans (Huh et al., 2012) and mice (Wrann et al., 2013), the highest level of FNDC5 gene expression was detected in muscles. Consistent with results from mice, FNDC5 expression was higher in the soleus muscles, which also contain higher levels of PGC-1α than gastrocnemius muscles (Wrann et al., 2013). In addition, we confirmed the previous observations in human (Huh et al., 2012, Moreno-Navarrete et al., 2013, Kurdiova et al., 2014)and rodent studies (Roca-Rivada et al., 2013) in which FNDC5 mRNA was expressed in subcutaneous and visceral adipose tissues at lower levels than in skeletal muscles.
4.4. Exercise significantly decreases the circulating irisin level in camels
To the best of our knowledge, no studies have investigated the regulation of irisin/FNDC5 in camel species. Surprisingly, the present study detected hypo-irisinemia in the camels after acute exercise (Fig. 4A). However, many studies on the effect of acute exercise on serum irisin levels in other species showed contradicting results. It has been reported that serum irisin levels increased after acute exercise in humans (Kraemer et al., 2014, Norheim et al., 2014, Huh et al., 2015, Nygaard et al., 2015, Löffler et al., 2015), rats (Aydin et al., 2014), and mice (Brenmoehl et al., 2014). Other studies reported that acute exercise did not influence serum irisin levels in humans (Kurdiova et al., 2014) and rats (Czarkowska-Paczek et al., 2014).
4.5. Exercise induces tissue-specific regulation of irisin, FNDC5, and PGC-1α in skeletal muscles and white adipose depots of camels
Interestingly, the present results clarified that in camels, there was tissue-specific regulation in FNDC5 and PGC-1α mRNA expression levels as well as irisin protein content in the skeletal muscles and white adipose tissues after acute exercise (Fig. 5, Fig. 6).
The ELISA measurement of tissue irisin content (Fig. 4B) and the results of real-time PCR for FNDC5 in the present study (Fig. 5) further confirmed the distinguishable differences in the immunoreactive intensity and number of immunopositive cells for irisin/FNDC5 protein in the skeletal muscles and white adipose tissues depots between the exercised and control camels.
We proved that the mRNA levels of PGC-1α were consistently parallel to FNDC5 mRNA and tissue irisin protein levels in the skeletal muscles and adipose tissues of the exercised camels (Table 1). Additionally, our results demonstrated that the expression of FNDC5 was significantly and positively correlated with the expression of PGC1-α in all of the examined tissues of the exercised camels (Table 2), consistent with studies on exercised human skeletal muscles and adipose tissues (Huh et al., 2012, Lecker et al., 2012, Moreno-Navarrete et al., 2013, Kurdiova et al., 2014) and exercised muscles of mice (Boström et al., 2012), suggesting the involvement of PGC1-α in the regulation of FNDC5 gene expression in the exercised camel tissues and that FNDC5 and PGC1-α genes are crucial factors for irisin protein regulation in camels.
The irisin protein content and mRNA expression levels of FNDC5 and PGC-1α in the camels’ soleus muscles were significantly elevated after exercise (Figs. 4B, 5, and 6). Moreover, the noticeable increase in the width and length of the camels’ soleus muscles after exercise in comparison to the controls was probably consistent with the increase in irisin content in the soleus muscles in response to exercise. One of the characteristic hallmarks of exercise is the induction of skeletal muscle hypertrophy (Marini and Veicsteinas, 2010). Irisin also induced hypertrophy in mice skeletal muscles (Reza et al., 2017).
Although the irisin content and FNDC5 mRNA expression in the gastrocnemius muscles was not statistically different between the exercised and control camels, the gastrocnemius of the exercised camels tended to present greater levels compared to the controls. Studies have shown no significant differences in skeletal muscle FNDC5 levels under the effect of acute exercise in rats and humans (Czarkowska-Paczek et al., 2014, Kurdiova et al., 2014). However, following acute exercise, FNDC5 mRNA in the muscles was upregulated in mice (Boström et al., 2012) and humans (Norheim et al., 2014, Nygaard et al., 2015). Our findings (Figs. 4B, 5, and 6 and Table 1) were in agreement with studies conducted on mice (Boström et al., 2012) and humans (Norheim et al., 2014) that concluded that the exercise-induced expression of FNDC5/irisin in muscles was dependent on increased PGC-1α mRNA.
Different white adipose depots may vary in their physiological functions and impact on metabolism (Pond, 1992, Bjørndal et al., 2011). Therefore, comparing the effect of exercise on white adipose tissues from different anatomical locations in camels may offer the possibility of differential contributions from these depots to the circulating irisin levels.
In the present study, the diverse gene expression profiles of irisin, FNDC5, and PGC-1α observed in the examined depots of camels’ white adipose tissues after exercise (Figs. 4B, 5, and 6) proved that the adipose cells in varying depots responded differently to exercise, which resulted in differences in the production and release of irisin from these depots and hence they may differ in their physiological tasks. Our study confirmed the results by Varela-Rodríguez et al. (2016), who showed that there were differences in FNDC5 expression depending on white adipose tissue depots (visceral, epididymal, and subcutaneous) in rats after long-term caloric restriction.
The significantly increased levels of irisin content in the soleus muscles and epididymal and perirenal adipose tissues of the camels post-exercise (Fig. 4B) were proportional to the increases observed in the FNDC5 and PGC-1α mRNA levels in these tissues after exercise (Table 1). Additionally, the lower irisin levels in the serum (Fig. 4A) and the higher irisin levels detected in the soleus muscles, epididymal, and perirenal adipose tissues of the camels under the effect of exercise (Fig. 4B) may also suggest the involvement of irisin uptake from the circulation into these tissues post-exercise. The mechanisms underlying the effect of exercise on irisin uptake remain unknown. However, we agreed with Kartinah and Sianipar (2018), who posited that exercise, as a physiological stress, would result in an increase in the transfer of irisin from the circulation into the adipose tissues of rats. Chen et al. (2017) suggested that under physiological stress, the presence of irisin uptake from the serum to the lungs was demonstrated in human patients with neonatal respiratory distress syndrome. In this context, Chen et al. (2017) confirmed an important role of lipid raft-mediated endocytosis in facilitating entry of irisin into mice alveolar cells subjected to ischemia-induced stress.
Although camels do not develop diabetes, they physiologically have high basal circulating glucose levels and low insulin sensitivity (Kaske et al., 2001, Ali et al., 2019), a state similar to that of type 2 diabetic patients. In camels, insulin does not develop a short- acting effect on glucose utilization (Elmahdi et al., 1997, Kaske et al., 2001, Ali et al., 2019). Thus, in the current study, the recognition of a greater decrease in insulin (Fig. 7A) and a greater increase in the circulating levels of glucose (Fig. 8A) and FFA (Fig. 8B) in the camels after exercise suggesting a partial or total interruption of the known actions of insulin. Consequently, this might contribute to decreasing the glucose uptake by the skeletal muscles and epididymal and perirenal adipose tissues under such physiological stress (exercise) in the camels and hence resulted in a higher increase in the circulating glucose levels (Fig. 8A).
Among the camel tissues examined in the present study, only the hump and subcutaneous adipose depots showed downregulation in their levels of irisin protein content and FNDC5 and PGC-1α mRNAs after exercise (Figs. 4B, 5, and 6) and hence positively correlated with the decreased circulating irisin levels (Table 1). No changes in the FNDC5/PGC-1α expression or irisin content could be seen in the visceral adipose post-exercise (Figs. 4B, 5, and 6). Therefore, the correlation analysis (Table 1) confirmed that the exercise-induced decrease in the circulating serum irisin levels was mainly due to the downregulated FNDC5/PGC-1α gene expression and irisin protein content in the camel hump and subcutaneous adipose tissues.
Our findings were in line with the results of earlier reports on humans and mice. Studies on FNDC5 gene expression in visceral and subcutaneous adipose tissues as well as skeletal muscles led Moreno-Navarrete et al. (2013) to suggest that it is the adipose tissue and not the skeletal muscle expression that correlates with circulating irisin levels in humans. It has been recognized that, in contrast to skeletal muscle, FNDC5 in adipose tissue and irisin in plasma decreased in type 2 diabetic patients (Kurdiova et al., 2014). Yang et al. (2015) concluded that FNDC5 protein expression in adipose tissue (subcutaneous), not skeletal muscle, contributed to changes in circulating irisin in high-fat diet-induced obese mice.
Our current data clearly presented consistent exercise-related downregulation of irisin protein in camel hump and subcutaneous white adipose tissue and circulating irisin, opposed by its increase in the skeletal muscles and epididymal and perirenal adipose depots of the camels after exercise. Therefore, the present study suggested the presence of tissue-specific mechanisms leading to the timely regulation of local and systemic irisin content under the effect of exercise, which is important for camels to tolerate and adapt to extreme conditions and maintain metabolic hemostasis.
4.6. Exercise induces hyper-glycemia, hypo-insulinemia, and hyper-cortisolemia in camels
Camels have exceptional carbohydrate metabolism. The normal high glucose and low insulin concentrations in camels could be physiologically related to different regulation factors than in true ruminants (Kaske et al., 2001). Our results proved that, in camels, exercise provoked an extra increase in the circulating glucose (Fig. 8A) along with a further decrease in the circulating insulin levels (Fig. 7A) than their basal control levels.
The present findings confirmed the presence of negative correlations between glucose and irisin in camels under the effect of exercise. Congruent with our results, serum irisin was negatively associated with hyper-glycemia and fasting glycemia in humans (Kurdiova et al., 2014). Our study revealed that insulin was found to be positively linked with irisin in camels under the effect of exercise (Table 3). In this context, Varela-Rodríguez et al. (2016) proved a relationship between insulin production and the circulating irisin levels in rats and demonstrated that exogenous irisin enhanced circulating insulin levels and that the reduction in FNDC5 gene expression in muscle and circulating irisin levels were associated with low endogenous insulin production. Moreover, serum irisin was reported to be increased after continuous subcutaneous insulin infusion in type 2 diabetes mellitus patients (Li et al., 2016). Our findings were also consistent with several reports that indicated that circulating irisin was significantly lower in type 2 diabetic humans (Liu et al., 2013, Moreno-Navarrete et al., 2013, Kurdiova et al., 2014, Hu et al., 2016) and rodents (Yang et al., 2015). In contrast, others showed irisin to be positively associated with insulin resistance (Park et al., 2013; Crujeiras et al., 2014, Ebert et al., 2014, Sesti et al., 2014).
The hypo-irisinemia-hyper-glycemia-hypo-insulinemia detected in the camels post-exercise in the present study (Figs. 4A, 8A, and 7A) might be considered adaptation mechanisms related to the specific physiological characteristics of the camel, suggesting a beneficial role of irisin in camel glucose homeostasis.
The present study confirmed that under the effect of exercise, the camels developed hyper-cortisolemia (Fig. 7B). This finding was in agreement with results reported by Saeb et al., 2010, El Khasmi et al., 2015, who reported hyper-cortisolemia in camels after a stressful event such as road transport and revealed that this increase was positively correlated with the transportation distance. Cortisol is released in response to stress in all mammals. Therefore, the significant increase in the circulating cortisol concentrations in our results may have been attributed to stress linked to exercise.
Based on our results (Fig. 7B), it is likely that the elevated serum cortisol levels may have contributed to increasing glycemia in the camels post-exercise, probably by increasing the gluconeogenesis rate. Glucose is nearly exclusively biosynthesized by gluconeogenesis in camels (Emmanuel, 1981). Moreover, it was indicated that cortisol resulted in a decrease in the cell glucose utilization rate (Hall, 2016). These findings could offer another explanation for the high increase in the camels’ blood glucose levels post-exercise.
Along with high gluconeogenesis, camels also have more efficient high lipo-mobilization (Ouajd and Kamel, 2009). Cortisol was also found to promote the mobilization of fatty acids from the adipose tissues (Hall, 2016). Consistent with this, our study demonstrated a significant increase in the camel blood FFA levels (Fig. 8B) accompanied by hyper-cortisolemia in the camels post-exercise (Fig. 7B).
4.7. Exercise increases FFA and triglycerides and decreases leptin levels in camels
Non-esterified fatty acids (NEFA) are important metabolic fuel. The basal blood FFA concentrations vary greatly between species. They are lower in camels than sheep and ponies (Kaske et al., 2001). Exercise increases white adipose tissue lipolysis and free fatty acid mobilization to supply the skeletal muscles with energy (Gollisch et al., 2009) The results of our study verified that exercise caused a significant increase in the camels’ circulating FFA and triglycerides (Fig. 8B and C), indicating that exercise induced lipolysis and mobilization of fatty acids from camel adipose depots due to the inhibition of glucose uptake.
It has been demonstrated that an increase in FFA blood levels decreased insulin-stimulated glucose uptake, with a subsequent increase in insulin resistance and development of type 2 diabetes in humans (Hoeks et al., 2006) and impaired insulin-stimulated glucose metabolism in rat skeletal muscle cells (Hirabara et al., 2010) In this regard, our results proved that the increase in the serum FFA levels observed in the camels post-exercise (Fig. 8A) was accompanied by increased serum glucose and cortisol levels and decreased serum irisin, insulin, and leptin levels (Figs. 4A, 7, and 8B). These findings were further confirmed by the correlation analysis (Table 3, Table 4).
In addition to insulin, leptin is considered one of the main hormones controlling glucose and lipid metabolism in ruminants. Leptin is mainly secreted by the adipose tissues of one-humped camels and plays an important role in the regulation of energy metabolism (Chilliard et al., 2005). The influence of acute exercise on leptin concentrations in humans and rats is conflicting; some studies revealed that exercise decreased leptin in humans (Koutsari et al., 2003, Voss et al., 2016) and others showed that exercise increased leptin levels in rats (Uysal et al., 2017), whereas Algul et al. (2017) found no change in leptin levels after exercise in male subjects.
At present, little is known about the regulatory effects of exercise on leptin levels in camel species. Our results, for the first time, proved that exercise produces hypo-leptinemia in camels (Fig. 8C). Moreover, we found a positive relationship between the circulating leptin and insulin levels in the camels under the effect of exercise (Table 4). It has been demonstrated that there is a synchronicity between insulin and leptin, in which changes in insulin precede those of leptin (Koutkia,et al., 2003) and that a decrease in leptin follows insulin declines during fasting (French and Castiglione, 2002). Roberts et al (2013) identified a positive association between the levels of plasma leptin and skeletal muscle FNDC5 mRNA in obese rats. Likewise, a positive correlation was detected between leptin and irisin levels in children after physical activity (Palacios-González et al., 2015). Consistent with these findings, we also demonstrated that the decrease in leptin levels (Fig. 7C) was accompanied by a decrease in the circulating levels of insulin (Fig. 7A) and irisin (Fig. 4A) in the camels after exercise.
5. Conclusion
Our study provides, for the first time, evidence of the synthesis, quantification, localization, and regulation of irisin/FNDC5 protein in camel species. Our findings indicated that exercise induced tissue-specific regulation of irisin, FNDC5, and PGC-1α levels in the camels’ skeletal muscles and white adipose tissues, which subsequently decreased the circulating irisin levels. However, other tissues that might contribute to this decrease in the circulating irisin levels in camels after exercise have yet to be identified. The hypo-irisinemia, hyper-glycemia, hypo-insulinemia, hyper-cortisolemia, and hypo-leptinemia detected in the camels post-exercise in the present study might be considered as adaptation mechanisms related to the specific physiological characteristics of camels. The presence of significant associations between irisin and energetic metabolites as well as the hormones regulating metabolism suggested a beneficial role of irisin in glucose and lipid metabolism homeostasis in Arabian camels. Collectively, our new findings extend the knowledge on irisin and may provide a basis to consider the PGC-1α/FNDC5/irisin signaling pathway under several regulators in camel species.
Funding
The study was funded by the author's research allowance of Doaa Kirat and Mohamed Hamada (Department of Physiology, Faculty of Veterinary Medicine, Zagazig University, Zagazig, Egypt) and Taku Miyasho (Laboratory of Animal Biological Responses, Department of Veterinary Medicine, Rakuno Gakuen University, Ebetsu, Hokkaido, Japan).
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Footnotes
Peer review under responsibility of King Saud University.
References
- Abdel-Fattah M., Amer H., Ghoneim M.A., Warda M., Megahed Y. Response of one-humped camel (Camelus dromedarius) to intravenous glucagon injection and to infusion of glucose and volatile fatty acids, and the kinetics of glucagon disappearance from the blood. Zentralbl. Veterinarmed. A. 1999;46(8):473–481. doi: 10.1046/j.1439-0442.1999.00237.x. [DOI] [PubMed] [Google Scholar]
- Algul S., Ozdenk C., Ozcelik O. Variations in leptin, nesfatin-1 and irisin levels induced by aerobic exercise in young trained and untrained male subjects. Biol. Sport. 2017;34(4):339–344. doi: 10.5114/biolsport.2017.69821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ali A., Baby B., Vijayan R. From desert to medicine: A review of camel genomics and therapeutic products. Front. Genet. 2019;10:17. doi: 10.3389/fgene.2019.00017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amengual J., García-Carrizo F.J., Arreguín A., Mušinović H., Granados N., Palou A., Bonet M.L., Ribot J. Retinoic acid increases fatty acid oxidation and irisin expression in skeletal muscle cells and impacts irisin in vivo. Cell Physiol. Biochem. 2018;46:187–202. doi: 10.1159/000488422. [DOI] [PubMed] [Google Scholar]
- Aydin S., Kuloglu T., Aydin S., Kalayci M., Yilmaz M., Cakmak T., Albayrak S., Gungor S., Colakoglu N., Ozercan I.H. A comprehensive immunohistochemical examination of the distribution of the fat-burning protein irisin in biological tissues. Peptides. 2014;61:130–136. doi: 10.1016/j.peptides.2014.09.014. [DOI] [PubMed] [Google Scholar]
- Bjørndal B., Burri L., Staalesen V., Skorve J., Berge R.K. Different adipose depots: their role in the development of metabolic syndrome and mitochondrial response to hypolipidemic agents. J. Obes. 2011:490650. doi: 10.1155/2011/490650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bornstein S. The ship of the desert. The dromedary camel (Camelus dromedarius), a domesticated animal species well adapted to extreme conditions of aridness and heat. Rangifer. 1990;10(3):231–236. doi: 10.7557/2.10.3.860. [DOI] [Google Scholar]
- Boström P., Wu J., Jedrychowski M.P., Korde A., Ye L., Lo J.C., Rasbach K.A., Boström E.A., Choi J.H., Long J.Z., Kajimura S., Zingaretti M.C., Vind B.F., Tu H., Cinti S., Højlund K., Gygi S.P., Spiegelman B.M. A PGC1-alpha-dependent myokine that drives brown-fat-like development of white fat and thermogenesis. Nature. 2012;481:463–468. doi: 10.1038/nature10777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brenmoehl J., Albrecht E., Komolka K., Schering L., Langhammer M., Hoeflich A., Maak S. Irisin is elevated in skeletal muscle and serum of mice immediately after acute exercise. Int. J. Biol. Sci. 2014;10:338–349. doi: 10.7150/ijbs.7972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen K., Xu Z., Liu Y., Wang Z., Li Y., Xu X., Chen C., Xia T., Liao Q., Yao Y., Zeng C., He D., Yang Y., Tan T., Yi J., Zhou J., Zhu H., Ma J., Zeng C. Irisin protects mitochondria function during pulmonary ischemia/reperfusion injury. Sci. Transl. Med. 2017;9(418):eaao6298. doi: 10.1126/scitranslmed.aao6298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chilliard Y., Delavaud C., Bonnet M. Leptin expression in ruminants: nutritional and physiological regulations in relation with energy metabolism. Domest. Anim. Endocrinol. 2005;29(1):3–22. doi: 10.1016/j.domaniend.2005.02.026. [DOI] [PubMed] [Google Scholar]
- Crujeiras A.B., Zulet M.A., Lopez-Legarrea P., de la Iglesia R., Pardo M., Carreira M.C., Martínez J.A., Casanueva F.F. Association between circulating irisin levels and the promotion of insulin resistance during the weight maintenance period after a dietary weight-lowering program in obese patients. Metabolism. 2014;63(4):520–531. doi: 10.1016/j.metabol.2013.12.007. [DOI] [PubMed] [Google Scholar]
- Czarkowska-Paczek B., Zendzian-Piotrowska M., Gala K., Sobol M., Paczek L. One session of exercise or endurance training does not influence serum levels of irisin in rats. J. Physiol. Pharmacol. 2014;65(3):449–454. [PubMed] [Google Scholar]
- Dun S.L., Lyu R.M., Chen Y.H., Chang J.K., Luo J.J., Dun N.J. Irisin-immunoreactivity in neural and non-neural cells of the rodent. Neuroscience. 2013;240:155–162. doi: 10.1016/j.neuroscience.2013.02.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ebert T., Focke D., Petroff D., Wurst U., Richter J., Bachmann A., Lössner U., Kralisch S., Kratzsch J., Beige J., Bast I., Anders M., Blüher M., Stumvoll M., Fasshauer M. Serum levels of the myokine irisin in relation to metabolic and renal function. Eur. J. Endocrinol. 2014;170(4):501–506. doi: 10.1530/EJE-13-1053. [DOI] [PubMed] [Google Scholar]
- El Khasmi M., Chakir Y., Bargaâ R., Barka K., Lektib I., El Abbadi N., Belhouari A., Faye B. Impact of transport distance on stress biomarkers levels in dromedary camel (Camelus dromedarius) Emir. J. Food Agric. 2015;27(6):507–512. doi: 10.9755/ejfa.2015.04.058. [DOI] [Google Scholar]
- Elmahdi B., Sallmann H.P., Fuhrmann H., von Engelhardt W., Kaske M. Comparative aspects of glucose tolerance in camels, sheep, and ponies. Comp. Biochem. Physiol. A Physiol. 1997;118:147–151. doi: 10.1016/s0300-9629(96)00449-5. [DOI] [PubMed] [Google Scholar]
- Emmanuel B. Further metabolic studies in the rumen of camel (Camelus dromedarius) and sheep (Ovis aries) Comp. Biochem. Physiol. 1981;63:155–158. [Google Scholar]
- French S., Castiglione K. Recent advances in the physiology of eating. Proc. Nutrit. Soc. 2002;61(4):489–496. doi: 10.1079/pns2002190. [DOI] [PubMed] [Google Scholar]
- Gebreyohanes G.M., Assen M.A. Adaptation mechanisms of camels (Camelus dromedarius) for desert environment: A review. J. Vet. Sci. Technol. 2017;8:486. doi: 10.4172/2157-7579.1000486. [DOI] [Google Scholar]
- Gollisch K.S., Brandauer J., Jessen N., Toyoda T., Nayer A., Hirshman M.F., Goodyear L.J. Effects of exercise training on subcutaneous and visceral adipose tissue in normal- and high-fat diet-fed rats. Am. J. Physiol. Endocrinol. Metab. 2009;297(2):E495–E504. doi: 10.1152/ajpendo.90424.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall J.E. Unit XIV: Endocrinology and reproduction, Chapter78: Adrenocortical hormones. In: Hall J.E., editor. Guyton and Hall Textbook of Medical Physiology. 13th ed. Saunders; Elsevier, Philadelphia, PA: 2016. pp. 965–982. [Google Scholar]
- Hirabara S.M., Curi R., Maechler P. Saturated fatty acid-induced insulin resistance is associated with mitochondrial dysfunction in skeletal muscle cells. J. Cell. Physiol. 2010;222(1):187–194. doi: 10.1002/jcp.21936. [DOI] [PubMed] [Google Scholar]
- Hoeks J., Hesselink M.K., Russell A.P., Mensink M., Saris W.H., Mensink R.P., Schrauwen P. Peroxisome proliferator-activated receptor-gamma coactivator-1 and insulin resistance: acute effect of fatty acids. Diabetologia. 2006;49(10):2419–2426. doi: 10.1007/s00125-006-0369-2. [DOI] [PubMed] [Google Scholar]
- Hu W., Wang R., Li J., Zhang J., Wang W. Association of irisin concentrations with the presence of diabetic nephropathy and retinopathy. Ann. Clin. Biochem. 2016;53(Pt 1):67–74. doi: 10.1177/0004563215582072. [DOI] [PubMed] [Google Scholar]
- Huh J.Y., Siopi A., Mougios V., Park K.H., Mantzoros C.S. Irisin in response to exercise in humans with and without metabolic syndrome. J. Clin. Endocrinol. Metab. 2015;100(3):E453–E457. doi: 10.1210/jc.2014-2416. [DOI] [PubMed] [Google Scholar]
- Huh J.Y., Panagiotou G., Mougios V., Brinkoetter M., Vamvini M.T., Schneider B.E., Mantzoros C.S. FNDC5 and irisin in humans: I. Predictors of circulating concentrations in serum and plasma and II. mRNA expression and circulating concentrations in response to weight loss and exercise. Metabolism. 2012;61:1725–1738. doi: 10.1016/j.metabol.2012.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kartinah N.T., Sianipar I.R., Nafiah Rabia. The effects of exercise regimens on irisin levels in obese rats model: Comparing high-intensity intermittent with continuous moderate-intensity training. BioMed. Res. Int. 2018;2018 doi: 10.1155/2018/4708287. Article ID 4708287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaske M., Elmahdi B., von Engelhardt W., Sallmann H.P. Insulin responsiveness of sheep, ponies, miniature pigs and camels: results of hyperinsulinemic clamps using porcine insulin. J. Comp. Physiol. B. 2001;171:549–556. doi: 10.1007/s003600100205. [DOI] [PubMed] [Google Scholar]
- Komolka K., Albrecht E., Schering L., Brenmoehl J., Hoeflich A., Maak S. Locus characterization and gene expression of bovine FNDC5: is the myokine irisin relevant in cattle? PloS One. 2014;9(1):e88060. doi: 10.1371/journal.pone.0088060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koutkia P., Canavan B., Johnson M.L., DePaoli A., Grinspoon S. Characterization of leptin pulse dynamics and relationship to fat mass, growth hormone, cortisol, and insulin. Am. J. Physiol. Endocrinol. Metab. 2003;285(2):E372–E379. doi: 10.1152/ajpendo.00097.2003. [DOI] [PubMed] [Google Scholar]
- Koutsari C., Karpe F., Humphreys S.M., Frayn K.N., Hardman A.E. Plasma leptin is influenced by diet composition and exercise. Int. J. Obes. Relat. Metab. Disord. 2003;27(8):901–906. doi: 10.1038/sj.ijo.0802322. [DOI] [PubMed] [Google Scholar]
- Kraemer R.R., Shockett P., Webb N.D., Shah U., Castracane V.D. A transient elevated irisin blood concentration in response to prolonged, moderate aerobic exercise in young men and women. Horm. Metab. Res. 2014;46(2):150–154. doi: 10.1055/s-0033-1355381. [DOI] [PubMed] [Google Scholar]
- Kurdiova T., Balaz M., Vician M., Maderova D., Vlcek M., Valkovic L., Srbecky M., Imrich R., Kyselovicova O., Belan V., Jelok I., Wolfrum C., Klimes I., Krssak M., Zemkova E., Gasperikova D., Ukropec J., Ukropcova B. Effects of obesity, diabetes and exercise on Fndc5 gene expression and irisin release in human skeletal muscle and adipose tissue: in vivo and in vitro studies. J. Physiol. 2014;592(5):1091–1107. doi: 10.1113/jphysiol.2013.264655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lecker S.H., Zavin A., Cao P., Arena R., Allsup K., Daniels K.M., Joseph J., Schulze P.C., Forman D.E. Expression of the irisin precursor FNDC5 in skeletal muscle correlates with aerobic exercise performance in patients with heart failure. Circ. Heart Fail. 2012;5(6):812–818. doi: 10.1161/CIRCHEARTFAILURE.112.969543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li L., Rampersad S., Wang X., Cheng X., Qu S. Serum irisin concentrations were increased after transient continuous subcutaneous insulin infusion in type 2 diabetes mellitus patients. Diabetes Res. Clin. Pract. 2016;113:44–47. doi: 10.1016/j.diabres.2016.01.030. [DOI] [PubMed] [Google Scholar]
- Liu J.J., Wong M.D., Toy W.C., Tan C.S., Liu S., Ng X.W., Tavintharan S., Sum C.F., Lim S.C. Lower circulating irisin is associated with type 2 diabetes mellitus. J. Diabetes Complicat. 2013;27:365–369. doi: 10.1016/j.jdiacomp.2013.03.002. [DOI] [PubMed] [Google Scholar]
- Löffler D., Müller U., Scheuermann K., Friebe D., Gesing J., Bielitz J., Erbs S., Landgraf K., Wagner I.V., Kiess W., Körner A. Serum irisin levels are regulated by acute strenuous exercise. J Clin. Endocrinol. Metab. 2015;100(4):1289–1299. doi: 10.1210/jc.2014-2932. [DOI] [PubMed] [Google Scholar]
- Mahgoub M.O., D’Souza C.D., Darmaki R.S., Baniyas M.M., Adeghate E.A. An update on the role of irisin in the regulation of endocrine and metabolic functions. Peptides. 2018;104:15–23. doi: 10.1016/j.peptides.2018.03.018. [DOI] [PubMed] [Google Scholar]
- Marini M., Veicsteinas A. The exercised skeletal muscle: a review. Eur. J. Translat. Myol. Rev. 2010;20(3):105–120. [Google Scholar]
- Moreno-Navarrete J.M., Ortega F., Serrano M., Guerra E., Pardo G., Tinahones F., Ricart W., Fernández-Real J.M. Irisin is expressed and produced by human muscle and adipose tissue in association with obesity and insulin resistance. J. Clin. Endocrinol. Metab. 2013;98:E769–E778. doi: 10.1210/jc.2012-2749. [DOI] [PubMed] [Google Scholar]
- Norheim F., Langleite T.M., Hjorth M., Holen T., Kielland A., Stadheim H.K., Gulseth H.L., Birkeland K.I., Jensen J., Drevon C.A. The effects of acute and chronic exercise on pgc-1alpha, irisin and browning of subcutaneous adipose tissue in humans. FEBS J. 2014;281:739–749. doi: 10.1111/febs.12619. [DOI] [PubMed] [Google Scholar]
- Nygaard H., Slettaløkken G., Vegge G., Hollan I., Whist J.E., Strand T., Rønnestad B.R., Ellefsen S. Irisin in blood increases transiently after single sessions of intense endurance exercise and heavy strength training. PLoS One. 2015;10(3):e0121367. doi: 10.1371/journal.pone.0121367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ouajd O., Kamel B. Physiological particularities of Dromedary (Camelus dromedarius) and experimental implications. Scand. J. Lab. Anim. Sci. 2009;36(1):19–29. doi: 10.23675/sjlas.v36i1.165. [DOI] [Google Scholar]
- Palacios-González B., Vadillo-Ortega F., Polo-Oteyza E., Sánchez T., Ancira-Moreno M., Romero-Hidalgo S., Meráz N., Antuna-Puente B. Irisin levels before and after physical activity among school-age children with different BMI: a direct relation with leptin. Obesity. 2015;23(4):729–732. doi: 10.1002/oby.21029. [DOI] [PubMed] [Google Scholar]
- Park K.H., Zaichenko L., Brinkoetter M., Thakkar B., Sahin-Efe A., Joung K.E., Tsoukas M.A., Geladari E.V., Huh J.Y., Dincer F., Davis C.R., Crowell J.A., Mantzoros C.S. Circulating irisin in relation to insulin resistance and the metabolic syndrome. J. Clin. Endocrinol. Metab. 2013;98(12):4899–4907. doi: 10.1210/jc.2013-2373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perakakis N., Triantafyllou G.A., Fernandez-Real J.M., Huh J.Y., Park K.H., Seufert J., Mantzoros C.S. Physiology and role of irisin in glucose homeostasis. Nat. Rev. Endocrinol. 2017;13:324–337. doi: 10.1038/nrendo.2016.221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pérez-Sotelo D., Roca-Rivada A., Baamonde I., Baltar J., Castro A.I., Domínguez E., Collado M., Casanueva F.F., Pardo M. Lack of adipocyte-Fndc5/irisin expression and secretion reduces thermogenesis and enhances adipogenesis. Sci. Rep. 2017;7(1):16289. doi: 10.1038/s41598-017-16602-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piya M.K., Harte A.L., Sivakumar K., Tripathi G., Voyias P.D., James S., Sabico S., Al-Daghri N.M., Saravanan P., Barber T.M., Kumar S., Vatish M., McTernan P.G. The identification of irisin in human cerebrospinal fluid: influence of adiposity, metabolic markers, and gestational diabetes. Am. J. Physiol. Endocrinol. Metab. 2014;306(5):E512–E518. doi: 10.1152/ajpendo.00308.2013. [DOI] [PubMed] [Google Scholar]
- Polyzos S.A., Mantzoros C.S. An update on the validity of irisin assays and the link between irisin and hepatic metabolism. Metabolism. 2015;64(9):937–942. doi: 10.1016/j.metabol.2015.06.005. [DOI] [PubMed] [Google Scholar]
- Pond C.M. An evolutionary and functional view of mammalian adipose tissue. Proc. Nutr. Soc. 1992;51:367–377. doi: 10.1079/pns19920050. [DOI] [PubMed] [Google Scholar]
- Reza M.M., Subramaniyam N., Sim C.M., Ge X., Sathiakumar D., McFarlane C., Sharma M., Kambadur R. Irisin is a pro-myogenic factor that induces skeletal muscle hypertrophy and rescues denervation-induced atrophy. Nat. Commun. 2017;8(1):1104. doi: 10.1038/s41467-017-01131-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts M.D., Bayless D.S., Company J.M., Jenkins N.T., Padilla J., Childs T.E., Martin J.S., Dalbo V.J., Booth F.W., Rector R.S., Laughlin M.H. Elevated skeletal muscle irisin precursor FNDC5 mRNA in obese OLETF rats. Metabolism. 2013;62(8):1052–1056. doi: 10.1016/j.metabol.2013.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roca-Rivada A., Castelao C., Senin L.L., Landrove M.O., Baltar J., Belén Crujeiras A., Seoane L.M., Casanueva F.F., Pardo M. FNDC5/irisin is not only a myokine but also an adipokine. PloS One. 2013;8(4):e60563. doi: 10.1371/journal.pone.0060563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saeb M., Baghshani H., Nazifi S., Saeb S. Physiological response of dromedary camels to road transportation in relation to circulating levels of cortisol, thyroid hormones and some serum biochemical parameters. Trop. Anim. Health Prod. 2010;42:55. doi: 10.1007/s11250-009-9385-9. [DOI] [PubMed] [Google Scholar]
- Sesti G., Andreozzi F., Fiorentino T.V., Mannino G.C., Sciacqua A., Marini M.A., Perticone F. High circulating irisin levels are associated with insulin resistance and vascular atherosclerosis in a cohort of nondiabetic adult subjects. Acta Diabetol. 2014;51(5):705–713. doi: 10.1007/s00592-014-0576-0. [DOI] [PubMed] [Google Scholar]
- Uysal N., Agilkaya S., Sisman A.R., Camsari U.M., Gencoglu C., Dayi A., Aksu I., Baykara B., Cingoz S., Kiray M. Exercise increases leptin levels correlated with IGF-1 in hippocampus and prefrontal cortex of adolescent male and female rats. J. Chem. Neuroanat. 2017;81:27–33. doi: 10.1016/j.jchemneu.2017.02.004. [DOI] [PubMed] [Google Scholar]
- Varela-Rodríguez B.M., Pena-Bello L., Juiz-Valiña P., Vidal-Bretal B., Cordido F., Sangiao-Alvarellos S. FNDC5 expression and circulating irisin levels are modified by diet and hormonal conditions in hypothalamus, adipose tissue and muscle. Sci. Rep. 2016;6:29898. doi: 10.1038/srep29898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voss S.C., Nikolovski Z., Bourdon P.C., Alsayrafi M., Schumacher Y.O. The effect of cumulative endurance exercise on leptin and adiponectin and their role as markers to monitor training load. Biol. Sport. 2016;33(1):23–28. doi: 10.5604/20831862.1180173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winn N.C., Grunewald Z.I., Liu Y., Heden T.D., Nyhoff L.M., Kanaley J.A. Plasma Irisin modestly increases during moderate and high-intensity afternoon exercise in obese females. PLoS One. 2017;12(1):e0170690. doi: 10.1371/journal.pone.0170690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wrann C.D. FNDC5/irisin - their role in the nervous system and as a mediator for beneficial effects of exercise on the brain. Brain Plast. 2015;1(1):55–61. doi: 10.3233/BPL-150019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wrann C.D., White J.P., Salogiannnis J., Laznik-Bogoslavski D., Wu J., Ma D., Lin J.D., Greenberg M.E., Spiegelman B.M. Exercise induces hippocampal BDNF through a PGC-1α/FNDC5 pathway. Cell Metab. 2013;18(5):649–659. doi: 10.1016/j.cmet.2013.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Z., Chen X., Chen Y., Zhao Q. Decreased irisin secretion contributes to muscle insulin resistance in high-fat diet mice. Int. J. Clin. Exp. Pathol. 2015;8(6):6490–6497. [PMC free article] [PubMed] [Google Scholar]