Abstract
Background
Decisions about lung cancer screening are inherently complex and create a need for methods to convey the risks and benefits of screening to patients.
Research Question
What kind of decision aids or tools are available to support shared decision-making for lung cancer screening? What is the current evidence for the effectiveness, acceptability, and feasibility of those tools?
Study Design and Methods
We conducted a systematic review of studies and searched PubMed, MEDLINE, EMBASE, Cochrane Clinical Trials Register, and ClinicalTrials.gov from inception to December 2019 for studies that evaluated the effectiveness and acceptability of tools to promote shared decision-making for patients who are considering lung cancer screening.
Results
After screening 2,427 records, we included one randomized control trial, two observational studies, 11 before/after studies of a decision aid or an educational tool. Fifteen distinct tools in various formats were evaluated in 14 studies. Most studies were of fair quality. Studies reported improvement in patients’ knowledge of lung cancer screening (n = 9 studies), but improvements in specific areas of knowledge were inconsistent. Decisional conflict was low or reduced after the administration of the tools (n = 7 studies). The acceptability of tools was rated as “high” by patients (n = 7 studies) and physicians (n = 1 study). Low dose CT scan completion rates varied among studies (n = 6 studies).
Interpretation
Evidence from 14 studies suggests that some elements of existing tools for lung cancer screening may help to prepare patients for decision-making by improving knowledge and reducing decisional conflict. Such tools generally are acceptable to patients and providers. Further studies that use consistent measures and reporting methods and assess relevant decisional and clinical outcomes are needed to determine the comparative effectiveness and feasibility of implementation of these tools.
Clinical Trial Registration
PROSPERO 2018 CRD4201874814
Key Words: cancer screening, decision aids, decision-making, lung neoplasms, shared decision-making
Abbreviations: DA, decision aid; IPDAS, International Patient Decision Aid Standards; LDCT, low-dose CT; RCT, randomized controlled trial; SDM, shared decision-making
Lung cancer is the leading cause of cancer death in the United States, accounting for approximately one-fourth of the estimated number of cancer deaths in both men and women in 2019.1 In 2013, the US Preventive Services Task Force recommended (grade B) annual lung cancer screening with low-dose CT (LDCT) scans for adults at high risk based on age and smoking history.2 Although LDCT lung cancer screening has been reported to reduce lung cancer mortality rates by 20% in high-risk populations, 365 per 1,000 high-risk patients who are screened will have a false-positive finding that will lead to additional tests, some of which may cause significant morbidity.3
Take-home Points.
Study Question: What kind of tools are available to support shared decision-making for lung cancer screening? What is the current evidence for the effectiveness, acceptability, and feasibility of those tools?
Results: Our systematic review identified 15 tools to support shared decision-making for lung cancer screening. Nine studies reported improvement in patients’ knowledge of lung cancer screening, but improvements in specific areas of knowledge were inconsistent. Decisional conflict was low or reduced after administration of the tools (n = 7 studies).
Interpretation: Existing tools to promote shared decision-making for lung cancer screening may help patients by improving knowledge and reducing decisional conflict.
The likelihood of receiving a benefit and the probability of experiencing harm from LDCT screening vary depending on each patient’s risk for lung cancer and on their comorbidities.4 The relatively low likelihood of benefit coupled with the relatively high likelihood of false-positive findings with associated harms makes the decision to undergo lung cancer screening preference-sensitive. To assure that patients are aware of their specific balance of benefit and harm and have an opportunity to consider the pros and cons of screening in the context of their preferences and values, the Centers for Medicare and Medicaid Services has mandated shared decision-making (SDM), with the use of at least one decision aid (DA), be performed by a physician or a qualified non-physician practitioner before a patient receives their first lung cancer screening.5,6
DAs may assist providers in presenting the benefits and harms of lung cancer screening and have been shown to improve patient knowledge and reduce decisional conflict when patients are faced with other cancer screening decisions.7, 8, 9, 10 Ideally the selection of a DA for use in a clinical setting should be based on evidence of DA effectiveness and be guided by knowledge of optimal modes and timing of delivery of DAs within typical workflows. The objectives of this systematic review were to (1) identify existing DAs and other educational tools that have been designed to support SDM for lung cancer screening and (2) summarize the evidence for their effectiveness, acceptability, and feasibility of implementation in clinical settings. To qualify formally as a DA, a tool had to meet the International Patient Decision Aid Standards (IPDAS) Collaboration definition of a DA. We refer to other decision support tools that do not conform to the IPDAS definition as educational tools. We used the term “tools” to refer to both DAs and educational tools.
Methods
Systematic Review Registration
We carried out a systematic review according to a prespecified protocol as registered at PROSPERO 2018 CRD42018074814. We searched PubMed, MEDLINE, EMBASE, Cochrane Clinical Trials Register, and ClinicalTrials.gov initially from inception to March 2018 to identify studies that reported on DAs and other educational tools that were designed to support SDM for lung cancer screening. Details of the search strategy for each database are shown in e-Appendix 1. We also manually reviewed the reference lists of included studies and reviewed other publications of the first and last authors of each article to identify other relevant studies. We later updated our search to identify studies that were published from March 2018 through December 2019. We followed the Preferred Reporting Items for Systematic Reviews and Meta-Analyses checklist for reporting the results.11 This review did not require approval by an institutional review board because it involved analysis of only previously published data.
Eligibility Criteria
Study Design
We included any randomized controlled trials (RCTs) controlled or uncontrolled studies, quasi experimental studies, and before/after studies. Qualitative studies were also included.
Participants
We included studies of adult participants who were considering lung cancer screening with LDCT scans in which a DA or other educational tool was used to support SDM. We included studies regardless of whether participants met any established lung cancer screening eligibility criteria, such as the US Preventive Services Task Force or Centers for Medicare and Medicaid Services criteria, given the small number of studies that focused exclusively on participants who met the established criteria.2,5 We excluded studies of patients who were considering lung cancer screening using modalities other than LDCT scanning, such as chest radiography.
Interventions
We evaluated DAs and educational tools that the authors identified as potentially supporting SDM for lung cancer screening, regardless of whether they met the IPDAS Collaboration definition for DAs.12 We included tools in any format, which included print, video, and web-based tools.
Comparators
We also included before/after designs and studies both with and without comparison groups that consisted of participants not receiving the DA or educational tool.
Outcomes
The outcomes that were evaluated included the impact of educational tools and DAs on patient knowledge, decisional conflict, preparation for decision-making, and intention to screen. Some studies also reported LDCT completion rates, acceptability among patients and providers, and measures of feasibility of delivery of the tool in clinical settings.
Data Extraction and Study Quality Assessment
Studies were selected for inclusion based on the review of titles and abstracts that were exported into a web-based data management repository.13 Two reviewers were involved independently in all stages of study selection, data extraction, and quality assessment. Discrepancies in reviewer assessments were addressed initially through joint review and discussion of the relevant text. A third reviewer was engaged to resolve persistent disagreements. We achieved full consensus prior to inclusion of the studies. All data were extracted into a prespecified Excel data sheet (Microsoft Corporation). Examination for clinical heterogeneity included examination for differences in population, intervention, follow-up duration, and other characteristics. We conducted a qualitative synthesis of the data because they were too heterogenous to be pooled in a meta-analysis.
We assessed the quality of included studies using the National Heart, Lung, and Blood Institute Quality Assessment Tools for randomized controlled trials, observational studies, and before/after designs.14 Studies were rated as good, fair, or poor based on their performance on the criterion. Two reviewers independently rated the quality of these studies, and a third reviewer adjudicated any discrepancies.
Results
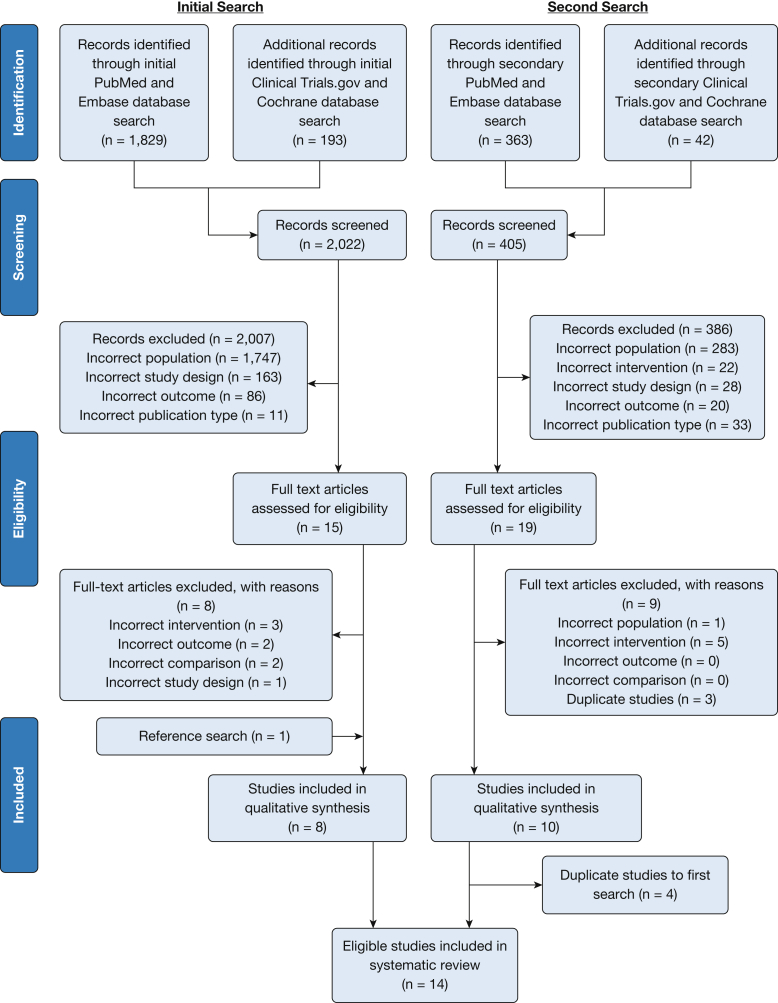
The flow of the study selection and review process is shown in the Preferred Reporting Items for Systematic Reviews and Meta-Analyses flow sheet (Fig 1). In total, 14 articles met all inclusion criteria,15, 16, 17, 18, 19, 20, 21, 22, 23, 24, 25, 26, 27, 28 including one RCT,26 two observational studies,22,28 and 11 studies with before/after designs (Table 1).15, 16, 17, 18, 19, 20, 21,23, 24, 25,27 The overall quality assessment of included studies is shown in Table 1, with details provided in e-Appendixes 2-5. Only one study, the RCT, was rated of good quality.26 The majority of studies were rated as fair.15, 16, 17, 18, 19, 20, 21, 22, 23, 24, 25,27 Only one study was rated as poor in quality because of incomplete reporting of participant eligibility criteria.28
Figure 1.
PRISMA flow sheet. From: Moher D, Liberati A, Tetzlaff J, Altman DG, The PRISMA Group (2009). Preferred Reporting Items for Systematic Reviews and Meta-Analyses: The PRISMA Statement. PLoS Med. 2009:6(7):e1000097. For more information, visit www.prisma-statement.org
Table 1.
Summary of Characteristics of Studies and Tools to Promote Shared Decision-Making in Lung Cancer Screening
| Study | Country | Study Setting and Source of Subjects | Tool | Delivery Location and Method | Purpose of a Tool | Study Quality |
|---|---|---|---|---|---|---|
| Randomized controlled trial: Ruparel et al26 (2019) | England | Nested in Lung Screening Uptake Trial | Booklet vs booklet + film | Study visit in the presence of health care professional | Preparation and facilitation | Good |
| Observational study with comparison: Tanner et al28 (2019) | United States | University hospital and Veterans Affairs hospital | Decision aid (print) | In-person vs telephone shared decision-making | Facilitation | Poor |
| Observational study without comparison: Dharod et al22 (2019) | United States | Large academic health system | Decision aid (web) | Patient portal/self-administered | Preparation | Fair |
| Before/after study | ||||||
| Volk et al15 (2014) | United States | Cancer center tobacco treatment program | Decision aid (video) | Online/self-administered | Preparation | Fair |
| Lau et al16 (2015) | United States | Convenient sample of volunteer | Decision aid (web) | Online/self-administered | Preparation | Fair |
| Crothers et al17 (2016) a | United States | Urban county hospital | Decision aid (web) | Focus group/self-administered with support | Preparation | Fair |
| Decision aid (print) | Reviewed in a focus group | Preparation | ||||
| Housten et al19 (2018) | United States | Cancer center tobacco treatment program | Decision aid (video) | Online/self-administered | Preparation | Fair |
| Mazzone et al18 (2017)a | United States | Lung cancer screening program | Decision aid (video) | Shared decision-making visit | Preparation | Fair |
| Decision aid (web) | Shared decision-making visit | Facilitation | ||||
| Reuland et al21 (2018) | United States | Academic internal medicine practice | Decision aid (video) | Study visit | Preparation | Fair |
| McDonnell et al20 (2018) | United States | Urban academic medical center | Decision aid (print) | Waiting room/self-administered/during an encounter with a provider | Preparation and facilitation | Fair |
| Hoffman et al24 (2018) | United States | Cancer center tobacco treatment program | Decision aid (video) | Online/self-administered | Preparation | Fair |
| Fagan et al23 (2020) | United States | Large primary care practice | Educational material (print) & online tool (web) | Telephone counseling | Facilitation | Fair |
| Manners et al25 (2020) | Australia | General outpatient medical clinic | Decision aid (print) | Study visit/self-administered | Preparation | Fair |
| Education pamphlet (print) | ||||||
| Sakoda et al27 (2020) | United States | Health care delivery system | Class | A group education class | Preparation | Fair |
Administered two decision aids to participants.
Type of Tools, Delivery Methods, and Study Settings
Table 1 and e-Appendix 6 show the characteristics of the selected studies and of the tools used in each study. One study was conducted in the United Kingdom26; one study was conducted in Australia,25 and the remaining studies were conducted in the United States. Three studies were conducted in the context of lung cancer screening programs.18,26,27 Fifteen distinct tools were used across all studies: four web-based tools in five studies,16, 17, 18,22,23 four video tools in six studies,15,18,19,21,24,26 and seven print tools in six-studies (Table 1).17,20,23,25,26,28 Nine tools were described as a DA by the authors,15, 16, 17, 18, 19, 20, 21, 22,24,25,28 five of which were reported to be developed in accordance with the IPDAS checklist.15, 16, 17, 18, 19, 20, 21,24,25 Ruparel et al26 described their tool as an “informational booklet and film.” Fagan et al23 used educational material and online software to guide a telephone SDM intervention. Sakoda et al27 used an educational class to prepare patients for the visit. Manners et al25 used an educational print tool for participants who were ineligible for lung cancer screening, along with a printed DA for eligible participants.
Eleven of the tools were used to assist patients in preparation for their SDM counseling visits15, 16, 17, 18, 19, 20, 21, 22,24, 25, 26, 27; only five tools were used during the SDM encounter.18,20,23,26,28 Two studies used the same web-based DA to prepare patients for the SDM visit that Mazzone et al18 used to facilitate SDM during a visit.16,17 Tools were used during telephone counseling in two studies.23,28 In five studies, SDM tools were used in conjunction with individual counseling.18,20,23,26,28 Dharod et al22 invited patients who were potentially eligible for lung cancer screening and who had been identified by an electronic medical record algorithm to view a web-based DA. Four studies administered two different formats of a tool to subjects.17,18,23,26
The included tools had largely similar content. They included information on risk factors for lung cancer, the eligibility criteria for lung cancer screening, benefits and harms of lung cancer screening, and the steps involved in performing lung cancer screening. Six DAs were reported to include a value clarification exercise.15, 16, 17, 18, 19, 20, 21,23, 24, 25
A web-based DA “Shouldiscreen.com” used calculations to explain patients’ personalized risk of experiencing the development of lung cancer and the benefits and risks of lung cancer screening.16, 17, 18 Crothers et al17 reported that at least one-fourth of study participants required assistance in using this web-based DA and that many participants preferred a paper-based tool rather than a web-based tool.
Characteristics of Participants
Table 2 presents participant characteristics. The largest number of participants included in a study was 1,000.22 The only RCT included 229 participants.26 The number of participants in most of the other studies was small, ranging from 20 to 137. Six studies included only participants who met established lung cancer screening eligibility criteria.18,20,21,23,26,28 Race/ethnicity, smoking status, and level of education among participants varied among studies.
Table 2.
Summary of Participant Characteristics
| Study | Participants, No. | Lung Cancer Screening Eligible, % | Mean Age, y | Female, % | White, % | Black, % | High School Education or Less, % | Current Smoker, % | Mean Pack-Years |
|---|---|---|---|---|---|---|---|---|---|
| Randomized controlled trial: Ruparel et al26 (2019) | |||||||||
| Booklet | 109 | 100a | 60-76 (Range) | 49.5 | 84.4 | 7.3 | 61.5 | NR | 35 (Median) |
| Booklet + film | 120 | 100a | 60-76 (Range) | 54.2 | 81.7 | 10.8 | 60.8 | NR | 38 (Median) |
| Observational study with comparison: Tanner et al28 (2019) | |||||||||
| In-person | 69 | 100 | 64.1 | 52.2 | 64.2 | 28.5 | 43.4 | NR | NR |
| Telephone | 68 | 100 | 65.2 | 5.9 | 63.2 | 27.9 | 36.8 | NR | NR |
| Observational study without comparison: Dharod et al22 (2019) | 1,000 | 28b,c | 65 | 51 | 84 | 14 | NR | 19 | NR |
| Before/after study | |||||||||
| Volk et al15 (2014) | 52 | 27 | 58.5 | 65.4 | 74.8 | 19.2 | 32.7 | 44.2 | 30 |
| Lau et al16 (2015) | 60 | 18 | 60.6 | 50 | 88 | 12 | 9 | 27 | 24.1 |
| Crothers et al17 (2016) | 45 | 44 | 61 | 29 | 58 | 31 | 57 | 76 | 37 (Median) |
| Housten et al19 (2018) | 30 | NR | 61.5 | 50 | 63.3 | 30 | 23.3 | 60.7 | 30.4 |
| Mazzone et al18 (2017) | 125 | 100 | 64.4 | 33.9 | NR | NR | 37.9 | 45.2 | 53 |
| Reuland et al21 (2018) | 50 | 100 | 63 | 48 | 58 | 30 | 50 | 46 | 52 |
| McDonnell et al20 (2018) | 20 | 100b | 64.5 | 70 | NR | 50 | NR | NR | NR |
| Hoffman et al24 (2018) | 30 | NR | 61.5 | 50 | 63.3 | 30 | 23.3 | 67 | 30.4 |
| Fagan et al23 (2020) | 28 | 100 | 62.6 | 53.6 | 78.6 | 17.9 | NR | 67.9 | 46.5 |
| Manners et al25 (2020) | |||||||||
| Eligible | 36 | 100d | 67.7 | 42 | NR | NR | 86 | 22 | 59.1 |
| Ineligible | 19 | 0d | 65.4 | 58 | NR | NR | 43 | 5 | 65.4 |
| Sakoda et al27 (2020) | 680 | 39.6 | 64.3 (Median) | 40.2 | 75.9 | 3.9 | NR | 53.7 | NR |
NR = not reported.
Eligibility criteria used in each study:
United States Preventive Services Task Force, lung-cancer risk-prediction model from Prostate, Lung, Colorectal, and Ovarian Cancer Screening Trial, ≥1.51%; Liverpool Lung Project prediction model for lung cancer model, ≥ 2.5%.
Center for Medicaid Services criteria
Percentage of eligible patients among 349 patients who completed an eligibility screening tool.
Lung-cancer risk-prediction model from Prostate, Lung, Colorectal, and Ovarian Cancer Screening Trial ≥ 1.5%, otherwise the United States Preventive Services Task Force criteria.
Evaluation of the Effect of DAs
Knowledge of lung cancer screening
Patients’ knowledge of lung cancer screening was reported to improve in nine studies after patient use of the tools (Table 3; e-Appendix 7).15, 16, 17, 18, 19,21,25, 26, 27 Seven of these studies reported a statistically significant improvement in knowledge.15,16,18,19,21,26,27 The studies used different questions and scales to measure patient knowledge and reported data in different formats, precluding any pooling of data across studies.
Table 3.
Summary of Patient Knowledge and Acceptability
| Study | Knowledgea | Acceptability |
|---|---|---|
| Randomized controlled trial: Ruparel et al26 (2019) | ||
| Control (booklet) | ↑b | High |
| Intervention (booklet + film) | ↑b | High |
| Before/after study | ||
| Volk et al15 (2014) | ↑b | High |
| Crothers et al17 (2016) | ↑ | High |
| Housten et al19 (2018) | ↑b | NR |
| Mazzone et al18 (2017) | ↑b | NR |
| Reuland et al21 (2018) | ↑b | High |
| McDonnell et al20 (2018) | NR | Highc |
| Manners et al25 (2020) | ↑ | High |
| Sakoda et al27 (2020) | ↑b | NR |
The upward arrow (↑) indicates improvement between the pre- and poststudy periods. See Table 2 legend for expansion of abbreviation.
The booklet + film group had greater statistically significant improvement in knowledge compared to booklet group.
Statistically significant improvement between the pre- and poststudy.
Acceptability among patients and providers.
The single RCT reported statistically significant improvements in knowledge in both the booklet-alone and booklet + film groups with significantly greater improvements in the booklet + film group.26 Mazzone et al18 evaluated patient knowledge before and immediately after the intervention and one month later. Although knowledge scores were higher immediately after engagement with the DA, there was a decline in knowledge scores one month later.
Despite overall improvements in knowledge scores, many patients could not answer key questions correctly, even after use of these tools. For example, three studies found that only a minority of participants (21-36%) could answer simple questions correctly about the eligibility criteria such as: “Should all smokers be screened for lung cancer?”15,17,27 Eight studies evaluated knowledge about lung cancer death reduction by LDCT screening (e-Appendix 8).15, 16, 17,19,21,25, 26, 27 Although a majority of participants of participants in five studies (61-95%) correctly responded that lung cancer mortality rates were reduced among screened patients,15, 16, 17,25,27 many fewer participants (15-48%) correctly answered a question regarding the magnitude of the lung cancer death benefit.15,19,21,26 A large majority of participants (74-97% across six studies)15, 16, 17,19,21,27 responded correctly that false-positive results can occur; however, many fewer participants (30-58% across two studies) responded correctly to a question regarding the frequency of a false-positive results (e-Appendix 9).16,26 Mazzone et al18 reported that those participants with the lowest level of formal education were most likely to provide incorrect answers in a knowledge test.
Decisional conflict
Decisional conflict was measured in seven studies (Tables 4 and 5).15,16,23, 24, 25, 26,28 It was low following all six interventions in four studies that reported only after intervention scores (Table 4). The RCT by Ruparel et al26 showed significantly higher decision certainty in the booklet + film group compared with the booklet group. The observational study by Tanner et al28 reported low decisional conflict in both the in-person and telephone SDM groups. In two studies that used the same video DA,15,24 scores on the value clarity scale were below the threshold score of 25, which suggested that participants were ready to make a decision.29
Table 4.
Summary of Result of Decision Conflict After Use of Tools
| Study | Measurement Tool | Control vs Intervention Score, mean (SD) |
|---|---|---|
| Randomized controlled trial: Ruparel et al26 (2019) (booklet vs booklet + film) | Adopted low-literacy Decisional Conflict Scale, with a maximum score of 9a | 8.5 (1.25) vs 8.24 (1.49)b |
| Observational study with comparison: Tanner et al28 (2019) (in-person vs telephone) | 10-Item Decisional Conflict Scale, with maximum score of 20c | 11.3 (3.4) vs 12.1 (3.4) |
| Before/after study | ||
| Volk et al15 (2014) | Decisional Conflict Scale value clarity subscale, with a maximum score of 100c | 7.84 (23.18) |
| Hoffman et al24 (2018) | Decisional Conflict Scale value clarity subscale, with a maximum score of 100c | 3.9 (NR) |
See Table 2 legend for expansion of abbreviation.
Higher point means less conflict.
Statistically significant.
Lower points means less conflict.
Table 5.
Summary of Decisional Conflict Scale Before and After the Use of Tools
| Study | Overall | Uncertainty | Informed | Values Clarity | Support | Effective Decision |
|---|---|---|---|---|---|---|
| Lau et al16 (2015) | ↓a | ↓a | ↓a | ↓a | ↓a | NR |
| Fagan et al23 (2020) | ↓ | ↓ | ↓ | ↓ | ↑ | NR |
| Manners et al25 (2020) | ↓a | ↓a | ↓a | ↓a | ↓a | ↓a |
The downward arrow (↓) indicates reduction; the upward arrow (↑) indicates increase of scale between the pre- and poststudy periods. Lower score indicates less decisional conflict. See Table 2 legend for expansion of abbreviation.
Statistically significant.
In three studies that reported before and after intervention scores, overall and most subscale scores were reduced after use of tools (Table 5).16,23,25 Lau et al16 reported a significant reduction in scores on all four subscales of the Decisional Conflict Scale (uncertainty, informed, value clarity, and support) to <25 after the use of a web-based DA. Although all subscales were reduced significantly after the use of a print DA in the study reported by Manners et al,25 the proportion of patients whose Decisional Conflict Scale score was <25 improved only from 28% to 39% (P = .1).
Behavioral intent and action
Intention to obtain an LDCT scan was reported in three studies (e-Appendix 10),20,24,27 and six studies reported LDCT screening completion (Table 6).18,21, 22, 23,26,28 Ruparel et al26 found no significant difference in in LDCT screening completion rates between booklet and booklet + film groups; Tanner et al28 also found no difference between screening rates in the two arms in their study (telephone and in-person SDM visits). Overall, the LDCT screening completion rates were high, ranging from 45% to 95% when tools were used with counseling.18,23,26,28 However, when tools were used without counseling, the LDCT screening completion rates were low at 2% to 20%.21,22
Table 6.
Summary of Result of Behavioral Action After the Use of Tools
| Study | Counseling Provided With Tool, Yes/No | Completion of Low-Dose CT Scan, % |
|---|---|---|
| Randomized controlled trial: Ruparel et al26 (2019) | Yesa | 78.9 vs 76.7 (Booklet vs booklet + film) |
| Observation study with comparison: Tanner et al28 (2019) | Yesb | 88.4 vs 88.2 (In-person vs telephone) |
| Observational study without comparison: Dharod et al22 (2019) | Noc | 2b |
| Before/after study | ||
| Mazzone et al18 (2017) | Yesd | 94.6 |
| Reuland et al21 (2018) | No | 20 |
| Fagan et al23 (2020) | Yese | 45 |
Health-care professionals were present during the use of tools and answered questions after use of tools.
In-person or telephone counseling was provided.
Percentage of patients who had a screening or nodule follow-up CT among 404 patients visited a web decision aid, regardless of their eligibility.
Decision aid was used in a lung cancer screening program during shared decision-making visit.
Telephone counseling was provided.
Acceptability of DA
Seven studies evaluated the acceptability of DAs among patients (Table 3).15, 16, 17,20,21,25,26 One study evaluated the acceptability among primary care providers.20 They all showed high acceptability among patients and providers.
Discussion
Our systematic review of DAs and educational tools to promote SDM for lung cancer screening identified 14 studies that included one RCT. Fifteen distinct tools in various formats were evaluated. Nine studies reported improvement in patients’ overall knowledge of lung cancer screening. Three studies that measured before and after intervention scores reported a reduction in decisional conflict after use of tools; four studies showed low decisional conflict after the use of tools. The acceptability of DAs was rated as high by both patients and physicians. A higher rate of LDCT screening completion was observed when tools were used with counseling.
Our review suggests that several tools can improve patients’ knowledge of lung cancer screening. Improvement of patient knowledge after use of tools is consistent with the effect of DAs in several other settings.7, 8, 9, 10 However, the improvements in knowledge were inconsistent across items. Many participants incorrectly answered questions regarding lung cancer screening eligibility, the magnitude of the false-positive rate, and the reduction in lung cancer morality rates associated with annual LDCT screening. It is difficult for patients and their providers to have discussions of the trade-offs of benefits and harms and make informed decisions that are consistent with patients’ values and preferences without a full understanding of the nature and likelihood of these benefits and risks.
Our findings on decisional conflict are also consistent with the effect of DAs in other settings that involve SDM discussions around prostate cancer and colon cancer screening.7, 8, 9, 10
Universal challenges in the implementation of SDM for lung cancer screening are the time needed to conduct SDM within the constraints of an office visit with multiple competing priorities and the limited resources in many clinical systems for developing and supporting a comprehensive SDM process that includes the use of a DA.30,31 Although primary care providers typically are expected to offer SDM during the regular clinic visits, no study evaluated the effectiveness, feasibility, and acceptability of tools that were in the context of a routine primary care visit.
The majority of the tools included in our review were designed to be delivered to patients who potentially are eligible for lung cancer screening prior to their encounter with a provider who would then engage the patient in SDM. However, the identification of patients who potentially are eligible for screening remains challenging, in part, because of inaccurate documentation of smoking history in many electronic medical records.32 When inaccurate information from an electronic medical record is used to identify potential candidates for lung cancer screening to receive a DA prior to a clinical encounter, many patients who ultimately are ineligible for lung cancer screening may be invited to engage with a DA. This occurred in the study reported by Dharod et al22 in which only 28% of subjects who visited a web-based DA ultimately were found to meet screening eligibility criteria. Although a group education class or a separate SDM visit in a lung cancer screening program could relieve the burden of SDM counselling from primary care providers, this would impose a burden on patients who would need to complete an additional visit. The findings on decisional conflict reduction reported by Tanner et al28 and Fagan et al23 for telephone SDM suggest that this approach to delivering DA-supported SDM is promising but will require further evaluation and changes in Centers for Medicare and Medicaid Services policy prior to widespread implementation.23,28
We received electronic notification of an RCT by Volk et al33 that became available after the updated search. Although this study is not included in our review, the findings of the RCT mirror those of our review. The authors reported that, among 516 smokers, patient use of a video-based DA compared with standard educational information led to better preparedness to decide about screening, greater likelihood of feeling informed and clear about screening choices, and greater knowledge of screening benefits and harms. These findings are in line with our findings that DAs increase knowledge and decrease decisional conflict. However, there was no difference in the rate of completion of LDCT screening.
There are some limitations of our review, which primarily reflect the limitations of the included studies. The number of studies is relatively small, and most of them included <100 subjects. Despite a comprehensive search of multiple databases, we may have missed a small number of studies, particularly those published in languages other than English. We did not have enough data to confirm that DA development was guided by the IPDAS checklist and relied on authors’ self-report. We were unable to perform any meta-analysis of study findings due to differences in measures of outcomes among the studies. Few studies compared different approaches and/or tools for supporting SDM, and only one study used a randomized design. We were unable to include some freely available DAs such as one developed by the Agency for Healthcare Research and Quality due to the absence of published studies that evaluated their effectiveness.34 The findings of three studies that were conducted in the setting of lung cancer screening programs are not generalizable to primary care practice.18,26,27
Our findings highlight opportunities for the improvement of the methodologic rigor and the focus of future studies. Future studies should use consistent measures of knowledge and decisional outcomes to allow meaningful comparisons across studies. Studies should evaluate the use of tools in the settings in which they are intended to be used (eg, primary care practice, telephone outreach) and should report cost and feasibility findings to compare different approaches to implement DAs. Studies should be powered adequately to achieve meaningful precision in results across subgroups. Future studies are needed to compare interventions that are less and more intensive and/or involve different types of DAs (eg, print vs web-based interactive). Such studies should also evaluate whether the effectiveness of specific DAs varies by patient subgroups based on age, smoking status, ethnicity, or literacy levels.
Evidence from 14 studies suggest that existing educational tools for lung cancer screening may help to prepare patients for decision-making by improving knowledge and reducing decisional conflict. Such tools are generally acceptable to participants. Future studies that use consistent measures and reporting methods are needed to determine the comparative effectiveness and feasibility of these tools on decisional and clinical outcomes.
Acknowledgments
Author contributions: M. I. F. and S. S. had full access to all the data in the study and take responsibility for the integrity of the data and the accuracy of data analysis; S. S. and M. I. F. participated in study concept and design; S. S., M. I. F., K. H., J. K., S. T., and V. F. F. participated in accusation, analysis, or interpretation of data; M. I. F., K. H., J. K., and S. S. participated in the drafting of the manuscript; M. I. F., R. L., P. K. J. H., K. M. M., and S. S. participated in critical revision of the manuscript for important intellectual content; M. I. F., J. K., and S. S. participated in the administrative, technical, or material support; and S. S. provided study supervision.
Financial/nonfinancial disclosures: The authors have reported to CHEST the following: P. K. J. H. received funding through the Maine Lung Cancer Coalition, supported jointly by the Bristol-Myers Squibb Foundation, Maine Cancer Foundation, and Maine Economic Improvement Fund. None declared (M. I. F., K. H., J. K., S. T., V. F. F., R. L., K. M. M., and S. S.).
Role of sponsors: The sponsor had no role in the design of the study, the collection and analysis of the data, or the preparation of the manuscript.
Other contributions: Angela Patterson, MS, edited the tables and figures. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH.
Additional information: The e-Appendixes can be found in the Supplemental Materials section of the online article.
Footnotes
FUNDING/SUPPORT: This study was funded in part by the National Heart, Lung, and Blood Institute, US [Grant 1K12HK138049-01 to M. I. F.].
Supplementary Data
References
- 1.Siegel R.L., Miller K.D., Jemal A. Cancer statistics, 2019. CA Cancer J Clin. 2019;69(1):7–34. doi: 10.3322/caac.21551. [DOI] [PubMed] [Google Scholar]
- 2.Moyer V.A., Force USPST Screening for lung cancer: U.S. Preventive Services Task Force recommendation statement. Ann Intern Med. 2014;160(5):330–338. doi: 10.7326/M13-2771. [DOI] [PubMed] [Google Scholar]
- 3.PDQ® Screening and Prevention Editorial Board. PDQ Lung Cancer Screening. Patient and physician guide: National Lung Screening Trial (NLST) Bethesda, MD: National Cancer Institute. Updated May 2019. https://www.cancer.gov/types/lung/patient/lung-screening-pdq. Accessed May 19, 2020.
- 4.Rivera M.P., Tanner N.T., Silvestri G.A. Incorporating coexisting chronic illness into decisions about patient selection for lung cancer screening: an official American Thoracic Society Research Statement. Am J Respir Crit Care Med. 2018;198(2):e3–e13. doi: 10.1164/rccm.201805-0986ST. [DOI] [PubMed] [Google Scholar]
- 5.Centers for Medicare and Medicaid Services Decision Memo for Screening for Lung Cancer with Low Dose Computed Tomography (LDCT) (CAG-00439N). 2015. https://www.cms.gov/medicare-coverage-database/details/nca-decision-memo.aspx?NCAId=274
- 6.National Academies of Sciences, Engineering, and Medicine; Health and Medicine Division; Board on Health Care Services; National Cancer Policy Forum . National Academies Press (US); Washington (DC): 2016. Implementation of Lung Cancer Screening: Proceedings of a Workshop. [PubMed] [Google Scholar]
- 7.O’Brien M.A., Whelan T.J., Villasis-Keever M. Are cancer-related decision aids effective? A systematic review and meta-analysis. J Clin Oncol. 2009;27(6):974–985. doi: 10.1200/JCO.2007.16.0101. [DOI] [PubMed] [Google Scholar]
- 8.Stacey D., Legare F., Lewis K. Decision aids for people facing health treatment or screening decisions. Cochrane Database Syst Rev. 2017;4:CD001431. doi: 10.1002/14651858.CD001431.pub5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Volk R.J., Linder S.K., Lopez-Olivo M.A. Patient decision aids for colorectal cancer screening: a systematic review and meta-analysis. Am J Prev Med. 2016;51(5):779–791. doi: 10.1016/j.amepre.2016.06.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ivlev I., Jerabkova S., Mishra M., Cook L.A., Eden K.B. Prostate cancer screening patient decision aids: a systematic review and meta-analysis. Am J Prev Med. 2018;55(6):896–907. doi: 10.1016/j.amepre.2018.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Moher D., Shamseer L., Clarke M. Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015 statement. Syst Rev. 2015;4:1. doi: 10.1186/2046-4053-4-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Joseph-Williams N., Newcombe R., Politi M. Toward minimum standards for certifying patient decision aids: a modified delphi consensus process. Med Decis Making. 2014;34(6):699–710. doi: 10.1177/0272989X13501721. [DOI] [PubMed] [Google Scholar]
- 13.Ouzzani M., Hammady H., Fedorowicz Z., Elmagarmid A. Rayyan-a web and mobile app for systematic reviews. Syst Rev. 2016;5(1):210. doi: 10.1186/s13643-016-0384-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.National Heart, Lung and Blood Institute Study Quality Assessment Tools. 2018. https://www.nhlbi.nih.gov/health-topics/study-quality-assessment-tools
- 15.Volk R.J., Linder S.K., Leal V.B. Feasibility of a patient decision aid about lung cancer screening with low-dose computed tomography. Prev Med. 2014;62:60–63. doi: 10.1016/j.ypmed.2014.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lau Y.K., Caverly T.J., Cao P. Evaluation of a personalized, web-based decision aid for lung cancer screening. Am J Prev Med. 2015;49(6):e125–e129. doi: 10.1016/j.amepre.2015.07.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Crothers K., Kross E.K., Reisch L.M. Patients’ attitudes regarding lung cancer screening and decision aids. a survey and focus group study. Ann Am Thorac Soc. 2016;13(11):1992–2001. doi: 10.1513/AnnalsATS.201604-289OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mazzone P.J., Tenenbaum A., Seeley M. Impact of a lung cancer screening counseling and shared decision-making visit. Chest. 2017;151(3):572–578. doi: 10.1016/j.chest.2016.10.027. [DOI] [PubMed] [Google Scholar]
- 19.Housten A.J., Lowenstein L.M., Leal V.B., Volk R.J. Responsiveness of a brief measure of lung cancer screening knowledge. J Cancer Educ. 2018;33(4):842–846. doi: 10.1007/s13187-016-1153-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.McDonnell K.K., Strayer S.M., Sercy E. Developing and testing a brief clinic-based lung cancer screening decision aid for primary care settings. Health Expect. 2018;21(4):796–804. doi: 10.1111/hex.12675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Reuland D.S., Cubillos L., Brenner A.T., Harris R.P., Minish B., Pignone M.P. A pre-post study testing a lung cancer screening decision aid in primary care. BMC Med Inform Decis Mak. 2018;18(1):5. doi: 10.1186/s12911-018-0582-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dharod A., Bellinger C., Foley K., Case L.D., Miller D. The reach and feasibility of an interactive lung cancer screening decision aid delivered by patient portal. Appl Clin Inform. 2019;10(1):19–27. doi: 10.1055/s-0038-1676807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fagan H.B., Fournakis N.A., Jurkovitz C. Telephone-based shared decision-making for lung cancer screening in primary care. J Cancer Educ. 2020;35(4):766–773. doi: 10.1007/s13187-019-01528-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hoffman A.S., Hempstead A.P., Housten A.J. Using a patient decision aid video to assess current and former smokers’ values about the harms and benefits of lung cancer screening with low-dose computed tomography. MDM Policy Pract. 2018;3(1) doi: 10.1177/2381468318769886. 2381468318769886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Manners D., Pettigrew S., Lake F.R., Piccolo F., McWilliams A.M., Brims F.J.H. Development and evaluation of a consumer information resource, including patient decision aid, for lung cancer screening: a quasi-experimental study. Transl Behav Med. 2020;10(2):404–412. doi: 10.1093/tbm/ibz029. [DOI] [PubMed] [Google Scholar]
- 26.Ruparel M., Quaife S.L., Ghimire B. Impact of a lung cancer screening information film on informed decision-making: a randomized trial. Ann Am Thorac Soc. 2019;16(6):744–751. doi: 10.1513/AnnalsATS.201811-841OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sakoda L.C., Meyer M.A., Chawla N. Effectiveness of a patient education class to enhance knowledge about lung cancer screening: a quality improvement evaluation. J Cancer Educ. 2020;35(5):897–904. doi: 10.1007/s13187-019-01540-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tanner N.T., Banas E., Yeager D., Dai L., Hughes Halbert C., Silvestri G.A. In-person and telephonic shared decision-making visits for people considering lung cancer screening: an assessment of decision quality. Chest. 2019;155(1):236–238. doi: 10.1016/j.chest.2018.07.046. [DOI] [PubMed] [Google Scholar]
- 29.O’Connor A. Decisional Conflict Scale User Manual. 2010. https://decisionaid.ohri.ca/docs/develop/User_Manuals/UM_Decisional_Conflict.pdf
- 30.Eberth J.M., McDonnell K.K., Sercy E. A national survey of primary care physicians: perceptions and practices of low-dose CT lung cancer screening. Prev Med Rep. 2018;11:93–99. doi: 10.1016/j.pmedr.2018.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wiener R.S. Point: Can shared decision-making of physicians and patients improve outcomes in lung cancer screening? Yes. Chest. 2019;156(1):12–14. doi: 10.1016/j.chest.2019.03.009. [DOI] [PubMed] [Google Scholar]
- 32.Modin H.E., Fathi J.T., Gilbert C.R. Pack-year cigarette smoking history for determination of lung cancer screening eligibility. comparison of the electronic medical record versus a shared decision-making conversation. Ann Am Thorac Soc. 2017;14(8):1320–1325. doi: 10.1513/AnnalsATS.201612-984OC. [DOI] [PubMed] [Google Scholar]
- 33.Volk R.J., Lowenstein L.M., Leal V.B. Effect of a patient decision aid on lung cancer screening decision-making by persons who smoke: a randomized clinical trial. JAMA Netw Open. 2020;3(1) doi: 10.1001/jamanetworkopen.2019.20362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Is Lung Cancer Screening Right for Me? A Decision Aid for People Considering Lung Cancer Screening with Low-dose Computed Tomography. Agency or Health Care Quality. 2016. https://effectivehealthcare.ahrq.gov/decision-aids/lung-cancer-screening/static/lung-cancer-screening-decision-aid.pdf
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.