Abstract
Neutrophils represent the first line of host cellular defense against various pathogens. The most recently described microbicidal mechanism of these cells is the release of neutrophil extracellular traps (NET). Currently, a wide range of chemical and biological stimuli are known to induce this response; however, the effect of short-chain fatty acids (SCFAs) on the induction of NET is still unknown. SCFAs are produced mainly by bacterial fermentation of dietary fiber and are found in host tissues and blood. This study aimed to determine whether physiological levels of SCFAs can induce the formation of NET. Previously reported concentrations of SCFAs (as found in the colonic lumen and peripheral blood in postprandial and basal states) were used to stimulate the neutrophils. In order to determine the signaling pathway utilized by SCFAs, we tested the inhibition of the Free Fatty Acid 2 Receptor (FFA2R) expressed in neutrophils using CATPB, the inhibitor of FFA2R, genistein, an inhibitor of the downstream Gα/q11 proteins and DPI, an inhibitor of the NADPH oxidase complex. The SCFAs at colonic intestinal lumen concentrations were able to induce the formation of NET, and when tested at concentrations found in the peripheral blood, only acetic acid at 100 μM (fasting equivalent) and 700 μM (postprandial equivalent) was found to induce the formation of NET. The administration of the competitive inhibitor against the receptor or blockade of relevant G protein signaling and the inhibition of NADPH oxidase complex decreased NET release. SCFAs stimulate NET formation in vitro and this effect is mediated, in part, by the FFA2R.
Keywords: FFA2R, NET, neutrophil extracellular traps induction, short-chain fatty acids
Introduction
Fatty acids that have between two to six carbon atoms in their structure are called short-chain fatty acids (SCFAs). Acetic, propionic, and butyric acid make up more than 95% of the total SCFAs in the intestinal lumen.1 These organic acids are produced exogenously by the bacterial fermentation of carbohydrates and proteins that escape digestion and absorption in the small intestine, and are endogenously formed by lipid oxidation and the metabolism of branched-chain amino acids.2 Therefore, amounts of SCFAs vary in the intestinal lumen and circulation depending on an individual’s diet, intestinal microbiota, and metabolism.3,4 SCFAs are of great importance due to their ability to not only modulate energy and endocrine response but also regulate immune cell function.1,5
Neutrophils belong to the initial line of immunological defense, and represent 60% to 70% of the total leukocytes in peripheral blood.6 SCFAs have been shown to be involved in cellular migration, cytokine expression, apoptosis, and increases in cytosolic calcium flow.7–10 The function of neutrophils in the presence of SCFAs varies according to the concentration, combination, and proportion of these acids. Their modulating effects on cells can be direct, through the activation of specific SCFAs receptors (Free fatty acid receptors, FFAR)7 or via histone deacetylase (HDAC),11 or indirect, through the modification of extracellular pH.12
The most recently reported microbicidal mechanism of neutrophils is the formation of neutrophil extracellular traps (NET).13 NET formation involves the release of chromatin structures in the form of an extracellular network containing nuclear or mitochondrial DNA, which is associated with microbicidal peptides; the objective of these structures is to trap pathogens and prevent their spread in the organism. The sequential steps of the molecular mechanism that induces NET formation involve the following: recognition of a stimulus by the receptors, production of reactive oxygen species (ROS), activation of the Raf-MEK-Erk pathway (Raf; Proto-oncogene serine/threonine-protein kinase, MEK; Mitogen-activated protein kinase kinase, Erk; Extracellular-signal-regulated kinase),14 translocation of elastase and myeloperoxidase into the nucleus, disassembly of nuclear membrane,15 dispersion and mixing of chromatin with cytoplasmic components, and finally the rupture of cytoplasmic membrane leading to the extracellular release of DNA associated with antimicrobial peptides.16,17
Depending on the stimulus, NET may require an increase of intracellular Ca2+ and activation of the peptidyl arginine deiminase 4 (PAD4) enzyme.18,19
The NET formation is a mechanism that is exacerbated by chronic inflammatory processes and autoimmune or metabolic complications.20,21 When the formation of NET does not occur, or its microbicidal capacity is insufficient, a greater vulnerability to recurrent bacterial or fungal infection is observed.22 In diabetes, for example, there is a defect in the formation of NET; this leads to recurrent secondary bacterial or fungal complications.23
Although the SCFAs can activate neutrophils, their role in the modulation of NET remains unknown. In the intestinal lumen, there are millimolar concentrations of SCFAs,24 while the amount is much lower in the peripheral blood.25,26 The objective of this study is to determine the effect of physiological concentrations of SCFAs on NET induction and whether FFA2R mediates this effect.
Materials and methods
Reagents
The following reagents were used in this study: 0.9% physiological saline solution (Solution sodium chloride 0.9%; PiSA®, Guadalajara, México), sterile-filtered density gradient solution1.077 g/mL (10771, Sigma Aldrich, Milwaukee, WS, USA), and 1.119 g/mL (Histopaque®-1119, Sigma Aldrich), calcium ionophore (A23187; Sigma Aldrich), CATBP ((S)-3-(2-(3-chlorophenyl) acetamido)-4-(4-(trifluoromethyl) phenyl) butanoic acid (SML1782 Sigma Aldrich), genistein (4′,5,7-trihydroxyisoflavone, 5,7-dihydroxy-3-(4-hydroxyphenyl)-4H-1-benzopyran-4-one (G6649, Sigma Aldrich), DAPI (2-(4-Amidino Phenyl)-6-indolecarbamidine dihydrochloride, 4′,6-Diamidino-2-phenylindole dihydrochloride) (D9542, Sigma Aldrich), DPI (Diphenyleneiodonium chloride) (D2926, Sigma Aldrich), DNAse I (AMPD1, Sigma Aldrich), Poly-L-lysine solution (P8920; Sigma Aldrich), and acetic acid, propionic acid, and butyric acid (A6283, 402907, B103500, Sigma Aldrich). SYTOX® Green (S7020; Thermo Fisher Scientific, Waltham, MA, USA), anti-neutrophil elastase antibody (ab21595; abcam, Cambridge, LDN, UK), and the secondary antibody (ab150077; abcam).
Neutrophil isolation
The study protocol was approved by the Research and Research Ethics Committee of the University Center for Health Sciences under the approval number, CI-03319. Written informed consent was obtained from all participants prior to sample collection, in accordance with the ethical guidelines of the 2013 Helsinki Declaration. Peripheral blood from clinically healthy subjects was collected in tubes with EDTA, and neutrophils were isolated by using a Ficoll Histopaque 1119/1077 density gradient (Sigma Aldrich) according to the manufacturer’s instructions. First, a layer of 3 mL of Histopaque 1119 was placed (density: 1.119 g/mL) followed by the careful addition of 3 mL of Histopaque 1077 (density: 1.077 g/mL), and finally 6 mL of peripheral blood. To achieve the separation of cells, the solution was centrifuged at 700 g for 30 min. The ring of granulocytes was taken and subsequently washed by centrifugation at 300 g for 10 min and resuspended in physiological saline solution. We evaluated the cell count and viability (>95%) in a Neubauer chamber using trypan blue.
Stimulation of NET with SCFAs
As a positive control for NET formation in vitro, we used 20 μM calcium ionophore. We used 250,000 neutrophils/mL that were freshly extracted and isolated for each sample. The standard medium of these experiments was 0.9% physiological saline solution with pH adjusted to 7.35 using NaOH.
We prepared acetic, butyric, and propionic acid in the physiological concentrations previously reported: intestinal lumen24 as well as peripheral blood in fasting25 and postprandial state26 (see Table 1). The pH of SCFAs solution was adjusted with NaOH to 7.35. The same experiments were carried at a pH of 6.00. Once the stimulus was added, the cells were incubated for 3 h at 37°C and 5% CO2.
Table 1.
Physiological concentration of SCFAs for induction of NET.
| SCFAs | Peripheral blood (µM) | Intestinal lumen (mM) | |
|---|---|---|---|
| Fasting | Postprandial | ||
| Acetic acid | 100 | 700 | 60 |
| Propionic acid | 4 | 80 | 20 |
| Butyric acid | 2 | 20 | 20 |
Competitive inhibition of FFA2R in neutrophils
Neutrophils were incubated with different inhibitors for the FFA2R and NADPH oxidase complex to determine if it has any role in the induction of NET by SCFAs. We used CATPB (500 nM), as an antagonist of the active site of the receptor,27,28 genistein (100 nM) to inhibit the phosphorylation of Gα/q11 proteins, which is necessary for intracellular signaling of FFA2R,28 and DPI (10 µM) as an inhibitor of the Heme groups and FAD of the NADPH oxidase complex.29 We incubated the neutrophils with each inhibitor 1 h before the induction of NET with SCFAs as described in section “Stimulation of NET with SCFAs.”
Staining of NET and microscopy
We prepared each sample in Petri dishes containing a round coverslip, which was washed and treated with poly-L-lysine to induce cell adherence. To the Petri dishes, we then added 1 mL of the corresponding stimuli in the conditions indicated in sections “Stimulation of NET with SCFAs” and “Competitive inhibition of FFA2R in neutrophils” and fixed the cells with 4% paraformaldehyde for 20 min. We stained the sample with DAPI (1 µg/mL) with an incubation time of 1 h at room temperature. We performed elastase staining using a specific procedure that has been previously described by Agraz-Cibrian et al.30 Briefly, after staining with DAPI, we performed two washes with PBS and incubated the samples with 0.5% Triton for 1 min at room temperature to permeabilize the cells. The coverslips were washed three times, and the primary anti-elastase antibody (ab21595, abcam) at a dilution of 1:500 in blocking solution was added followed by incubation for 1 h at 37°C. The coverslips were again washed three times, and the secondary antibody (ab150077 Goat anti-rabbit IgG H&L, abcam) at a dilution of 1:1000 in blocking solution was added followed by incubation for 1 h at 37°C. Finally, the coverslips were observed, and images captured using a fluorescence microscope at 488 nm (Carl ZEISS AXIO Imager 2, Germany).
NET quantification
In each well of a 24-well plate (costar® 3524), 250,000 neutrophils were added in a volume of 1 mL along with each of the evaluated stimuli, as noted above. The plates were incubated for 3 h at 37°C and 5% CO2. After the incubation, the samples were centrifuged at 300 g for 10 min, and a volume of 750 μL of supernatant was discarded from each well. Next, 1 unit/well of DNAse I was added, and the plate was incubated for 30 min. After this, the plate was centrifuged at 300 g for 10 min, and then 300 μL of the supernatant from each well was transferred to a dark 96-well plate. Each well of this new plate was added with 5 mM of SYTOX® Green and the plate was incubated at room temperature for 15 min. Quantification of the DNA/NET signals was performed using fluorometry. Fluorescence readings were realized using a Biotek Synergy HTX plate reader with 485 nm excitation/527 nm emission filters.
Statistics
All the experiments were performed at least twice with individual conditions repeated in duplicate or triplicate wells (specifics noted in the text for each experiment). For the calculation of the sample size we used the formula for two means. A unilateral hypothesis, error of 0.05 and 90% statistical power were proposed because the K value was 8.6. The standard deviation data and the mean value for the formula were obtained from our pilot study and Bukina et al.; using the above, giving n = 3.31
Data were compared with the untreated control. The analysis was done with the package of the GraphPad Prism 6 program, version 6.0c. We utilized non-parametric statistical analysis. The Kruskal-Wallis and Dunn’s test were used to analyze the experiments showing the effect of SCFAs on NET induction. The Mann-Whitney U test was used to analyze the rest of the experiments showing the regulation of inhibitors. For all the experiments, P < 0.05 was considered statistically significant.
Results
Intestinal concentrations of SCFAs stimulate the formation of NET
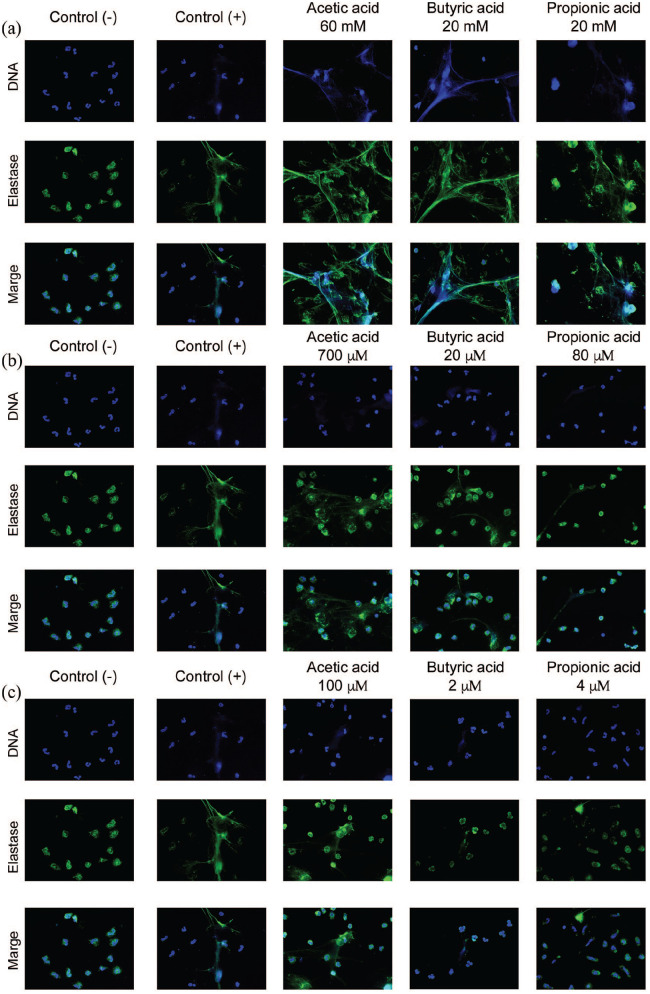
Each of the SCFAs administered at intestinal concentrations was able to induce the formation of NET. Acetic acid at 60 mM induced a more significant release of NET, as can be observed in the fluorescence microscopy analysis (Figure 1(a)) and through the quantification of the DNA released by fluorometry (Figure 2(a)).
Figure 1.
SCFAs stimulate the formation of NET. The SCFAs at a millimolar concentration in the gut (see Table 1) induce the formation of NET (a) in greater proportions than the micromolar concentration in the peripheral blood in the postprandial state (b) and fasting state (c). The neutrophils were fixed and stained using DAPI for DNA (blue color) and anti-elastase antibody for elastase (green color). The images are representative of four independent experiments for each stimulus. Fluorescence microscopy images are at 40× magnification. Negative control was SSF and positive control was calcium ionophore (20 μM).
Acetic acid at peripheral blood concentration can induce the formation of NET
We used the concentrations previously reported in peripheral blood in postprandial and fasting conditions to induce NET formation (Figure 1(b) and (c)). A significant effect was observed only when using acetic acid at postprandial (P < 0.01) (Figure 2(b)) and fasting peripheral blood concentrations (P < 0.001) (Figure2(c)).
Figure 2.
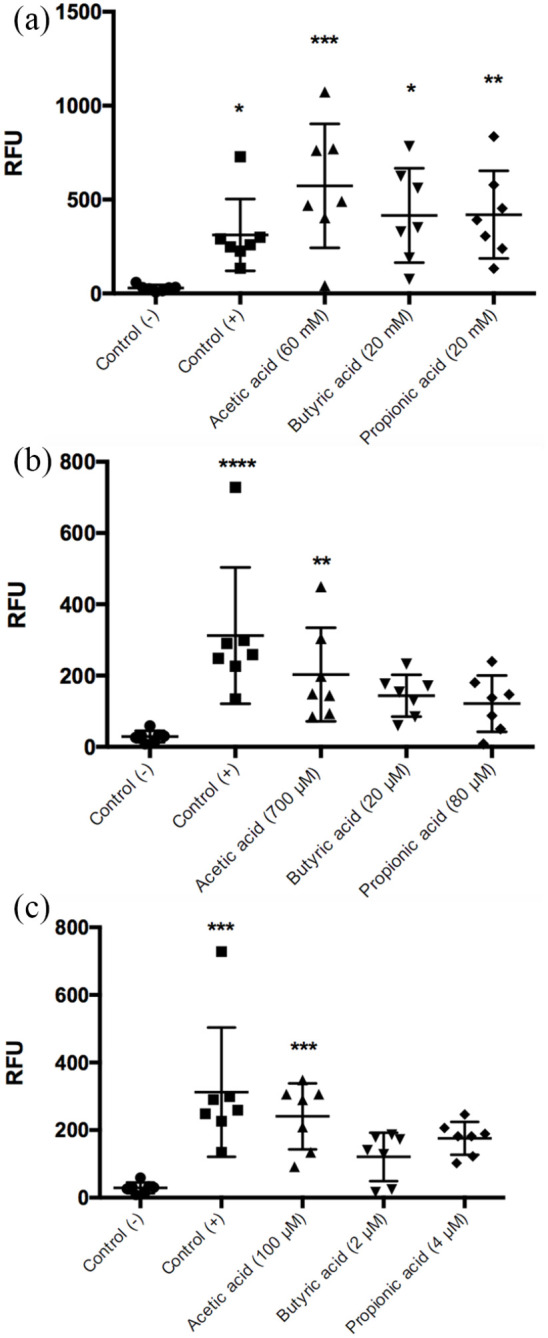
Quantification of DNA/NET induced by SCFAs. A greater effect on NET formation was observed in response to intestinal concentrations of acetic acid, 60 mM (***P < 0.001); butyric acid, 20 mM (*P < 0.5); and propionic acid, 20 mM (**P < 0.01) compared to the negative control (a). Acetic acid preserved this effect at the blood concentration in peripheral blood; 700 μM in the postprandial state (**P < 0.01) (b) and 100 μM in the fasting state (***P < 0.001) (c). Each bar represents the average ± SD of seven independent experiments; each experiment was performed in duplicate; the statistical analysis was performed with the Kruskal-Wallis test and Dunn’s test. Negative control was SSF and positive control was calcium ionophore (20 μM).
NET induction by SCFAs is affected by pH
Khan et al.32 reported that spontaneous NET induction occurs when neutrophils are incubated at an alkaline pH of 7.8, whereas an acidic pH of 6.0 prevents NET induction.33 To determine if the decrease in pH affects the formation of SCFAs-induced NET, we used a 100 μM stimulus of acetic acid at pH 6.0 and 7.35 adjusted with NaOH. Our results showed a significant decrease in the NET induction by acetic acid at pH 6.0 when compared to the induction of NET with acetic acid at pH 7.35. In these experiments NET induction was decreased up to 85% (P < 0.05*), suggesting that the pH regulates the induction of NET by SCFAs (Figure 3).
Figure 3.
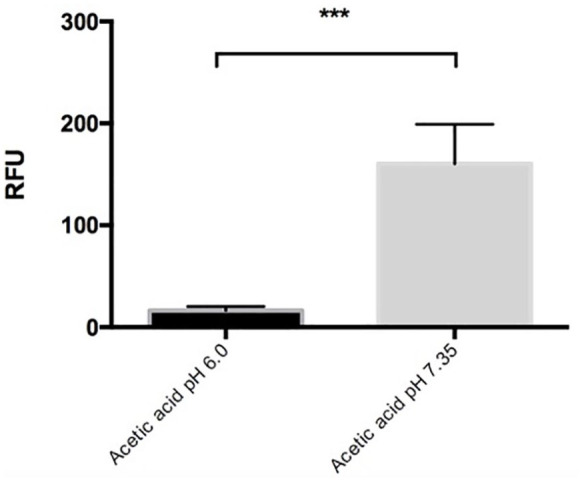
The induction of NET by SCFAs is modified by the pH. The percentage of NET released in response to 100 μM acetic acid when using the acid medium of pH 6.0 decreased significantly (***P < 0.001) compared to that when using a medium with pH 7.35; it was reported in Relative Fluorescence Units (RFU). Each bar represents the mean ± SD of four independent experiments in duplicate and was analyzed using Mann-Whitney U test.
SCFAs induce NET through the FFA2R and NADPH oxidase activity
To investigate whether the induction of NET by acetic acid is dependent upon FFA2R, CATPB was used as an antagonist of the active site of the receptor, genistein was used as an inhibitor of its intracellular signaling pathway, and DPI to block the NADPH oxidase activity. Our results showed that pre- incubation of neutrophils with CATPB resulted in a 2.4-fold decrease in the production of NET induced by 100 μM acetic acid (Figure 4(a)). Similarly, the pre-incubation of neutrophil with genistein inhibited the formation of NET by up to 2.5-fold (Figure 4(b)). Moreover, the pre-incubation with DPI showed 2.2-fold decrease in the NET production (Figure 4(c)).
Figure 4.
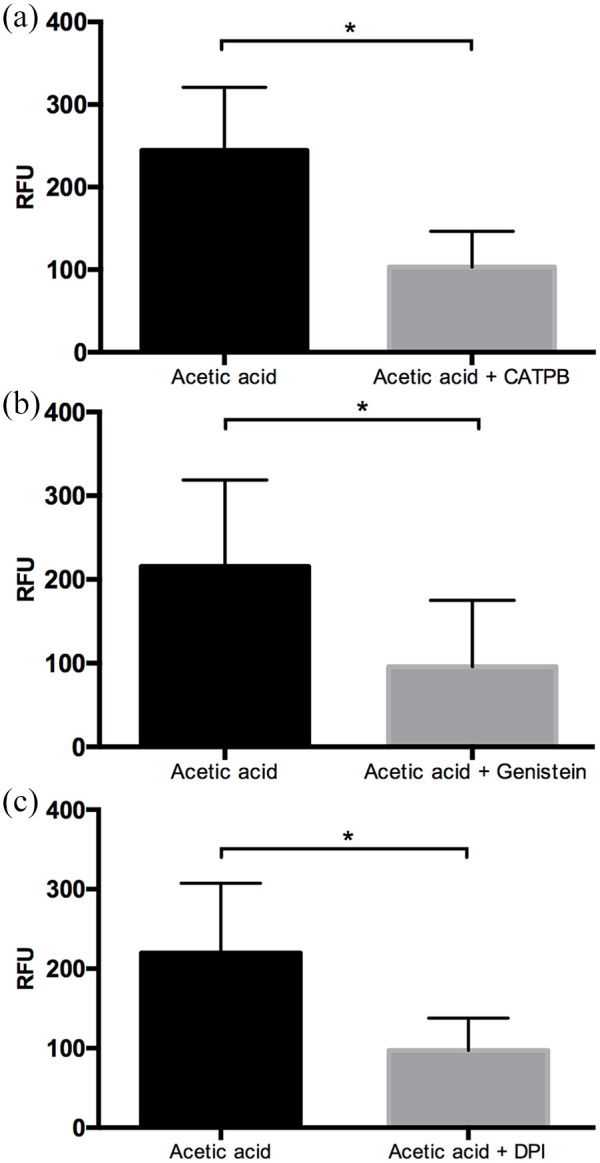
SCFAs induce NET formation through FFA2R and NADPH oxidase complex. The competitive inhibitor of FFA2R, CATPB, caused the inhibition of NET by acetic acid (*P < 0.05) (a). Under similar conditions of stimuli, the inhibitor of G protein, genistein, led to a diminution of NET formation (*P < 0.05) (b), and the inhibitor of NADPH oxidase complex, DPI, showed a decrease on NET production (*P < 0.05) (c). It was reported in Relative Fluorescence Units (RFU). Each bar represents the mean ± SD of three independent experiments in duplicate, and was analyzed using the Mann-Whitney U test.
Discussion
In this study, to the best of our knowledge, we demonstrated for the first time that SCFAs can induce the release of NET. The proportion and number of bacteria in the gut microbiota determine the amount of SCFAs and their proportion.1 Diverse pathologies such as obesity,34 metabolic syndrome,35 systemic lupus erythematosus,36 and salmonellosis37 are characterized by secondary symptoms known as intestinal dysbiosis, where the proportion and quantity of bacterial groups are altered and therefore, the production of SCFAs is likewise perturbed. Of the gut microbiota, the phylum Bacteroidetes is the primary producer of acetate and propionate, while the phylum Firmicutes produces mostly butyrate.1
Similar to our study, Carretta et al. in 2016 used 700 μM of butyric acid to induce NET formation in cells from ruminants. Additionally, this effect was associated with the activation of inflammatory processes in sub-acute ruminal acidosis in cattle; specifically, this group observed an effect on cellular activation and increases in intracellular calcium flow, ROS production, and the release of primary and secondary granule contents. The sum of their results suggested that butyric acid could contribute to the inflammatory response observed during digestive disorders in cattle that have high concentrations of butyric acid in the rumen.38
In another study, working with rats, Bukina et al. in 2018 showed that the administration of a probiotic strain of B. fragilis after previous treatment with vancomycin in the context of infection with S. typhimurium, led to the recovery of production of SCFAs and in turn increased the formation of NET in the blood and intestine.31
It has been reported that pH regulates several neutrophil functions, such as phagocytosis, ROS production, cell migration, and NET production.32,33 For this reason, we tested whether the pH could affect the formation of NET via SCFAs.
In this study, we demonstrated that acetic acid, even at 100 μM (peripheral blood concentration in the fasting state), serves as a stimulus for the in vitro formation of NET at pH 7.35; however, when we use an acidic pH of 6.0, NET production was decreased by up to 85%. Similarly, in a 2006 study, a pH shift from 6.7 to 5.5 during neutrophil stimulation with SCFAs decreased ROS by 85%.39
In line with the above, in 2016, Maueröder et al. proposed a potential in vivo model in an environment of local inflammation that hypothesized that NET are triggered only in situations of neutral and alkaline pH. The idea is that a pH gradient is formed in the inflamed area that is acidic at the center and is alkaline at the edges. The formation of NET would occur at the edges of the inflamed area, thereby forming a barrier and preventing the invasion of pathogens or the dissemination of molecular patterns associated with damage; meanwhile, in the acidic center, pathogens could still be phagocytosed and destroyed, as these are functions that are not inhibited at an acidic pH.40
Khan et al. demonstrated that histone cleavage by granular proteases was more active at a higher pH.32 On the other hand, the data obtained by Behnen et al. suggest that the inhibition of NET formation in acidic conditions is due to limited production of ROS and reduced glycolytic capacity.33
The weak organic acids can be found in an ionized or non-ionized form, and their solubility, transport, or binding to receptors depends on this. Acetic acid has a pKa = 4.8, and at physiological pH, it is mainly in its ionized form unable to cross membranes, and would require a receptor to carry out intracellular signaling. We performed experiments with lactic acid. This molecule has three carbons in its structure, like propionic acid, and pKA = 3.86 near to the pKa = 4.8 of acetic acid. Moreover, lactic acid is produced by bacteria, like SCFA, and also in muscle cells in anaerobic conditions. The concentration used was 34 mM, similar to Hartmann Solution utilized intravenously in humans. Lactic Acid did not stimulate NETs formation (data not shown).
Therefore, we demonstrated that SCFAs induce NET formation and that is in part, modulated by the FFA2R. Using CATPB as an inhibitor of FFA2R, the NET formation decreased by 2.4 times induced by using the stimuli of acetic acid at 100 μM. Björkman et al.28 showed the participation of FFA2R in neutrophils by demonstrating the increment of cytosolic calcium induced by agonists of FFA2R, acetic acid at 10 mM or Cmp1. This effect was blocked by CATPB; moreover, FFA2R is an orphan receptor and it needs to be coupled to G proteins to carry out its function. The pre-incubation of neutrophils with genistein, an inhibitor of Gα/q11 proteins, inhibited the formation of NET by up to 2.5-fold, thereby supporting the probable pathway induction of SCFAs, but the microenvironment is important too. In 2012, Paria Mirmonsef showed that only high concentrations of SCFAs (20 mM) induced the release of pro-inflammatory neutrophil cytokines in vitro; however, when cells were stimulated with combinations of ligands to TLR2 (Toll-like receptor 2) and TLR7 (Toll-like receptor 7), the production of cytokines was observed at SCFA concentrations of 0.02 to 2 mM.41
In the other hand, we used DPI an inhibitor of NADPH oxidase complex, to demonstrate that the released DNA upon acetic acid 100 μM stimulation is due to NET formation and not any other type of cell death, we observed a decrease of 2.2 times in NET formation, showing that SCFA need ROS for the release of NET.
It would be interesting to determine what combinations of different types of endotoxins, antioxidants, and pro-inflammatory cytokines could be developed to regulate or prime SCFA-induced NET formation. To fully understand this system, it will also be important to study the roles of other actors, such as the calcium chelator, BAPTA, a known inhibitor of NETs; Pertussis toxin, which inhibits the phosphorylation of the protein Gα/q11; and Cmp1, the FFAR2 agonist.
Our results showed that peripheral blood concentrations of acetic acid could induce the formation of NET in vitro. We suggest that our in vitro experiments do not directly translate to what happens in vivo, for if physiological levels of acetic acid induce NET formation even at fasting blood concentrations, this would present a problem at a systemic level with significant inflammatory immune consequences. Thus, we suspect that a more complicated interplay of additional mechanisms that constantly regulate the release of NET must exist. In this regard, inhibitory components of NET have recently been found in the serum. Some of these are currently the subject of new patent proposals, such as the neonatal NET inhibiting factor (nNIF) and related peptides (NRP), which are present in umbilical cord blood, the placenta, and the plasma of healthy adults.42 We also consider that other serum components, such as albumin, cytokines or antioxidants, could play an important role in moderating the neutrophil response. It has been suggested that antioxidants in human serum control, at least in part, NET formation.43 On the other hand, cytokines, such as IL-1β, IL-8 and TNF-α, have been shown to stimulate NET formation.28,44 The interplay of these various molecules together with SCFA has yet to be demonstrated, and will be one of the goals of our future investigations.
Ohbuchi in 2018 showed that acetic acid alone does not induce NET formation in vitro; in fact, acetic acid was capable of moderately inhibiting the formation of NET induced by phorbol 12-myristate 13-acetate (PMA).45 It appears that this study finding contradicts with our results; however, they used 5% inactivated autologous serum for the incubation of neutrophil and RPMI as the culture medium. In our study, RPMI spontaneously produced NETs (data not shown), as reported by Kamoshida, 2017.46 The 0.9% physiological saline solution at pH 7.35 showed a null effect on NET formation and did not affect cell viability; moreover, the response to LPS and ionomycin in our controls was adequate. We used physiological saline solution without serum to investigate the interaction between the SCFA and its receptor without interference from other components in the solution.
Conclusion
In this study, we demonstrate for the first time that SCFAs induce the formation of NET when neutrophils are exposed to the intestinal physiological concentrations of these acids. Additionally, acetic acid induced NET even at baseline blood concentration. In future studies we propose to conduct experiments aimed at finding components, microenvironmental conditions, and mechanisms that may prevent the formation of NET in response to endogenous stimuli.
Acknowledgments
We thank our colleagues who provided insightful inputs to achieve better results in this research: Karina Elizabeth Avila Arrezola, Karla Patricia Magaña López, and specially Jesse Haramati and Juan Manuel Agráz Cibrian for his comments that significantly improved our writing.
Footnotes
Declaration of conflicting interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Ethical approval: The protocol was submitted for evaluation and approval by the Research and Research Ethics Committee of the University Center for Health Sciences, and was approved under the opinion number CI-03319.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by the Universidad de Guadalajara PROINPEP (249518).
Informed consent: Written informed consent was obtained from all participants prior to sample collection, in accordance with the ethical guidelines of the 2013 Helsinki Declaration.
ORCID iD: Vidal Delgado-Rizo  https://orcid.org/0000-0002-2150-6890
https://orcid.org/0000-0002-2150-6890
References
- 1. den Besten G, van Eunen K, Groen AK, et al. The role of short-chain fatty acids in the interplay between diet, gut microbiota, and host energy metabolism. Journal of Lipid Research 2013; 54: 2325–2340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Macfarlane GT, Macfarlane S. Bacteria, colonic fermentation, and gastrointestinal health. Journal of AOAC International 2012; 95: 50–60. [DOI] [PubMed] [Google Scholar]
- 3. Rahim MBHA, Chilloux J, Martinez-Gili L, et al. Diet-induced metabolic changes of the human gut microbiome: Importance of short-chain fatty acids, methylamines and indoles. Acta Diabetologica 2019; 56: 493–500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Fredstrom SB, Lampe JW, Jung HJG, et al. Apparent fiber digestibility and fecal short-chain fatty acid concentrations with ingestion of two types of dietary fiber. Journal of Parenteral and Enteral Nutrition 1994; 18: 14–19. [DOI] [PubMed] [Google Scholar]
- 5. Ratajczak W, Rył A, Mizerski A, et al. Immunomodulatory potential of gut microbiome-derived short-chain fatty acids (SCFAs). Acta Biochimica Polonica 2019; 66: 1–12. [DOI] [PubMed] [Google Scholar]
- 6. Nauseef WM, Borregaard N. Neutrophils at work. Nature Immunology 2014; 15: 602–611. [DOI] [PubMed] [Google Scholar]
- 7. Le Poul E, Loison C, Struyf S, et al. Functional characterization of human receptors for short chain fatty acids and their role in polymorphonuclear cell activation. Journal of Biological Chemistry 2003; 278: 25481–25489. [DOI] [PubMed] [Google Scholar]
- 8. Nilsson NE, Kotarsky K, Owman C, et al. Identification of a free fatty acid receptor, FFA2R, expressed on leukocytes and activated by short-chain fatty acids. Biochemical and Biophysical Research Communications 2003; 303: 1047–1052. [DOI] [PubMed] [Google Scholar]
- 9. Maslowski KM, Vieira AT, Ng A, et al. Regulation of inflammatory responses by gut microbiota and chemoattractant receptor GPR43. Nature 2009; 461: 1282–1286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Vinolo MA, Rodrigues HG, Hatanaka E, et al. Short-chain fatty acids stimulate the migration of neutrophils to inflammatory sites. Clinical Science 2009; 117: 331–338. [DOI] [PubMed] [Google Scholar]
- 11. Licciardi PV, Ververis K, Karagiannis TC. Histone deacetylase inhibition and dietary short-chain fatty acids. ISRN Allergy 2011; 2011: 869647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Lupton JR, Kurtz PP. Relationship of colonic luminal short-chain fatty acids and pH to in vivo cell proliferation in rats. Journal of Nutrition 1993; 123: 1522–1530. [DOI] [PubMed] [Google Scholar]
- 13. Brinkmann V, Reichard U, Goosmann C, et al. Neutrophil extracellular traps kill bacteria. Science 2004; 303: 1532–1535. [DOI] [PubMed] [Google Scholar]
- 14. Hakkim A, Fuchs TA, Martinez NE, et al. Activation of the Raf-MEK-ERK pathway is required for neutrophil extracellular trap formation. Nature Chemical Biology 2011; 7: 75–77. [DOI] [PubMed] [Google Scholar]
- 15. Papayannopoulos V, Metzler KD, Hakkim A, et al. Neutrophil elastase and myeloperoxidase regulate the formation of neutrophil extracellular traps. Journal of Biological Chemistry 2010; 191: 677–691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Brinkmann V, Zychlinsky A. Beneficial suicide: Why neutrophils die to make NETs. Nature Reviews Microbiology 2007; 5: 577–582. [DOI] [PubMed] [Google Scholar]
- 17. Neeli I, Dwivedi N, Khan S, et al. Regulation of extracellular chromatin release from neutrophils. Journal of Innate Immunity 2009; 1: 194–201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Lewis HD, Liddle J, Coote JE, et al. Inhibition of PAD4 activity is sufficient to disrupt mouse and human NET formation. Nature Chemical Biology 2015; 11: 189–191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Li P, Li M, Lindberg MR, et al. PAD4 is essential for antibacterial innate immunity mediated by neutrophil extracellular traps. Journal of Experimental Medicine 2010; 207: 1853–1862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Delgado-Rizo V, Martínez-Guzmán MA, Iñiguez-Gutierrez L, et al. Neutrophil extracellular traps and its implications in inflammation: An overview. Frontiers in Immunology 2017; 8: 81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Yu Y, Su K. Neutrophil extracellular traps and systemic lupus erythematosus. Journal of Clinical & Cellular Immunology 2013; 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Bianchi M, Hakkim A, Brinkmann V, et al. Restoration of NET formation by gene therapy in CGD controls aspergillosis. Blood 2009; 114: 2619–2622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Wong SL, Demers M, Martinod K, et al. Diabetes primes neutrophils to undergo NETosis, which impairs wound healing. Nature Medicine 2015; 21: 815–819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Cummings J, Pomare E, Branch W, et al. Short chain fatty acids in human large intestine, portal, hepatic and venous blood. Gut 1987; 28: 1221–1227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Tarini J, Wolever TM. The fermentable fibre inulin increases postprandial serum short-chain fatty acids and reduces free-fatty acids and ghrelin in healthy subjects. Applied Physiology, Nutrition, and Metabolism 2010; 35: 9–16. [DOI] [PubMed] [Google Scholar]
- 26. Bray GA, Nielsen SJ, Popkin BM. Consumption of high-fructose corn syrup in beverages may play a role in the epidemic of obesity. The American Journal of Clinical Nutrition 2004; 79: 537–543. [DOI] [PubMed] [Google Scholar]
- 27. Bolognini D, Tobin AB, Milligan G, et al. The pharmacology and function of receptors for short-chain fatty acids. Molecular Pharmacology 2016; 89: 388–398. [DOI] [PubMed] [Google Scholar]
- 28. Björkman L, Mårtensson J, Winther M, et al. The neutrophil response induced by an agonist for free fatty acid receptor 2 (GPR43) Is primed by tumor necrosis factor alpha and by receptor uncoupling from the cytoskeleton but attenuated by tissue recruitment. Molecular and Cellular Biology 2016; 36: 2583–2595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Reis J, Massari M, Marchese S, et al. A closer look into NADPH oxidase inhibitors: Validation and insight into their mechanism of action. Redox Biology 2020; 32: 101466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Agraz-Cibrian JM, Segura-Ortega JE, Delgado-Rizo V, et al. Alterations in neutrophil extracellular traps is associated with the degree of decompensation of liver cirrhosis. The Journal of Infection in Developing Countries 2016; 10: 512–517. [DOI] [PubMed] [Google Scholar]
- 31. Bukina YV, Varynskyi B, Voitovich A, et al. The definition of neutrophil extracellular traps and the concentration of short-chain fatty acids in salmonella-induced inflammation of the intestine against the background of vancomycin and Bacteroides fragilis. Pathologia 2018; 15: 10–17. [Google Scholar]
- 32. Khan MA, Philip LM, Cheung G, et al. Regulating NETosis: Increasing pH promotes NADPH oxidase-dependent NETosis. Frontiers in Medicine 2018; 5: 19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Behnen M, Möller S, Brozek A, et al. Extracellular acidification inhibits the ROS-dependent formation of neutrophil extracellular traps. Frontiers in Immunology 2017; 8: 184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Fernandes J, Su W, Rahat-Rozenbloom S, et al. Adiposity, gut microbiota and faecal short chain fatty acids are linked in adult humans. Nutrition & Diabetes 2014; 4: e121–e121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Nishitsuji K, Xiao J, Nagatomo R, et al. Analysis of the gut microbiome and plasma short-chain fatty acid profiles in a spontaneous mouse model of metabolic syndrome. Scientific Reports 2017; 7: 1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Rodríguez-Carrio J, López P, Sánchez B, et al. Intestinal dysbiosis is associated with altered short-chain fatty acids and serum-free fatty acids in systemic lupus erythematosus. Frontiers in Immunology 2017; 8: 23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Ktsoyan ZA, Mkrtchyan MS, Zakharyan MK, et al. Systemic concentrations of short chain fatty acids are elevated in salmonellosis and exacerbation of familial mediterranean fever. Frontiers in Microbiology 2016; 7: 776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Carretta M, Hidalgo A, Burgos J, et al. Butyric acid stimulates bovine neutrophil functions and potentiates the effect of platelet activating factor. Veterinary Immunology and Immunopathology 2016; 176: 18–27. [DOI] [PubMed] [Google Scholar]
- 39. Mills SW, Montgomery SH, Morck DW. Evaluation of the effects of short-chain fatty acids and extracellular pH on bovine neutrophil function in vitro. American Journal of Veterinary Research 2006; 67: 1901–1907. [DOI] [PubMed] [Google Scholar]
- 40. Maueröder C, Mahajan A, Paulus S, et al. Menage-a-trois: The ratio of bicarbonate to CO2 and the pH regulate the capacity of neutrophils to form NETs. Frontiers in Immunology 2016; 7: 583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Mirmonsef P, Zariffard MR, Gilbert D, et al. Short-chain fatty acids induce pro-inflammatory cytokine production alone and in combination with toll-like receptor ligands. American Journal of Reproductive Immunology 2012; 67: 391–400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Yost CC, Schwertz H, Cody MJ, et al. Neonatal NET-inhibitory factor and related peptides inhibit neutrophil extracellular trap formation. Journal of Clinical Investigation 2016; 126: 3783–3798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Fuchs TA, Abed U, Goosmann C, et al. Novel cell death program leads to neutrophil extracellular traps. International Journal of Cell Biology 2007; 176: 231–241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Keshari RS, Jyoti A, Dubey M, et al. Cytokines induced neutrophil extracellular traps formation: Implication for the inflammatory disease condition. PLoS One 2012; 7: e48111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Ohbuchi A, Kono M, Takenokuchi M, et al. Acetate moderately attenuates the generation of neutrophil extracellular traps. Blood Research 2018; 53: 177–180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Kamoshida G, Kikuchi-Ueda T, Nishida S, et al. Spontaneous formation of neutrophil extracellular traps in serum-free culture conditions. FEBS Open Bio 2017; 7: 877–886. [DOI] [PMC free article] [PubMed] [Google Scholar]