Abstract
Objective
To assess the safety and effectiveness of a self-expandable metallic stent (SEMS) combined with Iodine-125 seeds strand to treat hilar malignant biliary obstruction (MBO).
Methods
This retrospective study included patients who had received SEMS with 125I seeds strand (seeds group) or SEMS alone (controls) to treat hilar MBO. Demographic, biochemical, stent patency, overall survival and complications data were extracted and analysed.
Results
A total of 76 patients were included (seeds group, n = 40; controls, n = 36), with a total of 608 seeds deployed in the seeds group (mean, 15.2 ± 4.1 [range, 8–25] seeds per patient). Statistically significant between-group differences were shown in median stent patency time (seeds group, 387.0 ± 27.9 days [95% confidence interval {CI} 332.4, 441.6] versus controls, 121.0 ± 9.1 days [95% CI 103.2, 138.8]) and in median overall survival (seeds group, 177.0 ± 17.9 days [95% CI 141.8, 212.2] versus controls, 123.0 ± 20.4 [95% CI 83.0, 163.0]). There were no statistically significant between-group differences in complication rates.
Conclusion
SEMS combined with 125I seeds strand is safe, feasible, and tolerable in treating patients with hilar MBO, and may be effective in prolonging stent patency time and overall survival.
Keywords: Biliary obstruction, self-expandable metallic stent, Iodine-125 seed
Introduction
Hilar malignant biliary obstruction (MBO) mainly invades the common hepatic duct, and bifurcation of the left and right hepatic ducts.1 Because it is asymptomatic at an early stage, most cases of cholangiocarcinoma are diagnosed at an advanced stage, at which the opportunity for radical surgery has been lost.2 For patients with unresectable MBO and life expectancy exceeding 3 months, self-expandable metallic stent (SEMS) deployment is the standard treatment option, and may effectively relieve jaundice and improve quality of life.3,4 However, stent restenosis can occur, mainly due to tumour overgrowth or ingrowth, biliary epithelial cell proliferation and biliary sludge formation and accumulation.5 Although several methods aim to improve biliary stent patency and therefore prolong survival in patients with hilar MBO, e.g. covered SEMS, stent combined with chemotherapy, photodynamic therapy and radiofrequency ablation,5–8 the results of such regimes are not optimal. The efficacy of endobiliary radiofrequency ablation remains controversial,5,9 and is associated with a risk of biliary perforation.10 The availability, photosensitivity in patients, and procedural cost limit the clinical application of photodynamic therapy.6 Research has shown that external beam radiotherapy or brachytherapy may be effective in the treatment of hilar cholangiocarcinoma, however, external beam radiotherapy may cause severe toxicities, including dehydration and mucositis.11 Interstitial Iodine-125 (125I) brachytherapy has been widely applied in tumour treatment to locally control lung cancer, prostate carcinoma, and malignant portal vein thrombus among others, all with promising results.12–14 125I seeds strands have also been applied to treat MBO,15,16 however, the use of 125I radioactive particles in hilar MBO has rarely been described.17–19
The aim of the present study was to perform retrospective analyses of the effectiveness and safety of SEMS combined with 125I seeds strand in the treatment of patients with Bismuth type I–IV hilar MBO.
Patients and methods
Study population
This retrospective study included data from sequential patients with hilar MBO who had undergone biliary SEMS with or without 125I seeds strand at Beijing Chaoyang Hospital, Capital Medical University between January 2017 and July 2018. Inclusion criteria were: (1) aged between 18 and 90 years; (2) hilar MBO diagnosis by clinical and radiological findings, or confirmed by histological examination; (3) total bilirubin increased three times higher than normal levels due to MBO; (4) ineligible for surgery, or declined resection due to poor Karnofsky performance status (score < 70); (5) life expectancy > 3 months. The following exclusion criteria were applied: (1) suspected benign biliary obstruction; (2) previous radiotherapy for treatment of hilar MBO; (3) previous metallic biliary stent insertion, surgery, chemotherapy or cancer-targeted treatment; (4) massive ascites; (5) uncontrolled coagulation disorder; (6) uncontrolled infection; (7) patients who refused biliary stent with or without 125I seeds to treat hilar MBO. Treatment with SEMS alone without 125I seeds was performed under the following conditions: (1) patient refusal to receive 125I seeds; and (2) the possibility of the patient having close contact with pregnant women, infants and children. Demographic and clinical data, including tumour type, Bismuth-Corlette classification1 and Karnofsky performance status score, were extracted from patient records and anonymized prior to analyses.
The study was approved by the ethics committee of Beijing Chaoyang Hospital, Capital Medical University, Beijing, China, and was conducted according to standards of the Declaration of Helsinki. Due to the retrospective characteristics of this study, the requirement for informed consent was waived by the ethics committee.
Interventional procedures
125I seeds strand preparation
The 125I seeds (CIAE-6711) were provided by Zhibo Gaoke Biotechnology (Beijing, China). The core radioactive source was a silver wire containing 125I, which was covered by a laser sealed medical titanium alloy tube. 125I seed activity was 0.6 millicurie (mCi) per particle. Each 125I seed had a half-life of 60.1 days, and was 4.5 ± 0.5 mm in length and 0.8 mm in diameter. The following formula was used to determine the number of 125I seeds (N) required: N = length of biliary stricture (mm)/4.5 + 4.16 The 125I seeds strand, used for loading the seeds, was made by inserting the appropriate number of 125I seeds into a 4F catheter (Cook Medical, Bloomington, IN, USA) that was then heat sealed at either end. The estimated radiation dose of the 125I seed strand at the reference points (5 mm from the source axis) was calculated according to the American Association of Physicists in Medicine (AAPM) Task Group No. 43 update report (TG-43U1).20 The incipient dose rate and the cumulative dose (1 half-life, measured at the dose reference points) were 8.03–8.59 cGy/h and 83.15–88.94 Gy, respectively.
SEMS and 125I seeds strand implantation
Two hydrophilic wires were arranged across the bile duct stricture. The SEMS (Cook Medical), usually 8 or 10 mm in diameter and 40–100 mm in length, was deployed via one wire. A 6F guiding catheter (Cook Medical), used for 125I seed deployment, was inserted via the other wire. After the stent was released, a prepared 125I seeds strand was inserted into the 6F guiding catheter and located at the bile duct stenosis. While withdrawing the 6F guiding catheter, the 125I seeds strand was released between the SEMS and bile duct wall. Finally, a 7.0–8.5F drainage catheter (Cook Medical) was placed indwelling above the SEMS. The 125I seeds strands were handled to prevent unintended radiation according to the International Commission on Radiological Protection recommendations.
Follow-up and definitions
Technical success was defined as follows: SEMS covered the biliary stricture segment with residual stricture < 30%; contrast agent passed smoothly through the SEMS; and the 125I seeds strand also covered the whole biliary stricture segment. Clinical success was defined as a decrease in bilirubin level by at least 75% after 1 month compared with the pre-procedural value. Procedural time (duration) was defined as the time from the start of the procedure to the stent release. Overall survival refers to the time interval from SEMS deployment to patient death or the last follow-up date. Stent patency time refers to the time interval from SEMS deployment to stent restenosis. If stent restenosis had not occurred when the patient died, the patency time was equal to the survival time but censored. Stent restenosis was defined as recurrence of jaundice and cholangitis symptoms, and an increase in total bilirubin, and was confirmed by imaging examination or cholangiography. Complications were divided into early or late complication according to whether they occurred within 30 days, or after 30 days, of stent deployment. All patients were followed up by telephone interview, outpatient appointment or inpatient examination every 3 months following the procedure. The follow-ups were concluded on 31 December 2018.
Statistical analyses
All clinical data were analysed using SPSS software, version 22.0 (IBM, Armonk, NY, USA). Continuous data conforming to normal distribution are presented as mean ± SD and were compared using Student’s t-test. Continuous data that were not normally distributed are presented as median (interquartile range) and were compared using Mann–Whitney U-test. Categorical variables were compared by χ2-test or Fisher’s exact test. Overall survival and stent patency time were analysed by the Kaplan-Meier estimator and log-rank test. A P value < 0 05 was considered statistically significant.
Results
Patient characteristics
Out of 76 patients in total, 40 had received SEMS combined with 125I seeds strand (seeds group) while 36 had received SEMS only (control group). The seeds group comprised a total of 21 male and 19 female patients (age, 70.2 ± 13.8 years; range, 30–90 years) and the control group comprised 21 male and 15 female patients (age, 68.1 ± 12.2 years; range, 42–89 years). Baseline clinical characteristics were balanced between the two groups (Table 1). A total of 71 out of 76 patients (93.42%) were diagnosed with hilar MBO by clinical and radiological findings, and five patients (6.58%) were diagnosed by histological examination. In the seeds group, three patients received 2–4 cycles of transarterial chemoembolization (TACE); two patients received 1–2 liver microwave ablations; and one patient was deployed a SEMS due to duodenal obstruction. In the control group, two patients received 1–2 cycles TACE and two patients had stents inserted due to intestinal obstruction. In the seeds group, a total of 608 seeds were deployed (mean, 15.2 ± 4.1 [range, 8–25] seeds per patient); 47 SEMSs were inserted in 40 patients, and seven cases received double biliary SEMSs. In the control group, 40 SEMSs were inserted in 36 patients, while four cases received double biliary SEMSs. Representative cases are shown in Figures 1 and 2.
Table 1.
Baseline demographic and clinical characteristics in patients with hilar malignant biliary obstruction treated with either self-expandable metallic stent (SEMS) plus 125I seeds strand (seeds group) or with SEMS alone (control group).
| Characteristic | Seeds group (n = 40) | Control group (n = 36) | Statistical significance |
|---|---|---|---|
| Sex, male/female | 21/19 | 21/15 | P = 0.610 |
| Age, years | 70.2 ± 13.8 | 68.1 ± 12.2 | P = 0.494 |
| Tumour type | |||
| Cholangiocarcinoma | 22 | 19 | P = 0.846 |
| Pancreatic cancer | 10 | 8 | P = 0.776 |
| Gallbladder cancer | 2 | 3 | P = 0.558 |
| Duodenal cancer | 2 | 1 | P = 0.619 |
| Metastatic cancer | 4 | 5 | P = 0.600 |
| Bismuth-Corlette classification | |||
| Type I–II | 26 | 28 | P = 0.516 |
| Type III–IV | 14 | 8 | |
| Performance status score | |||
| 70–90 | 33 | 29 | P = 0.827 |
| 50–69 | 7 | 7 | |
| Total bilirubin, µmol/l | 190.6 ± 123.5 | 208.5 ± 127.5 | P = 0.536 |
| Stent across ampulla | |||
| Yes | 16 | 13 | P = 0.727 |
| No | 24 | 23 | |
Data presented as mean ± SD or n patient prevalence.
No statistically significant between-group difference at P > 0.05 (Student’s t-test or Mann–Whitney U-test for continuous data; χ2-test or Fisher’s exact test for categorical data).
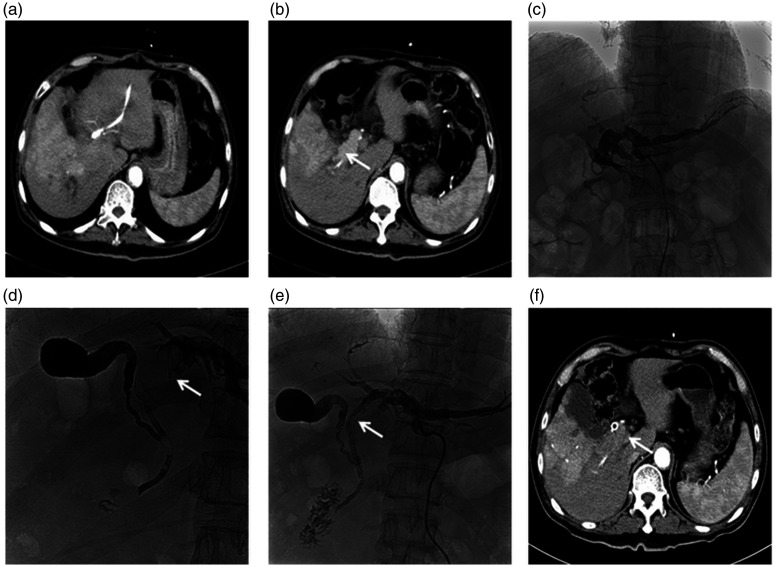
Figure 1.
Representative images from a 71-year-old male patient with cholangiocarcinoma: (a) Abdominal enhanced computed tomography (CT) showing a large tumour and local biliary branch expansion in the right hepatic lobe, (b) Abdominal enhanced CT showing a giant tumour embolus (arrow) in the right hepatic common biliary duct, (c) Percutaneous transhepatic cholangiography showing hilar malignant biliary obstruction (Bismuth type IV; right intrahepatic biliary duct, common hepatic duct and common bile duct were undetected), (d) Cholangiography showing KMP directional catheter (Cook Medical, Bloomington, IN, USA) crossing the biliary stricture segment, and giant tumour embolus (arrow), (e) A self-expandable metallic stent (diameter, 8 mm; length, 80 mm; Cook Medical) was inserted into the biliary stricture and a linearly arranged (arrow) 125I seeds strand with 22 seeds (0.6 mCi per seed) was fixed steadily between the stent and the malignant biliary duct wall and (f) Abdominal enhanced CT at 3 months following the procedure, showing a patent biliary stent and significantly reduced tumour thrombus (arrow).
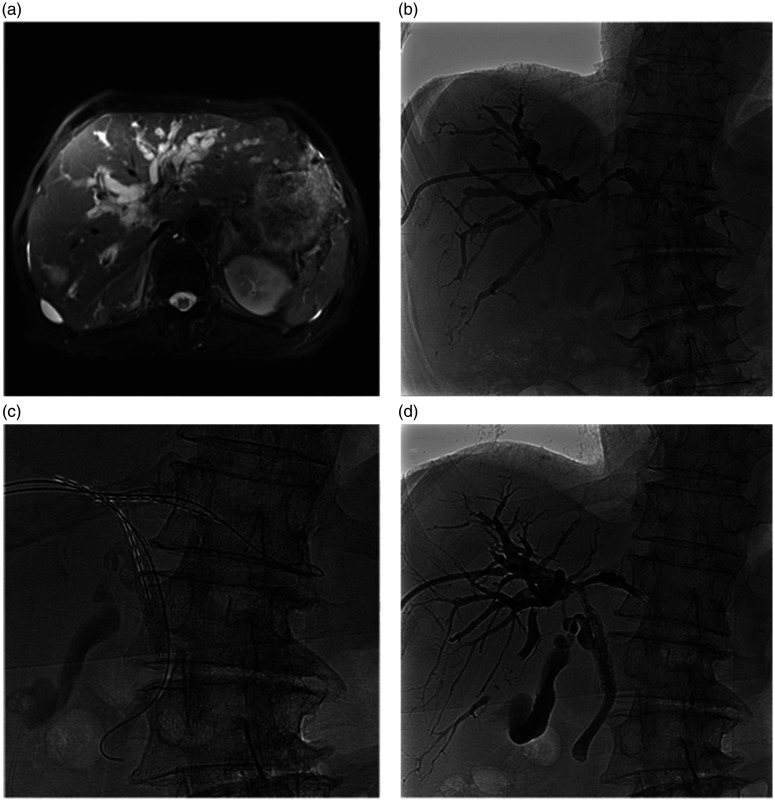
Figure 2.
Representative images from an 82-year-old male patient with hilar cholangiocarcinoma: (a) Magnetic resonance cholangiopancreatography showing widely expanded intrahepatic biliary duct, and hilar biliary obstruction with no communication between the right and left bile ducts, (b) Percutaneous transhepatic cholangiography showing hilar bile duct obstruction, Bismuth type III, (c and d) One self-expandable metallic stent (SEMS; diameter, 8 mm; length, 60 mm; Cook Medical, Bloomington, IN, USA) was inserted into the biliary stricture to connect the right hepatic and common bile ducts; the other SEMS (diameter, 8 mm; length, 40 mm; Cook Medical) was inserted into the biliary stricture to connect the right and left bile ducts; two linearly arranged 125I seeds strands with 12 seeds (0.6 mCi per seed) were fixed steadily between the two stents and the malignant biliary duct wall.
Procedural outcomes
The technical success rate of SEMS and 125I seeds strand release was 100% in all 76 patients. The clinical success rate was 95.0% (38/40) in the seeds group and 97.2% (35/36) in the control group (P = 0.619). Two patients in the seeds group and one patient in the control group died due to severe cholangitis and acute renal failure on days 10, 13 and 15 following the procedure. Procedural time was 46.4 ± 13.1 min and 39.4 ± 12.1 min in the seeds and control groups, respectively (P = 0.018).
Biochemical parameter improvement
Compared with pre-procedure values, there were statistically significant improvements in both groups regarding total bilirubin, direct bilirubin, alkaline phosphatase, glutamyl transferase, aspartate aminotransferase and alanine transaminase levels, following the procedure (all P < 0.05; Table 2).
Table 2.
Improvements in biochemical parameters at 1 month following the procedure in patients with hilar malignant biliary obstruction treated with either self-expandable metallic stent (SEMS) plus 125I seeds strand (seeds group) or with SEMS alone (control group).
| Parameter |
Seeds group |
Control group |
||||
|---|---|---|---|---|---|---|
| Pre-procedure | Post-procedure | Statistical significance | Pre-procedure | Post-procedure | Statistical significance | |
| TBIL, µmol/l | 190.6 ± 123.5 | 44.3 ± 50.7 | P < 0.01 | 220.9 ± 131.4 | 48.7 ± 38.3 | P < 0.01 |
| DBIL, µmol/l | 146.7 ± 93.4 | 31.0 ± 37.7 | P < 0.01 | 188.3 ± 116.7 | 34.3 ± 29.5 | P < 0.01 |
| GGT, U/l | 797.7 ± 787.5 | 185.1 ± 197.0 | P < 0.01 | 812.7 ± 693.5 | 229.4 ± 208.8 | P < 0.01 |
| ALP, U/l | 489.9 ± 269.6 | 185.9 ± 115.1 | P < 0.01 | 585.3 ± 370.3 | 220.8 ± 135.9 | P < 0.01 |
| AST, U/l | 116.6 ± 79.1 | 44.6 ± 52.7 | P < 0.01 | 120.8 ± 79.1 | 52.2 ± 60.2 | P < 0.01 |
| ALT, U/l | 122.8 ± 112.5 | 33.8 ± 27.3 | P < 0.01 | 128.4 ± 111.8 | 35.8 ± 24.5 | P < 0.01 |
Data presented as mean ± SD.
TBIL, total bilirubin; DBIL, direct bilirubin; ALP, alkaline phosphatase; GGT, glutamyl transferase; AST, aspartate aminotransferase; ALT, alanine transaminase.
Statistically significant at P < 0.05 (Student’s t-test or Mann–Whitney U-test).
Complications and re-intervention
The incidence of complications was 50.0% (20/40) in the seeds group versus 38.9% (14/36) in the control group, with no statistically significant differences (χ2 = 0.290, P = 0.590). Individual incidences of cholangitis, asymptomatic amylase increase, self-limited biliary haemorrhage, acute renal failure and seeds strand migration in each of the two treatment groups are summarised in Table 3. The rate of 125I seeds strand migration (2/40 [5%]) was low in this study. Irradiation from the migrated 125I seeds strand can still cover most of the MBO, so re-intervention is not required. However, if obvious migration exists, the 125I seeds strand should be drawn out by a trap. There were no cases of biliary perforation or massive intestinal bleeding in this study (Table 3).
Table 3.
Early and late complications in patients with hilar malignant biliary obstruction treated with either self-expandable metallic stent (SEMS) plus 125I seeds strand (seeds group) or with SEMS alone (control group).
| Complication | Seeds group (n = 40) | Control group (n = 36) | Statistical significance |
|---|---|---|---|
| Early complication | |||
| Cholangitis | 6 (15.0) | 6 (16.7) | P = 0.842 |
| Asymptomatic amylase increase | 6 (15.0) | 4 (11.1) | P = 0.617 |
| Self-limited biliary haemorrhage | 4 (10.0) | 3 (8.3) | P = 0.802 |
| Acute renal failure | 2 (5.0) | 1 (3.8) | P = 0.619 |
| Late complication | |||
| Seed strand migration | 2 (5.0) | 0 | NA |
Data presented as n (%) incidence of complications.
No statistically significant between-group difference at P > 0.05 (χ2-test or Fisher’s exact test).
Overall survival and stent patency time
Up to 31 December 2018, 10 patients remained alive and 30 patients had died in the seeds group; 19 of the 30 patients had died without jaundice and with patent biliary stents. Causes of death included multiple organ failure secondary to carcinoma progression (n = 20), extensive metastasis (n = 5), cardio- or cerebrovascular accident (n = 2), severe pneumonia (n = 2) and gastrointestinal haemorrhage (n = 1). Up to the same time-point, five patients remained alive while 31 had died in the control group; 11 of the 31 patients had died without jaundice and with patent biliary stents. Causes of death included multiple organ failure secondary to carcinoma progression (n = 21), extensive metastasis (n = 6), pulmonary embolism (n = 2), and severe pneumonia (n = 2). The median duration of stent patency (patency time) was significantly longer in the seeds group (387.0 ± 27.9 [95% CI 332.4, 441.6] days) compared with the control group (121.0 ± 9.1 [95% CI 103.2, 138.8] days; P < 0.001 between the two groups). The median overall survival was 177.0 ± 17.9 (95% CI 141.8, 212.2) days in the seeds group compared with 123.0 ± 20.4 (95% CI 83.0, 163.0) days in the control group, and the between-group difference was statistically significant (P = 0.041; Figure 3). Two patients in the seeds group one patient in the control group had died within 30 days, due to acute renal failure and severe cholangitis, as stated previously.
Figure 3.
Kaplan–Meier survival curves from patients with hilar malignant biliary obstruction treated with either self-expandable metallic stent (SEMS) plus 125I seeds strand (seeds group) or with SEMS alone (control group), showing: (a) cumulative overall survival, and (b) duration of stent patency (patency time).
Discussion
According to the Bismuth-Corlette system, hilar MBO can be classified into four types (Type I–IV).1 The percutaneous approach of biliary drainage is more effective and safer than endoscopic methods in hilar MBO, particularly in Bismuth type IV.21 Because of the silence and insidiousness in clinical progress of hilar MBO, only a few patients are suitable for radical resection,22 and the recurrence rate after surgery is also very high.23 Tumour overgrowth or ingrowth, biliary epithelial cell proliferation and biliary sludge formation and accumulation are the main reasons for stent restenosis.5
The half-life of 125I seed is about 60.1 days, with an effective radiation radius of 17 mm and dose attenuation according to the distance. Due to such characteristics, 125I seed is considered to be the first choice for intraluminal radiotherapy. 125I seed can induce a therapeutic effect by injury to the DNA double helix structure and induction of tumour cell apoptosis.24 In 1986, 125I seed was appoved by the US Food and Drug Administration for tumour treatment, and to date, it has been widely used in the treatment of various primary tumours and metastases, e.g. prostate and lung cancers.13,25 Chen et al.26 first reported the use of a 125I seeds strand in the pig bile tract, assessing technical feasibility in treating the human bile duct. The first encouraging results of 125I seeds stent in the clinical setting were reported by Zhu et al.15 who’s interim analysis showed that the 125I seeds stent was safe and feasible in the treatment of MBO, and appeared to extend overall survival compared with the conventional stent (7.40 months versus 2.50 months).15 Since then, low dose-rate 125I seeds combined with SEMS have been applied to treat MBO, with promising results,16–18,22,27 however, the application of 125I seeds in hilar biliary obstruction has rarely been reported.17,18 A prospective study on the treatment of hilar MBO (Bismuth I–II) with SEMS combined with 125I seeds, showed that overall survival and stent patency were significantly prolonged in the seeds group compared with control treatment.17 A retrospective analysis of 132 patients (95 with biliary obstruction located at the hilar region) who received SEMS with 125I seeds strand (seeds group) or SEMS alone (control group) for MBO, showed that, compared with controls, treatment with SEMS plus 125I seeds significantly prolonged median stent patency (194 days versus 86 days, P = 0.049) and median overall survival (194 days versus 96 days, P = 0.031).28 Similar results were reported in a study comparing radiation emitting metallic stent (REMS) and SEMS in the treatment of Bismuth type III or IV hilar cholangiocarcinoma.18 The first multicentre, randomized clinical trial, that included a total of 328 patients with Bismuth type I or II MBO who were enrolled from 20 Chinese centres, was conducted to further evaluate the effectiveness of radioactive stents in patients with unresectable hilar MBO.29 The results showed that radioactive stent deployment could improve stent patency and overall survival compared with SEMS alone in patients with hilar MBO.29
At present, there are three methods for deployment of 125I seeds: (1) 125I seeds strand inserted in a drainage catheter;16 (2) an 125I seeds-loaded stent;18,29 and (3) an 125I seeds strand fixed between SEMS and the bile duct wall.17,28 The advantage of the first method is replaceability of the 125I seeds strand, but a disadvantage is the limitation of external biliary drainage. A disadvantage of the second method is the composition of two-layer stents and larger diameter sheath. The third method was adopted in the present study for its simple procedural process and good tolerance, however, this method also has three disadvantages: (1) the 125I seeds strand is difficult to retrieve when 125I seeds stop functioning after 6 months; (2) pain medication should be administrated to relieve intra-procedural pain due to SEMS deployment without a sheath; and (3) the procedural time for delivering SEMS combined with 125I seeds strand may be longer than SEMS alone, as was shown in the present study (46.4 ± 13.1 min versus 39.4 ± 12.1 min, P = 0.018).
The 76 patients with hilar MBO were analysed retrospectively in the present study. Among the participants, there were 54 cases of Bismuth type I–II MBO, and 22 cases of Bismuth type III–IV MBO. A total of 608 seeds were deployed in 40 patients (seed group), with a mean of 15.2 ± 4.1 (range, 8–25) seeds per patient. The incipient dose rate and the cumulative dose (1 half-life, measured at the dose reference points and calculated according to the AAPM Task Group No. 43 update report (TG-43U1),20 were 8.03–8.59 cGy/h and 83.15–88.94 Gy, respectively. Such radiation doses satisfy the minimum threshold for treatment of adenocarcinoma, and have been reported as safe in animal experiments as well as in clinical trials.15,30 In the present study, compared with SEMS alone, SEMS combined with 125I seeds strand significantly prolonged stent patency (387.0 ± 27.9 days versus 121.0 ± 9.1 days, P < 0.001) and overall survival (177.0 ± 17.9 days versus 123.0 ± 20.4 days, P = 0.041). The complication rates did not differ between the two groups. Two patients in the seeds group died within 30 days, due to acute renal failure and severe cholangitis. Therefore, perioperative anti-infective and fluid replacement therapy are essentially important. Noteworthy technical features in the present study are the use of 6F guiding catheters combined with double guide wires, which can improve operative tolerance and technical success rate. External beam radiotherapy for hilar cholangiocarcinoma may cause haemorrhagic gastroduodenal ulcer.22 The advantage of the 125I seeds strand is that the local radiation dose is high but rapidly attenuated with distance. There were no fatal complications such as biliary or intestinal perforation, or massive haemorrhage in the present study.
The results of the present study may be limited by several factors. First, there was no treatment randomization due to this being a retrospective study, and thus, there may be bias in selection of the patient group. Secondly, there was no specific treatment planning system to guide the 125I seeds deployment into hollow organs. Thirdly, most of the patients with hilar MBO were diagnosed by clinical and radiological findings, and the histopathological diagnosis rate of hilar MBO was relatively low.
In conclusion, SEMS combined with 125I seeds strand represents a safe, feasible, and tolerable operation in patients with hilar MBO. Treatment with SEMS plus 125I seeds strand was shown to be effective in prolonging stent patency time and overall survival in patients with hilar MBO.
Declaration of conflicting interest
The authors declare that there is no conflict of interest.
Funding
This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
ORCID iD
Chuanguo Zhou https://orcid.org/0000-0002-0138-9068
References
- 1.Bismuth H, Corlette MB. Intrahepatic cholangioenteric anastomosis in carcinoma of the hilus of the liver. Surg Gynecol Obstet 1975; 140: 170–178. [PubMed] [Google Scholar]
- 2.Razumilava N, Gores GJ. Cholangiocarcinoma. Lancet 2014; 383: 2168–2179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kim JY, Ko GB, Lee THet al. Partially covered metal stents may not prolong stent patency compared to uncovered stents in unresectable malignant distal biliary obstruction. Gut Liver 2017; 11: 440–446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dumonceau JM, Tringali A, Blero Det al. Biliary stenting: indications, choice of stents and results: European Society of Gastrointestinal Endoscopy (ESGE) clinical guideline. Endoscopy 2012; 44: 277–298. [DOI] [PubMed] [Google Scholar]
- 5.Steel AW, Postgate AJ, Khorsandi Set al. Endoscopically applied radiofrequency ablation appears to be safe in the treatment of malignant biliary obstruction. Gastrointest Endosc 2011; 73: 149–153. [DOI] [PubMed] [Google Scholar]
- 6.Moole H, Tathireddy H, Dharmapuri Set al. Success of photodynamic therapy in palliating patients with nonresectable cholangiocarcinoma: a systematic review and meta-analysis. World J Gastroenterol 2017; 23: 1278–1288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Valle JW, Wasan H, Johnson Pet al. Gemcitabine alone or in combination with cisplatin in patients with advanced or metastatic cholangiocarcinomas or other biliary tract tumours: a multicentre randomised phase II study - The UK ABC-01 Study. Br J Cancer 2009; 101: 621–627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dhondt E, Vanlangenhove P, Geboes Ket al. No evidence of improved efficacy of covered stents over uncovered stents in percutaneous palliation of malignant hilar biliary obstruction: results of a prospective randomized trial. Eur Radiol Epub ahead of print 5 August 2019. DOI: 10.1007/s00330-019-06374-7. [DOI] [PubMed]
- 9.Mizandari M, Pai M, Xi Fet al. Percutaneous intraductal radiofrequency ablation is a safe treatment for malignant biliary obstruction: feasibility and early results. Cardiovasc Intervent Radiol 2013; 36: 814–819. [DOI] [PubMed] [Google Scholar]
- 10.Zhou C, Wei B, Gao Ket al. Biliary tract perforation following percutaneous endobiliary radiofrequency ablation: a report of two cases. Oncol Lett 2016; 11: 3813–3816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ghafoori AP, Nelson JW, Willett CGet al. Radiotherapy in the treatment of patients with unresectable extrahepatic cholangiocarcinoma. Int J Radiat Oncol Biol Phys 2011; 81: 654–659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lu J, Guo JH, Zhu HDet al. Safety and efficacy of irradiation stent placement for malignant portal vein thrombus combined with transarterial chemoembolization for hepatocellular carcinoma: a single-center experience. J Vasc Interv Radiol 2017; 28: 786–794.e3. [DOI] [PubMed] [Google Scholar]
- 13.Huo X, Wang H, Yang Jet al. Effectiveness and safety of CT-guided (125)I seed brachytherapy for postoperative locoregional recurrence in patients with non-small cell lung cancer. Brachytherapy 2016; 15: 370–380. [DOI] [PubMed] [Google Scholar]
- 14.Goy BW, Soper MS, Chang Tet al. Treatment results of brachytherapy vs. external beam radiation therapy for intermediate-risk prostate cancer with 10-year followup. Brachytherapy 2016; 15: 687–694. [DOI] [PubMed] [Google Scholar]
- 15.Zhu HD, Guo JH, Zhu GYet al. A novel biliary stent loaded with 125I seeds in patients with malignant biliary obstruction: preliminary results versus a conventional biliary stent. J Hepatol 2012; 56: 1104–1111. [DOI] [PubMed] [Google Scholar]
- 16.Chen Y, Wang XL, Yan ZPet al. The use of 125I seed strands for intraluminal brachytherapy of malignant obstructive jaundice. Cancer Biother Radiopharm 2012; 27: 317–323. [DOI] [PubMed] [Google Scholar]
- 17.Hasimu A, Gu JP, Ji WZet al. Comparative study of percutaneous transhepatic biliary stent placement with or without iodine-125 seeds for treating patients with malignant biliary obstruction. J Vasc Interv Radiol 2017; 28: 583–593. [DOI] [PubMed] [Google Scholar]
- 18.Lu J, Guo JH, Zhu HDet al. Palliative treatment with radiation-emitting metallic stents in unresectable Bismuth type III or IV hilar cholangiocarcinoma. ESMO Open 2017; 2: e000242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dechao J, Han X, Yanli Wet al. Y-configured metallic stent combined with (125)I seed strands cavity brachytherapy for a patient with type IV Klatskin tumor. J Contemp Brachytherapy 2016; 8: 356–360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rivard MJ, Coursey BM, DeWerd LAet al. Update of AAPM Task Group No. 43 Report: a revised AAPM protocol for brachytherapy dose calculations. Med Phys 2004; 31: 633–674. [DOI] [PubMed] [Google Scholar]
- 21.Guidi MA, Curvale C, Viscardi Jet al. Hilar bile duct tumors: endoscopic or percutaneous drainage? A prospective analysis. Rev Esp Enferm Dig 2015; 107: 488–494 [In Spanish, English abstract]. [DOI] [PubMed] [Google Scholar]
- 22.Isayama H, Tsujino T, Nakai Yet al. Clinical benefit of radiation therapy and metallic stenting for unresectable hilar cholangiocarcinoma. World J Gastroenterol 2012; 18: 2364–2370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kobayashi A, Miwa S, Nakata Tet al. Disease recurrence patterns after R0 resection of hilar cholangiocarcinoma. Br J Surg 2010; 97: 56–64. [DOI] [PubMed] [Google Scholar]
- 24.Monk BJ, Tewari KS, Puthawala AAet al. Treatment of recurrent gynecologic malignancies with iodine-125 permanent interstitial irradiation. Int J Radiat Oncol Biol Phys 2002; 52: 806–815. [DOI] [PubMed] [Google Scholar]
- 25.Langley SEM, Uribe J, Uribe-Lewis Set al. Comparative analysis of clinical outcomes and procedural costs between the conventional two-stage technique and 4D brachytherapy for early prostate cancer. Clin Oncol (R Coll Radiol) 2018; 30: 57–64. [DOI] [PubMed] [Google Scholar]
- 26.Chen Y, Wang XL, Yan ZPet al. Damage to pig bile duct caused by intraluminal brachytherapy using a (125)I ribbon. Acta Radiol 2013; 54: 272–277. [DOI] [PubMed] [Google Scholar]
- 27.Jiao D, Wu G, Ren Jet al. Study of self-expandable metallic stent placement intraluminal (125)I seed strands brachytherapy of malignant biliary obstruction. Surg Endosc 2017; 31: 4996–5005. [DOI] [PubMed] [Google Scholar]
- 28.Zhou WZ, Fu YM, Yang ZQet al. Study of percutaneous stent placement with iodine-125 seed strand for malignant biliary obstruction. Cardiovasc Intervent Radiol 2019; 42: 268–275. [DOI] [PubMed] [Google Scholar]
- 29.Zhu HD, Guo JH, Huang Met al. Irradiation stents vs. conventional metal stents for unresectable malignant biliary obstruction: a multicenter trial. J Hepatol 2018; 68: 970–977. [DOI] [PubMed] [Google Scholar]
- 30.Liu Y, Lu Z, Zou DWet al. Intraluminal implantation of radioactive stents for treatment of primary carcinomas of the peripancreatic-head region: a pilot study. Gastrointest Endosc 2009; 69: 1067–1073. [DOI] [PubMed] [Google Scholar]