Abstract
Background
Major depressive disorder (MDD) treatment is characterized by low remission rate and often involves weeks to months of treatment. Identification of pretreatment biomarkers of response may play a critical role in novel drug development, in enhanced prognostic predictions, and perhaps in providing more personalized medicine. Using a network restricted strength predictive modeling (NRS-PM) approach, the goal of the current study was to identify pretreatment functional connectome fingerprints (CFPs) that (1) predict symptom improvement regardless of treatment modality and (2) predict treatment specific improvement.
Methods
Functional magnetic resonance imaging and behavioral data from unmedicated patients with MDD (n = 200) were investigated. Participants were randomized to daily treatment of sertraline or placebo for 8 weeks. NRS-PM with 1000 iterations of 10 cross-validation were implemented to identify brain connectivity signatures that predict percent improvement in depression severity at week-8.
Results
The study identified a pretreatment CFP that significantly predicts symptom improvement independent of treatment modality but failed to identify a treatment specific CFP. Regardless of treatment modality, improved antidepressant response was predicted by high pretreatment connectivity between modules in the default mode network and the rest of the brain, but low external connectivity in the executive network. Moreover, high pretreatment internal nodal connectivity in the bilateral caudate predicted better response.
Conclusions
The identified CFP may contribute to drug development and ultimately to enhanced prognostic predictions. However, the results do not assist with providing personalized medicine, as pretreatment functional connectivity failed to predict treatment specific response.
Keywords: antidepressants, brain architecture, intrinsic connectivity networks, machine learning, major depressive disorders
Introduction
Major depressive disorder (MDD) is a severe mental illness, affecting about 300 million people annually,1 with at least 16% lifetime prevalence in the Unites States.2 Antidepressants are the most commonly used pharmacotherapy for acute MDD.3,4 In particular, selective serotonin reuptake inhibitors or selective serotonin norepinephrine reuptake inhibitors are currently recommended as first-line treatment.4 Nevertheless, the evidence of antidepressant treatment efficacy has been conflictive and less than 40% of patients have achieved remission with initial treatment.5–9 On the other hand, substantial placebo treatment effects in antidepressant trials have been increasingly demonstrated.10 These controversial findings seem to suggest improvement of symptoms in many patients with MDD might not be treatment-specific. Consequences of inadequate and ineffective treatments have led to extra economic costs and psychological burdens on patients.7,11 For more personalized treatment to be invented and assigned, it is important to identify pretreatment biomarkers that predicts future symptom improvement.
With the advent of resting-state functional magnetic resonance imaging (rs-fMRI), large-scale brain functional networks (FNs) have been identified.12 Altered functional connectivity in major FNs of default mode (DM) for internal attention processes, central-executive (CE) for external attention processes, and affective and salience networks for emotional processes have shown to be involved in the pathophysiology of MDD.13 These neurobiological markers were suggested to be useful for serving as treatment targets for MDD.14–16 Following the discovery of FNs, scientists were able to map functional connections of the entire brain, termed the “functional connectome.”17
Recently, connectome-based predictive modeling (CPM) approach was established using a data-driven protocol for developing predictive models of brain-behavior relationships from connectivity data using cross-validation.18 Behaviors were successfully predicted in healthy subjects as well as in patients suffering from neuropsychiatric disorders.19–23 In MDD, relevant research is scarce, one study showed that decreased brain dynamics in several brain regions predicted suicidal ideation in patients.24 Particularly in treatment response in MDD, Nemati, Akiki et al. (2020) identified a unique connectome fingerprint (CFP) at one-week post-treatment that predated and predicted response to the antidepressant sertraline. In comparison to placebo, the authors found reduction in DM connectivity at week one predicted better response to sertraline at week eight. Overall, compared to placebo, response to sertraline was predicted by a reduction in internal connectivity within the primary cortices and within the executive networks, but an increase in connectivity between the executive network and the rest of the brain.25
These new findings were derived by applying a network restricted strength (NRS) approach combined with the classic CPM method.18,26 There are two major advantages of the NRS approach: 1) it enables calculations of internal connectivity strength in all identifiable nodes within any FNs as well as external connectivity strength between all FNs; and 2) using the Akiki-Abdallah (AA) hierarchical atlas for whole-brain parcellation, “nodes” (modules or communities) are defined using subject-level clustering of functional networks and a reclustering network, allowing the large number of edges to be reduced, and thus reducing the number of statistical comparisons and leading to better interpretations of findings.27,28
Leveraging this promising new approach, the current study employed the NRS predictive modelling (PM) methods to identify a functional connectivity signature of treatment response in patients with MDD randomized to sertraline or placebo. In the present study, Aim 1 was to identify a pretreatment CFP that predicts antidepressant response, regardless of treatment modality and Aim 2 was to identify a CFP that predicts better response to sertraline, compared to placebo. To assess the brain localization of predictive connectivity, we also investigated the nodal fingerprint (NFP) along with the CFP. We hypothesized that pretreatment functional connectivity would successfully predict percent improvement in depression severity at week eight.
Methods
Demographic and Clinical Characteristics
The current study included 200 unmedicated patients with MDD with an age range between 18 to 65 years (Table S1). Inclusion and exclusion criteria for subjects were previously reported in detail.29 In brief, subjects met criteria for nonpsychotic MDD as per the Structured Clinical Interview for DSM-IV-TR (SCID) criteria,30 had a Quick Inventory of Depressive Symptomatology (QIDS)31 score equal or above 14 and were unmedicated for at least three weeks prior to the study.29 FMRI and behavior data were obtained. All subjects were randomly assigned to an eight-week treatment of either sertraline or placebo (up to 200 mg daily). The 17-item Hamilton Depression Rating Scale (HAMD) was rated for depression severity before and after treatment.32 HAMD scores were used as the primary clinical outcome of the parent trial. Scanning and assessments were conducted at baseline. Participants were treated with the study medication for a total of eight weeks. The HAMD scores were re-assessed at the end of week eight (Table S1). The antidepressant treatment response was defined as the percentage changes in HAMD scores before and after the MDD subjects were treated with sertraline or placebo.
Neuroimaging Acquisition and Processing
As previously reported, the structural (1x1x1 mm3) and functional (3.2x3.2x3.1 mm3; TR = 2000 ms; TE = 28 ms; 12 min) scans at baseline were collected using 3-Tesla magnets.33,34 MR scans were surface-based preprocessed by using a pipeline adapted from the HCP (https://github.com/Washington-University/HCPpipelines),35 as reported elsewhere.14,25,28,36,37
In the preprocessing pipeline, FreeSurfer parcellation was used for structural scans. Slice timing correction, motion correction, intensity normalization, brain masking, and registration of fMRI images to structural MRI and standard template were performed. The cortical gray matter ribbon voxels and each subcortical parcel were projected to a standard Connectivity Informatics Technology Initiative (CIFTI) 2 mm grayordinate space. ICA-FIX was used to identify and remove artifacts,38,39 followed by mean grayordinate time series regression (MGTR). The FIX and MGTR have been found to significantly reduce motion-correlated artifacts.40
Network Restricted Strength Predictive Modeling
The A424 atlas was used for gray matter whole-brain parcellation into 424 nodes and average time series within each node was computed.25,41–43 The network-affiliated parcellations based on the A424 nodes were then performed by using the AA hierarchical connectivity atlas at 50 (AA-50), 24 (AA-24), and 7 modules (AA-7) (https://github.com/emergelab/hierarchical-brain-networks).25,44 Fisher-z transformation was conducted on the pairwise correlation coefficients to produce the full connectome. The full details of A424 and AA atlases, along with the NRS-PM codes were previously reported,25 and are publicly available at https://github.com/emergelab. Here, we briefly introduce four key measures used in the NRS-PM: 1) NRS connectome is the pairwise connectivity of FNs affiliated modules at AA-24 and AA-50, 2) nodal strength (nS) is the average connectivity strength from one node to all other nodes in the brain, i.e., comparable to Global Brain Connectivity (GBC) as in Abdallah et al.,45 3) nodal internal NRS (niNRS) is the average connectivity strength from one node to all other nodes within the same canonical connectivity FN (i.e., at AA-7), and 4) nodal external NRS (neNRS) is the average connectivity strength from one node to all other nodes outside of its FN.25 The predictive modelling (PM) applied in this study was adapted from the CPM approach,18 as previously established.25 Using linear modeling, the CPM is a data-driven machine learning approach focusing on brain-behavior relationship with built-in cross-validation (CV).18 As in the previous study, 1000 iterations of ten-fold CV was used to determine the statistical significance and to guarantee the stability of the models in the current study.25 Full details of the NRS methods were previously reported in Nemati, Akiki et al. (2020).
Statistical Analyses
Normal probablity plots and test statistics were used to examine the normality of outcome measures. Estimates of variation were considered as the standard deviations of the sampling distribution of the mean. T-tests and chi-squares were performed to assess the difference in demographic data between the setraline and placebo groups. Multiple comparisons were corrected by using False Discovery Rate (FDR; p < 0.05). The statistical significance threshold was set at 0.05 (2-tailed tests). Analyses were conducted by using MATLAB (2017 b; Mathworks Inc.) and the Statistical Package for the Social Sciences (version 24; IBM).
The primary CFP analysis was conducted by using NRS-PM at AA-50 followed by two secondary CFPs at AA-24 and A424. The secondary CFPs were carried out to inspect the upstream modules (i.e., AA-24) and to test the model without network restrictions (i.e., A424).25 For the nodal fingerprint (NFP) analysis, nS-PM was considered as primary analysis, both niNRS-PM and neNRS-PM were performed as secondary analyses to investigate the shifts within and between canonical networks (i.e., at AA-7).25 The study codes and predictive models will be made publicly available at https://github.com/emergelab.
Results
The dataset was acquired from the National Institute of Mental Health Data Archive (NDA), Establishing Moderators and Biosignatures of Antidepressant Response for Clinical Care (EMBARC). We included all subjects (n = 200) with successful resting state fMRI and who completed depression assessment at week-8. There were no significant differences of clinical outcomes between treatment groups. Demographics and clinical characteristics are reported in Table S1. Detailed protocol and results of the EMBARC clinical trial were reported elsewhere.29,46
Aim 1: Pretreatment Connectivity Predicts Improvement Regardless of Treatment Modality
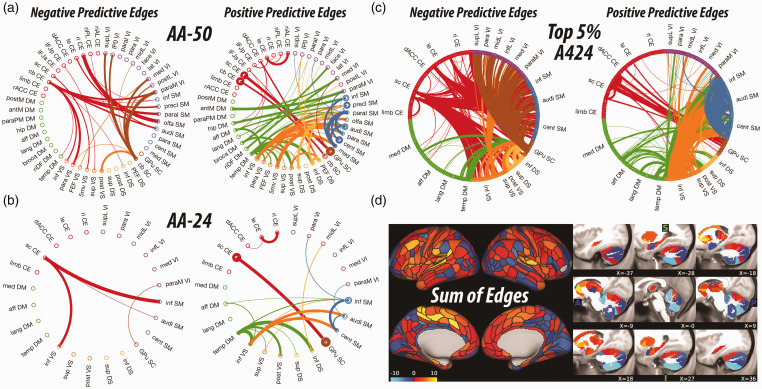
The primary CFP analysis based on AA-50 identified a pretreatment CFP that significantly predicted percentage of symptom improvement at week eight compared to baseline regardless whether patients received sertraline or placebo (r = 0.19, CV = 10, iterations = 1000, p = 0.03). As shown in Figure 1(a), enhanced treatment response was predicted by lower pretreatment connectivity between the executive and sensorimotor and salience modules, but increased connectivity between the DM modules and the rest of the brain. Secondary analyses also identified pretreatment CFP at higher resolution (A424), which significantly predicted percentage of symptom improvement (r = 0.19, CV = 10, iterations = 1000, p = 0.02; Figure 1) while pretreatment CFP at lower resolution (AA-24) showed a trend on the prediction (r = 0.14, CV = 10, iterations = 1000, p = 0.08) (Figure 1). Due to the large number of predictive edges, visualizing the full connectome (A424) PM often yields undiscernible CFP.25 However, inspecting the nodes with highest degree (i.e., top 2.5% of each of positive and negative predictive edges) revealed a pattern of connectivity signature comparable to the AA-50 CFP (Figure 1).
Figure 1.
Pretreatment connectome fingerprint (CFP). A–C, The circular graphs are labeled based on the Akiki-Abdallah (AA) whole-brain architecture at 50 modules (AA-50; primary CFP), 24 modules (AA-24), and the full connectome with 424 nodes (A424). Modules and nodes are colored according to their affiliation to the 7 canonical connectivity networks: central executive (CE), default mode (DM), ventral salience (VS), dorsal salience (DS), subcortical (SC), sensorimotor (SM), and visual (VI). Edges are colored based on the initiating module using a counter-clockwise path starting at 12 o’clock. Internal edges (i.e., within module) are depicted as outer circles around the corresponding module. Thickness of edges reflect their corresponding weight in the predictive model. The module abbreviations of AA-24 and AA-50, along with further details about the affiliation of each node are available at https://github.com/emergelab/hierarchical-brain-networks/blob/master/brainmaps/AA-AAc_main_maps.csv. Only edges of significant predictive models following correction are shown in A and C (all p ≤ 0.05). The model in B was at trend level (p = 0.08). C, For the full connectome, it is not possible to visually discern the underlying signature considering the large number of edges retained. Therefore, as in previous studies, the circular graph is thresholded using nodal strength within the full connectome fingerprint as cutoff to retain the highest top 2.5% negative predictive edges and top 2.5% positive predictive edges. D, Shows the nodal degree of the full connectome fingerprint edges without a threshold. The color bar unit is arbitrary, reflecting the sum of weighted edges. All predictive models will be made publicly available at https://github.com/emergelab.
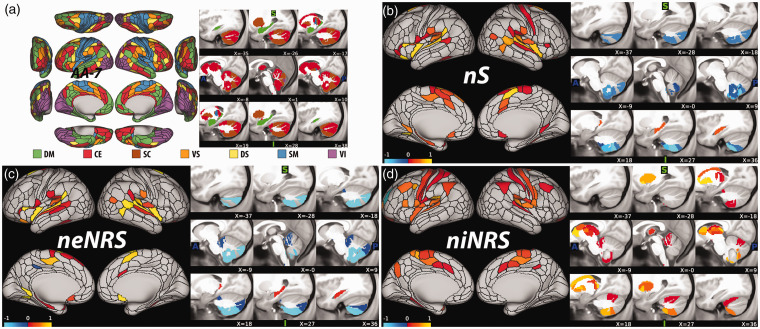
The primary NFP analysis, based on nS, identified a pretreatment NFP that is significantly associated with percent improvement of depression severity at week eight, regardless of treatment (i.e., sertraline or placebo) (r = 0.18, CV = 10, iterations = 1000, p = 0.03; Figure 2). Secondary analyses identified a significant NFP based on niNRS (r = 0.19, CV = 10, iterations = 1000, p = 0.02), but not on neNRS (r = 0.15, CV = 10, iterations = 1000, p = 0.08). As shown in Figure 2(a), percent improvement of depression severity was predicted by high global brain connectivity (i.e., nS) at baseline in various nodes within the sensorimotor network, particularly the auditory module. Enhanced response to sertraline and placebo was also predicted by higher global connectivity in nodes within DM network, particularly the posterior hippocampus. In contrast, lower global connectivity in nodes within the cerebellum predicted better response. Inspecting the results in Figure 2(d) shows higher pretreatment internal connectivity (i.e., niNRS) in primary cortices and subcortical regions, particularly the bilateral ventral caudate, as predictor of sertraline and placebo response.
Figure 2.
Pretreatment nodal fingerprint (NFP). A, The canonical networks nodal affiliation based on the Akiki-Abdallah (AA) hierarchical atlas at 7 modules (AA-7). The AA-7 affiliation was used to compute nodal external network restricted strength (neNRS) and nodal internal NRS (niNRS). B–D, Nodal predictive results using nodal strength (nS; primary NFP; B), neNRS (C), or niNRS (D) as input features. In (B) and (C), only nodes of significant predictive models following correction are shown (all p ≤ 0.05). The predictive model in (C) was at trend level (p = 0.08). The color bar unit is arbitrary, reflecting the sum of weighted nodes. All predictive models will be made publicly available at https://github.com/emergelab.
Aim 2: Pretreatment Connectivity Predicts Improvement Specific to Sertraline, Compared to Placebo
We failed to identify a significant pretreatment CFP or NFP that could predict percent improvement specific to sertraline, compared to placebo (all p > 0.05).
Discussion
By employing the newly validated NRS-PM approach,25 the current study provided evidence of a unique CFP that predicted symptom improvement regardless of treatment (sertraline vs. placebo). Our results revealed a unique connectomic signature evident at pretreatment that significantly predicted symptom improvement independent of treatment modality (i.e., sertraline or placebo) at week eight. An overall pattern emerged consistent with reduced connectivity between modules within the CE network and the visual (VI) network. The CE also showed reduced connectivity to the ventral salience (VS) and sensorimotor (SM) networks, along with enhanced connectivity found between the CE to the globus-pallidus-putamen subcortical (GPu SC) network and between the DM network to the dorsal salience (DS) and the ventral salience (VS) networks. Additionally, enhanced connectivity between modules within the SM was revealed. No CFP was identified at pretreatment that could predict the interaction between treatment and symptom improvement at week eight and neither was any correlation observed between pretreatment CFP and baseline disease severity.
The CE consists of important brain regions that have been strongly implicated in the pathophysiology of MDD such as the dorsal lateral prefrontal cortex (dlPFC) and dorsal medial PFC (dmPFC).47–49 Based on task and rs-fMRI studies, the CE has also been found to be one of the major FNs affected by MDD.49–51 Although findings have been inconsistent and possibly due to different methodologies used, a hypo-connected CE has been suggested in patients suffering from MDD.52,53 According to our findings, the CE was also decreasingly connected to VS and SM FNs. The CE is important for cognitive processing while the VS and SM are important for emotion and motor controls respectively.49 Zhi et al. found that patients with MDD compared to healthy controls showed decreased connectivity between CE and several FNs including the VS and SM.54 These results implicated that MDD patients before treatment showed weakening cognitive processing and might simultaneously interfered its adequate functional communication to emotional and motor controls when needed. Consistent rs-fMRI studies have also shown decreased connectivity within the VI in MDD patients and our observation on decreased connectivity between the modules within this FN is in line with the literature.49,54
On the other hand, the CE showed enhanced connectivity to the GPu SC FN. The GPu SC FN consists of the globus-pallidus and putamen, two important subcortical structures in the basal ganglia. Abnormalities in the basal ganglia including these two structures have been found in MDD patients compared to healthy controls.55–58 It is suggested that motor manifestations such as alteration in gait and posture observed in depressive states may derive from basal ganglia dysfunction.59 Though speculatively, the increased connectivity between the CE and the GPu SC might be compensatory to its weakening connectivity to the VS and SM and such neural mechanism seems to contribute to symptom improvement independent of treatment modality. Increased connectivity within the SM FN might have also reflected such mechanism for compensating declined ability in motor manifestations. Additionally, enhanced connectivity was found between the DM to the DS and the VS FNs. The DM represents brain function at rest while salience network represents emotional process. Alterations in both the FNs have been importantly implicated in pathophysiology of MDD.13,45,50,52,53,60,61 Major brain regions in the DM and salience network also anchor the frontal-limbic circuitry that is important for emotion regulation. Also, speculatively, increasing functional communication between these two FNs might be again compensatory to emotional dysregulation in MDD and thus such neural pattern could be an indicator of symptom improvement regardless of sertraline and placebo effects.
Using the same study sample, Nemati et al. revealed a unique CFP at one-week post-treatment significantly predicted response to sertraline compared to placebo. Specifically, three patterns of brain connectivity dynamic shifts emerged: 1) a reduction in internal connectivity among the CE and GPu SC modules, along with increased external connectivity between these modules and the rest of the brain; 2) reduced internal connectivity among modules within the SM and VI networks; 3) in DM/VS modules containing the amygdala and insula (i.e., affective DM and inferior VS, respectively). The reduced connectivity with perceptual and motor areas (i.e., SM and VI) was found and the authors suggested increased connectivity with higher order association regions might indicate an early shift toward enhanced executive control.25 Considering the findings from these two studies together, it seems that decreased functional connectivity within the CE may be involved in the pathophysiology of MDD independent of treatment effect. And it might be that at pretreatment, the stronger the functional circuitry shift to compensate neural deficits in MDD could better predict symptom improvement regardless of treatment. And after one week of treatment, the brain circuitry shifted again with the focus to adapt and to maximize the neurochemical effects brought by the sertraline in order to improve symptoms.
Nevertheless, in the current study, the CFP failed to predict interaction between treatment (i.e., sertraline vs. placebo) and symptom improvement and there was no correlation between pretreatment CFP and baseline disease severity. This could be attributed to the relatively small sample size which may lack the statistical power necessary for prediction. Alternatively, this could simply reflect the inability for CFP-PM to predict treatment response at baseline. If CFP represents the pattern of changes in the future, it may be less likely to be strongly linked to the present disease stage and thus may have resulted in no significant correlation findings between pretreatment CFP and baseline disease severity. On the other hand, since MDD patients with psychosis were excluded from the study, the current findings cannot be generalized to all patients suffering from depression.
In conclusion, the current study identified a unique pretreatment CFP that predicted symptom improvement in MDD patients. The pretreatment CFP revealed the reduced functional connectivity in the CE involved in the pathophysiology of MDD and the early brain circuitry shifted to enhance cognitive, emotional and motor processes and contributed to symptom improvement in the future. Longitudinal study needs to be carried out to further demonstrate changes in neural correlates related to symptom improvement independent of treatment modality.
Supplemental Material
Supplemental material, sj-pdf-1-css-10.1177_2470547020984726 for Pretreatment Brain Connectome Fingerprint Predicts Treatment Response in Major Depressive Disorder by Siyan Fan, Samaneh Nemati, Teddy J. Akiki, Jeremy Roscoe, Christopher L. Averill, Samar Fouda, Lynnette A. Averill and Chadi G. Abdallah in Chronic Stress
Acknowledgments
The authors would like to thank the subjects who participated in these studies for their invaluable contribution. Data used in the preparation of this manuscript were obtained and analyzed from the controlled access datasets distributed from the NIMH-supported National Database for Clinical Trials (NDCT). NDCT is a collaborative informatics system created by the National Institute of Mental Health to provide a national resource to support and accelerate discovery related to clinical trial research in mental health. Dataset identifier(s): STU 092010-151; Establishing Moderators and Biosignatures of Antidepressant Response for Clinical Care (EMBARC). This manuscript reflects the views of the authors and may not reflect the opinions or views of the NIMH, Department of Veterans Affairs or of the Submitters submitting original data to NDCT.
Footnotes
Declaration of Conflicting Interests: The author(s) declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: Dr Abdallah has served as a consultant and speaker and/or on advisory boards for Genentech, Janssen, Lundbeck, Psilocybin Labs, and FSV7 and editor of Chronic Stress for Sage Publications, Inc. and filed a patent for using mTORC1 inhibitors to augment the effects of antidepressants (filed on Aug 2,02,018). All other co-authors declare no conflict of interest.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship and/or publication of this article: Funding support was provided by the NIMH (C.G.A; K23MH101498), Department of Veterans Affairs Office of Research and Development (L.A.A. CSR&D CDA2 IK2CX001873), Association for Suicide Prevention (L. A.A.), the Brain and Behavior Foundation/National Alliance for Research on Schizophrenia and Depression (L.A.A.), the VA National Center for PTSD and the Beth K and Stuart Yudofsky Chair in the Neuropsychiatry of Military Post Traumatic Stress Syndrome.
ORCID iDs: Siyan Fan https://orcid.org/0000-0002-8331-0738
Teddy J. Akiki https://orcid.org/0000-0003-1988-9201
Lynnette A. Averill https://orcid.org/0000-0002-8985-9975
Chadi G. Abdallah https://orcid.org/0000-0001-5783-6181
Supplemental Material: Supplementary material for this article is available online.
References
- 1. WHO. World Health Organization: Depression and Other Common Mental Disorders: global Health Estimates. Geneva, Switzerland: World Health Organization; 2017. [Google Scholar]
- 2.Kessler RC, Angermeyer M, Anthony JC, et al. Lifetime prevalence and age-of-onset distributions of mental disorders in the world health organization’s world mental health survey initiative. World Psychiatry. 2007; 6(3): 168–176. [PMC free article] [PubMed] [Google Scholar]
- 3.Mojtabai R, Olfson M. National patterns in antidepressant treatment by psychiatrists and general medical providers: results from the national comorbidity survey replication. J Clin Psychiatry. 2008; 69(7): 1064–1074. [DOI] [PubMed] [Google Scholar]
- 4.Gartlehner G, Wagner G, Matyas N, et al. Pharmacological and non-pharmacological treatments for major depressive disorder: review of systematic reviews. BMJ Open. 2017; 7(6): e014912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gaynes BN, Warden D, Trivedi MH, et al. What did STAR*D teach us? Results from a large-scale, practical, clinical trial for patients with depression. Psychiatr Serv. 2009; 60(11): 1439–1445. [DOI] [PubMed] [Google Scholar]
- 6.Holtzheimer PE, Mayberg HS. Stuck in a rut: rethinking depression and its treatment. Trends Neurosci. 2011; 34(1): 1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.McGrath CL, Kelley ME, Holtzheimer PE, et al. Toward a neuroimaging treatment selection biomarker for major depressive disorder. JAMA Psychiatry. 2013; 70(8): 821–829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Coplan JD, Gopinath S, Abdallah CG, et al. A neurobiological hypothesis of treatment-resistant depression—mechanisms for selective serotonin reuptake inhibitor non-efficacy. Front Behav Neurosci. 2014; 8: 189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ionescu DF, Rosenbaum JF, Alpert JE. Pharmacological approaches to the challenge of treatment-resistant depression. Dialog Clin Neurosci. 2015; 17(2): 111–126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Furukawa TA, Cipriani A, Atkinson LZ, et al. Placebo response rates in antidepressant trials: a systematic review of published and unpublished double-blind randomised controlled studies. Lancet Psychiatry. 2016; 3(11): 1059–1066. [DOI] [PubMed] [Google Scholar]
- 11.Dunlop BW, Reddy S, Yang L, et al. Symptomatic and functional improvement in employed depressed patients: a double-blind clinical trial of desvenlafaxine versus placebo. J Clin Psychopharmacol. 2011; 31(5): 569–576. [DOI] [PubMed] [Google Scholar]
- 12.Damoiseaux JS, Rombouts SA, Barkhof F, et al. Consistent resting-state networks across healthy subjects. Proc Natl Acad Sci U S A. 2006; 103(37): 13848–13853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kaiser RH, Andrews-Hanna JR, Wager TD, et al. Large-Scale network dysfunction in major depressive disorder: a meta-analysis of resting-state functional connectivity. JAMA Psychiatry. 2015; 72(6): 603–611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Abdallah CG, Averill CL, Ramage AE, et al. ; the STRONG STAR Consortium. Reduced salience and enhanced central executive connectivity following PTSD treatment. Chronic Stress. 2019; 3: 247054701983897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pizzagalli DA. Frontocingulate dysfunction in depression: toward biomarkers of treatment response. Neuropsychopharmacology. 2011; 36(1): 183–206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Salomons TV, Dunlop K, Kennedy SH, et al. Resting-state cortico-thalamic-striatal connectivity predicts response to dorsomedial prefrontal rTMS in major depressive disorder. Neuropsychopharmacology. 2014; 39(2): 488–498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Biswal BB, Mennes M, Zuo XN, et al. Toward discovery science of human brain function. Proc Natl Acad Sci USA. 2010; 107(10): 4734–4739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Shen X, Finn ES, Scheinost D, et al. Using connectome-based predictive modeling to predict individual behavior from brain connectivity. Nat Protoc. 2017; 12(3): 506–518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Finn ES, Shen X, Scheinost D, et al. Functional connectome fingerprinting: identifying individuals using patterns of brain connectivity. Nat Neurosci. 2015; 18(11): 1664–1671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lake EMR, Finn ES, Noble SM, et al. The functional brain organization of an individual allows prediction of measures of social abilities transdiagnostically in autism and attention-deficit/hyperactivity disorder. Biol Psychiatry. 2019; 86(4): 315–326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yip SW, Scheinost D, Potenza MN, et al. Connectome-based prediction of cocaine abstinence. Am J Psychiatry. 2019; 176(2): 156–164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mellem MS, Liu Y, Gonzalez H, et al. Machine learning models identify multimodal measurements highly predictive of transdiagnostic symptom severity for mood, anhedonia, and anxiety. Biol Psychiatry Cogn Neurosci Neuroimaging. 2020; 5(1): 56–67. [DOI] [PubMed] [Google Scholar]
- 23.Jiang R, Calhoun VD, Zuo N, et al. Connectome-based individualized prediction of temperament trait scores. Neuroimage. 2018; 183: 366–374. [DOI] [PubMed] [Google Scholar]
- 24.Li J, Duan X, Cui Q, et al. More than just statics: temporal dynamics of intrinsic brain activity predicts the suicidal ideation in depressed patients. Psychol Med. 2019; 49(5): 852–860. [DOI] [PubMed] [Google Scholar]
- 25.Nemati S, Akiki TJ, Roscoe J, et al. A unique brain connectome fingerprint predates and predicts response to antidepressants. iScience. 2020; 23(1): 100800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Akiki TJ, Averill CL, Wrocklage KM, et al. Default mode network abnormalities in posttraumatic stress disorder: a novel network-restricted topology approach. Neuroimage. 2018; 176: 489–498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Akiki TJ, Abdallah CG. Determining the hierarchical architecture of the human brain using subject-level clustering of functional networks. Sci Rep. 2019; 9(1): 19290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nemati S, Abdallah CG. Increased cortical thickness in patients with major depressive disorder following antidepressant treatment. Chronic Stress (Thousand Oaks). 2020; 4: 247054701989996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Trivedi MH, McGrath PJ, Fava M, et al. Establishing moderators and biosignatures of antidepressant response in clinical care (EMBARC): rationale and design. J Psychiatr Res. 2016; 78: 11–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.First M, Spitzer R, Gibbon M, et al. Structured clinical interview for DSM-IV-TR axis I disorders, research version, patient edition (SCID-I/P). New York, NY: Biometrics Research, New York State Psychiatric Institute; 2002.
- 31.Rush AJ, Trivedi MH, Ibrahim HM, et al. The 16-Item quick inventory of depressive symptomatology (QIDS), clinician rating (QIDS-C), and self-report (QIDS-SR): a psychometric evaluation in patients with chronic major depression. Biol Psychiatry. 2003; 54(5): 573–583. [DOI] [PubMed] [Google Scholar]
- 32.Hamilton M. Development of a rating scale for primary depressive illness. Br J Soc Clin Psychol. 1967; 6(4): 278–296. [DOI] [PubMed] [Google Scholar]
- 33.Greenberg T, Chase HW, Almeida JR, et al. Moderation of the relationship between reward expectancy and prediction error-related ventral striatal reactivity by anhedonia in unmedicated major depressive disorder: findings from the EMBARC study. Am J Psychiatry. 2015; 172(9): 881–891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Downey D, Dutta A, McKie S, et al. Comparing the actions of lanicemine and ketamine in depression: key role of the anterior cingulate. Eur Neuropsychopharmacol. 2016; 26(6): 994–1003. [DOI] [PubMed] [Google Scholar]
- 35.Glasser MF, Sotiropoulos SN, Wilson JA, et al. ; WU-Minn HCP Consortium. The minimal preprocessing pipelines for the human connectome project. Neuroimage. 2013; 80: 105–124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Abdallah CG, Averill CL, Ramage AE, et al. ; the STRONG STAR Consortium. Salience network disruption in U.S. Army soldiers with posttraumatic stress disorder. Chronic Stress (Thousand Oaks). 2019; 3: 247054701985046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Abdallah CG, Dutta A, Averill CL, et al. Ketamine, but not the NMDAR antagonist lanicemine, increases prefrontal global connectivity in depressed patients. Chronic Stress (Thousand Oaks). 2018; 2: 247054701879610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Griffanti L, Salimi-Khorshidi G, Beckmann CF, et al. Ica-based artefact removal and accelerated fMRI acquisition for improved resting state network imaging. Neuroimage. 2014; 95: 232–247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Salimi-Khorshidi G, Douaud G, Beckmann CF, et al. Automatic denoising of functional MRI data: combining independent component analysis and hierarchical fusion of classifiers. Neuroimage. 2014; 90: 449–468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Burgess GC, Kandala S, Nolan D, et al. Evaluation of denoising strategies to address motion-correlated artifacts in resting-state functional magnetic resonance imaging data from the human connectome project. Brain Connect. 2016; 6(9): 669–680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Diedrichsen J, Balsters JH, Flavell J, et al. A probabilistic MR atlas of the human cerebellum. Neuroimage. 2009; 46(1): 39–46. [DOI] [PubMed] [Google Scholar]
- 42.Fan L, Li H, Zhuo J, et al. The human brainnetome atlas: a new brain atlas based on connectional architecture. Cereb Cortex. 2016; 26(8): 3508–3526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Glasser MF, Coalson TS, Robinson EC, et al. A multi-modal parcellation of human cerebral cortex. Nature. 2016; 536(7615): 171–178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Akiki TJ, Abdallah CG. Determining the hierarchical architecture of the human brain using subject-level clustering of functional networks. bioRxiv 2018. [DOI] [PMC free article] [PubMed]
- 45.Abdallah CG, Averill LA, Collins KA, et al. Ketamine treatment and global brain connectivity in major depression. Neuropsychopharmacol. 2017; 42(6): 1210–1219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Pizzagalli DA, Webb CA, Dillon DG, et al. Pretreatment rostral anterior cingulate cortex theta activity in relation to symptom improvement in depression: a randomized clinical trial. JAMA Psychiatry. 2018; 75(6): 547–554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Koenigs M, Grafman J. The functional neuroanatomy of depression: distinct roles for ventromedial and dorsolateral prefrontal cortex. Behav Brain Res. 2009; 201(2): 239–243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Fan S, Lippard ETC, Sankar A, et al. Gray and white matter differences in adolescents and young adults with prior suicide attempts across bipolar and major depressive disorders. J Affect Disord. 2019; 245: 1089–1097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Mulders PC, van Eijndhoven PF, Schene AH, et al. Resting-state functional connectivity in major depressive disorder: a review. Neurosci Biobehav Rev. 2015; 56: 330–344. [DOI] [PubMed] [Google Scholar]
- 50.Menon V. Large-scale brain networks and psychopathology: a unifying triple network model. Trends Cogn Sci. 2011; 15(10): 483–506. [DOI] [PubMed] [Google Scholar]
- 51.Evans JW, Szczepanik J, Brutsche N, et al. Default mode connectivity in major depressive disorder measured up to 10 days after ketamine administration. Biol Psychiatry. 2018; 84(8): 582–590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Liston C, Chen AC, Zebley BD, et al. Default mode network mechanisms of transcranial magnetic stimulation in depression. Biol Psychiatry. 2014; 76(7): 517–526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Alexopoulos GS, Hoptman MJ, Kanellopoulos D, et al. Functional connectivity in the cognitive control network and the default mode network in late-life depression. J Affect Disord. 2012; 139(1): 56–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Zhi D, Calhoun VD, Lv L, et al. Aberrant dynamic functional network connectivity and graph properties in major depressive disorder. Front Psychiatry. 2018; 9: 339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Lacerda AL, Nicoletti MA, Brambilla P, et al. Anatomical MRI study of basal ganglia in major depressive disorder. Psychiatry Res. 2003; 124(3): 129–140. [DOI] [PubMed] [Google Scholar]
- 56.Sacchet MD, Camacho MC, Livermore EE, et al. Accelerated aging of the putamen in patients with major depressive disorder. J Psychiatry Neurosci. 2017; 42(3): 164–171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Videbech P. PET measurements of brain glucose metabolism and blood flow in major depressive disorder: a critical review. Acta Psychiatr Scand. 2000; 101(1): 11–20. [DOI] [PubMed] [Google Scholar]
- 58.Lauterbach EC, Jackson JG, Wilson AN, et al. Major depression after left posterior globus pallidus lesions. Neuropsychiatry Neuropsychol Behav Neurol. 1997; 10(1): 9–16. [PubMed] [Google Scholar]
- 59.Cummings JL. The neuroanatomy of depression. J Clin Psychiatry. 1993; 54(Suppl): 14–20. [PubMed] [Google Scholar]
- 60.Manoliu A, Meng C, Brandl F, et al. Insular dysfunction within the salience network is associated with severity of symptoms and aberrant inter-network connectivity in major depressive disorder. Front Hum Neurosci. 2013; 7: 930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Sheline YI, Price JL, Yan Z, et al. Resting-state functional MRI in depression unmasks increased connectivity between networks via the dorsal nexus. Proc Natl Acad Sci USA. 2010; 107(24): 11020–11025. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, sj-pdf-1-css-10.1177_2470547020984726 for Pretreatment Brain Connectome Fingerprint Predicts Treatment Response in Major Depressive Disorder by Siyan Fan, Samaneh Nemati, Teddy J. Akiki, Jeremy Roscoe, Christopher L. Averill, Samar Fouda, Lynnette A. Averill and Chadi G. Abdallah in Chronic Stress