Abstract
Objectives
Spinal cord injury (SCI) is a disabling central nervous system disorder. This study aimed to explore the effects of repetitive trans-spinal magnetic stimulation (rTSMS) of different spinal cord segments on movement function and growth-associated protein-43 (GAP43) and 5-hydroxytryptamine (5-HT) expression in rats after acute SCI and to preliminarily discuss the optimal rTSMS treatment site to provide a theoretical foundation and experimental evidence for clinical application of rTSMS in SCI.
Methods
A rat T10 laminectomy SCI model produced by transient application of an aneurysm clip was used in the study. The rats were divided into group A (sham surgery), group B (acute SCI without stimulation), group C (T6 segment stimulation), group D (T10 segment stimulation), and group E (L2 segment stimulation).
Results
In vivo magnetic stimulation protected motor function, alleviated myelin sheath damage, decreased NgR and Nogo-A expression levels, increased GAP43 and 5-HT expression levels, and inhibited terminal deoxynucleotidyl transferase dUTP nick end labeling-positive cells and apoptosis-related protein expression in rats at 8 weeks after the surgery.
Conclusions
This study suggests that rTSMS can promote GAP43 and 5-HT expression and axonal regeneration in the spinal cord, which is beneficial to motor function recovery after acute SCI.
Keywords: Repetitive trans-spinal magnetic stimulation, acute spinal cord injury, motor function, growth-associated protein-43, 5-hydroxytryptamine, axonal regeneration
Introduction
Spinal cord injury (SCI) is a severely disabling disease caused by various events, including traffic accidents and natural disasters, and is characterized by disruption of the normal anatomic structure and function of the spinal cord, leading to sensory and motor dysfunction.1–3 The incidence rate of SCI exhibits an increasing trend each year, including younger patients.4,5 In addition, as one of the most important and prominent functional disorders of SCI, motor dysfunction greatly affects the life and work activities of patients. Therefore, promotion of motor function recovery after SCI is an important and urgent research topic. However, there is still no definite and effective treatment method for SCI in clinical practice.
Since the theory of spinal cord plasticity was proposed, it has become an important direction of SCI treatment.6 In recent decades, magnetic stimulation (MS) technology has gradually attracted wide attention from researchers and clinicians because of its safety and effectiveness. Studies have shown that transcranial magnetic stimulation (TMS) can change the excitability and accelerate regeneration of nerve cells, induce axon regeneration and lateral bud growth, enhance remodeling of the nervous system, and promote motor function recovery.7,8 In addition, repetitive transcranial magnetic stimulation (rTMS), as a new noninvasive technique, can induce an electrical current in the cerebral cortex, alter action potentials of cortical nerve cells, and produce long-term plasticity in the spinal cord.9 Other studies have found that 10 Hz rTMS improves motor function and relieves muscle spasms in SCI rats and that neurotransmitter levels are significantly altered after MS treatment.10 rTMS not only affects the central nervous system, but MS of peripheral nerves also produces excitatory changes in nerve cells. rTMS exhibits an obvious therapeutic effect on post-SCI respiratory dysfunction11 and the neurogenic rectum.12 Furthermore, domestic and foreign studies have reported that repetitive trans-spinal magnetic stimulation (rTSMS) also induces axon regeneration and functional reconstruction of spinal nerve cells and promotes motor function recovery after SCI.13 However, the stimulus parameters, stimulus sites, and intervention times selected in previous studies are not consistent, and corresponding comparative studies are not available. Thus, the spinal cord segment that produces the most significant improvement of motor function in response to rTSMS after acute SCI remains unknown. To solve this clinical problem, we intend to study the effect of rTSMS on motor function recovery after acute SCI in different spinal segments and preliminarily explore the optimal therapeutic sites for this treatment technology.
Materials and methods
Animals
Prior to the start of the study, approval was obtained from the Ethics/Review Committee for Experimental Animal Use and Care of Chengdu General Hospital of the Military Region (2013YG-B005). Eighty Sprague-Dawley (SD) rats (half male and half female; 285 ± 25 g) were purchased from Chengdu Dossy Experimental Animal Co. Ltd. (License number SCXK (Sichuan) 2015-030). The experimental protocol and the protocol for the care and use of laboratory animals were approved by the Local Ethics Committee for Animal Experiments in The General Hospital of Western Theater Command (China). All animals were maintained in the animal rooms under controlled conditions with a temperature of 24 ± 1°C, relative humidity of 55 ± 15%, and 12-hour light–dark cycle. The animals had free access to water and a standard diet. This study complied with the ethical principles for experimental animal use, care, and welfare, and animal suffering was minimized.
Establishment of a rat model of acute SCI
After rats were anesthetized with 3% pentobarbital sodium (30 mg pentobarbital sodium/kg rat body weight) by intraperitoneal injection,14 they were fixed on the surgery table. The surgical site was shaved and sterilized. Briefly, a longitudinal incision of 3 cm was made to expose the T9, T10, and T11 vertebral plates. Then, a laminectomy was performed at the T10 level to expose the dorsal epidural space of the spinal cord. A triangular needle was clamped with a needle-holding device so that the blunt end could completely pass through the space between the ventral dura and the vertebral body to avoid SCI and pulling. The aneurysm clip (Yasargil Titan Mini Clips, FT228T, Peter Lazic GmbH Tuttlingen, Germany, closing force 70 g) was fixed, and after opening, the clip was positioned at T10 through the channel to the opposite side to ensure that it completely crossed the spinal cord. Then, the clip was suddenly released, and the spinal cord was instantly compressed by the force, causing spasmodic tremor and oscillation in the rat body. When the clip was gently removed after 10 s, subdural congestion or hematoma was visible, with obvious clip marks outside of the dura.15 The wound was then sutured in layers. After the surgery, the rats were injected with penicillin for 7 successive days. Urination was initiated by rubbing the bladder of rats at 4 hours after the operation and at 8 am and 8 pm every day until the rats resumed spontaneous urination. For the sham-surgery controls, the rats underwent a T10 laminectomy without transient application of the aneurysm clip.
Animal grouping and intervention method
Eighty SD rats were randomly divided into five groups (n = 16 each), including group A (sham surgery), group B (acute SCI without stimulation), group C (T6 segment stimulation), group D (T10 segment stimulation), and group E (L2 segment stimulation). Our MS program followed established safety guidelines for repetitive MS.16,17 Rats in groups C, D, and E were treated with rTSMS at different spinal segments from postoperative day 4 using a Magstim Rapid Stimulator (Magstim Co. Ltd, Whitland, Wales, UK), with group C stimulated at the T6 segment, group D stimulated at the T10 segment, and group E stimulated at the L2 segment. The center of the “8”-shaped coil was cooled by a vacuum and was fixed close to the spine. The axis of the coil was perpendicular to the rat spinal segment to be stimulated. During the stimulus in group A (sham surgery), the magnetic coil was placed under the segments stimulated in the other groups, but MS was not transmitted. However, the magnetic coil vacuum cooling system still produced the typical auditory stimuli that were present during the actual stimulus. The rats underwent 20 minutes of baseline measurements and 10 minutes of post-stimulation measurements. During the 20-minute baseline measurement, rats were continuously subjected to rTSMS or sham stimulation. Treatment was performed daily at 3 pm, and the stimulation parameters were as follows: 5 Hz, 75% maximum output intensity (1.5 T), 5s for each sequence, 2s interstimulus interval, and each stimulus included 10 sequences, duration: one treatment per day, five treatments per week, continuous treatment for 8 weeks.
Collection of spinal cord tissue specimens
After 8 weeks, eight rats were randomly removed from each group and anesthetized by intraperitoneal injection of an excess of 10% chloral hydrate (6 mL/kg). The dorsal skin of the rats was then removed. While centering on segment T10 of the spinal cord, the surrounding vertebral plates and spinous processes were removed, the spinal cord was completely exposed, and the spinal nerve roots on both sides were removed and cut off. Then, centering on the damaged area, an approximately 1 cm length of spinal cord tissue was obtained, and four samples were randomly selected from each group and placed in a cryopreservation tube. The samples were labeled and stored in liquid nitrogen. The remaining four samples in each group were prefixed in 3% glutaraldehyde solution and observed by transmission electron microscopy (TEM). After the samples were stored, four rats were randomly selected from each group for spinal tissue extraction after cardiac perfusion. An approximately 1 cm length of spinal cord tissue was removed from the injured area as follows. First, 10% chloral hydrate solution (3 mL/kg) was injected intraperitoneally. After successful anesthesia, the abdomen was cut along the costal margin. The pericardium was removed to fully expose the heart and the ascending aorta. The left ventricle (apex) was rapidly perfused with 150 mL of normal saline. Subsequently, the ascending aorta was perfused with a universal tissue fixator (neutral, 4% paraformaldehyde + phosphate buffered saline (PBS)). Perfusion was stopped when the liver became pale and the limbs began to twitch. According to the modeling method, the skin, fascia, muscle, and other tissues on the back of the rat were cut along the direction of the spinal cord to expose the damaged spinal cord. After the spinal cord was fully exposed, the spinal nerve roots on both sides of the spinal cord were carefully dissected with a glass minute needle, and an approximately 1-cm length of the spinal nerve roots was cut off. Finally, the tissues were fixed in the prepared 4% paraformaldehyde fixative for 24 hours and then dehydrated, paraffin-embedded, and sliced.
Quantification of Basso, Beattie, and Bresnahan (BBB) scores
The BBB 22-point open-field locomotor rating scale was used to evaluate hind limb motor function; 0 points indicated total paralysis of the hind limbs, and 21 points indicated full functioning and normal locomotion. Behavioral analyses were conducted at the indicated time points (1 day before surgery and 1, 2 , 4, 6, and 8 weeks after surgery) by two investigators who were blinded to the treatment.
Transmission electron microscopy
The spinal cord tissues (1 cm) originally stored in 3% glutaraldehyde solution were removed and trimmed to 0.5 to 1.0 mm2. Then, the spinal cord tissue (0.5–1.0 mm2) was fixed with 3% glutaraldehyde for 2 hours, followed by dehydration in a series of acetone solutions. The dehydrated tissues were successively treated with a penetrant dehydrating agent and epoxy resin at ratios of 3:1, 1:1, and 1:3 for 30 to 60 minutes at each step. Next, the tissue was embedded for ultrathin sectioning for electron microscopy. The sections were stained with uranyl acetate and lead citrate for 15 minutes each at room temperature and then observed and photographed under a Hitachi H-600IV transmission electron microscope (Hitachi Medical Corporation, Tokyo, Japan).
Western blot assay
The total protein was extracted using radioimmunoprecipitation assay lysis buffer (Wuhan Boster Biological Technology, Ltd., Wuhan, Hubei, China). The protein was quantified with a BCA Protein Assay kit (NanJing KeyGen Biotech Co., Ltd., Nanjing, China). Protein samples were separated by 10% sodium dodecyl sulfate-polyacrylamide gel electrophoresis and then transferred to a poly-vinylidene fluoride (PVDF; Millipore, Billerica, MA, USA) membrane. Then, the PVDF membrane was incubated with 5% bovine serum albumin and the following primary antibodies at room temperature for 2 hours: rabbit anti-Nogo-A (ab180847; 1:1,000; Abcam, Cambridge, MA, USA), rabbit anti-Nogo (ab184556; 1:10,000; Abcam), mouse anti-growth-associated protein-43 (GAP43) (ab129990; 1:1000; Abcam), rabbit anti-caspase-3 (ab13847; 1:500; Abcam), rabbit anti-caspase-7 (ab255818; 1:1000; Abcam), rabbit anti-caspase-9 (ab2013; 1:1000; Abcam), rabbit anti-cleaved caspase-3 (ab214430; 1:5000; Abcam), rabbit anti-cleaved caspase-7 (ab256469; 1:1000; Abcam), rabbit anti-cleaved caspase-9 (ab2324; 1:1000; Abcam), rabbit anti-Bax (ab263897; 1:1000; Abcam), or mouse anti-β-actin (ab8226; 1:5000; Abcam). The membrane was then incubated with the following secondary antibodies at room temperature for 1 hour: goat anti-mouse IgG (ab205719; 1:10,000; Abcam) or goat anti-rabbit IgG (ab205718; 1:5000; Abcam). β-actin was used as the internal loading control. Protein bands were visualized using an ECL chemiluminescence kit (EMD Millipore) and analyzed by Image-Pro Plus software (Media Cybernetics, Rockville, MD, USA).
Immunohistochemical assay
Immunohistochemical staining was performed according to the manufacturer’s instructions. Briefly, paraffin-embedded spinal cord tissues were cut into 4-µm sections and deparaffinized with xylene. Then, antigen retrieval with 3% methanol and hydrogen peroxide was performed at room temperature for 10 minutes, and the sections were incubated with goat serum at room temperature for 20 minutes. The sections were incubated with mouse anti-GAP43 (ab129990; 1:500; Abcam) or rabbit anti-5-HT (ab140495; 1:1000; Abcam) at 4°C overnight and then incubated with goat anti-mouse IgG (ab205719; 1:5000; Abcam) or goat anti-rabbit IgG (ab205718; 1:5000; Abcam), respectively, for 30 minutes at 37°C. Then, the sections were incubated with diaminobenzidine (Boster, Wuhan, China) for 2 minutes and counterstained with hematoxylin. Positive immunostaining was defined as brown or yellow granules in the cytoplasm or nucleus. PBS was used instead of a primary antibody in the negative control group. Five visual fields were randomly selected and assessed for immunoreactive areas at 400× magnification using the BA200 Digital image system. The images were analyzed using Image-Pro Plus software.
Immunofluorescence staining of 5-HT
The spinal cord tissues were dissected from rats and rapidly frozen, and longitudinal sections (20 µm) were prepared with a cryostat (Leica, Wetzlar, Germany). Pretreatment, blocking, and primary and secondary antibody reactions were performed as described previously.18 Briefly, samples were incubated with rabbit anti-5-HT (ab221181; 1:400; Abcam) primary antibodies. Fluorochrome-conjugated anti-rabbit (ab221181; 1:1000; Abcam) secondary antibodies were added to the primary antibody-probed sections and incubated. After successive washes, the images were analyzed under a fluorescence microscope (Nikon, Kona, Japan).
Terminal deoxynucleotidyl transferase dUTP nick end labeling (TUNEL) staining
Apoptosis of spinal cord tissues from rats was measured by TUNEL staining using a TUNEL Apoptosis Assay kit (Beyotime Institute of Biotechnology, Jiangsu, China). TUNEL staining was used to identify apoptotic nuclei, and all nuclei were stained with 4′,6-diamidino-2-phenylindole and fluorescein-dUTP. The apoptotic index (AI) was calculated as follows: AI = number of TUNEL-positive cells/total number of cells. The AI was evaluated in 15 randomly selected fields.
Statistical analysis
Statistical analysis was performed using SPSS19.0 software (IBM Corp., Armonk, NY, USA). The Kolmogorov–Smirnov test was used to assess the normal distribution of variables, and normally distributed data are described as the mean ± standard error. Behavioral data were compared using repeated-measures analysis of variance (ANOVA). Multiple groups were compared by one-way ANOVA with Dunnett’s post hoc test or two-way ANOVA with Bonferroni’s post hoc test. Differences were considered statistically significant at P < 0.05 and P < 0.01.
Results
rTSMS treatment increased BBB scores in rats after acute SCI
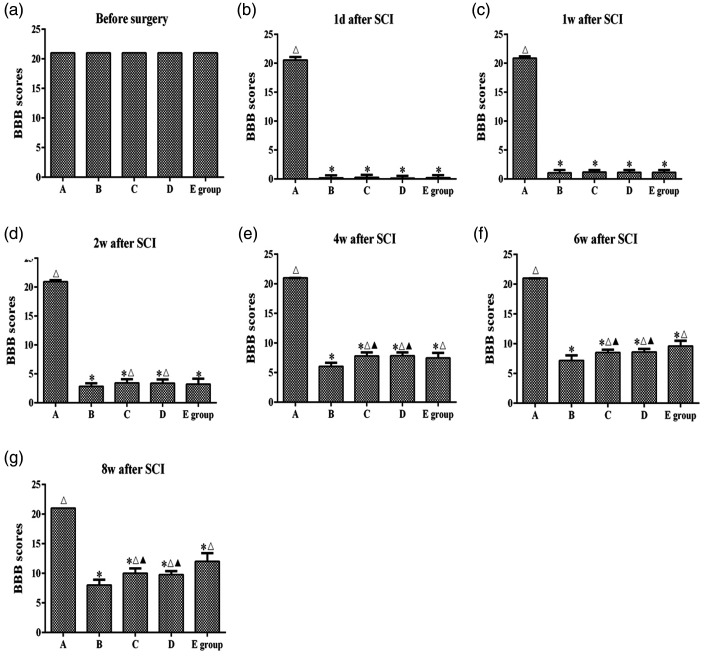
The BBB scores were determined to examine the motor function of post-surgery rats. As shown in Figure 1, the BBB scores of all rats were 21 at 1 day before surgery. After SCI, the BBB scores of rats in groups B, C, D, and E rapidly decreased to 0 to 2 points, and all rats showed typical paraplegia and limited hind limb movement during crawling. At 2, 4, 6, and 8 weeks after SCI, the BBB scores of groups C and D were significantly higher than those of group B (P < 0.05). At 4, 6, and 8 weeks after SCI, the BBB scores of group E were significantly higher than those of group B (P < 0.05). The BBB scores of groups C and D were higher than that of group E at 4 weeks after SCI (P < 0.05). However, the BBB scores of group E were higher than those of groups C and D at 6 and 8 weeks after SCI (P < 0.05). These results indicated significant motor function recovery of rats when MS was applied to the T6 and T10 segments at 0 to 6 weeks after surgery. Additionally, significant motor function recovery was observed when MS was applied to the L2 spinal segment at 6 to 8 weeks after surgery.
Figure 1.
Basso, Beattie, and Bresnahan (BBB) scores of rats in each group at different time points. (a–g) The BBB 22-point open-field locomotor rating scale was used to evaluate hind limb motor function in each group at different time points. A: sham surgery group, B: spinal cord injury group, C: T6 segment stimulation group, D: T10 segment stimulation group, E: L2 segment stimulation group. *P < 0.05, compared with group A; △P < 0.05, compared with group B; ★P < 0.05, compared with group D; ▲P < 0.05, compared with group E.
rTSMS alleviated myelin sheath damage in rats after acute SCI
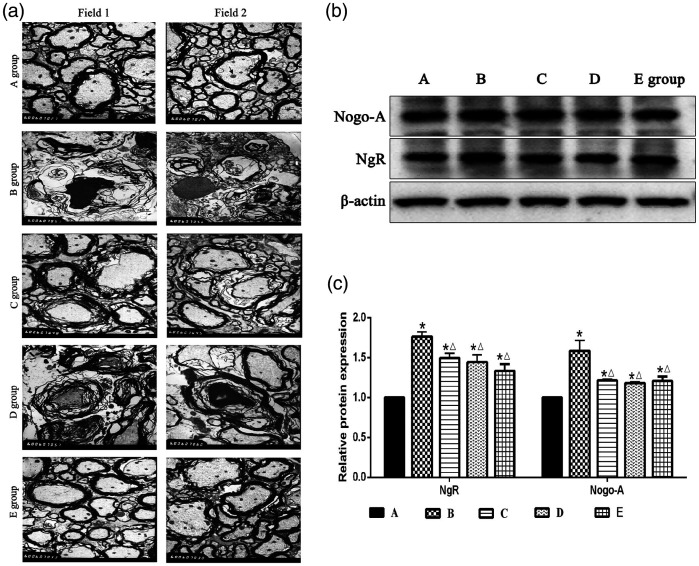
Morphological changes in the spinal cord were measured with TEM. As shown in Figure 2a, the myelin sheath structure in group A was complete and clear. The arrangement between the myelin sheath and axon was normal, and the mitochondria were evenly distributed and clearly structured. In group B, a large gap was formed between the myelin sheaths, and axons with normal structure were absent. Demyelination, myelin swelling, and structural disorder were observed. Myelin was disintegrated, broken, and missing, and serious demyelination was observed. In group E, the area between the myelin sheath and axon was relatively dense and regular, with mild demyelination. In group D, large gaps were formed between myelin sheaths, and myelin was swollen, exhibiting a disordered and unclear structure and demyelination. In group C, the myelin exhibited a loose lamellar structure, swelling, structural changes, and mild demyelination. These results indicated that rTSMS can alleviate the pathological injury of spinal cord tissue after SCI to a certain extent, and the greatest effect was observed when MS was applied to the L2 segment (Figure 2a).
Figure 2.
Effects of repetitive trans-spinal magnetic stimulation on myelin sheath damage in rats after acute spinal cord injury (SCI), (a) Images of dorsal spinal cord tissues in rats (approximately 1 cm in length) were observed by transmission electron microscopy at 8 weeks after SCI. Scale bars indicate 1 µm (6000×). The red arrow represents demyelination; the green arrow represents myelin disintegration, rupture, and loss; the blue arrows indicate clear mitochondrial structure. (b–c). A western blot assay was performed to detect NgR and Nogo-A expression levels at 8 weeks after SCI. A: sham surgery group, B: SCI group, C: T6 segment stimulation group, D: T10 segment stimulation group, E: L2 segment stimulation group. *P < 0.05, compared with group A; △P < 0.05, compared with group B.
Studies have confirmed that Nogo-A and NgR expression levels are increased after SCI.19 In addition, Nogo-A affects nerve function recovery after SCI in primates.20 Therefore, reduction of Nogo-A and NgR expression is crucial to promote recovery of the injured spinal cord. As shown in Figure 2b and c, the Nogo-A and NgR expression levels in groups B, C, D, and E were all significantly higher than those in group A (P < 0.05). In addition, the expression levels of these two proteins in groups C, D, and E were significantly lower than those in group B (P < 0.05) (Figure 2b–c).
rTSMS increased 5-HT expression in rats after acute SCI
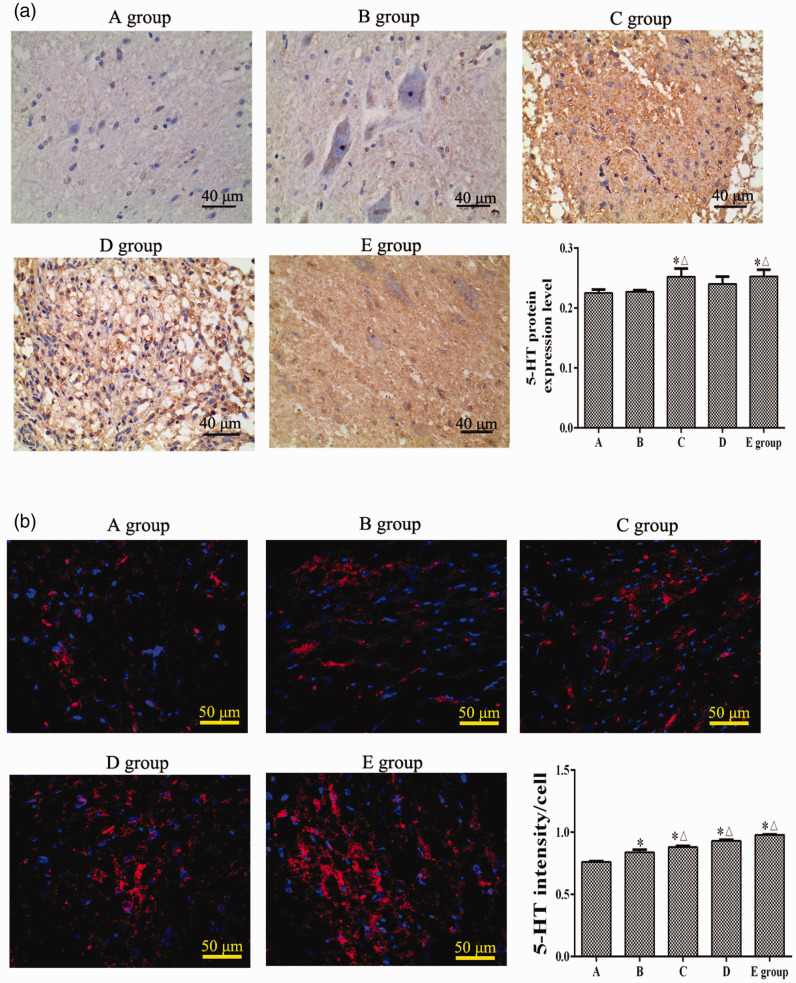
Immunohistochemistry and immunofluorescence staining were used to detect the 5-HT expression level in spinal cord tissues. The immunohistochemical staining results showed that, at 8 weeks after SCI, compared with group A, the 5-HT protein levels in the spinal cord in groups C and E were significantly increased (P < 0.05). Compared with group B, the 5-HT protein expression levels in groups C and E were significantly increased (P < 0.05) (Figure 3a). Furthermore, immunofluorescence staining results showed that, compared with group A, the 5-HT expression levels in groups B, C, D, and E were significantly increased (P < 0.05). However, compared with group B, the 5-HT expression levels in groups C, D, and E were significantly decreased (P < 0.05) (Figure 3b).
Figure 3.
Effects of repetitive trans-spinal magnetic stimulation on 5-hydroxytryptamine (5-HT) protein expression in rats after acute spinal cord injury (SCI). (a) An immunohistochemical assay was performed to detect the 5-HT expression level at 8 weeks after SCI. (b) Immunofluorescence staining was performed to detect the 5-HT expression level at 8 weeks after SCI. A: sham surgery group, B: SCI group, C: T6 segment stimulation group, D: T10 segment stimulation group, E: L2 segment stimulation group. *P < 0.05, compared with group A; △P < 0.05, compared with group B.
rTSMS increased GAP43 expression in rats after acute SCI
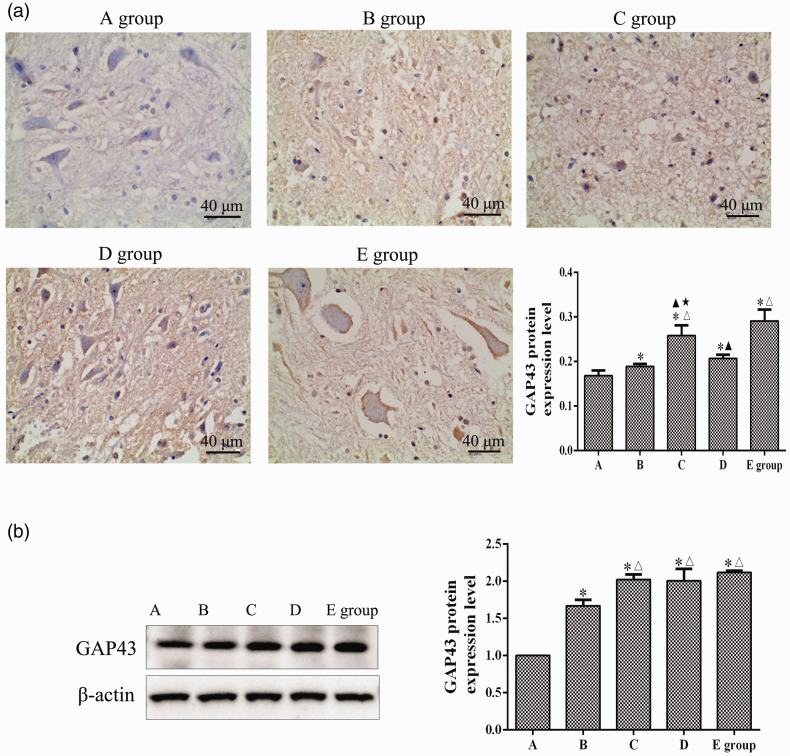
As shown in Figure 4a, at 8 weeks after SCI, a large number of cells with yellow or brown-yellow granules in the cytoplasm were observed in the injured spinal cord neurons in groups B, C, D, and E. Compared with group A, the GAP43 protein levels in the spinal cords of groups B, C, D, and E were significantly increased (P < 0.05). Compared with group B, the GAP43 protein expression levels in groups C and E were significantly increased (P < 0.05). The GAP43 protein expression level in group E was higher than those in groups C and D (P < 0.05). The GAP43 protein expression level in group C was higher than that in group D (P < 0.05). As shown in Figure 4b, at 8 weeks after SCI, compared with group A, the relative GAP43 protein expression levels were significantly increased in the spinal cord tissues of groups B, C, D, and E (P < 0.05). The relative expression levels of GAP43 protein in groups C, D, and E were significantly higher than that of group B (P < 0.05).
Figure 4.
Effects of repetitive trans-spinal magnetic stimulation on growth-associated protein-43 (GAP43) expression in rats after acute spinal cord injury (SCI). (a) An immunohistochemical assay was performed to detect the GAP43 expression level at 8 weeks after SCI. Scale bars indicate 40 µm (200×). (b) A western blot assay was performed to detect the GAP43 expression level at 8 weeks after SCI. A: sham surgery group, B: SCI group, C: T6 segment stimulation group, D: T10 segment stimulation group, E: L2 segment stimulation group. *P < 0.05, compared with group A; △P < 0.05, compared with group B; ▲P < 0.05, compared with group E; ★P < 0.05, compared with group D.
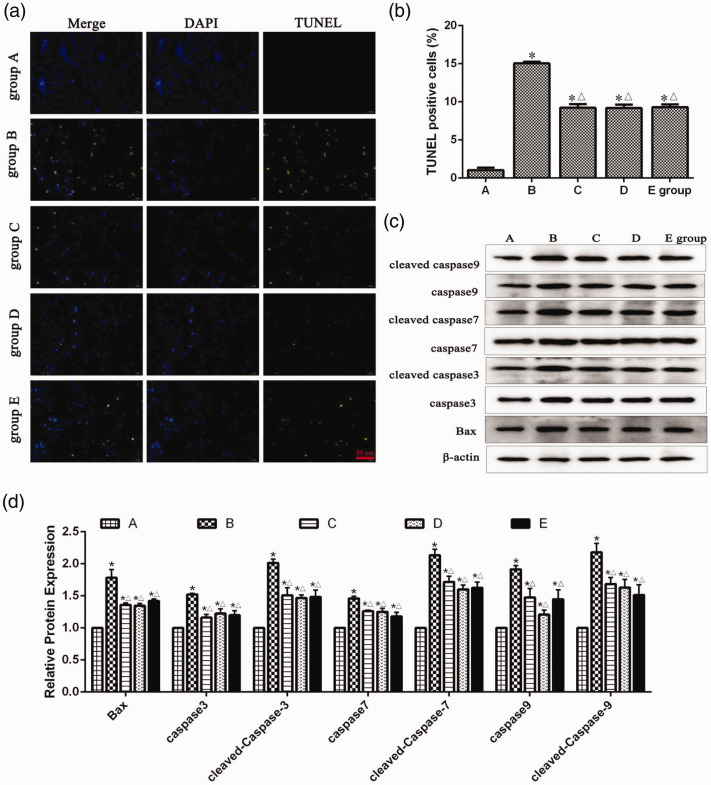
rTSMS inhibited apoptosis in the SCI areas in rats
TUNEL-positive cells and apoptosis-related proteins were detected in injured spinal cord tissues of rats. As shown in Figure 5a and b, at 8 weeks after SCI, compared with group A, TUNEL-positive cells were significantly increased in the spinal cord tissues in groups B, C, D, and E (P < 0.05). Compared with group B, TUNEL-positive cells were significantly decreased in the spinal cord tissues in groups C, D, and E (P < 0.05). In addition, as shown in Figure 5c and d, at 8 weeks after SCI, the expression levels of apoptosis-related proteins including Bax, caspase 3, cleaved-caspase 3, caspase 7, cleaved-caspase 7, caspase 9, and cleaved-caspase 9 in groups B, C, D, and E were significantly higher than those in group A (P < 0.05), while these proteins were significantly lower in groups C, D, and E than those in group B (P < 0.05).
Figure 5.
Effects of repetitive trans-spinal magnetic stimulation on terminal deoxynucleotidyl transferase dUTP nick end labeling (TUNEL)-positive cells of rats after acute spinal cord injury (SCI). (a–b) A TUNEL assay was performed to detect the TUNEL-positive cells of rats at 8 weeks after SCI. Scale bars indicate 50 µm (400×). (c–d) A western blot assay was performed to detect apoptosis-related protein expression levels at 8 weeks after SCI. A: sham surgery group, B: SCI group, C: T6 segment stimulation group, D: T10 segment stimulation group, E: L2 segment stimulation group. *P < 0.05, compared with group A; △P < 0.05, compared with group B.
Discussion
SCI is a traumatic disease that results in great disability and can cause severe dysfunction and even death.21 SCI is characterized by a high incidence, disability rate, and cost of treatment and a young age.22–24 Therefore, it is of great significance for patients, their families, and society to identify effective treatments. In recent decades, domestic and foreign studies have found that spinal cord MS can promote motor function recovery in rats after SCI. Mally et al.25 investigated nine patients with thoracic SCI who were treated with low-frequency MS in the motor cortex, spinal cord segments above the injury site, and lumbar nerve roots. After 6 months, motor function was significantly improved in some patients, and some patients could even stand on their own and walk short distances with walking aids. Ahemd et al.26 found that 1 Hz MS applied to acute SCI rats can effectively improve their motor function. Leydeker et al.13 also found that in addition to skill training, application of 15 Hz MS directly to SCI segments to treat T13 spinal cord contusion mice can effectively improve motor function in these mice. In the present study, rTSMS was applied to different spinal cord segments of rats with acute SCI, and behavioral observation over a period of 8 weeks confirmed that rTSMS could effectively promote recovery of motor function in these rats, which was consistent with previous reports. In addition, TEM results showed that, compared with the SCI group, the T6, T10, and L2 segment stimulation groups all showed different degrees of improvement in spinal cord ultrastructure, suggesting that rTSMS can alleviate the pathological changes of the spinal cord after acute SCI to some extent.
After SCI, the functional recovery ability of the spinal cord is very limited, which is mainly caused by severe inhibition of axon regeneration at the damaged end of the spinal segment after injury. With the development of SCI research, several factors related to inhibition of axon regeneration have been identified. At present, research results have confirmed that Nogo-A has an inhibitory effect on axonal regeneration, and NgR mediates the effect of Nogo-A.27 In addition, studies have confirmed that Nogo-A and NgR expression levels are increased after SCI.19 Furthermore, Nogo-A affects nerve function recovery after SCI in primates.20 Therefore, reduction of Nogo-A and NgR expression is crucial to promote recovery of the injured spinal cord. This study showed that Nogo-A and NgR proteins were expressed in spinal cord tissues in the sham group, and the results were consistent with those of earlier studies.27 The physiological significance of nerve fiber growth inhibitor protein expression in normal tissues may be that it acts as a boundary to the growing central nerve fibers to prevent the subsequent growth of lateral branches into the well-formed fibers. This study showed that after SCI, Nogo-A and NgR protein expression increased in both the injury group and the treatment groups. With application of rTSMS to different sites of the spinal cord, the expression levels of Nogo-A and NgR decreased, indicating that rTSMS can effectively reduce Nogo-A and NgR expression after SCI to promote recovery of function in the injured spinal cord in rats.
The recovery mechanisms of lower limb motor function after SCI include sprouting and compensation by the surviving descending pathway system, regeneration of the injured descending pathway, and compensatory mechanisms of the spinal cord intrinsic loop, namely the central pattern generator (CPG) theory.28,29 Normal rats can execute commands from the nerve centers above the spinal cord injury, while rats with complete SCI are more dependent on activation of the spinal cord locomotor CPG, which suggests that the compensatory mechanism of the CPG is particularly important for motor function improvement in rats with complete SCI.30 During exercise, spinal motor neurons are simultaneously regulated by a variety of neurotransmitters, such as 5-HT and norepinephrine.31 Medullary serotonergic neurons originate from the raphe nuclei of the medulla oblongata and the globus pallidus and can extend to the motor neurons in the anterior horn of the spinal cord to regulate neuron activity.32 5-HT release by the intrinsic spinal cord 5-HT neurons after SCI also regulates the electrophysiological activities of spinal cord neurons, affecting the motor neuron activation and signal output.33 The present study revealed that rTSMS significantly increased 5-HT expression in rats after acute SCI. This enhanced 5-HT expression may increase the continuous inward current by regulating the amplitude and threshold of the persistent inward current in neurons, thus amplifying the synaptic input signal, enhancing activation of CPG-related neurons, and promoting motor function recovery.34
Regeneration and budding of axons are affected by many factors, and GAP43 plays a key role in this process. In the field of neuroscience, GAP43 is known as a marker of axon regeneration and new connection formation in nerve cells.35 Therefore, changes in GAP43 in spinal cord tissue can accurately reflect nerve cell growth in the damaged spinal cord. In the present study, we found that GAP43 protein expression was significantly increased in SCI rats compared with the sham group, indicating that GAP43 was highly expressed after nerve injury to promote the rapid axon growth.36 In addition, our study showed that rTSMS effectively promoted GAP43 protein expression in the SCI area of rats, and L2 segment stimulation had the strongest effect on this expression, which may be related to the L2 segment being located in the CPG ring of rats. Under repeated MS, the spinal cord locomotor CPG is activated, which induces post-intervention plasticity and promotes the recombination of regenerated axons with spinal cord neuron circuits.37,38 An increased intracellular Ca2+ concentration is necessary for cell death and is an initiating factor for the secondary pathological changes of SCI.39 However, the content of Mg2+, a natural antagonist of Ca2+, decreases rapidly after SCI, which further aggravates the secondary pathological changes of spinal cord tissues.40,41 Therefore, we speculate that the improvement of motor function and promotion of axon regeneration after SCI by rTSMS might be related to improvement of the Ca2+ and Mg2+ imbalance in the microenvironment in the SCI area.
The pathophysiological changes and injury mechanism after SCI consist of a complex cascade reaction process, and irreversible injury (apoptosis) occurs to the nerve cells of the spinal cord after injury.42 At present, the exact molecular mechanism of apoptosis is unclear, but apoptosis involves the activation of a series of apoptotic proteins in the cytoplasm and their cleavage of substrates, and a current research focus is the caspase protein family and the Bax family proteins. The Bax, caspase-3, caspase-7, and caspase-9 genes are all closely related to apoptosis and have been confirmed to be involved in regulation of apoptosis in different studies. The caspase family plays a key role in apoptosis.43 In addition, as a component of ion channels on the mitochondrial membrane, Bax protein activates caspase-9, which is involved in initiating apoptosis, and further activate caspase-3, which is involved in executing apoptosis.44 All of these factors can lead to cell apoptosis. According to the detection of TUNEL-positive cells and apoptosis-related proteins, rTSMS could effectively inhibit apoptosis in the SCI area of rats.
In summary, our results demonstrated that rTSMS of the L2 segment led to the most obvious improvement of motor function and the highest expression level of GAP43 in rats with SCI. Our study provides an experimental basis for exploring the optimal stimulation site of rTSMS and theoretical support for the clinical application of rTSMS for SCI. However, because only the upper and lower spinal cord segments were included for comparative study, it is possible that stimulation of other segments may produce better results. Therefore, an optimal treatment site should be determined, and its mechanism should be further studied.
Footnotes
Authors' contributions: HL, DQX, ARZ, and WCW conceived and designed the experiments. HL, DQX, RZP, QD, NYS, and JQZ performed the experiments. WX and ZSC analyzed the data and contributed to the acquisition of reagents and materials. HL and DQX wrote the manuscript.
Declaration of conflicting interest: The authors declare that there is no conflict of interest.
Funding: The present study was supported by the Program for The General Hospital of Western Theater Command of the Chinese People’s Liberation Army (2013YG-B005), the National Research Talent Training Fund of Western Theater Command of the Chinese People’s Liberation Army (42412E1L), and the National Natural Science Foundation of China (81674044).
ORCID iD: Wenchun Wang https://orcid.org/0000-0001-9742-9089
References
- 1.Motiei-Langroudi R, Sadeghian H. Traumatic spinal cord injury: long-term motor, sensory, and urinary outcomes. Asian Spine J 2017; 11: 412–418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Li J, Liu G, Zheng Y, et al. The epidemiological survey of acute traumatic spinal cord injury (ATSCI) of 2002 in Beijing municipality. Spinal Cord 2011; 49: 777–782. [DOI] [PubMed] [Google Scholar]
- 3.Pérez K, Novoa AM, Santamariña-Rubio E, et al. Incidence trends of traumatic spinal cord injury and traumatic brain injury in Spain, 2000-2009. Accid Anal Prev 2012; 46: 37–44. [DOI] [PubMed] [Google Scholar]
- 4.Ramer LM, Ramer MS, Bradbury EJ. Restoring function after spinal cord injury: towards clinical translation of experimental strategies. Lancet Neurol 2014; 13: 1241–1256. [DOI] [PubMed] [Google Scholar]
- 5.Lee BB, Cripps RA, Fitzharris M, et al. The global map for traumatic spinal cord injury epidemiology: update 2011, global incidence rate. Spinal Cord 2014; 52: 110–116. [DOI] [PubMed] [Google Scholar]
- 6.Jutzeler CR, Streijger F, Aguilar J, et al. Sensorimotor plasticity after spinal cord injury: a longitudinal and translational study. Ann Clin Transl Neurol 2019; 6: 68–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lu H, Kobilo T, Robertson C, et al. Transcranial magnetic stimulation facilitates neurorehabilitation after pediatric traumatic brain injury. Sci Rep 2015; 5: 14769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Curt A, Van Hedel HJ, Klaus D, et al. Recovery from a spinal cord injury: significance of compensation, neural plasticity, and repair. J Neurotrauma 2008; 25: 677. [DOI] [PubMed] [Google Scholar]
- 9.Thickbroom GW, Byrnes ML, Edwards DJ, et al. Repetitive paired-pulse TMS at I-wave periodicity markedly increases corticospinal excitability: a new technique for modulating synaptic plasticity. Clin Neurophysiol 2006; 117: 61–66. [DOI] [PubMed] [Google Scholar]
- 10.Gao W, Yu LG, Liu YL, et al. Mechanism of GABA receptors involved in spasticity inhibition induced by transcranial magnetic stimulation following spinal cord injury. J Huazhong Univ Sci Technolog Med Sci 2015; 35: 241–247. [DOI] [PubMed] [Google Scholar]
- 11.Lin VW, Hsiao IN, Zhu E, et al. Functional magnetic stimulation for conditioning of expiratory muscles in patients with spinal cord injury. Arch Phys Med Rehabil 2001; 82: 162–166. [DOI] [PubMed] [Google Scholar]
- 12.Vallès M, Rodríguez A, Borau A, et al. Effect of sacral anterior root stimulator on bowel dysfunction in patients with spinal cord injury. Dis Colon Rectum 2009; 52: 986–992. [DOI] [PubMed] [Google Scholar]
- 13.Leydeker M, Delva S, Tserlyuk I, et al. The effects of 15 Hz trans-spinal magnetic stimulation on locomotor control in mice with chronic contusive spinal cord injury. Electromagn Biol Med 2013; 32: 155–164. [DOI] [PubMed] [Google Scholar]
- 14.Abematsu M, Tsujimura KM, Saito M, et al. Neurons derived from transplanted neural stem cells restore disrupted neuronal circuitry in a mouse model of spinal cord injury. J Clin Invest 2010; 120: 3255–3266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Euler MV, Seiger Å, Sundström E. Clip compression injury in the spinal cord: a correlative study of neurological and morphological alterations. Exp Neurol 1997; 145: 502–510. [DOI] [PubMed] [Google Scholar]
- 16.Wassermann EM. Risk and safety of repetitive transcranial magnetic stimulation: report and suggested guidelines from the International Workshop on the Safety of Repetitive Transcranial Magnetic Stimulation, June 5-7, 1996. Electroencephalogr Clin Neurophysiol 1998; 108: 1–16. [DOI] [PubMed] [Google Scholar]
- 17.Pascual-Leone A, Houser CM, Reese K, et al. Safety of rapid-rate transcranial magnetic stimulation in normal volunteers. Electroencephalogr Clin Neurophysiol 1993; 89: 120–130. [DOI] [PubMed] [Google Scholar]
- 18.Zhao P, Chao W, Li W. FBXW5 reduction alleviates spinal cord injury (SCI) by blocking microglia activity: a mechanism involving p38 and JNK. Biochem Biophys Res Commun 2019; 514: 558–564. [DOI] [PubMed] [Google Scholar]
- 19.Xiao WP, Ding LLQ, Min YJ, et al. Electroacupuncture promoting axonal regeneration in spinal cord injury rats via suppression of Nogo/NgR and Rho/ROCK signaling pathway. Neuropsychiatr Dis Treat 2019; 15: 3429–3442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Freund P, Schmidlin E, Wannier T, et al. Anti-Nogo-A antibody treatment promotes recovery of manual dexterity after unilateral cervical lesion in adult primates–re-examination and extension of behavioral data. Eur J Neurosci 2009; 29: 983–996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bowes AL, Yip PK. Modulating inflammatory cell responses to spinal cord injury: all in good time. J Neurotrauma 2014; 31: 1753–1766. [DOI] [PubMed] [Google Scholar]
- 22.Sabre L, Pedai G, Rekand T, et al. High incidence of traumatic spinal cord injury in Estonia. Spinal Cord 2012; 50: 755–759. [DOI] [PubMed] [Google Scholar]
- 23.Katoh S, Enishi T, Sato N, et al. High incidence of acute traumatic spinal cord injury in a rural population in Japan in 2011 and 2012: an epidemiological study. Spinal Cord 2014; 52: 264–267. [DOI] [PubMed] [Google Scholar]
- 24.Burke DA, Linden RD, Zhang YP, et al. Incidence rates and populations at risk for spinal cord injury: a regional study. Spinal Cord 2001; 39: 274–278. [DOI] [PubMed] [Google Scholar]
- 25.Mally J, Stone TW. New advances in the rehabilitation of CNS diseases applying rTMS. Expert Rev Neurother 2007; 2: 165–177. [DOI] [PubMed] [Google Scholar]
- 26.Ahmed Z, Wagdy M, Benjamin M, et al. Therapeutic effects of acrobatic exercise and magnetic field exposure on functional recovery after spinal cord injury in mice. Bioelectromagnetics 2015; 32: 49–57. [DOI] [PubMed] [Google Scholar]
- 27.Fournier AE, GrandPre T, Strittmatter SM. Identification of a receptor mediating Nogo-66 inhibition of axonal regeneration. Nature 2001; 409: 341–346. [DOI] [PubMed] [Google Scholar]
- 28.Grillner S. The spinal locomotor CPG: a target after spinal cord injury. Prog Brain Res 2002; 137: 97–108. [DOI] [PubMed] [Google Scholar]
- 29.Frigon A. Central pattern generators of the mammalian spinal cord. Neuroscientist 2012; 18: 56–69. [DOI] [PubMed] [Google Scholar]
- 30.Ballermann M, Fouad K. Spontaneous locomotor recovery in spinal cord injured rats is accompanied by anatomical plasticity of reticulospinal fibers. Eur J Neurosci 2010; 23: 1988–1996. [DOI] [PubMed] [Google Scholar]
- 31.Noga BR, Turkson RP, Xie S, et al. Monoamine release in the cat lumbar spinal cord during fictive locomotion evoked by the mesencephalic locomotor region. Front Neural Circuits 2017; 11: 59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Jacobs BL, Martín-Cora FJ, Fornal CA. Activity of medullary serotonergic neurons in freely moving animals. Brain Res Brain Res Rev 2002; 39: 45–52. [DOI] [PubMed] [Google Scholar]
- 33.Harvey PJ, Li X, Li Y, et al. Endogenous monoamine receptor activation is essential for enabling persistent sodium currents and repetitive firing in rat spinal motoneurons. J Neurophysiol 2006; 96: 1171–1186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tazerart S, Vinay L, Brocard F. The persistent sodium current generates pacemaker activities in the central pattern generator for locomotion and regulates the locomotor rhythm. J Neurosci 2008; 28: 8577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kawasaki A, Okada M, Tamada A, et al. Growth cone phosphoproteomics reveals that GAP-43 phosphorylated by JNK is a marker of axon growth and regeneration. iScience 2018; 4: 190–203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Alam M, Garcia-Alias G, Jin B, et al. Electrical neuromodulation of the cervical spinal cord facilitates forelimb skilled function recovery in spinal cord injured rats. Exp Neurol 2017; 291: 141–150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Solopova IA, Selionov VA, Kazennikov OV, et al. Effects of transcranial magnetic stimulation during voluntary and non-voluntary stepping movements in humans. Neurosci Lett 2014; 579: 64–69. [DOI] [PubMed] [Google Scholar]
- 38.Gordon IT, Whelan PJ. Deciphering the organization and modulation of spinal locomotor central pattern generators. J Exp Biol 2006; 209: 2007–2014. [DOI] [PubMed] [Google Scholar]
- 39.Zhang Y, Hou S, Wu Y. Changes of intracellular calcium and the correlation with functional damage of the spinal cord after spinal cord injury. Chin J Traumatol 2002; 5: 40–42. [PubMed] [Google Scholar]
- 40.Kostyuk P, Verkhratsky A. Calcium stores in neurons and glia. Neuroscience 1994; 63: 381–404. [DOI] [PubMed] [Google Scholar]
- 41.Mayer ML, Westbrook GL, Guthrie PB. Voltage-dependent block by Mg2+ of NMDA responses in spinal cord neurones. Nature 1984; 309: 261–263. [DOI] [PubMed] [Google Scholar]
- 42.Wang F, Zhou C, Gao ZC, et al. [ Depressant effect of lithium on apoptosis of nerve cells of adult rats after spinal cord injury]. Zhongguo Gu Shang 2018; 31: 379–385. [DOI] [PubMed] [Google Scholar]
- 43.Fan TJ, Han LH, Cong RS, et al. Caspase family proteases and apoptosis. Acta Biochim Biophys Sin (Shanghai) 2005; 37: 719–727. [DOI] [PubMed] [Google Scholar]
- 44.Schon EA, Manfredi G. Neuronal degeneration and mitochondrial dysfunction. J Clin Invest 2003; 111: 303–312. [DOI] [PMC free article] [PubMed] [Google Scholar]