Abstract
OBJECTIVE
Increasing evidence suggests that time spent sedentary predicts increasing cardiometabolic risk independent of other physical activity. We objectively measured activity levels in active older adults and examined the association between sedentary behavior and the continuous metabolic syndrome risk score (cMSy).
RESEARCH DESIGN AND METHODS
Older adults (age ≥65 years) were recruited from the Whistler Masters ski team, a group of active older adults who undergo organized group training. Daily activity levels were recorded with accelerometers (SenseWear) worn for 7 days. A compositional approach was used to determine proportion of the time spent sedentary as compared with all other nonsedentary behaviors (isometric log-ratio transformation for time spent sedentary [ILR1]). Waist circumference, triglycerides, HDL, systolic blood pressure, and fasting glucose were measured, and cMSy was calculated using principal component analysis (sum of eigenvalues ≥1.0).
RESULTS
Fifty-four subjects (30 women and 24 men, mean ± SE age 71.4 ± 0.6 years) were recruited. Subjects demonstrated high levels of physical activity (2.6 ± 0.2 h light activity and 3.9 ± 0.2 h moderate/vigorous activity). In our final parsimonious model, ILR1 showed a significant positive association with increasing cMSy (standardized β = 0.368 ± 0.110, R2 = 0.40, P = 0.002), independent of age and biological sex.
CONCLUSIONS
Despite high levels of activity, ILR1 demonstrated a strong association with cMSy. This suggests that even in active older adults, sedentary behavior is associated with increasing cardiometabolic risk.
Introduction
Metabolic syndrome is extremely common in the older adult population due to many factors, including an increase in sedentary time (ST) and a reduction in leisure-time physical activity (LTPA) (1). Metabolic syndrome is a cluster of risk factors that is associated with an increase in the incidence of cardiovascular disease and an increase in mortality (2). Current thinking has postulated that instead of a binary dichotomy (metabolic syndrome vs. not having metabolic syndrome), a more useful approach is to assign a continuous score to this cluster of risk factors called the continuous metabolic syndrome risk score (cMSy) (3). It has been well established that an increase in ST and an increase in LTPA show opposite associations with the development of metabolic syndrome in young and middle-aged populations (4).
However, more recent work has questioned examining activity levels as completely independent from each other. Given that there is only a finite amount of time in each day, any amount of time spent at one level of activity is by definition removed from time spent at another level of activity, a codependence that can be accounted for by a compositional approach (5,6). Due to this codependence, ST can be quite training resistant; previous work showed that some individuals compensate for increases in LTPA with a compensatory increase in ST (removed from the portion of the day that otherwise would have been spent in light activity) (7). Although not previously examined, it seems that this issue would be even more pronounced in the older adult population; it remains very much an open question how much of the cardiometabolic benefits of moderate/vigorous activity are offset by fatigue-related increases in ST in this vulnerable population.
Our study sought to address this knowledge gap by examining the association between the factors that make up the metabolic syndrome (as measured by cMSy) and the proportion of the day spent in ST using a compositional approach in an already extremely active group of older adult subjects. We hypothesized that even in a group vastly exceeding current activity guidelines, the proportion of the day spent sedentary would show strong positive associations with cMSy, despite the subject’s high levels of physical activity.
Research Design and Methods
Subjects
All subjects gave written consent, and our study protocol was approved by the Human Subject’s Committee of the University of British Columbia. Subjects were recruited via their affiliation with the Whistler Masters ski team (Whistler, British Columbia, Canada), a group that has organized training during the off-season; all measures were taken during the off-season (spring, summer, and fall) when the participants were not pursuing their primary sport (downhill skiing). Participants were approached through a series of information sessions and posters. All subjects were recruited in the context of a previous sleep study protocol (8).
Inclusion/Exclusion Criteria
All subjects had to be in good health; current smoking, the use of recreational drugs, having a diagnosis of cardiovascular disease (in the form of angina, myocardial infarction, or a history of coronary revascularization), having a diagnosis of diabetes, and having a previous stroke or transient ischemic attack were all exclusion criteria. All subjects had to be members of the Masters ski team, and had to be age 65 years or older.
Study Procedures
A single study visit was booked to collect anthropomorphic, blood pressure, laboratory, and clinical data. Each component of the metabolic syndrome was measured, as per current guidelines (9). During the same visit, the accelerometer was also applied, and a postage-paid envelope was given to each subject to allow them to return the device. The coordinator of the study was available 24 h per day by cell phone to answer any questions about the device. The research nurse recorded medication list and past medical history for each subject. Blood pressure was measured while the subject was seated quietly with a digital sphygmomanometer (ABPM 7100; Welch Allyn). After 20 min of quiet rest, an average of three readings separated by 5 min was recorded in the supine position. A stadiometer (Health-o-meter) was used to measure height to the nearest 0.1 cm. Weight was measured to the nearest 0.1 kg using a mechanical beam balance scale. Waist circumference was measured using a plastic tape measure held directly against the skin at the level of the umbilicus. Our tape measure was calibrated as per current guidelines (10), and all measures were performed by the same technician. Serum lipid measures (HDL and triglycerides) and fasting blood glucose were measured in a private-affiliated laboratory, first thing in the morning, with each subject in a fasted state (LifeLabs).
With use of an armband fitted snugly around the right upper triceps, triaxial accelerometers (SenseWear Pro; BodyMedia, Sword Medical, Blanchardstown, Dublin) were fitted and used to monitor levels of physical activity for seven full days, 24 h per day (8). Subjects were instructed to wear the accelerometers continuously, including during sleep, and to only remove them when bathing. The SenseWear Pro has a gyroscope (which allows the device to distinguish between upright and supine positions) and triaxial accelerometers (used to measure activity level). BodyMedia InnerView Research software (version 5.1; BodyMedia) uses proprietary algorithms to analyze minute-by-minute raw activity data from the SenseWear Pro (11); these algorithms have been shown to accurately measure sleep duration similar to polysomnography measurements (12).
Data Collection and Processing
Each subject had to wear the accelerometer for at least five valid days to be included in the analysis. A valid day was defined as at least 21 h of recorded activity on the accelerometer. The SenseWear Pro measures skin conductance, which allows the device to record when it is removed, in order to avoid confusion of an absence of activity with not the device not being worn (11).
Accelerometer Measures of Activity
The SenseWear Pro is a commercially available device that assesses energy expenditure from heat flux, triaxial accelerometers, galvanic skin response, and skin temperature. It estimates energy expenditure using proprietary equations developed by the manufacturer; ST, light activity time (LT), and time spent in moderate-to-vigorous activity (MT) were recorded as the average number of minutes per day. ST was defined as the average daily time spent in “any waking behavior characterized by an energy expenditure ≤1.5 METs while in a sitting or reclining posture” (11,13). LT was defined as an energy expenditure from 1.5 to 3.0 METs (13), and MT was categorized as energy expenditure >3.0 METs (11,13). Due to the very small amount of time our subjects spent in vigorous levels of activity (as defined by >6.0 METs), moderate-level activity and vigorous-level activity were combined into a single category, as per other previous studies using a compositional approach (5,6).
Statistical Methods
Calculation of the cMSy score (3) was performed using the five risk factors found in the National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (14). These risk factors consist of waist circumference, triglycerides, HDL cholesterol, blood pressure, and fasting plasma glucose (14). All risk factors were normalized (log 10), and a blood pressure index was calculated by averaging of systolic and diastolic blood pressure (3). A principal component (PC) analysis with varimax rotation was applied to the five normalized risk factors in order to derive PCs (eigenvalue 1.0) that represented a large fraction of the variance observed. As in previous studies (3), this analysis revealed PCs (eigenvalue 1.0) that represented a large fraction of the variance observed. cMSy was then computed by summing of the individual PC scores (after each one was weighted for by the relative contribution of each to the overall variance) (3).
As per current guidelines, measures of physical activity were normalized prior to analysis by the amount of time per day the accelerometer device was worn (15). Both standard and compositional descriptive statistics were calculated for all activity levels as previously described by partitioning the day into four periods: time spent sleeping, ST, LT, and MT (6,16). The compositional mean is calculated by first calculating the geometric mean for each of the four behaviors and then normalizing the geometric means of all movement behaviors so that they added up to 1,440 min/24 h (17).
Linear regression models were then fitted with an outcome variable of cMSy. For our standard analysis, we chose for our potential predictor variables age, biological sex, ST, LT, and MT. Prior to analysis, density plots for all variables were examined to identify data skewing; any variables that showed data skewing were logarithmically transformed (base 10) prior to both the univariate and multivariate analyses. For our compositional approach, we then used an isometric log-ratio transformation for time spent sedentary (ILR1) to adjust ST for the time spent in the other three periods (ILR1) (6,17); ILR1 expresses the ratio of ST to time spent in the other three (nonsedentary) time periods (6,17). We then fitted an initial model that used age, biological sex, and ILR1 as predictor variables. For model parsimony, we performed a stepwise regression. For each iteration of the stepwise regression model, the least significant predictor variable was removed until only significant correlates remained in the final model. After each predictor was removed from the model, Akaike information criterion was calculated, until the smallest Akaike information criterion was obtained indicating the best fit (18). Variance inflation factors and tolerance values were examined for multicollinearity for each model in order to ensure that the assumptions of the multivariate regression were met; generally a variance inflation factor >10 indicates issues with collinearity (18). Subjects were split into upper and lower halves of cardiometabolic risk by the median cMSy score. The R core software package, version 3.4.2, was used for statistical analysis with a significance level of P < 0.05 (19). All data analysis was done in a blinded fashion, and the mean ± SE was used to express results.
Results
Subject Recruitment
From the Masters ski team, 60 subjects were initially recruited and 55 subjects met our inclusion criteria. One subject did not meet our data collection criteria for successful accelerometer use, leaving a total of 54 subjects. The accelerometers were worn for a mean ± SE of 99 ± 0% of the study time. Data were otherwise complete.
Subject Characteristics
The demographic characteristics of participants are shown in Table 1, as well as biological sex differences.
Table 1.
Characteristics of subjects in the upper and lower halves of cMSy
| All subjects | Women | Men | P | |
|---|---|---|---|---|
| Age (years) | 71.4 ± 0.6 | 70.3 ± 0.7 | 72.6 ± 0.9 | 0.060 |
| Biological sex, n | 30 women, 24 men | 30 women | 24 men | N/A |
| Waist circumference (cm) | 86.6 ± 1.4 | 81.4 ± 1.5 | 92.9 ± 1.8 | <0.001* |
| Triglycerides (<1.7 mmol/L) | 1.00 ± 0.08 | 0.88 ± 0.07 | 1.15 ± 0.16 | 0.103 |
| HDL (>1.0 mmol/L men, >1.3 mmol/L women) | 1.85 ± 0.07 | 2.09 ± 0.07 | 1.57 ± 0.09 | <0.001 |
| Systolic blood pressure (mmHg) | 116 ± 2 | 114 ± 4 | 119 ± 3 | 0.366 |
| Diastolic blood pressure (mmHg) | 68 ± 1 | 67 ± 2 | 69 ± 2 | 0.333 |
| Mean arterial pressure (mmHg) | 84 ± 1 | 83 ± 2 | 86 ± 2 | 0.306 |
| Fasting blood glucose (4.0–7.0 mmol/L) | 5.1 ± 0.1 | 5.0 ± 0.2 | 5.2 ± 0.1 | 0.463 |
| Number of medications | 0.8 ± 0.1 | 0.8 ± 0.1 | 0.8 ± 0.1 | 0.943 |
| Daily ST (h) | 9.4 ± 0.2 | 9.2 ± 0.3 | 9.6 ± 0.3 | 0.378 |
| Daily LT (h) | 3.9 ± 0.2 | 4.3 ± 0.2 | 3.6 ± 0.2 | 0.029* |
| Daily MT (h) | 2.6 ± 0.2 | 2.5 ± 0.3 | 2.7 ± 0.2 | 0.580 |
| Number of daily steps | 10,852 ± 480 | 10,948 ± 604 | 10,734 ± 782 | 0.827 |
| Daily caloric expenditure | 1,633 ± 451 | 1,431 ± 398 | 1,876 ± 557 | <0.001* |
| cMSy (normalized units) | 0.00 ± 0.08 | 0.26 ± 0.07 | −0.31 ± 0.11 | <0.001* |
| Percent wear time, number of wear days | 98.5 ± 0.2, 7 days | 98.6 ± 0.3, 7 days | 98.3 ± 0.4, 7 days | 0.494 |
Data are means ± SEM unless otherwise indicated.
P value <0.05.
The mean ± SE cMSy for all subjects was 0.13 ± 0.08, similar to those found in previous studies of adult subjects (age range from 18 to 75 years old) (4). When we compared subjects who scored in the upper (0.44 ± 0.08 normalized units) versus lower (−0.42 ± 0.08 normalized units) halves of cMSy, subjects in the top half of cMSy tended to have significantly higher waist circumferences (91.5 ± 1.8 vs. 81.6 ± 1.7 cm, P < 0.001), higher triglycerides (1.31 ± 0.14 vs. 0.69 ± 0.04 mmol/L, P < 0.001), and lower HDL levels (1.51 ± 0.07 vs. 2.19 ± 0.06 mmol/L, P < 0.001). Subjects in the upper half of cMSy tended to be men (10 women and 17 men vs. 20 women and 7 men). There was no difference between the upper- and lower-half cMSy subjects with respect to age (72.0 ± 0.9 vs. 70.9 ± 0.8 years, respectively, P = 0.344), systolic blood pressure (118 ± 3 vs. 116 ± 4 mmHg, P = 0.805), diastolic blood pressure (68 ± 2 vs. 67 ± 2 mmHg, P = 0.807), mean arterial pressure (85 ± 2 vs. 84 ± 2 mmHg, P = 0.705), fasting blood glucose (5.2 ± 0.1 vs. 5.1 ± 0.2 mmol/L, P = 0.734), and number of medications (0.7 ± 0.1 vs. 0.8 ± 0.1, P = 0.756).
Activity Levels
Standard Means
Subjects had a mean ST of 9.7 ± 0.2 h per day (defined as sedentary behavior when not in the supine position). Study participants were quite active, performing light activities an average of 3.9 ± 0.2 h per day and moderate/vigorous activity an average of 2.6 ± 0.2 h per day. Overall, our subjects spent at least an average of 6.5 ± 0.2 h per day not engaged in sedentary behavior (not in the supine position). Subjects at higher cardiometabolic risk (upper half of cMSy) had significantly lower MT (2.2 ± 0.2 vs. 2.9 ± 0.3 h, P = 0.041), higher ST (10.1 ± 0.3 vs. 8.7 ± 0.3 h, P = 0.002), and lower step number (9,891 ± 672 vs. 11,777 ± 648 steps per day, P = 0.048) than those at lower cardiometabolic risk (lower half of cMSy). There was also a trend for subjects at lower cardiometabolic risk (lower half of cMSy) to expend more daily calories during physical activity (1,582 ± 639 vs. 1,737 ± 636, P = 0.091). There was no significant difference in LT (3.7 ± 636 vs. 4.1 ± 0.2 h, P = 0.157) or in the accelerometer wear time (98.7 ± 0.2 vs. 98.3 ± 0.2%, P = 0.425) between upper and lower cMSy subjects (Table 1).
Compositional Means
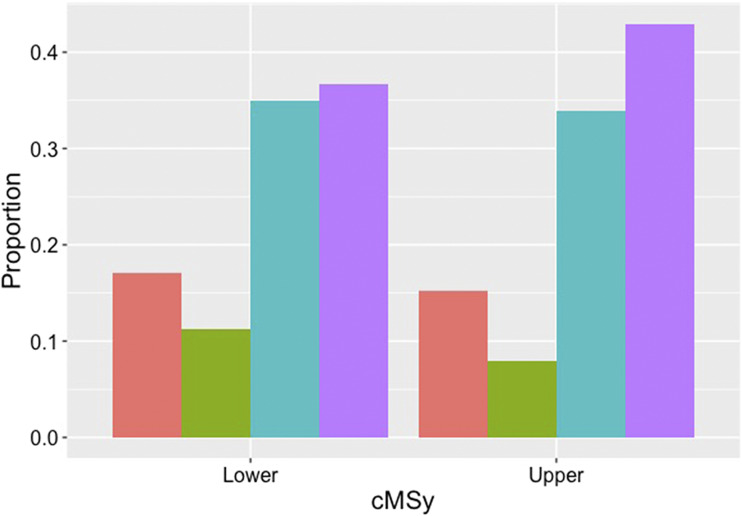
When a compositional approach was used, our subjects spent 39.8% (9.6 h) of each day sedentary, 16.2% (3.9 h) of each day engaged in light activities, 9.5% (2.3 h) of the day engaged in moderate/vigorous activity, and 34.5% (8.3 h) sleeping. With respect to our compositional analysis, as shown in Fig. 1, subjects in the top half of cMSy spent more time sedentary, resulting in a reduction in both LT and MT as compared with those subjects at lower cardiometabolic risk.
Figure 1.
Compositional activity analysis: composition of the day spent in light activity (red), moderate/vigorous activity (green), sleep (blue), and sedentary behavior (purple) in the upper and lower groups of cardiometabolic risk (cMSy).
Univariate Analysis
Of all our continuous predictor variables, only age (r = 0.283 ± 0.228, P = 0.038), ST (r = 0.368 ± 0.213, P = 0.007), and ILR1 (r = 0.399 ± 0.205, P = 0.003) showed significant associations with cMSy. LT (r = −0.225 ± 0.242, P = 0.106) and MT (r = −0.264 ± 0.235, P = 0.056) both showed a trend toward a negative association with cMSy, but they did not reach statistical significance.
Multivariable Analysis
Our initial multivariable regression model contained our logistic predictor variable (biological sex) and our continuous predictor variables (age, ST, LT, and MT) that explained 39% of the variance in cMSy (Table 2). In our final parsimonious model, the only remaining significant predictor variables were biological sex and ST; female sex and ST demonstrated a positive association with cMSy (Table 2).
Table 2.
Multivariable analysis (n = 54)
| R2 | Standardized β | P | |
|---|---|---|---|
| cMSy, model 1, F(5,48) = 6.074 | 0.39 | <0.001* | |
| Age (years) | 0.084 (0.123) | 0.499 | |
| Biological sex (female) | −0.945 (0.249) | <0.001* | |
| ST (min per day) | 0.113 (0.207) | 0.587 | |
| LT (min per day) | −0.095 (0.179) | 0.597 | |
| MT (min per day) | −0.245 (0.194) | 0.213 | |
| cMSy, model 1, MEM F(2,51) = 14.050 | 0.36 | <0.001* | |
| Biological sex (female) | −0.959 (0.229) | <0.001* | |
| ST (min per day) | 0.312 (0.115) | 0.009* | |
| cMSy, model 2, F(3,50) = 11.16 | 0.41 | <0.001* | |
| Age (years) | 0.078 (0.117) | 0.508 | |
| Biological sex (female) | −0.941 (0.227) | <0.001* | |
| ILR1 (proportion) | 0.350 (0.114) | 0.003* | |
| cMSy, model 2, MEM F(2,51) = 16.7 | 0.40 | <0.001* | |
| Biological sex (female) | −0.979 (0.218) | <0.001* | |
| ILR1 (proportion) | 0.368 (0.110) | 0.002* |
Data are results of multivariable analysis models of cMSy. Model 1 uses standard means of activity levels as predictors, while model 2 uses compositional means. Standard deviations are in parentheses. F, F-statistic; MEM, minimal effective model; R2, coefficient of determination; β, β-coefficient.
P value <0.05.
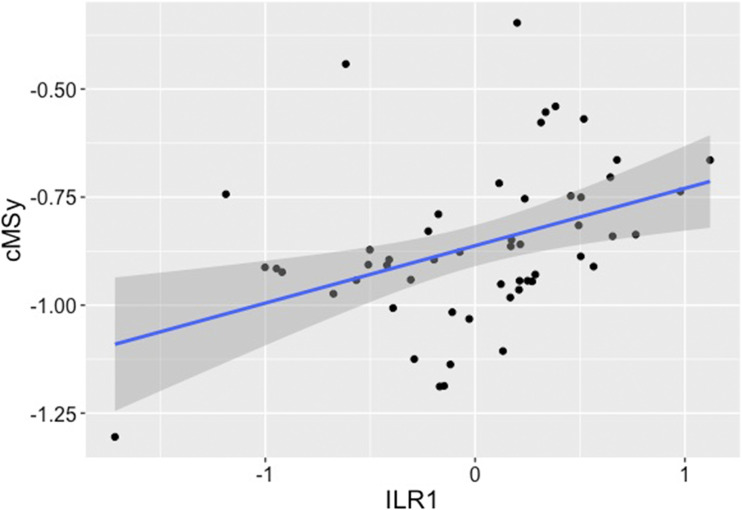
With use of a compositional approach to our predictor variables, our initial second model contained a logistic predictor variable (biological sex) and continuous predictor variables (age and ILR1) that explained 41% of the variance in cMSy (Table 2). In our final parsimonious model, the remaining significant predictor variables were biological sex and ILR1; female sex and proportion of time spent sedentary demonstrated a positive association with cMSy (Table 2 and Fig. 2).
Figure 2.
Association between cMSy and ILR1; ILR1 is a measure of the time spent sedentary as compared with all other nonsedentary behaviors. In our final model, the only significant predictor variables for ILR1 were biological sex (mean ± SE standardized β 0.979 ± 0.218, P < 0.001) and ILR1 (standardized β 0.367 ± 0.110, P = 0.002).
Conclusions
Principal Findings
Our subject population was an extremely active group, exercising 9.5% (2.3 h) of the day, vastly exceeding current physical activity guidelines (20). Despite very high levels of both light and moderate/vigorous activity, this subject pool engaged in sedentary behavior for 39.8% of the day (9.6 h). Although there are no universally recognized cutoffs for ST, ST >9 h per day has been shown to be at a level that increases all-cause mortality (21). Increased ILR1 per day showed a strong association with cMSy, even with correction for age and biological sex. In fact, subjects in the upper half of cMSy were sedentary for ∼1.5 more hours per day compared with subjects in the lower half (see Fig.1). This indicates that even in a population reaching “ceiling levels” of physical activity in an older population, time spent sedentary is still strongly associated with an increase in cardiometabolic risk as measured by cMSy. As shown in Fig. 1, those subjects at higher levels of cMSy had shifted time away from light activity, moderate/vigorous activity, and sleep in order to be sedentary for a larger proportion of the day.
Recent work focusing on sedentary behaviors (such as sitting watching television) has established that time spent sitting is an independent cardiometabolic risk factor (22) that is associated with increased mortality (23) in adults. There is a well-established association in younger subjects between metabolic syndrome and higher levels of ST (24), and prospective studies have shown that increased ST increases the risks for developing metabolic syndrome (25). A recent systematic review of sedentary behavior found that few studies have investigated sedentary behavior in older adults, and only three studies objectively measured ST using accelerometers to study associations with clinical parameters (26). To our knowledge, this is the first study to use objective measures of activity and demonstrate a positive association between ST and a continuous measure of cardiometabolic risk in an extremely active older population. Despite the fact that our population had high levels of physical activity, they spent a large amount of time sedentary (∼9.5 h per day), similar to the levels of ST seen in inactive older adults (27). These findings demonstrate that even a highly active population can have issues with excessive sitting and that this might be posing a significant cardiometabolic risk.
A previous study examining the relationship between cMSy and self-reported ST (4) showed a strong association in middle-aged women. Our results confirm these findings, by objective measurement of activity by accelerometer, and extend these findings to the active, older adult population. Previous work by Greer et al. (28) has also demonstrated in a young population that an increase in self-reported time spent sedentary predicted a much higher risk of developing metabolic syndrome, suggesting that ST is a modifiable determinant of increased cardiometabolic risk. We did not show (in our final model) any relationship between higher levels of LT/MT and cMSy, which differs from results of work in younger populations (4). Wijndaele et al. (4) showed a negative association between physical activity and cMSy; our study did not show such an association, likely due to the fact that we had already selected for highly active subjects that did not show much variability in either LT or MT.
Potential Mechanisms
The results of this study showed that subjects in the upper half of cardiometabolic risk (high cMSy, indicating higher waist circumferences, higher triglycerides, and lower HDL levels) were more sedentary. Previous bed rest studies in young athletes (29) have shown that increased time spent sedentary results in a reduction in skeletal muscle GLUT protein levels (GLUT-4) and a consequent decrease in insulin sensitivity (which manifests clinically as increased waist circumference) (30). In addition, muscle lipoprotein lipase (LPL) activity has been shown to be very sensitive to inactivity, a phenomenon that is prevented by even the minute contractile activity seen with standing (31). LPL is the rate-limiting enzyme that hydrolyzes circulating triglyceride-rich lipoproteins such as VLDLs and chylomicrons; reduced triglyceride hydrolysis results in an increase in plasma triglyceride levels, as well as a reduction in HDL cholesterol levels (32).
Previous investigations of older lifelong athletes have demonstrated that, along with higher aerobic capacity, they have higher levels of skeletal intramyocellular lipids (33) and higher mitochondrial content (34). Lower mitochondrial content is associated with increased insulin resistance that is not considered to be due to age per se but instead shows a tight relationship with aerobic capacity (35) (as measured by maximal oxygen uptake, one of the strongest predictors of mortality in older adults [36]). Although our subjects were obviously extremely active, ST was still associated with higher cMSy, possibly through an impact on maximal oxygen uptake, although this was not measured in our study. Subjects with higher cMSy (Fig. 1) had clearly shifted time away from physical activity to increase ST, which might have had an impact on aerobic fitness.
Clinical Implications
There is increasing consensus supporting a continuous score instead of a binary definition of metabolic syndrome. Not only is it well established that dichotomizing continuous outcome variables reduces statistical power, but also other work has shown that both diabetes risk and cardiovascular disease risk increase progressively with an increasing number of metabolic syndrome risk factors (37). In fact, an increase in 1 unit of cMSy (approximately the difference in the mean cMSy between our upper metabolic risk and lower metabolic risk groups) was found prospectively to have a harm ratio of 1.56 for cardiovascular events when subjects were followed prospectively (38).
The benefits of exercise with respect to successful aging is well established in older adults (39). Our study shows, however, that even older adults that meet current weekly physical activity recommendations on a daily basis still spend a significant quantity (>9 h) of the day completely sedentary. Some of these findings might merely reflect the fact that older athletes require longer recovery times than older adults who are active at lower intensity levels. Subjects in the upper half of ST spent ∼1.5 more hours per day engaged in sedentary behavior, and this was associated with a higher cMSy score due to higher waist circumferences, higher triglycerides, and lower HDL levels. These findings suggest that even very active older adults should have a further assessment of sedentary behaviors and attempts made to reduce time spent sitting.
Limitations and Future Research
The cross-sectional nature of our study limits our ability to infer causality. Any potential mechanisms linking ST to increases in the components that make up cMSy are purely speculative and require further study. The SenseWear device has some limitations compared with other devices (such as activePAL); specifically, the SenseWear device underestimates energy expenditure during exercise at high-intensity levels (40). In addition, we did not have any measures of maximal oxygen uptake in our subjects. The SenseWear device also has been shown to underestimate the amount of time spent at a vigorous activity level (for the purposes of our compositional analysis, time spent in moderate and vigorous activity was combined into a single variable, MT). Our study did not take into account any dietary/energy intake measures. More prospective studies need to be completed for determination of whether reducing sedentary behaviors will alter the development of metabolic syndrome in older adults or improve cardiometabolic risks in this population. In addition, the population studied was much more active than the broader older adult populations, so our findings might not be generalizable to all older adults.
Main Conclusions
Even in an extremely active older adult population, increased time spent sedentary was associated with an increase in cardiometabolic risk as measured by cMSy.
Article Information
Funding. This work was supported by the Allan M. McGavin Foundation and the Canadian Diabetes Association.
Duality of Interest. No potential conflicts of interest relevant to this article were reported.
Author Contributions. K.M. collected the data, analyzed the data, and wrote the manuscript. B.F. collected the data and managed the database. J.C. collected the data and edited the manuscript. K.M.M. is the guarantor of this work and, as such, had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Prior Presentation. Parts of this study were presented in abstract form at the 80th Scientific Sessions of the American Diabetes Association, 12–16 June 2020.
Footnotes
Clinical trial reg. no. NCT02088827, clinicaltrials.gov
See accompanying article, p. 17.
This article is featured in a podcast available at https://www.diabetesjournals.org/content/diabetes-core-update-podcasts.
References
- 1.Brug J, Chinapaw M. Determinants of engaging in sedentary behavior across the lifespan; lessons learned from two systematic reviews conducted within DEDIPAC. Int J Behav Nutr Phys Act 2015;12:134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lakka H-M, Laaksonen DE, Lakka TA, et al. . The metabolic syndrome and total and cardiovascular disease mortality in middle-aged men. JAMA 2002;288:2709–2716 [DOI] [PubMed] [Google Scholar]
- 3.Wijndaele K, Beunen G, Duvigneaud N, et al. . A continuous metabolic syndrome risk score: utility for epidemiological analyses. Diabetes Care 2006;29:2329. [DOI] [PubMed] [Google Scholar]
- 4.Wijndaele K, Duvigneaud N, Matton L, et al. . Sedentary behaviour, physical activity and a continuous metabolic syndrome risk score in adults. Eur J Clin Nutr 2009;63:421–429 [DOI] [PubMed] [Google Scholar]
- 5.Del Pozo Cruz B, Alfonso-Rosa RM, McGregor D, Chastin SF, Palarea-Albaladejo J, Del Pozo Cruz J. Sedentary behaviour is associated with depression symptoms: compositional data analysis from a representative sample of 3233 US adults and older adults assessed with accelerometers. J Affect Disord 2020;265:59–62 [DOI] [PubMed] [Google Scholar]
- 6.Chastin SFM, Palarea-Albaladejo J, Dontje ML, Skelton DA. Combined effects of time spent in physical activity, sedentary behaviors and sleep on obesity and cardio-metabolic health markers: a novel compositional data analysis approach. PLoS One 2015;10:e0139984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kozey-Keadle S, Staudenmayer J, Libertine A, et al. . Changes in sedentary time and physical activity in response to an exercise training and/or lifestyle intervention. J Phys Act Health 2014;11:1324–1333 [DOI] [PubMed] [Google Scholar]
- 8.Chase JM, Lockhart CK, Ashe MC, Madden KM. Accelerometer-based measures of sedentary behavior and cardio-metabolic risk in active older adults. Clin Invest Med 2014;37:E108–E116 [DOI] [PubMed] [Google Scholar]
- 9.Alberti KGMM, Zimmet P, Shaw J; IDF Epidemiology Task Force Consensus Group . The metabolic syndrome--a new worldwide definition. Lancet 2005;366:1059–1062 [DOI] [PubMed] [Google Scholar]
- 10.Higgins PB, Comuzzie AG. Measures of waist circumference. In Handbook of Anthropometry: Physical Measures of Human Form in Health and Disease. Preedy VR, Ed. New York, NY, Springer New York, 2012, pp. 881–891 [Google Scholar]
- 11.Jakicic JM, Marcus M, Gallagher KI, et al. . Evaluation of the SenseWear Pro Armband to assess energy expenditure during exercise. Med Sci Sports Exerc 2004;36:897–904 [DOI] [PubMed] [Google Scholar]
- 12.Roane BM, Van Reen E, Hart CN, Wing R, Carskadon MA. Estimating sleep from multisensory armband measurements: validity and reliability in teens. J Sleep Res 2015;24:714–721 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sedentary Behaviour Research Network Letter to the editor: standardized use of the terms “sedentary” and “sedentary behaviours”. Appl Physiol Nutr Metab 2012;37:540–542 [DOI] [PubMed] [Google Scholar]
- 14.Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults Executive summary of the Third Report of the National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III). JAMA 2001;285:2486–2497 [DOI] [PubMed] [Google Scholar]
- 15.Migueles JH, Cadenas-Sanchez C, Ekelund U, et al. . Accelerometer data collection and processing criteria to assess physical activity and other outcomes: a systematic review and practical considerations. Sports Med 2017;47:1821–1845 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.del Pozo Cruz B, McGregor DE, del Pozo Cruz J, et al. . Integrating sleep, physical activity, and diet quality to estimate all-cause mortality risk: a combined compositional clustering and survival analysis of the NHANES 2005-2006 cycle. Am J Epidemiol 2020;189:1057–1064 [DOI] [PubMed] [Google Scholar]
- 17.Gupta N, Mathiassen SE, Mateu-Figueras G, et al. . A comparison of standard and compositional data analysis in studies addressing group differences in sedentary behavior and physical activity. Int J Behav Nutr Phys Act 2018;15:53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chatterjee S, Hadi AS. Regression Analysis by Example: Wiley Series in Probability and Statistics. 4th ed Hoboken, NJ, Wiley-Interscience; 2006, pp. 375 [Google Scholar]
- 19.R Core Team R: a language and environment for statistical computing. Vienna, Austria, R Foundation for Statistical Computing, 2019. Accessed 22 September 2020. Available from https://mirror.rcg.sfu.ca/mirror/CRAN/ [Google Scholar]
- 20.Tremblay MS, Warburton DE, Janssen I, et al. . New Canadian physical activity guidelines. Appl Physiol Nutr Metab 2011;36:36–46, 47–58 [DOI] [PubMed] [Google Scholar]
- 21.Ku P-W, Steptoe A, Liao Y, Hsueh M-C, Chen L-J. A cut-off of daily sedentary time and all-cause mortality in adults: a meta-regression analysis involving more than 1 million participants. BMC Med 2018;16:74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Healy GN, Matthews CE, Dunstan DW, Winkler EA, Owen N. Sedentary time and cardio-metabolic biomarkers in US adults: NHANES 2003-06. Eur Heart J 2011;32:590–597 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.de Rezende LFM, Rodrigues Lopes M, Rey-López JP, Matsudo VKR, Luiz OdoC. Sedentary behavior and health outcomes: an overview of systematic reviews. PLoS One 2014;9:e105620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bankoski A, Harris TB, McClain JJ, et al. . Sedentary activity associated with metabolic syndrome independent of physical activity. Diabetes Care 2011;34:497–503 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Edwardson CL, Gorely T, Davies MJ, et al. . Association of sedentary behaviour with metabolic syndrome: a meta-analysis. PLoS One 2012;7:e34916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chastin SFM, Buck C, Freiberger E, et al.; DEDIPAC consortium . Systematic literature review of determinants of sedentary behaviour in older adults: a DEDIPAC study. Int J Behav Nutr Phys Act 2015;12:127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Shiroma EJ, Freedson PS, Trost SG, Lee I-M. Patterns of accelerometer-assessed sedentary behavior in older women. JAMA 2013;310:2562–2563 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Greer AE, Sui X, Maslow AL, Greer BK, Blair SN. The effects of sedentary behavior on metabolic syndrome independent of physical activity and cardiorespiratory fitness. J Phys Act Health 2015;12:68–73 [DOI] [PubMed] [Google Scholar]
- 29.Vukovich MD, Arciero PJ, Kohrt WM, Racette SB, Hansen PA, Holloszy JO. Changes in insulin action and GLUT-4 with 6 days of inactivity in endurance runners. J Appl Physiol (1985) 1996;80:240–244 [DOI] [PubMed] [Google Scholar]
- 30.Lee S, Bacha F, Gungor N, Arslanian SA. Waist circumference is an independent predictor of insulin resistance in black and white youths. J Pediatr 2006;148:188–194 [DOI] [PubMed] [Google Scholar]
- 31.Bey L, Hamilton MT. Suppression of skeletal muscle lipoprotein lipase activity during physical inactivity: a molecular reason to maintain daily low-intensity activity. J Physiol 2003;551:673–682 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Cheung MC, Sibley SD, Palmer JP, Oram JF, Brunzell JD. Lipoprotein lipase and hepatic lipase: their relationship with HDL subspecies Lp(A-I) and Lp(A-I,A-II). J Lipid Res 2003;44:1552–1558 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Dubé JJ, Broskey NT, Despines AA, et al. . Muscle characteristics and substrate energetics in lifelong endurance athletes. Med Sci Sports Exerc 2016;48:472–480 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Arribat Y, Broskey NT, Greggio C, et al. . Distinct patterns of skeletal muscle mitochondria fusion, fission and mitophagy upon duration of exercise training. Acta Physiol (Oxf) 2019;225:e13179. [DOI] [PubMed] [Google Scholar]
- 35.Broskey NT, Greggio C, Boss A, et al. . Skeletal muscle mitochondria in the elderly: effects of physical fitness and exercise training. J Clin Endocrinol Metab 2014;99:1852–1861 [DOI] [PubMed] [Google Scholar]
- 36.Myers J, Prakash M, Froelicher V, Do D, Partington S, Atwood JE. Exercise capacity and mortality among men referred for exercise testing. N Engl J Med 2002;346:793–801 [DOI] [PubMed] [Google Scholar]
- 37.Klein BEK, Klein R, Lee KE. Components of the metabolic syndrome and risk of cardiovascular disease and diabetes in Beaver Dam. Diabetes Care 2002;25:1790–1794 [DOI] [PubMed] [Google Scholar]
- 38.Agarwal S, Jacobs DR Jr., Vaidya D, et al. . Metabolic syndrome derived from principal component analysis and incident cardiovascular events: the Multi Ethnic Study of Atherosclerosis (MESA) and Health, Aging, and Body Composition (Health ABC). Cardiol Res Pract 2012;2012:919425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Chodzko-Zajko W, Schwingel A, Park CH. Successful aging: the role of physical activity. Am J Lifestyle Med 2009;3:20–28 [Google Scholar]
- 40.Powell C, Carson BP, Dowd KP, Donnelly AE. The accuracy of the SenseWear Pro3 and the activPAL3 Micro devices for measurement of energy expenditure. Physiol Meas 2016;37:1715–1727 [DOI] [PubMed] [Google Scholar]