Abstract
OBJECTIVE
To assess the diagnostic utility of corneal confocal microscopy (CCM) for diabetic peripheral neuropathy (DPN) and the risk factors for corneal nerve loss.
RESEARCH DESIGN AND METHODS
A total of 490 participants, including 72 healthy control subjects, 149 with type 1 diabetes, and 269 with type 2 diabetes, underwent detailed assessment of peripheral neuropathy and CCM in relation to risk factors.
RESULTS
Corneal nerve fiber density (CNFD) (P < 0.0001 and P < 0.0001), corneal nerve fiber branch density (CNBD) (P < 0.0001 and P < 0.0001), and corneal nerve fiber length (CNFL) (P < 0.0001 and P = 0.02) were significantly lower in patients with type 1 and type 2 diabetes compared with control subjects. CNFD (P < 0.0001), CNBD (P < 0.0001), and CNFL (P < 0.0001) were lower in type 1 diabetes compared with type 2 diabetes. Receiver operating characteristic curve analysis for the diagnosis of DPN demonstrated a good area under the curve for CNFD of 0.81, CNBD of 0.74, and CNFL of 0.73. Multivariable regression analysis showed a significant association among reduced CNFL with age (β = −0.27, P = 0.007), HbA1c (β = −1.1; P = 0.01), and weight (β = −0.14; P = 0.03) in patients with type 2 diabetes and with duration of diabetes (β = −0.13; P = 0.02), LDL cholesterol (β = 1.8, P = 0.04), and triglycerides (β = −2.87; P = 0.009) in patients with type 1 diabetes.
CONCLUSIONS
CCM identifies more severe corneal nerve loss in patients with type 1 diabetes compared with type 2 diabetes and shows good diagnostic accuracy for DPN. Furthermore, the risk factors for a reduction in corneal nerve fiber length differ between type 1 and type 2 diabetes.
Introduction
Diabetic peripheral neuropathy (DPN) is the most frequent long-term complication of diabetes (1). The diagnosis of DPN relies on abnormal symptoms and signs and electrophysiology. However, these tests do not reliably detect early damage to the small nerve fibers. Quantifying intraepidermal nerve fiber density is the gold standard for the assessment of small fiber damage but is an invasive procedure (2,3). Corneal confocal microscopy (CCM) is a rapid, noninvasive, ophthalmic imaging tool that is comparable to skin punch biopsy in the diagnosis of DPN (4) and allows objective assessment of early corneal nerve degeneration (5) and regeneration after an improvement in risk factors (6) and simultaneous pancreas and kidney transplantation (7). Corneal nerve fiber length (CNFL) is a valid predictor for incident DPN in patients with type 1 diabetes (8), and corneal nerve fiber density (CNFD) has the highest diagnostic performance to identify DPN in patients with type 1 and type 2 diabetes (4,9).
Several mechanisms may be involved in the development of DPN, including hyperglycemia-driven abnormalities of the polyol pathway, advanced glycation end products, and dyslipidemia (10). Furthermore, high BMI, hypertension, cholesterol, and triglycerides levels are associated with incident DPN in type 1 diabetes (11), and age, BMI, waist circumference, LDL cholesterol, and HDL cholesterol are associated with incident DPN in type 2 diabetes (12).
Small cohort studies have reported an association between clinical and metabolic variables and CCM measures (6,13–16), particularly with HbA1c (17), duration of diabetes (18), and HDL cholesterol (16) in both type 1 and type 2 diabetes (19) and with age, duration of diabetes (14,15), blood pressure, and HbA1c (20) in type 1 diabetes. However, in patients with type 2 diabetes, there was no association between corneal nerve loss and HbA1c (21), but there was an association with total and LDL cholesterol (13). Subjects with impaired glucose tolerance also develop corneal nerve loss (22), indicating the role of additional risk factors beyond elevated blood glucose. Corneal nerve loss has been associated with higher triglycerides in patients with idiopathic small fiber neuropathy (23).
We have undertaken CCM to assess its diagnostic utility, the relationship with other measures of neuropathy, and the risk factors for corneal nerve fiber loss in patients with type 1 and type 2 diabetes.
Research Design and Methods
Study Subjects
This study assessed 490 participants, including 72 healthy control subjects, 149 with type 1 diabetes, and 269 with type 2 diabetes, who took part in the Longitudinal Assessment of Neuropathy in Diabetes Using Novel Ophthalmic Markers (LANDMark) (16), Probing the Role of Sodium Channels in Painful Neuropathies (PROPANE) (24), and DEAMON (25) studies between 2007 and 2017. All participants underwent detailed assessment of neuropathy and CCM. Patients with a history of neuropathy (other than diabetes), current or active diabetic foot ulceration, chronic renal and liver failure, malignancy, systemic disease, deficiency of B12 or folate, previous corneal trauma, corneal disease, corneal surgery, and a history of or current contact lens wear were excluded from the study. The research adhered to the tenets of the Declaration of Helsinki and was approved by the Greater Manchester Research Ethics Committees (Manchester, U.K.). Written consent forms were obtained from all participants prior to their participation.
Clinical and Peripheral Neuropathy Assessment
All participants underwent an assessment of BMI, blood pressure, HbA1c, and lipid profile. The neuropathy symptom profile (NSP) was administered to assess symptoms of DPN, and the modified neuropathy disability score (NDS) was used to assess pinprick, vibration perception, temperature sensation, and ankle reflexes. The ANX 3.0 autonomic nervous system monitoring device (ANSAR Medical Technologies, Inc., Media, PA) was used to measure the heart rate response to deep breathing over two eight-cycle breathing series separated by a 5-min period of normal breathing and was reported as deep-breathing heart rate variability (DB-HRV) (9).
Vibration perception threshold (VPT) was evaluated using a Horwell Neurothesiometer (Scientific Laboratory Supplies Ltd., Nottingham, U.K.). The probe of the device was placed on the tip of the big toe, and the individual was asked to report if the vibration was felt as the intensity was gradually increased from 0 to 50 V. Cold perception threshold (CPT), warm perception threshold (WPT), and cold- and warm-induced pain (CIP and WIP) were assessed using a TSA 2 NeuroSensory Analyzer (Medoc, Ltd., Ramat Yishay, Israel) on the dorsum of the nondominant foot in the S1 dermatome. The temperature was changed using a staircase algorithm, and the individual was asked whether the warm or cold sensation and WIP or CIP were felt. Nerve conduction studies were undertaken using the Dantec Keypoint system (Dantec Dynamics Ltd., Bristol, U.K.), and a thermistor (temperature regulator; DISA Industries, Taastrup, Denmark) maintained the limb temperature between 32 and 35°C. A consultant neurophysiologist assessed sural nerve amplitude (SNAP), sural nerve conduction velocity (SNCV), peroneal motor nerve amplitude (PMNA), and peroneal motor nerve conduction velocity (PMNCV).
DPN was defined according to the Toronto consensus (3), which requires the presence of symptoms (abnormal NSP) or signs of neuropathy (NDS >2) and abnormal peroneal nerve conduction velocity (PMNCV <40 m/s).
Skin Biopsy
Three-millimeter skin punch biopsies were taken from the dorsum of the foot, 2 cm above the second metatarsal head under local anesthetic (1% lidocaine) in control subjects (n = 15) and patients with type 1 (n = 67) and type 2 diabetes (n = 50). The biopsy was fixed in 4% paraformaldehyde, cryoprotected in graded solutions of sucrose, frozen, and cut on a cryomicrotome (HM 450; Microm International GmbH, Walldorf, Germany). The 50-μm sections were immunostained using anti-human PGB 9.5 antibody (Abcam, Cambridge, U.K.), and nerve fibers were demonstrated using SG chromogen (Vector Laboratories, Peterborough, U.K.). Intraepidermal nerve fiber density (IENFD) was expressed as the number of nerve fibers per millimeter and quantified according to established criteria (4).
CCM
CCM was performed in both eyes using laser scanning CCM (Heidelberg Retina Tomograph 3 Rostock Cornea Module; Heidelberg Engineering, Heidelberg, Germany) to acquire images of the cornea (9), and six images (three per eyes) were selected according to the quality, position, and depth of the central subbasal nerve plexus (26). Manual analysis was undertaken using CCMetrics (The University of Manchester) in a masked and randomized fashion. CNFD (total number of main nerves per square millimeter), corneal nerve fiber branch density (CNBD; total number of branches per square millimeter), and CNFL (total length of main nerves and nerve branches per square millimeter) were quantified.
Statistical Analysis
The analysis was carried out using SPSS (Version 22.0 for windows; IBM Corporation, New York, NY). Data were tested for normality using the Shapiro-Wilk test, visualization of histograms, and Q-Q plots and presented as mean ± SEM. ANOVA with Bonferroni correction was used to compare means among groups. We performed an ANCOVA with least significant difference correction to control for age as a confounder in our neuropathy assessment comparisons among groups. Generalized linear models were used to explore the association between CCM measures and clinical findings. The Pearson correlation coefficient (parametric) or Spearman rank correlation coefficient (nonparametric) was used to determine the correlation between variables.
Receiver operating characteristic (ROC) curve analysis was performed for corneal nerve parameters. The area under the curve (AUC) for each parameter to diagnose DPN was determined by selecting the cutoff point for a concurrently optimized sensitivity and specificity at a ratio of 1:1. As the age of the control participants and patients with type 1 and type 2 diabetes differed, we also compared 199 patients with type 2 diabetes with 43 age-matched control subjects (Supplementary Table 1).
Results
Clinical Assessment
Age differed significantly between control subjects and patients with type 1 and type 2 diabetes (47.04 ± 1.61 vs. 48.81 ± 1.37 and 62.59 ± 0.59; P < 0.0001). There was a significant difference in BMI (P < 0.0001), waist circumference (P < 0.0001), HbA1c (P = 0.002), estimated glomerular filtration ratio (P < 0.0001), total cholesterol (P < 0.0001), HDL cholesterol (P < 0.0001), triglycerides (P < 0.0001), and LDL cholesterol (P < 0.0001), but no difference in smoking (P = 0.2) or alcohol consumption (P = 0.7) between groups (Table 1).
Table 1.
Clinical, demographic, and laboratory results in control subjects and patients with type 1 and type 2 diabetes
| Control subjects (n = 72) | Type 2 diabetes (n = 269) | Type 1 diabetes (n = 149) | ANOVA P value | |
|---|---|---|---|---|
| Age (years) | 47.04 ± 1.61 | 62.59 ± 0.59* | 48.81 ± 1.37$ | <0.0001 |
| Ethnicity (European) (%) | 63.9 | 63.2 | 94.00 | <0.0001 |
| Sex (female) (%) | 54.2 | 33.8 | 46.30 | 0.005 |
| Duration of diabetes (years) | NA | 11.03 ± 0.47 | 30.21 ± 1.44$ | <0.0001 |
| BMI (kg/m2) | 26.65 ± 0.58 | 32.08 ± 0.42* | 27.04 ± 0.41$ | <0.0001 |
| Waist circumference (cm) | 89.57 ± 1.72 | 107.55 ± 1.09* | 91.07 ± 1.46$ | <0.0001 |
| Smoking (number/day) | 0.42 ± 0.21 | 0.84 ± 0.33 | 1.38 ± 0.38 | 0.2 |
| Alcohol (units/week) | 4.27 ± 0.84 | 5.24 ± 0.71 | 4.97 ± 0.63 | 0.7 |
| HbA1c, % (mmol/mol) | 5.6 ± 0.05 (37.76 ± 0.42) | 7.8 ± 0.09 (62.03 ± 3.9)* | 8.1 ± 0.12 (65.28 ± 1.34)* | 0.002 |
| eGFR (mL/min/1.73 m2) | 84.64 ± 1.05 | 72.81 ± 1.53* | 77.22 ± 1.69* | <0.0001 |
| Total cholesterol (mmol/L) | 5.12 ± 0.11 | 4.04 ± 0.06* | 4.33 ± 0.07*$ | <0.0001 |
| HDL cholesterol (mmol/L) | 1.55 ± 0.05 | 1.23 ± 0.03* | 1.68 ± 0.04$ | <0.0001 |
| Triglycerides (mmol/L) | 1.48 ± 0.09 | 1.96 ± 0.07* | 1.18 ± 0.07$ | <0.0001 |
| LDL cholesterol (mmol/L) | 2.95 ± 0.09 | 1.97 ± 0.05* | 2.13 ± 0.06* | <0.0001 |
Data are mean ± SEM.
eGFR, estimated glomerular filtration rate; NA, not applicable.
Significant difference compared with control subjects.
$Significant difference compared with type 2 diabetes.
Peripheral Neuropathy Assessment
NDS was significantly higher in type 1 (4.29 ± 0.22; P < 0.0001) and type 2 diabetes (3.13 ± 0.17; P < 0.0001) compared with control subjects (1.37 ± 0.32) and was significantly higher in type 1 compared with type 2 diabetes (P < 0.0001). NSP was significantly higher in type 1 (5.3 ± 0.4; P < 0.0001) and type 2 diabetes (4.9 ± 0.5; P < 0.0001) compared with control subjects (0.8 ± 0.6) but did not differ between type 1 and type 2 diabetes (P = 0.8). DB-HRV was significantly lower in patients with type 1 (21.75 ± 1.03; P = 0.03) and type 2 diabetes (20.56 ± 1.05; P = 0.01) compared with healthy control subjects (25.96 ± 0.75) but did not differ between patients with type 1 and type 2 diabetes (P = 0.4). VPT was significantly higher in type 1 (18.98 ± 0.8 V; P < 0.0001) and type 2 diabetes (13.77 ± 0.61 V; P = 0.001) compared with control subjects (9.85 ± 1.14 V) and was higher in type 1 compared with type 2 diabetes (P < 0.0001). WPT was significantly higher in type 1 (41.23 ± 0.34°C; P < 0.0001) and type 2 diabetes (40.57 ± 0.25°C; P < 0.0001) compared with control subjects (38.00 ± 0.47°C), with no difference between type 1 and type 2 diabetes. WIP was significantly higher in type 1 (46.82 ± 0.25°C; P < 0.0001) and type 2 diabetes (47.6 ± 0.22°C; P < 0.0001) compared with control subjects (45.22 ± 0.3°C) and was lower in type 1 compared with type 2 diabetes (P = 0.03). CIP was significantly lower in type 1 (7.06 ± 0.75°C; P = 0.001) and type 2 diabetes (5.9 ± 0.66°C; P < 0.0001) compared with control subjects (10.69 ± 0.89°C), with no difference between type 1 and type 2 diabetes (P = 0.2). CPT was significantly lower in type 1 (23.47 ± 0.46°C; P < 0.0001) but not in type 2 diabetes (26.05 ± 0.34°C; P = 0.06) compared with control subjects (27.45 ± 0.6°C) and was significantly lower in type 1 compared with type 2 diabetes (P < 0.0001). IENFD was significantly lower in type 1 diabetes (5.46 ± 0.56/mm; P = 0.002) but not in type 2 diabetes (6.85 ± 0.7/mm; P = 0.1) compared with control subjects (8.6 ± 0.85/mm) and did not differ between type 1 and type 2 diabetes (P = 0.1). SNCV was lower in type 1 (39.26 ± 0.54 m/s; P < 0.0001) and type 2 diabetes (45.60 ± 0.41 m/s; P < 0.0001) compared with control subjects (48.82 ± 0.77 m/s) and was significantly lower in type 1 compared with type 2 diabetes (P < 0.0001). SNAP was lower in type 1 (6.35 ± 0.57 μV; P < 0.0001) and type 2 diabetes (10.86 ± 0.43 μV; P < 0.0001) compared with control subjects (17.16 ± 0.81 μV) and was significantly lower in type 1 compared with type 2 diabetes (P < 0.0001). PMNCV was significantly lower in type 1 (38.22 ± 0.55 m/s; P < 0.0001) and type 2 diabetes (44.07 ± 0.41 m/s; P = 0.002) compared with control subjects (46.89 ± 0.78 m/s) and was significantly lower in type 1 compared with type 2 diabetes (P < 0.0001). PMNA was significantly lower in type 1 diabetes (2.76 ± 0.19 mV; P < 0.0001) but not in type 2 diabetes (3.86 ± 0.14 mV; P = 0.06) compared with control subjects (4.44 ± 0.27 mV) and was significantly lower in type 1 compared with type 2 diabetes (P < 0.0001) (Table 2).
Table 2.
CCM and other measures of neuropathy in control subjects and patients with type 1 and type 2 diabetes
| Parameters | Control subjects (n = 72) | Type 2 diabetes (n = 269) | Type 1 diabetes (n = 149) | ANCOVA P value |
|---|---|---|---|---|
| CNFD (number/mm2) | 34.06 ± 0.95 | 25.98 ± 0.51* | 22.37 ± 0.67*$ | <0.0001 |
| CNBD (number/mm2) | 83.39 ± 4.39 | 63.22 ± 2.36* | 47.13 ± 3.11*$ | <0.0001 |
| CNFL (mm/mm2) | 24.76 ± 0.84 | 22.46 ± 0.45* | 17.84 ± 0.59*$ | <0.0001 |
| IENFD (number/mm) | 8.60 ± 0.85 | 6.85 ± 0.7 | 5.46 ± 0.56* | 0.007 |
| NDS (0–10) | 1.37 ± 0.32 | 3.13 ± 0.17* | 4.29 ± 0.22*$ | <0.0001 |
| NSP (0–38) | 0.8 ± 0.6 | 4.9 ± 0.5* | 5.3 ± 0.4* | <0.0001 |
| DB-HRV (bpm) | 25.96 ± 1.75 | 20.56 ± 1.05* | 21.75 ± 1.03* | 0.04 |
| VPT (V) | 9.85 ± 1.14 | 13.77 ± 0.61* | 18.98 ± 0.8*$ | <0.0001 |
| CPT (°C) | 27.45 ± 0.6 | 26.05 ± 0.34 | 23.47 ± 0.46*$ | <0.0001 |
| WPT (°C) | 38.00 ± 0.47 | 40.57 ± 0.25* | 41.23 ± 0.34* | <0.0001 |
| CIP (°C) | 10.69 ± 0.89 | 5.9 ± 0.66* | 7.06 ± 0.75* | <0.0001 |
| WIP (°C) | 45.22 ± 0.3 | 47.6 ± 0.22* | 46.82 ± 0.25*$ | <0.0001 |
| SNCV (m/s) | 48.82 ± 0.77 | 45.60 ± 0.41* | 39.26 ± 0.54*$ | <0.0001 |
| SNAP (μV) | 17.16 ± 0.81 | 10.86 ± 0.43* | 6.35 ± 0.57*$ | <0.0001 |
| PMNCV (m/s) | 46.89 ± 0.78 | 44.07 ± 0.41* | 38.22 ± 0.55*$ | <0.0001 |
| PMNA (mV) | 4.44 ± 0.27 | 3.86 ± 0.14 | 2.76 ± 0.19*$ | <0.0001 |
Data are mean ± SEM corrected for age using ANCOVA (least significant difference correction). All symbols represent statistically significant differences.
bpm, beats per minute.
Significant difference compared with control subjects.
$Significant difference compared with type 2 diabetes.
CCM
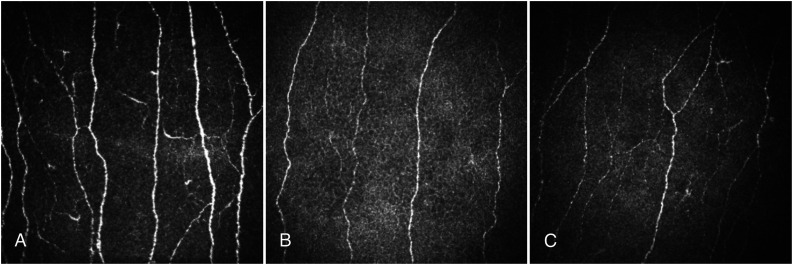
CNFD (22.37 ± 0.67 vs. 34.06 ± 0.95/mm2; P < 0.0001), CNBD (47.13 ± 3.11 vs. 83.39 ± 4.39/mm2; P < 0.0001), and CNFL (17.84 ± 0.59 vs. 24.76 ± 0.84 mm/mm2; P < 0.0001) were significantly lower in type 1 diabetes compared with control subjects. CNFD (25.98 ± 0.51 vs. 34.06 ± 0.95/mm2; P < 0.0001), CNBD (63.22 ± 2.36 vs. 83.39 ± 4.39/mm2; P < 0.0001), and CNFL (22.46 ± 0.45 vs. 24.76 ± 0.84 mm/mm2; P = 0.02) were significantly lower in type 2 diabetes compared with healthy control subjects. CNFD (P < 0.0001), CNBD (P < 0.0001), and CNFL (P < 0.0001) were significantly lower in type 1 compared with type 2 diabetes (Fig. 1 and Table 2).
Figure 1.
CCM images of the central cornea in a healthy control subject (A), an age-matched patient with type 2 diabetes (B), and an age-matched patient with type 1 diabetes (C).
Association Between CNFD and Other Measures of Neuropathy
In patients with type 2 diabetes, CNFD correlated with NDS (r = −0.1; P = 0.04), CPT (r = 0.2; P = 0.007), VPT (r = −0.2; P = 0.002), SNCV (r = 0.1; P = 0.03), SNAP (r = 0.2; P = 0.002), and PMNCV (r = 0.1; P = 0.003), but not IENFD (r = −0.02; P = 0.8), DB-HRV (r = 0.06; P = 0.4), WPT (r = −0.07; P = 0.2), WIP (r = −0.1; P = 0.2), CIP (r = 0.05; P = 0.5), and PMNA (r = 0.1; P = 0.06).
In patients with type 1 diabetes, CNFD correlated with NDS (r = −0.5; P < 0.0001), DB-HRV (r = 0.5; P < 0.0001), WPT (r = −0.4; P < 0.0001), CPT (r = 0.4; P < 0.0001), WIP (r = −0.4; P < 0.0001), CIP (r = 0.4; P < 0.0001), VPT (r = −0.6; P < 0.0001), SNCV (r = 0.5; P < 0.0001), SNAP (r = 0.5; P < 0.0001), PMNCV (r = 0.5; P < 0.0001), PMNA (r = 0.5; P < 0.0001), and IENFD (r = 0.2; P = 0.05).
Diagnostic Utility of CCM
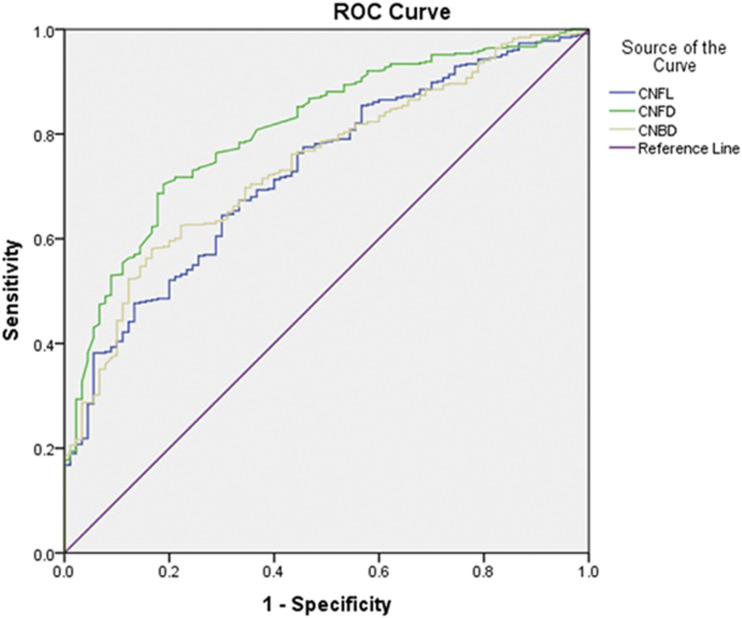
Based on the Toronto criteria, 27.7% of patients were diagnosed with DPN. The ROC curve analysis for CCM in the diagnosis of DPN showed that CNFD had the highest AUC at 0.81 with an optimal cutoff point of 29.40/mm2, sensitivity of 73.5%, and specificity of 74.4%. CNBD had an AUC of 0.74 with an optimal cutoff point of 64.58/mm2, sensitivity of 66.7%, and specificity of 66.7%. CNFL had the lowest AUC of 0.73, with an optimal cutoff point of 24.00 mm/mm2, sensitivity of 66.7%, and specificity of 66.4% (Supplementary Table 2 and Fig. 2).
Figure 2.
ROC curves for CNFL, CNFD, and CNBD.
Multivariable Regression Analysis
In patients with type 1 diabetes, the reduction in CNFL was associated with higher triglycerides (β = −2.87; P = 0.009), LDL cholesterol (β = 1.8; P = 0.04), and duration of diabetes (β = −0.13; P = 0.02). In patients with type 2 diabetes, reduced CNFL was associated with higher age (β = −0.27; P = 0.007), HbA1c (β = −1.1; P = 0.01), and weight (β = −0.14; P = 0.03) (Supplementary Table 3).
Conclusions
CCM has shown a reduction in corneal nerve fibers in small cohorts of patients with DPN (8,27). However, there is an inconsistency in the literature in relation to differences between patients with type 1 and type 2 diabetes and the risk factors associated with corneal nerve loss and DPN (6,14,16,20). In this large study of predominantly Europeans, we show greater corneal nerve loss in patients with type 1 compared with type 2 diabetes. We also demonstrate differences in the risk factors associated with corneal nerve loss in patients with type 1 and type 2 diabetes. This is consistent with data indicating that the natural history and risk factors for DPN may differ between type 1 and type 2 diabetes (28) and that components of the metabolic syndrome may contribute to DPN (29). Ethnicity may also contribute to differences in DPN, as we have previously shown that corneal nerves are more preserved in South Asians compared with Europeans with type 2 diabetes (25).
In type 1 diabetes, we show that corneal nerve loss is associated with the duration of diabetes, triglycerides, and LDL cholesterol. The association between duration of diabetes and corneal nerve loss may be attributed to the longer duration of diabetes and presence of early corneal nerve fiber loss even in children with type 1 diabetes (30). Dehghani et al. (15) also reported that a longer duration of diabetes was associated with reduced CNFD and CNBD in patients with type 1 diabetes. With regard to the relationship between lipids and corneal nerves, fibrate and statin therapy has been associated with a reduced incidence of DPN (31), and increased triglycerides are associated with incident DPN (32) and amputation (33). The Diabetes Control and Complications Trial (DCCT) also demonstrated that elevated triglycerides were a risk factor for the development of DPN in patients with type 1 diabetes (34). A univariate analysis has previously shown that LDL was related to CNFD and total cholesterol was related to CNFD and CNFL in patients with type 2 diabetes (13).
In type 2 diabetes, we show that corneal nerve loss is associated with HbA1c, age, and weight. Studies have shown an association between HbA1c and corneal nerve loss in type 2 diabetes (13). Furthermore, several interventional studies in patients with type 2 diabetes have demonstrated that a reduction in HbA1c was associated with an improvement in corneal nerve morphology (35–37). The association between older age and reduced CNFL agrees with Andersen et al. (13), who also reported an association between older age and reduced CNFD. With regard to weight, a recent study has shown that a reduction in weight in patients with type 2 diabetes was associated with an improvement in corneal nerve morphology (36).
We show that CCM, a marker of small fiber damage, has reasonable diagnostic utility for DPN in patients with type 1 and type 2 diabetes despite using the Toronto criteria, which is large fiber weighted for the diagnosis of DPN. Previously, Petropoulos et al. (27) demonstrated that CNFL had the highest diagnostic utility for DPN with an AUC of 0.75, sensitivity of 0.76, and specificity of 0.65. However, similar to the current study, Alam et al. (9) reported that CNFD had the highest AUC of 0.81 with a sensitivity of 0.77 and specificity of 0.79. In a large cohort of patients with type 1 and type 2 diabetes, Perkins et al. (38) reported comparable diagnostic utility for DPN using automated CNFL with an AUC of 0.77 in type 1 diabetes and 0.71 in type 2 diabetes. Given that the patients with type 2 diabetes were older than those with type 1 diabetes and control subjects, an age-adjusted ANCOVA was performed to compare means between groups, and further analysis was undertaken with an older control group. Furthermore, the patients had relatively well-controlled risk factors for DPN, which may impact on the relative strength of the associations between risk factors and severity of corneal nerve loss.
In conclusion, this study provides robust evidence from a large cohort of patients with type 1 and type 2 diabetes that CCM is a valid biomarker to evaluate neurodegeneration in human diabetic neuropathy. We also show that the severity and risk factors for corneal nerve loss and the relationship between CCM and other measures of neuropathy differ between patients with type 1 and type 2 diabetes. Further studies are required to establish the relationship between CCM and patient-oriented outcomes such as pain, disability, and quality of life. Longitudinal and interventional studies are also required to determine the natural history of corneal nerve fiber loss and the utility of CCM in clinical trials of DPN.
Article Information
Acknowledgments. The authors thank Dr. Mitra Tavakoli, who undertook CCM, and Dr. Hassan Fadavi, who undertook clinical assessment in a proportion of patients, both while working at the University of Manchester.
Funding. The research received funding from the European Union Seventh Framework Programme FP7/2007–2013 (602273), Diabetes UK (RD05/0003048), and JDRF (8-2008-362). This work was also supported by the Manchester Biomedical Research Centre and the Greater Manchester Comprehensive Local Research Network.
The authors alone are responsible for the content and writing of the paper.
Duality of Interest. No potential conflicts of interest relevant to this article were reported.
Author Contributions. All authors were involved in revising the manuscript critically for important intellectual content and for final approval of the version to be published. M.F. researched, analyzed, and interpreted data and wrote the manuscript. A.K. researched data and wrote the manuscript. S.A., I.N.P., G.P., U.A., O.A., A.M., M.J., and C.A. researched data. C.F. performed statistical analysis. G.L., C.G.F, H.S., N.E., and A.J.M.B. reviewed the manuscript. R.A.M. designed the study and reviewed and revised the manuscript. R.A.M. is the guarantor of this work and, as such, had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Footnotes
This article contains supplementary material online at https://doi.org/10.2337/figshare.13020023.
References
- 1.Boulton AJ, Vinik AI, Arezzo JC, et al.; American Diabetes Association . Diabetic neuropathies: a statement by the American Diabetes Association. Diabetes Care 2005;28:956–962 [DOI] [PubMed] [Google Scholar]
- 2.Petropoulos IN, Ponirakis G, Khan A, Almuhannadi H, Gad H, Malik RA. Diagnosing diabetic neuropathy: something old, something new. Diabetes Metab J 2018;42:255–269 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tesfaye S, Boulton AJ, Dyck PJ, et al.; Toronto Diabetic Neuropathy Expert Group . Diabetic neuropathies: update on definitions, diagnostic criteria, estimation of severity, and treatments. Diabetes Care 2010;33:2285–2293 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chen X, Graham J, Dabbah MA, et al. Small nerve fiber quantification in the diagnosis of diabetic sensorimotor polyneuropathy: comparing corneal confocal microscopy with intraepidermal nerve fiber density. Diabetes Care 2015;38:1138–1144 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Malik RA, Kallinikos P, Abbott CA, et al. Corneal confocal microscopy: a non-invasive surrogate of nerve fibre damage and repair in diabetic patients. Diabetologia 2003;46:683–688 [DOI] [PubMed] [Google Scholar]
- 6.Tavakoli M, Kallinikos P, Iqbal A, et al. Corneal confocal microscopy detects improvement in corneal nerve morphology with an improvement in risk factors for diabetic neuropathy. Diabet Med 2011;28:1261–1267 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Azmi S, Jeziorska M, Ferdousi M, et al. Early nerve fibre regeneration in individuals with type 1 diabetes after simultaneous pancreas and kidney transplantation. Diabetologia 2019;62:1478–1487 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pritchard N, Edwards K, Russell AW, Perkins BA, Malik RA, Efron N. Corneal confocal microscopy predicts 4-year incident peripheral neuropathy in type 1 diabetes. Diabetes Care 2015;38:671–675 [DOI] [PubMed] [Google Scholar]
- 9.Alam U, Jeziorska M, Petropoulos IN, et al. Diagnostic utility of corneal confocal microscopy and intra-epidermal nerve fibre density in diabetic neuropathy. PLoS One 2017;12:e0180175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Feldman EL, Nave KA, Jensen TS, Bennett DLH. New horizons in diabetic neuropathy: mechanisms, bioenergetics, and pain. Neuron 2017;93:1296–1313 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tesfaye S, Chaturvedi N, Eaton SE, et al.; EURODIAB Prospective Complications Study Group . Vascular risk factors and diabetic neuropathy. N Engl J Med 2005;352:341–350 [DOI] [PubMed] [Google Scholar]
- 12.Andersen ST, Witte DR, Dalsgaard EM, et al. Risk factors for incident diabetic polyneuropathy in a cohort with screen-detected type 2 diabetes followed for 13 years: ADDITION-Denmark. Diabetes Care 2018;41:1068–1075 [DOI] [PubMed] [Google Scholar]
- 13.Andersen ST, Grosen K, Tankisi H, et al. Corneal confocal microscopy as a tool for detecting diabetic polyneuropathy in a cohort with screen-detected type 2 diabetes: ADDITION-Denmark. J Diabetes Complications 2018;32:1153–1159 [DOI] [PubMed] [Google Scholar]
- 14.Dehghani C, Pritchard N, Edwards K, Russell AW, Malik RA, Efron N. Risk factors associated with corneal nerve alteration in type 1 diabetes in the absence of neuropathy: a longitudinal in vivo corneal confocal microscopy study. Cornea 2016;35:847–852 [DOI] [PubMed] [Google Scholar]
- 15.Dehghani C, Pritchard N, Edwards K, et al. Natural history of corneal nerve morphology in mild neuropathy associated with type 1 diabetes: development of a potential measure of diabetic peripheral neuropathy. Invest Ophthalmol Vis Sci 2014;55:7982–7990 [DOI] [PubMed] [Google Scholar]
- 16.Edwards K, Pritchard N, Vagenas D, Russell A, Malik RA, Efron N. Utility of corneal confocal microscopy for assessing mild diabetic neuropathy: baseline findings of the LANDMark study. Clin Exp Optom 2012;95:348–354 [DOI] [PubMed] [Google Scholar]
- 17.Su JB, Zhao LH, Zhang XL, et al. HbA1c variability and diabetic peripheral neuropathy in type 2 diabetic patients. Cardiovasc Diabetol 2018;17:47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tesfaye S Epidemiology and etiology of diabetic peripheral neuropathies. Adv Stud Med 2004;4:S1014–S1021 [Google Scholar]
- 19.Papanas N, Ziegler D. Risk factors and comorbidities in diabetic neuropathy: an update 2015. Rev Diabet Stud 2015;12:48–62 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ishibashi F, Okino M, Ishibashi M, et al. Corneal nerve fiber pathology in Japanese type 1 diabetic patients and its correlation with antecedent glycemic control and blood pressure. J Diabetes Investig 2012;3:191–198 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bitirgen G, Ozkagnici A, Malik RA, Kerimoglu H. Corneal nerve fibre damage precedes diabetic retinopathy in patients with type 2 diabetes mellitus. Diabet Med 2014;31:431–438 [DOI] [PubMed] [Google Scholar]
- 22.Azmi S, Ferdousi M, Petropoulos IN, et al. Corneal confocal microscopy identifies small-fiber neuropathy in subjects with impaired glucose tolerance who develop type 2 diabetes. Diabetes Care 2015;38:1502–1508 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tavakoli M, Marshall A, Pitceathly R, et al. Corneal confocal microscopy: a novel means to detect nerve fibre damage in idiopathic small fibre neuropathy. Exp Neurol 2010;223:245–250 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kalteniece A, Ferdousi M, Petropoulos I, et al. Greater corneal nerve loss at the inferior whorl is related to the presence of diabetic neuropathy and painful diabetic neuropathy. Sci Rep 2018;8:3283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Fadavi H, Tavakoli M, Foden P, et al. Explanations for less small fibre neuropathy in South Asian versus European subjects with type 2 diabetes in the UK. Diabetes Metab Res Rev 2018;34:e3044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kalteniece A, Ferdousi M, Adam S, et al. Corneal confocal microscopy is a rapid reproducible ophthalmic technique for quantifying corneal nerve abnormalities. PLoS One 2017;12:e0183040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Petropoulos IN, Ferdousi M, Marshall A, et al. The inferior whorl for detecting diabetic peripheral neuropathy using corneal confocal microscopy. Invest Ophthalmol Vis Sci 2015;56:2498–2504 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Partanen J, Niskanen L, Lehtinen J, Mervaala E, Siitonen O, Uusitupa M. Natural history of peripheral neuropathy in patients with non-insulin-dependent diabetes mellitus. N Engl J Med 1995;333:89–94 [DOI] [PubMed] [Google Scholar]
- 29.Grisold A, Callaghan BC, Feldman EL. Mediators of diabetic neuropathy: is hyperglycemia the only culprit? Curr Opin Endocrinol Diabetes Obes 2017;24:103–111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ferdousi M, Romanchuk K, Mah JK, et al. Early corneal nerve fibre damage and increased Langerhans cell density in children with type 1 diabetes mellitus. Sci Rep 2019;9:8758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Davis TM, Yeap BB, Davis WA, Bruce DG. Lipid-lowering therapy and peripheral sensory neuropathy in type 2 diabetes: the Fremantle Diabetes Study. Diabetologia 2008;51:562–566 [DOI] [PubMed] [Google Scholar]
- 32.Wiggin TD, Sullivan KA, Pop-Busui R, Amato A, Sima AA, Feldman EL. Elevated triglycerides correlate with progression of diabetic neuropathy. Diabetes 2009;58:1634–1640 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Callaghan BC, Feldman E, Liu J, et al. Triglycerides and amputation risk in patients with diabetes: ten-year follow-up in the DISTANCE study. Diabetes Care 2011;34:635–640 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Braffett BH, Gubitosi-Klug RA, Albers JW, et al.; DCCT/EDIC Research Group . Risk factors for diabetic peripheral neuropathy and cardiovascular autonomic neuropathy in the Diabetes Control and Complications Trial/Epidemiology of Diabetes Interventions and Complications (DCCT/EDIC) study. Diabetes 2020;69:1000–1010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ponirakis G, Abdul-Ghani MA, Jayyousi A, et al. Effect of treatment with exenatide and pioglitazone or basal-bolus insulin on diabetic neuropathy: a substudy of the Qatar Study. BMJ Open Diabetes Res Care 2020;8:e001420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ishibashi F, Taniguchi M, Kosaka A, Uetake H, Tavakoli M. Improvement in neuropathy outcomes with normalizing HbA1c in patients with type 2 diabetes. Diabetes Care 2019;42:110–118 [DOI] [PubMed] [Google Scholar]
- 37.Jia X, Wang X, Wang X, et al. In vivo corneal confocal microscopy detects improvement of corneal nerve parameters following glycemic control in patients with type 2 diabetes. J Diabetes Res 2018;2018:8516276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Perkins BA, Lovblom LE, Bril V, et al. Corneal confocal microscopy for identification of diabetic sensorimotor polyneuropathy: a pooled multinational consortium study. Diabetologia 2018;61:1856–1861 [DOI] [PMC free article] [PubMed] [Google Scholar]