Abstract
OBJECTIVE
To evaluate the effect of preoperative blood glucose (POBG) level on hospital length of stay (LOS) in patients undergoing appendectomy or laparoscopic cholecystectomy.
RESEARCH DESIGN AND METHODS
We conducted a retrospective cohort study of patients aged ≥18 years who had undergone appendectomy or laparoscopic cholecystectomy procedures between 2005 and 2016 at a tertiary medical center in Taiwan. The association between POBG level and LOS was evaluated using a multivariable quasi-Poisson regression with robust variance. Multiple imputations were performed to replace missing values.
RESULTS
We included 8,291 patients; 4,025 patients underwent appendectomy (appendectomy group) and 4,266 underwent laparoscopic cholecystectomy (laparoscopic cholecystectomy group). In the appendectomy group, patients with POBG levels of ≥123 mg/dL (adjusted relative risk [aRR] 1.19; 95% CI 1.06–1.33) had a 19% higher risk of having a LOS of >3 days than did those with POBG levels of <106 mg/dL. In the laparoscopic cholecystectomy group, patients with POBG levels of ≥128 mg/dL also had a significantly higher risk of having a LOS of >3 days (aRR 1.17; 95% CI 1.07–1.29) than did those with POBG levels of <102 mg/dL. A positive dose–response curve between POBG and an adjusted risk of a LOS of >3 days was observed, although the curve starts to flatten at a POBG level of ∼130 mg/dL.
CONCLUSIONS
We demonstrated that a higher POBG level was significantly associated with a prolonged LOS for patients undergoing appendectomy or laparoscopic cholecystectomy. The optimal POBG level may be lower than that commonly perceived.
Introduction
Growing evidence indicates the prognostic value of managing preoperative hyperglycemia in patients diagnosed as having diabetes or in older adults undergoing elective surgery (1). Nevertheless, no consensus has been reached on routine screening of preoperative blood glucose (POBG) levels because the evidence supporting the effectiveness of a specific blood glucose target range is scarce (2–6). A systematic review of studies published from February 2001 to March 2013 did not support the clinical effectiveness of routine screening of POBG or hemoglobin A1c (HbA1c) levels in otherwise healthy or asymptomatic adults receiving elective noncardiac surgery (6). Based on these findings, the latest European Society of Anaesthesiology (ESA) guidelines do not suggest routine POBG assessments for individuals undergoing elective noncardiac surgery (7).
Increasing evidence demonstrates that preoperative hyperglycemia has a detrimental effect on those undergoing surgery. A retrospective study of 904 patients who died within 30 days during hospitalization and 1,247 matched control patients who underwent noncardiovascular procedures reported that patients with POBG levels of >110 mg/dL had an increased risk of overall 30-day mortality and cardiovascular mortality compared with those with lower glucose levels and that this association was present in patients with prediabetes and diabetes (2). Moreover, among patients who received vascular and orthopedic surgery, the POBG level was associated with an increased risk of cardiovascular events within 30 days of surgery and infected total knee replacement within 1 year of surgery, respectively (8,9). In another study of 493 consecutive patients undergoing elective noncardiac surgery, ∼25% of patients without a diabetes diagnosis had fasting blood glucose levels of >110 mg/dL (3). Abdelmalak et al. (4) revealed that patients without diabetes with preoperative hyperglycemia (>153 mg/dL or 8.5 mmol/L) who underwent elective noncardiac surgery had higher 1-year mortality rates compared with patients with diabetes and similar blood glucose levels. However, no significant association was observed between preoperative hyperglycemia and short-term outcomes such as in-hospital postoperative complications and mortality. Wang et al. (5) observed that among 6,683 patients without diabetes who underwent nonemergent vascular and noncardiac surgery, patients with POBG levels between 100 and 139 mg/dL and between 140 and 179 mg/dL had a significantly increased risk of 30-day postoperative infection compared with patients with POBG levels between 70 and 99 mg/dL. Additionally, a linearly increasing trend between POBG level and the risk of postoperative infection was observed in patients with POBG levels between 100 and 179 mg/dL, which is generally accepted as a safe range in current surgical practice. In a recent intervention study, Garg et al. (10) demonstrated the benefits of preoperative diabetes management among patients with diabetes undergoing elective cardiovascular and noncardiovascular surgery. Optimizing POBG levels to below 200 mg/dL was associated with a reduction in the hospital length of stay (LOS) among patients with diabetes, although the clinical significance was unclear (10).
To clarify the association between preoperative hyperglycemia and postoperative outcomes, we conducted a large retrospective cohort study at the largest medical center in central Taiwan with a surgical volume of >48,000 per year to systematically evaluate the association between POBG level and hospital LOS. Appendectomy and cholecystectomy are among the most common noncritical surgical procedures in Taiwan (11,12). Both operations follow highly standardized clinical pathways that are rigorously regulated by Taiwan’s National Health Insurance Administration. Consequently, we focused on these noncritical surgical procedures (13–15).
Research Design and Methods
Study Population
China Medical University Hospital (CMUH) established the CMUH–Clinical Research Data Repository (CMUH–CRDR), which contains the medical records of 2,660,472 patients who sought care at CMUH between 2003 and 2016. The CMUH–CRDR includes verified and validated data on administrative and demographic information, diagnoses, medical and surgical procedures, prescriptions, laboratory measurements, physiological measurements, hospitalization, and catastrophic illness status (16). We identified 16,153 appendectomies or laparoscopic cholecystectomies from the CMUH–CRDR. After exclusion criteria were applied, the final study population comprised 4,025 patients who had undergone appendectomy (894 open and 3,131 laparoscopic) for the first time (hereafter referred to as the appendectomy group) and 4,266 patients who had undergone laparoscopic cholecystectomy for the first time (hereafter referred to as the laparoscopic cholecystectomy group) at CMUH (Supplementary Fig. 1). Supplementary Table 1 presents the characteristics of study population and patients who were excluded because of the absence of POBG measurements. This study was approved by the CMUH Research Ethical Committee/Institutional Review Board (CMUH105-REC3-068).
Glucose Measurement
The primary exposure of interest was the POBG level, which was measured within 48 h before surgery and closest to the surgical incision time. Plasma glucose levels were measured using the hexokinase enzymatic method at the CMUH Central Laboratory through a Beckman UniCel DxC 800 immunoassay system (Beckman Coulter, Brea, CA). We differentiated fasting glucose and nonfasting glucose according to the clinician’s order. We used all available data on preoperative fasting glucose levels and considered nonfasting glucose levels when data on fasting glucose levels were unavailable. Moreover, we evaluated two definitions of cutoff points for glucose: 1) clinical cutoff points for patients with prediabetes and diabetes (<100, 100–125, and >126 mg/dL) defined by the Classification and Diagnosis of Diabetes in the American Diabetes Association Standards of Medical Care in Diabetes (17), and 2) cutoff points based on the 25th and 75th percentiles of glucose levels.
Outcome Measurement and Covariates
The primary outcome of interest was hospital LOS, which was treated as a dichotomous variable (≤3 or >3 days). We did not model hospital LOS on a continuous scale for the following reasons: 1) the time to discharge is highly standardized by the diagnosis-related group reimbursement method for appendectomy and cholecystectomy in Taiwan, and a hospital LOS of >3 days is a significant deviation from clinical practice; and 2) studies have reported that the average hospital LOS of these two procedures is 1–2 days, despite the differences in their nature (emergent vs. elective) (18–20). Supplementary Table 2 details the biochemical- and procedure-related variables and provides the definition of hypertension and cardiovascular disease (CVD) history. We categorized patients into the following groups: diagnosed diabetes subgroup, comprising patients with an International Classification of Diseases-Ninth Revision, Clinical Modification diagnosis code of 250.xx or patients who took antidiabetic medications within 1 year before surgery; undiagnosed diabetes subgroup, comprising patients without diabetes who had a POBG level of ≥126 mg/dL within 1 year before surgery; and hyperglycemia subgroup, comprising patients without diabetes who had a blood glucose level of <126 mg/dL within a period of 1 year before surgery and a POBG level of ≥126 mg/dL within a period of 48 h before surgery. The highest white blood cell count (WBC), neutrophil-to-lymphocyte ratio (NLR), and C-reactive protein (CRP) values measured within 48 h before the incision time were defined as the baseline values.
Statistical Analysis
Data analysis was performed separately for the appendectomy group and cholecystectomy group. Continuous variables are expressed as medians and interquartile ranges and were compared using the nonparametric Kruskal-Wallis test. Categorical variables are expressed as a frequency (percentage) and were compared using the χ2 test. POBG level was modeled as both continuous and categorical (tertile) exposures and was categorized on the basis of common clinical cutoffs of 100 and 126 mg/dL or the first and third tertiles of POBG levels. Hospital LOS was modeled as a dichotomous outcome (LOS >3 days). Supplementary Table 3 provides information on missing data. Because of the high proportion of missing values for surgical parameters and NLR, we performed multiple imputations using an iterative Markov chain Monte Carlo procedure with 20 imputations and 100 iterations (21). We used the data from the multiple imputations in the subsequent multivariable analyses.
To determine the association between POBG level and dichotomized LOS outcomes, we used robust quasi-Poisson regression modeling with the classical sandwich estimator under a generalized estimating equation framework to obtain accurate SEs for the elements (22). We initially adjusted for age and sex. Subsequently, we adjusted for diabetes, hypertension, CVD, WBC, and NLR as well as for the following surgical parameters: American Society of Anesthesiologists (ASA) Physical Status Classification score, wound contamination class, surgical drain placement, and operation duration. We also characterized the dose–response relationship using a restricted cubic spline model with three knots located at the 10th, 50th, and 90th percentiles of the overall distribution for POBG levels. In the subgroup analyses, we stratified the study population by age (<65, ≥65 years), sex, diabetes, hypertension, CVD, ASA score (<3, ≥3), wound contamination class (clean or clean-contaminated, contaminated, or dirty), surgical drain (absence, presence), and operation duration (70 min [75th percentile] for appendectomy and 111 min [75th percentile] for cholecystectomy).
To evaluate the effectiveness of POBG levels in predicting a hospital LOS of >3 days, we constructed receiver operating characteristic (ROC) curves and obtained the areas under the curves (AUCs or C statistics) using logistic regression models (23,24). We also constructed the reference prediction model using variables of age, sex, diabetes, hypertension, CVD, WBC, and NLR to assess the predictive performance of the proposed model further incorporated with POBG levels. We plotted the observed risk probability against the predicted risk probability to demonstrate the differences in the calibration of all risk models for hospital LOS prediction. All statistical analyses were performed using SAS 9.4 software (SAS Institute, Cary, NC) and R 3.2.3 software (R Foundation for Statistical Computing, Vienna, Austria). The two-sided statistical significance level (α) was set at 0.05.
Results
Baseline Demographic and Clinical Characteristics
The median ages of the patients in the appendectomy group (n = 4,025) and laparoscopic cholecystectomy group (n = 4,266) were 38.0 and 53.4 years, respectively (Supplementary Table 4). Only 5.96% of the patients in the appendectomy group and 20.84% of those in the laparoscopic cholecystectomy group had received a diagnosis of diabetes before the procedures. Of the patients in the appendectomy and laparoscopic cholecystectomy groups, 4.07% and 14.63% had undiagnosed diabetes, respectively, and 24.07% and 16.08% had hyperglycemia, respectively. Complete blood cell count results revealed a baseline WBC count of 13.5 × 103/μL (neutrophils: 82.1%; NLR: 7.4) for the appendectomy group and 7.8 × 103/μL (neutrophils: 67.2%; NLR: 2.9) for the cholecystectomy group. The median ASA scores for the appendectomy and laparoscopic cholecystectomy groups were 1 and 2, respectively. Furthermore, 61.12% and 75.97% of the patients in the appendectomy and laparoscopic cholecystectomy had surgical wound class as clean or clean-contaminated, respectively (Supplementary Table 4). The average operation durations were 55 min for appendectomy and 85 min for laparoscopic cholecystectomy.
Clinical Characteristics Based on POBG Tertiles
At higher POBG levels, an upward trend was observed for median age and the prevalence of diagnosed or undiagnosed diabetes, hypertension, and CVD. Similarly, as POBG levels increased, elevated levels of WBC, NLR, CRP, body temperature, and pulse rate preoperatively for both surgical procedures were observed (Table 1). For both surgical procedures, patients whose POBG levels were in the highest tertile were more likely to have an ASA score of ≥3, a contaminated or dirty wound, and long operation duration compared with other patients; they were also more likely to require a surgical drain. Patients in higher POBG tertiles were significantly more likely to have a hospital LOS of >3 and >7 days. Nevertheless, the proportion of readmission and emergency department revisits within 30 days of discharge differed significantly across the POBG tertiles in the laparoscopic cholecystectomy group but not in the appendectomy group (Table 1). Because >90% of appendectomies and only 29.2% of cholecystectomies were emergent, we conducted a sensitivity analysis on emergent appendectomy and elective cholecystectomy and obtained similar results (Supplementary Table 5). Additionally, for both procedures, we noted a strong and positive correlation between POBG and postoperative glucose levels (Supplementary Fig. 2).
Table 1.
Baseline demographic and clinical characteristics by preoperative glucose level in patients undergoing appendectomy or laparoscopic cholecystectomy
| Appendectomy (n = 4,025) | Laparoscopic cholecystectomy (n = 4,266) | |||||||
|---|---|---|---|---|---|---|---|---|
| Glucose <106 (n = 1,320) | Glucose 106 to <123 (n = 1,291) | Glucose ≥123 (n = 1,414) | P value | Glucose <102 (n = 1,360) | Glucose 102 to <128 (n = 1,425) | Glucose ≥128 (n = 1,481) | P value* | |
| Age (years) | 30.8 (24.6, 40.6) | 36.7 (27.4, 47.8) | 48.5 (35.5, 60.8) | <0.001 | 44.7 (35.3, 57.3) | 52.3 (40.7, 63.3) | 61.6 (50.7, 71.2) | <0.001 |
| Age ≥65 | 62 (4.70) | 88 (6.82) | 258 (18.25) | <0.001 | 194 (14.26) | 322 (22.60) | 598 (40.38) | <0.001 |
| Female | 728 (55.15) | 598 (46.32) | 630 (44.55) | <0.001 | 819 (60.22) | 760 (53.33) | 689 (46.52) | <0.001 |
| Diabetes | <0.001 | <0.001 | ||||||
| Diagnosed diabetes | 23 (1.74) | 21 (1.63) | 196 (13.86) | 102 (7.50) | 174 (12.21) | 613 (41.39) | ||
| Undiagnosed diabetes | 25 (1.89) | 59 (4.57) | 80 (5.66) | 149 (10.96) | 241 (16.91) | 234 (15.80) | ||
| Hyperglycemia | 0 (0.00) | 0 (0.00) | 969 (68.53) | 0 (0.00) | 53 (3.72) | 633 (42.74) | ||
| Hypertension | 22 (1.67) | 19 (1.47) | 88 (6.22) | <0.001 | 99 (7.28) | 127 (8.91) | 271 (18.30) | <0.001 |
| CVD | 41 (3.11) | 39 (3.02) | 132 (9.34) | <0.001 | 96 (7.06) | 128 (8.98) | 237 (16.00) | <0.001 |
| ASA score | 1.00 (1.00, 2.00) | 1.00 (1.00, 2.00) | 2.00 (1.00, 2.00) | <0.001 | 2.00 (1.00, 2.00) | 2.00 (1.00, 2.00) | 2.00 (2.00, 3.00) | <0.001 |
| ASA score ≥3 | 61 (5.42) | 69 (6.43) | 160 (13.27) | <0.001 | 128 (10.72) | 171 (13.34) | 358 (26.84) | <0.001 |
| Operation duration (min) | 50.0 (39.5, 66.0) | 54.0 (40.0, 70.0) | 57.0 (45.0, 75.0) | <0.001 | 80.0 (62.0, 105.0) | 85.0 (64.0, 113.0) | 90.0 (69.0, 117.0) | <0.001 |
| >75th percentile† | 262 (19.85) | 308 (23.86) | 415 (29.35) | <0.001 | 273 (20.07) | 360 (25.26) | 429 (28.97) | <0.001 |
| EBL (mL) | 20.0 (20.0, 20.0) | 20.0 (20.0, 20.0) | 20.0 (20.0, 20.0) | 0.381 | 20.0 (20.0, 20.0) | 20.0 (20.0, 20.0) | 20.0 (20.0, 50.0) | <0.001 |
| Wound class | <0.001 | <0.001 | ||||||
| Clean | 51 (4.66) | 45 (4.33) | 33 (2.80) | 251 (21.34) | 194 (15.43) | 105 (8.00) | ||
| Clean contaminated | 826 (75.43) | 759 (73.05) | 746 (63.33) | 846 (71.94) | 889 (70.72) | 956 (72.87) | ||
| Contaminated | 191 (17.44) | 200 (19.25) | 309 (26.23) | 77 (6.55) | 172 (13.68) | 246 (18.75) | ||
| Dirty | 27 (2.47) | 33 (3.18) | 90 (7.64) | 2 (0.17) | 1 (0.08) | 5 (0.38) | ||
| Emergent | 1,015 (89.66) | 990 (91.58) | 1,131 (93.09) | 0.012 | 160 (13.36) | 388 (30.15) | 570 (42.54) | <0.001 |
| Drain | 213 (16.14) | 255 (19.75) | 436 (30.83) | <0.001 | 419 (30.81) | 628 (44.07) | 846 (57.12) | <0.001 |
| Glucose (mg/dL) | 98 (93, 102) | 113 (109, 117) | 139 (129, 158) | <0.001 | 92 (86, 97) | 113 (107, 119) | 152 (138, 182) | <0.001 |
| WBC (×103/µL) | 12.4 (9.8, 14.9) | 13.8 (11.4, 16.4) | 14.2 (11.2, 17.1) | <0.001 | 6.8 (5.7, 8.5) | 7.9 (6.2, 10.7) | 9.5 (6.9, 14.0) | <0.001 |
| Neutrophil segment (%) | 77.5 (71.0, 83.3) | 82.2 (76.7, 87.1) | 85.2 (79.9, 89.2) | <0.001 | 60.6 (53.4, 67.9) | 67.2 (58.1, 78.4) | 76.2 (63.2, 85.5) | <0.001 |
| Lymphocyte (%) | 15.0 (10.4, 20.6) | 11.4 (7.4, 16.1) | 9.2 (6.2, 13.6) | <0.001 | 29.8 (23.2, 35.9) | 23.9 (13.8, 31.8) | 16.7 (8.4, 27.9) | <0.001 |
| NLR | 5.3 (3.5, 8.2) | 7.6 (5.0, 12.4) | 9.8 (6.3, 15.0) | <0.001 | 2.0 (1.5, 2.9) | 2.9 (1.9, 6.2) | 5.1 (2.4, 10.9) | <0.001 |
| CRP (mg/dL) | 1.9 (0.4, 5.5) | 1.9 (0.4, 5.6) | 3.2 (0.7, 10.5) | <0.001 | 0.9 (0.3, 4.7) | 1.9 (0.4, 10.3) | 4.5 (0.6, 15.8) | <0.001 |
| Temperature (°C) | 37.0 (36.5, 37.4) | 37.1 (36.5, 37.7) | 37.4 (36.7, 38.2) | <0.001 | 36.7 (36.4, 37.0) | 36.9 (36.5, 37.4) | 37.1 (36.6, 37.8) | <0.001 |
| Temperature ≥38°C | 135 (10.59) | 228 (18.31) | 423 (30.88) | <0.001 | 24 (3.33) | 112 (11.45) | 265 (22.86) | <0.001 |
| Pulse (bpm) | 89.0 (79.0, 100.0) | 91.0 (80.0, 104.0) | 95.0 (83.0, 109.0) | <0.001 | 80.0 (71.0, 88.0) | 83.0 (73.0, 96.0) | 90.0 (79.0, 102.0) | <0.001 |
| Outcome | ||||||||
| Postoperative antibiotic use‡ | 340 (25.8) | 382 (29.6) | 569 (40.2) | <0.001 | 89 (6.54) | 218 (15.3) | 349 (23.6) | <0.001 |
| LOS (days) | 2.04 (1.64, 2.89) | 2.14 (1.66, 3.05) | 2.53 (1.77, 4.17) | <0.001 | 2.83 (1.99, 3.18) | 2.88 (2.05, 3.82) | 3.11 (2.37, 4.56) | <0.001 |
| LOS >3 days | 296 (22.42) | 334 (25.87) | 580 (41.02) | <0.001 | 437 (32.13) | 575 (40.35) | 823 (55.57) | <0.001 |
| LOS >7 days | 33 (2.50) | 55 (4.26) | 113 (7.99) | <0.001 | 28 (2.06) | 55 (3.86) | 145 (9.79) | <0.001 |
| Readmission in 30 days | 35 (2.65) | 45 (3.49) | 58 (4.10) | 0.113 | 32 (2.35) | 47 (3.30) | 70 (4.73) | 0.002 |
| Reemergency in 30 days | 107 (8.11) | 98 (7.59) | 99 (7.00) | 0.550 | 72 (5.29) | 94 (6.60) | 113 (7.63) | 0.042 |
Continuous data are presented as the median (interquartile range) and categorical data as n (%). EBL, estimated blood loss.
P values were calculated using a Kruskal-Wallis test for continuous variables and χ2 test for categorical variables.
The 75th percentile of operation duration was 70 min for appendectomy and 111 min for cholecystectomy.
Postoperative antibiotic use 24 h after surgical procedures until discharge.
Association Between POBG Level and Hospital LOS
When glucose was considered a continuous variable in a multivariable logistic regression, every 10 mg/dL increase in POBG levels was associated with 1% (95% CI 0–2) and 1% (95% CI 0–1) increases in the risk of an extended hospital LOS in the appendectomy and laparoscopic cholecystectomy groups, respectively (Table 2, model 4). As mentioned previously, we divided the patients in the appendectomy group into three tertiles according to their POBG levels and observed that patients with POBG levels of ≥123 mg/dL had a 19% (95% CI 6–33) higher risk of having a hospital LOS of >3 days than those with POBG levels of <106 mg/dL (Table 2, model 4). Similarly, we divided the patients in the laparoscopic cholecystectomy group into three tertiles according to their POBG levels. We noted that those with POBG levels of ≥128 mg/dL (the highest tertile) had a 17% (95% CI 7–29) higher risk of having an extended hospital LOS. When clinical cutoffs of 100 mg/dL and 126 mg/dL were used to define three POBG subgroups, the corresponding increases in the risk of an extended hospital LOS for appendectomy and cholecystectomy were 27% (95% CI, 10–46) and 13% (95% CI, 3–25), respectively (Table 2, model 4).
Table 2.
Relative risk of having a hospital LOS of >3 days according to POBG level on a continuous scale, in tertile groups, and with a clinical cutoff using the American Diabetes Association diagnostic criteria for the classification of prediabetes and diabetes based on fasting blood glucose levels
| Model 1* | Model 2† | Model 3‡ | Model 4§ | ||||
|---|---|---|---|---|---|---|---|
| N | Event | Crude RR‖ (95% CI) | aRR (95% CI) | aRR (95% CI) | aRR (95% CI) | aRR (95% CI) | |
| Appendectomy | |||||||
| Glucose (per 10 mg/dL) | 4,025 | 1,210 | 1.04 (1.03–1.05) | 1.02 (1.02–1.03) | 1.02 (1.02–1.03) | 1.02 (1.01–1.03) | 1.01 (1.00–1.02) |
| Glucose, mg/dL (tertiles) | |||||||
| Glucose <106 | 1,320 | 296 | 1.00 (Ref) | 1.00 (Ref) | 1.00 (Ref) | 1.00 (Ref) | 1.00 (Ref) |
| Glucose 106 to <123 | 1,291 | 334 | 1.15 (1.01–1.32) | 1.06 (0.93–1.22) | 1.07 (0.93–1.22) | 1.04 (0.91–1.19) | 1.02 (0.91–1.16) |
| Glucose ≥123 | 1,414 | 580 | 1.83 (1.63–2.06) | 1.43 (1.26–1.62) | 1.42 (1.25–1.61) | 1.34 (1.18–1.52) | 1.19 (1.06–1.33) |
| Glucose, mg/dL (clinical) | |||||||
| Glucose <100 | 784 | 165 | 1.00 (Ref) | 1.00 (Ref) | 1.00 (Ref) | 1.00 (Ref) | 1.00 (Ref) |
| Glucose 100 to <126 | 2,013 | 519 | 1.23 (1.05–1.43) | 1.13 (0.97–1.32) | 1.14 (0.98–1.33) | 1.11 (0.95–1.29) | 1.07 (0.93–1.23) |
| Glucose ≥126 | 1,228 | 526 | 2.04 (1.75–2.37) | 1.55 (1.32–1.81) | 1.54 (1.32–1.81) | 1.46 (1.25–1.71) | 1.27 (1.10–1.46) |
| Laparoscopic cholecystectomy | |||||||
| Glucose (per 10 mg/dL) | 4,266 | 1,835 | 1.04 (1.03–1.05) | 1.03 (1.02–1.03) | 1.02 (1.02–1.03) | 1.02 (1.01–1.02) | 1.01 (1.00–1.01) |
| Glucose, mg/dL (tertiles) | |||||||
| Glucose <102 | 1,360 | 437 | 1.00 (Ref) | 1.00 (Ref) | 1.00 (Ref) | 1.00 (Ref) | 1.00 (Ref) |
| Glucose 102 to <128 | 1,425 | 575 | 1.26 (1.14–1.39) | 1.16 (1.05–1.29) | 1.17 (1.06–1.29) | 1.14 (1.03–1.25) | 1.06 (0.97–1.16) |
| Glucose ≥128 | 1,481 | 823 | 1.73 (1.58–1.89) | 1.45 (1.32–1.59) | 1.38 (1.25–1.52) | 1.28 (1.16–1.42) | 1.17 (1.07–1.29) |
| Glucose, mg/dL (clinical) | |||||||
| Glucose <100 | 1,200 | 390 | 1.00 (Ref) | 1.00 (Ref) | 1.00 (Ref) | 1.00 (Ref) | 1.00 (Ref) |
| Glucose 100 to <126 | 1,505 | 588 | 1.20 (1.08–1.33) | 1.11 (1.00–1.23) | 1.11 (1.00–1.23) | 1.08 (0.98–1.2) | 1.02 (0.92–1.12) |
| Glucose ≥126 | 1,561 | 857 | 1.69 (1.54–1.85) | 1.41 (1.28–1.55) | 1.34 (1.22–1.48) | 1.24 (1.12–1.38) | 1.13 (1.03–1.25) |
Model 1: Adjusted for age and sex.
Model 2: Adjusted for age at entry, sex, diabetes, and hypertension.
Model 3: Adjusted for age at entry, sex, diabetes, hypertension, WBC, and NLR.
Model 4: Adjusted for age at entry, sex, diabetes, hypertension, CVD, WBC, NLR, ASA score of ≥3, wound classification, surgical drain, and operation duration above the 75th percentile.
RRs and corresponding 95% CIs are presented in bold when the 95% CI did not cross 1.
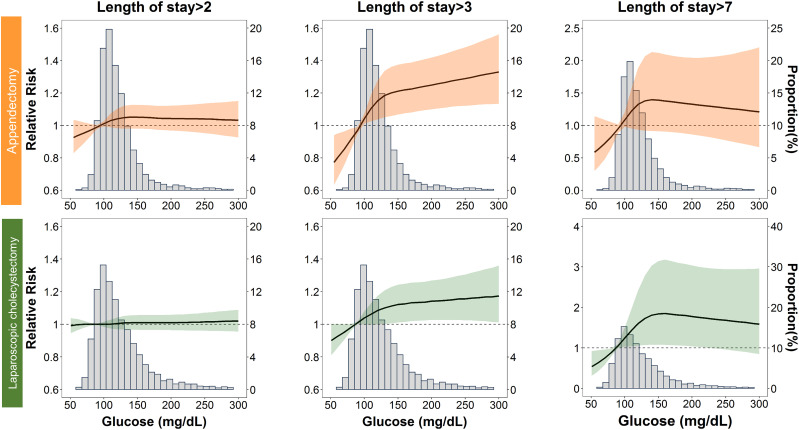
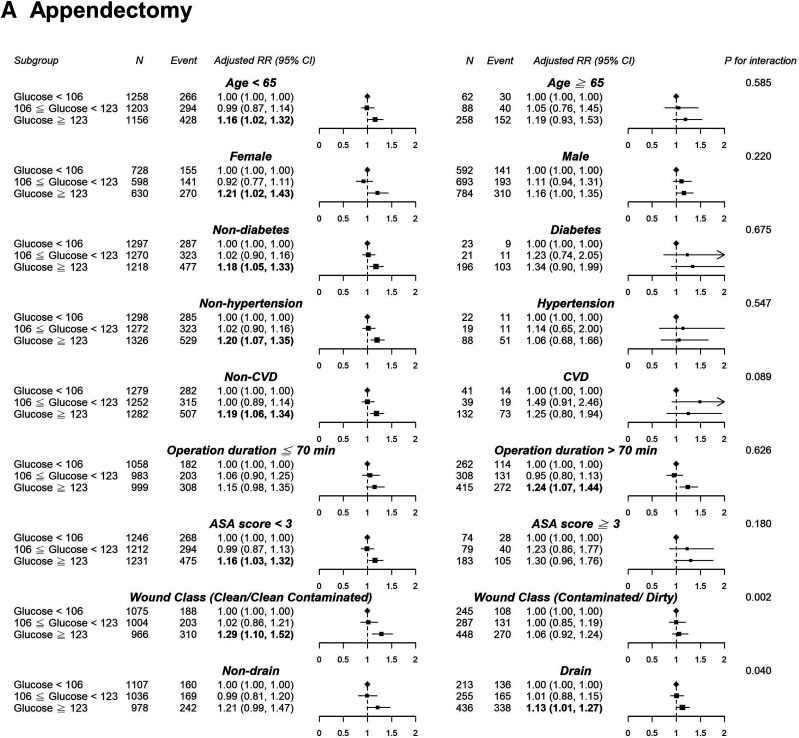
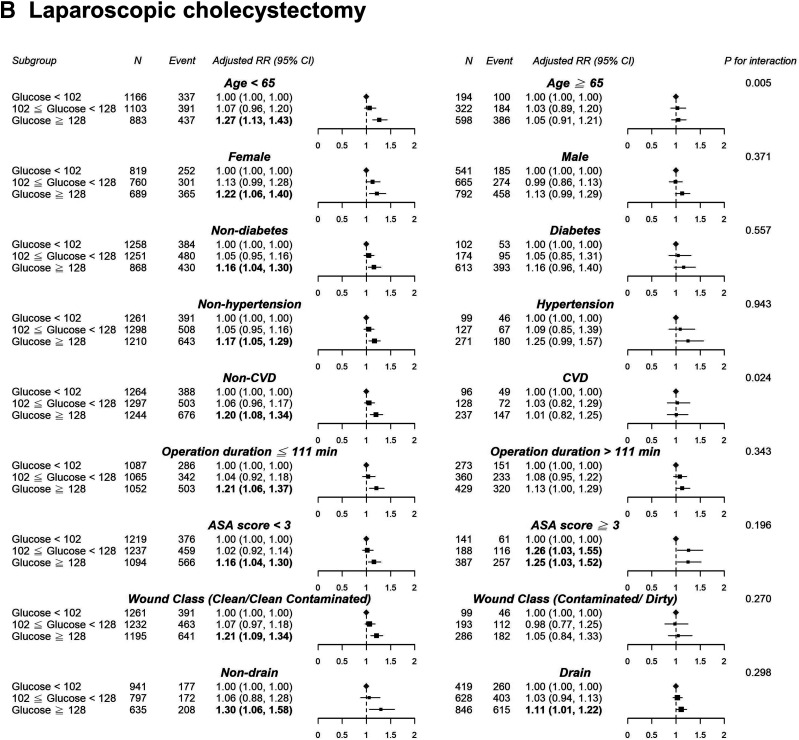
In the appendectomy or laparoscopic cholecystectomy group, we observed a nonlinear dose–response risk curve with an initial steep increase when the POBG levels ranged from 100 to 130 mg/dL, followed by a less pronounced rise when the POBG levels >130 mg/dL (Fig. 1). Subgroup analyses indicated no statistically significant interaction among most of the a priori variables of interest in the relationship between POBG level and a hospital LOS of >3 days, except for wound class and surgical drain in the appendectomy group as well as age and CVD in the cholecystectomy group (Fig. 2). Generally, the unfavorable effect of POBG on hospital LOS is more pronounced in younger and healthier patients. In addition, a dose–response relationship between increased glucose level and the relative risk of having a hospital LOS of >3 days was present in most subgroups. Supplementary Fig. 3 presents a summary of the discrimination performance after POBG levels (both continuous and categorical) were added to the seven-variable reference equation for hospital LOS prediction. Adding POBG tertiles significantly improved risk discrimination, with the AUC increasing from 0.657 to 0.663 (P = 0.04) and from 0.669 to 0.677 (P = 0.01) for the appendectomy and laparoscopic cholecystectomy groups, respectively; nevertheless, the overall predictive performance was only moderate (Supplementary Fig. 3A). The calibration plot demonstrated good agreement between observed and predicted probabilities, and adding the POBG tertiles slightly improved the agreement (Supplementary Fig. 3B).
Figure 1.
Relative risk for a hospital stay longer than 2, 3, and 7 days according to POBG level. The solid black lines represent aRRs based on restricted cubic splines for POBG level with knots at the 10th, 50th, and 90th percentiles (appendectomy: 94, 113, and 153 mg/dL; laparoscopic cholecystectomy: 87, 114, and 175 mg/dL, respectively). The shaded areas represent upper and lower 95% CIs. The reference was set at the 10th percentile of POBG levels. Adjustment factors are the same as those in model 4 of Table 2.
Figure 2.
Results of subgroup analyses of preoperative glucose level and a hospital stay of >3 days according to clinical characteristics. A, Appendectomy. B, Laparoscopic cholecystectomy. Adjustment factors are the same as those in model 4 of Table 2.
Conclusions
This large retrospective cohort study with robust analytic approaches demonstrated that increased POBG levels are significantly associated with a prolonged hospital LOS in a nonlinear dose–response manner in the appendectomy and laparoscopic cholecystectomy groups. A POBG level of ≥123 mg/dL was associated with a 19% higher risk of a hospital LOS of >3 days for appendectomy, and a POBG level of ≥128 mg/dL was associated with a 17% higher risk of a prolonged hospital LOS for cholecystectomy. Our study results indicate that the optimal POBG level may be lower than that in common surgical practice.
A consensus has not been reached on the risk threshold of POBG level for adverse outcomes. Our statistical cutoff points for both appendectomy (<106, 107–122, and ≥123 mg/dL) and cholecystectomy (<102, 102–127, and ≥128 mg/dL) are unexpectedly consistent with the preexisting diagnostic classification of diabetes for the general population (17). Davis et al. (25) identified a similar POBG cutoff of 120 mg/dL as being significantly associated with a longer hospital stay after neurosurgery. Noordzij et al. (2) reported that POBG levels of 110–199 mg/dL (prediabetes) and ≥200 mg/dL (diabetes) were significantly associated with increased mortality risk. Two recent studies of patients with cancer have demonstrated that higher POBG levels (≥140 mg/dL, HbA1c ≥6.5% [48 mmol/mol]) or poorly controlled diabetes (preoperative HbA1c ≥7.0% [53 mmol/mol]) were associated with decreased survival or tumor recurrence (26,27). However, the preoperative cutoff points reported by the aforementioned studies were empirically derived, representing a critical knowledge gap that requires urgent attention.
Clinical practice guidelines for patients undergoing surgery have largely focused on perioperative glycemic goals for patients with diabetes. The 2009 Practice Guidelines of The Society of Thoracic Surgeons suggest that the preoperative and intraoperative blood glucose levels should be maintained at ≤180 mg/dL for patients with diabetes undergoing cardiac surgery (28). According to the Society for Ambulatory Anesthesia Consensus statement in 2010, with insufficient evidence, the preoperative and intraoperative blood glucose levels should be maintained <180 mg/dL in ambulatory surgical patients with well-controlled diabetes (29). The 2012 Joint British Diabetes Societies Guidelines suggest that referral to a specialist diabetes team for advice should be considered if the preoperative HbA1c level is >8.5% (69 mmol/mol) for patients with diabetes undergoing elective surgery (30). According to the National Surgical Quality Improvement Program of the American College of Surgeons, glycemic control (HbA1c >7.0% [53 mmol/mol]) is emphasized only for patients with diabetes, with the aim of reducing the risk of surgical site infections and promoting wound healing (31). As no definitive POBG target range is indicated by relevant guidelines, health care providers may not be aware of the increased risks of adverse outcomes associated with preoperative hyperglycemia. Our study findings provide initial insight into blood glucose level optimization regardless of diabetic status. Further research focusing on the effect of optimal glucose control during the preoperative stage for patients without diabetes is required (32).
In our study, the high correlations observed among POBG and inflammatory markers such as WBC, NLR, and CRP indicate that blood glucose could be an indicator of the severity of surgical diseases and physiological status (Supplementary Fig. 4). This explains the potential role of POBG in hospital LOS prediction. However, an association does not guarantee causality. Further research is required to verify the prognostic role of POBG level during the entire surgical course. Such research should determine whether it is simply a surrogate marker, a trigger, or an effect modifier of subsequent acute local or systemic inflammation that leads to numerous complications, including poor wound healing and surgical site infection (33–37).
Strengths and Limitations
The strengths of this study include the careful efforts made to address confounders in the causal pathway between POBG level and prolonged hospital LOS, as illustrated in a directed acyclic graph (Supplementary Fig. 5). This was done to adjust for the measurable factors to estimate the independent effect of POBG level on hospital LOS and conduct multiple sensitivity analyses for robust inferences (Supplementary Tables 6 and 7). In addition, our results demonstrate the feasibility of using POBG level irrespective of a patient’s last meal because we analyzed both fasting and nonfasting glucose levels and their association with hospital LOS.
Despite these strengths, this study has several limitations. First, selection bias was present because we included only patients with POBG measurements taken within 48 h before the surgical procedures. Compared with our study population, patients without POBG measurements were less likely to have a diabetes diagnosis or undergo emergent procedures, but they were revealed to have longer hospitalization periods (Supplementary Table 1). The inclusion of only patients with POBG measurements could have resulted in an overestimation of the effect of POBG level on prolonged hospital LOS. However, the effect of this limitation on our inferences about patients undergoing appendectomy would be minimal, because only 6.3% of them did not have their POBG levels measured. In the laparoscopic cholecystectomy group, the proportion of patients without POBG measurements was 21.8%. Interestingly, our results revealed that among patients who received cholecystectomies and did not have POBG measurements, the attending surgeons who performed the operations were significantly younger than the surgeons who operated on patients who received POBG measurements (median age, 37.1 vs. 40.2 years) (Supplementary Table 1).
Second, residual and unmeasured confounders could not be entirely excluded. For instance, we may have slightly underestimated the prevalence of hypertension and CVD in the study population because this was a retrospective single-center study. Therefore, a slight overestimation of the effects of POBG level may have arisen due to the underestimation of these positive confounders. Additionally, surgeons’ individual performance records were not available; hence, their performance was an unmeasured factor.
Third, the measurement of POBG levels in this real-world observational study could not be standardized (i.e., fasting, nonfasting, or free of glucose water infusion), particularly for patients undergoing appendectomies. Misclassification of diabetes, undiagnosed diabetes, and stress hyperglycemia cannot be excluded because fasting status associated with blood samples cannot be routinely verified in the current surgical care flow in Taiwan. We could only differentiate fasting, nonfasting, or random glucose based on the title of clinician-ordered laboratory tests, which may not accurately reflect the patient’s clinical situation. However, this real-world practice constraint increases the likelihood of our study results being generalizable to a wide range of clinical conditions under which fasting glucose levels may be difficult to obtain.
Fourth, our findings were not verified in other populations under different health care systems and with different ethnic compositions. Nevertheless, the biological relationship between POBG levels and adverse outcomes may be similar across different populations as presented in prior studies, ensuring the generalizability of our findings.
Conclusion
In summary, our study results indicate that the POBG level is significantly associated with a prolonged hospital LOS in patients undergoing appendectomy or laparoscopic cholecystectomy procedures. The optimal POBG level may be much lower than the commonly applied level of 180 mg/dL (28,29) or HbA1c ≥7.0% (53 mmol/mol; glucose 154 mg/dL) or HbA1c >8.5% (69 mol/mol; glucose 197 mg/dL) (30,31). Additional studies are required to determine whether routine POBG screening or preoperative glucose control could improve the outcomes of patients, even those without diabetes, undergoing both emergent and elective surgery.
Article Information
Acknowledgment. The authors are grateful to the Health Data Science Center, China Medical University Hospital, for providing administrative, technical, and funding support. This manuscript was edited by Wallace Academic Editing.
Funding. This study was supported by the Ministry of Science and Technology of Taiwan (grant numbers MOST 108-2314-B-039-038-MY3, MOST 109-2321-B-468-001).
Duality of Interest. All authors have completed the International Committee of Medical Journal Editors uniform disclosure form at www.icmje.org/coi_disclosure.pdf (available on request from the corresponding author) and declare that they have no competing interests. No potential conflicts of interest relevant to this article were reported.
Author Contributions. H.-Y.C., K.-T.R.L., and C.-C.K. designed the study. H.-Y.C., K.-T.R.L., H.-C.H., C.-H.H., and Y.C. conducted data quality management and statistical analysis and drafted the manuscript. K.-T.R.L., Y.-L.H., S.-N.C., and C.-C.K. participated in the literature search, data preparation, and manuscript editing. H.-Y.C., Y.-L.H., Y.-C.W., and C.-C.K., critically revised the manuscript. All authors have read and approved the final manuscript. H.-Y.C. and C.-C.K. are the guarantors of this work and, as such, had full access to all the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis.
Footnotes
This article contains supplementary material online at https://doi.org/10.2337/figshare.13110221.
H.-Y.C. and K.-T.R.L. are co-first authors.
References
- 1.Duggan EW, Carlson K, Umpierrez GE. Perioperative hyperglycemia management: an update. Anesthesiology 2017;126:547–560 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Noordzij PG, Boersma E, Schreiner F, et al. Increased preoperative glucose levels are associated with perioperative mortality in patients undergoing noncardiac, nonvascular surgery. Eur J Endocrinol 2007;156:137–142 [DOI] [PubMed] [Google Scholar]
- 3.Hatzakorzian R, Bui H, Carvalho G, Shan WL, Sidhu S, Schricker T. Fasting blood glucose levels in patients presenting for elective surgery. Nutrition 2011;27:298–301 [DOI] [PubMed] [Google Scholar]
- 4.Abdelmalak BB, Knittel J, Abdelmalak JB, et al. Preoperative blood glucose concentrations and postoperative outcomes after elective non-cardiac surgery: an observational study. Br J Anaesth 2014;112:79–88 [DOI] [PubMed] [Google Scholar]
- 5.Wang R, Panizales MT, Hudson MS, Rogers SO, Schnipper JL. Preoperative glucose as a screening tool in patients without diabetes. J Surg Res 2014;186:371–378 [DOI] [PubMed] [Google Scholar]
- 6.Bock M, Johansson T, Fritsch G, et al. The impact of preoperative testing for blood glucose concentration and haemoglobin A1c on mortality, changes in management and complications in noncardiac elective surgery: a systematic review. Eur J Anaesthesiol 2015;32:152–159 [DOI] [PubMed] [Google Scholar]
- 7.De Hert S, Staender S, Fritsch G, et al. Pre-operative evaluation of adults undergoing elective noncardiac surgery: updated guideline from the European Society of Anaesthesiology. Eur J Anaesthesiol 2018;35:407–465 [DOI] [PubMed] [Google Scholar]
- 8.Jämsen E, Nevalainen P, Kalliovalkama J, Moilanen T. Preoperative hyperglycemia predicts infected total knee replacement. Eur J Intern Med 2010;21:196–201 [DOI] [PubMed] [Google Scholar]
- 9.Feringa HH, Vidakovic R, Karagiannis SE, et al. Impaired glucose regulation, elevated glycated haemoglobin and cardiac ischaemic events in vascular surgery patients. Diabet Med 2008;25:314–319 [DOI] [PubMed] [Google Scholar]
- 10.Garg R, Schuman B, Bader A, et al. Effect of preoperative diabetes management on glycemic control and clinical outcomes after elective surgery. Ann Surg 2018;267:858–862 [DOI] [PubMed] [Google Scholar]
- 11.Lin KB, Lai KR, Yang NP, et al. Epidemiology and socioeconomic features of appendicitis in Taiwan: a 12-year population-based study. World J Emerg Surg 2015;10:42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Huang J, Chang CH, Wang JL, et al. Nationwide epidemiological study of severe gallstone disease in Taiwan. BMC Gastroenterol 2009;9:63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Decker MR, Dodgion CM, Kwok AC, et al. Specialization and the current practices of general surgeons. J Am Coll Surg 2014;218:8–15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sweeney JF Postoperative complications and hospital readmissions in surgical patients: an important association. Ann Surg 2013;258:19–20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gani F, Hundt J, Daniel M, Efron JE, Makary MA, Pawlik TM. Variations in hospitals costs for surgical procedures: inefficient care or sick patients? Am J Surg 2017;213:1–9 [DOI] [PubMed] [Google Scholar]
- 16.Tsai CW, Huang HC, Tien N, et al. Longitudinal progression trajectory of random urine creatinine as a novel predictor of ESRD among patients with CKD. Clin Chim Acta 2019;489:144–153 [DOI] [PubMed] [Google Scholar]
- 17.American Diabetes Association 2. Classification and diagnosis of diabetes: Standards of Medical Care in Diabetes—2020. Diabetes Care 2020;43(Suppl. 1):S14–S31 [DOI] [PubMed] [Google Scholar]
- 18.Peitzman AB, White MT, Childs N, Blake DP, Frazee R. Outpatient laparoscopic appendectomy should be the standard of care for uncomplicated appendicitis [discussion]. J Trauma Acute Care 2014;76:82–83 [DOI] [PubMed] [Google Scholar]
- 19.Cross W, Chandru Kowdley G. Laparoscopic appendectomy for acute appendicitis: a safe same-day surgery procedure? Am Surg 2013;79:802–805 [PubMed] [Google Scholar]
- 20.Akoh JA, Watson WA, Bourne TP. Day case laparoscopic cholecystectomy: reducing the admission rate. Int J Surg 2011;9:63–67 [DOI] [PubMed] [Google Scholar]
- 21.Sterne JA, White IR, Carlin JB, et al. Multiple imputation for missing data in epidemiological and clinical research: potential and pitfalls. BMJ 2009;338:b2393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chen W, Qian L, Shi J, Franklin M. Comparing performance between log-binomial and robust Poisson regression models for estimating risk ratios under model misspecification. BMC Med Res Methodol 2018;18:63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Harrell F, Institute SAS. The PHGLM Procedure. Raleigh, NC, SAS Institute, 1979 [Google Scholar]
- 24.Guo C, So Y, Jang W. Paper SAS462-2017: Evaluating Predictive Accuracy of Survival Models with PROC PHREG. Cary, NC, SAS Institute, 2017 [Google Scholar]
- 25.Davis MC, Ziewacz JE, Sullivan SE, El-Sayed AM. Preoperative hyperglycemia and complication risk following neurosurgical intervention: a study of 918 consecutive cases. Surg Neurol Int 2012;3:49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sandini M, Strobel O, Hank T, et al. Pre-operative dysglycemia is associated with decreased survival in patients with pancreatic neuroendocrine neoplasms. Surgery 2020;167:575–580 [DOI] [PubMed] [Google Scholar]
- 27.Liang SH, Shen YC, Wu JY, Wang LJ, Wu MF, Li J. Impact of poor preoperative glycemic control on outcomes among patients with cervical cancer undergoing a radical hysterectomy. Oncol Res Treat 2020;43:10–18 [DOI] [PubMed] [Google Scholar]
- 28.Lazar HL, McDonnell M, Chipkin SR, et al.; Society of Thoracic Surgeons Blood Glucose Guideline Task Force . The Society of Thoracic Surgeons practice guideline series: blood glucose management during adult cardiac surgery. Ann Thorac Surg 2009;87:663–669 [DOI] [PubMed] [Google Scholar]
- 29.Joshi GP, Chung F, Vann MA, et al.; Society for Ambulatory Anesthesia . Society for Ambulatory Anesthesia consensus statement on perioperative blood glucose management in diabetic patients undergoing ambulatory surgery. Anesth Analg 2010;111:1378–1387 [DOI] [PubMed] [Google Scholar]
- 30.Dhatariya K, Levy N, Kilvert A, et al.; Joint British Diabetes Societies . NHS Diabetes guideline for the perioperative management of the adult patient with diabetes. Diabet Med 2012;29:420–433 [DOI] [PubMed] [Google Scholar]
- 31. American College of Surgeons. Glycemic Control. Accessed 5 September 2019. Available from https://www.facs.org/quality-programs/strong-for-surgery/clinicians/glycemic.
- 32.Levy N, Dhatariya K. Pre-operative optimisation of the surgical patient with diagnosed and undiagnosed diabetes: a practical review. Anaesthesia 2019;74(Suppl. 1):58–66 [DOI] [PubMed] [Google Scholar]
- 33.Centers for Disease Control and Prevention Surgical Site Infection (SSI) event, 2019. Accessed 5 September 2019. Available from https://www.cdc.gov/nhsn/pdfs/pscmanual/9pscssicurrent.pdf
- 34.Kotagal M, Symons RG, Hirsch IB, et al.; SCOAP-CERTAIN Collaborative . Perioperative hyperglycemia and risk of adverse events among patients with and without diabetes. Ann Surg 2015;261:97–103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kwon S, Thompson R, Dellinger P, Yanez D, Farrohki E, Flum D. Importance of perioperative glycemic control in general surgery: a report from the Surgical Care and Outcomes Assessment Program. Ann Surg 2013;257:8–14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Frisch A, Chandra P, Smiley D, et al. Prevalence and clinical outcome of hyperglycemia in the perioperative period in noncardiac surgery. Diabetes Care 2010;33:1783–1788 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ramos M, Khalpey Z, Lipsitz S, et al. Relationship of perioperative hyperglycemia and postoperative infections in patients who undergo general and vascular surgery. Ann Surg 2008;248:585–591 [DOI] [PubMed] [Google Scholar]