Abstract
OBJECTIVE
To investigate the association of folate and vitamin B12 in early pregnancy with gestational diabetes mellitus (GDM) risk.
RESEARCH DESIGN AND METHODS
The data of this study were from a subcohort within the Shanghai Preconception Cohort Study. We included pregnancies with red blood cell (RBC) folate and vitamin B12 measurements at recruitment (between 9 and 13 gestational weeks) and those with three samples available for glucose measurements under an oral glucose tolerance test. GDM was diagnosed between 24 and 28 weeks’ gestation. Odds ratio (OR) and 95% CI of having GDM was used to quantify the association.
RESULTS
A total of 1,058 pregnant women were included, and GDM occurred in 180 (17.01%). RBC folate and vitamin B12 were significantly higher in pregnancies with GDM than those without GDM (P values were 0.045 and 0.002, respectively) and positively correlated with 1-h and 2-h serum glucose. Daily folic acid supplementation in early pregnancy increases the risk of GDM; OR (95% CI) was 1.73 (1.19–2.53) (P = 0.004). Compared with RBC folate <400 ng/mL, pregnancies with RBC folate ≥600 ng/mL were associated with ∼1.60-fold higher odds of GDM; the adjusted OR (95% CI) was 1.58 (1.03–2.41) (P = 0.033). A significant trend of risk effect on GDM risk across categories of RBC folate was observed (Ptrend = 0.021). Vitamin B12 was significantly associated with GDM risk (OR 1.14 per 100 pg/mL; P = 0.002). No significant association of serum folate and percentile ratio of RBC folate/vitamin B12 with GDM was observed.
CONCLUSIONS
Higher maternal RBC folate and vitamin B12 levels in early pregnancy are significantly associated with GDM risk, while the balance of folate/vitamin B12 is not significantly associated with GDM.
Introduction
As one of the most common pregnancy complications, gestational diabetes mellitus (GDM) affects ∼17% of pregnancies worldwide (1). In China, ∼2.9 million pregnant women suffer from this disorder (2). GDM has long-term adverse outcomes in both mothers and offspring (3). Despite its serious complications, the diagnosis of GDM is not performed until the late second or early third trimester (4). Investigating modifiable risk factors in early pregnancy stage would more significantly contribute to the early prevention of GDM.
Folate and vitamin B12, metabolically entwined during one-carbon metabolism, are both key nutrients in early pregnancy and involved in the DNA methylation and cell metabolism (5–7). Folic acid supplementation (FAS) of 0.4 mg/day is conventionally recommended for women of the childbearing age before and during the first trimester of pregnancy for the essential role of folate in the prevention of neural tube defects (NTDs) (8,9). The mandatory folic acid fortification aimed to alleviate micronutrient deficiencies has been implemented by >50 countries (10). Nevertheless, the relationship between folate and GDM risk with inconsistent findings has emerged as a field of interest. The Nurses’ Health Study II including 14,533 women has observed that FAS before pregnancy is associated with a lower risk of GDM (11); in contrast, another cohort study gave opposite conclusions that daily folic acid intake in early pregnancy increased the risk of GDM (12), reminding us that the association of folate with GDM is still equivocal.
Compared with serum folate, red blood cell (RBC) folate responds slower to changes in folate intake and represents the long-term folate status, because the erythrocytes have a 120-day life span and only accumulate folate during erythropoiesis (13). As serum folate is an indicator of recent folate intake and is dramatically affected by FAS, these case-control studies are subject to bias derived from dietary folic acid (13). As such, investigating the association of RBC folate with GDM will be more helpful to elucidate the long-term exposure of folate and GDM risk. A 15-year national cohort observed higher RBC folate is associated with an increased risk of death among adults with diabetes (14). Xie et al. (15) found maternal RBC folate concentrations during the second trimester significantly in association with an increased risk of GDM, which is diagnosed in the same period of gestation. Whether maternal RBC folate exposure in early pregnancy associated with the incidence of GDM has not been investigated.
Vitamin B12 deficiency has been associated with risk of metabolic abnormalities in pregnant women, such as insulin resistance, fatty acids, and GDM (16). Imbalanced levels of folate and vitamin B12 increase the risk of being born small for gestational age (17). Increasing concerns have risen about supplementing high doses of folic acid to women of reproductive age with low vitamin B12 intake, although this issue has not been well investigated yet (5). Several case-control studies demonstrated that higher serum folate/vitamin B12 ratio was positively associated with GDM risk (18,19). However, to our knowledge, no studies have investigated the relationship between maternal vitamin B12 in early pregnancy and GDM. In this study, we aimed to evaluate the association of maternal folate, vitamin B12, and percentile ratio of folate/vitamin B12 in early pregnancy with GDM using a prospective cohort study. The findings of this study would be useful to provide a theoretical basis for early prevention of GDM.
Research Design and Methods
Study Population
This study was conducted within the Shanghai Preconception Cohort (SPCC; NCT02737644) that aims to investigate the associations between periconceptional maternal key nutrients and neonatal health outcomes, including congenital heart disease (20). Two subcohorts consisting of 938 and 458 subjects were derived for studying the associations of RBC folate with congenital heart diseases and infant atopic dermatitis. These two subcohorts thus formed the source of our study population. We restricted our study sample to pregnancies with folate and vitamin B12 measurements examined at recruitment between 9 and 13 gestational weeks and with three glucose measurements under an oral glucose tolerance test (OGTT), resulting in 1,058 pregnancies included in the final analysis.
Baseline characteristics were obtained by a standardized self-reported questionnaire via face-to-face interviewers. The preconceptional BMI was calculated through self-report prepregnancy weight and height. Smoking exposure was defined as self-smoker or exposed to secondhand cigarette smoking during pregnancy. Alcohol drinking was defined as drinking any alcoholic beverages 3 months before or during pregnancy. FAS was defined as a regular intake of folic acid tablets or compound vitamin supplements after the last menstrual period and during pregnancy. The folic acid tablet contains 400 μg/pill of folic acid, and the compound vitamin supplements contain 400 μg/pill or 800 μg/pill depending on the brands. Written informed consent was obtained from each study subject before recruitment. This study was approved by the Ethics Committee of the Children’s Hospital of Fudan University (Shanghai, China).
Diagnosis of GDM
All the pregnant women underwent a 75-g OGTT during 24–28 gestational weeks, and diagnosis of GDM was made when any of the following criteria were met according to the recommendations of the International Association of the Diabetes and Pregnancy Study Groups Consensus Panel (4): fasting glucose ≥5.1 mmol/L, 1-h glucose ≥10.0 mmol/L, or 2-h glucose ≥8.5 mmol/L.
Blood Sample Collection and Nutritious Biomarker Measurements
The details of blood sample collection and nutritious biomarker measurements were described elsewhere (20). Briefly, the remaining blood samples after routine examinations at the first antenatal visit were collected by trained staff. These samples were temporarily stored in a refrigerator at 4°C for dispensing within 6 h and were transferred to another refrigerator at −20°C. Serum and whole blood were then transported by trained investigators to the central laboratory for storage in freezers at −80°C. Given the effect of light on folate measurements, lightproof tubes completely protected from light were used when transported and stored. RBC folate, serum folate, and vitamin B12 levels were measured in an ETDA anticoagulated blood sample according to a standard protocol. These biomarkers were measured using electrochemiluminescence assays (ARCHITECT i2000SR Analyzer; Abbott Laboratories) by professional staff. Three standard solutions (quality controls [QC]1, QC2, and QC3) were used as daily QC. The interassay coefficient of variation of QC1–QC3 for serum folate, vitamin B12, and RBC folate was <7.5%, and the intra-assay coefficient of variation was <6.5% for the entire study population.
Statistical Analysis
Continuous data were summarized as mean (SD) or median (interquartile range), and categorical data were displayed as percentages. An unpaired Student t test was used to test the differences in quantitative variables with a normal distribution. Otherwise, the equivalent nonparametric test was used. Categorical variables were compared with χ2 tests. RBC folate concentrations >400 ng/mL (906 nmol/L) have been recommended as the optimal cutoff level for preventing NTDs in women of reproductive age (21). Additionally, RBC folate concentrations >600 ng/mL have been chosen as a cutoff for high RBC folate according to the Canadian Health Measures Survey (22). Accordingly, RBC folate concentrations were categorized as <400, 400–600, and ≥600 ng/mL in the current study. Vitamin B12 and serum folate were categorized into tertiles because there is no specified cutoff for pregnancy. We used multivariable logistic regression analyses to investigate the associations of RBC folate, serum folate, and vitamin B12 with GDM and treated these biomarkers as continuous and categorical variables, with adjustment for age, preconceptional BMI, family history of diabetes, smoking exposure, and drinking status. To further examine the effects of combinations of folate and vitamin B12 status on the risk of GDM, we used percentiles of RBC folate, serum folate, and vitamin B12 to derive ratios of RBC folate/vitamin B12 and serum folate/vitamin B12. Percentile ratios were categorized into tertiles for the association analyses with the GDM risk. According to previous studies (19), the folate/vitamin B12 ratio was also calculated based on their raw data. Moreover, the restricted cubic spline (RCS) regression model with assumed three knots was used to outline the potential nonlinear relations among continuous RBC folate, vitamin B12, and GDM risk. The association of serum folate at the time of OGTT with GDM based on a subgroup of our study subjects (n = 458) was also conducted. Serum folate change was calculated by the levels in early pregnancy subtracted by the levels in midgestation. Odds ratio (OR) and 95% CI were reported. Correlation analysis was used to evaluate the relationship among RBC folate, vitamin B12, fasting serum glucose, and 1-h and 2-h serum glucose. Stata version 16.0 and R package (version 3.6.1) were used for all statistical analyses. A two-tailed P value of 0.05 was defined as statistical significance.
Results
Baseline Characteristics
The demographic characteristics and nutrient levels of the study population are summarized in Table 1. The mean (± SD) age and preconceptional BMI of the pregnant women were 30.24 (± 3.97) years and 21.03 (± 2.81) kg/m2, respectively. Among the pregnant women, 38.75%, 15.60%, and 13.42%, respectively, had a family history of diabetes, smoking exposure, and alcohol consumption during gestation. FAS was found in 63.70% (674 out of 1,058) of the pregnant women, of whom 96.43% (650 out of 674) took compound vitamin supplements. The median (interquartile range) concentrations of RBC folate, serum folate, and vitamin B12 were 385.92 (289.97–529.68), 14.39 (12.60–16.80), and 405.93 (298.00–485.00) (Supplementary Fig. 1).
Table 1.
Demographic characteristics of the study population (N = 1,058)
| Characteristics | Summary |
|---|---|
| Age (years) | 30.2 ± 4.0 |
| Preconception BMI (kg/m2) | 21.0 ± 2.8 |
| <18.5 | 170 (16.1) |
| 18.5–24 | 748 (70.7) |
| >24 | 140 (13.2) |
| Family history of diabetes [n (%)] | |
| Yes | 410 (38.7) |
| No | 648 (61.3) |
| Smoking exposure [n (%)] | |
| Yes | 165 (15.6) |
| No | 893 (84.4) |
| Alcohol consumption [n (%)] | |
| Yes | 142 (13.4) |
| No | 916 (86.6) |
| FAS [n (%)] | |
| Compound vitamin supplements | 650 (61.4) |
| Folic acid tablet | 24 (2.3) |
| No | 384 (36.3) |
| RBC folate (ng/mL) | 385.92 (290.0–529.7) |
| <400 | 557 (52.6) |
| 400–600 | 301 (28.5) |
| ≥600 | 200 (18.9) |
| Serum folate (ng/mL) | 14.39 (12.6–16.8) |
| <25.5 | 1,045 (98.8) |
| ≥25.5 | 13 (1.2) |
| Vitamin B12 (pg/mL) | 405.93 (298.0–485.0) |
| <200 | 113 (10.7) |
| ≥200 | 945 (89.3) |
Continuous variables are presented as means ± SD or median (interquartile range), and categorical variables are listed as n (%). No missing data existed in the current analysis.
Comparison of RBC Folate, Serum Folate, and Vitamin B12
Among 1,058 pregnancies, GDM occurred in 180 women (17.01%) with a mean follow-up time of 11.1 ± 1.5 gestational weeks. Compared with the group without GDM, median RBC folate and vitamin B12 levels in early pregnancy were significantly higher in the group with GDM (for RBC folate: 426.44 vs. 380.95 ng/mL, P = 0.045; for vitamin B12: 421.00 vs. 364.00 pg/mL, P = 0.002). No significant differences in serum folate concentration between GDM and non-GDM were found (Supplementary Fig. 2).
Associations of Maternal Folate Status and Vitamin B12 With GDM Risk
FAS was associated with an increased risk of GDM (adjusted OR [aOR] 1.73 [95% CI 1.19–2.53]; P = 0.004). No significant association was found between RBC folate and serum folate levels as continuous variables with GDM (for RBC folate: aOR 1.07 [95% CI 0.99–1.15]; P = 0.08; for serum folate: aOR 1.01 [95% CI 0.97–1.05]; P = 0.43) (Table 2). Compared with pregnant women who had RBC folate levels <400 ng/mL, those with RBC folate ≥600 ng/mL were associated with ∼1.60-fold higher odds of GDM (aOR 1.58 [95% CI 1.03–2.41]; P = 0.033). An obvious increased risk trend across RBC folate groups with GDM risk was observed (Ptrend = 0.021).
Table 2.
Association of maternal FAS, RBC folate, and vitamin B12 in early pregnancy with GDM risk
| Variables | GDM/total (%) | Model 1† | Model 2‡ | ||||
|---|---|---|---|---|---|---|---|
| OR | 95% CI | P | OR | 95% CI | P | ||
| FAS | |||||||
| No | 43/340 (12.7) | Reference | Reference | ||||
| Yes | 137/718 (19.1) | 1.62 | 1.12–2.35 | 0.010 | 1.73 | 1.19–2.53 | 0.004 |
| RBC folate (ng/mL)* | 1.08 | 1.00–1.17 | 0.033 | 1.07 | 0.99–1.15 | 0.08 | |
| <400 | 80/557 (14.4) | Reference | Reference | ||||
| 400–600 | 56/301 (18.6) | 1.36 | 0.93–1.98 | 0.10 | 1.39 | 0.94–2.04 | 0.09 |
| ≥600 | 44/200 (22.0) | 1.68 | 1.11–2.53 | 0.013 | 1.58 | 1.03–2.41 | 0.033 |
| Trend test | 0.009 | 0.021 | |||||
| Serum folate (ng/mL) | 1.01 | 0.97–1.04 | 0.53 | 1.01 | 0.97–1.05 | 0.43 | |
| Q1 (<13.9) | 64/401 (16.0) | Reference | Reference | ||||
| Q2 (13.9–16.0) | 36/255 (14.1) | 0.86 | 0.55–1.34 | 0.52 | 0.91 | 0.58–1.44 | 0.71 |
| Q3 (≥16.0) | 80/402 (20.0) | 1.30 | 0.91–1.87 | 0.14 | 1.36 | 0.94–1.99 | 0.10 |
| Trend test | 0.13 | 0.09 | |||||
| Vitamin B12 (pg/mL)* | 1.11 | 1.02–1.20 | 0.012 | 1.14 | 1.04–1.24 | 0.002 | |
| Q1 (<330) | 51/397 (12.9) | Reference | Reference | ||||
| Q2 (330–429) | 45/254 (17.7) | 1.46 | 0.94–2.25 | 0.08 | 1.49 | 0.95–2.33 | 0.07 |
| Q3 (≥429) | 84/407 (20.6) | 1.76 | 1.20–2.57 | 0.003 | 2.00 | 1.35–2.96 | 0.001 |
| Trend test | 0.003 | 0.001 | |||||
RBC folate and vitamin B12 were divided by 100.
Univariate model.
Adjusted for age, preconceptional BMI, family history of diabetes, smoking exposure, and drinking status.
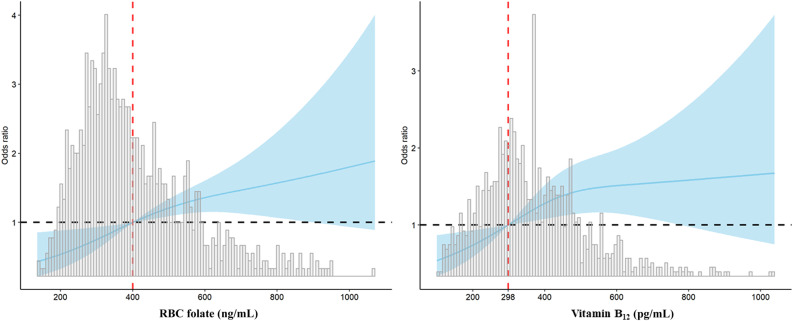
Vitamin B12 level was significantly associated with GDM risk (OR 1.14 per 100 pg/mL [95% CI 1.04–1.24]; P = 0.002). Of note, pregnant women in the upper tertile of vitamin B12 had approximately twofold higher odds of GDM (aOR 2.00 [95% CI 1.35–2.96]; P = 0.001). We also investigated the nonlinear relationship among RBC folate, vitamin B12, and GDM risk. The RCS regression model revealed that higher levels of RBC folate and vitamin B12 were associated with an increased risk of GDM in a nonlinear fashion (Fig. 1).
Figure 1.
RCS regression analysis of RBC folate and vitamin B12 with GDM risk. A: RBC folate 400 ng/mL was selected as the reference level. B: The median of vitamin B12 298 pg/mL was selected as the reference level. The lines indicate estimated ORs, and the light blue areas represent 95% CI.
Moreover, RBC folate concentration in early pregnancy was positively correlated with OGTT 1-h and 2-h serum glucose levels, with the correlation coefficient r values being 0.19 and 0.11, respectively (both P < 0.001). Similar correlations were observed between vitamin B12 and 1-h and 2-h serum glucose level (both r values were 0.13; P < 0.001).
Associations of Folate/Vitamin B12 Balance With GDM Risk
A greater serum folate/vitamin B12 ratio was associated with a decreased GDM risk (aOR 0.98 [95% CI 0.97–0.99]; P = 0.009) (Supplementary Table 1). The risk of GDM became significantly lower in the Q3 tertile compared with that in the Q1 tertile (aOR 0.60 [95% CI 0.41–0.98]; P = 0.042) and showed a significant trend (Ptrend = 0.041). However, no significant association was found between RBC folate/vitamin B12 ratio and GDM. When analyzed as percentile ratios, neither RBC folate/vitamin B12 nor serum folate/vitamin B12 was significantly associated with GDM (Table 3).
Table 3.
Association of percentile folate/vitamin B12 ratio in early pregnancy with GDM risk
| Variables | GDM/total (%) | Model 1† | Model 2‡ | ||||
|---|---|---|---|---|---|---|---|
| OR | 95% CI | P | OR | 95% CI | P | ||
| RBC folate/vitamin B12* | 0.99 | 0.97–1.00 | 0.39 | 0.99 | 0.97–1.00 | 0.33 | |
| Q1 (<0.68) | 72/409 (17.60) | Reference | Reference | ||||
| Q2 (0.68–1.62) | 59/354 (16.67) | 0.93 | 0.64–1.36 | 0.73 | 0.89 | 0.60–1.31 | 0.56 |
| Q3 (≥1.62) | 49/295 (16.61) | 0.93 | 0.62–1.38 | 0.73 | 0.84 | 0.55–1.26 | 0.41 |
| Trend test | 0.71 | 0.40 | |||||
| <1 | 111/613 (18.10) | Reference | Reference | ||||
| ≥1 | 69/445 (15.50) | 0.82 | 0.59–1.15 | 0.26 | 0.77 | 0.55–1.09 | 0.15 |
| Serum folate/vitamin B12* | 0.99 | 0.94–1.03 | 0.70 | 0.98 | 0.94–1.03 | 0.51 | |
| Q1 (>0.76) | 63/336 (18.75) | Reference | Reference | ||||
| Q2 (0.76–1.24) | 61/322 (18.94) | 1.01 | 0.68–1.49 | 0.94 | 1.01 | 0.68–1.51 | 0.93 |
| Q3 (≥1.24) | 56/400 (14.00) | 0.70 | 0.47–1.04 | 0.08 | 0.66 | 0.44–1.01 | 0.05 |
| Trend test | 0.07 | 0.05 | |||||
| <1 | 98/529 (18.52) | Reference | Reference | ||||
| ≥1 | 82/529 (15.50) | 0.80 | 0.58–1.11 | 0.19 | 0.78 | 0.56–1.09 | 0.15 |
Based on percentile of concentrations.
Univariate model.
Adjusted for age, preconceptional BMI, family history of diabetes, smoking exposure, and drinking status.
Associations of Serum Folate in Midgestation With GDM Risk
Among a subgroup of our study cohort with available serum folate examined at midgestation, we found that higher serum folate levels at the time of OGTT were associated with a higher risk of GDM (aOR 1.08 [95% CI 1.03–1.14]; P = 0.002) (Supplementary Table 2). More pronounced effects were found when analyzed using serum folate tertiles (highest vs. lowest tertiles: aOR 2.54 [95% CI 1.28–5.03]; P = 0.007). However, the change in serum folate was negatively associated with GDM risk (aOR 0.95 [95% CI 0.90–0.99]; P = 0.019), indicating an association of greater decrease of serum folate from early to midgestation with a lower risk of GDM.
Conclusions
In this prospective cohort, we investigated the association of maternal folate, vitamin B12, and percentile ratios of folate/vitamin B12 in early pregnancy with the incidence of GDM. We found an increased risk of GDM in a dose-response manner across RBC folate and vitamin B12 concentrations and across their corresponding categories during early pregnancy. Supportive findings include positive correlations between the two biomarkers with OGTT 1-h and 2-h serum glucose levels. However, we did not find a significant impact of the balance of the two biomarkers measured by percentile ratio with GDM.
Folate is one of the key nutrients for pregnant women for its protective effect in preventing birth defects. Two large studies have assessed the association of periconceptional FAS with subsequent GDM risk (11,12) but with inconsistent findings. While preconception FAS was associated with reduced GDM risk in the Nurses’ Study (11), opposite findings were found for FAS during early pregnancy in the China-Anhui Birth Cohort (12). However, due to variations in individual folate metabolism, periconceptional folate status evaluated by FAS via questionnaire cannot directly reflect the folate levels in the body. In a case-control study of 2,282 Chinese pregnancies, Xie et al. (15) found that RBC folate during midpregnancy (19–24 weeks’ gestation) was linked with a 1.16-fold increased risk of GDM. Nevertheless, it is hard to infer that higher folate levels result in GDM, because both the RBC folate and GDM diagnosis were achieved in almost the same period of gestation. In the current study, we were able to directly measure RBC folate levels in early pregnancy. We found that the relationship between RBC folate status with GDM was nonlinear based on our RCS model, and RBC folate of >400 ng/mL, especially >600 ng/mL, conferred as much as a 1.6-fold higher odds of GDM. The findings were supported by the positive correlations between RBC folate with 1-h and 2-h serum glucose, which was consistent with recently published results (15,19). To our knowledge, our study provides the first evidence that the higher RBC folate concentration in early pregnancy may confer subsequent GDM risk. Given the protective role of RBC folate for NTDs and other folate-sensitive defects (21), it is justifiable to maintain an optimal level of ∼400 ng/mL for women with baseline RBC levels less than this value. However, for those with a RBC folate level of >400 ng/mL, attention should be paid to the potential increased risk of GDM as well as other health outcomes (23).
The association between serum vitamin B12 levels and GDM has been explored in two small prospective studies (24,25), in which vitamin B12 was measured after 24 weeks of gestation. In both studies, lower vitamin B12 levels were associated with an increased risk of GDM at 28–30 gestational weeks. On the contrary, we observed a positive association between vitamin B12 concentration in early pregnancy and GDM risk. Although the reason for this discrepancy is unknown, it may be because of the differences in the gestational time points when determinations of vitamin B12 were made between the studies. Research has documented physiological changes influencing maternal vitamin B12 requirements and status during pregnancy, including a gradual decrease in vitamin B12 concentrations as pregnancy processed from preconception to midgestation (26,27). In addition, the proper preparation and storage of vitamin B12 are critical to obtaining accurate results. In the study by Krishnaveni et al. (24), vitamin B12 was examined in samples that were stored for 8 years and under no lightproof conditions, which might affect the association of vitamin B12 deficiency and GDM risk.
The mechanisms by which folate and vitamin B12 may confer an increased GDM risk are not well understood. Evidence from an animal model revealed that higher maternal folate concentration was positively associated with insulin resistance in offspring (28). A positive correlation between RBC folate and insulin resistance was observed in the obese population (29). In addition, folate was reported to play a role in the progression of type 1 diabetes via NK cell dysfunction in human subjects (30). Regarding vitamin B12, higher levels of its circulating form in our study may be partially due to liver function impairment. About 50% of absorbed vitamin B12 is stored in the liver, and elevated serum vitamin B12 levels have been found in chronic viral hepatitis and cirrhosis and severe alcoholic liver disease (31,32). In our additional subgroup analysis, we found that higher γ-glutamyl transferase levels, which were an indicator of mild liver dysfunction and were associated with GDM risk (33), were higher in our group with GDM (Supplementary Fig. 3). We thus assumed pregnancies with GDM might have concurrent mild liver dysfunction. Consequently, the observed association of serum vitamin B12 in early pregnancy with GDM might reflect differences in liver function between the pregnancies with or without GDM.
Among a subgroup of the study sample, we also observed associations of higher serum folate levels at the time of the OGTT with a higher risk of GDM (Supplementary Table 2), which confirmed the findings of previous studies (19). Furthermore, greater decrease of serum folate from early to midgestation was associated with a lower risk of GDM, supporting our hypothesis that high folate levels in early pregnancy could be a risk factor for GDM. Our novel findings raise an important question regarding the potential mechanism or causality for the development of GDM in subjects with vitamin insufficiency. Future studies longitudinally assessing the relationship of early pregnancy vitamin status in women with low folate and B12 levels late in gestation with GDM may be helpful to explore the mechanism behind the positive associations of folate and vitamin B12 with GDM.
Considering that both folate and vitamin B12 are essential for nucleic acid synthesis, methyl group generation, and conversion of homocysteine to methionine, their balance may have important implications for maternal and neonatal health (5–7). A recent case-control study of 406 pregnant women reported that a higher serum folate/vitamin B12 ratio midgestation (24–28 weeks) calculated based on raw values was associated with a higher risk of GDM (19). We extended their work to using RBC folate/vitamin B12 ratio at early gestation by using a percentile ratio that is robust to differences in measurement scales. Intriguingly, we did not find any significant association between this ratio at early pregnancy and GDM. Unlike the work by Li et al. (19), in which directionally opposite associations were found for folate and vitamin B12, these two markers showed directionally similar associations with GDM in our study. Besides, >95% of the pregnant women took multivitamins in which proportions of the two nutrients were fixed, and the levels of these two biomarkers correlated significantly in our population (r = 0.12; P < 0.001; data not shown). It is thus likely that the effects of RBC folate and vitamin B12 on GDM may be confounded, and this may partially explain the disappeared association for GDM when the percentile ratio of RBC folate and vitamin B12 was used.
This study has several limitations. First, selection bias may exist because we only included pregnancies with measurements of RBC folate, serum folate, and vitamin B12 in early pregnancy from the large-scale SPCC, and the generalizability of our findings to other regions needs further validation. Second, residual confounding cannot be ruled out, as we did not have data on covariates, including physical activities, diet, medication use, and lipid levels. Third, the exploratory nature of our study without a prespecified power calculation precludes us from drawing any confirmative conclusions regarding the association among folate, vitamin B12, and GDM.
In conclusion, we report that higher maternal RBC folate and vitamin B12 levels in early pregnancy are significantly associated with GDM risk, while the balance of folate/vitamin B12 is not significantly associated with GDM. Given the exploratory nature of this study, more investigations are needed to figure out the appropriate levels of folate and vitamin B12 in early pregnancy that are essential for maternal and offspring health.
Article Information
Acknowledgments. The authors thank all of the participants in this study. The authors also thank the physicians, interviewers, laboratory technicians, and the whole SPCC team for their work on this study.
Funding. This study was supported by the National Key Research and Development Program (grant number 2016YFC1000500), Chinese Academy of Medical Sciences Research Unit (grant number 2018RU002), and the Three-Year Action Plan for Strengthening the Construction of Public Health System in Shanghai (grant number GWIV-24).
Duality of Interest. No potential conflicts of interest relevant to this article were reported.
Author Contributions. X.C. contributed to the statistical analysis, interpretation of data, and manuscript writing. Y.Z., D.W., and M.L. were involved in pregnancy recruitment, questionnaires, and sample collection. X.C., Y.W., and X.S. contributed to sample measurement and data management. All authors contributed to results interpretation. H.C. and W.Y. revised the manuscript. G.H. and W.Y. obtained funding and contributed to the conception, design of the study, and study supervision. G.H. and W.Y. are the guarantors of this work and, as such, had full access to all of the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis.
Footnotes
This article contains supplementary material online at https://doi.org/10.2337/figshare.13079519.
References
- 1.Guariguata L, Linnenkamp U, Beagley J, Whiting DR, Cho NH. Global estimates of the prevalence of hyperglycaemia in pregnancy. Diabetes Res Clin Pract 2014;103:176–185 [DOI] [PubMed] [Google Scholar]
- 2.Xu T, Dainelli L, Yu K, et al. . The short-term health and economic burden of gestational diabetes mellitus in China: a modelling study. BMJ Open 2017;7:e018893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Reece EA, Leguizamón G, Wiznitzer A. Gestational diabetes: the need for a common ground. Lancet 2009;373:1789–1797 [DOI] [PubMed] [Google Scholar]
- 4.Metzger BE, Gabbe SG, Persson B, et al.; International Association of Diabetes and Pregnancy Study Groups Consensus Panel . International Association of Diabetes and Pregnancy Study Groups recommendations on the diagnosis and classification of hyperglycemia in pregnancy. Diabetes Care 2010;33:676–682 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Obeid R, Heil SG, Verhoeven MMA, van den Heuvel EGHM, de Groot LCPGM, Eussen SJPM. Vitamin B12 intake from animal foods, biomarkers, and health aspects. Front Nutr 2019;6:93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Crider KS, Yang TP, Berry RJ, Bailey LB. Folate and DNA methylation: a review of molecular mechanisms and the evidence for folate’s role. Adv Nutr 2012;3:21–38 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Paul L, Selhub J. Interaction between excess folate and low vitamin B12 status. Mol Aspects Med 2017;53:43–47 [DOI] [PubMed] [Google Scholar]
- 8.Czeizel AE Is folic acid a risk factor for oral clefts? Eur J Epidemiol 2013;28:841–843 [DOI] [PubMed] [Google Scholar]
- 9.Czeizel AE, Vereczkey A, Szabó I. Folic acid in pregnant women associated with reduced prevalence of severe congenital heart defects in their children: a national population-based case-control study. Eur J Obstet Gynecol Reprod Biol 2015;193:34–39 [DOI] [PubMed] [Google Scholar]
- 10.Burdge GC, Lillycrop KA. Folic acid supplementation in pregnancy: are there devils in the detail? Br J Nutr 2012;108:1924–1930 [DOI] [PubMed] [Google Scholar]
- 11.Li M, Li S, Chavarro JE, et al. . Prepregnancy habitual intakes of total, supplemental, and food folate and risk of gestational diabetes mellitus: a prospective cohort study. Diabetes Care 2019;42:1034–1041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhu B, Ge X, Huang K, et al. . Folic acid supplement intake in early pregnancy increases risk of gestational diabetes mellitus: evidence from a prospective cohort study. Diabetes Care 2016;39:e36–e37 [DOI] [PubMed] [Google Scholar]
- 13.Chanarin I Folate deficiency. In Folates and Pterins. Nutritional, Pharmacological, and Physiological Aspects Vol. 3 Blakley RL, Whitehead VM, Eds. New York, John Wiley & Sons, 1986, pp. 75–146 [Google Scholar]
- 14.Kyte B, Ifebi E, Shrestha S, Charles S, Liu F, Zhang J. High red blood cell folate is associated with an increased risk of death among adults with diabetes, a 15-year follow-up of a national cohort. Nutr Metab Cardiovasc Dis 2015;25:997–1006 [DOI] [PubMed] [Google Scholar]
- 15.Xie K, Xu P, Fu Z, et al. . Association of maternal folate status in the second trimester of pregnancy with the risk of gestational diabetes mellitus. Food Sci Nutr 2019;7:3759–3765 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kouroglou E, Anagnostis P, Daponte A, Bargiota A. Vitamin B12 insufficiency is associated with increased risk of gestational diabetes mellitus: a systematic review and meta-analysis. Endocrine 2019;66:149–156 [DOI] [PubMed] [Google Scholar]
- 17.Gadgil M, Joshi K, Pandit A, et al. . Imbalance of folic acid and vitamin B12 is associated with birth outcome: an Indian pregnant women study. Eur J Clin Nutr 2014;68:726–729 [DOI] [PubMed] [Google Scholar]
- 18.Lai JS, Pang WW, Cai S, et al. . High folate and low vitamin B12 status during pregnancy is associated with gestational diabetes mellitus. Clin Nutr 2018;37:940–947 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Li S, Hou Y, Yan X, et al. . Joint effects of folate and vitamin B12 imbalance with maternal characteristics on gestational diabetes mellitus. J Diabetes 2019;11:744–751 [DOI] [PubMed] [Google Scholar]
- 20.Wang D, Zhang Y, Jiang Y, et al.; SPCC Group . Shanghai Preconception Cohort (SPCC) for the association of periconceptional parental key nutritional factors with health outcomes of children with congenital heart disease: a cohort profile. BMJ Open 2019;9:e031076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cordero AM, Crider KS, Rogers LM, Cannon MJ, Berry RJ. Optimal serum and red blood cell folate concentrations in women of reproductive age for prevention of neural tube defects: World Health Organization guidelines. MMWR Morb Mortal Wkly Rep 2015;64:421–423 [PMC free article] [PubMed] [Google Scholar]
- 22.Colapinto CK, O’Connor DL, Tremblay MS. Folate status of the population in the Canadian Health Measures Survey [published correction appears in CMAJ 2011;183:1519]. CMAJ 2011;183:E100–E106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Colapinto CK, O’Connor DL, Sampson M, Williams B, Tremblay MS. Systematic review of adverse health outcomes associated with high serum or red blood cell folate concentrations. J Public Health (Oxf) 2016;38:e84–e97 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Krishnaveni GV, Hill JC, Veena SR, et al. . Low plasma vitamin B12 in pregnancy is associated with gestational ‘diabesity’ and later diabetes. Diabetologia 2009;52:2350–2358 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tarim E, Bagis T, Kilicdag E, et al. . Elevated plasma homocysteine levels in gestational diabetes mellitus. Acta Obstet Gynecol Scand 2004;83:543–547 [DOI] [PubMed] [Google Scholar]
- 26.Costantine MM Physiologic and pharmacokinetic changes in pregnancy. Front Pharmacol 2014;5:65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Looman M, Geelen A, Samlal RAK, et al. . Changes in micronutrient intake and status, diet quality and glucose tolerance from preconception to the second trimester of pregnancy. Nutrients 2019;11:460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Huang Y, He Y, Sun X, He Y, Li Y, Sun C. Maternal high folic acid supplement promotes glucose intolerance and insulin resistance in male mouse offspring fed a high-fat diet. Int J Mol Sci 2014;15:6298–6313 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Li Z, Gueant-Rodriguez RM, Quilliot D, et al. . Folate and vitamin B12 status is associated with insulin resistance and metabolic syndrome in morbid obesity. Clin Nutr 2018;37:1700–1706 [DOI] [PubMed] [Google Scholar]
- 30.Bayer AL, Fraker CA. The folate cycle as a cause of natural killer cell dysfunction and viral etiology in type 1 diabetes. Front Endocrinol (Lausanne) 2017;8:315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ermens AA, Vlasveld LT, Lindemans J. Significance of elevated cobalamin (vitamin B12) levels in blood. Clin Biochem 2003;36:585–590 [DOI] [PubMed] [Google Scholar]
- 32.Solomon LR Disorders of cobalamin (vitamin B12) metabolism: emerging concepts in pathophysiology, diagnosis and treatment. Blood Rev 2007;21:113–130 [DOI] [PubMed] [Google Scholar]
- 33.Sridhar SB, Xu F, Darbinian J, Quesenberry CP, Ferrara A, Hedderson MM. Pregravid liver enzyme levels and risk of gestational diabetes mellitus during a subsequent pregnancy. Diabetes Care 2014;37:1878–1884 [DOI] [PMC free article] [PubMed] [Google Scholar]