Abstract
Background
Runs of homozygosity (ROH) have become the state-of-the-art method for analysis of inbreeding in animal populations. Moreover, ROH are suited to detect signatures of selection via ROH islands and are used in other applications, such as genomic prediction and genome-wide association studies (GWAS). Currently, a vast amount of single nucleotide polymorphism (SNP) data is available online, but most of these data have never been used for ROH analysis. Therefore, we performed a ROH analysis on large medium-density SNP datasets in eight animal species (cat, cattle, dog, goat, horse, pig, sheep and water buffalo; 442 different populations) and make these results publicly available.
Results
The results include an overview of ROH islands per population and a comparison of the incidence of these ROH islands among populations from the same species, which can assist researchers when studying other (livestock) populations or when looking for similar signatures of selection. We were able to confirm many known ROH islands, for example signatures of selection for the myostatin (MSTN) gene in sheep and horses. However, our results also included multiple other ROH islands, which are common to many populations and not identified to date (e.g. on chromosomes D4 and E2 in cats and on chromosome 6 in sheep).
Conclusions
We are confident that our repository of ROH islands is a valuable reference for future studies. The discovered ROH island regions represent a unique starting point for new studies or can be used as a reference for future studies. Furthermore, we encourage authors to add their population-specific ROH findings to our repository.
Background
Runs of homozygosity (ROH) are long continuous homozygous stretches in the genome and are formed by the combination of two identical haplotypes in an individual [1]. Broman and Weber [2] first identified these long homozygous segments in the human genome and Gibson et al. [3] described their potential for inbreeding assessment. A genomic inbreeding coefficient based on ROH (FROH) was first defined by McQuillan et al. [4]. Since 2010, analysis of ROH has become a standard approach to study inbreeding and detect signatures of selection in animal populations with the first reported studies in 2010 for cattle [5], in 2010 for dogs [6], in 2012 for pigs [7], in 2013 for horses [8], in 2014 for goats [9], in 2015 for sheep [10], in 2016 for cats [11] and in 2020 for water buffaloes [12]. Moreover, ROH analyses are complementary to genome-wide association studies (GWAS), inbreeding depression studies, genomic prediction and detection of deleterious variants and population-specific major genes [1, 13].
ROH analyses allow the accurate estimation of FROH on both the population and the individual level. It is commonly accepted that short ROH are indicators of distant consanguinity, whereas long ROH are more likely the result of recent inbreeding [14]. However, ROH may also result from small inversions that suppress recombination [1] or demographic events [15], such as a population bottleneck, genetic drift, or (artificial) selection. Moreover, it has been shown that ROH are more likely to arise in genomic regions with a low recombination rate and high linkage disequilibrium (LD), such as for the X-chromosome and near the centromere of chromosomes [1, 16]. In addition, copy number variants (CNV) and/or coverage gaps may lead to artefacts in ROH analyses [17]. The effect of gaps in SNP coverage can be reduced by adjusting the parameters set for the ROH analysis, as discussed by Meyermans et al. [18].
ROH facilitate the investigation of highly inbred genomic regions within a population, first referred to as ROH islands by Nothnagel et al. [19]. These ROH islands can provide important insights into the studied population and are likely to be signatures of positive selection due to LD [15, 19–21]. However, currently many populations have not been studied for the identification of ROH islands, although several large online genotype datasets are available for various species. Furthermore, even when such populations have been investigated for ROH islands, it is often difficult to compare the results between studies, for example because of differences in analysis methods or detection criteria.
In this paper, we provide an overview of ROH islands in 442 populations (18,633 individuals) from eight animal species (cat, cattle, dog, goat, horse, pig, sheep and water buffalo) using medium-density SNP data, which were all analyzed using a standardized protocol. The outcome and R-script of our analyses are made online available and can be used as a reference for future studies (https://doi.org/10.17605/OSF.IO/XJTKV). Since ROH islands are potential signatures of selection, overlapping ROH islands across populations and species are a valuable tool in comparative genomic studies and may reveal important genetic regions.
Methods
Medium-density SNP data from eight species (cat, cattle, dog, goat, horse, pig, sheep, and water buffalo) and 797 populations were collected from online available datasets (Table 1). Detailed information and background on these data are described in the corresponding studies.
Table 1.
Data overview per species
| Animal species | Populations before QC | Animals before QC | Populations after QC | Animals after QC | SNPs before QC | References |
|---|---|---|---|---|---|---|
| Cat | 47 | 2078 | 26 | 1657 | 58,888 | [23] |
| Cattle | 144 | 4103 | 50 | 2263 | 45,926 | [28, 49–58] |
| Dog | 146 | 5406 | 49 | 4414 | 160,432 | [24] |
| Goat | 143 | 4653 | 96 | 4327 | 49,943 | [25, 47] |
| Horse | 37 | 795 | 35 | 774 | 50,042 | [26, 48] |
| Pig | 146 | 2113 | 78 | 1438 | 52,783 | [27] |
| Sheep | 118 | 3609 | 100 | 3490 | 52,413 | [28–33] |
| Water buffalo | 16 | 346 | 8 | 270 | 53,830 | [34] |
| Total | 797 | 23,103 | 442 | 18,633 |
The number of populations and individuals are shown for the raw datasets and after applying quality control. The number of autosomal SNPs available per species before quality control is also shown. The main cause for excluding a population was a lack of a sufficient number of individuals (n < 15)
QC quality control, SNPs single nucleotide polymorphisms
Quality control and ROH analyses were performed using the PLINK v1.9 software [22]. Each population was subjected to the following quality control (PLINK commands in brackets). For SNPs, only autosomal SNPs were retained, and neither minor allele frequency pruning (--maf), no Hardy–Weinberg equilibrium test (--hwe), nor LD pruning were performed [18]. Individual call rate was set to 0.90 (--mind 0.10) and possibly duplicated individuals were removed (--genome; PI_HAT > 0.95). Minimal SNP call rate was set to 0.95 (--geno 0.05) and only populations with more than 15 individuals were retained after quality control.
For the ROH analysis (--homozyg), no heterozygous SNP was allowed (--homozyg-window-het and --homozyg-het) and one SNP could be missing (--homozyg-window-missing) [35, 36]. The minimal number of SNPs per window (--homozyg-window-snp) and in the final ROH segment (--homozyg-snp) were breed specifically calculated by the L-parameter [18, 35, 37] and a window had a minimal size of 1000 kb (--homozyg-kb). The density was set to 150 kb/SNP, thus 1 SNP every 150 kb (--homozyg-density), the maximal gap was 1000 kb (--homozyg-gap), and the window threshold set to two outer SNPs [18]. Average SNP density was at least one SNP per 55 kb for all populations. Consequently, genome coverage was higher than 97% for all breeds, which means that the given settings allowed ROH detection for more than 97% of the autosomal genome.
ROH incidence was calculated as the percentage of animals with a SNP within an ROH segment for a given population and were visualised via Manhattan plots using the qqman package [38]. ROH islands were defined as regions where SNPs had a P-value for ROH incidence higher than a population specific threshold. This threshold was calculated based on standard normal z-scores derived from the distribution of ROH incidences. The top 0.1% of SNPs with a P-value higher than 0.999 using a z-score table for ROH incidence were considered to form ROH islands, as specified by Purfield et al. [15] and Gorssen et al. [39]. As an additional restriction, the minimal threshold for detection of ROH islands was set to 30%, which means that a ROH has to be present in at least 30% of the population to be included in a ROH island. For populations with high levels of inbreeding (e.g. Boxer dogs with a mean FROH of 45%), no SNP reached a P-value > 0.999 for ROH incidence. Therefore, a maximal threshold for ROH island detection was set at 80%, meaning that all ROH with an incidence higher than 80% were marked as ROH islands.
Results
An R-script was developed for standardized breed-by-breed quality control and ROH analysis. This script uses a PLINK-format genotype file (.bim,.bed and.fam), with a unique family ID (FID) for each population. First, parameter settings are specified for quality control and ROH analysis. Second, the R-script performs quality control and a ROH analysis per population (FID). Third, the script creates Manhattan plots based on ROH incidence per SNP for every investigated population, and a summary table. For the 442 populations studied here, all figures and the R-script are deposited at Open Science Framework (OSF) (https://doi.org/10.17605/OSF.IO/XJTKV).
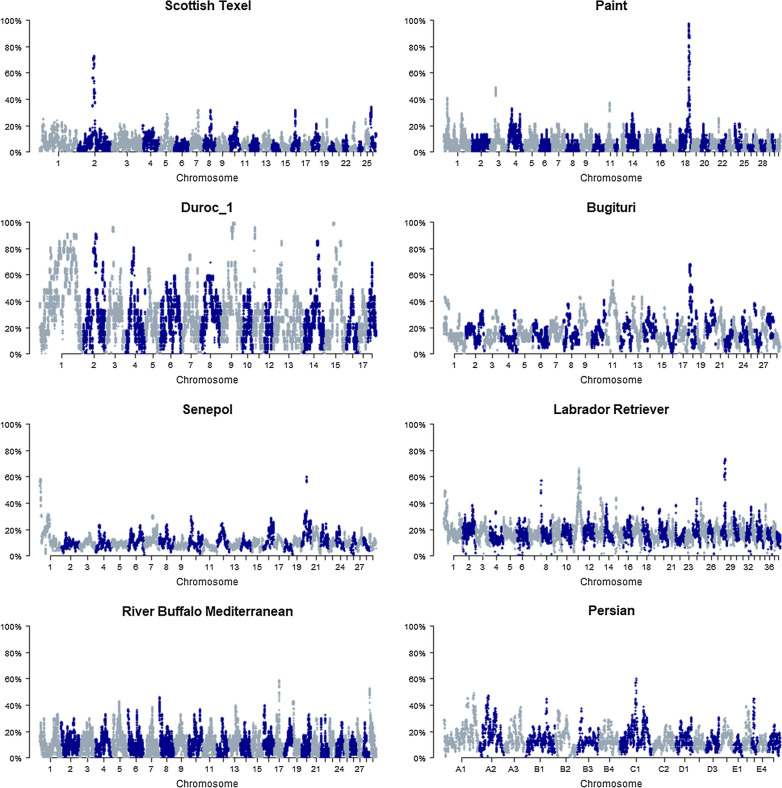
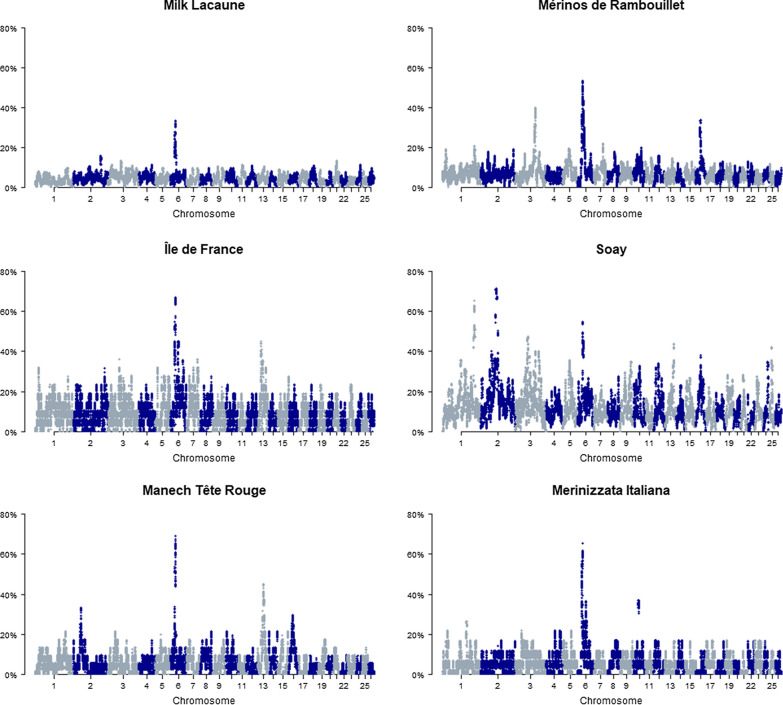
Figures 1 and 2 show an example of the ROH incidence plots. These Manhattan plots provide a quick overview of population-specific baseline ROH levels and ROH islands. For example, in Paint horses, ROH incidence levels are generally low (0 to 15%) but one remarkable ROH island is observed on Equus caballus chromosome (ECA)18 at 68 Mb. This ROH island was found in 23 out of the 24 studied horses in the Paint population (Fig. 1). Duroc pigs have higher baseline ROH levels (0 to 60%), with several ROH islands having an incidence higher than 80% (Fig. 1). To investigate ROH islands in more detail, we created tables with ROH island locations (bins of one Mb) per population, which are in Additional file 1: Tables S1, Additional file 2: Table S2, Additional file 3: Table S3, Additional file 4: Table S4, Additional file 5: Table S5, Additional file 6: Table S6, Additional file 7: Table S7, Additional file 8: Table S8, Additional file 9: Table S9, Additional file 10: Table S10, Additional file 11: Table S11, Additional file 12: Table S12, Additional file 13: Table S13, Additional file 14: Table S14, Additional file 15: Table S15, Additional file 16: Table S16 and in the repository. As an example, detailed information on the ROH islands per breed and chromosomal region for cats is provided in Table 2. These figures and tables can be used to detect overlapping ROH island regions in multiple populations. In cats, for example, 19 out of the 26 studied populations (73%) have a ROH island on chromosome B3 around 27–28 Mb (Table 2). In sheep, 15 of the 100 studied populations show a ROH island on Ovis aries chromosome (OAR)6 at ~ 38 Mb (Fig. 2, Additional file 13: Table S13 and Additional file 14: Table S14).
Fig. 1.
Incidence plots of SNPs in ROH for a population of each species studied in this analysis. Incidence plots of SNPs in ROH are given for a population of sheep (ScottishTexel), horse (Paint), pig (Duroc_1), goat (Bugituri), cattle (Senepol), dog (Labrador Retriever), water buffalo (Mediterranean River Buffalo) and cat (Persian). Clear ROH islands are visible: for example for Scottish Texel sheep (OAR2) and Paint horses (ECA18) around the MSTN gene. Details of ROH islands and populations are in Additional file 1: Tables S1, Additional file 2: Table S2, Additional file 3: Table S3, Additional file 4: Table S4, Additional file 5: Table S5, Additional file 6: Table S6, Additional file 7: Table S7, Additional file 8: Table S8, Additional file 9: Table S9, Additional file 10: Table S10, Additional file 11: Table S11, Additional file 13: Table S13, Additional file 14: Table S14, Additional file 15: Table S15, Additional file 16: Table S16
Fig. 2.
Incidence plots of SNPs in ROH for six sheep populations. These incidence plots show a remarkable ROH island on OAR6 around 38 Mb. This ROH island was seen in 15 of the studied sheep populations. Details of ROH islands and populations are shown in Additional file 13: Table S13, Additional file 14: Table S14
Table 2.
ROH island regions (bins of one Mb) for 26 cat populations listed per chromosome
| Population | Chromosome | |||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| A1 | A2 | A3 | B1 | B2 | B3 | B4 | C1 | C2 | D1 | D2 | D3 | D4 | E1 | E2 | E3 | F1 | F2 | |
| Abyssinian | 34–42 | 27–30 | 65–91 | |||||||||||||||
| American Curl | 17–18 | 143–146 | 15–41 | |||||||||||||||
| Bengal | 87–90 | 82–89 | 39–40 | 60–77 | 37–38 | |||||||||||||
| British Shorthair | 83–87 | 24–30 | 32–37 | 33–33 | ||||||||||||||
| Burmese | 35–49 | 22–30 | 26–55 | 28–32 | ||||||||||||||
| Colony | 25–25 | 37–38 | ||||||||||||||||
| Devon Rex | 35–83 | |||||||||||||||||
| Domestic | 26–29 | |||||||||||||||||
| LaPerm | 83–84 | 27–29 | 31–34 | 25–38 | ||||||||||||||
| Lykoi | 26–29 | 23–38 | ||||||||||||||||
| Maine Coon | 0–4 | 27–28 | 35–38 | 15–17 | ||||||||||||||
| Munchkin | 25–29 | 30–33 | ||||||||||||||||
| Norwegian Forest Cat | 127–129 | 98–99 | 23–30 | 33–35 | 37–38 | |||||||||||||
| Oriental | 83–87 | 23–30 | 32–34 | 166–174 | 91–93 | |||||||||||||
| Oriental Toygers | 157–159 | 130–131 | 27–30 | 114–119 | 27–30 | 49–49 | ||||||||||||
| Persian | 160–203 | 58–61 | 149–150 | 104–112 | 15–17 | |||||||||||||
| Peterbald | 181–193 | 23–58 | 166–168 | 91–122 | ||||||||||||||
| Ragdoll | 54–88 | 40–61 | 26–29 | 46–46 | ||||||||||||||
| Scottish Fold | 101–103 | 49–50 | 25–30 | 37–39 | ||||||||||||||
| Selkirk Rex | 24–29 | 103–110 | 37–40 | |||||||||||||||
| Siamese | 152–186 | 49–52 | 73–73 | 25–30 | 32–34 | 166–174 | 91–93 | 43–45; 92–92 | ||||||||||
| Siberian | 186–186 | 24–30 | 33–33 | |||||||||||||||
| Sphynx | 136–140 | 53–57 | 15–17 | |||||||||||||||
| Tenessee Rex | 25–31 | 22–41 | ||||||||||||||||
| Turkish Van | 34–34 | 31–33 | ||||||||||||||||
| Wildcat | ||||||||||||||||||
Within each cell of the table, the size of genomic region(s) with a ROH island is indicated in Mb. ROH islands were detected for multiple populations on e.g. chromosome B3 (25–29 Mb), E2 (37–38 Mb) and D4 (31–33 Mb)
Discussion
We created a repository of ROH-islands for 442 populations from eight animal species (cat, cattle, dog, goat, horse, pig, sheep and water buffalo). These results are available online via OSF (https://doi.org/10.17605/OSF.IO/XJTKV) and examples are provided in Figs. 1 and 2, Table 2 and Additional file 1: Tables S1, Additional file 2: Table S2, Additional file 3: Table S3, Additional file 4: Table S4, Additional file 5: Table S5, Additional file 6: Table S6, Additional file 7: Table S7, Additional file 8: Table S8, Additional file 9: Table S9, Additional file 10: Table S10, Additional file 11: Table S11, Additional file 12: Table S12, Additional file 13: Table S13, Additional file 14: Table S14, Additional file 15: Table S15, and Additional file 16: Table S16.
For many populations, the figures of ROH incidence show interesting ROH islands. For example, previously known ROH islands are observed around the myostatin (MSTN) gene in Texel sheep (OAR2, 129 Mb) and Paint and Quarter horses (ECA18, 66 Mb) (Fig. 1) and Additional file 9: Table S9, Additional file 10: Table S10, Additional file 13: Table S13, and Additional file 14: Table S14. Both Purfield et al. [15] and Fariello et al. [40] found a signature of selection in the region spanning the MSTN gene in Texel sheep using different methods [15, 40]. Petersen et al. [26] showed the existence of a clear signature of selection in the genomic region around MSTN in Paint and Quarter Horses using FST-based statistics [26]. MSTN is a major gene involved in muscle development. In sheep, selection on conformation has apparently led to a common ROH island, spanning the MSTN gene. In cats, we found that 19 of the 26 populations showed a ROH island on chromosome B3 between 20 and 30 Mb (Table 2). Montague et al. [41] found strong signatures of selection in cats in these regions on chromosome B3 using FST-based analysis [41]. They suggested that the ARID3B gene—which is known to impact neural crest cell survival—might be the driving factor for the selection signature on chromosome B3 and they linked it to the domestication syndrome hypothesis [42].
Although the above-mentioned ROH islands had an assumed/known underlying biological factor driving positive selection, we detected multiple ROH islands in different species without a clear link to the underlying (biological) mechanisms, so far. These ROH islands might indicate genetic regions under positive selection, although they could also be the result of population bottlenecks, regions with repressed recombination, or artefacts caused by CNV or SNP gaps [1, 13, 17]. We observed that ROH and ROH islands occured more frequently near the centromere of chromosomes, possibly due to a difference in recombination levels [7, 17]. We minimized ROH artefacts that are caused by large SNP gaps, by optimizing ROH detection in PLINK as described in Meyermans et al. [18], in particular by adjusting the minimal SNP density for ROH detection. However, hemizygous deletions that are especially large might still resemble a ROH signal in SNP data [1, 4, 17]. To address this and also the possible interference between ROH and CNV via SNP data, raw genotyping data (e.g. Illumina final report files) are required. These are often not available for such large datasets and could not be examined in this study. Moreover, ascertainment bias might also impact ROH analysis as genotyping arrays can be less suitable for populations that are only distantly related to the populations used for array development. In this study, ascertainment bias was minimized by calculating the L-parameter, which takes potential heterozygosity differences into account [18, 35, 37] and by setting the minimum ROH length to 1000 kb. Furthermore, we would like to note that the presence of a ROH island does not implicate an identical underlying haplotype. For example, in the context of selection, ROH are likely to arise in genomic regions that contain important genes, but the underlying genotypes might differ among populations and individuals [39].
Regardless of their origin, ROH islands can provide a valuable clue for future research. For example, 15 sheep populations in this study show a ROH island on OAR6 at 38 Mb (Fig. 2). OAR6 appears to harbour multiple genes that are linked with milk production in sheep, but we also found several QTL for fat-tail, growth and bone-related traits near the region around 38 Mb [43, 44]. As another example, seven cat populations showed a ROH island on chromosome E2 (37–38 Mb) and six cat populations on chromosome D4 (33 Mb) (Table 2). However, to our knowledge, these regions have never been reported in the literature as regions of interest. Therefore, these overlapping ROH islands can be a starting point for researchers investigating signatures of selection but also for studies on islands of speciation, recombination hot- and coldspots and population history. Furthermore, researchers can compare the outcome of their (ROH) studies with our repository. Comparing (ROH) results among studies reported in the literature is often difficult, due to differences in quality control, software and parameter settings. The advantage of our repository is that it combines information on multiple populations and species which were analysed using a standardized method.
To define ROH islands, we implemented a population-specific threshold based on the ROH incidence distribution, which allows a comparison of ROH islands across different populations. In this study, we used PLINK for ROH detection as it is still the most frequently used software for these analyses [13]. Especially when using medium-density SNP data, it is essential to optimize (PLINK) ROH detection settings [18]. For example, the default value for density setting in PLINK is one SNP per 50 kb, which is higher than the mean density for our sheep (one SNP per 50.5 kb) and cattle (one SNP per 54.5 kb) SNP data even before quality control. Thus, using the default PLINK values can dramatically underestimate the number of ROH detected. Our recommendation for future studies using PLINK is to carefully consider these parameters, to make sure results are as correct and comparable as possible. To facilitate this, we share our R-script with other researchers upon proper citation (https://doi.org/10.17605/OSF.IO/XJTKV). We would like to draw attention to the fact that the dog data had a higher average SNP density (one SNP per 14 kb). However, differences in SNP density were accounted for by following Meyermans et al. [18]. Besides PLINK, other algorithms are also available for ROH detection, for example RzooRoH [45, 46]. Results obtained by using these software can also be included in our collection, since differences between rule-based (e.g., PLINK) and model-based (e.g., RzooRoH) approaches should be small when performed correctly on medium-density SNP data [39, 45, 46].
Conclusions
We have shown that important ROH islands can be detected by scanning multiple populations simultaneously for ROH islands using a standardized detection method. We provide our script for standardized ROH island analyses and make all the results publicly available via OSF (https://doi.org/10.17605/OSF.IO/XJTKV). By sharing our results, our aim is to give researchers a useful reference to compare with their own analyses or to provide a unique starting point to investigate specific signatures of selection. Moreover, we encourage authors of future ROH studies to add their Manhattan plots of ROH incidence to our collection. We strongly believe that this ROH island repository will be very valuable for comparisons with future (ROH) studies or as a starting point for new studies.
Supplementary Information
Additional file 1: Table S1. ROH island regions (bins of one Mb) for the studied cat populations: overview for all chromosomes. Within each cell, the genomic region(s) with an ROH island are given (in Mb).
Additional file 2: Table S2. ROH island regions (bins of one Mb) for the studied cat populations: details per chromosome. Within the Excel file, there is one tab for each chromosome with ROH island details per population. Cells are marked with an ‘x’ if an ROH island appeared in the specific region.
Additional file 3: Table S3. ROH island regions (bins of one Mb) for the studied cattle populations: overview for all chromosomes. Within each cell, the genomic region(s) with an ROH island are given (in Mb).
Additional file 4: Table S4. ROH island regions (bins of one Mb) for the studied cattle populations: details per chromosome. Within the Excel file, there is one tab for each chromosome with ROH island details per population. Cells are marked with an ‘x’ if an ROH island appeared in the specific region.
Additional file 5: Table S5. ROH island regions (bins of one Mb) for the studied dog populations: overview for all chromosomes. Within each cell, the genomic region(s) with an ROH island are given (in Mb).
Additional file 6: Table S6. ROH island regions (bins of one Mb) for the studied dog populations: details per chromosome. Within the Excel file, there is one tab for each chromosome with ROH island details per population. Cells are marked with an ‘x’ if an ROH island appeared in the specific region.
Additional file 7: Table S7. ROH island regions (bins of one Mb) for the studied goat populations: overview for all chromosomes. Within each cell, the genomic region(s) with an ROH island are given (in Mb).
Additional file 8: Table S8. ROH island regions (bins of one Mb) for the studied goat populations: details per chromosome. Within the Excel file, there is one tab for each chromosome with ROH island details per population. Cells are marked with an ‘x’ if an ROH island appeared in the specific region.
Additional file 9: Table S9. ROH island regions (bins of one Mb) for the studied horse populations: overview for all chromosomes. Within each cell, the genomic region(s) with an ROH island are given (in Mb).
Additional file 10: Table S10. ROH island regions (bins of one Mb) for the studied horse populations: details per chromosome. Within the Excel file, there is one tab for each chromosome with ROH island details per population. Cells are marked with an ‘x’ if an ROH island appeared in the specific region.
Additional file 11: Table S11. ROH island regions (bins of one Mb) for the studied pig populations: overview for all chromosomes. Within each cell, the genomic region(s) with an ROH island are given (in Mb).
Additional file 12: Table S12. ROH island regions (bins of one Mb) for the studied pig populations: details per chromosome. Within the Excel file, there is one tab for each chromosome with ROH island details per population. Cells are marked with an ‘x’ if an ROH island appeared in the specific region.
Additional file 13: Table S13. ROH island regions (bins of one Mb) for the studied sheep populations: overview for all chromosomes. Within each cell, the genomic region(s) with an ROH island are given (in Mb).
Additional file 14: Table S14. ROH island regions (bins of one Mb) for the studied sheep populations: details per chromosome. Within the Excel file, there is one tab for each chromosome with ROH island details per population. Cells are marked with an ‘x’ if an ROH island appeared in the specific region.
Additional file 15: Table S15. ROH island regions (bins of one Mb) for the studied water buffalo populations: overview for all chromosomes. Within each cell, the genomic region(s) with an ROH island are given (in Mb).
Additional file 16: Table S16. ROH island regions (bins of one Mb) for the studied water buffalo populations: details per chromosome. Within the Excel file, there is one tab for each chromosome with ROH island details per population. Cells are marked with an ‘x’ if an ROH island appeared in the specific region.
Acknowledgements
Not applicable.
Authors’ contributions
WG and RM analyzed the data and wrote the manuscript. WG, RM, SJ and NB designed and conceived this study. SJ and NB critically reviewed the analyses and the manuscript. All authors read and approved the final manuscript.
Funding
This study was partially funded by an SB PhD fellowship (1S37119N) and an FR PhD fellowship (1104320N) of the Research Foundation Flanders (FWO). The funding bodies played no role in the design of the study and collection, analysis, and interpretation of data and in writing the manuscript.
Availability of data and materials
The datasets analysed during the current study are available in the following repositories.
Cat
Gandolfi et al. [23]. Data available on: https://www.nature.com/articles/s41598-018-25438-0#Sec26.
Cattle
Sempéré et al. [28]. Data available on: http://widde.toulouse.inra.fr/widde/.
Batch selection of all populations with SNP data from Illumina Bovine SNP50v1, Illumina Bovine SNP50v2 and Illumina BovineHD. Only common markers (46,387) were selected from all chromosomes.
Dog
Shannon et al. [24]. Data available on: https://datadryad.org/stash/dataset/doi:10.5061/dryad.v9t5h.
Goat
Colli et al. [25] and data from Bertolini et al. [47]. Dryad Digital Repository. https://doi.org/10.5061/dryad.v8g21pt.
Horse
Petersen et al. [26] and data from Petersen et al. [48]. NAGPR Community Data Repository. https://www.animalgenome.org/repository/pub/UMN2013.0125/.
Pig
Yang et al. [27]. Data at the Dryad Digital Repository. https://doi.org/10.5061/dryad.30tk6.
Sheep
Sempéré et al. [28]. Data available on: http://widde.toulouse.inra.fr/widde/.
Batch selection of all populations with SNP data from Illumina OvineSNP50v1 and AgResearch OvineHD. Only common markers (42,439) were selected from all chromosomes.
Water buffalo
Colli et al. [34]. Data available at: https://datadryad.org/stash/dataset/doi:10.5061/dryad.h0cc7.
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Wim Gorssen and Roel Meyermans share first authorship
Contributor Information
Wim Gorssen, Email: wim.gorssen@kuleuven.be.
Roel Meyermans, Email: roel.meyermans@kuleuven.be.
Steven Janssens, Email: steven.janssens@kuleuven.be.
Nadine Buys, Email: nadine.buys@kuleuven.be.
Supplementary Information
The online version contains supplementary material available at 10.1186/s12711-020-00599-7.
References
- 1.Ceballos FC, Joshi PK, Clark DW, Ramsay M, Wilson JF. Runs of homozygosity: Windows into population history and trait architecture. Nat Rev Genet. 2018;19:220–234. doi: 10.1038/nrg.2017.109. [DOI] [PubMed] [Google Scholar]
- 2.Broman KW, Weber JL. Long homozygous chromosomal segments in reference families from the Centre d’Étude du Polymorphisme Humain. Am J Hum Genet. 1999;65:1493–1500. doi: 10.1086/302661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gibson J, Morton NE, Collins A. Extended tracts of homozygosity in outbred human populations. Hum Mol Genet. 2006;15:789–795. doi: 10.1093/hmg/ddi493. [DOI] [PubMed] [Google Scholar]
- 4.McQuillan R, Leutenegger AL, Abdel-Rahman R, Franklin CS, Pericic M, Barac-Lauc L, et al. Runs of homozygosity in European populations. Am J Hum Genet. 2008;83:359–372. doi: 10.1016/j.ajhg.2008.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sölkner J, Ferenčaković M, Fürst C, Čurik I. Genomic metrics of individual autozygosity, applied to a cattle population. In Proceedings of the 61st Annual Meeting of the European Association for Animal Production: 23–27 August 2010; Heraklion. 2010.
- 6.Boyko AR, Quignon P, Li L, Schoenebeck JJ, Degenhardt JD, Lohmueller KE, et al. A simple genetic architecture underlies morphological variation in dogs. PLoS Biol. 2010;8:e1000451. doi: 10.1371/journal.pbio.1000451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bosse M, Megens HJ, Madsen O, Paudel Y, Frantz LAF, Schook LB, et al. Regions of homozygosity in the porcine genome: Consequence of demography and the recombination landscape. PLoS Genet. 2012;8:e1003100. doi: 10.1371/journal.pgen.1003100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Khanshour AM. Genetic diversity and population structure of the Arabian horse populations from Syria and other countries. PhD thesis, Texas A&M University. 2013.
- 9.Guangul SA. Design of community based breeding programs for two indigenous goat breeds of Ethiopia. PhD thesis, University of Natural Resources and Life Sciences Vienna. 2014.
- 10.Beynon SE, Slavov GT, Farré M, Sunduimijid B, Waddams K, Davies B, et al. Population structure and history of the Welsh sheep breeds determined by whole genome genotyping. BMC Genet. 2015;16:65. doi: 10.1186/s12863-015-0216-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bertolini F, Gandolfi B, Kim ES, Haase B, Lyons LA, Rothschild MF. Evidence of selection signatures that shape the Persian cat breed. Mamm Genome. 2016;27:144–155. doi: 10.1007/s00335-016-9623-1. [DOI] [PubMed] [Google Scholar]
- 12.Ghoreishifar SM, Moradi-Shahrbabak H, Fallahi MH, Jalil Sarghale A, Moradi-Shahrbabak M, Abdollahi-Arpanahi R, et al. Genomic measures of inbreeding coefficients and genome-wide scan for runs of homozygosity islands in Iranian river buffalo Bubalus bubalis. BMC Genet. 2020;21:16. doi: 10.1186/s12863-020-0824-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Peripolli E, Munari DP, Silva MVGB, Lima ALF, Irgang R, Baldi F. Runs of homozygosity: current knowledge and applications in livestock. Anim Genet. 2017;48:255–271. doi: 10.1111/age.12526. [DOI] [PubMed] [Google Scholar]
- 14.Curik I, Ferenčaković M, Sölkner J. Inbreeding and runs of homozygosity: a possible solution to an old problem. Livest Sci. 2014;166:26–34. doi: 10.1016/j.livsci.2014.05.034. [DOI] [Google Scholar]
- 15.Purfield DC, McParland S, Wall E, Berry DP. The distribution of runs of homozygosity and selection signatures in six commercial meat sheep breeds. PLoS One. 2017;12:e0176780. doi: 10.1371/journal.pone.0176780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ma L, O’Connell JR, VanRaden PM, Shen B, Padhi A, Sun C, et al. Cattle sex-specific recombination and genetic control from a large pedigree analysis. PLoS Genet. 2015;11:e1005387. doi: 10.1371/journal.pgen.1005387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nandolo W, Utsunomiya YT, Mészáros G, Wurzinger M, Khayadzadeh N, Torrecilha RBP, et al. Misidentification of runs of homozygosity islands in cattle caused by interference with copy number variation or large intermarker distances. Genet Sel Evol. 2018;50:43. doi: 10.1186/s12711-018-0414-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Meyermans R, Gorssen W, Buys N, Janssens S. How to study runs of homozygosity using plink? a guide for analyzing medium density snp data in livestock and pet species. BMC Genomics. 2020;21:94. doi: 10.1186/s12864-020-6463-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nothnagel M, Lu TT, Kayser M, Krawczak M. Genomic and geographic distribution of snpdefined runs of homozygosity in Europeans. Hum Mol Genet. 2010;19:2927–2935. doi: 10.1093/hmg/ddq198. [DOI] [PubMed] [Google Scholar]
- 20.Peripolli E, Stafuzza NB, Munari DP, Lima ALF, Irgang R, Machado MA, et al. Assessment of runs of homozygosity islands and estimates of genomic inbreeding in Gyr (Bos indicus) dairy cattle. BMC Genomics. 2018;19:34. doi: 10.1186/s12864-017-4365-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sabeti PC, Reich DE, Higgins JM, Levine HZ, Richter DJ, Schaffner SF, et al. Detecting recent positive selection in the human genome from haplotype structure. Nature. 2002;419:832–837. doi: 10.1038/nature01140. [DOI] [PubMed] [Google Scholar]
- 22.Chang CC, Chow CC, Tellier LC, Vattikuti S, Purcell SM, Lee JJ. Second-generation PLINK: rising to the challenge of larger and richer datasets. Gigascience. 2015;4:7. doi: 10.1186/s13742-015-0047-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gandolfi B, Alhaddad H, Abdi M, Bach LH, Creighton EK, Davis BW, et al. Applications and efficiencies of the first cat 63K DNA array. Sci Rep. 2018;8:7024. doi: 10.1038/s41598-018-25438-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Shannon LM, Boyko RH, Castelhano M, Corey E, Hayward JJ, McLean C, et al. Genetic structure in village dogs reveals a Central Asian domestication origin. Proc Natl Acad Sci USA. 2015;112:13639–13644. doi: 10.1073/pnas.1516215112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Colli L, Milanesi M, Talenti A, Bertolini F, Chen M, Crisà A, et al. Genome-wide SNP profiling of worldwide goat populations reveals strong partitioning of diversity and highlights post-domestication migration routes. Genet Sel Evol. 2018;50:58. doi: 10.1186/s12711-018-0422-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Petersen JL, Mickelson JR, Rendahl AK, Valberg SJ, Andersson LS, Axelsson J, et al. Genome-wide analysis reveals selection for important traits in domestic horse breeds. PLoS Genet. 2013;9:e1003211. doi: 10.1371/journal.pgen.1003211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yang B, Cui L, Perez-Enciso M, Traspov A, Crooijmans RPMA, Zinovieva N, et al. Genome-wide SNP data unveils the globalization of domesticated pigs. Genet Sel Evol. 2017;49:71. doi: 10.1186/s12711-017-0345-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sempéré G, Moazami-Goudarzi K, Eggen A, Laloë D, Gautier M, Flori L. WIDDE: A Web-Interfaced next generation database for genetic diversity exploration, with a first application in cattle. BMC Genomics. 2015;16:940. doi: 10.1186/s12864-015-2181-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ciani E, Lasagna E, D’Andrea M, Alloggio I, Marroni F, Ceccobelli S, et al. Merino and Merino-derived sheep breeds: a genome-wide intercontinental study. Genet Sel Evol. 2015;47:64. doi: 10.1186/s12711-015-0139-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kijas JW, Lenstra JA, Hayes B, Boitard S, Neto LR, Cristobal MS, et al. Genome-wide analysis of the world’s sheep breeds reveals high levels of historic mixture and strong recent selection. PLoS Biol. 2012;10:e1001258. doi: 10.1371/journal.pbio.1001258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rochus CM, Tortereau F, Plisson-Petit F, Restoux G, Moreno-Romieux C, Tosser-Klopp G, et al. Revealing the selection history of adaptive loci using genome-wide scans for selection: an example from domestic sheep. BMC Genomics. 2018;19:71. doi: 10.1186/s12864-018-4447-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Pickering NK, Young EA, Kijas JW, Scobie DR, Mcewan JC. Genetic origin of Arapawa sheep and adaptation to a feral lifestyle. Proc Assoc Advmt Anim Breed Genet. 2011;20:451–454. [Google Scholar]
- 33.Young EA, Kijas JW, McCulloch R, Scobie DR, McRae KM, Pickering NK, et al. Arapawa: a novel New Zealand sheep breed of distinct origin. Proc New Zeal Soc Anim Prod. 2011;71:248–250. [Google Scholar]
- 34.Colli L, Milanesi M, Vajana E, Iamartino D, Bomba L, Puglisi F, et al. New insights on water buffalo genomic diversity and post-domestication migration routes from medium density SNP chip data. Front Genet. 2018;9:53. doi: 10.3389/fgene.2018.00053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Purfield DC, Berry DP, McParland S, Bradley DG. Runs of homozygosity and population history in cattle. BMC Genet. 2012;13:70. doi: 10.1186/1471-2156-13-70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ferenčaković M, Sölkner J, Curik I. Estimating autozygosity from high-throughput information: Effects of SNP density and genotyping errors. Genet Sel Evol. 2013;45:42. doi: 10.1186/1297-9686-45-42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lencz T, Lambert C, DeRosse P, Burdick KE, Morgan TV, Kane JM, et al. Runs of homozygosity reveal highly penetrant recessive loci in schizophrenia. Proc Natl Acad Sci USA. 2007;104:19942–19947. doi: 10.1073/pnas.0710021104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Turner DS. qqman: an R package for visualizing GWAS results using Q-Q and manhattan plots. J Open Source Softw. 2018;3:731. doi: 10.21105/joss.00731. [DOI] [Google Scholar]
- 39.Gorssen W, Meyermans R, Buys N, Janssens S. SNP genotypes reveal breed substructure, selection signatures and highly inbred regions in Piétrain pigs. Anim Genet. 2020;51:32–42. doi: 10.1111/age.12888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Fariello MI, Boitard S, Naya H, SanCristobal M, Servin B. Detecting signatures of selection through haplotype differentiation among hierarchically structured populations. Genetics. 2013;193:929–941. doi: 10.1534/genetics.112.147231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Montague MJ, Li G, Gandolfi B, Khan R, Aken BL, Searle SMJ, et al. Comparative analysis of the domestic cat genome reveals genetic signatures underlying feline biology and domestication. Proc Natl Acad Sci USA. 2014;111:17230–17235. doi: 10.1073/pnas.1410083111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wilkins AS, Wrangham RW, Tecumseh FW. The, “domestication syndrome” in mammals: A unified explanation based on neural crest cell behavior and genetics. Genetics. 2014;197:795–808. doi: 10.1534/genetics.114.165423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ruiz-Larrañaga O, Langa J, Rendo F, Manzano C, Iriondo M, Estonba A. Genomic selection signatures in sheep from the Western Pyrenees. Genet Sel Evol. 2018;50:9. doi: 10.1186/s12711-018-0378-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Matika O, Riggio V, Anselme-Moizan M, Law AS, Pong-Wong R, Archibald AL, et al. Genome-wide association reveals QTL for growth, bone and in vivo carcass traits as assessed by computed tomography in Scottish Blackface lambs. Genet Sel Evol. 2016;48:11. doi: 10.1186/s12711-016-0191-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Bertrand AR, Kadri NK, Flori L, Gautier M, Druet T. RZooRoH: An R package to characterize individual genomic autozygosity and identify homozygous-by-descent segments. Methods Ecol Evol. 2019;10:860–866. doi: 10.1111/2041-210X.13167. [DOI] [Google Scholar]
- 46.Druet T, Gautier M. A model-based approach to characterize individual inbreeding at both global and local genomic scales. Mol Ecol. 2017;26:5820–5841. doi: 10.1111/mec.14324. [DOI] [PubMed] [Google Scholar]
- 47.Bertolini F, Servin B, Talenti A, Rochat E, Kim ES, Oget C, et al. Signatures of selection and environmental adaptation across the goat genome post-domestication. Genet Sel Evol. 2018;50:57. doi: 10.1186/s12711-018-0421-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Petersen JL, Mickelson JR, Cothran EG, Andersson LS, Axelsson J, Bailey E, et al. Genetic diversity in the modern horse illustrated from genome-wide SNP data. PLoS One. 2013;8:e54997. doi: 10.1371/journal.pone.0054997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Gautier M, Laloë D, Moazami-Goudarzi K. Insights into the genetic history of French cattle from dense SNP data on 47 worldwide breeds. PLoS ONE. 2010;5(9):e13038. doi: 10.1371/journal.pone.0013038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Decker JE, McKay SD, Rolf MM, Kim JW, Molina Alcalá A, Sonstegard TS, et al. Worldwide patterns of ancestry, divergence, and admixture in domesticated cattle. PLoS Genet. 2014;10(3):e1004254. doi: 10.1371/journal.pgen.1004254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Flori L, Thevenon S, Dayo GK, Senou M, Sylla S, Berthier D, et al. Adaptive admixture in the West African bovine hybrid zone: Insight from the Borgou population. Mol Ecol. 2014;23(13):3241–3257. doi: 10.1111/mec.12816. [DOI] [PubMed] [Google Scholar]
- 52.Flori L, Gonzatti MI, Thevenon S, Chantal I, Pinto J, Berthier D, et al. A quasi-exclusive European ancestry in the Senepol tropical cattle breed highlights the importance of the slick locus in tropical adaptation. PLoS One. 2012;7(5):e36133. doi: 10.1371/journal.pone.0036133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Gao Y, Gautier M, Ding X, Zhang H, Wang Y, Wang X, et al. Species composition and environmental adaptation of indigenous Chinese cattle. Sci Rep. 2017;7(1):16196. doi: 10.1038/s41598-017-16438-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Gautier M, Flori L, Riebler A, Jaffrézic F, Laloë D, Gut I, et al. A whole genome Bayesian scan for adaptive genetic divergence in West African cattle. BMC Genomics. 2009;10:550. doi: 10.1186/1471-2164-10-550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Iso-Touru T, Tapio M, Vilkki J, Kiseleva T, Ammosov I, Ivanova Z, et al. Genetic diversity and genomic signatures of selection among cattle breeds from Siberia, eastern and northern Europe. Anim Genet. 2016;47(6):647–657. doi: 10.1111/age.12473. [DOI] [PubMed] [Google Scholar]
- 56.Matukumalli LK, Lawley CT, Schnabel RD, Taylor JF, Allan MF, Heaton MP, et al. Development and characterization of a high density SNP genotyping assay for cattle. PLoS One. 2009;4(4):e5350. doi: 10.1371/journal.pone.0005350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Flori L, Moazami-Goudarzi K, Alary V, Araba A, Boujenane I, Boushaba N, et al. A genomic map of climate adaptation in Mediterranean cattle breeds. Mol Ecol. 2019;28(5):1009–1029. doi: 10.1111/mec.15004. [DOI] [PubMed] [Google Scholar]
- 58.Illumina Agrigenomics. Available from: https://emea.illumina.com/areas-of-interest/agrigenomics.html?langsel=/fr/. Accessed 15 Jan 2020.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional file 1: Table S1. ROH island regions (bins of one Mb) for the studied cat populations: overview for all chromosomes. Within each cell, the genomic region(s) with an ROH island are given (in Mb).
Additional file 2: Table S2. ROH island regions (bins of one Mb) for the studied cat populations: details per chromosome. Within the Excel file, there is one tab for each chromosome with ROH island details per population. Cells are marked with an ‘x’ if an ROH island appeared in the specific region.
Additional file 3: Table S3. ROH island regions (bins of one Mb) for the studied cattle populations: overview for all chromosomes. Within each cell, the genomic region(s) with an ROH island are given (in Mb).
Additional file 4: Table S4. ROH island regions (bins of one Mb) for the studied cattle populations: details per chromosome. Within the Excel file, there is one tab for each chromosome with ROH island details per population. Cells are marked with an ‘x’ if an ROH island appeared in the specific region.
Additional file 5: Table S5. ROH island regions (bins of one Mb) for the studied dog populations: overview for all chromosomes. Within each cell, the genomic region(s) with an ROH island are given (in Mb).
Additional file 6: Table S6. ROH island regions (bins of one Mb) for the studied dog populations: details per chromosome. Within the Excel file, there is one tab for each chromosome with ROH island details per population. Cells are marked with an ‘x’ if an ROH island appeared in the specific region.
Additional file 7: Table S7. ROH island regions (bins of one Mb) for the studied goat populations: overview for all chromosomes. Within each cell, the genomic region(s) with an ROH island are given (in Mb).
Additional file 8: Table S8. ROH island regions (bins of one Mb) for the studied goat populations: details per chromosome. Within the Excel file, there is one tab for each chromosome with ROH island details per population. Cells are marked with an ‘x’ if an ROH island appeared in the specific region.
Additional file 9: Table S9. ROH island regions (bins of one Mb) for the studied horse populations: overview for all chromosomes. Within each cell, the genomic region(s) with an ROH island are given (in Mb).
Additional file 10: Table S10. ROH island regions (bins of one Mb) for the studied horse populations: details per chromosome. Within the Excel file, there is one tab for each chromosome with ROH island details per population. Cells are marked with an ‘x’ if an ROH island appeared in the specific region.
Additional file 11: Table S11. ROH island regions (bins of one Mb) for the studied pig populations: overview for all chromosomes. Within each cell, the genomic region(s) with an ROH island are given (in Mb).
Additional file 12: Table S12. ROH island regions (bins of one Mb) for the studied pig populations: details per chromosome. Within the Excel file, there is one tab for each chromosome with ROH island details per population. Cells are marked with an ‘x’ if an ROH island appeared in the specific region.
Additional file 13: Table S13. ROH island regions (bins of one Mb) for the studied sheep populations: overview for all chromosomes. Within each cell, the genomic region(s) with an ROH island are given (in Mb).
Additional file 14: Table S14. ROH island regions (bins of one Mb) for the studied sheep populations: details per chromosome. Within the Excel file, there is one tab for each chromosome with ROH island details per population. Cells are marked with an ‘x’ if an ROH island appeared in the specific region.
Additional file 15: Table S15. ROH island regions (bins of one Mb) for the studied water buffalo populations: overview for all chromosomes. Within each cell, the genomic region(s) with an ROH island are given (in Mb).
Additional file 16: Table S16. ROH island regions (bins of one Mb) for the studied water buffalo populations: details per chromosome. Within the Excel file, there is one tab for each chromosome with ROH island details per population. Cells are marked with an ‘x’ if an ROH island appeared in the specific region.
Data Availability Statement
The datasets analysed during the current study are available in the following repositories.
Cat
Gandolfi et al. [23]. Data available on: https://www.nature.com/articles/s41598-018-25438-0#Sec26.
Cattle
Sempéré et al. [28]. Data available on: http://widde.toulouse.inra.fr/widde/.
Batch selection of all populations with SNP data from Illumina Bovine SNP50v1, Illumina Bovine SNP50v2 and Illumina BovineHD. Only common markers (46,387) were selected from all chromosomes.
Dog
Shannon et al. [24]. Data available on: https://datadryad.org/stash/dataset/doi:10.5061/dryad.v9t5h.
Goat
Colli et al. [25] and data from Bertolini et al. [47]. Dryad Digital Repository. https://doi.org/10.5061/dryad.v8g21pt.
Horse
Petersen et al. [26] and data from Petersen et al. [48]. NAGPR Community Data Repository. https://www.animalgenome.org/repository/pub/UMN2013.0125/.
Pig
Yang et al. [27]. Data at the Dryad Digital Repository. https://doi.org/10.5061/dryad.30tk6.
Sheep
Sempéré et al. [28]. Data available on: http://widde.toulouse.inra.fr/widde/.
Batch selection of all populations with SNP data from Illumina OvineSNP50v1 and AgResearch OvineHD. Only common markers (42,439) were selected from all chromosomes.
Water buffalo
Colli et al. [34]. Data available at: https://datadryad.org/stash/dataset/doi:10.5061/dryad.h0cc7.